Dear editor,
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a novel coronavirus emerging first in December 2019 in the city of Wuhan and rapidly spreading across most China and even worldwide, causes Coronavirus Disease 2019 (COVID-19).1, 2 – 3 Most studies mainly focused on clinical features, epidemiological characteristics, CT scanning and RT-PCR assay findings.1 – 3 Quick diagnosis is most crucial in the most emergent period while detection of antibodies in blood of the potential patients is less supportive for the quick diagnosis and prompt treatment. Detection of the specific antibodies, typically IgG, will be very helpful for tracking the infected population after the disease is controlled, but not for the emergent time. Here, we read with great interest the Letter to the Editor of COVID-19 by Xiao et al.4 We are also interested in the specific antibodies against SARS-CoV-2.
Twenty-seven hospitalized patients with COVID-19 and 36 healthy volunteers were included to analyze the profile of specific antibodies against SARS-CoV-2. Two respective enzyme-linked immunosorbent assay kits (Zhuhai Livzon Diagnostics INC.) were adopted to test the levels of SARS-CoV-2 specific IgM and IgG in these 27 patients with COVID-19 from day 3 to 39 after the onset of COVID-19 dynamically.
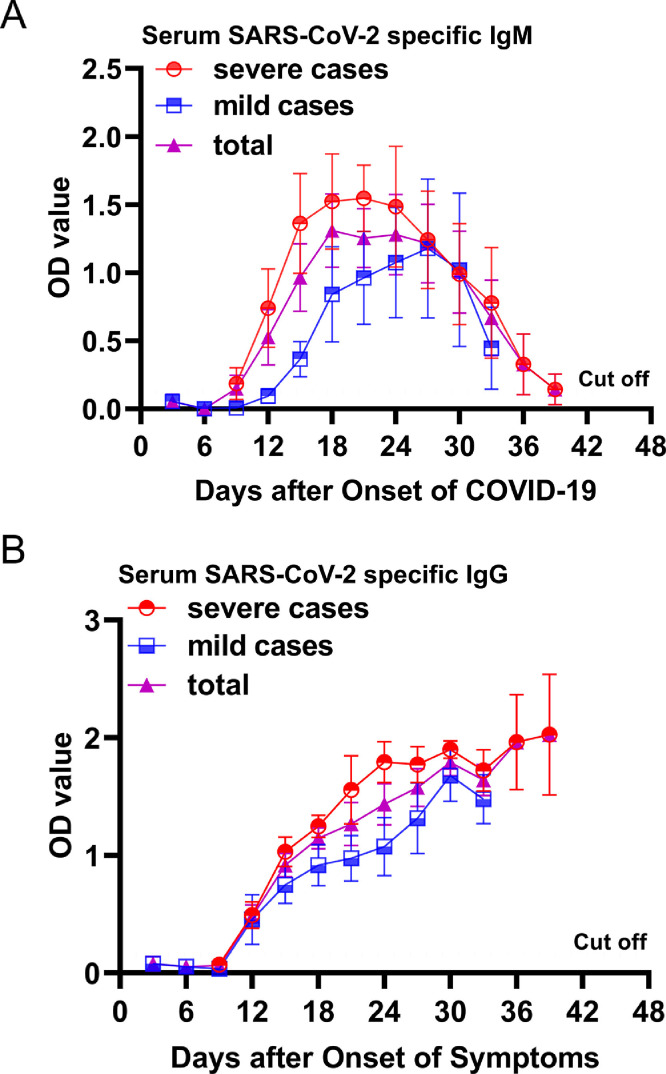
Of the 27 hospitalized patients with COVID-19, the median age was 62 years (interquartile range, 46–67; range, 29–87 years) and 14 were men. All of them produced SARS-CoV-2 specific IgM and IgG and detailed results were showed in Fig. 1 . In brief, at day 6 after the onset of COVID-19, all patients tested negative for SARS-CoV-2 specific IgM and IgG. At day 9 after the onset of COVID-19, the mean level of SARS-CoV-2 specific IgM increased to cut off value (OD=0.105), then increased rapidly and peaked at day 18. The SARS-CoV-2-specific IgM antibody persisted a high level for about 9 days, then rapidly decreased to 0.215 (∼2 × cut off value) at day 39 after the onset of COVID-19. At day 9 after the onset of COVID-19, the mean level of SARS-CoV-2 specific IgG was still lower than cut off value (OD=0.105), then rapidly increased within day 9 to 15 and slowly increased during day 15 to 39. Of the 27 hospitalized patients with COVID-19, 17 were severe cases. Comparing to the mild cases, the severe cases generally had an earlier SARS-CoV-2 specific IgM response, and higher SARS-CoV-2 specific IgM and IgG levels. All 36 healthy volunteers tested negative for SARS-CoV-2 specific IgM and IgG.
Fig. 1.
Changing Titers of SARS-CoV-2 Specific antibody. (A) Serum SARS-CoV-2 specific IgM levels were tested within day 3 to 39 after the onset of COVID-19 every 3 days using enzyme-linked immunosorbent assay kits. All severe patients were transferred from other hospital to Zhongnan Hospital in range of day 6 to 9 of the onset of COVID-19, so we could not collect blood for testing serum SARS-CoV-2 specific IgM levels at day 9 before the onset of COVID-19. All mild patients were discharged at day 33 after the onset of COVID-19, no blood was collected for testing serum SARS-CoV-2 specific IgM levels at day 33 after the onset of COVID-19. (B) Serum SARS-CoV-2 specific IgG levels were tested within day 3 to 39 after the onset of COVID-19 every 3 days using enzyme-linked immunosorbent assay kits. All severe patients were transferred from other hospital to Zhongnan Hospital in range of day 6 to 9 of the onset of COVID-19, so we could not collect blood for testing serum SARS-CoV-2 specific IgG levels at day 9 before the onset of COVID-19. All mild patients were discharged at day 33 after the onset of COVID-19, no blood was collected for testing serum SARS-CoV-2 specific IgG levels at day 33 after the onset of COVID-19.
In present study, dynamics changes of SARS-CoV-2 specific IgM and IgG levels were found in patients with COVID-19. It was first found that the time to cut off and peak values of mean level of SARS-CoV-2 specific IgM were day 9 and 18 after the onset of COVID-19, respectively, which was consistent with findings with regard to acute SARS-CoV infectious diseases, where the mean SARS-specific IgM titer was 0 at week 1, 1:120 at week 2, 1:320 at week 3, and 1:160 at week 4 (cut off value, 1:10).5 The SARS-CoV-2-specific IgM persisted a high level in patients with COVID-19 for about 9 days, then rapidly decreased to ∼2 × cut off value at day 39 after the onset of COVID-19. A previous study showed that the SARS-CoV-specific IgM also persisted a high level for about two weeks, then decreased to ∼2 × cut off value within next 6 weeks5, which indicating that the SARS-CoV-2 specific IgM remained measurable for a much shorter period than the SARS-CoV specific IgM. Therefore, SARS-CoV-2 specific IgM may be helpful in the early diagnosis of acute COVID-19.
At day 9 after the onset of illness, SARS-CoV-2 specific IgG was tested to be negative in patients with COVID-19, then rapidly increased and obviously higher than the cut off value at day 12 after the onset of COVID-19, which indicating the production time of SARS-CoV-2 specific IgG was in range of day 9 to 12 after the onset of COVID-19, and also consistence with these findings with regard to acute SARS-CoV infectious diseases, where mean SARS-CoV-specific IgG titer was 0 at week 1, 1:40 at week 2, 1:256 at week 3, 1:368 at week 4, 1:640 at week 8, and 1:640 at week 12 (cut off value, 1:10).5 We also found that SARS-CoV-2 specific IgG kept going up in patients with COVID-19 in range of day 9 to 39 after the onset of illness as well as SARS-CoV specific IgG. Therefore, the SARS-CoV-2 specific IgG may also be helpful in the diagnosis and in epidemiologic surveys of COVID-19. In addition, our study showed the severe cases generally had earlier IgM response and higher IgM and IgG levels against SARS-CoV-2 than the mild cases, which indicating that the humoral immunity to the SARS-CoV-2 was stronger in the severe cases than that in the mild cases. Supporting our finding, it was found that SARS-CoV patients with a more severe clinical course had earlier and higher antibody responses.6 , 7
In conclusion, the profile of specific antibodies to the SARS-COV-2 was similar to the common findings from acute viral infectious diseases such as SARS-CoV and hepatitis A. The profile of anti-SARS-CoV-2 may be conducive to the diagnosis as well as epidemiologic surveys of COVID-19. The presence of high titers of IgG antibody to SARS-CoV-2 in the patients with COVID-19 at the convalescent stage also suggests that passive immunization could be developed for the treatment of the severe COVID-19 patients.
Declaration of Competing Interest
The authors have nothing to disclose.
Acknowledgments
Acknowledgment
This study was supported in part by grants from the Emergency Science and Technology Project of Hubei Province, China (2020FCA008). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
Z.Z. and L.C. designed and performed research, analyzed data and prepared figures. Z.Z., L.C., Y.P., Q.D., G.Y., Y.L. and X.W. performed and analyzed research. Y.L. and X.W. supervised the study. Z.Z., L.C. and Y.P. provided patient samples. Z.Z. wrote the paper and L.C. and X.W. edited the manuscript. All authors approved the final version of the manuscript.
Ethics
All the patients provided written informed consent. All study procedures were performed in accordance with the ethical standards of the Institutional Ethics Review Committee.
References
- 1.Chen L. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg Microbes Infect. 2020;9:313–319. doi: 10.1080/22221751.2020.1725399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lan L. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao A., Gao C., Zhang S. Profile of specific antibodies to SARS-CoV-2: the first report. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G., Chen X., Xu A. Profile of specific antibodies to the SARS-associated coronavirus. N Engl J Med. 2003;349:508–509. doi: 10.1056/NEJM200307313490520. [DOI] [PubMed] [Google Scholar]
- 6.Ho M.S. Neutralizing antibody response and sars severity. Emerg Infect Dis. 2005;11:1730–1737. doi: 10.3201/eid1111.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J., Subbarao K. The immunobiology of SARS*. Annu Rev Immunol. 2007;25:443–472. doi: 10.1146/annurev.immunol.25.022106.141706. [DOI] [PubMed] [Google Scholar]