Abstract
Background & Aims
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), which has been characterized by fever, respiratory, and gastrointestinal symptoms as well as shedding of virus RNA into feces. We performed a systematic review and meta-analysis of published gastrointestinal symptoms and detection of virus in stool and also summarized data from a cohort of patients with COVID-19 in Hong Kong.
Methods
We collected data from the cohort of patients with COVID-19 in Hong Kong (N = 59; diagnosis from February 2 through February 29, 2020),and searched PubMed, Embase, Cochrane, and 3 Chinese databases through March 11, 2020, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We analyzed pooled data on the prevalence of overall and individual gastrointestinal symptoms (loss of appetite, nausea, vomiting, diarrhea, and abdominal pain or discomfort) using a random effects model.
Results
Among the 59 patients with COVID-19 in Hong Kong, 15 patients (25.4%) had gastrointestinal symptoms, and 9 patients (15.3%) had stool that tested positive for virus RNA. Stool viral RNA was detected in 38.5% and 8.7% among those with and without diarrhea, respectively (P = .02). The median fecal viral load was 5.1 log10 copies per milliliter in patients with diarrhea vs 3.9 log10 copies per milliliter in patients without diarrhea (P = .06). In a meta-analysis of 60 studies comprising 4243 patients, the pooled prevalence of all gastrointestinal symptoms was 17.6% (95% confidence interval [CI], 12.3–24.5); 11.8% of patients with nonsevere COVID-19 had gastrointestinal symptoms (95% CI, 4.1–29.1), and 17.1% of patients with severe COVID-19 had gastrointestinal symptoms (95% CI, 6.9–36.7). In the meta-analysis, the pooled prevalence of stool samples that were positive for virus RNA was 48.1% (95% CI, 38.3–57.9); of these samples, 70.3% of those collected after loss of virus from respiratory specimens tested positive for the virus (95% CI, 49.6–85.1).
Conclusions
In an analysis of data from the Hong Kong cohort of patients with COVID-19 and a meta-analysis of findings from publications, we found that 17.6% of patients with COVID-19 had gastrointestinal symptoms. Virus RNA was detected in stool samples from 48.1% patients, even in stool collected after respiratory samples had negative test results. Health care workers should therefore exercise caution in collecting fecal samples or performing endoscopic procedures in patients with COVID-19, even during patient recovery.
Keywords: Fecal-to-Oral Transmission, PRISMA, SARS, Viral Persistence
Abbreviations used in this paper: ACE2, angiotensin-converting enzyme 2; CI, confidence interval; CoV, coronavirus; cpm, copies per milliliter; IQR, interquartile range; MERS, Middle East respiratory syndrome; SARS, severe acute respiratory syndrome
Graphical abstract
See Covering the Cover synopsis on page 4.
What You Need to Know.
Background and Context
Infection with SARS-Co-2 virus, which causes COVID-19, results in respiratory as well as gastrointestinal symptoms; virus RNA has been detected in fecal samples.
New Findings
A meta-analysis of publications found that gastrointestinal symptoms have been reported in 17.6% of patients with COVID-19. Stool samples from 48.1% of patients tested positive for virus RNA; stool samples from 70.3% of these patients tested positive for virus RNA even after respiratory specimens tested negative.
Limitations
This study analyzed mostly data from reported cases from China; systematic data collection was lacking for most studies.
Impact
Gastrointestinal symptoms occur in almost 18% of patients with COVID-19. Virus RNA can be detected in fecal samples—even those collected after respiratory samples test negative.
In December 2019, a cluster of an unidentified form of viral pneumonia cases was first reported in Wuhan, China, and swiftly spread to the rest of China and then the rest of the world within a very short period. The virus was subsequently identified to be a novel coronavirus (CoV) that belongs to the beta-coronavirus lineage B, with more than 80% resemblance to the previously reported severe acute respiratory syndrome (SARS) CoV in 2003. Through March 16, 2020, more than 150,000 cases had been reported from more than 150 countries or regions across the globe, with more than 81,000 cases in China, 21,000 cases in Italy, 12,000 cases in Iran, and 8100 cases in Korea. Although the number of new cases seems to be declining in China, the numbers of cases are rising in an exponential manner in Europe, North America, and the Middle East. The death toll has already reached more than 5700 globally, with more than 3000 from the Hubei Province of China, where Wuhan city is located. In response to the emerging threat posed by this virus, the World Health Organization (WHO) declared a Public Health Emergency of International Concern on January 30, 2020 and further labeled it as a pandemic on March 11, 2020.
The disease was named coronavirus disease 2019 (COVID-19) by the World Health Organization, and the virus was termed SARS-CoV-2 by the International Committee on Taxonomy of Viruses. SARS-CoV-2 is a positive-sense, single-stranded RNA virus and has strong genetic similarity to bat coronaviruses, but the intermediate reservoir has yet to be identified.1 Together with the other 2 previously identified coronaviruses, SARS-CoV and Middle East respiratory syndrome (MERS) CoV, which cause SARS and MERS, this is the third coronavirus identified to cause severe viral pneumonia in humans (Table 1 ). Similar to the other 2 coronaviruses, SARS-CoV-2 has very high infectivity because no one has immunity, resulting in an ongoing global health crisis.
Table 1.
Comparison of SARS, MERS, and COVID-19
| Characteristics | SARS | MERS | COVID-19 |
|---|---|---|---|
| Genus |
Betacoronavirus B lineage |
Betacoronavirus C lineage |
Betacoronavirus B lineage |
| Virus | SARS-CoV | MERS-CoV | SAR-CoV-2 |
| Presumed reservoir host | Asian civet cat (Paguma larvata) | Dromedary camel | Bat (?) |
| First reported | November 2002 in China | 2012 in Saudi Arabia | December 2019 in China |
| Incubation period, d | Median: 4–5 Maximum: 14 |
Median: 5–7 Range: 2–14 |
Mean: 6.4 Range (2.5th–97.5th percentile)2: 2.1–11.1 |
| Mode of transmission | Human to human Hospital (Direct mucous membrane contact with respiratory droplets and/or through exposure to fomites) |
Human to human Hospital Zoonotic |
Human to human Hospital |
| Reproductive number (R0) | 2–4 | 3.9 (Choi et al3) (range: 2–5) |
Average: 3.3 (Liu et al4) Median: 2.8 |
| Countries and regions affected | 29 | 27 | >110 |
| Number of cases | 8096 | 2494 | >140,000 |
| Mechanical ventilation rate, % | — | 50–89 | — |
| Case fatality ratio, % | 9.6 | 34.4 | 2.4 |
| Risk factors for severe disease | Age >65 years Comorbidities (eg, DM, malignancy, chronic lung/kidney/liver/heart disease) |
Age High SOFA score High D-dimer levels |
|
| Stool RT-PCR positive rate, % | Days 6–14 from illness onset: 86–100 Days 21–23: 43 |
15 Up to 24 days |
52.7 Up to ≥33 days |
DM, diabetes mellitus; SOFA, Sequential Organ Failure Assessment; RT-PCR, reverse transcription polymerase chain reaction.
Based on existing observations, the case fatality rate of COVID-19 is lower than that of SARS and MERS; it is estimated to be about 1%–2% but is much higher in older patients. In addition to age, a high Sequential Organ Failure Assessment score and D-dimer level >1 μg/L on admission are associated with poor prognosis.5 Apart from respiratory symptoms, gastrointestinal manifestations are common in patients with SARS, MERS, and the latest COVID-19. We previously reported the high prevalence of enteric symptoms in patients with SARS and demonstrated acute viral replication in the small intestinal mucosa of patients with SARS.6 It is estimated that 16%–73% of patients had diarrhea during the course of SARS illness. Fecal shedding of SARS-CoV RNA was found in 86%–100% of patients during days 6–14 of illness and could persist for >30 days of illness.7 , 8 It was subsequently found that SARS-CoV binds to angiotensin-converting enzyme 2 (ACE2) receptors of the intestinal and respiratory tracts, which is the entry point for the virus to the epithelial cells.9 Similarly, up to a quarter of patients with MERS also reported gastrointestinal symptoms such as diarrhea or abdominal pain.10 Again, MERS-CoV could be detected in 15% of stool samples and could persist for up to 24 days after diagnosis.11 It was shown that the human intestinal tract, including primary intestinal epithelial cells, small intestine explants, and intestinal organoids, is highly susceptible to MERS-CoV.12
Enteric manifestations of SAR-CoV-2 not only pose important diagnostic challenge to clinicians when facing patients with mild COVID-19 symptoms on initial presentation but also signify potential fecal transmission of this virus. With the increasing number of reported cases of COVID-19, there is a pressing need to systemically summarize the enteric manifestations of COVID-19 and the temporal pattern of fecal shedding of the SARS-CoV-2 virus, particularly to gastroenterologists and endoscopists who may not be familiar with this disease.
This study aimed to summarize the existing data on gastrointestinal manifestations of COVID-19 and the temporal pattern of fecal shedding of SARS-CoV-2 based on published data as well as the data from our recent cohort of COVID-19 patients in Hong Kong.
Methods
Coronavirus Disease 2019 Cohort From Hong Kong
We included a cohort of 59 patients with virologically confirmed COVID-19 diagnosed between February 2 and 29, 2020 in Hong Kong. The prevalence of gastrointestinal symptoms (including nausea/vomiting, diarrhea, and abdominal pain/discomfort) and viral load in stool collected on admission was reported.
Study Selection
Three databases, PubMed, Embase, and Cochrane Library, were searched following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines13 from December 1, 2019, through March 11, 2020. Keywords were 2019-nCoV-2, coronavirus, COVID-19, SARS-CoV-2, or novel coronavirus. The search details are listed in the Supplementary Material. Additional related articles were retrieved from 3 Chinese electronic databases (Chongqing VIP [CQVIP], Wanfang Data, and Chinese National Knowledge Infrastructure). Potential studies were retrieved after title/abstract screening by 1 investigator (KSC). All articles were imported to Endnote X9.2 (Thompson and Reuters, Philadelphia, PA), and duplicates were removed.
Selection Criteria
Two authors (KSC and IFH) determined the eligibility of studies independently, and dissonance was resolved by a third author (WKL). The inclusion criteria included (1) study population: patients with COVID-19 (including adult or pediatric patients and pregnant women); (2) study design: case reports/case series, prospective/retrospective cohort studies, case-control studies, and randomized controlled trials. There was no language restriction. The exclusion criteria were (1) patients without virologic proof of SARS-CoV-2 infection; (2) asymptomatic patients infected with SARS-CoV-2; (3) studies that did not report gastrointestinal symptoms; and (4) review articles, meta-analyses, editorials, and other forms (eg, commentaries).
If all gastrointestinal symptoms were not reported and the number of events of any individual gastrointestinal symptom was less than 1, this was considered not available and was excluded from the meta-analysis of all gastrointestinal symptoms. However, the study was still included in the meta-analysis of individual gastrointestinal symptom if the proportion of patients with that symptom was reported. Two additional studies14 , 15 that did not report on gastrointestinal symptoms but provided data on stool viral RNA was included in the meta-analysis of stool viral RNA only.
Data Extraction
For eligible articles, we recorded items including first authors, site of study, inclusion/exclusion criteria, sample size, age, sex, disease severity, any gastrointestinal symptoms (anorexia, nausea/vomiting, diarrhea, or abdominal pain), and other symptoms (fever, cough, expectoration, and dyspnea). Severe disease was defined according to the American Thoracic Society and Infectious Disease Society of America guidelines for community-acquired pneumonia,16 need for intensive care unit admission, and death.
Data Analysis
All statistical analyses were performed using R, version 3.2.3, statistical software (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables are expressed as median (interquartile range [IQR]) or mean ± standard deviation. The prevalence of gastrointestinal symptoms is expressed as proportion and 95% confidence interval (CI) using the random effects model and is presented as a forest plot. We used the Cochran Q test to detect heterogeneity among studies, with a P value of <.10 indicating significant heterogeneity. We calculated the I 2 statistic to measure the proportion of total variation in study estimates attributed to heterogeneity. I 2 values of <25%, 25%–75%, and >75% indicate low, moderate, and high heterogeneity, respectively.17
Subgroup analysis was performed according to whether studies were from China or other countries, in or outside of the Hubei province, disease severity, and patient group (adults, pediatric patients, and pregnant women).
Results
Corona Virus Disease 2019 Cohort in Hong Kong
A total of 59 patients with confirmed COVID-19 in Hong Kong were recruited. The median age was 58.5 years (IQR, 43.5–68.0; range, 22–96 years) with 27 (45.8%) men. Fever was present in 56 (94.9%), cough in 22 (37.3%), dyspnea in 4 (6.8%) patients. Thirty-six (61.0%) patients did not have respiratory symptoms of cough or dyspnea on presentation. Among 15 (25.4%) patients who had gastrointestinal symptoms (vomiting, 1 [1.7%]; diarrhea, 13 [22.0%]; and abdominal pain/discomfort, 7 [11.9%]), all had fever, but 8 (53.5%) did not have cough or dyspnea.
On presentation, stool viral RNA test results were positive in 9 (15.3%) patients, and the median viral load was 4.7 log10 copies per milliliter (cpm) (range, 3.4–7.6 log10cpm). The proportion of patients with detectable stool viral RNA was higher among those with diarrhea than those without diarrhea (38.5% vs 8.7%; P = .019). There was also a trend for higher stool viral load in patients with diarrhea (median, 5.1 [IQR, 4.8–5.6] vs 3.9 [IQR, 3.5–4.4] log10cpm; P = 0.06). Of the 44 patients without gastrointestinal symptoms, 4 (9.1%) had positive stool viral RNA.
Study Characteristics of Meta-analysis
Figure 1 depicts the study selection process. Of the 2034 studies identified, 69 were included in the meta-analysis (60 studies with data on all gastrointestinal symptoms and 11 on stool viral load).
Figure 1.
Study selection flow diagram. If all gastrointestinal symptoms were not reported and the number of events of any individual gastrointestinal symptom was >1, it was regarded as not available and was excluded from the meta-analysis of all gastrointestinal symptoms. However, the study was still included in the meta-analysis of individual gastrointestinal symptom if the proportion of patients with that symptom was reported.
The characteristics of the included studies are shown in Table 2 , including the hospital admission period, places in which the patients were recruited, sample size, age, sex, disease severity, nongastrointestinal symptoms (fever and respiratory symptoms) on presentation, and gastrointestinal symptoms (anorexia, nausea/vomiting, diarrhea, and abdominal pain/discomfort). The median age of patients was 45.1 years (IQR, 41.0–54.8), and 57.3% were male. Among studies that reported disease severity, severe disease accounted for 1.3%–62.3%.
Table 2.
Characteristics of the Studies Included for Meta-analysis:
| Study | Study date | Study location | No. | Age, y (unless otherwise noted) | Male, n/total (%) or n (%) | Severe disease,a n (%) |
Fever, n/total (%) or n (%) | Respiratory symptoms, n/total (%) or n (%) | All GI symptoms,b n (%) | Anorexia, n/total (%) or n (%) | Nausea/vomiting, n/total (%) or n (%) | Diarrhea, n/total (%) or n (%) | Abdominal pain/discomfort, n/total (%) or n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| China | |||||||||||||
| Guan et al18 | December 11, 2019–29 January 2020 | 552 hospitals in China | 1099 | Median: 47 IQR 35–58 | 637/1096 (58.1) | 173 (15.7) | 975/1099 (88.7) | C: 745 (67.8) E: 370 (33.7) D: 205 (18.7) |
≥55 (5.0) | NA | 55 (5.0) | 42 (3.8) | NA |
| Cheng et al19 | Up to February 19 | Henan | 1079 | Mean: 46 Range: 3 mo–94 y |
573 (53.2) | 72 (5.7) | 553/605 (91.4) | C: 110/605 (18.2) E: 19/605 (3.1) |
21/605 (3.5) | NA | NA | NA | NA |
| Fang et al20 | January 27–February 14, 2020 | Hubei | 305 | Median: 57 | 146 (47.9) | 46 (15.1) | 163/201 (81.1) | C: 79/201 (39.3) | 159/201 (79.1) | 101/201 (50.2) | N: 59/201(29.4) V: 32/201(15.9) |
66/295 (22.4) | 12/201 (6.0) |
| Zhou et al5 | December 29, 2020–January 31, 2020 | Hubei | 191 | Median: 56 IQR: 46–67 |
119 (62.3) | 119 (62.3) | 180 (94.2) | C: 151 (79.1) E: 44 (23.0) |
≥9 (4.7) | NA | 7 (3.7) | 9 (4.7) | NA |
| Yang et al21 | January 17–February 10, 2020 | Zhejiang | 149 | Mean ± SD: 45.1 ± 13.4 | 81 (54.4) | 2 (1.3) | 114 (76.5) | C: 87 (58.4) E: 48 (32.2) D: 2 (1.3) |
≥11 (7.4) | NA | 2 (1.3) | 11 (7.4) | NA |
| Zhang et al22 | January 16–February 3, 2020 | Hubei | 140 | Median: 57 IQR: 25–87 | 71 (50.7) | 58 (41.4) | 110/120 (91.7) | C: 90/120 (75.0) D: 44/120 (36.7) |
55/139 (39.6) | 17/139 (12.2) | N: 24/139 (17.3) V: 7/139 (5.0) |
18/139 (12.9) | 8/139 (12.9) |
| Wang et al23 | January 1–28, 2020 | Hubei | 138 | Median: 56 IQR: 42–68 |
75 (54.3) | ICU: 36 (26.1) | 136 (98.6) | C: 82 (59.4) E: 37 (26.8) D: 43 (31.2) |
≥14 (10.1) | 55 (9.9) | N: 14 (10.1) V: 5 (3.6) |
14 (10.1) | 3 (2.2) |
| Liu et al24 | December 30, 2019–January 24, 2020 | Hubei | 137 | Median: 57 Range: 20–83 | 61 (44.5) | 34 (24.8) | 112 (81.8) | C: 66 (48.2) E:6 (4.4) D: 26 (19.0) |
11 (8.0) | NA | NA | 11 (8.0) | NA |
| Peng et al25 | January 20, 2020–February 15, 2020 | Hubei | 112 | Median: 67 IQR: 55–67 |
53 (47.3) | ICU: 16 (14.3) | 101 (90.2) | C: 76 (67.9) D: 13 (11.6) |
15 (13.4) | NA | NA | 15 (13.4) | NA |
| Zhao et al26 | NA | Hunan | 101 | Mean: 44.4 Range: 17–75 |
56 (55.4) | 14 (13.9) | 79 (78.2) | C: 63 (62.4) D: 1(1.0) |
≥3 (3.0) | NA | 2 (2.0) | 3 (3.0) | NA |
| Chen et al27 | January 1–20, 2020 | Hubei | 99 | Mean ± SD: 55.5 ± 13.1 Range: 21–82 |
67 (67.7) | 17 (17.2) | 82 (82.8) | C: 81 (81.8) D: 31 (31.3) |
≥2 (2.0) | 1 (1.0) | 2 (2.0) | NA | NA |
| Xu et al28 | January 23–February 4, 2020 | Guangdong | 90 | Median: 50 Range: 18–86 | 39 (43.3) | NA | 70 (77.8) | C: 57 (63.3) E: 11 (12.2) |
≥5 (5.6) | NA | N: 5 (5.6) V: 2 (2.2) |
5 (5.6) | NA |
| Li et al29 | January–February 2020 | Chongqing | 83 | Mean ± SD: 45 ± 12.3 | 44 (53.0) | 25 (30.1) | 7 2 (86.7) | C: 65 (78.3) E: 15 (18.1) D: 9 (10.8) |
7 (8.4) | NA | NA | 7 (8.4) | |
| Shi et al30 | December 20, 2019–January 23, 2020 | Hubei | 81 | Mean ± SD: 49.5 ± 11.0 | 42 (51.9) | NA | 59 (72.8) | C: 48 (59.2) E: 15 (18.5) D: 34 (42.0) |
≥4 (4.9) | 1 (1.2) | V: 4 (4.9) | 3 (3.7) | NA |
| Wu et al31 | January 22–February 14, 2020 | Jiangsu | 80 | Mean ± SD: 46.1 ± 15.4 | 39 (48.8) | 3 (3.8) | 63 (78.8) | C: 51 (63.8) D: 30 (37.5) |
≥1 (1.3) | NA | 1 (1.3) | 1 (1.3) | NA |
| Wu et al32 | January–February 2020 | Chongqing | 80 | Mean ± SD: 44 ± 11 | 42 (52.5) | NA | 61 (76.3) | C: 58 (72.5) E: 11 (13.8) D: 7 (8.8) |
7 (8.8) | NA | NA | 7 (8.8) | |
| Fang et al33 | January 22–February 18, 2020 | Anhui | 79 | Mean ± SD: 45.1 ± 16.6 | 45 (60.0) | 24 (30.4) | 67 (84.8) | C: 45 (57.0) E: 10 (12.7) D: 9 (11.4) |
≥5 (6.3) | 5 (6.3) | NA | 4 (5.1) | NA |
| Xiao et al34 | February 1–14, 2020 | Guangdong | 73 | 43 Range: 10 mo–78 y |
41 (56.2) | ICU: 4 (5.5) | NA | 53 (72.6) | 26 (35.6) | NA | NA | 26 (35.6) | NA |
| Xu et al35 | January 10–26, 2020 | Zhejiang | 62 | Median: 41 IQR: 32–52 |
35 (56.5) | ICU: 1 (1.6) | 48 (77.4) | C: 50 (80.6) E: 35 (56.5) |
3 (4.8) | NA | NA | 3 (4.8) | NA |
| Zhou et al36 | January 16–30, 2020 | Hubei | 62 | Mean ± SD: 52.8 ± 12.2 Range: 30–70 |
39 (62.9) | NA | 54 | C: 28 D: 15 |
9 (14.) | NA | NA | 9 (14.) | |
| Yang et al37 | December 24, 2019–January 26, 2020 | Hubei | 52 | Mean ± SD: 59.7 ± 13.3 | 35 (67.3) | ICU: 52 (100) | 51 (98.1) | C: 40 (46.9) D: 33 (63.5) |
2 (3.8) | NA | 2 (3.8) | NA | NA |
| Xu et al38 | January–February 2020 | Beijing and Hebei | 50 | Mean ± SD: 43.9 ± 16.8 Range: 3–85 |
29 (58.0) | 13 (26) | 43 (86.0) | C:20 (40.0) E: 7 (14.0) D: 4 (8.0) |
1 (2.0) | NA | NA | NA | NA |
| Xiong et al39 | January 11–February 5, 2020 | Hubei | 42 | Mean ± SD: 49.5 ± 14.1 Range: 26–75 |
25 (59.5) | NA | 36 (85.7) | C: 27 (64.3) D: 8 (19.0) |
10 (23.8) | NA | NA | 10 (23.8) | NA |
| Huang et al40 | December 16, 2019–January 2, 2020 | Hubei | 41 | Median: 49 IQR: 41–58 |
30 (73.2) | ICU: 13 (31.7) | 40 (97.5) | C: 31 (75.6) E: 11/39 (28.2) D: 22/40 (55.0) |
1/38 (2.6) | NA | NA | 1/38 (2.6) | NA |
| Wu et al41 | January 19–25, 2020 | Tianjin | 40 | Mean: 45 Range: 10–76 |
13 (32.5) | 17 (42.5) | 38 (95.0) | C: 14 (35.0) E: 3 (7.5) D: 2 (5.0) |
≥6 (15.0) | NA | 3 (7.5) | 6 (15.0) | 3 (7.5) |
| Huang et al42 | December 21, 2019–January 8, 2020 | Hubei | 34 | Mean ± SD: 56.2 ± 17.1 Range: 26–88 |
14 (41.2) | 8 (23.5) | 32 (94.1) | C: 17 (50.0) E: 8 (23.5) D: 5 (14.7) |
5 (14.7) | NA | NA | 5 (14.7) | NA |
| Li et al43 | January–February 2020 | Hubei | 31 | Mean ± SD: 54 ± 13 | 18 (58.1) | 11 (35.5) | 25 (80.6%) | C: 25 (80.6) E: 16 (51.6) D: 10 (32.3) |
≥13 (41.9) | 13 (41.9) | 5 (16.1) | 3 (9.7) | NA |
| Liu et al44 | January 11–February 3, 2020 | Hubei | 30 | Mean ± SD: 35 ± 8 Range: 21–59 |
10 (33.3) | 4 (13.3) | 23 (76.7) | C: 25 (83.3) D: 14 (46.7) |
9 (30) | NA | N/V/D: 9 (30.0) | NA | |
| Pan et al45 | January 12–February 6, 2020 | Hubei | 21 | Mean ± SD: 40 ± 9 Range: 25–63 |
6 (28.6) | 0 | 18 (85.7) | C: 12 (57.1) E: 6 (28.6) |
9 (42.9) | 9 (42.9) | NA | NA | NA |
| Zou et al46 | January 7–26, 2020 | Guangdong | 18 | Median: 59 Range: 26–76 | 9 (50.0) | ICU: 3 (16.7) | 10 (55.6) | C: 10 (55.6) D: 3 (16.7) |
3 (16.7) | 1 (5.6) | N: 1 (5.6) | 1 (5.6) | 0 |
| Wang et al47 | January 21–February 5, 2020 | Henan | 18 | Median: 39 IQR: 29–55 |
10 (55.6) | ICU: 2 (11.1) | 17 (94.4) | C:10 (55.6) D: 4 (22.2) |
3 (16.7) | NA | 1 (5.6) | 3 (16.7) | NA |
| Zhang et al48 | January 27–February 10, 2020 | Zhejiang | 14 | Median: 41 IQR: 18–87 |
7 (50.0) | NA | 13 (92.9) | C: 10 (71.4) | NA | NA | 0 | 0 | NA |
| Chang et al49 | January 16–29, 2020 | Beijing | 13 | Median: 34 IQR: 34–78 |
10 (76.9) | 0 | 12 (92.3) | C: 6 (46.2) E: 2 (15.4) |
1 (7.7) | NA | NA | 1 (7.7.) | NA |
| Liu et al50 | Up to January 21, 2020 | Shenzen | 12 | Range: 10–72 | 8 (66.7) | 6 (50.0) | 10 (83.3) | C: 11 (91.7) | ≥3 (25.0) | NA | 2 (16.7) | 2 (16.7) | NA |
| Huang et al51 | January 21–February 1, 2020 | Jiangsu | 11 | NA | 4 (36.4) | NA | 9 (90.0) | C: 8 (80.0) | 1 (10.0) | NA | NA | 1 (10.0) | NA |
| Shen et al52 | February 2020 | Liaoning | 10 | Range: 33–85 | 6 (60) | 2 (20.0) | 9 (90) | C: 9 (90) | 4 (40.0) | NA | NA | 4 (40.0) | NA |
| Zhang et al53 | January 18–February 3, 2020 | Beijing | 9 | Median: 36 Range: 15–49 |
5 (55.6) | NA | 8 (88.9) | C: 5 (55.6) E: 1 (11.1) |
1 (11.1) | NA | NA | 1 (11.1) | NA |
| An et al54 | January 17–24, 2020 | Hubei | 9 | Median: 35.8 IQR: 28–45 | 4 (44.4) | 0 | After admission:5 (55.6) | After admission: 5 (55.6) |
9 (100) | 6 (66.7) | 1 (11.1) | 1 (11.1) | 0 |
| Qiu et al55 | February 3, 2020 | Henan | 8 | Range: 4–53 | 4 (50.0) | 0 | 5 (62.5) | C: 3 (37.5) | 1 (12.5) | NA | NA | 1 (12.5) | NA |
| Chan et al56 | January 10, 2020 | Shenzhen | 6 | Range: 36–66y | 3 (50) | 0 | 5 (83.3) | C: 4 (66.7) E: 1 (16.7) |
2 (33.3) | NA | NA | 2 (33.3) | NA |
| Ren et al57 | December 18–29, 2020 | Hubei | 5 | Range: 41–65 | 3 (60.0) | 4 (80.0) | 5 (100) | C: 5 (100) E: 1 (20.0) D: 4 (80.0) |
NA | NA | NA | 0 | NA |
| Wang et al58 | January 21–24, 2020 | Shanghai | 4 | Range: 19–63 | 3 (75.0) | 0 | 4 (100) | C: 3 (75.0) | NA | NA | NA | 0 | NA |
| Yu et al59 | January 20–23, 2020 | Shanghai | 4 | Range: 65–88 | 2 (50.0) | 1 (25.0) | 4 (100) | C: 1 (25.0) | 1 (25.0) | 1 (25.0) | NA | NA | NA |
| Yang et al60 | January 23–25, 2020 | Guandong | 3 | Range: 25–62 | 2 (66.7) | 0 | 2 (66.7) | C: 2 (66.7) E: 1 (33.3) |
2 (66.7) | 0 | 0 | 2 (66.7) | 0 |
| Huang et al61 | NA | Taiwan | 2 | Range: 73–74 | 0 | 0 | 2 (100) | C: 1 (50.0) | 2 (100) | 2 (100) | NA | 0 | 0 |
| Cheng et al62 | January 20, 2020 | Taiwan | 1 | 55 | 0 | 0 | 1 (100) | C: 1 (100) D: 1 (100) |
1 (100) | NA | NA | NA | 1 (100) |
| Li et al63 | January 24, 2020 | Sichuan | 1 | 33 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 1 (100) | NA | NA | NA |
| Han et al64 | January 21, 2020 | Gansu | 1 | 47 | 1 (100) | 1 (100) | 1 (100) | C: 1 (100) | 1 (100) | NA | N: 1 (100) | NA | NA |
| Song et al65 | January 29, 2020 | Shandong | 1 | 22 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | NA | NA | 1 (100) | NA |
| Zhang et al66 | January 18, 2020 | Hubei | 1 | 57 | 1 (100) | 0 | 1 (100) | C: 1 (100) D: 1 (100) |
1 (100) | NA | NA | 1 (100) | NA |
| Wu et al67 | February 8, 2020 | Hubei | 1 | 48 | 1 (100) | 0 | 1 (100) | C: 1 (100) | NA | NA | 0 | NA | NA |
| Lin et al68 | January 25, 2020 | Gansu | 1 | 61 | 1 (100) | 0 | 0 | D: 1 (100) | NA | NA | NA | 0 | NA |
| Outside of China | |||||||||||||
| Kong et al69 | Up to February 14, 2020 | South Korea | 28 | Mean: 42.6 IQR: 20–73 |
15 (53.6) | NA | 9 (32.1) | Cough: 5 (17.9) | 2/18 (11.1) | NA | NA | 2/18 (11.1) (days 3 and 4) |
NA |
| Young et al70 | January 23– February 3, 2020 |
Singapore | 18 | Median: 41 Range: 31–73 | 9 (50.0) | 3 (16.7) | 13 (72.2) | C 15 (83.3) D: 2 (94.4) |
3 (16.7) | NA | NA | 3 (16.7) | NA |
| Kim et al71 | January 10–19, 2020 | South Korea | 2 | Range: 35–55 | 1 (50) | 1 (50.0) | 2 (100) | 2 (100) | 2 (50.0) | 0 | 0 | 2 (100) | 0 |
| Lillie et al72 | January 30–3,1 2020 | United Kingdom | 2 | Range: 23–50 | 1 (50.0) | 0 | 2 (100) | C: 2 (100) | 1 (50.0) | NA | NA | NA | NA |
| Phan et al73 | January 22, 2020 | Vietnam | 2 | Range: 27–65 | 2 (100) | 0 | 2 (100) | C: 1 (100) | 1 (50.0) | NA | 1 (50.0) | 1 (50.0) | NA |
| Yan et al74 | February 9–13 2020 | Singapore | 2 | Both 57 | 1 (50.0) | NA | 2 (100) | C: 2 (100) D: 1 (50.0) |
1 (50.0) | NA | NA | 1 (50.0) | NA |
| Holshue et al75 | January 20, 2020 | United States | 1 | 35 | 1 (100) | 1 (100) | 1 (100) | C: 1 (100) | 1 (100) | NA | 1 (100) | 1 (100) | 1 (100) |
| Pediatric patients | |||||||||||||
| Wang et al76 | January 25–February 21, 2020 | 6 provinces (northern China) | 31 | Median: 7.1 Range: 6 mo–17 y |
15 (48.4) | 0 | 20 (64.5) | C: 14 (45.2) E: 6 (19.4) |
5 (16.1) | NA | 2 (6.5) (1 as first presenting symptom) |
3 (9.7) (all as first presenting symptom) |
NA |
| Xia et al77 | January 23– February 8, 2020 | Hubei | 20 | Median: 2.1 Range: 1 d–14.6 y |
13 (65.0) | NA | 12 (60.0) | C: 13 (65.0) | ≥3 (15.0) | NA | 2 (10.0) | 3 (15.0) | NA |
| Cai et al78 | January 19, 2020– February 3, 2020 | Shanghai, Hainan, Anhui, and Shandong | 10 | Mean: 74 mo Range: 3–131 mo |
4 (40.0) | 0 | 8 (80.0) | C: 6 (60.0) | NA | NA | NA | 0 | NA |
| Chen et al79 | January 27, 2020 | Hubei | 1 | 13 mo | 1 (100) | 1 (100) | 1 (100) | 0 | 1 (100) | 1 (100) | 1 (100) | 1 (100) | NA |
| Zeng et al80 | February 5, 2020 | Hubei | 1 | 17 d | 1 (100) | 0 | 0 | 0 | 1 (100) | 1 (100) | 1 (100) | 1 (100) | NA |
| Zhang et al81 | January 26, 2020 | Hainan | 1 | 3 mo | 0 | 0 | 1 (100) | 0 | NA | 0 | 0 | 0 | NA |
| Pregnant women | |||||||||||||
| Chen et al82 | January 20–31, 2020 | Hubei | 9 | Range: 26–40 | 0 | NA | 7 (77.8) | C: 4 (44.4) D: 1 (11.1) |
1 (11.1) | NA | NA | 1 (11.1) | NA |
| Zhu et al83 | January 20–February 5, 2020 | Hubei | 9 | Range: 25–35 | 0 | NA | 8 (88.9) | C: 4 (44.4) | 1 (11.1) | NA | NA | 1 (11.1) | NA |
| Chen et al84 | January 21–February 4, 2020 | Hubei | 3 | Range: 23–34 | 0 | 0 | 1 (33.3) | D: 1 (33.3) | NA | NA | NA | 0 | NA |
| Liu et al85 | February 5, 2020 | Shandong | 1 | 38 | 0 | 0 | 0 | 0 | 1 (100) | 1 (100) | N: 1 (100) | 1 (100) | 0 |
C, cough; D, dyspnea; E, expectoration; GI, gastrointestinal; ICU, intensive care unit; N, nausea; NA, not available; SD, standard deviation; V, vomiting.
Severe disease was defined based on the American Thoracic Society and Infectious Disease Society of America guidelines for community-acquired pneumonia, need of ICU admission, and death.
If all gastrointestinal symptoms were not reported and the number of events of any individual gastrointestinal symptom was less than 1, it was regarded as not available and was excluded from the meta-analysis of all gastrointestinal symptoms. However, this study was still included in the meta-analysis of individual gastrointestinal symptoms if the proportion of patients with that symptom was reported.
Meta-analysis of Gastrointestinal Symptoms
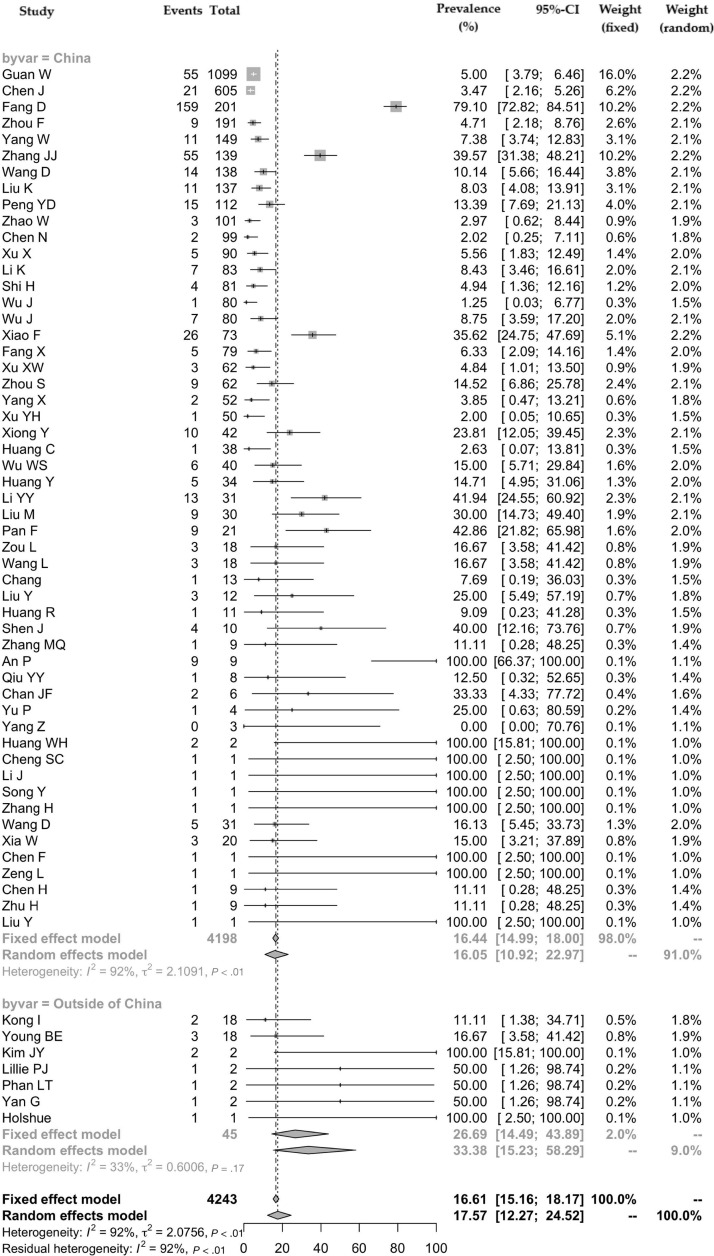
For the meta-analysis of all gastrointestinal symptoms (60 studies), there was a total of 4243 patients with COVID-19. Fifty-three (88.3%) studies were from China, and 7 (11.7%) were from other countries (South Korea, n = 2; Singapore, n = 2; Vietnam, n = 1; United States, n = 1; and United Kingdom, n = 1). Of the 53 studies from China, 27 (50.9%) were from Hubei Province where Wuhan is located. One study by Guan et al18 used the data reported to the National Health Commission of China from 552 hospitals across the country. The pooled prevalence of all gastrointestinal symptoms was 17.6% (95% CI, 12.3–24.5) (Figure 2 ), with significant heterogeneity noted among studies (P < .001; I 2 = 91.5%).
Figure 2.
Pooled prevalence of all gastrointestinal symptoms in patients with COVID-19 (all studies and according to geographical variation—China vs outside of China).
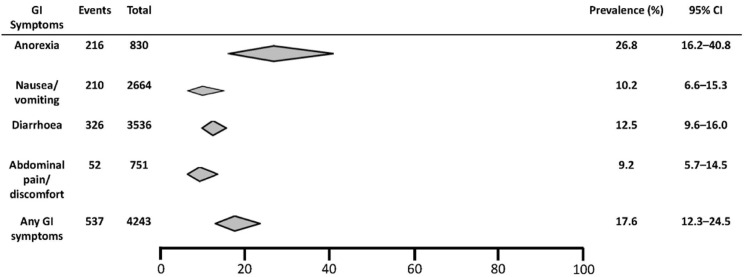
For individual gastrointestinal symptoms, there were 18 studies reporting on the prevalence of loss of appetite, 32 on nausea/vomiting, 58 on diarrhea, and 12 on abdominal pain. The pooled prevalence of loss of appetite was 26.8% (95% CI, 16.2–40.8) (Supplementary Figure 1), of nausea/vomiting was 10.2% (95% CI, 6.6–15.3) (Supplementary Figure 2), of diarrhea was 12.5% (95% CI, 9.6–16.0) (Supplementary Figure 3), and of abdominal pain/discomfort was 9.2% (95% CI, 5.7–14.5) (Supplementary Figure 4). Figure 3 shows the summary estimates for the prevalence of individual and all gastrointestinal symptoms. Significant heterogeneity among studies was seen for loss of appetite, nausea/vomiting, and diarrhea (P < .001; I 2 = 74.6%–85.2%), whereas the heterogeneity was less for abdominal pain/discomfort (P = .008; I 2 = 57.0%).
Figure 3.
Summary estimates of the prevalence of individual and all gastrointestinal symptoms in patients with COVID-19. GI, gastrointestinal.
Subgroup Analysis
Geographic Variations and Gastrointestinal Symptoms
The pooled prevalences of all gastrointestinal symptoms were 16.1% (95% CI, 10.9–23.0) and 33.4% (95% CI, 15.2–58.3) in studies from China and other countries, respectively (Figure 2). There was no significant subgroup difference between the studies based on country origin (P = .09). However, there was significant heterogeneity among the studies conducted in China (P < .001; I 2 = 92.4%) but not among the studies from other countries (P = .174; I 2 = 33.2%).
Among studies from China, the prevalence of all gastrointestinal symptoms in the single study of 1099 patients from 552 hospitals by Guan et al18 was 5.0% (95% CI, 3.9–6.5) (Figure 2). For studies from Hubei Province, the pooled prevalence of all gastrointestinal symptoms was 16.2% (95% CI, 9.3–26.7), whereas for those from outside Hubei Province, it was 18.6% (95% CI, 12.2–27.2). There was a significant subgroup difference between the studies from and outside of Hubei Province (P < .001), and there was also significant heterogeneity among the studies (P < .001; I 2 = 93.5% and I 2 = 76.8%, respectively).
Disease Severity and Gastrointestinal Symptoms
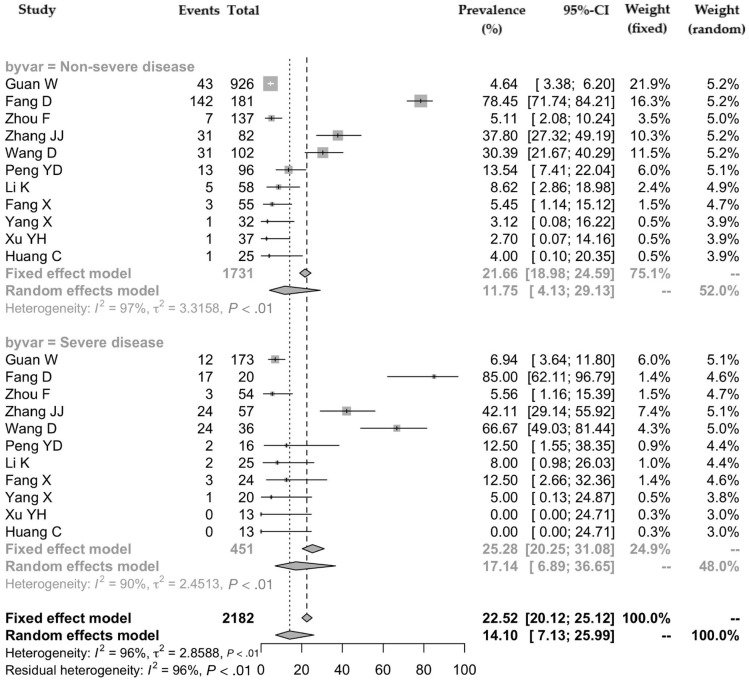
There were 11 studies that compared the prevalence of all gastrointestinal symptoms according to the severity of COVID-19 (the numbers of patients with severe and nonsevere disease were 451 and 1731, respectively) (Supplementary Table 1). The pooled prevalence of all gastrointestinal symptoms was 17.1% (95% CI, 6.9–36.7) and 11.8% (95% CI, 4.1–29.1) in patients with severe and nonsevere disease, respectively (Figure 4 ). There was significant heterogeneity among the studies (P < .001; I 2 = 90.9% and I 2 = 97.7%).
Figure 4.
Pooled prevalence of all gastrointestinal symptoms according to the severity of COVID-19.
Adult, Pediatric Patients, and Pregnant Women
There were 53 studies on adults, 4 on pediatric patients, and 3 on pregnant women. The corresponding pooled prevalences of all gastrointestinal symptoms in adults, pediatric patients, and pregnant women were 16.7% (95% CI, 11.4–23.9), 24.8% (95% CI, 9.6–50.4), and 20.0% (95% CI, 4.3–58.2). There was no significant subgroup difference (P = .717).
Detection of Viral RNA in Stool
None of the studies tested stool viral RNA on the day of hospitalization except our current study. There were 12 studies that tested for viral RNA in stool; the study by Wang et al86 reported stool viral RNA positivity rate according to number of stool specimens (44/153; 28.8%) rather than number of patients but reported the stool viral RNA results among 13 patients who tested positive for respiratory specimens. Of 138 patients, 68 (pooled prevalence, 48.1%; 95% CI, 38.3–57.9) tested positive for both respiratory and stool specimens (R+S+) after hospitalization (Supplementary Figure 5). In 9 studies with serial viral RNA test results of R+S+ patients, 87 of 124 patients (pooed prevalence, 70.3%; 95% CI, 49.6–85.1) had persistent positive stool viral RNA despite negative respiratory samples (R–S+) (Supplementary Figure 6). Ling et al15 reported that the stool viral clearance was longer in patients with steroid use compared to those without steroid use (20 vs 11 days; P < .001).
Figure 5 shows the timeline of the symptoms and viral test results (nasopharyngeal/throat swab, sputum, and stool samples) in 38 patients with available details. Based on the available data, none of the studies reported patients presenting with diarrhea on presentation (except for the study by Young et al,70 which did not report the association between stool viral RNA and diarrhea). Persistence of viral RNA in stool was longer than respiratory specimens (R–S+) in 13, including 7 pediatric, patients. Viral RNA was detected as early as day 3 of illness onset in these patients and remained positive in a 78-year-old patient for ≥33 days from illness onset.
Figure 5.
Timeline of the symptomatology and viral test results (respiratory and stool specimens) of 38 patients with COVID-19. A filled circle represents a positive result, and an empty circle represents a negative result. Gastrointestinal symptoms are color coded as shown (abdominal pain/discomfort, orange; vomiting, yellow; diarrhea, green). The details of 13 R–S+ patients are shown in case numbers 2, 5, 8, 10, 11, 14, 15, 18, 24, 26, 27, 28, and 29. Nasopharyngeal/oropharyngeal and stool samples were tested for viral RNA within 4–48 hours and 3–13 days after illness onset, respectively, in the study by Cai et al78; the authors did not state the exact day from illness onset on which the respiratory and stool samples were tested for individual patients; in addition, all patients tested negative for 2 consecutive respiratory specimens, but the exact day on which the second consecutive respiratory specimen tested negative for viral RNA was not stated. The sample size of Young et al70 was 18 (3 had diarrhea on presentation); the authors did not state which particular patient who tested for stool viral RNA (n = 8) had diarrhea. The number of days (D) represents the days from symptom onset (fever, cough, dyspnea, sore throat, nasal congestion, rhinorrhea, sneezing, loss of appetite) and was not reported in the study by Zhang J et al48; hence, the first day on which respiratory specimens were tested was regarded as day 1 in this graph.
Discussion
In this meta-analysis of 4243 patients with COVID-19 from 6 countries, the pooled prevalence of all gastrointestinal symptoms (including loss of appetite, nausea/vomiting, diarrhea, or abdominal pain) was 17.6%. Loss of appetite was the most common gastrointestinal symptom (26.8%), followed by diarrhea (12.5%), nausea/vomiting (10.2%), and abdominal pain/discomfort (9.2%). In the Hong Kong cohort, viral RNA was detected in the stool of 15.3% of patients on presentation, including patients without any gastrointestinal symptoms. Moreover, patients with diarrhea on presentation had higher stool RNA positivity and viral load than those without diarrhea. We also noted that 48.1% of patients had detectable stool viral RNA during the course of illnesses. More importantly, prolonged shedding of viral RNA in stool rather than respiratory samples was observed in 70.3% of patients, which could be up to ≥33 days from illness onset.
Although diarrhea is one of the common gastrointestinal manifestations, the presence of constipation could not rule out COVID-19, as a case report of 4 patients reported that constipation was noted in 2.58 Despite the inclusion of >60 reports, the actual prevalence of any gastrointestinal symptoms could be underestimated because many earlier studies did not report other gastrointestinal symptoms except for diarrhea.19 , 24 , 34 , 35 , 40 , 42 Moreover, the majority of studies reported gastrointestinal symptoms only on the day of admission but not throughout the disease course. The issue is further complicated by differences in the criteria for diagnosing diarrhea in various hospitals.87
With more than 80% resemblance to SARS-CoV, infection of the gastrointestinal tract by SARS-CoV-2 is not unexpected and is proposed to be mediated via the ACE2 cell receptors. ACE2 receptors are highly expressed in the small intestine, especially in proximal and distal enterocytes,9 , 87 and the binding affinity of ACE2 receptors determines infectivity. Because ACE2 modulates intestinal inflammation,88 SARS-CoV-2 may cause disruption of ACE2 function and result in diarrhea. A recent study showed the intracellular staining of viral nucleocapsid protein and ACE2 protein expression in the human gastric, duodenal, and rectal epithelial cells, further suggesting that the ACE2 receptors could act as the entry point of the SARS-CoV-2 virus in the intestinal tract.34
Gastrointestinal manifestations were also commonly reported during the SARS and MERS outbreaks. In the previous SARS outbreak in Hong Kong, 16% of patients reported diarrhea.8 Similarly, up to a quarter of patients with MERS also reported gastrointestinal symptoms such as diarrhea or abdominal pain.10 In our COVID-19 cohort in Hong Kong, 22% of patients reported diarrhea, which was slightly higher than our previous SARS cohort. However, many of these patients contracted the virus in a large outbreak during a dinner gathering in the Lunary New Year, probably via both fecal-oral and respiratory routes, thus partly explaining the higher frequency of gastrointestinal manifestations. Previous studies during SARS showed that viral load in the stool was strongly associated with presence of diarrhea.89 In our COVID-19 cohort, patients with diarrhea also had higher prevalence of detectable stool viral RNA on presentation. Importantly, gastrointestinal manifestations may be the only initial symptoms in some patients with COVID-19. In the study by An et al,54 9 patients reported only gastrointestinal symptoms (predominantly loss of appetite [66.7%]) in the absence of fever or respiratory symptoms on presentation.
Subgroup analysis showed that the pooled prevalence of all gastrointestinal symptoms was lower in studies from China than other countries (16.1% vs 33.4%). Although any true difference between countries remains to be investigated, this observation could be due to the smaller number of patients in studies from outside of China. Also, it is noteworthy that many of these early reports from outside of China included visitors from China. Because China was the first country affected by the COVID-19 outbreak with a large number of patients, the gastrointestinal manifestations may have been overlooked in the beginning of the outbreak, particularly Wuhan city, leading to underreporting of gastrointestinal symptoms in earlier studies.
Our meta-analysis showed that the prevalence of severe disease was more common in patients who had gastrointestinal symptoms than those who did not (17.1% vs 11.8%). Wang et al reported that abdominal pain was more frequent in patients who required ICU care than those who did not.23 Health care professionals should be aware of the potential prognostic implications in patients with gastrointestinal symptoms, who may require closer monitoring.
In our COVID-19 cohort in Hong Kong, we found that 15.3% of patients tested positive for stool viral RNA on the day of admission. As for the meta-analysis, we found that 48.1% of patients had stool samples ever tested positive for viral RNA during the illness. Because of the lack of systematic stool collection protocol in currently published studies, the full extent of the stool positivity rate remains to be characterized, particularly the peak timing and extent of fecal shedding. It is, however, alarming to note that 70.3% of patients had stool viral RNA remaining positive despite negative respiratory specimens. Although it is uncertain at this moment whether these are live virus particles or just RNA fragments released from the intestinal cells, this finding could raise a serious concern about the isolation policy for patients with COVID-19, particularly during the recovery phase. During the SARS outbreak in 2003, it was reported that the sewage system of the Amoy Gardens in Hong Kong served as the major source of infection from patients excreting coronavirus RNA.7 The sewage concentrates of 2 hospitals receiving patients with SARS in Beijing were also found to have SARS-CoV RNA detected at that time.90 Intuitively, proper handling of the excreta of patients with COVID-19 should still be strongly enforced despite repeatedly negative results in respiratory specimens.
Another interesting feature of COVID-19 is the recurrent infection in some patients, that is, recurrent symptoms after apparent recovery with positive respiratory specimens for viral RNA again after initial clearance. It remains to be determined whether the persistence of viral RNA in stool may be used as a surrogate monitor for recurrent infection in some patients.
There are several strengths of our study. To our knowledge, this is the first meta-analysis to summarize the rapidly emerging and sometimes confusing literature on COVID-19 on the prevalence of the overall and individual gastrointestinal manifestations. The comprehensive inclusion of >60 studies allows a more precise estimation of the prevalence of gastrointestinal symptoms. Subgroup analysis found that the presence of gastrointestinal symptoms was associated with a more severe disease course, highlighting the importance of more detailed inquiry into gastrointestinal symptoms for both diagnostic and prognostic purposes. The alarmingly high prevalence of viral shedding in stool, particularly after viral RNA negativity in respiratory specimens, prompts further research into the viral shedding dynamics in different systems, as well as the potential transmission risk via the fecal-oral route, which carries significant infection control and public health implications. A few limitations of this study should be noted. As mentioned, gastrointestinal symptoms may be underreported in some studies, which may lead to a lower pooled prevalence rate. Second, studies with large sample sizes of ethnic groups other than Chinese are currently lacking, precluding a more precise estimate of the prevalence of gastrointestinal manifestations in other ethnic groups.
Conclusion
In this study, we found that gastrointestinal symptoms were present in 17.6% of patients diagnosed with COVID-19. Moreover, viral shedding in stool was detected in 48.1% of patients and could persist for up to ≥33 days from illness onset even after viral RNA negativity in respiratory specimens.
Acknowledgments
CRediT Authorship Contributions
Ka Shing Cheung, MBBS, MPH (Data curation: Equal; Formal analysis: Lead; Methodology: Lead; Writing – original draft: Lead; Writing – review & editing: Supporting); Ivan F.N. Hung, MD (Conceptualization: Equal; Data curation: Equal; Formal analysis: Supporting; Investigation: Lead; Methodology: Lead; Supervision: Equal; Writing – review & editing: Equal); Pierre P.Y. Chan, MBBS FRCP (Investigation: Equal); K.C. Lung, MBBS MRCP (Investigation: Equal); Eugene Tso, MBBS, FRCP, (Investigation: Equal); Raymond Liu, MBBS FRCP (Investigation: Equal); Y.Y. Ng, MBChB MRCP (Investigation: Equal) Man Y Chu, MBBS MRCP (Investigation: Equal); Tom W.H. Chung, MBBS MRCP (Investigation: Equal); Anthony Raymond Tam, MBBS MRCP (Investigation: Equal); Cyril C.Y. Yip, PhD (Investigation: Equal); Kit Hang Leung, MSc (Investigation: Equal); Agnes Yim Fong Fung, BSc (Investigation: Equal); Ricky R. Zhang, MSc (Investigation: Equal); Yansheng Lin, MD (Data curation: Supporting); Ho Ming Cheng, PhD (Data curation: Supporting); Anna J.X. Zhang, PhD (Investigation: Equal); Kelvin K.W. To, MD (Investigation: Equal); Kwok H. Chan, PhD (Investigation: Equal); Kwok Y. Yuen, MD (Investigation: Lead); Wai K. Leung, MD (Methodology: Lead; Supervision: Lead; Validation: Lead; Writing – review & editing: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Author names in bold designate shared co-first authorship.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.03.065.
Supplementary Material
References
- 1.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Euro Surveill. 2020;25:2000062. doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi S., Jung E., Choi B.Y. High reproduction number of Middle East respiratory syndrome coronavirus in nosocomial outbreaks: mathematical modelling in Saudi Arabia and South Korea. J Hosp Infect. 2018;99:162–168. doi: 10.1016/j.jhin.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y., Gayle A.A., Wilder-Smith A. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):taaa021. doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung W.K., To K.F., Chan P.K. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan K.H., Poon L.L., Cheng V.C. Detection of SARS coronavirus in patients with suspected SARS. Emerg Infect Dis. 2004;10:294–299. doi: 10.3201/eid1002.030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO issues consensus document on the epidemiology of SARS. Wkly Epidemiol Rec. 2003;78:373–375. [PubMed] [Google Scholar]
- 9.Wan Y., Shang J., Graham R. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. e00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corman V.M., Albarrak A.M., Omrani A.S. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis. 2016;62:477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J., Li C., Zhao G. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci Adv. 2017;3(11) doi: 10.1126/sciadv.aao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W., Du R.H., Li B. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ling Y., Xu S.B., Lin Y.X. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metlay J.P., Waterer G.W., Long A.C. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng J.L., Huang C., Zhang G.J. Epidemiological characteristics of novel coronavirus pneumonia in Henan. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:327–331. doi: 10.3760/cma.j.cn112147-20200222-00148. [DOI] [PubMed] [Google Scholar]
- 20.Fang D., Ma J.D., Guan J.L. Manifestations of digestive system in hospitalized patients with novel coronavirus pneumonia in Wuhan, China: a single-center, descriptive study. Chin J Dig. 2020;40:151–156. [Google Scholar]
- 21.Yang W., Cao Q., Qin L. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. In press. [DOI] [PubMed]
- 23.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu K., Fang Y.Y., Deng Y. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng Y.D., Meng K., Guan H.Q. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E004. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 26.Zhao W., Zhong Z., Xie X. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 27.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X., Yu C., Qu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47:1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li K., Wu J., Wu F. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55:327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of COVID-19 in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed]
- 32.Wu J., Wu X., Zeng W. Chest CT findings in patients with coronavirus disease 2019 and its relationship with clinical features. Invest Radiol. 2020;55:257–261. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang X., Mei Q., Yang T. Clinical characteristics and treatment strategies of 79 patients with COVID-19. Chin Pharm Bulletin. 2020;36:1–7. [Google Scholar]
- 34.Xiao F., Tang M., Zheng X. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X.W., Wu X.X., Jiang X.G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou S., Wang Y., Zhu T. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;214:1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 37.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Y.H., Dong J.H., An W.M. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect. 2020;80:394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong Y., Sun D., Liu Y. Clinical and high-resolution CT features of the COVID-19 infection: comparison of the initial and follow-up changes. Invest Radiol. 2020;55:332–339. doi: 10.1097/RLI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu W.S., Li Y.G., Wei Z.F. Investigation and analysis on characteristics of a cluster of COVID-19 associated with exposure in a department store in Tianjin. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:489–493. doi: 10.3760/cma.j.cn112338-20200221-00139. [DOI] [PubMed] [Google Scholar]
- 42.Huang Y, Tu M, Wang S, et al. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: a retrospective single center analysis. Travel Med Infect Dis. In press. [DOI] [PMC free article] [PubMed]
- 43.Li Y.Y., Wang W.N., Lei Y. Comparison of the clinical characteristics between RNA positive and negative patients clinically diagnosed with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(5):427–430. doi: 10.3760/cma.j.cn112147-20200214-00095. [DOI] [PubMed] [Google Scholar]
- 44.Liu M., He P., Liu H.G. Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):209–214. doi: 10.3760/cma.j.issn.1001-0939.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 45.Pan F., Ye T., Sun P. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou L., Ruan F., Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L., Gao Y.H., Lou L.L. The clinical dynamics of 18 cases of COVID-19 outside of Wuhan, China. Eur Respir J. 2020;55:2000398. doi: 10.1183/13993003.00398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Wang S, Xue Y. Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia. J Med Virol. In press. [DOI] [PMC free article] [PubMed]
- 49.Chang, Lin M., Wei L. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323:1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang R., Xia J., Chen Y. A family cluster of SARS-CoV-2 infection involving 11 patients in Nanjing, China. Lancet Infect Dis. 2020;20:534–535. doi: 10.1016/S1473-3099(20)30147-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen J., Yu J., Yan Y. Clinical and chest HRCT characteristics in family group outbreak of novel coronavirus pneumonia. J Dalian Med Uni. 2020;42:32–36. [Google Scholar]
- 53.Zhang M.Q., Wang X.H., Chen Y.L. Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:215–218. doi: 10.3760/cma.j.issn.1001-0939.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 54.An P., Chen H., Jiang X. Clinical features of 2019 novel coronavirus pneumonia presented gastrointestinal symptoms but without fever onset. Preprints with The Lancet. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3532530 Published February 6, 2020.
- 55.Qiu Y.Y., Wang S.Q., Wang X.L. Epidemiological analysis on a family cluster of COVID-19. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:506–509. doi: 10.3760/cma.j.cn112338-20200221-00147. [DOI] [PubMed] [Google Scholar]
- 56.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren L.L., Wang Y.M., Wu Z.Q. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 2020;133:1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Z., Chen X., Lu Y. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 59.Yu P., Zhu J., Zhang Z. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. J Infect Dis. 2020;221(11):1757–1761. doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Z., Li G., Dai X.C. Three cases of novel coronavirus pneumonia with viral nucleic acids still positive in stool after throat swab detection turned negative. Chi J Dig. 2020;40(2):77–79. [Google Scholar]
- 61.Huang W.H., Teng L.C., Yeh T.K. 2019 novel coronavirus disease (COVID-19) in Taiwan: reports of two cases from Wuhan, China. J Microbiol Immunol Infect. 2020;53(3):481–484. doi: 10.1016/j.jmii.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng S.C., Chang Y.C., Fan Chiang Y.L. First case of coronavirus disease 2019 (COVID-19) pneumonia in Taiwan. J Formos Med Assoc. 2020;119:747–751. doi: 10.1016/j.jfma.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J., Li W.Q., Lie B. Shu ru xing nan bian xing xin xing guan zhuang bing du fei yan yi li ji chuan bo mo shi fen xi. West China Med J. 2020;35:137–140. [Google Scholar]
- 64.Han W., Quan B., Guo Y. The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019. J Med Virol. 2020;92:461–463. doi: 10.1002/jmv.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song Y., Liu P., Shi X.L. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020;69:1143–1144. doi: 10.1136/gutjnl-2020-320891. [DOI] [PubMed] [Google Scholar]
- 66.Zhang H., Xie C., Huang Y. The treatment and outcome of a lung cancer patient infected with SARS-CoV-2. J Thorac Oncol. 2020;15(5):e63–e64. doi: 10.1016/j.jtho.2020.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu T., Kang S.C., Feng W. Biological characters analysis of COVID-19 patient accompanied with aplastic anemia. Zhonghua Xue Ye Xue Za Zhi. 2020;41(0):E003. doi: 10.3760/cma.j.issn.0253-2727.2020.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin C., Ding Y., Xie B. Asymptomatic novel coronavirus pneumonia patient outside Wuhan: the value of CT images in the course of the disease. Clin Imaging. 2020;63:7–9. doi: 10.1016/j.clinimag.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kong I., Park Y., Woo Y. Early epidemiological and clinical characteristics of 28 cases of coronavirus disease in South Korea. Osong Public Health Res Perspect. 2020;11:8–14. doi: 10.24171/j.phrp.2020.11.1.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Young B.E., Ong S.W.X., Kalimuddin S. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15) doi: 10.1001/jama.2020.3204. 1488-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J.Y., Ko J.H., Kim Y. Viral load kinetics of SARS-CoV-2 infection in first two patients in Korea. J Korean Med Sci. 2020;35(7):e86. doi: 10.3346/jkms.2020.35.e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lillie P.J., Samson A., Li A. Novel coronavirus disease (Covid-19): the first two patients in the UK with person to person transmission. J Infect. 2020;80:578–606. doi: 10.1016/j.jinf.2020.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phan L.T., Nguyen T.V., Luong Q.C. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382:872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan G., Lee C.K., Lam L.T.M. Covert COVID-19 and false-positive dengue serology in Singapore. Lancet Infect Dis. 2020;20:536. doi: 10.1016/S1473-3099(20)30158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang D., Ju X.L., Xie F. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua Er Ke Za Zhi. 2020;58(4):E011. doi: 10.3760/cma.j.cn112140-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 77.Xia W., Shao J., Guo Y. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020;55:1169–1174. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cai J, Xu J, Lin D, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed]
- 79.Chen F., Liu Z.S., Zhang F.R. First case of severe childhood novel coronavirus pneumonia in China. Zhonghua Er Ke Za Zhi. 2020;58:179–182. doi: 10.3760/cma.j.issn.0578-1310.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 80.Zeng L.K., Tao X.W., Yuan W.H. First case of neonate infected with novel coronavirus pneumonia in China. Zhonghua Er Ke Za Zhi. 2020;58(0):E009. doi: 10.3760/cma.j.issn.0578-1310.2020.0009. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Y.H., Lin D.J., Xiao M.F. 2019-novel coronavirus infection in a three-month-old baby. Zhonghua Er Ke Za Zhi. 2020;58:182–184. doi: 10.3760/cma.j.issn.0578-1310.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 82.Chen H., Guo J., Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu H., Wang L., Fang C. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen S., Huang B., Luo D.J. Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi. 2020;49(5):418–423. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 85.Liu Y., Ren X., Sun Y. Diagnosis and treatment of novel coronavirus pneumonia in pregnancy with gastrointestinal symptoms as first manifestations. J Jilin Uni. 2020;46:410–414. [Google Scholar]
- 86.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liang W., Feng Z., Rao S. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69:1141–1143. doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- 88.Hashimoto T., Perlot T., Rehman A. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487(7408):477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hung I.F., Cheng V.C., Wu A.K. Viral loads in clinical specimens and SARS manifestations. Emerg Infect Dis. 2004;10:1550–1557. doi: 10.3201/eid1009.040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X.W., Li J., Guo T. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital of the Chinese People’s Liberation Army. Water Sci Technol. 2005;52:213–221. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.