Abstract
Background & Aims
Recent data on the coronavirus disease 2019 (COVID-19) outbreak caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has begun to shine light on the impact of the disease on the liver. But no studies to date have systematically described liver test abnormalities in patients with COVID-19. We evaluated the clinical characteristics of COVID-19 in patients with abnormal liver test results.
Methods
Clinical records and laboratory results were obtained from 417 patients with laboratory-confirmed COVID-19 who were admitted to the only referral hospital in Shenzhen, China from January 11 to February 21, 2020 and followed up to March 7, 2020. Information on clinical features of patients with abnormal liver tests were collected for analysis.
Results
Of 417 patients with COVID-19, 318 (76.3%) had abnormal liver test results and 90 (21.5%) had liver injury during hospitalization. The presence of abnormal liver tests became more pronounced during hospitalization within 2 weeks, with 49 (23.4%), 31 (14.8%), 24 (11.5%) and 51 (24.4%) patients having alanine aminotransferase, aspartate aminotransferase, total bilirubin and gamma-glutamyl transferase levels elevated to more than 3× the upper limit of normal, respectively. Patients with abnormal liver tests of hepatocellular type or mixed type at admission had higher odds of progressing to severe disease (odds ratios [ORs] 2.73; 95% CI 1.19–6.3, and 4.44, 95% CI 1.93–10.23, respectively). The use of lopinavir/ritonavir was also found to lead to increased odds of liver injury (OR from 4.44 to 5.03, both p <0.01).
Conclusion
Patients with abnormal liver tests were at higher risk of progressing to severe disease. The detrimental effects on liver injury mainly related to certain medications used during hospitalization, which should be monitored and evaluated frequently.
Lay summary
Data on liver tests in patients with COVID-19 are scarce. We observed a high prevalence of liver test abnormalities and liver injury in 417 patients with COVID-19 admitted to our referral center, and the prevalence increased substantially during hospitalization. The presence of abnormal liver tests and liver injury were associated with the progression to severe pneumonia. The detrimental effects on liver injury were related to certain medications used during hospitalization, which warrants frequent monitoring and evaluation for these patients.
Keywords: 2019-nCoV, SARS-Cov-2, Pneumonia, Critical care, Liver injury, Liver tests, Bilirubin
Graphical abstract
Highlights
-
•
Of 417 patients with COVID-19, 76.3% had abnormal liver tests and 21.5% had liver injury during hospitalization.
-
•
Patients with abnormal liver tests had significantly higher odds of developing severe pneumonia.
-
•
The use of lopinavir/ritonavir increased the odds of liver injury by 4-fold.
Introduction
Coronaviruses are a family of viruses that are known to cause both respiratory and intestinal diseases in various animal species and humans.1 These viruses tend to target the upper respiratory tract, causing anywhere from moderate to severe illnesses, such as the cold or in more extreme cases, pneumonia. To date, 7 human coronaviruses have been identified, including the 3 epidemic viruses of severe acute respiratory syndrome (SARS)-CoV, middle east respiratory syndrome (MERS)-CoV and the newest, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).2 These 3 epidemic viruses have similar sequence identity, sharing more than 50% genome sequences.3 In December of 2019, a series of pneumonia cases of unknown origin began to spread in the central city of Wuhan, China. Now identified as SARS-CoV-2, the virus had gone on to infect more than 300,000 people worldwide by March 2020.4 The coronavirus disease (COVID-19) has been labelled a pandemic by the World Health Organization (WHO) having led to thousands of deaths and hospitalizations worldwide. While most COVID-19 cases have been identified as mild, more extreme diagnoses have led to respiratory failure, septic shock, and/or multiple organ dysfunction.5 As this infectious disease continues to spread, further clinical and epidemiological characteristics must be elucidated to improve our understanding of the true extent of the virus, in order to improve diagnostic and treatment capabilities and reduce its overall impact on morbidity and mortality.
Recently, there has been some insight into the impact of COVID-19 on other organs, as a number of reports have indicated that more than half of patients with COVID-19 showed varying levels of liver disease.6 A new study found that the SARS-CoV-2 virus may bind to angiotensin-converting enzyme 2 (ACE2) on cholangiocytes, leading to cholangiocyte dysfunction and inducing a systemic inflammatory response leading to liver injury.7 As of March 10, 2020, 7 relatively large-scale hospital-based studies have reported the clinical characteristics of patients with COVID-19, including some insights into other factors, which may lead to COVID-19 induced liver damage.[8], [9], [10], [11], [12], [13], [14] In these studies, elevated levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were reported, ranging from 14% to 53%.8 , 9 , 11 , 14 Additionally, a pathological study of liver biopsy specimens from a patient who died from COVID-19 showed moderate microvesicular steatosis and mild lobular and portal activity, indicating that SARS-CoV-2 may have led to this liver damage.15 However, little data exists that has comprehensively analyzed other liver enzymes and clinical characteristics of liver failure among patients with COVID-19. Hence, the aim of this study was to report the clinical course and liver test parameters in patients with COVID-19 admitted to the only referral hospital in Shenzhen, China. With better knowledge of pathogenesis, more targeted therapies and holistic care models could be developed, which may help to prevent severe liver injury or failure in patients with COVID-19.
Patients and methods
Study design and participant criteria
This was a cross-sectional study among patients recruited from the Third People's Hospital of Shenzhen, which is the only referral hospital in Shenzhen, China. From January 11, 2020 to February 21, 2020, 417 patients diagnosed with COVID-19 based on the WHO interim guidance16 were identified. Those that had ≥1 abnormal liver test result from admission to the end of February 2020 were enrolled in the study. This study was approved by the Ethics Committee of The Third People's Hospital of Shenzhen (2020 019). All patients provided signed informed consent. It was not appropriate or possible to involve patients or the public in the design, conduct, or reporting of our research.
Confirmation of COVID-19
The presence of SARS-CoV-2 was detected by real-time reverse transcription PCR.17 Two pairs of primers targeting the open reading frame 1ab (ORF1ab) and the nucleocapsid protein (N) were amplified and examined. The corresponding sequences for ORF1ab were 5′-CCCTGTGGGTTTTACACTTAA-3′ (F), 5′-ACGATTGTGCATCAGCTGA-3′ (R), and 5′-CY3-CCGTCTGCGGTATGTGGAAAGGTTATGG-BHQ1-3′ (probe), and those for N were 5′-GGGGAACTTCTCCTGCTAGAAT-3′ (F), 5′-CAGACATTTTGCTCTCAAGCTG-3′ (R), and 5′-FAM-TTGCTGCTGCTTGACAGATT-TAMRA-3′ (probe). Each sample was run in triplicate with positive and negative control sets, as suggested. These diagnostic criteria were based on the recommendations by the National Centers for Disease Control and Prevention of China (China CDC). The Shenzhen CDC reconfirmed samples that were identified as positive for SARS-CoV-2 by the local laboratory.
Liver test parameters and abnormalities
Liver test abnormalities were defined as the elevation of the following liver enzymes in serum: ALT >40 U/L, AST >40 U/L, gamma-glutamyltransferase (GGT) >49 U/L, alkaline phosphatase (ALP) >135 U/L, and total bilirubin (TBIL) >17.1 μmol/L. As COVID-19 is a new, emerging infectious disease, guidance or consensus on liver injury classifications are lacking. Thus, we classified the pattern of these abnormalities as hepatocellular, cholestatic, or mixed. Patients who had raised ALT and/or AST more than 3× the upper limit unit of normal (ULN) were classified as hepatocyte type; patients who had raised ALP or GGT twice the ULN were classified as cholangiocyte type; and patients who had a combination of both ALT/AST elevated more than 3× the ULN and ALP/GGT twice the ULN were classified as mixed type (Abnormality type (1)). To further describe liver test characteristics, we defined ALT and/or AST over 3× ULN, ALP, GGT, and/or TBIL over 2× ULN as liver injury. Moreover, as the magnitude of the liver test elevations in our patients ranged from mild to moderate, we also defined patterns of liver abnormality according to another criteria. Patients were classified as hepatocyte type when the AST/ALT activity was higher than the ALP/GGT activity, with the liver enzyme activities calculated by multiples of their ULN, respectively, and were classified as cholangiocyte type when the reverse occurred (Abnormality type (2)).
Severity of COVID-19
As per the national guidelines for community-acquired pneumonia and the diagnosis and treatment plan for the new coronavirus in China,18 , 19 all patients were classified into severe or mild cases based on results from chest radiography, clinical examination, and symptoms. Patients with mild symptoms (i.e., fever, cough, expectoration, and other upper respiratory tract symptoms), and without abnormalities, or with mild changes on chest radiography, were classified as non-severe types.8 A mild change in chest radiography is defined by multiple small patchy shadows and interstitial changes, mainly in the outer zone of the lung and under the pleura. Severe pneumonia was defined by the presence of any of the following conditions: i) significantly increased respiration rate (RR): RR ≥30 times/minute; ii) hypoxia: oxygen saturation (resting state) ≤93%; iii) blood gas analysis: partial pressure of oxygen/fraction of inspired oxygen (PaO2) /FiO2) ≤300 mmHg (millimeters of Mercury); or iv) the occurrence of respiratory or other organ failure that requires intensive care unit (ICU) monitoring and treatment, or shock.
We also investigated the pathological characteristics of a patient who died from COVID-19 by obtaining biopsy samples at autopsy.
Statistical analysis
Categorical variables were described as frequency and percentages, and continuous variables as mean and SD or median and IQR. Means for continuous variables were compared using independent group t tests when the data were normally distributed; otherwise, the Mann-Whitney U test was used. Comparison of categorical variables was done using the χ2 test or the Fisher exact test, if the cell counts were small. Multivariable logistic regression was used to explore the association between liver test abnormalities and the severity of disease, and the association between drugs and liver injury, using odds ratios (ORs) and 95% CIs. As the presence of underlying liver disease may also play a role in the association of liver tests with disease severity, we also conducted sensitivity analyses excluding patients with underlying liver disease, including chronic hepatitis B and alcoholic/non-alcoholic fatty liver disease (NAFLD). NAFLD was defined by ultrasonographic detection or CT measurements of steatosis, with the exclusion of both secondary causes and of a daily alcohol consumption ≥30 gram in men and ≥20 gram in women.20 HBV infection was defined by the positive test for hepatitis B surface antigen.21 Furthermore, we used inverse probability weighting (IPW) to adjust for potential confounders and to account for possible selection bias induced by the severity of disease at admission when examining the effects of drugs on liver injury. All statistical analyses were performed using STATA/SE version 16.0 software (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC). A 2-sided α of less than 0.05 was considered statistically significant.
Results
Clinical features of patients with COVID-19
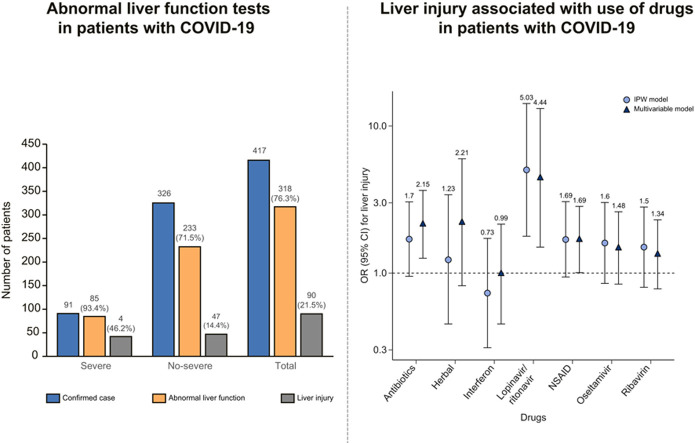
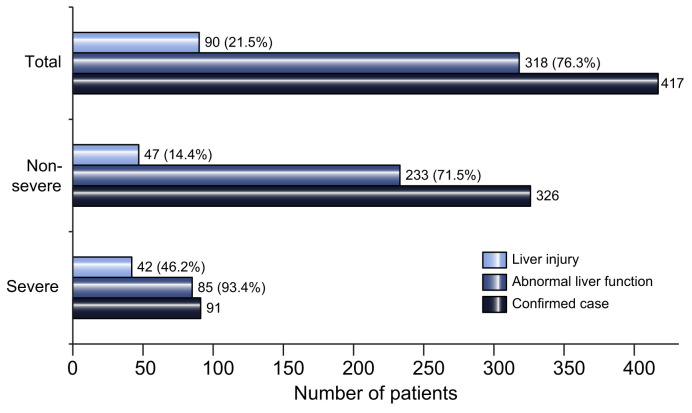
Of 417 patients with COVID-19, 318 (76.3%) had abnormal liver test results and 90 (21.5%) had liver injury during hospitalization. Ninety-one (21.8%) developed severe disease and 326 (78.2%) had mild disease during hospitalization (Fig. 1 ).
Fig. 1.
Liver test abnormality during hospitalization in patients with COVID-19 by severity of disease.
(Bars represent number of patients).
Clinical features of patients with COVID-19 at admission
Table 1 shows that at admission, about half of the patients had abnormal liver test results and 21 (5%) had liver injury. Patients with abnormal liver test results were older, had a higher body mass index (BMI), were male, and had no clear contact history (p <0.05). Patients also tended to have underlying liver diseases, including NAFLD, alcoholic liver disease, and chronic hepatitis B (p = 0.001), and had cough as an initial symptom (p = 0.04) (Table 1). Most of the patients had abnormal liver test results within 1–2× ULN at admission and only a few (<4%) had abnormal liver test results higher than 2× ULN. The increase in GGT at admission appeared to be more pronounced, with 53 (12.71%) having 1–2× ULN, 5 (1.2%) having 2–3× ULN, and 10 (2.4%) having more than 3× ULN (Table 1).
Table 1.
Characteristics of 417 patients with COVID-19 at admission by liver tests.
| Characteristics | Liver tests |
Total | p value | ||
|---|---|---|---|---|---|
| Normal | Abnormal | Injury | |||
| Number (%) | 225 (54.0) | 170 (41.0) | 22 (5.0) | 417 | |
| Age, year, median (IQR) | 47 (33–59) | 47 (33–61) | 53 (42–64) | 47 (34–60) | 0.04 |
| <10 | 5 (2.22) | 15 (8.77) | 0 (0) | 20 (4.8) | 0.03 |
| 10–19 | 7 (3.11) | 7 (4.09) | 0 (0) | 14 (3.36) | |
| 20–39 | 75 (33.33) | 50 (29.24) | 3 (14.29) | 128 (30.7) | |
| 40–49 | 39 (17.33) | 20 (11.7) | 5 (23.81) | 64 (15.35) | |
| ≥50 | 99 (44) | 79 (46.2) | 13 (61.9) | 191 (45.8) | |
| Males, n (%) | 81 (36) | 103 (60.2) | 14 (66.7) | 198 (47.5) | <0.001 |
| BMI, kg/m2, median (IQR) | 22.6 (20.6–25) | 23.7 (21.4–26.2) | 25.8 (22.2–27) | 23.1 (21.2–25.6) | 0.004 |
| Epidemiology information, n (%) | |||||
| From Hubei | 135 (60) | 82 (47.95) | 9 (42.86) | 226 (54.2) | 0.03 |
| Not been to Hubei, but infected by individuals from Hubei | 80 (35.56) | 70 (40.94) | 9 (42.86) | 159 (38.13) | |
| Without any clear contact history | 10 (4.44) | 19 (11.11) | 3 (14.29) | 32 (7.67) | |
| Comorbidities, n (%) | |||||
| Diabetes | 12 (5.33) | 10 (5.85) | 1 (4.76) | 23 (5.52) | 0.96 |
| Hypertension | 25 (11.11) | 27 (15.79) | 6 (28.57) | 58 (13.91) | 0.06 |
| Liver disease† | 4 (1.78) | 14 (8.19) | 3 (14.29) | 21 (5.04) | 0.001 |
| Initial symptoms, n (%) | |||||
| Fever | 147 (65.3) | 118 (69.0) | 14 (66.7) | 279 (66.9) | 0.74 |
| Cough | 73 (32.4) | 73 (42.7) | 11 (52.4) | 157 (37.7) | 0.04 |
| Chest radiography, n (%) | |||||
| No change | 29 (12.89) | 35 (20.47) | 2 (9.52) | 66 (15.83) | 0.18 |
| Mild | 36 (16) | 20 (11.7) | 1 (4.76) | 57 (13.67) | |
| Advanced | 140 (62.2) | 96 (56.14) | 15 (71.4) | 251 (60.2) | |
| Severe | 20 (8.89) | 20 (11.7) | 3 (14.29) | 43 (10.31) | |
| ALT, U/L, median (IQR) | 17 (12–24) | 27 (18–39.5) | 47 (38–65.2) | 21 (15–31) | <0.001 |
| Normal | 225 (100) | 132 (77.19) | 6 (28.57) | 363 (87.1) | <0.001 |
| 1–2 ULN, n (%) | 0 (0) | 35 (20.47) | 13 (61.9) | 48 (11.51) | |
| 2–3 ULN, n (%) | 0 (0) | 4 (2.34) | 1 (4.76) | 5 (1.2) | |
| >3 ULN, n (%) | 0 (0) | 0 (0) | 1 (4.76) | 1 (0.24) | |
| AST, U/L, median (IQR) | 23 (19–28) | 34 (24.3–43) | 47.2 (30.9–63.8) | 26.5 (21–35) | <0.001 |
| Normal | 225 (100) | 108 (63.16) | 8 (38.1) | 341 (81.77) | <0.001 |
| 1–2 ULN, n (%) | 0 (0) | 58 (33.92) | 11 (52.38) | 69 (16.55) | |
| 2–3 ULN, n (%) | 0 (0) | 5 (2.92) | 1 (4.76) | 6 (1.44) | |
| >3 ULN, n (%) | 0 (0) | 0 (0) | 1 (4.76) | 1 (0.24) | |
| TBIL, μmol/L, median (IQR) | 9.55 (7.6–12.6) | 16.8 (9.3–22) | 17.2 (9.2–34.3) | 10.9 (8.3–16.3) | <0.001 |
| Normal | 222 (100) | 86 (50.29) | 10 (47.62) | 318 (76.81) | <0.001 |
| 1–2 ULN, n (%) | 0 (0) | 85 (49.71) | 5 (23.81) | 90 (21.74) | |
| 2–3 ULN, n (%) | 0 (0) | 0 (0) | 5 (23.81) | 5 (1.21) | |
| >3 ULN, n (%) | 0 (0) | 0 (0) | 1 (4.76) | 1 (0.24) | |
| ALP, U/L, median (IQR) | 59 (48–70.5) | 63 (52–79) | 68 (56–81) | 61 (50.5–74.5) | <0.001 |
| Normal | 180 (100) | 120 (88.89) | 16 (94.12) | 316 (95.18) | <0.001 |
| 1–2 ULN, n (%) | 0 (0) | 14 (10.37) | 1 (5.88) | 15 (4.52) | |
| 2–3 ULN, n (%) | 0 (0) | 1 (0.74) | 0 (0) | 1 (0.3) | |
| >3 ULN, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| GGT, U/L, median (IQR) | 21.71 (9.04) | 36.45 (21.94) | 134.91 (108.04) | 33.45 (37.41) | <0.001 |
| Normal | 225 (100) | 120 (70.18) | 4 (19.05) | 349 (83.69) | <0.001 |
| 1–2 ULN, n (%) | 0 (0) | 51 (29.82) | 2 (9.52) | 53 (12.71) | |
| 2–3 ULN, n (%) | 0 (0) | 0 (0) | 5 (23.81) | 5 (1.2) | |
| >3 ULN, n (%) | 0 (0) | 0 (0) | 10 (47.62) | 10 (2.4) | |
ALT, alanine aminotransferase; AST, aspartate transaminase; ALP, alkaline phosphatase; GGT, gamma-glutamyltransferase; NSAIDs, non-steroidal anti-inflammatory drugs; TBIL, total bilirubin abnormal; ULN, upper limit of normal.
Liver comorbidities include non-alcoholic fatty liver disease, alcoholic liver disease and chronic hepatitis B.
Clinical features of patients with COVID-19 and abnormal liver tests during hospitalization
Table 2 shows that, of the 318 patients with COVID-19 and abnormal liver test results during hospitalization, 26.7% progressed to severe pneumonia. Regarding the patterns of abnormal liver test results, 20.75% were hepatocyte type, 29.25% were cholestatic type, and 43.4% were mixed type (Table 2). The presence of liver test abnormalities became more pronounced during hospitalization for ALT, AST, TBIL and GGT, with 33 (10.38%), 18 (5.66%), 9 (2.83%), and 37 (11.64%) patients exhibiting abnormal levels more than 3× ULN, respectively (Table 2).
Table 2.
Clinical characteristics of 318 patients with COVID-19 and abnormal liver test results during hospitalization.
| Characteristics | Disease severity |
Total | p value | |
|---|---|---|---|---|
| Non-severe | Severe | |||
| N (%) | 233 (73.27) | 85 (26.73) | 318 | – |
| Abnormality type, n (%) | ||||
| Hepatocellular | 50 (21.46) | 16 (18.82) | 66 (20.75) | <0.001 |
| Cholestatic | 86 (36.91) | 7 (8.24) | 93 (29.25) | |
| Mixed | 78 (33.48) | 60 (70.59) | 138 (43.4) | |
| ALT, U/L, Median (IQR) | 41 (23–65) | 67 (47–100) | 46 (27–76) | 0.003 |
| Normal | 116 (49.79) | 15 (17.65) | 131 (41.19) | <0.001 |
| 1–2 ULN, n (%) | 75 (32.19) | 38 (44.71) | 113 (35.53) | |
| 2–3 ULN, n (%) | 27 (11.59) | 14 (16.47) | 41 (12.89) | |
| >3 ULN, n (%) | 15 (6.44) | 18 (21.18) | 33 (10.38) | |
| AST, U/L, Median (IQR) | 34 (27–45) | 58 (41–93) | 38 (28–52) | 0.005 |
| Normal | 147 (63.09) | 21 (24.71) | 168 (52.83) | <0.001 |
| 1–2 ULN, n (%) | 74 (31.76) | 39 (45.88) | 113 (35.53) | |
| 2–3 ULN, n (%) | 10 (4.29) | 9 (10.59) | 19 (5.97) | |
| >3 ULN, n (%) | 2 (0.86) | 16 (18.82) | 18 (5.66) | |
| TBIL, μmol/L, Median (IQR) | 19 (13–26) | 22 (18–28) | 20 (14–27) | <0.001 |
| Normal | 93 (39.91) | 21 (24.71) | 114 (35.85) | <0.001 |
| 1–2 ULN, n (%) | 123 (52.79) | 58 (68.24) | 181 (56.92) | |
| 2–3 ULN, n (%) | 14 (6.01) | 0 (0) | 14 (4.4) | |
| >3 ULN, n (%) | 3 (1.29) | 6 (7.06) | 9 (2.83) | |
| ALP, U/L, Median (IQR) | 69 (57–89) | 79 (62–101) | 73 (59–92) | 0.31 |
| Normal | 180 (89.55) | 72 (87.8) | 252 (89.05) | 0.78 |
| 1–2 ULN, n (%) | 20 (9.95) | 9 (10.98) | 29 (10.25) | |
| 2–3 ULN, n (%) | 1 (0.5) | 1 (1.22) | 2 (0.71) | |
| >3 ULN, n (%) | 0 (0) | 0 (0) | 0 (0) | |
| GGT, U/L, Median (IQR) | 40 (25–61) | 92 (53–161) | 47.5 (28–83) | <0.001 |
| Normal | 142 (60.94) | 21 (24.71) | 163 (51.26) | <0.001 |
| 1–2 ULN, n (%) | 67 (28.76) | 25 (29.41) | 92 (28.93) | |
| 2–3 ULN, n (%) | 12 (5.15) | 14 (16.47) | 26 (8.18) | |
| >3 ULN, n (%) | 12 (5.15) | 25 (29.41) | 37 (11.64) | |
| Drug-use, n (%) | ||||
| Antibiotics | 14 (29.79) | 33 (76.74) | 47 (52.22) | <0.001 |
| NSAIDs | 78 (33.48) | 57 (67.06) | 135 (42.45) | <0.001 |
| Ribavirin | 57 (24.46) | 38 (44.71) | 95 (29.87) | <0.001 |
| Oseltamivir | 55 (23.61) | 27 (31.76) | 82 (25.79) | 0.14 |
| Herbal medications | 189 (81.12) | 83 (97.65) | 272 (85.53) | <0.001 |
| Interferon | 196 (84.12) | 83 (97.65) | 279 (87.74) | 0.001 |
| Lopinavir/ritonavir | 210 (90.13) | 78 (91.76) | 288 (90.57) | 0.66 |
| Multi-organ failure, n (%) | 0 (0) | 10 (11.76) | 10 (3.14) | – |
| Liver failure | 0 | 2 | 2 | |
ALT, alanine aminotransferase; AST, aspartate transaminase; ALP, alkaline phosphatase; GGT, gamma-glutamyltransferase; NSAIDs, non-steroidal anti-inflammatory drugs; TBIL, total bilirubin abnormal; ULN, upper limit of normal.
Table 2 also shows that, in the 318 patients with abnormal liver test results, those who progressed to severe cases tended to be mixed type (p = 0.01), had higher ALT, AST, TBIL and GGT (p <0.005), but not ALP (Table 2). The use of drugs that may induce liver injury including antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs), ribavirin, herbal medications, and interferon were associated with severity of disease (all p ≤0.001) in patients with abnormal liver test results, except for lopinavir/ritonavir (p = 0.66) and oseltamivir (p = 0.14).
Clinical features of patients with COVID-19 and liver injury during hospitalization
Table 3 shows that in 90 patients with COVID-19 and liver injury during hospitalization, about half were severe cases. Increases in ALT and GGT were frequent (elevated to more than 3× ULN in 37% and 41% of patients, respectively), while increases in AST and TBIL were slightly less frequent (elevated to more than 3× ULN in 20% and 10% of patients, respectively). The increase in ALP was not pronounced, with only 1 patient exhibiting an elevation to more than 3× ULN. The use of antibiotics, NSAIDs, Chinese herbal medications, and interferon were associated with progression to severe disease (p from <0.001 to 0.04). Moreover, 10 (23.26%) of the severe cases presented with multi-organ failure. Of the 10 patients who developed multiple organ failure, 3 patients died (1 patient had liver failure). In addition to respiratory failure, these 10 patients had other complications, including liver failure (2/10), septic shock (9/10), heart failure (4/10), renal failure (8/10), gastrointestinal hemorrhage (1/10), and disseminated intravascular coagulation (2/10). Except for respiratory failure, most of these complications, including liver failure, were related to severe secondary infections in the ICU.
Table 3.
Clinical characteristics of patients with COVID-19 and liver injury.
| Characteristics | Disease severity |
Total | p value | |
|---|---|---|---|---|
| Non-severe | Severe | |||
| N (%) | 47 (52.22) | 43 (47.78) | 90 | – |
| Abnormality type (1),† n (%) | ||||
| Hepatocellular | 8 (17.02) | 0 (0) | 8 (8.89) | 0.001 |
| Cholestatic | 8 (17.02) | 2 (4.65) | 10 (11.11) | |
| Mixed | 28 (59.57) | 41 (95.35) | 69 (76.67) | |
| Abnormality type (2),† n (%) | ||||
| Hepatocellular | 20 (42.55) | 17 (39.53) | 37 (41.11) | 0.77 |
| Cholestatic | 27 (57.45) | 26 (60.47) | 53 (58.89) | |
| ALT, U/L, Median (IQR) | 84 (42–136) | 96 (63–159) | 90.5 (53–145) | 0.17 |
| Normal | 10 (21.28) | 2 (4.65) | 12 (13.33) | 0.14 |
| 1–2 ULN, n (%) | 12 (25.53) | 13 (30.23) | 25 (27.78) | |
| 2–3 ULN, n (%) | 10 (21.28) | 10 (23.26) | 20 (22.22) | |
| >3 ULN, n (%) | 15 (31.91) | 18 (41.86) | 33 (36.67) | |
| AST, U/L, Median (IQR) | 45 (29–81) | 80 (56–145) | 63 (36–101) | 0.1 |
| Normal | 20 (42.55) | 4 (9.3) | 24 (26.67) | <0.001 |
| 1–2 ULN, n (%) | 15 (31.91) | 16 (37.21) | 31 (34.44) | |
| 2–3 ULN, n (%) | 10 (21.28) | 7 (16.28) | 17 (18.89) | |
| >3 ULN, n (%) | 2 (4.26) | 16 (37.21) | 18 (20) | |
| TBIL, μmol/L, Median (IQR) | 22 (15–41) | 25 (19–32) | 23.5 (16–37) | 0.13 |
| Normal | 16 (34.04) | 9 (20.93) | 25 (27.78) | <0.001 |
| 1–2 ULN, n (%) | 14 (29.79) | 28 (65.12) | 42 (46.67) | |
| 2–3 ULN, n (%) | 14 (29.79) | 0 (0) | 14 (15.56) | |
| >3 ULN, n (%) | 3 (6.38) | 6 (13.95) | 9 (10) | |
| ALP, U/L, Median (IQR) | 77 (65–97) | 92 (76–130) | 83 (66–114.5) | 0.01 |
| Normal | 39 (95.12) | 33 (76.74) | 72 (85.71) | 0.05 |
| 1–2 ULN, n (%) | 2 (4.88) | 9 (20.93) | 11 (13.1) | |
| 2–3 ULN, n (%) | 0 (0) | 1 (2.33) | 1 (1.19) | |
| >3 ULN, n (%) | 0 (0) | 0 (0) | 0 (0) | |
| GGT, U/L, Median (IQR) | 101 (39–148) | 161 (122–211) | 130.5 (77–187) | <0.001 |
| Normal | 16 (34.04) | 0 (0) | 16 (17.78) | <0.001 |
| 1–2 ULN, n (%) | 7 (14.89) | 4 (9.3) | 11 (12.22) | |
| 2–3 ULN, n (%) | 12 (25.53) | 14 (32.56) | 26 (28.89) | |
| >3 ULN, n (%) | 12 (25.53) | 25 (58.14) | 37 (41.11) | |
| Drug-use, n (%) | ||||
| Antibiotics | 14 (29.79) | 33 (76.74) | 47 (52.22) | <0.001 |
| NSAIDs | 18 (38.3) | 29 (67.44) | 47 (52.22) | 0.006 |
| Ribavirin | 16 (34.04) | 17 (39.53) | 33 (36.67) | 0.59 |
| Oseltamivir | 11 (23.4) | 17 (39.53) | 28 (31.11) | 0.10 |
| Herbal | 42 (89.36) | 43 (100) | 85 (94.44) | 0.03 |
| Interferon | 38 (80.85) | 41 (95.35) | 79 (87.78) | 0.04 |
| Lopinavir/ritonavir | 45 (95.74) | 41 (95.35) | 86 (95.56) | 0.93 |
| Multi-organ failure, n (%) | 0 (0) | 10 (23.26) | 10 (11.11) | – |
ALT, alanine aminotransferase; AST, aspartate transaminase; ALP, alkaline phosphatase; GGT, gamma-glutamyltransferase; NSAIDs, non-steroidal anti-inflammatory drugs; TBIL, total bilirubin abnormal; ULN, upper limit of normal.
Abnormality type (1): Patients who had raised ALT and/or AST more than 3× the ULN were classified as hepatocyte type; patients who had raised ALP or GGT twice the ULN were classified as cholangiocytes type; and patients who had a raised combination of both ALT/AST more 3 time the ULN and ALP/GGT twice the ULN were classified as mixed type. Abnormality type (2): patients were classified as hepatocyte type when the AST/ALT activity was higher than the ALP/GGT activity and were classified as cholangiocyte type when the reverse occurred.
Association of abnormal liver function tests and COVID-19 disease severity by multivariate analysis
Table 4 shows that, after adjustment for age, sex, epidemiological history, liver comorbidities, and initial symptoms, abnormality or injury as indicated by liver tests at admission was not associated with disease severity. Using the definition of abnormality type (1), patients with hepatocellular type were at almost 3-fold greater risk of severe COVID-19 compared to those without liver test abnormalities (OR 2.73; CI 1.19–6.30; p = 0.02) and those with a mixed abnormality were at 4.44 higher odds of severe disease (OR 4.44; CI 1.93–10.23; p <0.001). Moreover, when using the definition of abnormality type (2), patients who were classified as hepatocellular type and cholestatic type were at ∼3-fold greater risk of developing severe COVID-19 (OR 3.83; 95% CI 1.45–10.11; p = 0.007 and OR 3.45; 95% CI 1.25–9.5; p = 0.02, respectively). Sensitivity analyses excluding 21 patients with preexisting liver disease showed similar results, with the adjusted ORs (95% CIs) for severity being 1.73 (0.94–3.16) and 1.86 (0.58–5.92) in patients with abnormal liver tests and liver injury, respectively (Table not shown).
Table 4.
Association of abnormal liver test results with COVID-19 severity (severe vs. non-severe).
| Crude OR (95% CIs) | p value | Adjusted OR (95% CIs)† | p value | |
|---|---|---|---|---|
| At admission | ||||
| Liver tests | ||||
| Normal | 1.00 | 1.00 | ||
| Abnormal | 1.93 (1.18–3.16) | 0.009 | 1.64 (0.91–2.95) | 0.10 |
| Injury | 3.94 (1.55–10.03) | 0.004 | 2.03 (0.69–5.98) | 0.20 |
| Abnormality type (1)‡ | ||||
| Normal | 1.00 | 1.00 | ||
| Hepatocellular | 5.26 (2.58–10.75) | <0.001 | 2.73 (1.19–6.3) | 0.02 |
| Cholestatic | 1.07 (0.54–2.13) | 0.84 | 1.28 (0.58–2.82) | 0.55 |
| Mixed | 4.82 (2.42–9.6) | <0.001 | 4.44 (1.93–10.23) | <0.001 |
| Abnormality type (2)‡ | ||||
| Normal | 1.00 | 1.00 | ||
| Hepatocellular | 2.34 (1.36–4.03) | 0.002 | 1.64 (0.86–3.12) | 0.14 |
| Cholestatic | 1.83 (1.00–3.35) | 0.049 | 1.75 (0.84–3.64) | 0.14 |
| Peak values of liver test parameters during hospitalization | ||||
| Liver tests | ||||
| Normal | 1.00 | 1.00 | ||
| Abnormal | 3.5 (1.44–8.53) | 0.006 | 2.48 (0.94–6.55) | 0.07 |
| Injury | 14.18 (5.63–35.7) | <0.001 | 9.04 (3.19–25.6) | <0.001 |
| Abnormality type (1)‡ | ||||
| Normal | 1.00 | 1.00 | ||
| Hepatocellular | 4.48 (1.8–11.15) | 0.001 | 3.19 (1.15–8.84) | 0.03 |
| Cholestatic | 1.14 (0.4–3.26) | 0.81 | 1.09 (0.34–3.49) | 0.88 |
| Mixed | 10.77 (4.88–23.78) | <0.001 | 11.22 (4.42–28.45) | <0.001 |
| Abnormality type (2)‡ | ||||
| Normal | 1.00 | 1.00 | ||
| Hepatocellular | 6.05 (2.49–14.71) | <0.001 | 3.83 (1.45–10.11) | 0.007 |
| Cholestatic | 5.17 (2.08–12.83) | <0.001 | 3.45 (1.25–9.5) | 0.02 |
ALT, alanine aminotransferase; AST, aspartate transaminase; ALP, alkaline phosphatase; GGT, gamma-glutamyltransferase; OR, odds ratio; ULN, upper limit of normal.
Adjusted for age, sex, epidemiological history, body mass index, liver comorbidity and cough.
Abnormality type (1): Patients who had raised ALT and/or AST more than 3× the ULN were classified as hepatocyte type; patients who had raised ALP or GGT twice the ULN were classified as cholangiocytes type; and patients who had a raised combination of both ALT/AST more 3 time the ULN and ALP/GGT twice the ULN were classified as mixed type. Abnormality type (2): patients were classified as hepatocyte type when the AST/ALT activity was higher than the ALP/GGT activity, and were classified as cholangiocyte type when the reverse occurred.
After similar adjustment, patients with liver injury were at a 9-fold greater risk of severe COVID-19 (OR 9.04; 95% CI 3.19–25.6; p <0.001). Having hepatocyte type or a mixed type (OR 3.19; 95% CI 1.15–8.84, and 11.22; 95% CI 4.42–28.45, respectively) also increased the odds of developing severe disease. Sensitivity analyses excluding 21 patients with preexisting liver disease showed similar results, with the adjusted ORs (95% CIs) for severity being 2.41 (0.91–6.42) and 9.62 (3.34–27.7) in patients with abnormal liver tests and liver injury during hospitalization, respectively (Table not shown).
Moreover, after similar adjustment, patients treated with ACE-inhibitors/angiotensin II receptor blockers (ARBs) did not show differential odds for severe disease compared to patients taking other antihypertensive drugs (i.e., nifedipine), with the adjusted OR being 0.70 (95% CI 0.20–2.36; p = 0.56) (Table not shown).
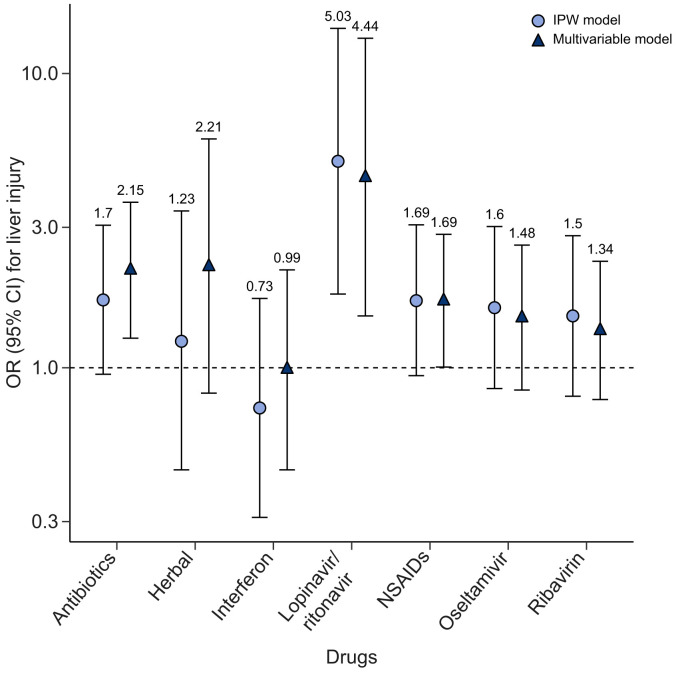
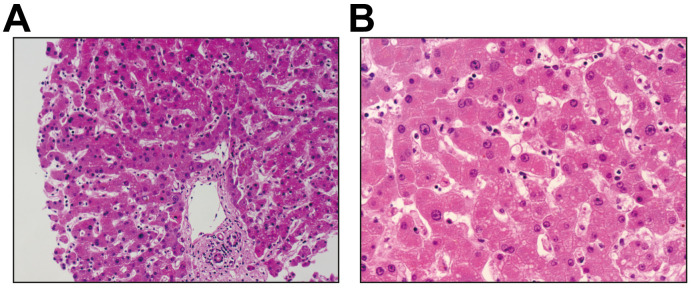
Compared to those without the use of the suspected drugs that may lead to liver dysfunction (including antibiotics, NSAIDs, ribavirin, herbal medications, and interferon), no significant evidence showed the use of such drugs led to a higher risk of liver injury (p >0.05 in IPW estimation), except for lopinavir/ritonavir (OR 4.44; 95% CI 1.50–13.17 in multivariable model and 5.03, 1.78–14.23 in the IPW estimation; p <0.01) (Fig. 2 ). Patients who used lopinavir/ritonavir had much higher levels of TBIL and GGT during hospitalization (p from <0.004) (Table S1). One patient aged 69 years who died from COVID-19 had a liver biopsy. Histological examination showed no obvious inflammation in the portal area (Fig. 3 A). The structure of the interlobular bile duct, interlobular vein, and interlobular artery were clear; the hepatocytes in the interlobular region were orderly, and a few hepatocytes were observed to have slightly vesicular steatosis and watery degeneration (probably due to ischemia and hypoxia). In Fig. 3B, slight vesicular steatosis and watery degeneration were observed in the hepatocytes, and a few inflammatory cells (neutrophils, plasma cells, and Kupffer cells) were found in the hepatic sinuses.
Fig. 2.
Adjusted odds ratios (95% CIs) for liver injury associated with use of drugs in patients with COVID-19.
All results were adjusted for radiography image grade at admission, age, sex, body mass index and comorbidities. Triangle legends for results from multivariable regression and circle legends for inverse probability weighting. Levels of significance: both p values <0.01 for lopinavir/ritonavir; all p values >0.05 for other drugs (multivariable logistic regression). IPW, inverse probability weighting; NSAIDs, non-steroidal anti-inflammatory drugs.
Fig. 3.
Liver biopsy of 1 patient aged 69 years who died from the COVID-19.
(A) (20×) There was no obvious inflammation in the portal area. The structure of interlobular bile duct, interlobular vein and interlobular artery was clear; the hepatocytes in the interlobular were arranged orderly, and a few hepatocytes were observed slightly vesicular steatosis and watery degeneration (possibly related to ischemia and hypoxia). (B) (40×) The hepatocytes were observed slightly vesicular steatosis and watery degeneration, and a few inflammatory cells (neutrophils, plasma cells and Kupffer cells) were found in hepatic sinuses.
Discussion
Our study is the first, and perhaps most comprehensive, to describe the liver test results in patients with COVID-19. Compared to patients with normal liver tests at admission, those who had abnormal liver test results, especially in hepatocyte type or mixed type, had significantly higher risks of developing severe pneumonia. As almost all patients had liver tests at admission, liver test abnormalities can be used as a predictor for the severity of the disease. More than 90% of patients with abnormal liver tests were mild at admission (i.e., with <2× ULN), and about 24% of them developed increased ALT and GGT levels to substantially more than 3× ULN during hospitalization. However, the increase in AST and TBIL to more than 3× ULN were moderate (12% and 15%, respectively), and no increase in ALP was found. Patients with elevated liver enzymes classified as hepatocyte type at admission or during hospitalization had significantly higher odds of progressing to severe COVID-19. After admission, the use of drugs, especially lopinavir and ritonavir, was the most important risk factor for liver damage. The use of lopinavir/ritonavir increased the odds of liver injury by 4-fold. Thus, it is suggested to closely monitor patients who used these particular therapies, especially in those who had abnormal liver test results at admission.
The liver test abnormality rate in our study was higher than previously reported and only a small portion had underlying liver disease, suggesting that liver damage in patients with coronavirus infection might be directly caused by the viral infection of liver cells.22 Two recent studies showed that ACE2 was the key receptor for SARS-CoV-2 cell entry,23 , 24 which was mainly localized in the heart, kidney, and testes, and expressed at a low level in many other tissues, especially in the colon and lung.25 Another recent study showed that SARS-CoV-2 might directly bind to ACE2 positive cholangiocytes and cause liver damage,7 which may partially explain the contribution of SARS-CoV-2 infection to the liver test dysfunction in our patients. Moreover, the use of ACE-inhibitors and ARBs might also affect liver tests. All patients in this study with hypertension used ACE-inhibitors/ARBs at admission, subsequently, these medications may have influenced their abnormal liver tests. However, although we found that those receiving ACE-inhibitors/ARBs appeared to have a higher percentage of abnormal liver tests at admission (15.6–28.6% vs. 11.1%), the difference was not statistically significant. Additionally, we excluded these 58 patients with hypertension at admission and found that the prevalence of abnormal liver function tests remained similar (from 46% to 44%), suggesting that the influence of ACE-Is/ARBs drugs on liver tests at admission, if any, should be minor. Moreover, our study showed that patients treated with ACE-inhibitors/ARBs were not at increased odds of progressing to severe disease compared to patients taking other antihypertensive drugs. The prevalence of abnormal liver tests increased to 76% during hospitalization, which could be due to a more frequent examination (i.e., every 3 to 5 days) and drugs used during hospitalization. Note that 84% of patients used lopinavir/ritonavir during hospitalization, drugs which have been reported to cause liver damage and affect liver tests.26
In our study, GGT was elevated substantially at admission and increased to a much higher level during hospitalization, whereas the increase in ALP was not pronounced. Both GGT and ALP were considered as “cholangiocyte-related enzymes”. However, besides the bile duct, ALP is present in bone, intestine, kidney, and placenta, while GGT is mainly distributed in the cell membranes of many tissues including kidneys, bile duct, pancreas, gallbladder, spleen, heart, brain, and seminal vesicles. Hence, for bile duct injury, ALP is more sensitive than GGT. For patients with elevated GGT level and normal ALP, drug-induced liver injury and injury in other organs should be considered, but cannot be classified into the bile duct type.27 , 28
We observed a substantial increase in the incidence of liver injury after admission. In our study, about 90% patients with abnormal liver test results were mild at admission (i.e., with <2× ULN), and more than 10% of them had increased levels of ALT and GGT (more than 3× ULN) during hospitalization. The increases in AST and TBIL to more than 3× ULN were moderate (about 6% and 3%, respectively), and no increase in ALP was found. For these patients, there were few other factors affecting liver test abnormality, such as underlying liver disease and drug usage, hence, we speculate that the disease itself is the most likely cause of this change. However, for hospitalized patients, more attention should be paid to drug-induced liver damage.
In addition, the liver biopsy specimens of the patient who died from COVID-19 showed that the raised liver enzymes during hospitalization could be partly due to the drugs used for treatment, and the observed liver test abnormality might be due to sepsis and shock. The 2 cases of liver failure occurring in patients with sepsis and multiple organ failure also support this hypothesis.
As it may take years to develop new agents specifically for SARS-CoV-2, an efficient approach is to test whether existing antiviral drugs are effective in treating the SARS-CoV-2 infection. Our study showed that the use of lopinavir/ritonavir was associated with 4× higher odds of liver injury. Lopinavir/ritonavir have previously been used to treat patients with SARS23 and HIV24 infections in China and are widely used (84%) in our patients with COVID-19. However, given the potentially high risk of liver injury, efficacy and safety of these therapies warrant further investigation. Moreover, in China, drug-induced liver impairment is most frequently reported with antibiotics and Chinese herbal medicine.29 The present study suggested that the use of antibiotics, but not Chinese herbal medicine, was associated with liver injury in patients with SARS-CoV-2 infection in the multivariable regression model. Although the results from the IPW model did not show statistical significance, it is also important for clinicians to be aware of these cases, which may need to be carefully monitored.
Compared to patients with normal liver tests, those who had abnormal liver test results, especially in hepatocyte or mixed type (i.e., raised ALT/AST, or both ALT/AST and ALP/GGT) at admission or during hospitalization had significantly higher odds of progressing to severe COVID-19. Exacerbation to severe pneumonia is an important clinical endpoint, indicating a higher mortality rate, requiring ICU support, or mechanical ventilation. In previous studies, the risk factors of severe COVID-19 included age, gender, and underlying diseases.8 , 9 , [30], [31], [32] This is one of the first reports to highlight abnormal liver tests related to severe disease. It is speculated that the SARS-CoV-2 virus is not only highly transmissible, but may also cause severe multi-organ dysfunction in humans,33 , 34 and our results, to some extent, support this hypothesis.
The present study has some limitations. First, as patients of this study were from a single, large city in China, these findings cannot be generalized to rural communities or other regions of varying epidemiological characteristics. Second, as very few patients died from the disease in our study (only 3 patients died during the course of the study), the potential influence of liver abnormality or injury on mortality cannot be assessed. As new cases are emerging globally, further studies in large patient series are warranted. In addition, data on other causes of liver injury in the patients progressing to liver injury, such as herbal medicines or other drugs used as self-medication before developing COVID-19 pneumonia, were not available from our patients. However, since the start of the COVID-19 outbreak in China in December 2019, discussion about COVID-19 has spread rapidly on the Internet and has quickly become the focus of worldwide attention. Shenzhen is one of the most developed cities in China and access to the Internet is widespread, meaning many in the population would have a high degree of awareness of the disease. Most (i.e., >70%) patients went to the hospital within 5 days after the onset disease symptoms and rarely used over-the-counter medicine to self-treat because medical insurance is widely available in Shenzhen. Hence, although the data on herbs and other drugs used as self-medication before developing COVID-19 pneumonia was not available in our study, their influence on the results might not be substantial. Lastly, we did not apply the definition of drug-induced liver injury from the European Association for the Study of the Liver clinical practice guidelines,35 as currently there was no evidence indicating that the abnormal liver injury during hospitalization was fully induced by the drugs used.
In conclusion, we estimated the clinical characteristics of COVID-19 pneumonia in patients with abnormal liver test results. Patients with abnormal liver tests were at increased risk of progressing to severe disease. The detrimental effects on liver injury mainly related to certain medications used during hospitalization, and should be monitored and evaluated frequently.
Abbreviations
ACE, angiotensin-converting enzyme; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ARBs, angiotensin II receptor blockers; AST, aspartate aminotransferase; BMI, body mass index; COVID-19, coronavirus disease 2019; GGT, gamma-glutamyltransferase; ICU, intensive care unit; IPW, inverse probability weighting; NAFLD, non-alcoholic fatty liver disease; NSAID, non-steroidal anti-inflammatory drugs; OR, odds ratio; SARS-CoV2, severe acute respiratory syndrome coronavirus 2; TBIL, total bilirubin; ULN, upper limit unit of normal; WHO, World Health Organization.
Financial support
This work is supported by the National Clinical Research Center for Infectious Diseases, Funds for the construction of key medical disciplines in Shenzhen and the Sanming Project of Medicine in Shenzhen (SZSM201612014).
Authors' contributions
JC and LL had the idea for and designed the study, received the grant supports and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. QC, DH, HY and XL contributed to the writing of the report. LX, HY, ZZ and QH contributed to the critical revision of the report. QC, and XL contributed to the statistical analysis. All authors contributed to data acquisition, data analysis, or data interpretation, and reviewed and approved the final version.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank Sarah Robbins Scott, an independent consultant for her help in English writing.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2020.04.006.
Supplementary data
References
- 1.Dong Y., Liang X., Yu X. Prognostic value of the dynamic changes in extra vascular lung water index and angiopoietin-2 in severe multiple trauma patients with acute respiratory distress syndromeZhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31:571–576. doi: 10.3760/cma.j.issn.2095-4352.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Niu P., Shen J., Zhu N., Lu R., Tan W. Two-tube multiplex real-time reverse transcription PCR to detect six human coronaviruses. Virologica Sinica. 2016;31:85–88. doi: 10.1007/s12250-015-3653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia Z., Yan L., Ren Z., Wu L., Wang J., Guo J. Delicate structural coordination of the Severe Acute Respiratory Syndrome coronavirus Nsp13 upon ATP hydrolysis. Nucleic Acids Res. 2019;47:6538–6550. doi: 10.1093/nar/gkz409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. Published online February 24, 2020. [DOI] [PubMed] [Google Scholar]
- 6.Chau T.N., Lee K.C., Yao H., Tsang T.Y., Chow T.C., Yeung Y.C. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chai X., Hu L., Zhang Y., Han W., Lu Z., Ke A. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020 doi: 10.1101/2020.02.03.931766. [DOI] [Google Scholar]
- 8.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian S., Hu N., Lou J., Chen K., Kang X., Xiang Z. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80:401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. Published online February 28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. Published online 19 February 2020. [DOI] [PubMed] [Google Scholar]
- 14.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. Published online February 18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Available at.
- 17.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L. Presumed Asymptomatic Carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. Published online February 21, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metlay J.P., Waterer G.W., Long A.C., Anzueto A., Brozek J., Crothers K. Diagnosis and treatment of Adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Health Commission of the People's Republic of China Handbook of Prevention and Treatment of the Pneumonia Caused by the Novel Coronavirus (2019-nCoV) (in Chinese) 2020. http://en.nhc.gov.cn/2020-02/06/c_76295.htm Updated: February 6, 2020. Available at.
- 20.European Association for the Study of the Liver, European Association for the Study of Diabetes EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Obes Facts. 2016;9:65–90. doi: 10.1159/000443344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020 doi: 10.1016/S2468-1253(20)30057-1. Published online March 04, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. Published online March 05, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke N.E., Turner A.J. Angiotensin-converting enzyme 2: the first decade. Int J Hypertens. 2012;2012:307315. doi: 10.1155/2012/307315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meraviglia P., Schiavini M., Castagna A., Viganò P., Bini T., Landonio S. Lopinavir/ritonavir treatment in HIV antiretroviral-experienced patients: evaluation of risk factors for liver enzyme elevation. HIV Med. 2004;5:334–343. doi: 10.1111/j.1468-1293.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 27.Dillon J.F., Miller M.H. SAGE Publications Sage UK; London, England: 2016. Gamma glutamyl transferase ‘To be or not to be’a liver function test? [DOI] [PubMed] [Google Scholar]
- 28.Fernandez N.J., Kidney B.A. Alkaline phosphatase: beyond the liver. Vet Clin Pathol. 2007;36:223–233. doi: 10.1111/j.1939-165x.2007.tb00216.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y., Niu M., Chen J., Zou Z-s, Ma Z-j, Liu S-h. Hepatobiliary and pancreatic: comparison between Chinese herbal medicine and Western medicine-induced liver injury of 1985 patients. J Gastroenterol Hepatol. 2016;31:1476–1482. doi: 10.1111/jgh.13323. [DOI] [PubMed] [Google Scholar]
- 30.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacLaren G., Fisher D., Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020 doi: 10.1001/jama.2020.2342. Published online February 19, 2020. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W. Imaging changes of severe COVID-19 pneumonia in advanced stage. Intensive Care Med. 2020:1–3. doi: 10.1007/s00134-020-05990-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrade R.J., Aithal G.P., Bjornsson E.S., Kaplowitz N., Kullak-Ublick G.A., Karlsen T.H. EASL clinical practice guidelines: drug-induced liver injury. J Hepatol. 2019;70:1222–1261. doi: 10.1016/j.jhep.2019.02.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.