Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was declared a pandemic by the World Health Organization on March 11, 2020.1 This virus is responsible for a clinical condition, defined as coronavirus disease 2019 (COVID-19), with a clinical spectrum from mild respiratory and/or gastrointestinal symptoms to interstitial pneumonia with acute respiratory distress syndrome, possible multiorgan failure, and death.2 COVID-19 severity directly correlates with patient’s age and presence of comorbidities.3 Proinflammatory cytokines may play an important role, especially in the more severe form of COVID-19. As a consequence, patients undergoing immunosuppressive therapy might be protected to some extent from the SARS-CoV-2 infection and its complications.4 Indeed, COVID-19 has not been described in solid organ transplant recipients so far.5 Current American Association for the Study of Liver Diseases recommendations acknowledge that post-transplant immunosuppression is not a risk factor for mortality associated with SARS-CoV-2 infection “per se,” but the recommendations underline that post-transplant recipients older than 60 years are more likely to acquire SARS-CoV-2 infection. Therefore, the American Association for the Study of Liver Diseases suggests adoption of stringent prevention measures for liver transplant recipients.6
In this article, we report the results of a survey administered to a large series of transplanted patients who were followed at the Maggiore Hospital Policlinico, Milan.
Materials and Methods
At the beginning of the outbreak in Italy, we recommended to all liver transplant recipients the following measures to prevent SARS-CoV-2 infection: frequent handwashing and sanitization, avoid public places and overcrowded situations, and wear a surgical mask in every public place. We later minimized in-person visits by improving the use of telemedicine.
In March, we performed a survey of our liver transplant patients to verify adherence to the preventive measures against SARS-CoV-2 and compliance with the seasonal anti-flu vaccination. We also inquired about possible symptoms or signs of COVID-19. All suspected cases were further investigated for a diagnosis.
Results
In total, 640 patients completed the survey. The epidemiologic and clinical features of this population are reported in Supplementary Table 1. From the beginning of the pandemic in Italy as of April 4, 2020, our data show that 516 recipients (81%) adhered to at least 2 preventive measures, and 455 (71%) received the seasonal anti-flu vaccination. Thirty-four patients (5.3%) experienced a flu-like syndrome, complicated by bronchitis or bacterial pneumonia in 6; all recovered with antibiotic therapy. COVID-19 was diagnosed in 8 symptomatic recipients (overall prevalence, 1.25%), including 1 patient previously classified as having a flu-like syndrome. Epidemiologic and clinical features are reported in Table 1 .
Table 1.
Epidemiologic and Clinical Features of the Eight Liver Transplant Recipients With a Diagnosis of COVID-19
| #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | |
|---|---|---|---|---|---|---|---|---|
| Age, y | 60 | 78 | 65 | 57 | 57 | 62 | 75 | 50 |
| Sex | M | M | M | M | M | F | M | F |
| Region | Lombardy | Lombardy | Lombardy | Lombardy | Lombardy | Lombardy | Lombardy | Lombardy |
| Province | Brescia | Brescia | Lecco | Lecco | Milano | Milan | Brescia | Milan |
| Time since LT, mo | 36 | 230 | 65 | 187 | 96 | 137 | 211 | 3 |
| Immunosuppression | CNI + MMF | CNI + MMF | Steroids | CNI + MMF | CNI + MMF | CNI + MMF | CNI + MMF | CNI + steroids |
| Seasonal anti-flu vaccination | Yes | Yes | No | No | Yes | Yes | No | No |
| Cardio-pulmonary comorbidities | No/no | Yes/no | No/yes | Yes/yes | No/no | No/yes | Yes/no | Yes/yes |
| Start of symptoms | February 28 | March 11 | March 15 | March 15 | March 18 | March 23 | March 24 | March 25 |
| COVID-19 diagnosis | Nasal swab chest x-ray | Nasal swab chest-CT scan | — Chest-CT scan | Nasal swab chest x-ray | Nasal swab chest x-ray | Nasal swab chest-CT scan | Nasal swab chest x-ray | Nasal swab chest-CT scan |
| Fever | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Respiratory symptoms | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Diarrhea | No | No | No | No | No | No | Yes | No |
| Pneumonia | No | Yes | Yes (mild) | No | Yes (mild) | Yes (mild) | Yes (mild) | Yes |
| Hospitalization | No | Yes (noninvasive ventilation) | No | No | Yes (discharged) | Yes (discharged) | Yes (discharged) | Yes (noninvasive ventilation) |
CNI, calcineurin inhibitor; COVID-19, corona virus disease 2019; CT, computed tomography; LT, liver transplantation; MMF, mycophenolate.
Discussion
In this study we report a real-life “snapshot” of a large cohort of liver transplanted patients during the SARS-CoV-2 outbreak in Italy. Our data show an excellent adherence rate to the recommended preventive measures (84%); this high compliance with preventive strategies might have contributed to the relatively low prevalence of SARS-CoV-2 infection in our population. Nevertheless, some patients from our cohort of liver transplant recipients developed COVID-19, with an observed 1.25% infection rate so far. Noteworthy, most of the cases (75%) had a mild disease (3 quarantined and 3 rapidly discharged), whereas 2 patients are still hospitalized.
These findings slightly differ from those reported by D’Antiga7 in which some infected patients but no cases of COVID-19 were described. The different populations investigated by the 2 groups could explain the disparities; our cohort in Milan concerns only adult patients, whereas D’Antiga’s cohort in Bergamo mainly included pediatric transplanted patients. Results may also be affected by the different time frames in the 2 reports. However, both studies showed that transplant settings may differ from the general population, possibly in regard to a high degree of surveillance at individual patient level and a milder disease expression that could be related to immune-suppressed status of liver transplant patients. Notably, all patients with COVID-19 live in Lombardy (Supplementary Figure 1), the region with the highest prevalence of infection in Italy: 10 million inhabitants with 63,094 ascertained infected people and 11,608 virus-related deaths. However, these data are not exhaustive for a correct estimation of the virus spreading in the general population because of the limited number of diagnoses performed (overall nasopharyngeal swabbing, 232,674).8
Supplementary Figure 1.
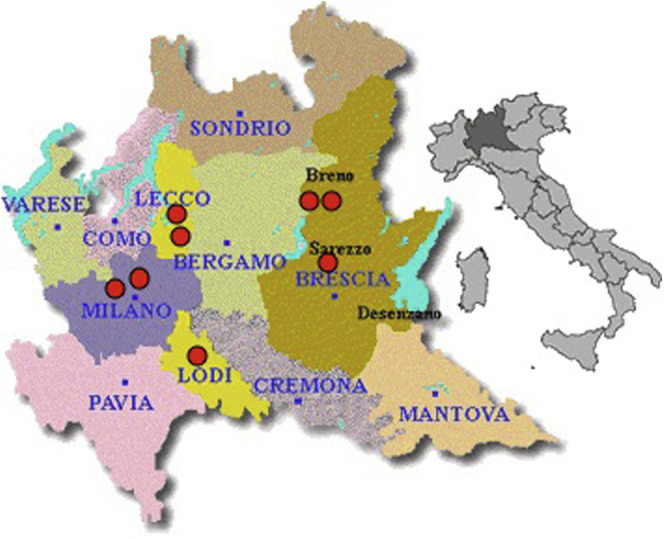
Geographical distribution of the 8 transplanted recipients with a diagnosis of COVID-19 in Lombardy. COVID-19, corona virus disease 2019.
The main merit of this preliminary study is the large sample size, with a homogeneous collection of data from a single center. The main limitations are related to the observational nature of the study and the short duration of follow-up. Overall, our study provides support for the use of telemedicine to deliver care to liver transplant recipients. Long-term clinical and epidemiologic studies in the transplant setting will be of great utility in the field.
Acknowledgments
The authors thank Miss Donatella Dessì for secretarial assistance and support in taking care of post-transplant patients.
Footnotes
Conflicts of interest This author discloses the following: Dr Lampertico advises and is on the speakers’ bureau for Janssen, MYR Pharmaceuticals, GlaxoSmithKline, Gilead Sciences, AbbVie, Roche, Eiger, Alnylam, Bristol-Myers Squibb, and Merck Sharp & Dohme. The remaining authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2020.04.041.
Supplementary Material
Supplementary Table 1.
Demography of the 640 Liver Transplant Recipients Enrolled in the Survey
| Overall, N = 640 | |
|---|---|
| Age, y, median (range) | 63 (20–83) |
| Age >60 y, N (%) | 378 (59) |
| Male, N (%) | 455 (71) |
| Region, N (%) | |
| - Lombardy | 523 (82) |
| - North Italy | 31 (5) |
| - Central-South Italy | 86 (13) |
| HCV etiology, N (%) | 241 (38) |
| Time since LT, mo, median (range) | 98 (3–410) |
| CNI-based immunosuppression, N (%) | 627 (98) |
| Season anti-flu vaccination, N (%) | 455 (71) |
| Preventive measures, N (%) | |
| One | 614 (96) |
| Two to three | 516 (81) |
CNI, calcineurin inhibitor; HCV, hepatitis C virus; LT, liver transplantation.
References
- 1.WHO Director-General’s opening remarks at the media briefing on COVID-19: March 11, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Available online:
- 2.Wu Z., McCoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 Mar 13 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michaels G.M., La Hoz R.M., Danzider-Isakov L. Coronavirus disease 2019: implications of emerging infections for transplantation. Am J Transplant. 2020 Feb 24 doi: 10.1111/ajt.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Association for the Study of Liver Diseases Clinical insights for hepatology and liver transplant providers during the Covid-19 pandemic. 2020. https://www.aasld.org/sites/default/files/2020-03/AASLD-COVID19-ClinicalInsights-3-23-2020FINAL-v2.pdf Available at:
- 7.D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020 Mar 20 doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 8.Ministry of Health Covid-19, situation in Italy 2020. http://www.salute.gov.it Available at:


