Abstract
Whether patients with atrial fibrillation (AF) and thyroid disease are clinically distinct from those with AF and no thyroid disease is unknown. Furthermore, the effectiveness of anticoagulation for prevention of AF-related thromboembolic events in patients with thyroid disease has not been adequately studied. Patients enrolled in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation, which compared apixaban with warfarin in patients with AF (n = 18,201), were categorized by thyroid disease history at randomization (hypothyroidism, hyperthyroidism, and no thyroid disease). Adjusted hazard ratios derived from Cox models were used to compare outcomes by thyroid disease history. Associations between randomized treatment and outcomes by thyroid disease history were examined using Cox models with interaction terms. A total of 18,021/18,201 (99%) patients had available thyroid disease history at randomization: 1,656 (9%) had hypothyroidism, 321 (2%) had hyperthyroidism, and 16,044 (89%) had no thyroid disease. When compared with those without a history of thyroid disease, patients with hypo- or hyperthyroidism were more likely to be female (60.4% vs 32.1%; 52.0% vs 32.1%; both p <0.0001). Patients with hypothyroidism were older (73 vs 70 years, p <0.0001) and more likely to have had previous falls (8.7% vs 4.3%, p <0.0001). There was no difference in clinical outcomes by thyroid disease history. The benefit of apixaban compared with warfarin was similar regardless of thyroid disease history (interaction p >0.10). In conclusion, despite differences in baseline characteristics of patients with and without thyroid disease, their clinical outcomes were similar. The benefit of apixban compared with warfarin was preserved regardless of thyroid disease history.
Atrial fibrillation (AF) is associated with an increased risk of cerebrovascular events and systemic embolism.1 The risk of embolic events associated with AF can be significantly reduced with oral anticoagulation, and randomized controlled trials have confirmed the efficacy and safety of nonvitamin K antagonist oral anticoagulants compared with warfarin.2–6 A close relation exists between AF and thyroid disease. Hyperthyroidism is strongly associated with the development of AF.7,8 Although hypothyroidism does not portend an increased risk of AF, there is considerable overlap between these conditions due to common risk factors.9 Thyroid function influences the hemostatic system, with response to systemic anticoagulation being unpredictable.10–13 Despite the known effects of thyroid disease on the development and treatment of AF, there remains a paucity of data describing the characteristics, outcomes and response to therapy of patients with AF and thyroid disease. In this secondary analysis of the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE), we compared clinical characteristics of patients with AF with and without thyroid disease; evaluated clinical outcomes according to thyroid disease history; and analyzed the treatment effect of apixaban compared with warfarin according to thyroid disease history.
Methods
Data for this analysis were obtained from the ARISTOTLE trial. The design and results of this trial have been previously published.2,14 Briefly, ARISTOTLE was a multinational, randomized, double-blind, controlled trial that evaluated whether apixaban, a direct factor Xa inhibitor, was non-inferior to warfarin for the prevention of stroke or systemic embolism in patients with AF enrolled 18,201 patients with AF or atrial flutter and at least 1 additional risk factor for stroke. If noninferiority of apixaban to warfarin was established, the trial was designed to examine superiority of apixaban over warfarin. Patients with moderate or severe mitral stenosis, prosthetic mechanical heart valves, AF due to a reversible cause, previous intracranial hemorrhage, severe renal insufficiency, stroke within 7 days before randomization, or need for aspirin at a dose of >165 mg per day or dual antiplatelet therapy were excluded. Patients were randomized to receive either dose-adjusted warfarin (target international normalized ratio (2.0 to 3.0) or apixaban (5 mg twice daily). Apixaban dosing was reduced to 2.5 mg twice daily in patients with 2 or more of the following criteria: age ≥80 years, weight ≤60 kg, or creatinine level ≥1.5 mg/dl. Median follow-up was 1.8 years. Ethics committees at all participating sites approved the protocol and all patients provided written informed consent.
Of the 18,201 patients enrolled in ARISTOTLE, we identified 18,021 with available thyroid disease history (hypothyroidism, hyperthyroidism, or no thyroid disease) at the time of randomization; 43 patients were excluded due to unavailable thyroid history, and 137 patients with thyroid disease were excluded due to unavailable thyroid disease type.
The classification of thyroid disease history (hypo- vs hyper- vs no disease) was determined based on investigator responses on the ARISTOTLE data intake form. Patients in whom the presence or type of thyroid disease was not available, but who were noted to be taking thyroid replacement hormone or antithyroid therapy were included as hypo- and hyperthyroid, respectively. Patients who were designated as having hyperthyroidism but were documented to be taking thyroid replacement hormone were categorized as being hypothyroid.
The primary outcome of this analysis was a combined end point of stroke (ischemic or hemorrhagic) or systemic embolism. The primary safety outcome was major bleeding, according to the criteria defined by the International Society on Thrombosis and Haemostasis. Secondary outcomes included any stroke, ischemic stroke (including unknown type of stroke), hemorrhagic stroke, all-cause death, myocardial infarction, hospitalization for heart failure, and any bleeding event.
Patients were grouped by categories of thyroid disease history (hypothyroidism, hyperthyroidism, and no thyroid disease) for all analyses. In each analysis, patients with hypothyroidism were compared with patients with no thyroid disease; patients with hyperthyroidism were compared with patients with no thyroid disease. Baseline characteristics of the 3 groups were summarized as medians (twenty-fifth and seventy-fifth percentiles) for continuous variables and as percentages (frequencies) for categorical variables. Continuous and categorical variables were compared across the 3 groups using the Kruskal-Wallis and chi-square tests. Pairwise comparisons between hypo- and hyperthyroidism and the no thyroid disease group were performed using the Wilcoxon and chi-square tests. Given the exploratory nature of these comparisons, corrections for multiplicity were not applied. Unadjusted and adjusted hazard ratios (HRs) comparing the hypothyroidism and hyperthyroidism groups with the no thyroid disease group were derived using Cox models. Variables for adjusted results were selected from previously developed models. Adjusted HRs derived from Cox models were used to compare outcomes of interest by thyroid disease category. Pairwise comparisons between each thyroid disease history group and the no thyroid disease history group were adjusted for multiplicity using simulations. Associations between randomized treatment (apixaban vs warfarin) and outcomes, stratified by thyroid disease history, were examined using a Cox model with interaction terms. All analyses were performed using SAS v9.4 TS1M4 (SAS, Inc., Cary, North Carolina).
Results
Of the 18,021 patients who met the inclusion criteria and had available thyroid disease history at randomization, 1,656 (9%) had a history of hypothyroidism, 321 (2%) had a history of hyperthyroidism, and 16,044 (89%) as had no history of thyroid disease. Baseline characteristics of the study population are shown in Table 1.
Table 1.
Baseline characteristics by history of thyroid disease status
| Characteristic | History of thyroid disease |
p value* |
||||
|---|---|---|---|---|---|---|
| Hypothyroidism (n = 1,656) | Hyperthyroidism (n = 321) | No Disease (n = 16,044) | Hypo vs No | Hyper vs No | Overall | |
| Age, median (25th, 75th) (years) | 73 (66, 79) | 69 (62, 76) | 70 (62, 76) | <0.0001 | 0.8382 | <0.0001 |
| Women | 1001 (60.4%) | 167 (52.0%) | 5145 (32.1%) | <0.0001 | <.0001 | <0.0001 |
| Region of enrollment | <0.0001 | <.0001 | <0.0001 | |||
| North America | 695 (42.0%) | 56 (17.4%) | 3710 (23.1%) | |||
| Latin America | 290 (17.5%) | 36 (11.2%) | 3131 (19.5%) | |||
| Europe | 544 (32.9%) | 177 (55.1%) | 6477 (40.4%) | |||
| Asia | 127 (7.7%) | 52 (16.2%) | 2726 (17.0%) | |||
| Systolic BP, median (25th, 75th) (mm Hg) | 130 (120, 140) | 130 (120, 140) | 130 (120, 140) | 0.0028 | 0.4882 | 0.0099 |
| Weight, median (25th, 75th) (kilograms) | 80 (69, 95) | 78 (66, 90) | 82 (70, 96) | 0.0526 | <.0001 | <0.0001 |
| Prior Myocardial Infarction | 235 (14.2%) | 46 (14.3%) | 2276 (14.2%) | 0.9884 | 0.9451 | 0.9976 |
| Prior stroke, transient ischemic attack, or systemic embolism | 319 (19.3%) | 74 (23.1%) | 3110 (19.4%) | 0.9056 | 0.1002 | 0.2540 |
| Heart failure or reduced left ventricular ejection fraction | 478 (28.9%) | 108 (33.6%) | 5764 (35.9%) | <.0001 | 0.3988 | <0.0001 |
| Hypertension requiring treatment | 1472 (88.9%) | 289 (90.0%) | 13981 (87.1%) | 0.0420 | 0.1250 | 0.0435 |
| Prior clinically relevant or spontaneous bleeding | 378 (22.8%) | 66 (20.6%) | 2582 (16.1%) | <.0001 | 0.0315 | <0.0001 |
| History of fall within previous year | 132 (8.7%) | 13 (4.3%) | 601 (4.1%) | <.0001 | 0.8573 | <0.0001 |
| Diabetes Mellitus | 473 (28.6%) | 81 (25.2%) | 3956 (24.7%) | 0.0005 | 0.8125 | 0.0022 |
| End-organ damage | 96 (5.8%) | 18 (5.6%) | 650 (4.1%) | 0.0007 | 0.1644 | 0.0017 |
| Type of Atrial fibrillation | <.0001 | 0.0140 | <0.0001 | |||
| Paroxysmal | 312 (18.8%) | 63 (19.6%) | 2359 (14.7%) | |||
| Persistent or permanent | 1344 (81.2%) | 258 (80.4%) | 13682 (85.3%) | |||
| Atrial fibrillation/Flutter | 1333 (81.1%) | 256 (80.3%) | 13670 (85.7%) | <.0001 | 0.0066 | <0.0001 |
| Heart rate in atrial fibrillation/Flutter, median (25th, 75th) (beats per minute) | 77 (67, 90) | 80 (68, 92) | 79 (69, 90) | 0.0005 | 0.7391 | 0.0020 |
| Sinus rhythm | 262 (15.9%) | 56 (17.6%) | 2043 (12.8%) | 0.0003 | 0.0121 | 0.0001 |
| HR in sinus rhythm, median (25th, 75th) (beats per minute) | 61 (55, 67) | 64 (56, 71) | 62 (56, 70) | 0.0542 | 0.7259 | 0.1391 |
| Prior vitamin K antagonist use for >30 days | 1101 (66.5%) | 241 (75.1%) | 8978 (56.0%) | <0.0001 | <0.0001 | <0.0001 |
| CHADS2 score, mean (standard deviation) | 2.21 (1.12) | 2.20 (1.17) | 2.11 (1.10) | 0.0002 | 0.2773 | 0.0005 |
| CHADS2 score | 0.0005 | 0.6900 | 0.0033 | |||
| ≤1 | 493 (29.8%) | 108 (33.6%) | 5532 (34.5%) | |||
| 2 | 622 (37.6%) | 110 (34.3%) | 5718 (35.6%) | |||
| ≥3 | 541 (32.7%) | 103 (32.1%) | 4794 (29.9%) | |||
| Medications at randomization | ||||||
| ACE inhibitor or angiotensin receptor blocker | 1116 (67.8%) | 235 (74.4%) | 11331 (71.9%) | 0.0005 | 0.3263 | 0.0013 |
| Amiodarone | 185 (11.2%) | 18 (5.7%) | 1824 (11.6%) | 0.6915 | 0.0012 | 0.0050 |
| Beta-blocker | 1067 (64.8%) | 226 (71.5%) | 10076 (63.9%) | 0.4583 | 0.0052 | 0.0166 |
| Aspirin | 447 (27.0%) | 86 (26.8%) | 5048 (31.5%) | 0.0002 | 0.0741 | 0.0002 |
| Clopidogrel | 41 (2.5%) | 7 (2.2%) | 289 (1.8%) | 0.0533 | 0.6135 | 0.1427 |
| Digoxin | 483 (29.3%) | 106 (33.5%) | 5189 (32.9%) | 0.0033 | 0.8118 | 0.0126 |
| Any calcium channel blocker | 573 (34.8%) | 107 (33.9%) | 4842 (30.7%) | 0.0006 | 0.2292 | 0.0017 |
| Statin | 808 (49.1%) | 141 (44.6%) | 6481 (41.1%) | <.0001 | 0.2083 | <0.0001 |
| Nonsteroidal anti-inflammatory agent | 206 (12.5%) | 31 (9.8%) | 1278 (8.1%) | <.0001 | 0.2724 | <0.0001 |
| Gastric antacid drugs | 430 (26.1%) | 80 (25.3%) | 2828 (17.9%) | <.0001 | 0.0007 | <0.0001 |
| Antithyroid preparations | 2 (0.1%) | 71 (22.1%) | 0 (0.0%) | |||
| Thyroid preparations | 1200 (72.5%) | 0 (0.0%) | 0 (0.0%) | |||
| Iodine preparations | 7 (0.4%) | 0 (0.0%) | 1 (0.0%) | |||
| Any thyroid therapy | 1202 (72.6%) | 71 (22.1%) | 1 (0.0%) | |||
| Renal function | <.0001 | 0.3563 | <0.0001 | |||
| Normal (80 ml/min) | 535 (32.3%) | 129 (40.2%) | 6765 (42.2%) | |||
| Mild impairment (>50–80 ml/min) | 710 (42.9%) | 140 (43.6%) | 6667 (41.6%) | |||
| Moderate impairment (>30–50 ml/min) | 356 (21.5%) | 47 (14.6%) | 2326 (14.5%) | |||
| Severe impairment (≤30 ml/min) | 48 (2.9%) | 1 (0.3%) | 220 (1.4%) | |||
Exploratory.Data presented as no. (%), unless otherwise indicated.ACE = angiotensin converting enzyme; AF = atrial fibrillation; ARB = angiotensin receptor blocker; BP = blood pressure; bpm = beats per minute; HF = heart failure; HR = heart rate; LVEF = left ventricular ejection fraction; MI = myocardial infarction; SD = standard deviation; SE = systemic embolism; TIA = transient ischemic attack; VKA = vitamin K antagonist.
Table 2 shows the association between types of thyroid disease and the end points of interest, expressed as unadjusted and adjusted HRs. Covariates included in the adjusted models are provided as a footnote to Table 2. Clinical outcomes of patients with hypo- or hyperthyroidism were similar to those with no thyroid disease, including stroke/systemic embolism (adjusted HR 0.76, 95% confidence interval [CI] 0.51 to 1.14, p = 0.24; adjusted HR 0.59, 95% CI 0.21 to 1.60, p = 0.41), all-cause death (adjusted HR 0.91, 95% CI 0.72 to 1.14, p = 0.57; adjusted HR 0.95, 95% CI 0.57 to 1.61, p = 0.97), and major bleeding (adjusted HR 1.00, 95% CI 0.76 to 1.31, p = 1.00; adjusted HR 0.81, 95% CI 0.41 to 1.60, p = 0.73).
Table 2.
Association between types of thyroid disease and end points
| End point | Hypothyroidism |
Hyperthyroidism |
No thyroid disease | Overall p value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted‡ |
Unadjusted |
Adjusted‡ |
Unadjusted | Adjusted‡ | ||||||||
| Rate (events) | hazard ratio* (95% confidence Interval) | p value | hazard ratio* (95% confidence Interval) | p value | Rate (events) | hazard ratio* (95% confidence Interval) | p value | hazard ratio* (95% confidence Interval) | p value | Rate (events) | |||
| Stroke/systemic embolism | 1.13 (34) | 0.76 (0.51–1.13) | 0.23 | 0.76 (0.51–1.14) | 0.24 | 0.84 (5) | 0.57 (0.21–1.55) | 0.38 | 0.59 (0.21–1.60) | 0.41 | 1.48 (435) | 0.14 | 0.16 |
| Any stroke | 1.09 (33) | 0.78 (0.53–1.16) | 0.31 | 0.79 (0.53–1.20) | 0.36 | 0.50 (3) | 0.36 (0.10–1.308) | 0.15 | 0.38 (0.10–1.38) | 0.17 | 1.40 (410) | 0.09 | 0.11 |
| Ischemic stroke† | 0.99 (30) | 0.96 (0.63–1.47) | 0.98 | 0.96 (0.62–1.48) | 0.97 | 0.50 (3) | 0.49 (0.14–1.78) | 0.39 | 0.50 (0.14–1.83) | 0.40 | 1.03 (302) | 0.47 | 0.48 |
| Hemorrhagic stroke | 0.10 (3) | 0.26 (0.07–0.94) | 0.04 | 0.30 (0.08–1.11) | 0.08 | 0.00 (0) | – | – | – | – | 0.38 (114) | 0.07 | 0.12 |
| All-cause death | 3.48 (107) | 0.92 (0.73–1.15) | 0.61 | 0.91 (0.72–1.14) | 0.57 | 3.15 (19) | 0.83 (0.49–1.39) | 0.65 | 0.95 (0.57–1.61) | 0.97 | 3.81 (1145) | 0.50 | 0.63 |
| Myocardial infarction | 0.99 (30) | 1.82 (1.17–2.83) | 0.01 | 1.43 (0.90–2.26) | 0.16 | 0.17 (1) | 0.31 (0.03–2.88) | 0.42 | 0.33 (0.04–3.15) | 0.47 | 0.54 (161) | 0.01 | 0.11 |
| Heart failure hospitalization | 2.36 (71) | 1.03 (0.78–1.36) | 0.96 | 0.99 (0.74–1.31) | 0.99 | 3.27 (19) | 1.44 (0.86–2.42) | 0.22 | 1.45 (0.85–2.46) | 0.23 | 2.28 (670) | 0.29 | 0.30 |
| International Society on Thrombosis and Haemostasis major bleeding | 3.08 (82) | 1.19 (0.91–1.54) | 0.27 | 1.00 (0.76–1.31) | 1.00 | 2.22 (12) | 0.86 (0.45–1.64) | 0.84 | 0.81 (0.41–1.60) | 0.73 | 2.59 (694) | 0.29 | 0.79 |
| Any bleeding | 26.58 (558) | 1.22 (1.11–1.35) | <.0001 | 1.07 (0.96–1.19) | 0.29 | 22.79 (102) | 1.07 (0.86–1.34) | 0.75 | 1.05 (0.83–1.32) | 0.88 | 21.35 (4713) | <.0001 | 0.34 |
Hazard ratios vs no thyroid disease.
Includes unknown type of stroke.Pairwise p values and 95% confidence intervals corrected by multiplicity
Covariates included in adjusted models by outcome: Any Stroke and Ischemic Stroke: Adjusted by region, diabetes, hypertension, moderate or severe valvular heart disease, prior stroke, transient ischemic attack or systemic embolism, prior use of vitamin K antagonist >30 days, congestive heart failure, age, and weight (nonlinear).Hemorrhagic Stroke, ISTH Major Bleeding, and Any Bleeding: Adjusted by sex, region, coronary artery disease, prior myocardial infarction, history of bleeding, anemia, CHADS2 score, renal disease, and age.All-cause death: Adjusted by sex, region, hypertension, moderate or severe valvular heart disease, prior myocardial infarction, prior stroke, transient ischemic attack or systemic embolism, anemia, current smoker, prior use of vitamin K antagonist >30 days, New York Heart Association class, CHADS2 score, renal disease, congestive heart failure, age (nonlinear), systolic blood pressure (nonlinear), diastolic blood pressure (nonlinear), and weight.Myocardial Infarction: Adjusted by region, diabetes, coronary artery disease, prior myocardial infarction, New York Heart Association class, renal disease, congestive heart failure, and age (nonlinear).Heart failure hospitalization: Adjusted by diabetes, moderate or severe valvular heart disease, prior myocardial, renal disease, left bundle branch block, current smoker, New York Heart Association class, congestive heart disease, age (nonlinear), systolic blood pressure (nonlinear), and weight (nonlinear).
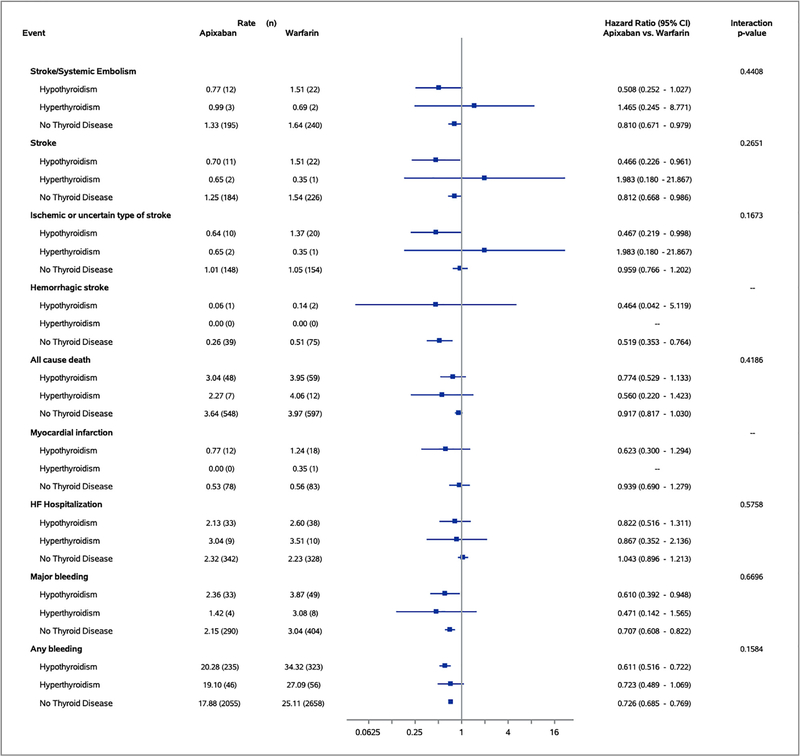
Figure 1 shows the associations between randomized treatments (apixaban vs warfarin) and end points, stratified by thyroid disease history. Interaction tests between randomized treatment and thyroid disease history were >0.10 for the primary outcome, primary safety end point, and all secondary outcomes including stroke/systemic embolism (interaction p value = 0.44), all-cause death (interaction p value = 0.42), and major bleeding (interaction p value = 0.67). These results demonstrate that apixaban was superior to warfarin in patients with and without thyroid disease, a finding that is consistent with the overall results of the ARISTOTLE trial.
Figure 1.
Association between randomized treatments and end points by thyroid disease status.
Discussion
The relation between thyroid disease and AF has been well described. The risk of AF is proportional to circulating levels of free thyroxine and inversely proportional to thyroid stimulating hormone level, with an increase in risk of AF present even in patients with subclinical hyperthyroidism.15 Furthermore, new onset AF in patients without a history of thyroid disease is an independent predictor of subsequent development of hyperthyroidism.16 Hypothyroidism is not associated with an increased risk of AF, but considerable overlap exists between these conditions given their high prevalence and similar risk factors.9
In general, hyperthyroidism is considered a prothrombotic condition whereas hypothyroidism may lead to a hypocoagulable state with an increased risk of bleeding.17,18 Additionally, thyroid dysfunction may affect response to oral anticoagulation, potentially making prevention of AF-related thrombotic events without the development of bleeding complications difficult.12,13,17 Despite this, there is a paucity of literature describing patients with both diagnoses. Furthermore, whether it is safe and effective to treat patients with AF and thyroid disease with traditional anticoagulation strategies is unknown. The current analysis addresses these issues through the following notable findings. Baseline characteristics of patients with AF and a history of thyroid disease differ significantly from those with AF without a history of thyroid disease. When compared with patients without a history of thyroid disease, those with AF and a history of thyroid disease have similar rates of stroke/systemic embolism, all-cause death, and major bleeding in the setting of oral anticoagulation. Finally, apixaban is superior to warfarin in patients with and without a history of thyroid disease for all investigated efficacy and safety end points.
We demonstrated differences in the baseline characteristics of patients with AF and a history of thyroid disease compared with those without thyroid disease. Patients with any thyroid disease history were more likely to live in North America, likely representing ascertainment bias associated with more frequent testing in this region. Additionally, patients with a history of thyroid disease were more likely to have previous use of a vitamin K antagonist for >30 days and have previous clinically relevant or spontaneous bleeding, possibly related to the effect of thyroid disease on the coagulation system. Finally, patients with a history of hyperthyroidism were less commonly taking amiodarone at the time of enrollment, likely due to concern that amiodarone use had caused or might exacerbate underlying hyperthyroidism and the potential development of thyroid storm. Interestingly, despite a known association between worsening of hypothyroidism and amiodarone use, use of this medication was similar in those with a history of hypothyroidism compared with those without a history of thyroid disease. This may be attributed to the fact that management of new or existing hypothyroidism in the amiodarone-treated patient is far less challenging than drug-induced hyperthyroidism.
Because hypo- and hyperthyroidism are believed to alter the coagulation system and potentially affect responses to oral anticoagulation, it has been hypothesized that thyroid disease may affect the efficacy and safety of AF-related stroke prevention. Furthermore, older age and increased falls seen in patients with hypothyroidism in our analysis may also increase the risk of bleeding complications. Despite this, our analysis showed that, with regard to the evaluated efficacy and safety end points, patients with AF and either hypo- or hyperthyroidism treated with oral anticoagulation had similar clinical outcomes compared with those without thyroid disease. Additionally, the finding in the larger ARISTOTLE trial that apixaban was superior to warfarin was preserved in this subgroup analysis, irrespective of thyroid disease history. Our findings suggest that patients with AF and thyroid disease should be managed with oral anticoagulation similarly to patients with AF and no thyroid disease.
To our knowledge, this is the first study examining differences in patient characteristics, clinical outcomes, and anticoagulation strategy in patients with AF and thyroid disease. Current American College of Cardiology/American Heart Association guidelines have few recommendations regarding the management of AF in patients with thyroid disease, and do not address anticoagulation strategies in this patient group.8 This novel analysis provides important insight regarding patients with AF and thyroid disease that can be used to further our understanding of how to clinically manage this important patient population. Given the significant differences in patient characteristics identified, however, future prospective and randomized trials should be aimed at validating and expanding upon these results.
Because this analysis was limited to patients enrolled in a clinical trial, the results may not be generalizable to all patients with AF and thyroid disease. Although we adjusted for baseline differences using multivariable analyses, patients were not randomized based on baseline thyroid disease history and thus our results represent associations as opposed to causal relations. Inherent to a secondary analysis is that some important information may not have been collected in the original trial and therefore was not available for our analysis. Specifically, we were only able to analyze patients based on baseline thyroid disease history at the time of study entry; details regarding changes in thyroid disease history and treatment during the study period were not available. Also, classification of thyroid disease may not have been accurate. Patients who were initially diagnosed with hypothyroidism could have developed hyperthyroidism due to over-supplementation. Likewise, some patients may have initially been diagnosed with hyperthyroidism and developed hypothyroidism as a result of therapy. Finally, patients with a history of thyroid disease who were adequately treated may have been functionally euthyroid while enrolled in the study. Thyroid hormone levels were not available to confirm the accuracy of the reported diagnosis. An analysis using hormone levels and specific thyroid treatments could yield different results. Finally, because only patients without a reversible cause of AF were included in ARISTOTLE, our study included a comparatively small number of patients with hyperthyroidism compared with hypothyroidism. Patients with hyperthyroidism who were included likely had very well controlled disease. Thus, our results are likely not generalizable to patients with clinically important hyperthyroidism.
In conclusion, significant differences exist between patients with AF and thyroid disease history compared with those without thyroid disease history. Despite these differences, AF-related clinical outcomes and anticoagulation treatment response were similar irrespective of thyroid disease history. These results suggest that patients with AF and thyroid disease should be treated with oral anticoagulation similarly to those with AF and no thyroid disease.
Acknowledgments
Disclosures
Goldstein, Wojdyla, Al-Khatib: None. Green: Institutional research grants from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Sanofi-Aventis, Intarcia; Consulting fees from Boehringer Ingelheim, Daiichi-Sankyo, Merck, Novo-Nordisk, Bioscientificia, Horizon/PriMed CME.
Goldstein: Huber: Research grants from Bristol-Myers Squibb, Eli Lilly, Medtronic, Sanofi-Aventis; Consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Fibrex, Eli Lilly, Portola, Sanofi-Aventis, Schering-Plough, The Medicines Company, Boston Scientific, Cordis/Johnson & Johnson, GlaxoSmithKline, Pfizer.
Lopes: Institutional research grants from Bristol-Myers Squibb, Pfizer, GlaxoSmithKline, Medtronic PLC, Sanofi-Aventis; Consulting fees from Bristol-Myers Squibb, Pfizer, Amgen, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Medtronic PLC, Sanofi-Aventis.
Alexander: Institutional research grants from Bristol-Myers Squibb, Boehringer Ingelheim, AstraZeneca, Cyro-Life, CSL Behring, US FDA, NIH, Sanofi, VoluMetrix; Consulting fees from Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, AbbVie Pharmaceuticals, CSL Behring, Novo-Nordisk, Portola Pharmaceuticals, Quantum Genomics, Teikoku Pharmaceuticals, VA Cooperative Studies, Zafgen.
Vinereanu: Research grants from Bristol-Myers Squibb/Pfizer, Boehringer Ingelheim, Bayer, Daiichi Sankyo; Consulting fees from Boehringer Ingelheim, Bayer, Daiichi Sankyo.
Wallentin: Institutional research grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, Roche Diagnostics, and Merck & Co; Consulting fees from Abbott; holds two patents involving GDF-15 licensed to Roche Diagnostics.
Granger: Institutional research grants from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen, Pfizer, Armetheon, AstraZeneca, US FDA, GlaxoSmithKline, The Medicines Company, Medtronic Foundation, Medtronic, Inc., Novartis; Consulting fees from Bayer, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Janssen, Pfizer, AbbVie, Armetheon, AstraZeneca, Eli Lilly, Gilead, GlaxoSmithKline, Hoffmann-LaRoche, The Medicines Company, NIH, Novartis, Sirtex, Verseon, Apple, Medscape, LLC, Merck, Novo Nordisk, Roche Diagnostics, Rho Pharmaceuticals.
Funding Source: The ARISTOTLE trial was funded by Bristol-Myers Squibb, Princeton, NC and Pfizer, Inc., New York, NY. The ARISTOTLE trial was designed and led by a steering committee that included representatives of the sponsors. The sponsor was not involved in the planning of the analysis presented herewith, the interpretation of the data, writing the manuscript, or in the decision to submit the manuscript for publication. Dr. Goldstein was also supported by NIH training grant T-32-HL069749-15.
Footnotes
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim Y-H, McAnulty JH, Zheng Z-J, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJL. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation 2013:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation.N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 3.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM. Edoxaban versus warfarin in patients with atrial fibrillation.N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 4.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation.N Engl J Med 2011;365: 883–891. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation.N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 6.Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study.Lancet 1989;1:175–179. [DOI] [PubMed] [Google Scholar]
- 7.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001;344:501–509. [DOI] [PubMed] [Google Scholar]
- 8.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 9.Kim EJ, Lyass A, Wang N, Massaro JM, Fox CS, Benjamin EJ, Magnani JW. Relation of hypothyroidism and incident atrial fibrillation (from the Framingham Heart Study).Am Heart J 2014;167:123–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elbers LPB, Fliers E, Cannegieter SC. The influence of thyroid function on the coagulation system and its clinical consequences.J Thromb Haemos 2018;16:634–645. [DOI] [PubMed] [Google Scholar]
- 11.Howard-Thompson A, Luckey A, George C, Choby BA, Self TH. Graves’ disease and treatment effects on warfarin anticoagulation. Case Rep Med 2014;2014:292468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bucerius J, Joe AY, Palmedo H, Reinhardt MJ, Biersack HJ. Impact of short-term hypothyroidism on systemic anticoagulation in patients with thyroid cancer and coumarin therapy. Thyroid 2006;16:369–374. [DOI] [PubMed] [Google Scholar]
- 13.Kellett HA, Sawers JS, Boulton FE, Cholerton S, Park BK, Toft AD. Problems of anticoagulation with warfarin in hyperthyroidism. Q J Med 1986;58:43–51. [PubMed] [Google Scholar]
- 14.Lopes RD, Alexander JH, Al-Khatib SM, Ansell J, Diaz R, Easton JD, Gersh BJ, Granger CB, Hanna M, Horowitz J, Hylek EM, McMurray JJ, Verheugt FW, Wallentin L. Apixaban for reduction in stroke and other ThromboemboLic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J 2010;159:331–339. [DOI] [PubMed] [Google Scholar]
- 15.Selmer C, Olesen JB, Hansen ML, Lindhardsen J, Olsen AM, Madsen JC, Faber J, Hansen PR, Pedersen OD, Torp-Pedersen C, Gislason GH. The spectrum of thyroid disease and risk of new onset atrial fibrillation: a large population cohort study. BMJ 2012;345:e7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selmer C, Hansen ML, Olesen JB, Merie C, Lindhardsen J, Olsen AM, Madsen JC, Schmidt U, Faber J, Hansen PR, Pedersen OD, Torp-Pedersen C, Gislason GH. New-onset atrial fibrillation is a predictor of subsequent hyperthyroidism: a nationwide cohort study. PLoS One 2013;8:e57893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elbers LPB, Fliers E, Cannegieter SC. The influence of thyroid function on the coagulation system and its clinical consequences. J Thromb Haemost 2018;16:634–645. [DOI] [PubMed] [Google Scholar]
- 18.Haynes JH 3rd, Kageler WV. Thyrocardiotoxic embolic syndrome. South Med J 1989;82:1292–1293. [DOI] [PubMed] [Google Scholar]