Therapeutic approaches to mitigate the severe acute lung injury associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have rapidly entered clinical trials primarily on anecdotal observations and few clinical studies. Along with the clinical symptoms related to viral invasion, the reported molecular response known as the cytokine storm has attracted the greatest attention, in both the scientific and the lay press, as a cause of organ injury. The hypothesis that quelling this storm with anti-inflammatory therapies directed at reducing interleukin-6 (IL-6), IL-1, or even tumour necrosis factor α (TNFα) might be beneficial has led to several ongoing trials. Anecdotal evidence from non-controlled clinical trials has suggested a possible beneficial effect, and anti-IL-6 has been shown to be effective in chimeric antigen receptor T (CAR-T) and cytokine response syndrome (CRS).1, 2 However, past attempts in randomised clinical trials to block the cytokine storm associated with other microbial infections and with sepsis have not been successful and, in some cases, have worsened outcomes.3, 4 Redundancy of cytokine action, delayed intervention, and the essential role of these cytokines in recovery and immune surveillance have all been proposed as possible explanations for these findings.
The first reports from China emphasised elevated plasma concentrations of IL-6 and provided a rationale for the introduction of anti-IL-6 therapies (tocilizumab and sarulimab) in randomised clinical trials.5, 6 However, closer examination of plasma IL-6 concentrations has provided conflicting data. Results from early studies suggested that plasma IL-6 concentrations, although elevated (hundreds of picograms per μL) above values obtained from healthy control patients, were modest, especially when compared with the cytokine storm associated with septic shock, in which concentrations might be in the high hundreds to thousands of picograms per μL. Although more recent controlled studies indicate that plasma IL-6 concentrations can be in the range seen in bacterial infections, the time course of change is very different; in some cases, concentrations in patients with coronavirus disease 2019 (COVID-19) seem to increase over time with illness severity and worsening lung function.6 These dynamics clearly distinguish the SARS-CoV-2 host response from that seen in sepsis. Additionally, previous sepsis studies established that IL-6 concentrations might be an indicator of the magnitude of the inflammatory response rather than the cause of organ injury.7 Therefore, it is important to ask whether current therapeutic approaches are only targeting symptoms or are modulating the disease itself.
Little is known about the concentrations of other proinflammatory or anti-inflammatory mediators in patients with COVID-19, the landscape of the cytokine storm, and especially the chemokines that regulate the distribution and activity of effector cell populations. Interpreting changes in cytokine concentrations—all seem to be elevated—without additional immune cellular parameters does not provide clarity about the molecular basis of COVID-19 or potential treatment strategies. Indeed, when measured in patients infected with SARS-CoV-2, IL-10 concentrations (the most immunosuppressant cytokine in the body) are also elevated, which might lead to a different conclusion for therapeutic approaches and in understanding the disease pathophysiology. Similarly, there is concern that suppressing the innate and adaptive immune system to address increased cytokine concentrations, such as elevated IL-6, could enable unfettered viral replication, suppress adaptive immunity, and delay recovery processes.
Lost in the current enthusiasm for anti-inflammatory approaches to SARS-CoV-2 infection is the growing recognition that potent immunosuppressive mechanisms are also prevalent in such patients. This focus is reminiscent of that seen in the early investigations of sepsis-induced inflammation, since it was nearly a decade later that the contribution of immune suppression to sepsis pathology was generally accepted. Profound lymphopenia (low absolute lymphocyte counts, ALC), often to levels seen in septic shock, is a near uniform finding in severely ill patients with COVID-19 and correlates with increased secondary infections and mortality.8, 9 This loss of immune effector cells occurs in all lymphocyte subsets, including CD8+ and natural killer cells, which have important antiviral roles, and B cells, which are essential for making antibodies that inactivate the virus.10, 11, 12 Autopsy results have revealed a near complete dissolution of some secondary lymphoid organs.13 Unsurprisingly, secondary nosocomial infections, often with pathogens usually associated with immune suppression, are present in up to 50% of hospitalised patients.8
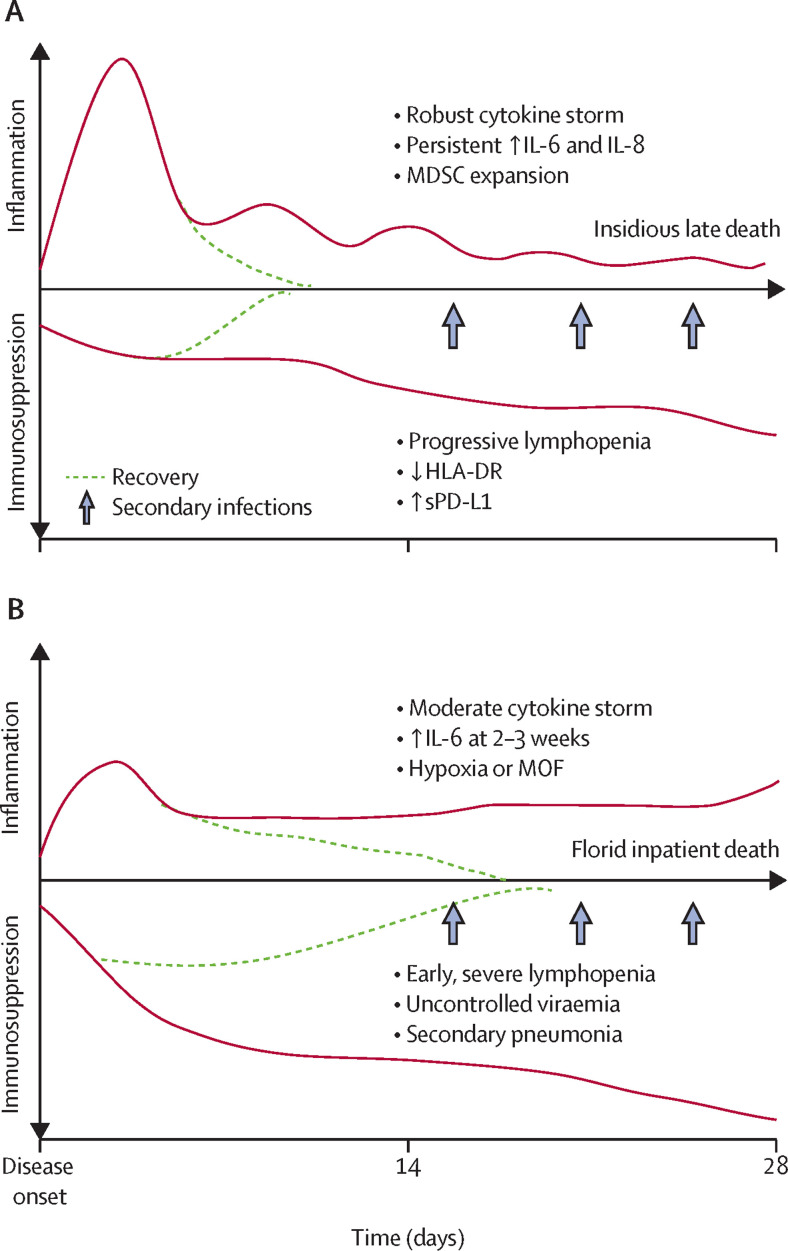
This early immunological picture of SARS-CoV-2 infection is one that shares many similarities with bacterial sepsis, but some unique differences should be noted (figure ). In particular, the modest inflammatory response and the progressive and profound suppression of adaptive immunity in COVID-19 relative to sepsis argues for perhaps a different therapeutic approach. Supporting host protective immunity must be considered as an essential component of any therapeutic intervention, of equal importance to or perhaps greater importance than targeting the cytokine storm.
Figure.
Immunological landscape in polymicrobial sepsis (A) and COVID-19 (B)
Bullet points refer to the symptoms seen throughout disease progression. MOF=multiorgan failure. COVID-19=coronavirus disease 19. MDSC=myeloid-derived suppressor cells. HLA-DR=human leukocyte antigen-DR. sPD-L1=soluble programmed cell death protein 1.
What is the most rational approach to supporting host protective immunity? Several immune stimulants in the clinical armamentarium are available for patients infected with SARS-CoV-2. Focusing on agents that target adaptive immunity in general, and T-cell function in particular, appears to be the most rational approach, based on the observation of progressive loss of T cells.12, 14 Programmed death ligand pathway (eg, PD-1) inhibitors, such as nivolumab and pembrolizumab, have been game changers in cancer and some other viral infections.15, 16 T cells from patients with COVID-19 show evidence of T-cell exhaustion associated with increased CD279 (PD-1) expression.11, 12
In addition to checkpoint inhibitors, the pluripotent cytokine IL-7 has been effective in multiple other viral infections.17, 18, 19 Early clinical trials of both treatments have been initiated in sepsis and shown to be safe and to have biological activity.20 IL-7 has shown benefit in raising lymphocyte counts in septic patients with low ALC20 and in restoring protective immunity in JC virus-induced progressive multifocal leukoencephalopathy.18, 19, 21, 22 Its effectiveness, and that of other immune stimulants, has only begun to be explored in sepsis, and should be considered in SARS-CoV-2 infection. Although immune stimulants such as IL-7 or nivolumab could theoretically feed the cytokine storm, both have been given to patients with sepsis with IL-6 concentrations similar to that in patients with COVID-19, without exacerbation of inflammatory responses.15
Randomised clinical trials based on the best observational findings remain paramount to moving forward, and we would propose starting with IL-7. Because of the complexity of the host response and the fact that monotherapies have not worked in sepsis trials in the past, we suggest that priority be given to biological response modifiers that are pluripotent (such as IL-7) or combination therapies that target multiple immunological pathways simultaneously (IL-7 and anti-PD-1).
What has treating patients with sepsis taught us about treatment approaches for patients with COVID-19? Like sepsis, antimicrobials (antivirals in this case) and supportive therapies are likely to remain the bedrock of therapeutic interventions for SARS-CoV-2 infection. However, if SARS-CoV-2 infection is similar to other chronic inflammatory and immune suppressive diseases, such as sepsis, we argue that immune stimulants, and not anti-inflammatory agents, should be considered as the first-line treatment option. However, we fully recognise that the pathophysiology and mechanisms of SARS-CoV-2 are still being elucidated, and that there is great uncertainty in predicting the efficacy of current therapeutic approaches. We are only just starting to explore the interplay of virus-mediated endothelial damage, pathogen–receptor signalling effects (including ACE2), and alterations in haemostasis and coagulation as a basis for the heterogeneous clinical pathologies seen in patients. Undeniably, there might be a subset of patients with exaggerated proinflammatory cytokine release that could derive benefit from anti-IL-6 or anti-IL-1 therapies. However, until better methods are available to determine (among the heterogeneity in clinical phenotypes) which patients meet these criteria, it will be difficult to establish a benefit. Observations from clinical centres dealing with large volumes of patients with COVID-19, show compelling evidence that patient mortality is directly related to multiorgan failure, including coagulopathy and probably damage to the endothelium. These patients also have altered immune function, as shown by lymphopenia. We suspect that a balanced, biologically plausible approach would be to provide anti-inflammatory treatment early in the disease course coupled with antiviral therapies, such as remdesivir. However, as the disease transitions to a suppressed state, therapies that restore host protective immunity should be considered a high priority for patients in intensive care with progressing lung injury.
What else needs to be considered? First, better methods are needed to assess the functional status of immune cells in patients with COVID-19. Circulating cytokine concentrations might reflect the degree of systemic inflammation but are not indicative of the functional state of individual lymphocyte and myeloid populations. Readily applicable tests that inform on whether the adaptive immune system is exhausted or whether myeloid cells are activated or tolerant would better guide application of drugs that can appropriately modulate the immune response. It would also enable balanced immune therapies targeted to either innate or adaptive immune cells. This approach is being used in cancer immunotherapy today and is being tested in the treatment of sepsis. This balanced therapeutic approach will allow precise deployment of inhibitory (anti-IL-6 and anti-IL-1) or restorative (IL-7 and checkpoint inhibitors) therapies, probably all as adjuvants to antiviral drugs.
Second, we need better measures of viral load with a rapid turnaround time. We recognise that our ability to identify and quantitate bacterial infections in patients with sepsis is still quite rudimentary, and quantifying viral loads by qPCR has not provided the required precision, which has hindered our ability to assess the effectiveness of interventions.
Most importantly, in designing and conducting interventional trials in patients with COVID-19, we need to remember the lessons learned from the ongoing sepsis epidemic that kills 250 000 people annually in the USA. Inflammation is often transitory, and the Surviving Sepsis Campaign has shown that earlier recognition and more immediate implementation of best practices can reduce early mortality and organ injury due to the cytokine storm. Conversely, immune suppression is prolonged, progressive, and ultimately lethal. Effective treatment of patients in this pandemic needs to be balanced, to be administered with precision to individual patients, and to build on our knowledge of past failures so that we can achieve future successes.
Acknowledgments
SCB reports other support from Revimmune and Bristol Myers Squibb, outside of the submitted work. BF reports personal fees from Biomérieux, Aridis, Ashai-Kasai, Polyphor, AM-Pharma, Ferring, Inotrem, Enlivex, and Transgene, outside of the submitted work. CSD reports grants from National Institute of General Medical Sciences (NIGMS), other support from Enlivex, and non-financial support from La Jolla Pharmaceuticals, outside of the submitted work. RSH is the principal investigator on a clinical trial of IL-7 in sepsis, has received reimbursement for travel and lodging expenses for a steering committee meeting, and reports grants from NIGMS. LLM reports grants from NIGMS, outside of the submitted work. KER, RJ, TD, GM, and P-FL declare no competing interests.
References
- 1.Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15:813–822. doi: 10.1080/1744666X.2019.1629904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le RQ, Li L, Yuan W, et al. FDA Approval Summary: Tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23:943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 4.Giamarellos-Bourboulis EJ. Failure of treatments based on the cytokine storm theory of sepsis: time for a novel approach. Immunotherapy. 2013;5:207–209. doi: 10.2217/imt.13.8. [DOI] [PubMed] [Google Scholar]
- 5.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020 doi: 10.1002/jmv.25801. published online April 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105954. published online March 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Y, Huang Z, Guoping Y, et al. Study of the lymphocyte change between COVID-19 and non-COVID-19 pneumonia cases suggesting other factors besides uncontrolled inflammation contributed to multi-organ injury. medRxiv. 2020 doi: 10.1101/2020.02.19.20024885. published online Feb 23. (preprint). [DOI] [Google Scholar]
- 11.Zheng HY, Zhang M, Yang CX, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0401-3. published online March 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0402-2. published online March 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? Lancet. 2020;395 doi: 10.1016/S0140-6736(20)30691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardiner D, Lalezari J, Lawitz E, et al. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N Engl J Med. 2014;371:380–383. doi: 10.1056/NEJMcibr1404664. [DOI] [PubMed] [Google Scholar]
- 17.Alstadhaug KB, Croughs T, Henriksen S, et al. Treatment of progressive multifocal leukoencephalopathy with interleukin 7. JAMA Neurol. 2014;71:1030–1035. doi: 10.1001/jamaneurol.2014.825. [DOI] [PubMed] [Google Scholar]
- 18.Levy Y, Lacabaratz C, Weiss L, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu H, Zhao Z, Kalina T, et al. Interleukin-7 improves reconstitution of antiviral CD4 T cells. Clin Immunol. 2005;114:30–41. doi: 10.1016/j.clim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Francois B, Jeannet R, Daix T, et al. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight. 2018;3 doi: 10.1172/jci.insight.98960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuller MJ, Callendret B, Zhu B, et al. Immunotherapy of chronic hepatitis C virus infection with antibodies against programmed cell death-1 (PD-1) Proc Natl Acad Sci U S A. 2013;110:15001–15006. doi: 10.1073/pnas.1312772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minter S, Willner I, Shirai K. Ipilimumab-induced hepatitis C viral suppression. J Clin Oncol. 2013;31:e307–e308. doi: 10.1200/JCO.2012.46.5831. [DOI] [PubMed] [Google Scholar]