Abstract
This randomized trial will evaluate the mechanisms of three chronic pain treatments: cognitive therapy (CT), mindfulness meditation (MM), and activation skills (AS). We will determine the extent to which late-treatment improvement in primary outcome (pain interference) is predicted by early-treatment changes in cognitive content, cognitive process, and/or activity level. The shared versus specific role of these mechanisms across the three treatments will be evaluated during treatment (Primary Aim), and immediately post-treatment to examine relapse mechanisms (Secondary Aim).
We will enroll 300 individuals with chronic pain (with low back pain as a primary or secondary condition), with 240 projected to complete the study. Participants will be randomly assigned to eight, 1.5 h telehealth group sessions of CT, MM, or AS. Mechanisms and outcomes will be assessed twice daily during 2-week baseline, 4-week treatment period, and 4-week post-treatment epoch via random cue-elicited ecological momentary assessment (EMA); activity level will be monitored during these time epochs via daily monitoring with ActiGraph technology. The primary outcome will be measured by the PROMIS 5-item Pain Interference scale. Structural equation modeling (SEM) will be used to test the primary aims. This study is pre-registered on clinicaltrials.gov (Identifier: NCT03687762).
This study will determine the temporal sequence of lagged mediation effects to evaluate rates of change in outcome as a function of change in mediators. The findings will provide an empirical basis for enhancing and streamlining psychosocial chronic pain interventions. Further, results will guide future efforts towards optimizing maintenance of gains to effectively reduce relapse risk.
Keywords: Chronic pain, Chronic low back pain, Mechanisms, Cognitive therapy, Mindfulness meditation, Activation skills
1. Introduction
Chronic pain is debilitating, pervasive, and costly [1,2]. A number of interventions have demonstrated efficacy [[3], [4], [5]] for chronic pain management, including chronic low back pain (CLBP): (1) Cognitive Therapy (CT); (2) Mindfulness Meditation (MM); and (3) Activation Skills (AS) [6,7]. Each of these approaches has a unique theoretical rationale underpinning its application.
CT teaches patients to notice maladaptive thoughts and their influence on pain, and targets these for change. Thus, changing what people think (i.e., cognitive content) to more adaptive thoughts is a focus of CT [6]. MM encourages patients to disengage from automatic thinking and to mindfully place attention on different perceptive experiences [8]. MM therefore targets how people think (i.e., cognitive process). AS targets reductions in maladaptive pain behaviors and uses reinforcement principles to increase well behaviors. Hence, what people do (i.e., behavior) is targeted in AS [9].
Understanding the mechanisms of psychosocial pain treatments has been identified as critical [10,11]. While equivalent efficacy is typically obtained on average when active treatments are compared, e.g., [12,13] the theories underlying specific treatments argue that effects of different treatments work through unique mechanisms. It is also possible that these unique mechanisms underlie post-treatment changes. However, minimal research has examined whether CT, MM, and AS engender benefit via their specific theorized pathways – the Specific Mechanism Model – or if benefit is obtained via a combination of shared pathways – the Shared Mechanism Model [[14], [15], [16]].
If a mechanism factor plays a causal role in outcome, change in that factor must precede change in outcome [17]. Actigraphy and ecological momentary assessment (EMA) are repeated measures methodologies ideally suited to assess such mechanism relations in real-time [18]. EMA technology affords the capacity to disentangle temporal precedence of mechanism-outcome changes. These data will allow us to determine, for the first time, the timing of changes in mechanism variables during treatment, the effects of these changes on subsequent changes in outcome variables, and the extent to which these changes and effects are specific to, versus shared across, the three treatment conditions.
The purpose of this randomized trial is to identify the mechanisms of CT, MM, and AS for chronic pain, with low back pain experienced as a primary or secondary condition. Given past research showing equivalent efficacy for active treatments, we hypothesize no significant group level outcome differences.e.g.,12,13 However, we do hypothesize individual variability in treatment response and maintenance of gains.1
1.1. Aim 1 (primary)
Identify the mechanisms of CT, MM, and AS. We will determine the extent to which late-treatment improvement in pain interference is predicted by early-treatment changes in three primary mechanism variables: cognitive content (i.e., catastrophizing), cognitive process (i.e., mindful non-judgment), and/or activity level (i.e., ActiGraph “activity counts”).
Hypothesis 1a
Early treatment changes in mechanisms will be significantly associated with late treatment improvement in pain interference.
Hypothesis 1b
The Shared Mechanisms Model hypothesizes that if the mechanisms are shared, there would be small, non-significant between-treatment differences in early mechanism changes.
Hypothesis 1c
The Specific Mechanisms Model hypothesizes that if changes in mechanisms are specific to CT, MM, and AS, then treatment condition will have a significant effect on early changes in the mechanisms, which will then be associated with subsequent outcome change.
1.2. Aim 2 (secondary)
We will evaluate the post-treatment mechanisms that explain relapse (i.e., return to baseline levels – or worse – on pain interference), maintenance, and continued gains associated with these treatments. The Shared (Hypothesis 2a) and Specific (Hypothesis 2b) Mechanism models will be applied to test post-treatment mechanisms.
2. Methods
2.1. Design overview
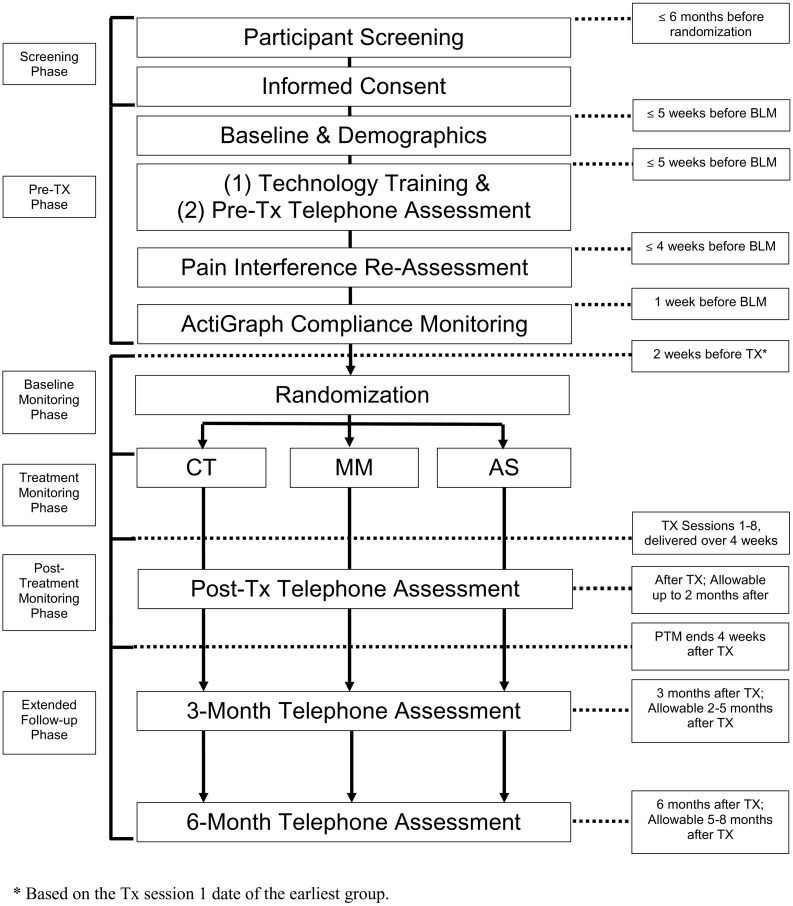
In this study, we will use a 3-group parallel (1:1:1), single-blind design (see Fig. 1 ). We will recruit and enroll 300 individuals with chronic pain, with a low back pain problem experienced as a primary or secondary pain condition in the past 6-months. Participants will be randomly assigned to eight, 1.5 h, group Zoom videoconference sessions of CT, MM, or AS. Mechanism and outcome variables will be assessed twice daily during the 2-week baseline, 4-week treatment period, and 4-week post-treatment epoch via random cue-elicited EMA; physical activity level will be monitored during these time epochs via daily monitoring using ActiGraph technology. Follow-up assessments of mechanism and outcome variables will be conducted at 3- and 6-months post-treatment. The primary endpoint for the primary study aim (Aim 1) is the post-treatment pain interference score, operationalized as an average of pain interference ratings made on the twice-daily diaries during the first four days after treatment. The endpoint for the secondary study aim (Aim 2) is the post-treatment score at 28 days follow-up, as operationalized as the average of pain interference ratings on the diaries from the final four days of the immediate post-treatment follow-up period. All study procedures were piloted and developed in preliminary work by the investigative team. Specifically germane to this proposal, we have conducted numerous clinical trials examining psychological interventions based on the techniques investigated in this study for CLBP and other pain conditions [[19], [20], [21], [22]], including telehealth assessment and treatment delivery [[23], [24], [25], [26], [27]]. We have also published multiple studies examining treatment mechanisms and have a great deal of experience in implementing EMA, with compliance rates exceeding 85% [14,15,21,[28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48]]. This study is pre-registered on clinicaltrials.gov (Identifier: NCT03687762). The trial protocol follows Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines [49]. Informed consent will be obtained from all participants prior to enrollment.
Fig. 1.
Study design and trial flowchart. *Based on the Tx session 1 date of the earliest group.
3. Study sample and recruitment
Potential participants (N = 300 enrolled for N = 240 completers; see power analysis below) will be identified via diagnostic codes in the UW Medicine medical records. Recruitment will also occur via other strategies, including posting flyers in the relevant pain and rehabilitation clinics, clinician referrals, announcements on the hospitals-wide electronic reader boards and national recruitment strategies, including social media.
3.1. Participant inclusion and exclusion criteria
Study inclusion criteria include: (1) age ≥ 18 years; (2) endorse having low back pain as a primary or secondary pain problem in the past 6 months; (3) meet criteria for having a chronic pain problem (≥3 months, with pain experienced on ≥50% of days in the past 6 months) [1]; (4) average intensity of chronic pain ≥3 on a 10-point scale for most days of the previous 3 months; (5) chronic pain interference for general activities ≥3 on a 10-point scale for the past 3 months; (6) able to read, speak, and understand English to comprehend the worksheets, measures, and interventions implemented; (7) if currently taking analgesic or psychotropic medication, medications must have been stabilized for ≥4 weeks prior to this study; and (8) availability of a telephone, webcam, and microphone through computer or telephone, as well as daily internet access.
Exclusion criteria include: (1) primary pain condition is headache; (2) severe cognitive impairment; (3) current alcohol or substance dependence; (4) active malignancy (e.g., cancer not in remission), terminal illnesses, or serious medical conditions that may interfere with either study participation or with receiving potential treatment benefits (e.g., severe lupus); (5) inability to walk at least 50 yards, which would limit the ability of participants to benefit from the activation skills intervention; (6) significant pain from a recent surgery or injury; (7) pain condition for which surgery has been recommended and is planned; (8) any planned surgery, procedure, or hospitalization that may conflict with or otherwise influence participation in the study; (9) currently or recently receiving other psychosocial treatments for any pain condition (as this may influence these treatment results); (10) current or past participation in a UW Department of Rehabilitation Medicine research study with treatment components that may overlap those in the current study; (11) current or history of diagnosis of primary psychotic or major thought disorder within the past 5 years; (12) psychiatric hospitalization within the past 6 months; (13) psychiatric or behavioral conditions in which symptoms were unstable or severe within the past 6 months; (14) any psychiatric or behavioral issues as noted in the medical record or disclosed/observed during self-report screening that would indicate participant may be inappropriate in a group setting; and (15) presenting symptoms at the time of screening that would interfere with participation, specifically active suicidal or homicidal ideation with intent to harm oneself or others, or active delusional or psychotic thinking.
3.2. Randomization
Assignment to one of the three groups will be accomplished using a covariate-adaptive randomization scheme. We will use a procedure proposed by Pocock and Simon, with the objective of balancing the covariate in the marginal distributions [50]. The covariates for the covariate-adaptive randomization will be sex, baseline pain interference score (mild/moderate or severe, as assessed via the 11-item Roland-Morris Disability Scale [51] with cutoff for severe being a score of ≥7), and low back pain type (primary or secondary pain).
3.3. Study interventions
3.3.1. Overview
Participants will be offered eight 1.5-h group treatment sessions scheduled twice per week for four weeks. This treatment format was selected based on our successful application of these format specifications in prior clinical trials, which have evaluated eight session, group-delivered treatment programs, and programs with session duration length of 1.5 h (both in person and via telehealth). e.g., [19,23,25,52,53]. During their study participation, all participants will continue to receive their usual medical, psychiatric, and psychotherapeutic care. Clinicians will be expected to follow closely the treatment manuals to ensure all scheduled material is covered, and to ensure the consistency and replicability of treatment. In all three treatment conditions, group sessions will be conducted via the online, HIPAA-compliant Zoom videoconferencing platform (https://zoom.us/). Zoom videoconferences allow participants to see and hear each other, and also allow screen sharing, giving clinicians the opportunity to display visual information (e.g., PowerPoint slides) during sessions. All participants will receive a treatment workbook specific to their treatment allocation to refer to during the group sessions as well as additional content to read between sessions. In all conditions, home practice activities will be assigned to build skills in the coping techniques taught in sessions.
3.3.2. Cognitive Therapy (CT) condition
Cognitive-restructuring techniques will be used to help patients recognize the relationships between thoughts and the connection between thoughts with feelings, behaviors and pain [54]. These techniques will help patients: identify negative or unrealistic automatic thoughts; evaluate automatic thoughts for accuracy, identify sources of distorted thoughts, recognize the connection between automatic thoughts and emotional/physical shifts; challenge negative, distorted automatic thoughts via “weighing the evidence”; develop new realistic alternative cognitive appraisals; and practice applying new appraisals and beliefs.
3.3.3. Mindfulness Meditation (MM) condition
Participants will receive training in mindfulness meditation, specifically Vipassana, which is the form of meditation typically implemented in mindfulness research [55]. With this technique, the emphasis is placed upon developing focused attention on an object of awareness, e.g., the breath. This focus is then expanded to include a more open, non-judgmental monitoring of any sensory, emotional, or cognitive events. A standard script will be used by the clinician as a guide. The clinician will however lead the practice “from within,” in the sense that they will not simply just “read” the scripts, but will use their own language to guide the meditation taking into account his/her own experiences (for example, when describing sounds in the virtual room). Participants will be seated in a comfortable yet alert position. A guided inquiry of the participant's experiences will follow each in-person exercise, and will also be implemented in relation to discussing participant's at-home practice.
3.3.4. Activation Skills (AS) condition
Participants will be educated about the role of inactivity and behavioral avoidance in chronic pain and functioning [56]. They will learn how to be aware of the activities they avoid because of pain, and how to set effective goals so that, step by step, they can start being more active and resume some activities they enjoyed in the past but are currently avoiding. Explanation and practice of a set of specific skills – including appropriate pacing skills – to facilitate an increase in appropriate activity level will be provided.
3.4. Therapists and therapist training
The sessions will be conducted by a postdoctoral psychology fellow or licensed psychologist (the “clinician”) with at least two years of clinical experience, including experience working within the context of chronic pain treatment. The clinicians will be trained and supervised by the investigators who have considerable experience in the study treatments. During training, clinicians will be assigned reading materials [[54], [55], [56]] and will complete three, 6-h treatment workshops led by the investigators; all therapists will be trained in, and will deliver, all three treatments. MM therapists will be strongly encouraged to engage in their own personal practice of mindfulness, including discussions around how this personal practice is important for being able to respond genuinely and to have insight into the processes of meditation. Clinicians will also be trained in the use of Motivational Interviewing (MI) for enhancing motivation to engage in treatment, including reading Miller and Rollnick's text [57], and engaging in at least 3 h of MI instruction and practice. Clinicians will also receive training in group leadership techniques, including strategies for enhancing group cohesion. The clinicians will be provided with a detailed treatment manual and protocol, and will be provided with regularly scheduled supervision.
3.5. Fidelity monitoring
Adherence and fidelity will be monitored using session audio recordings. Masters-level or above clinicians supervised by the investigators will review a random selection of 25% of the recordings (2 randomly selected sessions per group) to ensure procedures are followed. Delivery quality and protocol adherence criteria will be developed for each session, adapted from the CT Adherence and Competence Scale [58] and the Mindfulness-Based CT-Adherence Appropriateness and Quality Scale [55]. Corrective feedback will be provided to the clinician during supervision; didactics and role plays to correct “drift” will be implemented if needed.
3.6. Participant retention and adherence to study procedures
We will implement a number of strategies to maximize participant retention. For example, across cohorts, sessions will be offered at different times on a recurrent basis (e.g., a morning session cohort will be offered, and then in the next cohort an evening session will be offered), giving participants scheduling flexibility; however, once a participant commences treatment, a participant cannot change groups/clinicians. We will track session attendance and reasons for missed sessions. Reasons for attrition will be assessed for participants who withdraw. Clinician-rated participant engagement will be assessed following each session for each participant [52]. Enactment of treatment-specific skills will be assessed by homework practice, assessed via EMA. To minimize possible missed extended surveys, EMA, and ActiGraph data, we will provide financial incentives.
3.7. Measures
The descriptive, primary and secondary outcome variables, covariates (variables to control for in planned analyses if needed), and mechanism (mediator and moderator) variables for this study are listed in Table 1 . Specific measures by time point are provided in Table 2 . Assessments will be undertaken via a combination of extended telephone assessments and EMA monitoring. Participants will have the option to complete the EMA surveys via smart phone, tablet, laptop, or desktop computer. The cue-elicited EMAs will be administered via software programmed to randomly alert participants daily within two pre-set 120-min blocks (via notifications for smart phone users and email messages for tablet, laptop, or desktop users) to complete the EMA surveys in the morning and evening. All participants will be given 3 pre-determined options for the morning and evening blocks for receiving surveys: for example, 5–7 AM and PM, 6–8 AM and PM, or 7–9 AM and PM. Notification cues to complete the EMAs will be administered during a randomly selected time within these blocks. The number of items for each measure in the EMAs was selected on the basis of content validity, factor loadings established during initial measure development and validation studies, brevity, and pilot data. Building on this, the minimum number of items was then selected that achieved at least good internal consistency reliability (α ≥ .80) for the mechanism variables and excellent reliability (α ≥ .90) for the primary outcome variable of pain interference in our pilot data. The Actigraph will be worn throughout all EMA phases.2 All outcome measures will be administered by staff blind to group allocation.
Table 1.
Descriptive, Primary, Secondary, Co-Variate, and Mechanism Variables.
| Variable type | Domain | Measure (# items EMA, # items Extended) |
|---|---|---|
| Descriptive/Demographics | Patient Characteristics | Purpose built demographics, patient characteristics, and pain history questionnaire [[42], [43], [44], [45]] |
| Primary Outcome | Pain Interference | PROMIS Pain Interference (5, 5) [46,47] |
| Primary Mechanisms and Moderators | Cognitive Content | Pain Catastrophizing – Items from Pain Appraisal Scale (3, 5), Coping Strategy Questionnaire (2, Extended only) [48,49] |
| Cognitive Process | Pain-Related Cognitive Process Questionnaire (PCPQ) Non-Judgment Scale (2, 6) [50] | |
| Activity Level | Actigraphy + Godin Leisure-Time Exercise Questionnaire (3, 3), hours spent sitting without exercising (EMA only) [51] | |
| Secondary Outcomes | Average Pain Intensity | Numerical Rating Scale (NRS), 0–10 (1,1) [52] |
| Mood | Positive and Negative Affect Schedule (PANAS) (2, 10) [53,54] | |
| Physical Function | PROMIS Physical Function (4, Extended only) [46,55] | |
| Sleep Quality | Actigraphy, PROMIS Sleep Disturbance (4, Extended only) [46,55] | |
| Depression | PROMIS Depression (4, Extended only) [46,55] | |
| Anxiety | PROMIS Anxiety (4, Extended only) [46,55] | |
| Medication Use | Purpose built Medication Use Questionnaire (Extended only) | |
| Medication Use Attitudes | Survey of Pain Attitudes (SOPA) Medication Beliefs Scale (6, Extended only) [56] and Pain Medication Questionnaire (PMQ) (26, Extended Baseline assessment only) [57] | |
| Post-Traumatic Stress Disorder | PTSD Checklist Civilian Version (PCL-C) (17, Extended only) [58] | |
| Secondary Mechanisms | Pain Self-Efficacy | UW Pain-Related Self-Efficacy Scale (3, 6) [48] |
| Patient Engagement | Clinician rated patient engagement (5, rated by clinician) [23] | |
| Therapeutic Alliance | Working Alliance Inventory (WAI) (12, EMA only) [59] | |
| Group Cohesion | Group Climate Questionnaire (GSQ-S) Engagement Scale (5, EMA only) [60] | |
| Skills Engagement | Purpose built duration and number of days/times practicing skills (EMA); number of days and duration of time practicing skills (Extended) | |
| Tertiary Outcomes | Health Care Use | # visits to health care professional in last month (1, Extended only) |
| Pleasurable Activity | Pleasant Events Schedule SF (10, Extended only) [61,62] | |
| Behavior Activation | Behavioral Activation for Depression Scale (BADS) (9, Extended only) [63] | |
| Quality of Life | Global quality of life (1, Extended only) [64] | |
| Employment Status | Employment question (1, Extended only) | |
| Weight Change | Weight question (1, Extended only) | |
| Patient Global Impressions of Change | Patient Global Impressions of Change (PGIC) (6, Extended post-treatment and follow-up assessment only); and Patient Global Assessment of Treatment Satisfaction (PGATS) (1, Extended post-treatment and follow-up assessment only) | |
| Tertiary Mechanisms | Mindfulness | Mindful Attention Awareness Scale (MAAS) (15, Extended only) [65] |
| Resilience | Pain Resilience Scale (14, Extended only) [66] | |
| Other Cognitive Processes | All other PCPQ items (47 additional, Extended only) [50] | |
| Pain Beliefs | Survey of Pain Attitudes (SOPA) Harm, Control and Disability Scales (18, Extended only) [56] | |
| Exploratory Moderators | Cognitive Abilities | PROMIS Cognitive Function Abilities (6, Extended only) [46,55] |
| Treatment Credibility | Treatment Credibility & Expectancies items [67] (5, EMA only) | |
| COVID-19 | As of 2020: Investigator-developed items on COVID-19’s effects (6 items ext. & 1 item Qualitative Interview) | |
| Covariate | Primary Problem (LBP primary or secondary) | Purpose built screening, self-report |
| Optional Assessments | Responses to Pain | Positive & Negative Response to Pain Scales (85, Extended Optional only) [68] |
| Future Self | Future Self Questionnaire (FSQ) (16, Extended Optional only) | |
| Values-Consistent Goals | Valued Living Scale (VLS) [69] (8, Extended Optional only) | |
| Qualitative Outcomes | Experiences in group & program feedback | 15″–30″ of investigator-developed qualitative items |
Table 2.
Study assessment schedule.
| Measures | EMA | Baseline | Pre-TX | During Tx | Post-Tx | 3-Month | 6-Month |
|---|---|---|---|---|---|---|---|
| Demographic Information | X | ||||||
| Pain and Treatment History | X | ||||||
| Start Back Tool | X | ||||||
| Pain Medication Questionnaire (PMQ) | X | ||||||
| Roland Morris Disability Questionnaire SF (RMDQ) | X | ||||||
| PROMIS Pain Interference | X | X | X | X | X | ||
| Pain Appraisal Scale (PAS) | X | X | X | X | X | ||
| 2-item Catastrophizing Scale from the Coping Strategy Questionnaire (CSQ) | X | X | X | X | |||
| Non-Judgment Scale from the Pain-Related Cognitive Process Questionnaire (PCPQ) | X | X | X | X | X | ||
| Godin Leisure-Time Exercise Questionnaire | X | X | X | X | X | ||
| Hours Spent Sitting w/o Exercising | X | ||||||
| Pain Intensity NRS | X | X | X | X | X | X | |
| Positive and Negative Affect Schedule (PANAS) | X | X | X | X | X | ||
| PROMIS Sleep Disturbance | X | X | X | X | |||
| PROMIS Physical Function | X | X | X | X | |||
| PROMIS Depression | X | X | X | X | |||
| PROMIS Anxiety | X | X | X | X | |||
| PTSD Checklist (PCL-C) | X | X | X | X | |||
| Medication Use | X | X | X | X | |||
| UW Pain-Related Self-Efficacy Scale | X | X | X | X | X | ||
| Participant Engagement | Xa | ||||||
| Working Alliance Inventory (WAI) | Xb | ||||||
| Group Climate Questionnaire (GSQ-S) | Xb | ||||||
| Duration and Times Practicing Skills | X | X | X | X | |||
| Sleep/Wake Times | X | ||||||
| Health Care Utilization | X | X | X | X | |||
| Pleasant Events Schedule SF | X | X | X | X | |||
| Behavioral Activation for Depression Scale (BADS) | X | X | X | X | |||
| Global Quality of Life | X | X | X | X | |||
| Employment Status | X | X | X | X | |||
| Weight | X | X | X | X | |||
| Mindful Awareness and Attention Scale (MAAS) | X | X | X | X | |||
| Pain Resilience Scale | X | X | X | X | |||
| Pain-Related Cognitive Process Questionnaire (PCPQ) – Full | X | X | X | X | |||
| Control, Harm, Disability and Medication scales from the Survey of Pain Attitudes (SOPA) | X | X | X | X | |||
| PROMIS Cognitive Function Abilities | X | X | X | X | |||
| COVID-19 Impact Questions (as of 2020) | X | X | X | X | |||
| Treatment Credibility and Expectancies | Xc | ||||||
| Patient Global Impression of Change (PGIC) | X | X | X | ||||
| Patient Global Assessment of Treatment Satisfaction (PGATS) | X | X | X | ||||
| Treatment Modality & Preferences | X | ||||||
| Qualitative Outcomes | X | ||||||
| Optional: Positive & Negative Response to Pain Scales, Future Self Questionnaire (FSQ), Valued Living Scale (VLS) | X | X | X | X |
Will be assessed for each participant and reported by the clinician following every treatment session.
Will be assessed during the evening EMA following Sessions 4 & 8 only.
Will be assessed once before Session 1 and once following Session 1 but before Session 2.
3.8. Statistical analyses
Due to space limitations, here we provide only a brief description of the planned analyses. Readers interested in more details can find them on clinicaltrials.gov (Identifier: NCT03687762). Only analyses planned to address the primary study aims are described here. Briefly, we plan to test the primary study hypotheses using a 3-wave structural equation modeling (SEM) approach.
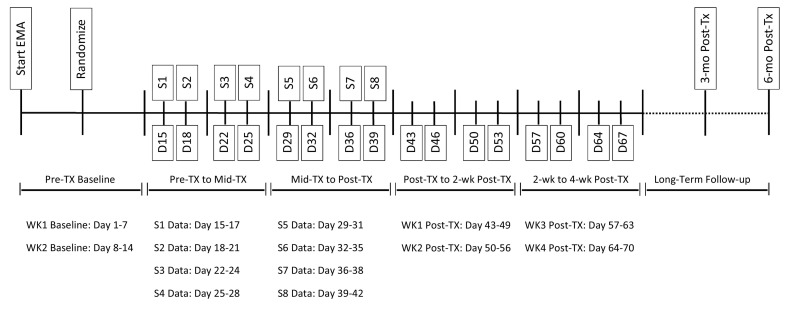
We will first calculate slope coefficients representing the linear change in each outcome and mechanism variable during the first two weeks of treatment (early treatment) and second two weeks of treatment (late treatment), using regression. See Fig. 2 for the data time points included in these calculations. We will then enter these variables, along with control variables and variables representing treatment condition in a series of SEM models.
Fig. 2.
EMA data collection and data time points used in statistical analyses. Note: The specific day counts are approximate and might slightly vary in the instance of extenuating circumstances for a given cohort.
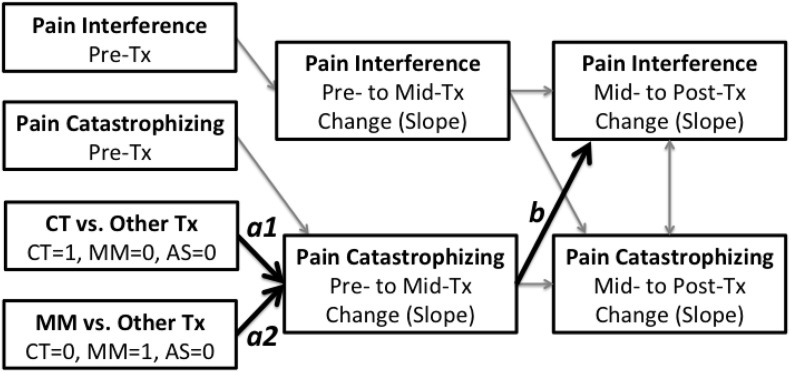
The model depicted in Fig. 3 represents the initial model we plan to test for the catastrophizing mechanism variable, providing that it evidences at least a small effect size for change over time for at least one treatment condition during the first two weeks of treatment. However, the model will be simplified (by removing treatment condition as a predictor, and the paths associated with treatment condition) if non-significant between treatment condition effects are found for change during the early treatment phase. Up to two additional SEM models will also be tested, with non-judgment and activity level as the mechanism variables.
Fig. 3.
Initial model testing the mechanism role of pain catastrophizing during treatment.
In these models, the a1 and a2 coefficients represent the treatment condition effect (two dummy IVs) on early change in the mechanism variable being examined. The b coefficient in this model represents the effect of early treatment change in the mechanism variable on subsequent late treatment change in outcome. A significant b coefficient would support Hypothesis 1a for that mechanism variable. Significant a1 or a2 coefficients, indicating between-treatment condition differences in early changes in mechanism variables, would support Hypothesis 1c (the Specific Mechanisms Model). Post-hoc analyses would then be performed to determine which treatment resulted in greater changes in the mechanism variable being examined. If both the a1 and a2 coefficients are not statistically significant, this would be consistent with Hypothesis 1b (i.e., the Shared Mechanisms Model) for that mechanism variable.
4. Power analysis
We will conduct six primary statistical tests (described above) to test the primary aim for three mechanism variables, in order to better understand the effects of the treatments on pain interference. Data from prior research – including means and standard deviations – supports the anticipated medium to large effects of (1) the causal effects of the treatments on the mechanism variables [52,[59], [60], [61]], (2) the association between the mechanism variables and pain interference [[61], [62], [63]], as well as (3) the mechanism paths that we propose to test [59,61,64], which form the basis of our assumptions for the power analyses. Although we were unable to identify any studies that examined the effect sizes associated with any of these treatments on behavioral activity, as a group these studies are consistent with our assumptions that CT, MM, and AS treatments have medium to strong effects on key mechanism variables.
Assuming at least medium effects (i.e., rs ≥ 0.30 and/or ds ≥ 0.50) [65] we then computed the sample sizes needed to detect significant effects for the planned primary analyses, using the Benjamini-Hochberg procedure to control for alpha inflation in these analyses [66]. The sample sizes needed to detect significant effects for each of these analyses is presented in Table 3 , using the tests for the three direct mediation effects of the mechanism variables for three of these analyses, and a test for the three Treatment Condition X Mediation (representing moderated mediation) effects for the other three analyses, assuming at least medium effects for each of these effects. Sample size estimates needed to detect the primary mediation effects were conducted based on the joint significance method of testing mediated effects, using the PowMedR program in R Version 3.0.2 statistical software with the following assumptions: (1) alpha levels consistent with the Benjamini-Hochberg procedure (see Table 3); (2) power of 0.80; and (3) at least medium effects. Power calculations for the interaction (mediated moderation) analyses were conducted using G*Power3 with the same assumptions.
Table 3.
Sample size estimates for the six planned analyses, assuming medium effects for the causal paths (a and b) and at least a medium interaction effect (f2) for the three planned Treatment Condition X Mediation effects tests.
| Alpha | Power | Effect size path a | Effect size path b | Interaction effects | n-required/n-planned |
|---|---|---|---|---|---|
| 0.050 | 0.80 | Medium (r = 0.30) | Medium (r = 0.30) | 109/240 | |
| 0.025 | 0.80 | Medium (r = 0.30) | Medium (r = 0.30) | 129/240 | |
| 0.017 | 0.80 | Medium (r = 0.30) | Medium (r = 0.30) | 140/240 | |
| 0.013 | 0.80 | Medium (f2 = 0.15) | 93/240 | ||
| 0.010 | 0.80 | Medium (f2 = 0.15) | 98/240 | ||
| 0.008 | 0.80 | Medium (f2 = 0.15) | 102/240 |
Thus, by employing the analytic models experiment-wise, and by integrating treatment-condition into the model(s) as an interaction effect (rather than running separate analyses for each treatment condition), we will be able to take advantage of the power afforded by running the entire sample of participants (n = 240) through the planned tests. This, combined with the less stringent Benjamini-Hochberg type-I error adjustment, has left us well-powered to detect the hypothesized effects if they exist. That said, we plan to enroll 300 participants with a goal of obtaining complete data for 240 participants (n = 80 per condition).
5. Protection of human subjects: ethics
This study was reviewed and approved by the UW Human Subjects Division. An independent Data Safety Monitoring Committee (DSMC) comprised of an occupational therapist (Chair), biostatistician, and physical therapist with experience in treating CLBP has been appointed. The DSMC will monitor safety of participants throughout all phases of the trial. Per UW Human Subjects Division guidelines, we will monitor for and track possible adverse events (AEs) throughout the study. Reviewing and reporting of AEs to the DSMC, the UW IRB, and NCCIH will be undertaken in accordance with requirements.
This study will be stopped prior to its completion if: (1) one of the interventions is associated with adverse effects that call into question the safety of the intervention; (2) any new information becomes available during the trial that necessitates stopping the trial; or (3) other situations occur that warrant stopping the trial.
6. Discussion
This study is designed to isolate the effects of coping skills typically taught in integrated, multi-modal treatments (i.e., CBT, MBIs) to determine their specific role in chronic pain management. Testing the mediators of these specific pain techniques – CT, MM, and AS – will identify if theorized mechanisms are unique to a specific treatment or are shared, trans-therapy mechanisms. Results of this study will determine the relative importance of targeting change in what people think, how people think, or what people do in relation to chronic pain management. Identification of treatment mediators will bring order and parsimony to psychotherapeutic theory [67,68].
Research has underscored the problem of patients relapsing back to baseline levels of pain/function following psychosocial pain interventions, with relapses found in as little as one month following treatment [69,70]. Thus, the time period immediately post-treatment might be critical. This study will examine the mechanisms that possibly precede continued improvement, maintenance, and relapse in the one month immediately post-treatment, and will investigate how these factors may relate to longer-term (i.e., 3- and 6-month) outcomes.
Although some prior research has used EMA or actigraphy to evaluate treatment outcomes,e.g.,71,72 to the best of our knowledge, this study will be the first to utilize both actigraphy and EMA during pain treatment and during the critical month following administration of treatment to evaluate mechanisms. In planned secondary analyses, this methodological advancement of the inclusion of actigraphy and EMA will afford the capacity to determine precisely (1) when and how sudden gains might occur, (2) how long it takes for “slow and steady” gains to become meaningful and what processes underlie these gains, (3) the earliest point at which it is possible to conclusively determine that the treatment is not well suited to a given individual and that an alternative approach should be offered, and (4) the processes and temporal sequence underlying post-treatment relapse, maintenance, and continued gain. These findings could lead to streamlined interventions and informed relapse-prevention approaches that distill the most critical change factors into an efficient and cost-effective treatment package.
Trial status
Recruitment started in August 2018. The trial is underway; it is expected to be completed May 2022.
Conflicts of interest and sources of funding
Research reported in this manuscript was supported by the National Center for Complementary and Integrative Health of the National Institutes of Health under the Award Number 1 R01 AT008559. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no conflicts of interest to report.
Footnotes
The exploratory aims of the study are not described in detail herein, due to space limitations. These exploratory tests will include: (1) evaluating the moderators of response per the Limit, Activate, and Enhance (LAE) moderation model from pre- to post-treatment (i.e., to test individual differences in treatment response), as well as moderators of change from pre-treatment to follow-up (i.e., to test individual differences related to maintenance of gains); (2) utilizing the ActiGraph and EMA data to explore the nature of the time course of micro-level changes in mechanisms and outcomes during and following treatment; (3) effects related to secondary outcomes (e.g., pain intensity) and non-specific mechanisms (e.g., therapeutic alliance), as well as changes at 3- and 6-month follow-up.
The gold standard for assessment of movement (i.e., what people actually do) in the real world is Actigraph technology, which is why we elected to use this assessment approach in this trial. That this mechanism is not measured via self-report like the primary hypothesized mechanisms of the other two conditions is a potential methodological confound that was considered. Hence, to address this, we elected to also concurrently administer a self-report measure of what people do (i.e., GODIN, and self-reported amount of time spent sitting). This will allow us to test whether the findings differ as a function of form of assessment (i.e., self-report versus objective assessment).
References
- 1.Crombie I.K., Croft P.R., Linton S.J., Leresche L., Von Korff M. IASP Press; Seattle, WA: 1999. Epidemiology of Pain. [Google Scholar]
- 2.Rogerson M.D., Gatchel R.J., Bierner S.M. A cost utility analysis of interdisciplinary early intervention versus treatment as usual for high risk acute low back pain patients. Pain Practice. 2009;10(5):382–395. doi: 10.1111/j.1533-2500.2009.00344.x. [DOI] [PubMed] [Google Scholar]
- 3.Morone N.E., Greco C.M., Weiner D.K. Mindfulness meditation for the treatment of chronic low back pain in older adults: a randomized controlled pilot study. Pain. 2008;134(3):310–319. doi: 10.1016/j.pain.2007.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morley S. Efficacy and effectiveness of cognitive behaviour therapy for chronic pain: Progress and some challenges. Pain. 2011;152(3 Suppl):S99–106. doi: 10.1016/j.pain.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 5.Goyal M., Singh S., Sibinga E. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA. 2014;174:357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turk D.C., Meichenbaum D., Genest M. Guilford Press; New York: 1983. Pain and Behavioral Medicine: A Cognitive-Behavioral Perspective. [Google Scholar]
- 7.Kabat-Zinn J. Delacourt; New York: 1990. Full Catastrophe Living: Usinf the Wisdom of your Body and Mind to Face Stress, Pain and Illness. [Google Scholar]
- 8.Kabat-Zinn J. Mindfulness-based interventions in context: past, present, and future. Clin. Psychol. Sci. Pract. 2003;10:144–156. [Google Scholar]
- 9.Fordyce W.E. Mosby; St. Louis: 1976. Behavioral Methods for Chronic Pain and Illness. [Google Scholar]
- 10.Jensen M.P. Psychosocial approaches to pain management: an organizational framework. Pain. 2011;152(4):717–725. doi: 10.1016/j.pain.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Thorn B.E., Burns J.W. Common and specific treatment mechanisms in psychosocial pain interventions: the need for a new research agenda. Pain. 2011;152(4):705–706. doi: 10.1016/j.pain.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Glombiewski J.A., Hartwich-Tersek J., Rief W. Two psychological interventions are effective in severely disabled, chronic back pain patients: a randomised controlled trial. Int. J. Behav. Med. 2010;17(2):97–107. doi: 10.1007/s12529-009-9070-4. [DOI] [PubMed] [Google Scholar]
- 13.Redondo J.R., Justo C.M., Moraleda F.V., Velayos Y.G., Puche J.J., Zubero J.R., Hernández T.G., Ortells L.C., Pareja M.A. Long-term efficacy of therapy in patients with fibromyalgia: a physical exercise-based program and a cognitive-behavioral approach. Arthritis Rheum. 2004;51(2):184–192. doi: 10.1002/art.20252. [DOI] [PubMed] [Google Scholar]
- 14.Burns J., Nielson W.R., Jensen M.P., Heapy A., Czlapinski R., Kerns R.D. Does change occur for the reasons we think it does? A test of specific therapeutic operations during cognitive-behavioral treatment of chronic pain. Clin. J. Pain. 2015;31:603–611. doi: 10.1097/AJP.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 15.Burns J.W., Day M.A., Thorn B.E. Is reduction in pain catastrophizing a therapeutic mechanism specific to cognitive-behavioral therapy for chronic pain? Transl. Behav. Med. 2012;2:22–29. doi: 10.1007/s13142-011-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns J. Mechanisms of Psychosocial Chronic Pain Treatments. 2014. https://clinicaltrials.gov/ct2/show/NCT02133976?term=John+Burns&rank=11 ClinicalTrials.gov Identifier: NCT02133976. Accessed February 8, 2019.
- 17.Kazdin A.E. Mediators and mechanisms of change in psychotherapy research. Annu. Rev. Clin. Psychol. 2007;3:1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432. [DOI] [PubMed] [Google Scholar]
- 18.Shiffman S., Stone A.A., Hufford M.R. Ecological momentary assessment. Annu. Rev. Clin. Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 19.Ehde D.M., Jensen M.P. Feasibility of a cognitive restructuring intervention for treatment of chronic pain in persons with disabilites. Rehabil. Psychol. 2004;49:254–258. [Google Scholar]
- 20.Jensen M.P., Ehde D.M., Gertz K.J. Effects of self-hypnosis training and cognitive restructuring on daily pain intensity and catastrophizing in individuals with multiple sclerosis and chronic pain. Int. J. Clin. Exp. Hypn. 2011;59(1):45–63. doi: 10.1080/00207144.2011.522892. [DOI] [PubMed] [Google Scholar]
- 21.Thorn B.E., Pence L.B., Ward L.C. A randomized clinical trial of targeted cognitive behavioral treatment to reduce catastrophizing in chronic headache sufferers. J. Pain. 2007;8(12):938–949. doi: 10.1016/j.jpain.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Turner J.A., Jensen M. Efficacy of cognitive therapy for chronic low back pain. Pain. 1993;52:169–177. doi: 10.1016/0304-3959(93)90128-C. [DOI] [PubMed] [Google Scholar]
- 23.Bombardier C.H., Ehde D.M., Gibbons L.E. Telephone-based physical activity counseling for major depression in people with multiple sclerosis. J. Consult. Clin. Psychol. 2013;81(1):89–99. doi: 10.1037/a0031242. [DOI] [PubMed] [Google Scholar]
- 24.Guihan M., Bombardier C.H., Ehde D.M. Comparing multicomponent interventions to improve skin care behaviors and prevent recurrence in veterans hospitalized for severe pressure ulcers. Arch. Phys. Med. Rehabil. 2014;95:1246–1253. doi: 10.1016/j.apmr.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Ehde D.M., Elzea J.L., Verrall A.M., Gibbons L.E., Smith A.E., Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: a randomized controlled trial with a one-year follow-up. Arch. Phys. Med. Rehabil. 2015;96(11):1945–1958. doi: 10.1016/j.apmr.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Thorn B.E., Day M.A., Burns J. Randomized trial of group cognitive-behavioral therapy compared to a pain education control for low literacy rural people with chronic pain. Pain. 2011;152(12):2710–2720. doi: 10.1016/j.pain.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehde D.M. Seattle, WA; Patient-Centered Outcomes Research Institute: 2013. Improving the Quality of Care for Pain and Depression in Prsons iwht Multiple Sclerosis. In. [Google Scholar]
- 28.Jensen M., Turner J.A., Romano J.M. Changes in beliefs, catastrophizing and coping are associated with improvement in multidisciplinary pain treatment. J. Consult. Clin. Psychol. 2001;69:655–662. doi: 10.1037//0022-006x.69.4.655. [DOI] [PubMed] [Google Scholar]
- 29.Jensen M.P., Turner J.A., Romano J.M. Correlates of improvement in multidisciplinary treatment of chronic pain. J. Consult. Clin. Psychol. 1994;62(1):172–179. doi: 10.1037//0022-006x.62.1.172. [DOI] [PubMed] [Google Scholar]
- 30.Jensen M.P., Nielson W.R., Turner J.A., Romano J.M., Hill M.L. Changes in readiness to self-manage pain are associated with improvement in multidisciplinary pain treatment and pain coping. Pain. 2004;111(1–2):84–95. doi: 10.1016/j.pain.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Jensen M., Turner J.A., Romano J.M. Changes after multidisciplinary pain treatment in patient pain beliefs and coping are associated with concurrent changes in patient functioning. Pain. 2007;131:38–47. doi: 10.1016/j.pain.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burns J.W., Glenn B., Bruehl S., Harden R.N., Lofland K. Cognitive factors influence outcome following multidisciplinary chronic pain treatment: a replication and extension of a cross-lagged panel analysis. Behav. Res. Ther. 2003;41(10):1163–1182. doi: 10.1016/s0005-7967(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 33.Burns J.W., Kubilus A., Bruehl S., Harden R.N., Lofland K. Do changes in cognitive factors influence outcome following multidisciplinary treatment for chronic pain? A cross-lagged panel analysis. J. Consult. Clin. Psychol. 2003;71(1):81–91. doi: 10.1037//0022-006x.71.1.81. [DOI] [PubMed] [Google Scholar]
- 34.Day M.A., Thorn B.E., Burns J. Mindfulness-based cognitive therapy for the treatment of headache pain: a mixed-methods analysis comparing treatment responders and treatment non-responders. Complement Ther. Med. 2014;22(2):278–285. doi: 10.1016/j.ctim.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Anderson B.S., Kavanagh K., Borckardt J.J. Decreasing procedural pain over time of left prefrontal rTMS for depression: initial results from the open-label phase of a multi-site trial (OPT-TMS) Brain Stimul. 2009;2(2):88–92. doi: 10.1016/j.brs.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borckardt J.J., Nahas Z.H., Teal J. The painfulness of active, but not sham, transcranial magnetic stimulation decreases rapidly over time: results from the double-blind phase of the OPT-TMS Trial. Brain Stimul. 2013;6(6):925–928. doi: 10.1016/j.brs.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borckardt J.J., Nash M.R., Murphy M.D., Moore M., Shaw D., O’Neil P. Clinical practice as natural laboratory for psychotherapy research: a guide to case-based time-series analysis. Am. Psychol. 2008;63(2):77–95. doi: 10.1037/0003-066X.63.2.77. [DOI] [PubMed] [Google Scholar]
- 38.Borckardt J.J., Reeves S.T., Beam W. A randomized, controlled investigation of motor cortex transcranial magnetic stimulation (TMS) effects on quantitative sensory measures in healthy adults: evaluation of TMS device parameters. Clin. J. Pain. 2011;27(6):486–494. doi: 10.1097/AJP.0b013e31820d2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glaser J., Reeves S.T., Stoll W.D. Motor/prefrontal transcranial direct current stimulation (tDCS) following lumbar surgery reduces postoperative analgesia use. Spine (Phila Pa 1976) 2016;41(10):835–839. doi: 10.1097/BRS.0000000000001525. [DOI] [PubMed] [Google Scholar]
- 40.Short E., Borckardt J., George M., Beam W., Reeves S. Non-invasive brain stimulation approaches to fibromyalgia pain. J. Pain Manag. 2009;2(3) [PMC free article] [PubMed] [Google Scholar]
- 41.Short E.B., Borckardt J.J., Anderson B.S. Ten sessions of adjunctive left prefrontal rTMS significantly reduces fibromyalgia pain: a randomized, controlled pilot study. Pain. 2011;152(11):2477–2484. doi: 10.1016/j.pain.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Day M.A., Thorn B.E., Ward L.C. Mindfulness-based cognitive therapy for the treatment of headache pain: a pilot study. Clin. J. Pain. 2014;30(2):152–161. doi: 10.1097/AJP.0b013e318287a1dc. [DOI] [PubMed] [Google Scholar]
- 43.Borckardt J.J., Nash M.R. How practitioners (and others) can make scientifically viable contributions to clinical-outcome research using the single-case time-series design. Int. J. Clin. Exp. Hypn. 2002;50(2):114–148. doi: 10.1080/00207140208410095. [DOI] [PubMed] [Google Scholar]
- 44.Borckardt J.J., Nash M.R. Simulation modelling analysis for small sets of single-subject data collected over time. Neuropsychol. Rehabil. 2014;24(3–4):492–506. doi: 10.1080/09602011.2014.895390. [DOI] [PubMed] [Google Scholar]
- 45.Borckardt J.J., Reeves S.T., Robinson S.M. Transcranial direct current stimulation (tDCS) reduces postsurgical opioid consumption in total knee arthroplasty (TKA) Clin. J. Pain. 2013;29(11):925–928. doi: 10.1097/AJP.0b013e31827e32be. [DOI] [PubMed] [Google Scholar]
- 46.Borckardt J.J., Reeves S.T., Weinstein M. Significant analgesic effects of one session of postoperative left prefrontal cortex repetitive transcranial magnetic stimulation: a replication study. Brain Stimulat. 2008;1(2):122–127. doi: 10.1016/j.brs.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borckardt J.J., Smith A.R., Reeves S.T. A pilot study investigating the effects of fast left prefrontal rTMS on chronic neuropathic pain. Pain Med. 2009;10(5):840–849. doi: 10.1111/j.1526-4637.2009.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borckardt J.J., Weinstein M., Reeves S.T. Post-operative left prefrontal repetitive transcranial magnetic stimulation (rTMS) reduces patient-controlled analgesia use. Anesthesiology. 2006;105:557–562. doi: 10.1097/00000542-200609000-00020. [DOI] [PubMed] [Google Scholar]
- 49.Chan A.W., Tetzlaff J.M., Altman D.G. SPIRIT 2013 statement: Defininf standard protocol items for clinical trials. Ann. Intern. Med. 2013;158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenberger W.F., Lachin J.M. 2nd edition. Wiley; New Jersey: 2016. Randomization in Clinical Trials: Theory and Practice. [Google Scholar]
- 51.Stroud M.W., McKnight P.E., Jensen M. Assessment of self-reported physical activity in chronic pain patients: development of an abbreviated Roland-Morris Disability scale. J. Pain. 2004;5:257–263. doi: 10.1016/j.jpain.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Day M.A., Thorn B.E., Ward L.C. Mindfulness-based cognitive therapy for the treatment of headache pain: a pilot study. Clin. J. Pain. 2014;22(2):278–285. doi: 10.1097/AJP.0b013e318287a1dc. [DOI] [PubMed] [Google Scholar]
- 53.Day M.A., Ward L.C., Ehde D.M. A pilot randomized controlled trial comparing mindfulness meditation, cognitive therapy, and mindfulness-based cognitive therapy for chronic low back pain. Pain Med. 2019;20(11):2134–2148. doi: 10.1093/pm/pny273. [DOI] [PubMed] [Google Scholar]
- 54.Thorn B.E. 2nd ed. The Guilford Press; New York: 2017. Cognitive Therapy for Chronic Pain: A Step-by-step Guide. [Google Scholar]
- 55.Day M.A. Wiley; Chichester, UK: 2017. Mindfulness-Based Cognitive Therapy for Chronic Pain: A Clinical Manual and Guide. [Google Scholar]
- 56.Martell C.R., Dimidjian S., Herman-Dunn R. Guilford; New York: 2010. Behavioral Activation for Depression: A clinician’s Guide. [Google Scholar]
- 57.Miller W.R., Rollnick S. 3rd ed. Guilford Press; New York, NY: 2012. Motivational Interviewing: Helping People Change. [Google Scholar]
- 58.Barber J.P., Liese B.S., Abrams M.J. Development of the cognitive therapy adherence and competence scale. Psychother. Res. 2003;13(2):205–221. [Google Scholar]
- 59.Plagge J.M., Lu M.W., Lovejoy T.I., Karl A.I., Dobscha S.K. Treatment of comorbid pain and PTSD in returning veterans: a collaborative approach utilizing behavioral activation. Pain Med. 2013;14:1164–1172. doi: 10.1111/pme.12155. [DOI] [PubMed] [Google Scholar]
- 60.Turner J.A., Anderson M.L., Balderson B.H., Cook A.J., Sherman K.J., Cherkin D.C. Mindfulness-based stress reduction and cognitive-behavioral therapy for chronic low back pain: similar effects on mindfulness, catastrophizing, self-efficacy, and acceptance in a randomized controlled trial. Pain. 2016;157(11):2434–24444. doi: 10.1097/j.pain.0000000000000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Day M.A. Coping Skills Training for Living With Chronic Low Back Pain. 2015. https://clinicaltrials.gov/ct2/show/NCT02478307?term=mindfulness-based+cognitive+therapy&cond=chronic+low+back+pain&cntry=AU&city=Brisbane&rank=1 ClinicalTrials.gov Identifier: NCT02478307. Accessed February 8, 2019.
- 62.Poulin P.A., Romanow H.C., Rahbari N. The relationship between mindfulness, pain intensity, pain catastrophizing, depression, and quality of life among cancer survivors living with chronic neuropathic pain. Support Care Cancer. 2016;24(10):4167–4175. doi: 10.1007/s00520-016-3243-x. [DOI] [PubMed] [Google Scholar]
- 63.Thomas E.A., Garland E.L. Mindfulness is associated with increased hedonic capacity among chronic pain patients receiving extended opioid pharmacotherapy. Clin. J. Pain. 2017;33:166–173. doi: 10.1097/AJP.0000000000000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veehof M.M., Trompetter H.R., Bohlmeijer E.T., Schreurs K.M.G. Acceptance- and mindfulness-based interventions for the treatment of chronic pain: a meta-analytic review. Cogn. Behav. Ther. 2016;45(1):5–31. doi: 10.1080/16506073.2015.1098724. [DOI] [PubMed] [Google Scholar]
- 65.Cohen J. 2nd ed. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 66.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57(1):289–300. [Google Scholar]
- 67.Kraemer H.C., Wilson G.T., Fairburn C.G., Agras W.S. Mediators and moderators of treatment effects in randomized clinical trials. Arch. Gen. Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 68.Kazdin A.E. Understanding how and why psychotherapy leads to change. Psychother. Res. 2009;19(4–5):418–428. doi: 10.1080/10503300802448899. [DOI] [PubMed] [Google Scholar]
- 69.Turk D.C., Rudy T.E. Neglected topics in the treatment of chronic pain patients - relapse, noncompliance, and adherence enhancement. Pain. 1991;44:5–28. doi: 10.1016/0304-3959(91)90142-K. [DOI] [PubMed] [Google Scholar]
- 70.Turner J.A. Comparison of group progressive-relaxation training and cognitive-behavioral group therapy for chronic low back pain. J. Consult. Clin. Psychol. 1982;50(5):757–765. doi: 10.1037//0022-006x.50.5.757. [DOI] [PubMed] [Google Scholar]