Abstract
Objectives
To describe the prevalence, clinical features and complications of human metapneumovirus (hMPV) infections in a population of adults hospitalized with influenza-like illness (ILI).
Methods
This was a retrospective, observational, multicenter cohort study using prospectively collected data from adult patients hospitalized during influenza virus circulation, for at least 24 h, for community-acquired ILI (with symptom onset <7 days). Data were collected from five French teaching hospitals over six consecutive winters (2012–2018). Respiratory viruses were identified by multiplex reverse transcription polymerase chain reaction (RT-PCR) on nasopharyngeal specimens. hMPV + patients were compared with hMPV– patients, influenza+ and respiratory syncytial virus (RSV)+ patients using multivariate logistic regressions. Primary outcome was the prevalence of hMPV in patients hospitalized for ILI.
Results
Among the 3148 patients included (1449 (46%) women, 1988 (63%) aged 65 and over; 2508 (80%) with chronic disease), at least one respiratory virus was detected in 1604 (51%, 95% confidence interval (CI) 49–53), including 100 cases of hMPV (100/3148, 3% 95% CI 3–4), of which 10 (10%) were viral co-infection. In the hMPV + patients, mean length of stay was 7 days, 62% (56/90) developed a complication, 21% (14/68) were admitted to intensive care unit and 4% (4/90) died during hospitalization. In comparison with influenza + patients, hMPV + patients were more frequently >65 years old (adjusted odds ratio (aOR) = 3.3, 95% CI 1.9–6.3) and presented more acute heart failure during hospitalization (aOR = 1.8, 95% CI 1.0–2.9). Compared with RSV + patients, hMPV + patients had less cancer (aOR = 0.4, 95% CI 0.2–0.9) and were less likely to smoke (aOR = 0.5, 95% CI 0.2–0.9) but had similar outcomes, especially high rates of respiratory and cardiovascular complications.
Conclusions
Adult hMPV infections mainly affect the elderly and patients with chronic conditions and are responsible for frequent cardiac and pulmonary complications similar to those of RSV infections. At-risk populations would benefit from the development of antivirals and vaccines targeting hMPV.
Keywords: Adults, Human metapneumovirus, Influenza, Influenza-like-illness, Respiratory syncytial virus
Introduction
During winter, community-acquired influenza-like illness (ILI), mostly caused by respiratory viruses, is very common. The most frequent viruses seen in primary care are influenza viruses A/B, rhinovirus, coronavirus, respiratory syncytial virus (RSV) and human metapneumovirus (hMPV) [1,2]. In the hospital setting, adults with ILI are commonly tested only for influenza, resulting in limited data concerning other respiratory viruses. The use of multiplex reverse transcription polymerase chain reaction (RT-PCR) allows identification of multiple viruses simultaneously but remains a second-line test in non-immunocompromised patients in emergency departments because of its cost and the limited therapeutic options [3].
Human MPV, discovered in 2001, is phylogenetically similar to RSV and has been frequently found to be associated with respiratory tract illnesses [1,4,5]. Its circulation occurs with a seasonal distribution from January to March in the Northern hemisphere, often overlapping or following RSV infection season [6,7]. Human MPV is a major paediatric respiratory pathogen [8] but can affect all age groups, especially persons with chronic conditions [9], leading to diverse clinical presentation from upper respiratory tract symptoms to severe pneumonia [10,11].
While hMPV is known to contribute substantially to the burden of wintertime respiratory illnesses in adults [12], data on its frequency and comparison with other respiratory viruses in large adult populations are scarce.
We aimed to (a) describe the clinical characteristics and outcome of hMPV infection and (b) compare hMPV with influenza and RSV infections in adults hospitalized for community-acquired ILI in France in winter between 2012 and 2018.
Methods
Study design
We performed a post hoc, retrospective analysis of the FLUVAC study. FLUVAC is a French prospective case-test negative design study evaluating influenza vaccine effectiveness on influenza-associated hospitalization conducted in five (2012/13, 2015/16 to 2017/18) or six (2013/14 to 2014/15) teaching hospitals. All adults hospitalized for at least 24 h for ILI during the influenza circulation period, with symptom onset <7 days before screening, were included in FLUVAC. ILI was defined according to the European Centre for Disease Prevention and Control (ECDC) definition [13] as a combination of the following: (a) at least one of the following systemic symptoms – fever or feverishness, headache, myalgia or malaise; and (b) at least one of the following respiratory symptoms – cough, sore throat or dyspnea. Patients with contra-indication for influenza immunization, those who had previously tested positive for influenza virus in the same season and those without a French social security affiliation were excluded. Each participant was interviewed, and nasopharyngeal samples were obtained at enrolment to screen for influenza and other respiratory viruses.
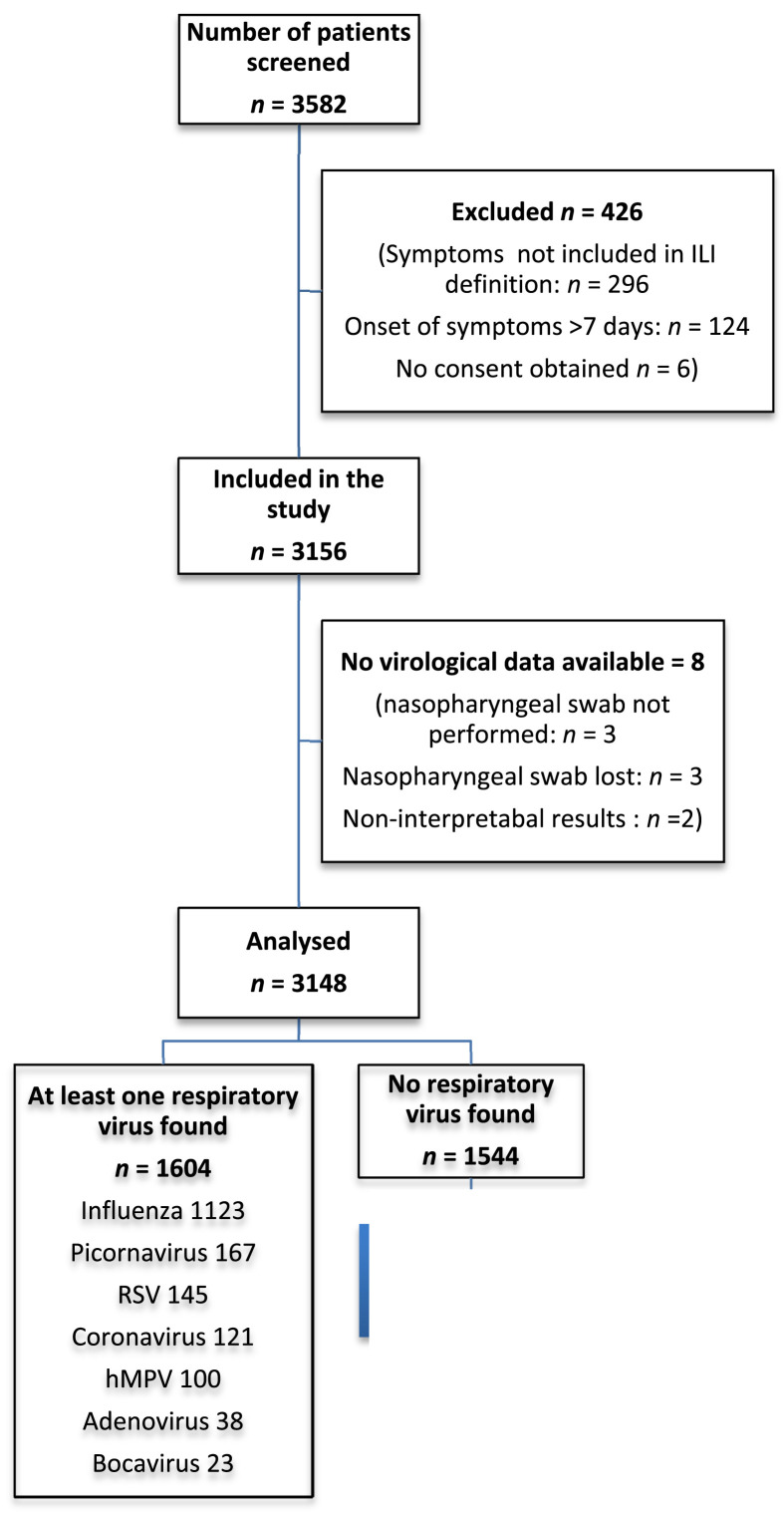
In the present study, we included all the patients from the first six FLUVAC seasons (2012/13, 2013/14, 2014/15, 2015/16, 2016/17, 2017/18) for whom virologic results on respiratory viruses were available (Fig. 1 ).
Fig. 1.
Flowchart. hMPV, human metapneumovirus; ILI, influenza-like illness; RSV, respiratory syncytial virus.
Outcomes
The primary outcome was the prevalence of confirmed hMPV infection in patients hospitalized with ILI. Secondary outcomes were the demographic characteristics, chronic underlying diseases and treatments, clinical presentation of the ILI episode and complications, intensive care unit admission or death in patients with hMPV infections.
Microbiological data
Respiratory viruses were identified using multiplex RT-PCR performed on nasopharyngeal swabs. Clinical bronchoalveolar lavage fluid samples and tracheal aspirates were also tested. Samples were first tested in the virology laboratory of each participating hospital using real-time (RT) influenza A and B PCR after manual nucleic acid extraction. All samples were then sent to the French National Influenza Reference Centre (CNR-Lyon) for influenza confirmation and screening for other respiratory viruses (adenoviruses, bocaviruses, hMPV, picornavirus, RSV, coronaviruses, parainfluenza viruses (since 2013)) by RT-PCR using the Respiratory Multiwell System r-gene® on an ABI 7300 analyser between 2012/13 and 2014/15. From 2015/16, all the virology laboratories of the five sites used the same Eurobio kit and only B lineage was provided by the CNR-Lyon.
Statistical analysis
Quantitative variables were expressed as mean and standard deviation (SD) or median and interquartile range (IQR), and qualitative variables as number and percentage. Qualitative variables were compared using the χ2 and Fisher's exact tests, as appropriate. Quantitative variables were compared by Wilcoxon rank sum test. Missing data for each variable were excluded from the denominator.
Patients with multiple viral infections were excluded from the analyses. Univariate analysis was used to asses risk factors for the detection of hMPV infection, influenza infection, RSV infection and acute heart failure. We performed two multivariate analyses using a backward stepwise logistic regression model using hMPV test result (positive/negative) and acute heart failure (yes/no) as the dependent variable in the first and second model, respectively. Covariates with a p-value <0.2 in univariate analysis were tested in the multivariate model. Results from regression models are expressed as crude odds ratios (OR) and adjusted ORs (aOR) with 95% CI. A p-value of <0.05 was considered statistically significant. Analyses were performed using R software (Version 1.1.463).
Ethics
The FLUVAC study (clinicaltrials.gov NCT02027233) followed Good Epidemiological and Clinical Practices in Clinical Research, and the Declaration of Helsinki, and was approved by the regional ethics committees. All the study participants gave their informed consent for respiratory virus testing.
Results
Comparison of hMPV+ and hMPV- patients
Of the 3148 patients included in the FLUVAC study, 1604 (51%, 95% CI 49–53) tested positive for at least one respiratory virus. Most had influenza (1123/1604, 70%, 95% CI 68–72), while 167 had picornavirus (10%, 95% CI 9–12), 145 had RSV (9%, 95% CI 8–10), 100 had hMPV (6%, 95% CI 5–7), 38 had adenovirus (2%, 95% CI 2–3) and 23 had bocavirus (1%, 95% CI 1–2) (Fig. 1, Supplementary Table S1). Ten of the 100 hMPV infections (10%) were co-infection cases with at least one other virus: three with influenza A virus, one with coronavirus, one with both influenza A virus and coronavirus, two with picornavirus, one with parainfluenza virus, one with adenovirus and one with RSV.
Overall, 3% (95% CI 3–4) of people with ILI symptoms (90/3148) tested positive for hMPV. Peak hMPV detection occurred between mid-January and mid-February (Supplementary Figs S1, S2). While patients with hMPV infections were generally older than those without (median age 78 years (IQR 70–86) vs 71 years (IQR 57–83), p < 0.001), other demographic characteristics were similar. Most patients with hMPV presented at least one chronic condition (81%, 73/90), mostly cardiac (50%, 45/90) and respiratory (44%, 40/90) chronic diseases, 17% under immunosuppressive treatment (15/90), 14% active smokers (12/87) and 41% (37/90) hospitalized in the 12 months preceding the study (Table 1 ).
Table 1.
Clinical characteristics and outcomes of hospitalized patients infected with human Metapneumovirus compared with patients without human metapneumovirus, 2012–2018
| hMPV-(n = 3048) | hMPV+ (n = 90) | Univariate analysis | Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude OR | p | Adjusted OR | p | ||||||
| Baseline characteristics | |||||||||
| Gender | |||||||||
| Women, n (%) | 1390/3048 (46%) | 50/90 (56%) | 0.06 | 1.5 | (1.0; 2.3) | 0.08 | |||
| Men, n (%) | 1658/3048 (54%) | 40/90 (44%) | |||||||
| Age | |||||||||
| Median age, years (IQR) | 71 (56–82) | 78 (70–86) | <0.001 | ||||||
| Age >65 years, n (%) | 1904/3048 (62%) | 76/90 (84%) | <0.001 | 2.9 | (1.6; 5.7) | 0.001 | 3.3 | (1.9; 6.1) | <0.001 |
| Median BMI, kg/m2 (IQR) | 24.8 (21.5–28.5) | 25.4 (21.1–29.3) | |||||||
| Smoking status | |||||||||
| Smoker, n (%) | 641/2938 | 12/87 (14%) | 0.07 | 0.9 | (0.5; 1.7) | 0.84 | |||
| Ex-smoker, n (%) | 973/2938 | 28/87 (32%) | 0.93 | ||||||
| Non-smoker, n (%) | 1324/2938 | 47/87 (54%) | 0.12 | ||||||
| Chronic diseases (at least one), n (%) | 2428/3048 (80%) | 73/90 (81%) | 0.74 | ||||||
| Chronic respiratory disease, n (%) | 1361/3048 (45%) | 40/90 (44%) | 0.96 | ||||||
| Chronic heart disease, n (%) | 1267/3048 (42%) | 45/90 (50%) | 0.12 | 1.1 | (0.7; 1.8) | 0.57 | |||
| Diabetes, n (%) | 714/3048 (23%) | 18/90 (20%) | 0.52 | ||||||
| Chronic renal failure, n (%) | 468/3048 (15%) | 9/90 (10%) | 0.17 | 0.5 | (0.2; 1.0) | 0.08 | |||
| Cancer, n (%) | 515/3048 (17%) | 13/90 (14%) | 0.45 | ||||||
| Cirrhosis, n (%) | 109/3035 (4%) | 4/90 (4%) | 0.55 | ||||||
| Immunosuppressive treatment, n (%) | 514/3043 (17%) | 15/90 (17%) | 0.85 | ||||||
| Pregnancy, n (% of women of childbearing age) | 28/339 (8%) | 0 | 1 | ||||||
| Influenza vaccination, n (%) | 1418/3011 (47%) | 52/90 (58%) | 0.03 | 1.1 | (0.7; 1.7) | 0.69 | |||
| Hospitalization in the previous 12 months, n (%) | 1405/3031 (46%) | 37/90 (41%) | 0.33 | ||||||
| Presence of child/children <5 years in the household, n (%) | 182/3025 (6%) | 5/90 (6%) | 0.80 | ||||||
| Clinical presentation | |||||||||
| Median time from symptom onset to hospitalization, days (IQR) | 2 (1–3) | 2 (1–3) | 0.7 | ||||||
| Symptoms | |||||||||
| Fever or feverishness, n (%) | 2562/3045 (84%) | 79/90 (88%) | 0.40 | ||||||
| Cough, n (%) | 2360/3045 (78%) | 75/90 (83%) | 0.18 | 1.5 | (0.9; 2.7) | 0.18 | |||
| Dyspnea, n (%) | 1739/2199 (79%) | 76/90 (84%) | 0.54 | ||||||
| Weakness/malaise, n (%) | 821/3041 (27%) | 13/90 (14%) | 0.007 | 0.5 | (0.2; 0.8) | 0.01 | 0.4 | (0.2; 0.8) | 0.008 |
| Headache, n (%) | 755/3032 (25%) | 16/90 (18%) | 0.08 | 0.8 | (0.4; 1.4) | 0.44 | |||
| Myalgia, n (%) | 734/3029 (24%) | 19/90 (21%) | 0.45 | ||||||
| Sore throat, n (%) | 439/3024 (15%) | 8/90 (9%) | 0.10 | 0.8 | (0.3; 1.5) | 0.48 | |||
| Outcome | |||||||||
| At least one complication during the hospital stay, n (%) | 1648/3148 (52%) | 56/90 (62%) | 0.13 | ||||||
| Pneumonia∗, n (%) | 918/3124 (29%) | 32/89 (36%) | 0.27 | ||||||
| Respiratory failure, n (%) | 873/3124 (28%) | 32/89 (36%) | 0.17 | ||||||
| Acute heart failure, n (%) | 420/3122 (13%) | 22/89 (25%) | 0.004 | ||||||
| Acute respiratory distress syndrome, n (%) | 257/3123 (8%) | 8/89 (9%) | 0.85 | ||||||
| Median length of stay, days (IQR) | 5 (3–9) | 7 (4–13) | 0.50 | ||||||
| ICU admission after hospitalization in acute care, n (%) | 543/2283 (24%) | 15/90 (17%) | 0.60 | ||||||
| Death, n (%) | 137/3131 (4%) | 4/90 (4%) | 1 | ||||||
BMI, body mass index; hMPV, human metapneumovirus; ICU, intensive care unit; IQR, interquartile range; OR, odds ratio.
The median time from symptom onset to admission was similar between hMPV+ and hMPV- patients (2 days (IQR 1–3)) as well as the main symptoms at inclusion except for weakness/malaise that was less frequent in hMPV + patients (13/90 (14%) vs 27%, p < 0.007).
There was no difference between hMPV+ and hMPV- groups in terms of median length of stay, number of complications during hospitalization, intensive care unit (ICU) admission and death. However, hMPV + patients were more likely to have an acute heart failure during hospitalization (25% (22/89) vs 14% (377/2722), p < 0.004).
There was no difference in sociodemographic characteristics, clinical presentation or outcomes between hMPV and viral coinfection and patients with hMPV infections alone.
In the multivariate analysis, when comparing hMPV + patients to all hMPV- patients, age >65 years (aOR 95% CI 3.3 (1.9; 6.1), p < 0.001) was significantly associated with hMPV detection. In contrast, the sudden onset of symptoms, defined as the occurrence of malaise/weakness, was associated with the absence of hMPV infection (aOR 95% CI 0.4 (0.2; 0.8), p = 0.008) (Table 1).
After adjustment for chronic heart disease, age, gender, smoking status and influenza vaccination, hMPV infection was significantly associated with occurrence of acute heart failure during hospitalization (aOR 95% CI 1.8 (1.1; 3.0), p = 0.02).
Comparison of hMPV and influenza + patients
In univariate analysis, in comparison with influenza + patients, hMPV + patients were older (median age 78 IQR (70–86) vs 69 IQR (54–82) years, respectively, p < 0.001), more vaccinated against influenza (58% vs 39% respectively, p < 0.001), presented more dyspnea at inclusion (p = 0.05) but less weakness (p = 0.01), headache (p = 0.01) and sore throat (p = 0.008). They were more likely to present acute heart failure during the hospital stay (p = 0.002). Other outcomes were not significantly different (Table 2 ).
Table 2.
Sociodemographic, clinical characteristics and outcome of hospitalized patients infected with human metapneumovirus, compared with respiratory syncytial virus and influenza virus infected patients, 2012–2018
| hMPV+ (n = 90) | Influenza+ (n = 908) | p | RSV+ (n = 129) | p | |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Gender | |||||
| Women, n (%) | 50/90 (56%) | 430/908 (47%) | 0.14 | 67/129 (52%) | 0.60 |
| Men, n (%) | 40/90 (44%) | 478/908 (53%) | 62/129 (48%) | ||
| Age | |||||
| Median age, years (IQR) | 78 (70–86) | 69 (54–82) | <0.001 | 74 (64–84) | 0.06 |
| Age >65 years, n (%) | 76/90 (84%) | 538/908 (59%) | <0.001 | 95/129 (74%) | 0.06 |
| Median BMI, kg/m2 (IQR) | 24.8 (21.5–28.5) | 25.1 (22.1–28.4) | 0.85 | 24.6 (21.6–28.8) | 0.91 |
| Smoking status | |||||
| Smoker, n (%) | 12/87 (14%) | 178/874 (21%) | 0.12 | 31/126 (25%) | 0.05 |
| Ex-smoker, n (%) | 28/87 (32%) | 260/874 (30%) | 0.72 | 44/126 (35%) | 0.70 |
| Non-smoker, n (%) | 47/87 (54%) | 420/874 (49%) | 0.37 | 51/126 (40%) | 0.05 |
| Chronic diseases (at least one), n (%) | 73/90 (81%) | 678/908 (75%) | 0.18 | 117/129 (91%) | 0.04 |
| Chronic respiratory disease, n (%) | 40/90 (44%) | 344/908 (38%) | 0.22 | 70/129 (54%) | 0.15 |
| Chronic heart disease, n (%) | 45/90 (50%) | 362/908 (40%) | 0.06 | 64/129 (50%) | 0.96 |
| Diabetes, n (%) | 18/90 (20%) | 204/908 (22%) | 0.59 | 30/129 (23%) | 0.57 |
| Chronic renal failure, n (%) | 9/90 (10%) | 116/908 (13%) | 0.45 | 26/129 (20%) | 0.04 |
| Cancer, n (%) | 13/90 (14%) | 131/908 (14%) | 0.99 | 33/129 (26%) | 0.05 |
| Cirrhosis, n (%) | 4/90 (4%) | 28/922 (3%) | 0.52 | 4/129 (3%) | 0.60 |
| Immunosuppressive treatment, n (%) | 15/90 (17%) | 151/904 (17%) | 0.99 | 26/128 (20%) | 0.50 |
| Pregnancy, n (% of women of childbearing age) | 0 | 13/123 (11%) | 1.00 | 0 | 1.00 |
| Influenza vaccination, n (%) | 52/90 (58%) | 351/919 (39%) | <0.001 | 70/126 (56%) | 0.68 |
| Hospitalization in the previous 12 months, n (%) | 37/90 (41%) | 360/908 (40%) | 0.79 | 64/129 (50%) | 0.21 |
| Presence of child/children <5 years in the household, n (%) | 5/90 (6%) | 69/915 (8%) | 0.67 | 8/129 (6%) | 0.84 |
| Clinical presentation | |||||
| Median time from symptom onset to hospitalization, days (IQR) | 2 (1–3) | 2 (1–4) | 0.23 | 2 (1–3) | 0.7 |
| Symptoms | |||||
| Fever or feverishness, n (%) | 79/90 (88%) | 811/907 (89%) | 0.63 | 109/128 (85%) | 0.58 |
| Cough, n (%) | 75/90 (83%) | 784/908 (86%) | 0.43 | 106/128 (83%) | 0.92 |
| Dyspnea, n (%) | 76/90 (84%) | 650/908 (71%) | 0.05 | 112/128 (88%) | 0.42 |
| Weakness/malaise, n (%) | 13/90 (14%) | 244/904 (27%) | 0.01 | 29/128 (23%) | 0.13 |
| Headache, n (%) | 16/90 (18%) | 277/902 (31%) | 0.01 | 30/128 (23%) | 0.31 |
| Myalgia, n (%) | 19/90 (21%) | 260/897 (29%) | 0.11 | 19/128 (15%) | 0.23 |
| Sore throat, n (%) | 8/90 (9%) | 184/901 (20%) | 0.008 | 22/126 (17%) | 0.07 |
| Outcome | |||||
| At least one complication during the hospital stay, n (%) | 56/90 (62%) | 473/908 (52%) | 0.07 | 85/129 (66%) | 0.58 |
| Pneumonia, n (%) | 32/89 (36%) | 257/904 (28%) | 0.14 | 50/128 (39%) | 0.64 |
| Respiratory failure, n (%) | 32/89 (36%) | 254/904 (28%) | 0.12 | 47/128 (37%) | 0.91 |
| Acute heart failure, n (%) | 22/89 (25%) | 114/903 (13%) | 0.002 | 21/128 (16%) | 0.13 |
| Acute respiratory distress syndrome, n (%) | 8/89 (9%) | 83/904 (9%) | 0.95 | 15/128 (12%) | 0.52 |
| Median length of stay, days (IQR) | 7 (4–13) | 6 (3–10) | 0.06 | 7 (5–14) | 0.50 |
| ICU admission after hospitalization in acute care, n (%) | 15/90 (17%) | 155/908 (17%) | 0.88 | 33/128 (26%) | 0.20 |
| Death, n (%) | 4/90 (4%) | 36/906 (4%) | 0.78 | 9/129 (7%) | 0.44 |
BMI, body mass index; hMPV, human metapneumovirus; ICU, intensive care unit; IQR, interquartile range; RSV, respiratory syncytial virus.
In the final model from multivariate analysis, in comparison with influenza + patients, hMPV + patients were more frequently >65 years old (aOR 3.3 (1.9–6.3), P < 0.001).
Comparison of hMPV+ and RSV + patients
In univariate analysis, hMPV + patients had less chronic disease (p = 0.04), including chronic renal failure (p = 0.04) and cancer (p = 0.05) and were less likely to smoke (p = 0.05) than RSV + patients.
In the final multivariate model, in comparison with RSV + patients, hMPV + patients had less cancer (aOR = 0.4 (0.2–0.9), p = 0.02) and were less likely to smoke (aOR = 0.5 (0.2–0.9), P = 0.04). Clinical presentation and outcomes were similar between the two groups (Table 2).
Discussion
In our post hoc analysis of 3148 hospitalized adult patients with community-acquired ILI, hMPV was found in 3% of the samples. These patients were older, had chronic conditions, frequent respiratory and cardiac chronic diseases, and frequently presented complications. This prevalence is consistent with several studies that found hMPV in 3–6% of adult patients with lower respiratory tract infection in primary care [1,[14], [15], [16]] and in 6% of patients hospitalized for acute respiratory infection (ARI) [17]. This frequency may vary according to the inclusion criteria, especially temperature cut-off, as hMPV infection frequently causes non-febrile illness [5].
Our hospitalized hMPV + adults were mostly older and/or high-risk patients as previously described in the literature [5,10,[18], [19], [20]]. Complications were frequent (62%), as well as ICU admission (17%) and death (4%). These rates were similar to those of the 91 hospitalized patients with hMPV from the Walsh et al. study in 2008 in the USA [10], but lower than those of the 128 critically ill adults with hMPV infection (31% required ICU admission and 8% died) from the Hasvold et al. study in 2016 [20].
The majority of hMPV + patients presented several respiratory signs (cough, dyspnea), whereas sudden onset of symptoms was associated with the absence of hMPV infection. There were differences in clinical presentation between hMPV + patients and influenza + patients (less frequent constitutional symptoms (headache, weakness, myalgia) but more dyspnea) but not between hMPV + patients and RSV + patients. These points emphasize the difficulty in distinguishing between respiratory viruses based on clinical signs alone and question the relevance of the current ILI definition to detect hMPV infection.
Interestingly, we also found that hMPV + patients were older and presented more chronic cardiac conditions and acute heart failure during the hospitalization than influenza + patients. Although, influenza [21] and RSV [22] are known to worsen heart failure, no study has specifically assessed hMPV [21,22]. In our study, hMPV infection was independently associated with the occurrence of acute heart failure.
Several limitations should be acknowledged. First, we enrolled patients during influenza virus circulation but hMPV circulation does not always match that of influenza. Second, we had no data to support causality between the detection of hMPV in nasopharyngeal samples and the ILI hospital admission. Other pathogens such as respiratory bacteria may have been involved and hMPV may have been a concomitant infectious agent with no role in the symptoms reported. The absence of data on bacteriological results prevents us from addressing this issue. Finally, although asymptomatic carriage of hMPV appears to be uncommon [23], we had no control population (i.e. hospitalized adults without ILI symptoms) to help evaluate hMPV pathogenicity.
In conclusion, adult hMPV infections concerned 3% of patients hospitalized with ILI in tertiary care hospitals over six consecutive influenza seasons in France. Most of the patients were older, had associated chronic conditions and developed pulmonary and cardiac complications. The relation between hMPV infection and worsening of heart failure needs further investigation. These at-risk populations would benefit from the development of antivirals and vaccines targeting hMPV.
Transparency declaration
The authors declare no competing interest related to the study. P.L. has received personal fees and non-financial support from Pfizer and Sanofi Pasteur. O.L. is an investigator for clinical trials sponsored by Janssen, GSK, Pfizer, Sanofi Pasteur and MSD and received travel support to attend scientific meetings from pharmaceutical companies. The current work received no funding. However, the study sites received funding from Sanofi Pasteur, Sanofi Pasteur MSD and Janssen for the FLUVAC study. Vaccine producers had no role in the study design, data analysis, decision to publish or preparation of the manuscript.
Authors' contributions
P.L. and O.L. conceptualized the study. P.L. developed the methodology. P.M. did the analysis. P.L. and P.M. wrote the original manuscript. N.L., F.G., F.L., Z.L., P.V., X.D., D.P., S.A., S.R., G.L., A.S.L., V.F., N.H., B.L. and F.C. critically reviewed the manuscript. O.L. supervised the research.
Acknowledgements
We thank Sarah Kabani for her editing assistance.
Editor: A. Huttner
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.04.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ieven M., Coenen S., Loens K., Lammens C., Coenjaerts F., Vanderstraeten A., et al. Aetiology of lower respiratory tract infection in adults in primary care: a prospective study in 11 European countries. Clin Microbiol Infect. 2018;24:1158–1163. doi: 10.1016/j.cmi.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Souty C., Masse S., Valette M., Behillil S., Bonmarin I., Pino C., et al. Baseline characteristics and clinical symptoms related to respiratory viruses identified among patients presenting with influenza-like illness in primary care. Clin Microbiol Infect. 2019;25:1147–1153. doi: 10.1016/j.cmi.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uyeki T.M., Bernstein H.H., Bradley J.S., Englund J.A., File T.M., Fry A.M., et al. Clinical practice guidelines by the infectious diseases society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenzaa. Clin Infect Dis. 2019;68:e1-47. doi: 10.1093/cid/ciy866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stockton J., Stephenson I., Fleming D., Zambon M. Human metapneumovirus as a cause of community-acquired respiratory illness. Emerg Infect Dis. 2002;8:5. doi: 10.3201/eid0809.020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falsey A.R., Erdman D., Anderson L.J., Walsh E.E. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–790. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 6.Panda S., Mohakud N.K., Pena L., Kumar S. Human metapneumovirus: review of an important respiratory pathogen. Int J Infect Dis. 2014;25:45–52. doi: 10.1016/j.ijid.2014.03.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Reeves R.M., Wang X., Bassat Q., Brooks W.A., Cohen C., et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health. 2019;7 doi: 10.1016/S2214-109X(19)30264-5. [DOI] [PubMed] [Google Scholar]
- 8.Principi N., Esposito S. Paediatric human metapneumovirus infection: epidemiology, prevention and therapy. J Clin Virol. 2014;59:141–147. doi: 10.1016/j.jcv.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Boivin G., Abed Y., Pelletier G., Ruel L., Moisan D., Côté S., et al. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186:1330–1334. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- 10.Walsh E.E., Peterson D.R., Falsey A.R. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168:2489. doi: 10.1001/archinte.168.22.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidaur L., Totorika I., Montes M., Vicente D., Rello J., Cilla G. Human metapneumovirus as cause of severe community-acquired pneumonia in adults: insights from a ten-year molecular and epidemiological analysis. Ann Intensive Care. 2019;24:86. doi: 10.1186/s13613-019-0559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Hoogen B.G. Respiratory tract infection due to human metapneumovirus among elderly patients. Clin Infect Dis. 2007;44:1159–1160. doi: 10.1086/513295. [DOI] [PubMed] [Google Scholar]
- 13.European Centre for Disease Prevention and Control (ECDC) Influenza case definition. https://ecdc.europa.eu/en/surveillance-and-disease-data/eu-case-definitions Available from:
- 14.Van den Hoogen B.G., Osterhaus A.D., Fouchier R.A. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr Infect Dis J. 2004;23:S25–S32. doi: 10.1097/01.inf.0000108190.09824.e8. [DOI] [PubMed] [Google Scholar]
- 15.Sentilhes A.-C., Choumlivong K., Celhay O., Sisouk T., Phonekeo D., Vongphrachanh P., et al. Respiratory virus infections in hospitalized children and adults in Lao PDR. Influenza Other Respir. Viruses. 2013;7:1070–1078. doi: 10.1111/irv.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim Y.K., Kweon O.J., Kim H.R., Kim T.-H., Lee M.-K. Clinical features, epidemiology, and climatic impact of genotype-specific human metapneumovirus infections: long-term surveillance of hospitalized patients in South Korea. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz697. https://academic-oup-com.proxy.insermbiblio.inist.fr/cid/advance-article/doi/10.1093/cid/ciz697/5540161 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefebvre A., Manoha C., Bour J.-B., Abbas R., Fournel I., Tiv M., et al. Human metapneumovirus in patients hospitalized with acute respiratory infections: a meta-analysis. J Clin Virol. 2016;81:68–77. doi: 10.1016/j.jcv.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haas L.E.M., de Rijk N.X., Thijsen S.F.T. Human metapneumovirus infections on the ICU: a report of three cases. Ann Intensive Care. 2012;2:30. doi: 10.1186/2110-5820-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamelin M.E., Cotu S., Laforge J., Lampron N., Bourbeau J., Weiss K., et al. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin Infect Dis. 2005;41:498–502. doi: 10.1086/431981. [DOI] [PubMed] [Google Scholar]
- 20.Hasvold J., Sjoding M., Pohl K., Cooke C.R., Hyzy R.C. The role of human metapneumovirus in the critically ill adult patient. J Crit Care. 2016;31:233–237. doi: 10.1016/j.jcrc.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel N.J., Nalluri N., Deshmukh A., Pant S., Shah N., Badheka A.O., et al. Seasonal trends of heart failure hospitalizations in the United States: a national perspective from 2000 to 2011. Int J Cardiol. 2014;173:562–563. doi: 10.1016/j.ijcard.2014.03.122. [DOI] [PubMed] [Google Scholar]
- 22.Ivey K.S., Edwards K.M., Talbot H.K. Respiratory syncytial virus and associations with cardiovascular disease in adults. J Am Coll Cardiol. 2018;71:1574–1583. doi: 10.1016/j.jacc.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Falsey A., Criddle M., Walsh E. Detection of respiratory syncytial virus and human metapneumovirus by reverse transcription polymerase chain reaction in adults with and without respiratory illness. J Clin Virol. 2006;35:46–50. doi: 10.1016/j.jcv.2005.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.