Abstract
Novel coronavirus disease 2019 (COVID-19) emerged in late December 2019 in Wuhan, China. Since then, COVID-19 has become a pandemic affecting more than 4.1 million people worldwide. Patients with COVID-19 have a wide spectrum of manifestations, one being cytokine release syndrome (CRS) and its fatal correlate, secondary hemophagocytic lymphohistiocytosis (sHLH). Anti-cytokine therapy such as tocilizumab, an IL-6 receptor antagonist, is a potential treatment for COVID-19; however, data regarding the efficacy of this anti-IL-6 therapy are currently lacking. We report two cases of patients who received a diagnosis of COVID-19 complicated by CRS and were treated with tocilizumab. Both patients progressed to sHLH despite treatment with tocilizumab, and one developed viral myocarditis, challenging the safety and clinical usefulness of tocilizumab in the treatment of COVID-19-induced CRS. These cases highlight the need for clinical trials to determine optimal patient selection and timing for the use of tocilizumab during this disease process.
Key Words: COVID-19, critical care, cytokines, viral disease
Abbreviations: COVID-19, novel coronavirus disease 2019; CRP, C-reactive protein; CRS, cytokine release syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; sHLH, secondary hemophagocytic lymphohistiocytosis; TNF-α, tumor necrosis factor α
In December 2019, a novel coronavirus emerged as the cause of a cluster of pneumonia cases in Wuhan, China. This virus was later designated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease or syndrome was named COVID-19. As of May 17, 2020, COVID-19 has affected more than 4.1 million people, resulting in more than 285,000 deaths worldwide.1 SARS-CoV-2 infection causes a spectrum of disease ranging from mild to hypoxic respiratory failure, ARDS, and multiorgan failure and shock. A potential etiology of these severe manifestations of COVID-19 is cytokine release syndrome (CRS) and its most severe form, secondary hemophagocytic lymphohistiocytosis (sHLH).2 These syndromes are characterized by an excessive production of inflammatory cytokines (IL-6, IL-10, and tumor necrosis factor-α [TNF-α]).3, 4, 5, 6 Thus, anticytokine therapy has been suggested as a treatment for COVID-19, although its safety and efficacy in this population are yet to be established.2 Tocilizumab is a US Food and Drug Administration-approved IL-6 receptor antagonist that is commonly used to treat CRS secondary to chimeric antigen receptor T-cell therapy.7 Tocilizumab is theorized to treat the CRS that can occur in patients with COVID-19, similar to its use in CRS secondary to chimeric antigen receptor T-cell therapy.8 Thus far, reports detailing the outcomes of patients with COVID-19 undergoing this treatment strategy are sparse. We report two cases detailing poor outcomes of patients with COVID-19 CRS after treatment with tocilizumab.
Case Reports
Case 1
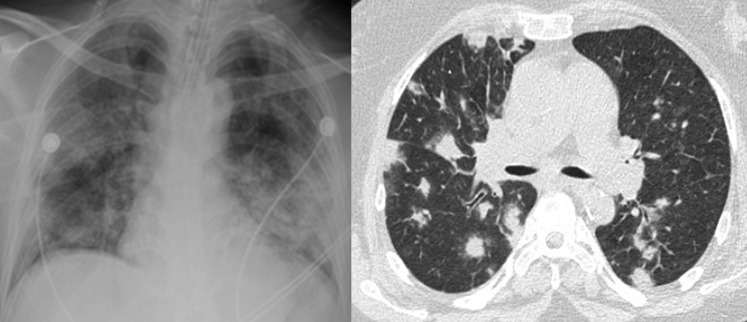
A 40-year-old man with no medical history presented with 5 days of fever, dry cough, and dyspnea on exertion. On examination, he was febrile to 39.4°C (102.9°F), in moderate respiratory distress, and had oxyhemoglobin desaturation. COVID-19 was confirmed by a positive PCR test result obtained via a nasopharyngeal swab, and he was started on hydroxychloroquine and azithromycin. Over the next 2 days, he continued to develop worsening hypoxemia, bilateral chest infiltrates (Fig 1 ), and accessory muscle fatigue requiring intubation and transfer to the medical ICU. There, he was diagnosed with ARDS and treated with low tidal volume ventilation, prone positioning, and a bumetanide drip. On day 4 of admission (9 days after symptom onset), he developed septic shock and was started on a norepinephrine drip. Given this decompensation and increasing inflammatory markers (Table 1 ), he was treated with a dose of tocilizumab (400 mg IV). The next day the patient developed ST segment depression in leads V4-V6 on ECG and increased troponin levels, which peaked at 30.39 ng/mL. ECG revealed mild global hypokinesis, and a diagnosis of viral myocarditis was made. Swan-Ganz catheter measurements confirmed a reduced cardiac index. The next day the patient developed a fever of 42.8°C and indexes of septic shock (normal cardiac index and reduced systemic vascular resistance) refractory to four vasopressor agents and passed away. Repeat laboratory measures (Table 1) suggested the development of sHLH (cytopenias, hypertriglyceridemia, elevated ferritin and lactate dehydrogenase, hypofibrinogenemia). This decompensation occurred despite a decrease in C-reactive protein (CRP) after tocilizumab therapy.
Figure 1.
Chest imaging of patients with COVID-19 pneumonia. Left: Chest radiograph from patient 1. There is evidence of bilateral interstitial opacifications consistent with ARDS. Right: Chest CT scan from patient 2. There is evidence of nodular opacities homogeneously distributed throughout all lung fields.
Table 1.
Clinical Laboratory Trends in Patient 1
| Laboratory Value | D1 | D2 | D3 | D4a | D5 | D6 | D7 |
|---|---|---|---|---|---|---|---|
| WBC count, × 103/μL | 7.0 | 7.0 | 9.0 | 11.1 | 5.9 | 5.8 | 6.9 |
| Platelet count, × 103/μL | 199 | 217 | 244 | 252 | 272 | 328 | 25 |
| Neutrophil count, × 103/μL | 5.7 | 6.4 | 7.8 | 7.7 | 4.7 | 5.5 | 5.5 |
| Lymphocyte count, × 103/μL | 0.8 | 0.5 | 0.7 | 2.1 | 0.8 | 0.2 | 0.7 |
| NLR | 7.5 | 12.7 | 11.1 | 3.6 | 5.7 | 27.7 | 7.9 |
| Fibrinogen, mg/dL | … | … | … | 885 | > 1,000 | > 1,000 | 247 |
| D-dimer, ng/mL | 673 | 1117 | 982 | 7,044 | 17,060 | 29,979 | 30,233 |
| Creatinine, mg/dL | 0.7 | 0.5 | 0.5 | 0.7 | 0.6 | 1.3 | 5.3 |
| Alanine aminotransferase, IU/L | 107 | 68 | 56 | 62 | … | 167 | 1,159 |
| Aspartate aminotransferase , IU/L | 112 | 48 | 42 | 64 | … | 980 | 4,174 |
| Ferritin, ng/mL | 1,385 | 1,179 | 1,412 | 1,529 | 1,849 | 38,299 | |
| Lactate dehydrogenase, IU/L | 368 | 369 | 377 | 494 | 436 | 1,857 | 5,517 |
| C-reactive protein, mg/dL | … | … | 9.0 | 18.3 | 44.1 | 29.0 | 7.7 |
| IL-6, pg/mL | … | … | … | 74.3 | 345 | … | … |
| Triglycerides, mg/dL | … | … | … | 229 | 276 | 390 | 811 |
| Troponin T, ng/mL | … | … | … | < 0.01 | 5.21 | 17.91 | 30.39 |
D = day; NLR = neutrophil-to-lymphocyte ratio.
Tocilizumab administered on day 4.
Case 2
A 69-year-old woman with a history of type 2 diabetes mellitus, rheumatoid arthritis, and aplastic anemia presented with 6 days of productive cough, pleuritic chest pain, fever, fatigue, and abdominal pain. On examination, she was febrile to 38.1°C, not in any respiratory distress, and her oxyhemoglobin saturation was 96% on room air. A CT scan revealed diffuse bilateral nodular opacities (Fig 1). COVID-19 was confirmed by a positive PCR test result obtained via a nasopharyngeal swab and she was started on hydroxychloroquine and azithromycin. On hospital day 2 (7 days after symptom onset), she rapidly progressed into acute hypoxemic respiratory failure and septic shock. She was intubated, started on norepinephrine, and treated with a dose of tocilizumab (560 mg IV). On day 3, her shock worsened, requiring the maximum dose of three vasopressor agents, and she was started on stress dose steroids. Concurrently, she went into acute kidney injury requiring continuous venovenous hemodialysis. On day 4 (9 days after symptom onset), her inflammatory markers continued to increase (Table 2 ) and she was treated with a second dose of tocilizumab (700 mg IV). Despite the second dose, her clinical status worsened as she developed sHLH (cytopenias, hypertriglyceridemia, elevated ferritin and lactate dehydrogenase, hypofibrinogenemia) and passed away. As in case 1, these developments occurred despite a decrease in CRP after tocilizumab therapy.
Table 2.
Clinical Laboratory Trends in Patient 2
| Laboratory Value | D1 | D2a | D3 | D4a | D5 | D6 | D7 |
|---|---|---|---|---|---|---|---|
| WBC count, × 103/μL | 2.1 | 3.6 | 2.6 | 1.5 | 1.6 | 2.2 | 5.3 |
| Platelet count, × 103/μL | 397 | 286 | 329 | 102 | 60 | 45 | 26 |
| Neutrophil count, × 103/μL | 0.6 | 0.8 | 1.2 | … | … | 1.1 | 2.3 |
| Lymphocyte count, × 103/μL | 1.0 | 2.4 | 0.6 | … | … | 0.3 | 0.3 |
| NLR | 0.6 | 0.3 | 0.5 | … | 3.7 | 7.7 | |
| Fibrinogen, mg/dL | … | … | … | … | 183 | 104 | 35 |
| D-dimer, ng/mL | 830 | … | 5,964 | … | 60,983 | 87,206 | 67,094 |
| Creatinine, mg/dL | 0.9 | 1.5 | 2.9 | 3.7 | 2.6b | 1.1b | 0.9b |
| Alanine aminotransferase, IU/L | 154 | 99 | 66 | 161 | 1,438 | 1,316 | 932 |
| Aspartate aminotransferase, IU/L | 254 | 131 | 97 | 837 | > 7,000 | 4,432 | 2,963 |
| Ferritin, ng/mL | 2,052 | … | … | … | 33,315 | 63,378 | 40,934 |
| Lactate dehydrogenase, IU/L | … | … | … | … | … | … | … |
| C-reactive protein, mg/dL | … | 20.8 | … | 33.0 | 20.4 | 12.6 | 8.4 |
| IL-6, pg/mL | … | … | > 400 | … | … | … | … |
| Triglycerides, mg/dL | … | … | … | … | … | … | … |
| Troponin T, ng/mL | < 0.01 | … | … | … | … | 0.03 | 0.04 |
See Table 1 legend for expansion of abbreviations.
Tocilizumab administered on days 2 and 4.
Value obtained after initiation of dialysis.
Discussion
In this report, we have presented two cases of COVID-19-induced CRS with elevated IL-6 levels and progression to sHLH, despite treatment with tocilizumab. In addition, we report viral myocarditis development in one patient posttreatment. Interestingly, the clinical decompensations occurred despite a dramatic decrease in the IL-6 surrogate, CRP, posttreatment. These findings substantiate a report from Wuhan, China in which four of seven critically ill patients treated with tocilizumab died or experienced disease aggravation despite improvement in CRP.9 Our report further challenges the clinical usefulness of anti-IL-6 therapy in the treatment of COVID-19-induced CRS.
Tocilizumab may have worsened the clinical course of the patients by adding to immunosuppression. Evidence suggests that a deficient T-cell response underlies the severity of COVID-19. CD8+ and CD4+ T-cell levels are decreased in patients with COVID-19 and correlate with severity of disease.6 Moreover, diabetes, which is associated with T-cell-mediated immunosuppression, is a risk factor for COVID-19.10 IL-6 promotes immature thymocyte differentiation into cytotoxic T cells and is needed for B-cell production of IgM and IgG.8 Thus, it is possible that elevated IL-6 levels are a compensatory mechanism for an impaired, virally directed cytotoxic T-cell response. Thus decreasing IL-6 levels in COVID-19 may promote increased viral replication if tocilizumab is used too early in the disease course. In fact, viral replication has been found as late as 22 days after symptom onset.11 In our first case, the patient developed viral myocarditis, an established presentation of severe COVID-19 on day 10 after symptom onset.12 , 13 We fear that tocilizumab may have been responsible for the development of viral myocarditis. This notion is further supported by decreased lymphocyte counts posttreatment in both the patients, suggesting decreased IL-6-mediated lymphocyte maturation.
The progression to sHLH in the patients suggests that excessive IL-6 production may only be a small component of the CRS seen in COVID-19.5 , 14 This is supported by the fact that the patients decompensated despite decreased levels of the IL-6 correlate, CRP. Along with IL-6, circulating levels of IL-2, IL-10, and TNF-α are all elevated in patients with severe COVID-19.6 Of these, IL-10 and TNF-α are classically involved in CRS and sHLH. Immunomodulating therapies may need to target multiple cytokines to treat COVID-19 CRS. Given the fear of increased viral replication, there is a need to know when it would be safe to give alternative, stronger, and/or broader immunosuppressant therapies.
There are currently no data from rigorously conducted clinical trials evaluating tocilizumab use in COVID-19. Although off-label use based on hypothetical benefit may drive the use of this and other drugs during the COVID-19 pandemic, there must be recognition that the true clinical benefits of untested medications are unknown. However, several clinical trials are actively recruiting subjects to determine the safety and efficacy of tocilizumab in the treatment of severe COVID-19 pneumonia in adult patients.15, 16, 17, 18 These clinical trials will further elucidate the effects of tocilizumab on COVID-19-induced organ failure and mortality and correlate these outcomes with inflammatory marker trends. Our report highlights that off-label drugs are best used in the context of a randomized clinical trial, where the timing of tocilizumab administration in the disease course and indications based on clinical parameters and biomarkers can be determined.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: P. J. B. receives funding from Gilead for NCT0429899 and NCT04292730, but these sources of funding were not used for this research. She also receives internal Rutgers funding (not external), but this is also not for the purposes of this research. None declared (J. R., N. N.).
References
- 1.Johns Hopkins Coronavirus Resource Center COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU): Global Cases. https://coronavirus.jhu.edu/map.html Updated April 8, 2020. Accessed April 8, 2020.
- 2.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen S.F., Ho Y.-C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimabukuro-Vornhagen A., Gödel P., Subklewe M. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen G., Wu D., Guo W. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frey N., Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant. 2019;25(4):e123–e127. doi: 10.1016/j.bbmt.2018.12.756. [DOI] [PubMed] [Google Scholar]
- 8.Zhang C, Wu Z, Li J-W, Zhao H, Wang G-Q. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. In press. https://doi.org/10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed]
- 9.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. In press. https://doi.org/10.1002/jmv.25801. [DOI] [PMC free article] [PubMed]
- 10.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lescure F-X, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. In press. https://doi.org/10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed]
- 12.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. In press. https://doi.org/10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed]
- 13.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramos-Casals M., Brito-Zerón P., López-Guillermo A., Khamashta M.A., Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503–1516. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 15.National Institutes of Health Clinical Center . National Institutes of Health; Bethesda, MD: 2020. Tocilizumab for SARS-CoV2 (COVID-19) severe pneumonitis. NCT04315480. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT04315480 [Google Scholar]
- 16.National Institutes of Health Clinical Center . National Institutes of Health; Bethesda, MD: 2020. Tocilizumab in COVID-19 pneumonia (TOCIVID-19). NCT04317092. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT04317092 [Google Scholar]
- 17.National Institutes of Health Clinical Center . National Institutes of Health; Bethesda, MD: 2020. A study to evaluate the safety and efficacy of tocilizumab in patients with severe COVID-19 pneumonia (COVACTA). NCT04320615. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT04320615 [Google Scholar]
- 18.Chinese Clinical Trial Registry A multicenter, randomized controlled trial for the efficacy and safety of tocilizumab in the treatment of new coronavirus pneumonia (COVID-19). ChiCTR2000029765. 2020. http://www.chictr.org.cn/showprojen.aspx?proj=49409