Abstract
Patients with Coronavirus Disease 2019 (COVID-19) often have clinical characteristics, such as chest tightness and dyspnea. Continuous, unresolved dyspnea often indicates the progression of lung lesions. The mechanism that underlies the chest distress and dyspnea in patients with COVID-19 is still unclear. Chest CT has a higher sensitivity and can play an essential role in the diagnosis and treatment of the disease. However, our clinical observations showed that although some patients had significant chest distress and dyspnea, the lesions that were observed in the lungs during computed tomography were milder and not completely consistent with clinical symptoms. We analyzed the clinical characteristics, laboratory test results, and imaging findings of these patients. We found that extensive inflammation of the bilateral and respiratory bronchioles in patients with COVID-19 due to excessive activation of proinflammatory cytokines and chemotactic aggregation of T-lymphocytes at the site of inflammation are possible mechanisms underlying chest distress and dyspnea in patients with COVID-19. Short-time and lose-dose use of corticosteroid may be helpful to treat chest tightness and dyspnea in mild COVID-19 patients. Through this study, we aimed to improve our understanding of the pathogenesis of COVID-19.
Keywords: COVID-19, Chest CT, Dyspnea, Chest tightness, Corticosteroid use
1. Introduction
The novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first discovered in Wuhan in December 2019 [1],and the disease caused was named COVID-19. As of March 23, there have been 31,4627 confirmed infections, resulting in 13,533 deaths worldwide. No effective treatments are currently available [2].Analysing the possible pathogenesis of existing cases could help in identifying appropriate interventions.
It has been confirmed that SARS-CoV-2 can enter cells through angiotensin converting enzyme 2 (ACE2) receptor and subsequently cause injury. ACE2 receptor is mainly distributed in type Ⅱ alveolar cells (AT2 cell) in the lung tissues. Therefore, the primary target organ of SARS-CoV-2 in the human body is the lung [3], the primary presentation of patients include respiratory tract symptoms, such as cough, fever, and dyspnea [2]. About 50% of patients with severe cases of COVID-19 (age > 60 years; lung or cardiovascular disease) develop dyspnea one week after the onset of the disease, which can progress rapidly to acute respiratory distress syndrome, difficult-to-correct metabolic acidosis, coagulopathy, and even death [4]. Patients with severe dyspnea have increased lung lesions with larger extent in advanced stages, involving multiple lung lobes, and some cases exhibit the ‘white lung’. These are primarily consolidation opacities together with ground-glass opacities and are often accompanied by multiple linear opacities and air bronchograms [5]. Therefore, sudden or exacerbated dyspnea in the clinic often indicates an increase in lung lesions and further aggravation of the disease.
During the clinical diagnosis and treatment of patients with COVID-19, we discovered a special phenomenon: the extent of lesions on chest computed tomography (CT) images in some patients was not consistent with their clinical symptoms, such as severe chest distress and dyspnoea. These symptoms are severe in these patients; however, the lesions in the lung observed during CT are different from the common and extensive consolidation opacities and large number of ground-glass opacities. Instead, the lesions primarily manifested as inflammatory changes in the bilateral and respiratory bronchioles. Use of short-time and low-dose corticosteroid could rapidly attenuate the chest tightness and dyspnea symptoms. We believe that our findings are helpful to better understand the mechanisms and treatment of COVID-19.
2. Case presentation
2.1. Case 1
A 25-year-old female patient was admitted to hospital on 24 February 2020 because of intermittent chest distress for over a month and relapse since two days. During the onset, she was admitted to a square-cabin hospital in Wuhan for treatment. She experienced night sweats, fever, cough, and expectoration of a small amount of white sputum. After treatment, her symptoms eased, she showed improvement on her chest CT, and she was discharged. On February 22, chest distress and shortness of breath, which was exacerbated by activity, reappeared. Therefore, she was admitted to the hospital again. She complained of right-sided back pain but had no chills, fever, cough, sputum, haemoptysis, or joint pain. Normal diet was tolerated, sleep was poor, and urination and defecation were normal. There was no significant weight loss. The patient was previously healthy and had no underlying disease. Physical examination at admission revealed the following: body temperature, 36.4 °C; pulse, 82 beats/min; respiration, 20 breaths/min; blood pressure, 123/72 mmHg; and oxygen saturation, < 93%. The patient was conscious. The respiratory sounds in both lungs were slightly coarse, and moist rales could be heard at the base of the lungs. The heart rate was 82 beats/min, heart rhythm was normal, the abdomen was soft, and there were no obvious abnormalities in limb movements and no oedema in the lower limbs.
Supplemental examination after admission revealed the following: Blood routine test (2020-2-24): WBC 7.83 × 109/L (normal range: 3.5–9.5), lymphocyte count 2.59 × 109/L (normal range: 1.1–3.2), RBC 4.47 × 1012/L (normal range: 3.8–5.1), hemoglobin 135g/L (normal range: 115–150), PLT: 217 × 109/L (normal range: 125–350). The creatine kinase MB (CK-MB), myoglobin, ultrasensitive troponin I (ultra-TnI), lactate dehydrogenase, CRP and procalcitonin (PCT) were all within the normal ranges. Cytokines (2020-2-24): IL-6 26.62pg/mL (≤20.0), TNF- α 44.87pg/mL (≤5.5), other cytokines (IL-2, IL-4, IL-5, IL-10 and IFN-γ) were within the normal ranges. Electrocardiogram was normal. To figure out the potential pathogen of her infection, a panel of extra laboratory tests was performed, and the results are shown in Table 1 . During the hospitalization, the CT scans of lungs were closely monitored. On February 22, the pulmonary lesion of CT was mild, and only single thin opacities in the posterior basal segment of the bilateral inferior lobes were found (Fig. 1 A). However, chest CT showed that the segmental bronchial wall thickened, and the lumen widened, indicating overactive inflammation response of pulmonary bronchial system (Fig. 1C). Summary reports of chest CT scans of patient 1 were shown in Fig. 1.
Table 1.
Laboratory tests for infectious respiratory pathogens of patient 1.
| Throat swab, nucleic acid detection | Results | Serum, antibody detection (IgM) | Results | Coronavirus IgM and IgG detection | Results(normal range: <10) |
|---|---|---|---|---|---|
| Influenza A virus, InfA-RNA | Negative | Legionella pneumophila serum type 1, LPN1-IgM | Negative | 2019-nCoV, IgG-NCOV | 173.65 AU/mL |
| Influenza A H1N1 (2009), H1N1-RNA | Negative | Mycoplasma pneumoniae, MP-IgM | Negative | 2019-nCoV, IgM-NCOV | 16.58 AU/mL |
| Influenza A H3N2, H3N2-RNA | Negative | Q fever Rickettsia, COX-IgM | Negative | ||
| Influenza B virus, InfB-RNA |
Negative | Chlamydia pneumonia, CP-IgM | Negative | ||
| Parainfluenza virus, HPIV-RNA | Negative | Adenovirus, ADV-IgM | Negative | ||
| Respiratory syncytial virus, HRSV-RNA | Negative | Respiratory syncytial virus, RSV-IgM | Negative | ||
| Metapneumovirus, HMPV-RNA | Negative | Influenza A virus, FluA-IgM | Negative | ||
| Coronavirus, HCOV-RNA | Negative | Influenza B virus, FluB-IgM | Negative | ||
| Rhinovirus, HRV-RNA | Negative | Parainfluenza virus type 1/2/3, PIV-IgM | Negative | ||
| Adenovirus, ADV-DNA | Negative | ||||
| Bocaparvovirus, Boca-DNA | Negative | ||||
| Pneumonia mycoplasma, Mp-DNA | Negative | ||||
| Pneumonia chlamydia, Ch-DNA | Negative |
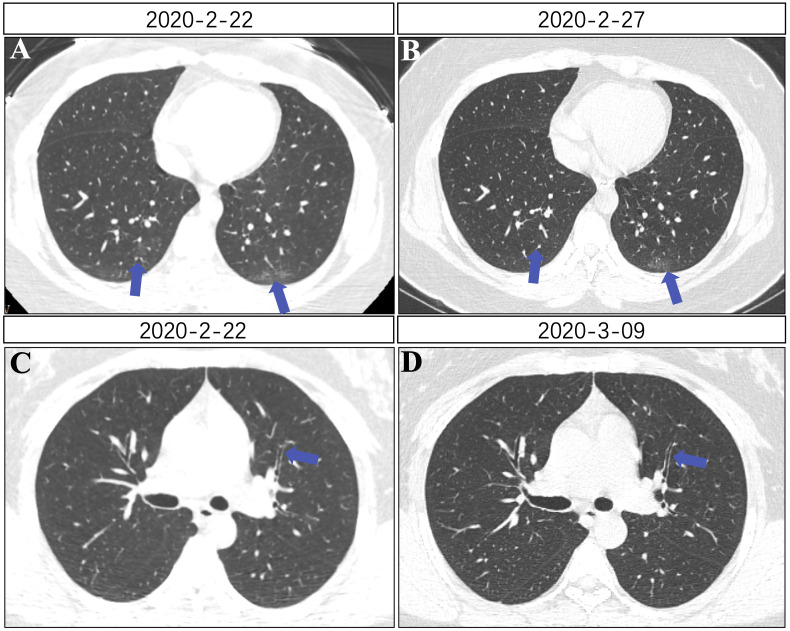
Fig. 1.
Chest CT images of patient 1. (A) A little thin shadow in the posterior basal segment of the double lower lobe (blue arrow). (B) Reduced lesions in the bilateral inferior lobes compared with the previous image on February 22. (C) Thickening of subsegmental bronchial walls, no gradual luminal narrowing (blue arrow). (D) Thinning of subsegmental bronchial walls compared with before, gradual luminal narrowing (blue arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
After admission, she was given symptomatic support treatments such as oxygen inhalation and hepatic functional protection (diammonium glycyrrhizinate capsules:150mg,po,tid, 1 week), antiviral therapies (ribavirin:250mg twice daily, arbidol: 0.2g,po,tid, 2 weeks) and anti-inflammatory treatments (methylprednisolone: 40mg once daily, 5 days). Her chest tightness and dyspnea symptoms were significantly improved after 3 days. On March 9, the wall of subsegment bronchus became thinner than before, and the lumen became thinner gradually on chest CT when she was discharged (Fig. 1D).
2.2. Case 2
A 31-year-old woman was admitted to the hospital on 20 January 2020 because of coughing and chest distress for four days and fever for three days. During the course of the disease, she developed myalgia, nausea and vomiting, and diarrhea. The patient had a history of debridement for right alveolar osteonecrosis and had no history of allergies. Physical examination revealed the following: body temperature, 38.8 °C; pulse, 112 beats/min; respiration, 20 breaths/min; and blood pressure, 111/77 mmHg. The patient was conscious and was in a good mood. There was no jaundice of the skin or sclera. No swelling of the superficial lymph nodes was found upon palpation. There was no coarse respiratory sound in both lungs and no moist or dry rales. The heart rate was 112 beats/min, heart rhythm was normal, and there was no murmur. The abdomen was soft with no tenderness or rebound pain. The liver and spleen could not be palpated below the ribs. The shifting dullness sign was negative. Borborygmi was found. There was no pain in the bilateral kidney areas and no oedema in the lower limbs.
Supplemental examination after admission revealed the following: Blood routine test (2020-1-21): WBC, 2.84 × 109/L (normal range: 3.5–9.5); lymphocyte count, 0.73 × 109/L (normal range: 1.1–3.2); RBC, 4.67 × 1012/L (normal range: 3.8–5.1); hemoglobin, 120g/L (normal range: 115–150); PLT, 224 × 109/L (normal range: 125–350). CRP, 11.8mg/L (normal range: 0–10). Myocardial enzymes including CK-MB and ultra-TnI, D-Dimer, and PCT (2020-1-21) were all within the normal ranges. Cellular immunity (2020-1-21): CD3, 53.00% (normal range: 56–86); CD3 count, 406 cells/uL (normal range: 723–2737); CD4, 25.32% (normal range: 33–58); CD4 count, 192 cells/uL(normal range: 404–1612), CD8 count, 182 cells/uL (normal range: 220–1129); CD16+56, 29.89% (normal range: 5–26). All cytokines (IL-2, IL-4, IL-5, IL-6, IL-10, TNF-α and IFN-γ) were normal. The laboratory examinations of infectious respiratory pathogens were performed and shown in Table 2 .
Table 2.
Laboratory tests for infectious respiratory pathogens of patient 2.
| Throat swab, antigen detection | Results | Throat swab, nucleic acid detection | Results | Serum, antibody detection (IgM) | Results | Coronavirus, Nucleic acid detection |
Results |
|---|---|---|---|---|---|---|---|
| Influenza A virus, FluA-Ag | Negative | Influenza A virus, InfA-RNA | Negative | Legionella pneumophila serum type 1, LPN1-IgM | Negative | 2019nCoV nucleocapsid protein gene, nCov-NP | Positive |
| Influenza B virus, FluB-Ag | Negative | Influenza A H1N1 (2009), H1N1-RNA | Negative | Mycoplasma pneumoniae, MP-IgM | Negative | 2019nCoV open reading frame 1ab, nCovORF1ab | Positive |
| Adenovirus, ADV-Ag | Negative | Influenza A H3N2, H3N2-RNA | Negative | Q fever Rickettsia, COX-IgM | Negative | 2019nCoV envelope protein gene, nCov-EP | Positive |
| Respiratory syncytial virus, RSV-Ag | Negative | Influenza B virus, InfB-RNA |
Negative | Chlamydia pneumonia, CP-IgM | Negative | ||
| Parainfluenza virus (type 1), PIV1–Ag | Negative | Parainfluenza virus, HPIV-RNA | Negative | Adenovirus, ADV-IgM | Negative | ||
| Parainfluenza virus (type 2), PIV2–Ag | Negative | Respiratory syncytial virus, HRSV-RNA | Negative | Respiratory syncytial virus, RSV-IgM | Negative | ||
| Parainfluenza virus (type 3), PIV3–Ag | Negative | Metapneumovirus, HMPV-RNA | Negative | Influenza A virus, FluA-IgM | Negative | ||
| Coronavirus, HCOV-RNA | Negative | Influenza B virus, FluB-IgM | Negative | ||||
| Rhinovirus, HRV-RNA | Negative | Parainfluenza virus type 1/2/3, PIV-IgM | Negative | ||||
| Adenovirus, ADV-DNA | Negative | ||||||
| Bocaparvovirus, Boca-DNA | Negative | ||||||
| Pneumonia mycoplasma, Mp-DNA | Negative | ||||||
| Pneumonia chlamydia, Ch-DNA | Negative |
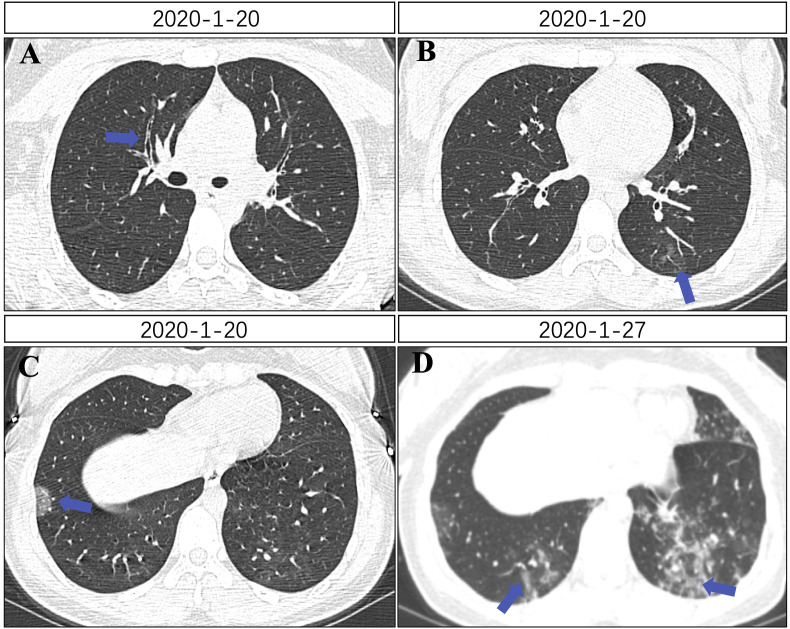
During hospitalization, the dynamics of the pulmonary CT scans were also closely monitored. On January 20, her pulmonary CT after admission showed subsegment bronchial wall thickening (Fig. 2 A), sparse lesion in the left inferior lobe (Fig. 2B) and leaf-like shadow on the lower right lung (Fig. 2C). She was given symptomatic support treatment such as oxygen inhalation. She also received treatments of anti-infection: moxifloxacin (400mg, once daily, 3 days) for mycoplasma and chlamydia, antiviral therapies (arbidol: 0.2g, po, tid, 2 weeks) and anti-inflammatory (methylprednisolone: 40mg once daily, 5 days). Then the patient was given human gamma globulin 10g (iv gtt, qd) for 5 days. The symptoms of chest tightness and dyspnea were completely relieved after 1 week. On January 27, the lesions of chest CT increased, and diffuse ground-glass shadows were found in bilateral lower lungs (Fig. 2D), suggesting the inconsistence with the complete relief of clinical symptoms. The levels of CD3%, CD3 count, CD4%, CD4 count, CD8 count returned to normal, and the percentage and cell counts of CD16+56 decreased on January 29. CD16+56% was 3.47% (normal range: 5–26), the cell count of CD16+56 was 69 cells/uL (normal range: 84–724). Blood routine test and CRP were normal.
Fig. 2.
(A) Subsegment bronchial wall thickening (blue arrow). (B) Sparse lesion in the left inferior lobe (blue arrow). (C) Flaky sparse opacity in the right inferior lobe (blue arrow). (D) Sparse, diffuse opacities in the bilateral inferior lobes (blue arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
The CT image for our first patient on 22 February showed that the extent of lung lesions was extremely mild, and the symptoms resolved. Five days later, on 27 February, the lung lesions were further reduced. Clearly, it was difficult to explain the persistent chest distress in the patient at admission based on the size of the lung lesions in that case. Our second patient had obvious chest distress at admission. Chest CT on 20 January revealed reduced extent of the lung lesions. Chest distress resolved after seven days, but diffuse chest lesions appeared during chest CT re-examination on 27 January in the absence of chest distress. The apparent deviation between clinical symptoms and chest CT findings was confusing. We know that chest distress and dyspnea are affected by several factors including cardiovascular and hematological diseases. Both patients were young women without previous histories of cardiovascular or hematological diseases. Examination of the cardiovascular and hematologic systems at admission did not reveal any abnormalities. It was clear that chest distress and dyspnea were due to respiratory system involvement.
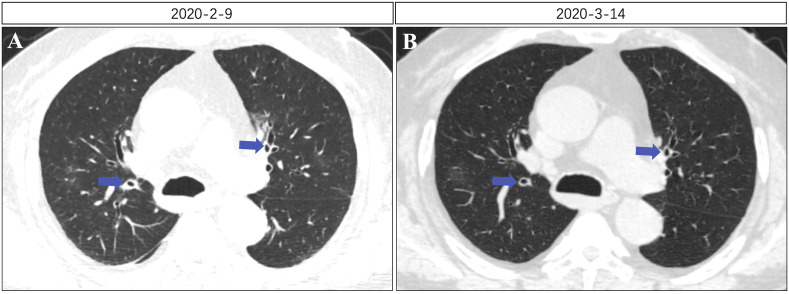
In the first case, we compared lung CT findings from 22 February, when the patient was admitted to the hospital, to those from 9 March, when the symptoms were relieved and found that the subsegmental bronchial wall became thinner and the lumen became narrower after relief of symptoms. In the second case, chest CT on 20 February also showed a thickened bronchial tube wall and a slightly enlarged lumen. This definitively demonstrated that the bronchioles were affected in both patients during the development and progression of the disease. In addition, we observed in our clinical practice that a large number of patients with COVID-19 had thickened subsegmental bronchial walls and enlarged lumen at multiple sites on chest CT images and exhibited extensive bronchial inflammatory changes (Fig. 3 ). Based on the ‘Radiological Diagnosis of New Coronavirus Infected Pneumonitis: Expert Recommendation from the Chinese Society of Radiology (First edition)’ published by the Chinese Society of Radiology of the Chinese Medical Association [6], the present study further showed that air bronchograms often appear in consolidation opacities, the bronchial walls were thickened, and the bronchi were distended locally during novel coronavirus pneumonia. Our observations indicated that bronchi with thickened walls and luminal expansion are not necessarily a part of the lesion opacities. This suggests that inflammation in the respiratory system of some patients involves visible local lesions of the lung parenchyma, and diffused inflammatory changes may exist in bilateral and respiratory bronchioles, which may be the cause of chest distress in patients.
Fig. 3.
(A) Subsegmental bronchial wall thickening, expanded lumen. (B) Recovery of subsegmental bronchial wall and lumen to normal condition.
Extensive respiratory tract inflammation may be associated disorders caused by inflammatory factors. Our first patient admitted had a TNF-α level of 44.87 pg/mL, which was much higher than normal (≤5.5 pg/mL). TNF-α is a polypeptide factor secreted by immune cells (mainly macrophages) in our body. It can induce the synthesis of acute phase proteins and mediate endotoxin and tissue injury in the inflammatory and stress responses [7]. In addition, TNF-α has important immunoregulatory effects, such as activating neutrophils to kill pathogenic microorganisms, stimulating monocytes and other cells to produce IL-1, IL-6, IL-8, and other cytokines, and activating T and B cells to produce antibodies [8]. TNF-α may be involved in the chronic inflammation of the respiratory tract. The second possible cause may be related to the migration of lymphocytes to the tracheobronchial lesions. In our second patient, the cellular immunity panel at admission showed a significant decrease in CD3, CD4, and CD8 levels. After treatment, the CD3, CD4, and CD8 cells returned to normal. Meanwhile, our previous clinical observations suggested that the decrease in these T-lymphocytes was mainly due to the chemotactic accumulation of these cells at the site of COVID-19 lung inflammation and their resulting depletion [9]. Therefore, we believe that the bilateral bronchial inflammation in the second patient may be associated with lymphocyte accumulation in bronchial lesions.
Our observations showed that the mechanism that underlies chest distress or dyspnea, which is inconsistent with lung lesions observed on CT images in patients with COVID-19 whose lung lesions are difficult to explain, may be due to the involvement of the air conduction system, such as the trachea, bronchi, and bronchioles. This may also explain why younger patients with mild lesions on lung imaging may have increased partial pressure of carbon dioxide on the arterial blood gas test. Apparently, inflammation of the bilateral air conduction system caused insufficient pulmonary ventilation, leading to dyspnea and chest distress. This also explains why these patients had reduced symptoms of chest distress after receiving oxygen and low-dose hormone therapy. This further indicated that the use of short-term hormone therapy in patients with common cases of COVID-19 with chest distress and dyspnea as the main symptoms is reasonable and that this therapy should not be limited to patients with severe cases.
Animal and human rights statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethical Review Committee of Renmin Hospital of Wuhan University. Informed consent was obtained from all participants enrolled in the study.
Declaration of competing interest
All authors had no conflict of interests to declare.
Acknowledgements
This study was supported by the emergency science and technology projects of COVID-19 of Hubei Province (No.2020FCA005).
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L., et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin L., Li T.S. [Interpretation of "guidelines for the diagnosis and treatment of novel coronavirus (2019-nCoV) infection by the national health commission (trial version 5)"] Zhonghua Yixue Zazhi. 2020;100:E001. doi: 10.3760/cma.j.issn.0376-2491.2020.0001. 0. [DOI] [PubMed] [Google Scholar]
- 5.Lu X.F.G.W., Wang L., et al. Clinical features and high resolution CT imaging findings of preliminary diagnosis novel coronavirus pneumonia. Chin. J. Radiol. 2020;(54):E006. 00. [Google Scholar]
- 6.CSo Radiology. Radiological diagnosis of New coronavirus infected pneumonitis: Expert recommendation from the Chinese society of Radiology (first edition) Chin. J. Radiol. 2020;55:E001. 00. [Google Scholar]
- 7.Croft M., Benedict C.A., Ware C.F. Clinical targeting of the TNF and TNFR superfamilies. Nat. Rev. Drug Discov. 2013;12(2):147–168. doi: 10.1038/nrd3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans H.G., Roostalu U., Walter G.J., Gullick N.J., Frederiksen K.S., Roberts C.A., et al. TNF-alpha blockade induces IL-10 expression in human CD4+ T cells. Nat. Commun. 2014;5:3199. doi: 10.1038/ncomms4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z., Li X., Zhang W., Shi Z.L., Zheng Z., Wang T. Clinical features and treatment of 2019-nCov pneumonia patients in wuhan: report of A couple cases. Virol. Sin. 2020 doi: 10.1007/s12250-020-00203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]