Abstract
In the event of a global infectious pandemic, drastic measures may be needed that limit or require adjustment of ambulatory allergy services. However, no rationale for how to prioritize service shut down and patient care exists. A consensus-based ad-hoc expert panel of allergy/immunology specialists from the United States and Canada developed a service and patient prioritization schematic to temporarily triage allergy/immunology services. Recommendations and feedback were developed iteratively, using an adapted modified Delphi methodology to achieve consensus. During the ongoing pandemic while social distancing is being encouraged, most allergy/immunology care could be postponed/delayed or handled through virtual care. With the exception of many patients with primary immunodeficiency, patients on venom immunotherapy, and patients with asthma of a certain severity, there is limited need for face-to-face visits under such conditions. These suggestions are intended to help provide a logical approach to quickly adjust service to mitigate risk to both medical staff and patients. Importantly, individual community circumstances may be unique and require contextual consideration. The decision to enact any of these measures rests with the judgment of each clinician and individual health care system. Pandemics are unanticipated, and enforced social distancing/quarantining is highly unusual. This expert panel consensus document offers a prioritization rational to help guide decision making when such situations arise and an allergist/immunologist is forced to reduce services or makes the decision on his or her own to do so.
Key words: SARS-CoV-2, COVID-19, Allergy, Allergy immunotherapy, Asthma, Food allergy, Venom allergy, Allergic rhinitis, Primary immunodeficiency, Urticaria, Angioedema, Atopic dermatitis
Abbreviations used: CDC, Centers for Disease Control and Prevention; COPD, Chronic obstructive lung disease; COVID-19, Coronavirus disease 2019; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
Executive Summary
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has exhibited a pattern of pandemic spread in a few short months, as countries and communities struggle to rapidly design effective strategies to prevent spread of the novel coronavirus. The virus has been named SARS-CoV-2 and the disease it causes “coronavirus disease 2019” (COVID-19).1 , 2 Community transmission is now evident, and it is clear that SARS-CoV-2 is a highly contagious virus.3 , 4 The spectrum of disease ranges from severe respiratory illness and fatality from these complications (particularly in the elderly and those with comorbidities) to asymptomatic spread,1 , 5 , 6 with the proclivity of SARS-CoV-2 for person-to-person transmission in asymptomatic individuals presenting one of the most vexing problems from a public health standpoint.1 Given the rapid and pervasive spread, the World Health Organization declared SARS-CoV-2 a pandemic on March 11, 2020, and on March 13, 2020, the president of the United States declared a national emergency in the United States, consistent with similar actions taken in several other countries7 , 8 (Figure 1 ).
Figure 1.
Theoretic model of pandemic caseload vs health care infrastructure capacity.9
Prevention and control
Although vaccine development is underway, it is unlikely that a vaccine will be available in 2020.1 The Centers for Disease Control and Prevention has recommended use of personal protective equipment by health care workers including standard, contact, and airborne precautions and with the use of eye protection. This means health care workers caring for a patient with suspected COVID-19 should wear a gown, gloves, and either an N95 respirator plus face shield and goggles or a powered air-purifying respirator; however, the Centers for Disease Control and Prevention also notes that a face mask may be substituted for an N95 respirator if one is not available, and a negative pressure room may be reserved for patients undergoing aerosol-generating procedures.1 , 2 However, this information is fluid and may continue to change. Commonsense strategies to control the spread of SARS-CoV-2 are detailed in Table E1 and frequently asked questions in Table E2 in this article's Online Repository at www.jaci-inpractice.org.2 , 9 During the COVID-19 pandemic the concept of social distancing has also been incorporated into prevention strategies, with the Centers for Disease Control and Prevention recommending avoiding close contact (<6 ft).2 , 9
Emergency social distancing—Prioritizing care in the event of ambulatory service rationing
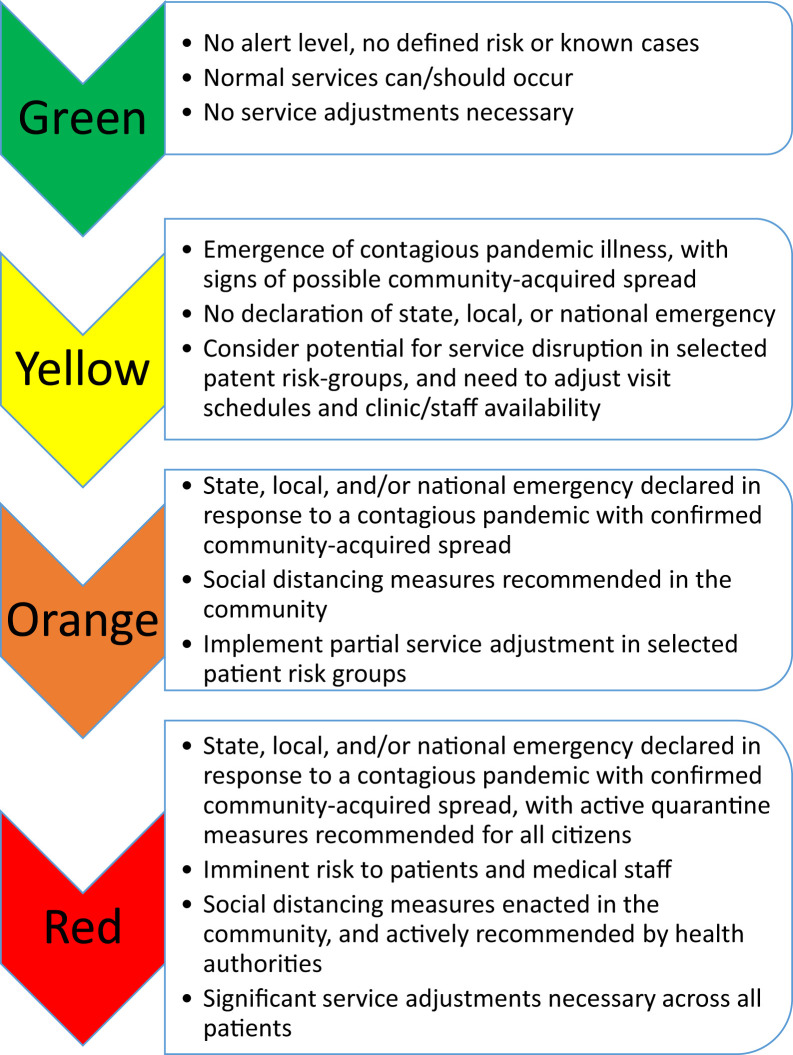
In the presence of a highly contagious global pandemic, decisions will need to be considered regarding the short-term rationing of services, keeping a perspective that many allergy/immunology services are elective and can be managed without face-to-face interaction, or deferred outright for short periods of time. This is prefaced by stating that such measures would be for emergency purposes only, such as at the present time, where the US president has declared a national state of emergency.8 As COVID-19 becomes more pervasive, recommended and mandated social distancing becomes more pronounced. Several countries have initiated widespread quarantine measures to try to contain and mitigate the spread of SARS-CoV-2. During a pandemic where a national state of emergency has been declared and quarantine measures are recommended or mandated, “red zone” measures must be considered.2 , 7 , 8 A helpful view of a stratified approach is presented in Figure 2 . Much of what follows relates to “red zone” operations. Some of the suggestion below may be most appropriate for a greater level of social distancing and quarantine than exists in the moment, and, as such, the clinician must view these as conditional recommendations to be incorporated within context-specific, evolving situations.
Figure 2.
Proposed paradigm of pandemic threat levels affecting normal allergy/immunology.
Again, we want to ensure that all readers understand that this is a suggested framework, and furthermore a framework only to be considered in the setting of a global emergency during a time when nations, societies, and institutions are facing drastic pandemic measures in a “red zone” situation. Ultimately, any decision to reduce or shift service resides within the sole autonomy of the clinician, their practice, their health care system, and their community.
Telehealth—Expanding services during the pandemic
Telehealth and virtual patient encounters can be central in delivering allergy services within a risk-stratified context of the SARS-CoV-2 pandemic. The ability to integrate telecommunications, information systems, and patient care has been in place for more than 4 decades and has been gaining traction across medical specialties, even before the emergence of COVID-19.10 , 11
Acute services reduction: Guidance for service reduction/prioritization by specific conditions
Recommendations for condition-specific guidance for service reduction and patient prioritization are presented for the following conditions:
-
•
Asthma
-
•
Allergic rhinitis
-
•
Immunotherapy and biologics
-
•
Food allergy, food protein induced enterocolitis syndrome, eosinophilic esophagitis, drug allergy, and anaphylaxis
-
•
Allergic skin disorders
-
•
Immunodeficiency
Also described below is an approach to shared decision making in these circumstances and tips for communication with patients.
Conclusions
A pandemic response during a global emergency is a highly unusual and atypical circumstance from business as usual. The framework described herein represents a course of action in a highly specific and temporary situation, necessary only in a most extreme and improbable circumstance, where there is a state of emergency and a pandemic risk that outweighs the risk of deferral of an office visit for the allergic condition. Please keep in mind that these are suggestions that must be conditioned on individual “on the ground” circumstances. They are not mandates or forced actions. The decision to enact any of these measures rests with the clinician and the individual health care system. These suggestions are intended to help provide a logical approach to quickly adjust services to mitigate risk to both medical staff and patients during the ongoing pandemic while social distancing is being encouraged. Importantly, individual community circumstances may be unique and require contextual consideration. We acknowledge that taking actions to limit face-to-face access may have financial implications in terms of lost revenue, fixed operating costs, and unclear reimbursement for telehealth and that advocacy on the part of professional organizations may be both appropriate and necessary to leverage some share of federal resources during this pandemic.8 If nothing else, we can fall back on the old adage “remember your training.” We are some of the most highly trained and adept medical specialists in the world. We can and will persevere through any challenge that the specialty faces.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has exhibited a pattern of pandemic spread in a few short months, as countries and communities struggle to rapidly design effective strategies to prevent spread of the novel coronavirus. The virus has been named SARS-CoV-2 and the disease it causes “coronavirus disease 2019” (COVID-19).1 , 2 China first notified the World Health Organization of several cases of a human respiratory illness that were linked to an open seafood and livestock market in the city of Wuhan in December 2019, which appears to have originated in chrysanthemum bats.1 , 3 Worldwide community transmission is now evident, and it is clear that SARS-CoV-2 is a highly contagious virus.3 Cases have been identified across the globe, and on 1 cruise ship alone more than 700 infections were reported, demonstrating the high level of potential contagion.1 , 4 The spectrum of disease ranges from severe respiratory illness and fatality from these complications (particularly in the elderly and those with comorbidities) to asymptomatic spread,1 , 5 , 6 with the proclivity of SARS-CoV-2 for person-to-person transmission in asymptomatic individuals presenting one of the most vexing problems from a public health standpoint.1 Of note, based on data at the time of drafting this document, serious illness appears to occur in approximately 14% to 16% of cases.2 , 6 However, we cannot stress enough that these are fluid situations that may change hourly. Given the rapid and pervasive spread, the World Health Organization declared SARS-CoV-2 a public health emergency of international concern on January 30, 2020, and a pandemic on March 11, 2020, and on March 13, 2020, the president of the United States declared a national emergency in the United States, consistent with similar actions taken in several other countries.7 , 8 Information is sparse in some instances, and inconsistent in others, but there is anticipated widespread caseload across the North America and an urgent need to match the pace of the outbreak to the capacity of national health care systems to serve the needs of affected individuals in an urgent and timely manner (Figure 1). It is incumbent on each physician to monitor the day-to-day evolution of the pandemic in their region, and to be prepared to implement the recommendations of authorities and experts. The situation is changing quickly and requires a rapid, flexible, and informed response.
Biology, Epidemiology, Clinical Presentation, and Management
Although this is more fully summarized elsewhere, the biology of the virus is of some interest. Please see this article's Online Repository at www.jaci-inpractice.org for additional information on biology, epidemiology, clinical presentation, and management of COVID-19. The practicing allergist should keep in mind that there is overlap with allergic rhinitis, influenza, viral upper respiratory tract infection, and asthma in the early stages with respect to certain upper respiratory tract symptoms that only later progress to more clearly defined COVID-19 symptoms.
The overall case-fatality rate of patients with COVID-19 presenting for medical evaluation has been estimated to be around 2.3%, but it is highly variable and may be as high as 8% to 15% in higher risk populations.1 , 6 , 10 Health care workers are not immune, as noted by the finding that 3.8% of cases occurred in health care workers. Of 1716 COVID-19 infections in health care workers, 14.8% were classified as severe, and 5 deaths were reported (case-fatality rate, 0.3%).6 There is some speculation that insufficient access to testing and intensive care services (secondary to equipment and space shortages) may contribute to some of the fatality rate variation. Again, it should be emphasized that data reporting and event rates are fluid and changing rapidly. Of note, fatality rates may actually be much lower when mild and asymptomatic cases are considered.
Prevention and Control Measures for Health Care Workers
Although vaccine development is underway, it is unlikely that a vaccine will be available in 2020.1 Key strategies for containing the virus and limiting its spread include identifying and quarantining infected individuals and those at high risk for infection. However, this approach is limited by a lack of timely and accurate testing and overlap of mild COVID-19 with seasonal viral infections. The Centers for Disease Control and Prevention (CDC) has recommended the use of personal protective equipment by health care workers including standard, contact, and airborne precautions and the use of eye protection. This means health care workers caring for a patient with suspected COVID-19 should wear a gown, gloves, and either an N95 respirator plus face shield and goggles or a powered air-purifying respirator; however, the CDC also notes that a face mask may be substituted for an N95 respirator if one is not available. At present a negative pressure room may be reserved for patients undergoing aerosol-generating procedures.1 , 2 As is with any of this information, this is fluid and may continue to change. Commonsense strategies are important in controlling the spread of SARS-CoV-2 and are detailed in Table E1 in this article's Online Repository at www.jaci-inpractice.org.2 , 11 During the COVID-19 pandemic the concept of social distancing has also been incorporated into prevention strategies, with the CDC recommending avoiding close contact (<6 ft).2 , 11
During the pandemic, self-quarantine of asymptomatic health care providers resulting from exposure to sick patients or family members may limit their ability to provide care. Telehealth may provide an additional resource in these circumstances, and providers may consider implementing systems to facilitate virtual care from home.
Emergency Social Distancing—Prioritizing Care in the Event of Ambulatory Service Rationing
In the presence of a highly contagious global pandemic, decisions will need to be considered regarding the short-term rationing of services. It is important to note that many allergy/immunology services are elective and can be managed without face-to-face interaction or deferred outright for short periods of time. Not only will these considerations be important for patient health and safety, but it will also be important to consider those health care workers who are within the high-risk group as specified by the CDC. A strong argument can be made that we must diligently protect our workforce by realigning present priorities to limit face-to-face patient interactions where possible, particularly for health care workers.12 Relevant, though admittedly 16-year-old, data from a US allergy/immunology 2004 survey found that the average age of the allergy and immunology physician workforce was 53 years in 2004 versus 51 years in 1999, with physicians working longer before retiring.12
To provide an approach to triaging allergy/immunology services during the COVID-19 pandemic, a consensus-based ad-hoc expert panel of allergy/immunology specialists from the United States and Canada developed a service and patient prioritization schematic to temporarily adjust allergy/immunology services. Recommendations and feedback were developed iteratively, using an adapted modified Delphi methodology to achieve consensus.
A hierarchy for understanding these scenarios is detailed in Figure 2, which depicts a graded approach to how allergy and immunology services may need to be adjusted during an emerging pandemic. As COVID-19 becomes more pervasive, recommended and mandated social distancing become more pronounced. Several countries have initiated widespread quarantine measures to try to contain and mitigate the spread of SARS-CoV-2. Drastic measures were initially taken in Wuhan, limiting travel, and on March 9, 2020, the Italian government released a decree prohibiting movement in public places except for “justifiable reasons” such as commuting to work, obtaining basic necessities (ie, food shopping), and for health emergencies. The decree cancelled sporting events and public gatherings and closed schools, universities, and recreational facilities through April 3.13 On March 13, France announced plans to close nonessential businesses and Spain announced a nationwide lockdown.14 Currently throughout North America, there have already been widespread cancellations and postponements of large gatherings, including most major sporting events and leagues.
A helpful view of a stratified approach is presented in Figure 2. In this context, “green zone” represents normal operations, “yellow zone” defines operations during emergence of a contagious pandemic illness with signs of possible community spread, “orange zone” relates to a pandemic with a state, local, and/or national emergency declared, and “red zone” would be implemented in the setting of a declared emergency with full or partial quarantine measures recommended for all citizens (ie, school closings or government-imposed social distancing restrictions).
During a pandemic in which a global health emergency has been declared, “red zone” measures must be considered.2 , 7 , 8 The remainder of this document deals with a rationale to enact such “red zone” measures. It must be explicitly stated that the following framework serves only as a suggestion and should only be considered within the context of a global emergency during a time when nations, societies, and institutions are facing drastic pandemic measures in a “red zone” situation. The recommendations must also be considered with the understanding that normal services will eventually resume, and that such recommendations only represent contingency plans for prioritization of staff, space, and patients, with an expected timeline of 6 months or less. Thus, the remainder of this document aims to make recommendations regarding how clinicians can consider prioritizing who needs to be seen, weighing the risks and benefits of what that may involve in terms of risk of infection, space constraints, and staff availability. Ultimately, any decision to reduce or shift service resides within the sole autonomy of the clinician, their practice, their health care system, and their community.
Much of what follows relates to “red zone” operations. Some of the suggestion below may not be required at the moment, and as such the clinician must view these as conditional recommendations to be incorporated within context-specific, evolving situations.
Telehealth and Other Methods of Virtual Encounters—Expanding Services During the Pandemic
Telehealth can be central in delivering allergy/immunology services within a risk-stratified context of the SARS-CoV-2 pandemic. Telehealth has the potential to help with social distancing. Several advantages that telehealth offers are as follows: (1) it can limit exposure of providers to potentially infected patients, particularly if they are older or have health problems, (2) it can reduce exposure of patients, many of whom have conditions such as asthma or immunodeficiency disorders, to other infected patients, and (3) it can provide access to rapid evaluation for potential COVID-19 infection, reducing the likelihood that they will go to an urgent care clinic or emergency department where they have increased risk of virus exposure. To provide telehealth services to patients it is important to remember that the provider must be licensed to practice medicine in the state where the patient is located (although some regulations are in flux during the national emergency). Please see this article's Online Repository at www.jaci-inpractice.org for additional information on telehealth.15, 16, 17, 18, 19, 20, 21, 22, 23 The American Academy of Allergy, Asthma & Immunology and the American College of Allergy, Asthma & Immunology telemedicine/telehealth toolkits can be valuable resources.17 , 24
Although telehealth may be a valuable and critical resource, challenges will include triaging patient diagnoses and severity to provide immediate access to services to patients with more acute need.25, 26, 27, 28, 29 For example, a patient requiring assessment of possible idiopathic anaphylaxis would likely require more immediate access to this service than a patient needing follow-up for well-controlled asthma or allergic rhinitis. In other circumstances discussed below, patients with well-controlled allergic disease may be able to appropriately defer both face-to-face and telehealth visits. Clinicians will also need to be aware of potential pitfalls of virtual care. For example, the case of unstable asthma in a patient with poor perception of dyspnea or during a significant exacerbation. However, telehealth can also be an excellent tool for many allergic conditions for those with less severe and stable conditions, such as in those with seasonal allergies who need a brief reassessment and refill of their prescriptions before their allergy season. In the current context, especially if formal telehealth services are not available, virtual care may also need to be dispensed using telephone, electronic medical record patient portal messaging, and e-visits, including in the event of absolute emergencies. For many situations, incorporating phone triage as a first step may be helpful, particularly in areas where the clinical situation may lack clarity as to the acuity or pressing need for the patient to be seen, and then working toward maximizing telehealth or other means of virtual care where social distancing can be preserved and health care needs can still be met. This document seeks to provide a rationale to be considered for such instances.
As a general approach, no recommendations will be an ideal fit for every unique clinician, situation, or practice setting. Each clinician must use their judgment in making decisions about which services may be deferred, which may be offered using virtual care, and which will require in-person care. The goal of this document is to provide a resource for consideration in rather unusual circumstances, rather than to give any directives. Specific conditions are discussed below. Tables are provided for suggested service adjustments for patients with specific conditions.
Specific conditions
Asthma
For asthma-specific recommendations on service reduction, please seeTable I, and for an approach to triaging an asthma exacerbation in this setting, seeFigure 3. Asthma is a major health problem around the globe.30 Because SARS-CoV-2 is a respiratory pathogen, it is important to know what risk patients with asthma have in this time of a COVID-19 pandemic. There are relatively few data at this time to demonstrate a specific increased risk for COVID-19 from asthma, or an increased disease pathology in patients with asthma infected with SARS-CoV-2. However, this association could evolve. Early published data from China noted that asthma was not a strong risk factor for severe COVID-19 disease. One study of 140 patients with COVID-19 found none with asthma,31 and in a larger study of 1099 hospitalized patients, asthma was not described.32 In this larger study, chronic obstructive lung disease (COPD) was noted in patients hospitalized with COVID-19, but the rate of patients with COPD who had COVID-19 (1.1%) was lower than the rate of COPD in the general Chinese population (which is at least 10%).33 Data from Korea also indicate that asthma is not a relevant comorbidity.10 Together these data suggest that the risk of severe COVID-19 may not be dramatically elevated in those with asthma or COPD. However, these data are based on hospitalized patients and may have significant limitations due to selection and reporting bias. It is also important to note that asthma appears underdiagnosed and underreported in China, with an estimated prevalence of only 4.2%.34 The actual risk of disease in those with asthma or COPD within the broader Chinese population or those of non-Chinese background is not known, and may evolve with additional data reporting.
Table I.
Service adjustment for asthma
The following hierarchy of service adjustments could be considered:
|
ED, Emergency department; WHO, World Health Organization.
Figure 3.
Triage approach to the patient with an asthma exacerbation during a pandemic. PPE, Personal protective equipment.
Beyond the direct risk of the infection itself, there is also a risk of experiencing an asthma exacerbation triggered by coronavirus infection. Previous pandemic coronaviruses (SARS-CoV and Middle East respiratory syndrome coronavirus) have not been associated with asthma exacerbations, but there are nonpandemic coronaviruses that circulate annually and have been reported to cause asthma exacerbations.35 , 36 Nonetheless, it is imperative that patients with asthma implement appropriate steps to ensure their asthma is under control to limit the chance for a more serious exacerbation.
Knowledge about the potential use of corticosteroids in treating COVID-19 is evolving. Currently, the World Health Organization and the CDC recommend that in the general population with COVID-19, systemic corticosteroids should be avoided because of a potential for prolonged viral replication that was observed in patients with Middle East respiratory syndrome coronavirus. However, there is also acknowledgment that there may be a role for systemic corticosteroids when indicated for other reasons, such as septic shock.2 , 37, 38, 39 For example, in a study of 309 intensive care unit patients with Middle East respiratory syndrome coronavirus, 151 received corticosteroids acutely, and those who received corticosteroids were more likely to receive mechanical ventilation (93.4% vs 76.6%; P < .0001), had higher 90-day crude mortality (74.2% vs 57.6%; P = .002), and had delayed viral clearance. Of note, mortality rates did not differ by corticosteroid use when adjusted by time-varying confounders.38 Approximately 20% to 30% of hospitalized patients with COVID-19 have pneumonia and may require intensive care for respiratory support2 , 39; thus, it is important to appreciate that corticosteroids may have distinct roles in treating lung injury versus airway inflammation. Recently, Russel et al40 summarized current evidence in relation to the use of corticosteroids for mitigating lung injury from coronaviruses and concluded that there is likely to be a lack of efficacy in COVID-19 lung injury.
However, it is important to differentiate between the use of corticosteroids for the treatment of COVID-19 and their use as a controller medication for the management of a chronic disease, such as asthma. As mentioned, it is most important to maintain asthma control, and the lack of patients with comorbid asthma being noted in COVID-19 studies or data reporting suggests that patients with asthma may not be at a greatly increased risk of more serious disease—even with the use of inhaled corticosteroids as part of a controller regimen. In fact, it may be more likely that a patient with asthma would have an exacerbation from other causes, including seasonal pollen exposure or a virus other than SARS-CoV-2 if they stopped regular use of indicated controller therapy based on best evidence. An exacerbation could require them to enter the health care system, which would put them at increased risk of being exposed to SARS-CoV-2 during the current pandemic. Until studies in patients with asthma with SARS-CoV-2 have been performed and show evidence to the contrary, a prudent recommendation would be to continue to manage asthma according to current asthma guideline–based recommendations.30
Of note, nebulizer use is discouraged unless essential during this pandemic, because the use of nebulized therapy is more likely to aerosolize SARS-CoV-2 and increase the risk of contagion. As such, asthma therapy delivered by metered dose inhaler would be most appropriate both in the health care setting and at home.41, 42, 43
Methodologically sound and high-quality evidence supports the administration of a number of biologic agents—targeting IL-5, IL-4/IL-13, and IgE—for appropriately selected patients with refractory moderate to severe persistent asthma.43 There is no evidence that suggests that immune response to COVID-19 will be impaired in patients with asthma treated with anti–IL-5, anti–IL-5Ra, anti–IL-4/IL-13, or anti-IgE medications. In the absence of any data indicating a potential for harm, it would be reasonable to continue administration of biologic agents during the COVID-19 pandemic in patients for whom such agents are clearly indicated and have been effective.44 , 45
In summary, understanding of the intersection between asthma and COVID-19 is evolving. There are currently scant data to indicate the degree of risk (or protection) from disease, and no data to support strong recommendations for or against specific asthma treatments. Until more information suggests otherwise, it is strongly recommended that physicians continue to manage asthma according to existing accepted asthma guidelines.30 Ensuring that those with asthma have their condition under optimal control is the best deterrent against a poor outcome from any viral respiratory tract infection, and there is a high likelihood that this recommendation also extends to SARS-CoV-2.
Allergic rhinitis
Under red zone circumstances, there are no recommendations for prioritizing the evaluation of new patients or return visits of established patients with allergic rhinitis. Face-to-face visits for evaluation and management of patients with allergic rhinitis can generally be postponed or shifted to telehealth visits for initiation or monitoring of care as an alternative. Therefore, with rare exception (or “unless there are extenuating circumstances”), service reduction for allergic rhinitis would be strongly recommended as pandemic management and isolation measures continue to escalate. Although telehealth and phone triage do remain as available options, telehealth utilization comes with the caveat that other diagnoses may need these limited resources with higher priority. Skin testing to inhalants may not be appropriate; it may be prudent to postpone such testing or to perform in vitro serum specific IgE testing as an alternative, with the understanding that this would also entail entering a health care environment for performance of phlebotomy. Such patients may be better managed via avoidance measures and administration of medication(s) as indicated on the basis of best evidence.46
Immunotherapy and biologics
Allergen immunotherapy and biologic therapy are valued treatment options for the care of many allergic/immunologic disorders.47 However, in some cases they represent alternatives to other front-line medical management, and in some settings are a preference-sensitive care option as a first-line therapy. For specific recommendations on service reduction for immunotherapy and biologics, please see Table II .
Table II.
Service adjustment for immunotherapy and biologics
The following hierarchy of service adjustments could be considered:
|
VIT, Venom immunotherapy.
Food allergy, food protein induced enterocolitis syndrome, eosinophilic esophagitis, drug allergy, and anaphylaxis
For specific recommendations on service reduction for food allergy, food protein induced enterocolitis syndrome, eosinophilic esophagitis, drug allergy, and anaphylaxis, please seeTable III. Many patients with food allergy, eosinophilic esophagitis, and anaphylaxis are generally healthy, with the exception of other allergic comorbidities such as asthma, allergic rhinitis, or eczema. With limited exception, most of the care of these conditions would reasonably qualify under temporarily nonessential ambulatory elective services, which could be delayed or deferred in the short- to intermediate-term (a few weeks to even a few months) with no anticipated significant serious untoward effects. Most of the care for patients with these conditions could forego any face-to-face visits in the short-term, and if necessary be addressed through virtual care until the pandemic subsides. Many such patients could likely forego any care in this time interval. When considering what is critically necessary, routine food allergy follow-up visits and many new referrals should be considered to fall under a more elective category, where such visits could be handled via telehealth, potentially. Food challenges, with limited exceptions, would also follow suit. In the setting of a pandemic with quarantine measures, unless there is a critical acute nutritional need for introduction of a key nutrient, it is likely that all food challenges would be deferred. Research visits for ongoing study protocols and food allergy immunotherapy visits for initiation and escalation could also be delayed, with the possible exception of food challenge visits at the end of a study interval where delay would risk influencing the primary/secondary outcomes. However, sponsors are likely issuing their own directives for handling this, which should be followed unless the local facility issues guidance that supersedes that of the sponsor with regard to access to space or staff. Where possible, it is recommended that there be planning to provide telehealth visits without testing to provide essential diagnostic management and make medication adjustments, or a plan to address this through phone triage.
Table III.
Service adjustment for food allergy, food protein induced enterocolitis syndrome, eosinophilic esophagitis, drug allergy, and anaphylaxis
The following hierarchy of service adjustments could be considered:
|
EoE, Eosinophilic esophagitis; FPIES, food protein–induced enterocolitis syndrome; GI, gastrointestinal; G-tube, gastrostomy-tube.
Allergic skin disorders
For specific recommendations on service reduction for allergic skin disorder, please see Table IV . In patients with urticaria, angioedema, and atopic dermatitis, most visits can be considered under the nonurgent category where face-to-face care can be postponed or conducted via phone triage or telehealth.52 , 55 Nearly all follow-up visits could fall under this guidance. Use of telehealth, e-visits, or digital photography can be of use to help visualize any rash, which can reduce the need for face-to-face visits. For patients with known hereditary angioedema who develop an acute episode, triage to region-specific urgent or emergency care facilities is appropriate. If it is possible to obtain on-demand therapy for home administration, this would also be recommended.
Table IV.
Service adjustment for allergic skin disorders
The following hierarchy of service adjustments could be considered:
|
Immunodeficiency
For specific recommendations on service reduction for immunodeficiency, please seeTable V. Immunodeficiency is one of the few potential areas of service where exceptions may have to be made to continue to provide routine face-to-face services. These patients may be at a higher baseline increased risk from COVID-19 complications, community-acquired infections, and nosocomial infections; however, the degree of this risk is still a matter of speculation.61,62 As is the rationale with other conditions, telehealth should be encouraged and certain care can be postponed, but face-to-face care may be necessary for more severe illness. Many of the deprioritizations of other routine care is to preserve unfettered access to care for patients with higher acuity conditions. The International Patient Organization for Primary Immunodeficiencies has recently published a joint statement together with European Society of Infectious Diseases, International Nursing Group for Immunodeficiencies, Asian Pacific Society for Immunodeficiencies, Arab Society for Primary Immunodeficiency, African Society for Immunodeficiency, Clinical Immunology Society, Latin American Society for Immunodeficiencies, and South East Asia Primary Immunodeficiency Network on the COVID-19 epidemic. The statement covers general and primary immunodeficiency–specific precautions.61 It is essential for patients on immunoglobulin therapy to continue their regular treatment. These products are safe and will protect from other infections. According to a statement from the Plasma Protein Therapeutics Association, there is no risk of transmission of this virus in these products.63
Table V.
Service adjustment for immunodeficiency
The following hierarchy of service adjustments could be considered:
|
BMT, Bone marrow transplant; IV, intravenous; PFT, pulmonary function test; PJP, Pneumocystis jiroveci pneumonia; SC, subcutaneous; SCID, severe combined immunodeficiency.
Shared decision making
Shared decision making is a patient-centered process whereby the patient and their clinician have a discussion regarding care or treatment options, in which patient values and preferences are considered in the context of the medical decision-making process to determine the best management option.64 , 65 Please see this article's Online Repository at www.jaci-inpractice.org for additional information on shared decision making during the pandemic.
Communication with patients
The vast majority of patients use the internet and social media to find health-related information.66 , 67 Please see this article's Online Repository at www.jaci-inpractice.org for additional information on communicating with patients during the pandemic.
Practice implications
With the declaration of reduction of nonessential medical services, physicians in private small or solo practices may have significant concerns about practice sustainability in times of uncertainty. Please see this article's Online Repository at www.jaci-inpractice.org for practice implications of COVID-19 reduction in services.
Conclusions
The new decade has begun with unprecedented challenges. Although we each hope the COVID-19 pandemic will be contained and mitigated as soon as possible, we all have personal roles and professional duties to our patients and our larger society. A pandemic response during a global emergency is a highly unusual and atypical circumstance from business as usual. The framework described herein represents a course of action in a highly specific and temporary situation, necessary only in a most extreme and improbable circumstance, where there is a state of emergency and a pandemic risk that outweighs the risk of deferral of an office visit for conditions within the spectrum of allergic/immunologic disorders.
Please keep in mind that these are suggestions that must be conditioned on individual “on the ground” circumstances. They are not mandates or forced actions. The decision to enact any of these measures rests with the judgment of each clinician and individual health system. These suggestions are intended to help provide a logical approach to quickly adjust service to mitigate risk to both medical staff and patients during the ongoing pandemic while social distancing is being encouraged. Importantly, individual community circumstances may be unique and require contextual consideration. The expert panel acknowledges that taking actions to limit face-to-face access may have financial implications in terms of lost revenue, fixed operating costs, and unclear reimbursement for telehealth and that advocacy on the part of professional organizations may be both appropriate and necessary to leverage some share of federal resources during this pandemic.8 However, the broader financial implications and economic impacts of the COVID-19 pandemic are beyond the scope of this document.
Although SARS-CoV-2 presents the allergy/immunology community with a challenge on an unprecedented scale, it is not the first coronavirus we have encountered in the last few decades.68 , 69 It is likely that this will not be the last pandemic we encounter, and strategies that may be proven effective for COVID-19 may inform our future approach in unexpected disasters that we hope will never come to pass. Still, as we meet this challenge with compassion, humility, and common sense, it will again be evident that an ounce of prevention is worth a pound of cure—in our clinic, community, nation, and world. If nothing else, we can fall back on the old adage “remember your training.” We are some of the most highly trained and adept medical specialists in the world. We can and will persevere through any challenge that the specialty faces.
Footnotes
M.G. is supported by the Agency for Healthcare Research and Quality (grant no. 5K08HS024599-02).
Conflicts of interest: M. S. Shaker is a member of the Joint Taskforce on Allergy Practice Parameters; has a family member who is CEO of Altrix Medical; and serves on the Editorial Board of the Journal of Food Allergy and the Annals of Allergy, Asthma, and Immunology. J. Oppenheimer has received research support and provided adjudication for AstraZeneca, GlacoSmithKline, Sanofi, and Novartis; is a consultant for GlaxoSmithKline, AstraZeneca, and Sanofi; is an associate editor for Annals of Allergy Asthma Immunology, AllergyWatch; is a section editor for Current Opinion of Allergy; receives royalties from UptoDate; is Board Liaison American Board of Internal Medicine for American Board of Allergy and Immunology; and is a member of the Joint Taskforce on Allergy Practice Parameters. M. Grayson is a medical advisory board participant for Aimmune, DBV, and Genzyme; director and treasurer of the ABAI; associate editor of the Annals of Allergy, Asthma, and Immunology; Chair of the Medical Scientific Council of the Asthma and Allergy Foundation of America; and member of the Scientific Advisory Committee of the American Lung Association. D. Stukus is a consultant for DBV Therapeutics, Before Brands, and Abbott Nutrition. N. Hartog is a speaker and on the advisory board for Horizon Pharmaceuticals; is a speaker for Takeda; and is on the Orchard Therapeutics advisory board. E. W. Y. Hsieh is supported by the National Institutes of Health National Institutes of Arthritis and Musculoskeletal and Skin Diseases (grant no. K23AR070897), the Boettcher Foundation Webb-Waring Biomedical research grant, the Childhood Arthritis and Rheumatology Research Alliance large grant, the Jeffrey Modell Foundation Translational Award, and Takeda Pharmaceuticals. N. Rider is a consultant and is on scientific advisory boards for Horizon Therapeutics, CSL Behring, and Takeda Pharmaceuticals; receives royalties from Kluwer Wolters; is an UpToDate topic contributor; and receives grant funding from the Jeffrey Model Foundation. T. K. Vander Leek has served on advisory boards for Aralez and Pediapharm; has served on speaker bureaus for and received honoraria from Aralez, Pediapharm, and Pfizer; and currently serves as vice president for the Canadian Society of Allergy and Clinical Immunology. H. Kim has served on speakers' bureau and advisory boards for AstraZeneca, Aralez, Boehringer Ingelheim, CSL Behring, Kaleo, Merck, Mylan, Novartis, Pediapharm, Sanofi, Shire, and Teva; and has received research funding from AstraZeneca, Shire, Sanofi, and Novartis. E. S. Chan has received research support from DBV Technologies; has been a member of advisory boards for Pfizer, Pediapharm, Leo Pharma, and Kaleo; is a member of the scientific advisory board for Food Allergy Canada; and was an expert panel and coordinating committee member of the National Institute of Allergy and Infectious Diseases (NIAID)-sponsored Guidelines for Peanut Allergy Prevention. D. Mack is a member of the Board of Directors for the Canadian Society of Allergy and Clinical Immunology; serves on the Editorial Board of the Journal of Food Allergy; has provided consultation and speaker services for Pfizer, Aimmune, Merck, Covis, and Pediapharm; and has been part of an advisory board for Pfizer and Bausch Health. D. Lang is on the Editorial Board for Allergy and Asthma Proceedings; is topic editor for DynaMed; is associate editor for Journal of Asthma; and is delegate to National Quality Forum representing the American Academy of Allergy, Asthma & Immunology (AAAAI). J. Lieberman has received research support (money to institution) from DBV, Aimmune, and Regeneron; is on the advisory boards for DBV, Genentech, and Covis; and is a consultant for Kaleo. D. Fleischer received institutional research funding from DBV Technologies and Aimmune Therapeutics; has served as a consultant and received personal fees from DBV Technologies, Aimmune Therapeutics, Kaleo Pharmaceutical, INSYS Therapeutics, Abbott, Allergenis, Acquestive, and Nestle; is a nonpaid member of the Scientific Advisory Council for the National Peanut Board and a nonpaid member of clinical advisory boards for Food Allergy Research and Education and Food Allergy and Anaphylaxis Connectivity Team. D. B. K. Golden has received financial support from Aquestive, Sandoz, ALK-Abelló, Genentech, Stallergenes Greer, and UpToDate. D. Wallace has received financial support from Mylan, Kaleo, Optinose, ALK, Bryan, and Sanofi. J. Portnoy has received financial support from Thermofisher, Kaleo, Teva, Novartis, Hycor, and Boehringer Ingelheim. G. Mosnaim has received research grant support from AstraZeneca and GlaxoSmithKline; currently receives research grant support from Propeller Health; owned stock in Electrocore; and has served as a consultant and/or member of a scientific advisory board for GlaxoSmithKline, Sanofi-Regeneron, Teva, Novartis, Astra Zeneca, Boehringer Ingelheim, and Propeller Health. M. Greenhawt is supported by the Agency for Healthcare Research and Quality (grant no. 5K08HS024599-02); is an expert panel and coordinating committee member of the NIAID-sponsored Guidelines for Peanut Allergy Prevention; has served as a consultant for the Canadian Transportation Agency, Thermo Fisher, Intrommune, and Aimmune Therapeutics; is a member of physician/medical advisory boards for Aimmune Therapeutics, DBV Technologies, Sanofi/Genzyme, Genentech, Nutricia, Kaleo Pharmaceutical, Nestle, Acquestive, Allergy Therapeutics, Allergenis, Aravax, and Monsanto; is a member of the Scientific Advisory Council for the National Peanut Board; has received honorarium for lectures from Thermo Fisher, Aimmune Therapeutics, DBV Technologies, Before Brands, multiple state allergy societies, the American College of Allergy, Asthma & Immunology, and the European Academy of Allergy and Clinical Immunology; is an associate editor for the Annals of Allergy, Asthma, and Immunology; and is a member of the Joint Taskforce on Allergy Practice Parameters. The rest of the authors declare that they have no relevant conflicts of interest.
Online Repository. Biology, Epidemiology, Clinical Presentation, and Management
The biology of SARS-CoV-2 is of interest as it uses densely glycosylated spike (S) protein to enter host cells and bind to the angiotensin-converting enzyme 2 receptor (expressed in type II alveolar cells), similar to the 2003 coronavirus that caused severe acute respiratory syndrome (SARS-CoV). Preliminary data suggest that the coronavirus may have originated in bats and undergone recombination in the pangolin (a scaly anteater), an endangered and commonly trafficked mammal.E1 But in contrast to the 2003 epidemic SARS, which spread to more than 2 dozen countries before it was contained, global spread has been more dramatic with SARS-Cov-2. Between November 2002 and July 2003, a total of 8090 probable SARS cases were reported to the World Health Organization, with only 8 US cases having laboratory evidence of SARS-CoV.E1, E2 Similarly, although the Middle East respiratory syndrome coronavirus first reported in Saudi Arabia in 2012 was associated with a high mortality rate, only 2 cases in the United States ever tested positive.E3 However, SARS-CoV-2 has proven more infectious and elusive than its cousins SARS-CoV-1 and Middle East respiratory syndrome coronavirus, with 2952 COVID-19 cases and 57 deaths occurring in the United States alone, as of March 15, 2020. In Canada, there were 250 confirmed cases and 1 death as of March 15, 2020.E4 As of this date there have been 156,400 confirmed COVID-19 cases with 5833 deaths worldwide, though variations in testing availability may suggest this is a potential underestimation of the true caseload. On a positive note, however, there are currently 73,968 total recovered cases reported worldwide as of March 15, 2020.E5 These numbers are expected to rise.
Although the incubation period of SARS-CoV-2 was initially reported at 1 to 14 days, with a median of 5 to 6 days, it may be as long as 24 days.E1, E6 The virus is spread by large droplets, but also possibly stool and blood.E1, E5 Of note, health care transmission is high, with one study indicating 41% of 138 cases to be presumptively health care acquired.E7 Clinical presentation involves fever (77%-98% of patients), dry cough (46%-82% of patients), shortness of breath (3%-31%), and fatigue or myalgia (11%-52%). Symptoms may also include headache, sore throat, abdominal pain, and diarrhea.E1, E4 Laboratory features include lymphopenia (70%) and eosinopenia (52.9%), and imaging often reveals bilateral patchy infiltrates on chest x-ray and ground-glass opacities on chest computed tomography.E1, E8 Certain upper respiratory tract symptoms overlap with allergic rhinitis and influenza in the early stages that only later progress to more clearly defined COVID-19 symptoms, a point that the practicing allergist/immunologist should keep in mind.
The overall case-fatality rate (CFR) has been estimated at around 2.3% of patients with COVID-19 presenting for medical evaluation, but it is highly variable and may be as high as 8% to 15% in higher risk populations.E1, E9 For example, in a recent report of 72,314 COVID-19 cases in China, no deaths occurred in children younger than age 9 years, but the CFR for patients 70 to 79 years was 8.0%. The age-adjusted CFR was highest in patients 80 years and older (14.8%). In patients with critical illnesses, the CFR was 49.0%. Preexisting conditions also increase risk, with a CFR of 10.5% for cardiovascular disease, 7.3% for diabetes, 6.3% for chronic respiratory disease, 6.0% for hypertension, and 5.6% for cancer.E9 Data from Korea are similar.E10 Health care workers are not immune, as 3.8% of cases occurred in health care workers. However, of 1716 COVID-19 infections in health care workers, though 14.8% were classified as severe, only 5 deaths were reported (CFR, 0.3%). There is some speculation that insufficient access to testing and intensive care services (secondary to equipment and space shortages) may contribute to some of the fatality rate variation. Again, it should be emphasized that data reporting and event rates are very fluid and rapidly changing. Of note, fatality rates may actually be much lower when mild and asymptomatic cases are considered.
There have been limitations to timely and accurate testing for COVID-19 at the onset of this pandemic. It is important to note that as access to accurate and timely testing becomes more widely available, a larger number of patients will be identified, including those with mild and asymptomatic disease, thereby potentially causing the calculated CFR to fall. Before and initially after the declaration of a pandemic, barriers included recommendations by the World Health Organization and national and regional public health agencies to limit testing based on a combination of travel and exposure history together with symptoms, though these recommendations have markedly evolved to become sufficiently inclusive as the pandemic spread has become more rapid. In addition, current testing requires a laborious and time-consuming process available only in specialized laboratories, using multiple steps and with limitations that inherently slow the process and increase the risk for errors. Access to rapidly deployed field test kits available at the point-of-care, currently in development, will significantly improve public health efforts to contain the virus and limit its spread.
Management of COVID-19 is currently limited primarily to supportive care. Antiviral agents with effectiveness against SARS-CoV-2 are not yet known, though the nucleoside prodrug remdesivir is under investigation, in addition to other agents including lopinavir, ritonavir, and favilavir, and both chloroquine and hydroxychloroquine.E1, E7
Telehealth—Expanding Services During the Pandemic
Telehealth can be central in delivering allergy services within a risk-stratified context of the SARS-CoV-2 pandemic. The ability to integrate telecommunications, information systems, and patient care has been in place for more than 4 decades and has been gaining traction across medical specialties, even before the emergence of COVID-19.E11, E12 Both the American Academy of Allergy, Asthma, & Immunology (AAAAI) and the American College of Allergy, Asthma, & Immunology (ACAAI) have been strong advocates to advance telehealth to allow allergy/immunology services to expand and most directly serve patients where they are needed.E11, E13 An excellent example of telehealth in practice is its use in penicillin allergy delabeling.E14, E15 Telehealth services have also been shown to be a viable alternative option to face-to-face visits for the management of patients with various conditions in the spectrum of allergic/immunologic disorders, including asthma.E16
Providers who wish to limit their exposure to infected patients may choose to see patients from their home. To do this, patients would go to the allergist's office where they could be seen by the allergist using facilitated telehealth. This type of facilitated visit should be done using 2-way video using a Health Insurance Portability and Accountability Act–compliant platform.E17 To perform a physical examination, which is required only for an initial visit, digital examination equipment including a stethoscope and high-resolution camera with an otoscope adaptor would be required.E18 Established patients do not require a physical examination unless medically necessary, so if the practice were limited to such patients, it is not necessary to acquire digital examination equipment. With appropriate training, a nurse in the allergist's office could serve as the tele-facilitator. If other providers are present in the office during the visit, procedures such as skin testing could be performed. This type of visit limits the provider's exposure to infectious diseases; however, it does not reduce the patient's exposure.
To reduce patient exposure, established patients could be seen from their home.E19 This can be done if the visit is performed either with a 2-way video connection or by telephone. Since 2018, Medicare has paid for virtual visits with patients who have an established relationship with a physician provided that the communication is (1) not related to a medical visit within the previous 7 days and (2) does not lead to a medical visit within the next 24 hours.E20 One requirement is that the patient must verbally consent to virtual check-ins in advance, and the consent must be documented in the medical record prior to the patient using the service. Billing for these virtual check-ins is specific to the technology used such as telephone (Healthcare Common Procedure Coding System code G2012 or Current Procedural Terminology codes 99441-99433) or by video (Healthcare Common Procedure Coding System code G2010). Another option is to charge patients a flat fee for service (typically $49.95 for general services and $79.95 for specialty services) to use direct-to-consumer telehealth from their home. This avoids the need to meet requirements set out in the Current Procedural Terminology codes but is not reimbursable by health plans.
Medicare also pays for patients to communicate with their doctors without going to the doctor's office using online patient portals. These types of individual communications, like the virtual check-ins, must be initiated by the patient; however, practitioners may educate beneficiaries on the availability of this kind of service before patient initiation. The communications can occur over a 7-day period. The services may be billed using Current Procedural Terminology codes 99421-99423 and Healthcare Common Procedure Coding System codes G2061-G206, as applicable.
One advantage of setting up a telehealth service during COVID-19 is that it can establish the infrastructure for an ongoing telehealth service after the current situation is over. Telehealth has been shown to be effective for managing patients with chronic conditions,E21 and it is as effective for managing asthma as in-person visits.E16 Although there is nothing good about a pandemic with COVID-19, it can be seen as an opportunity to introduce telehealth into an allergy practice.
In the setting of a US national emergency, US Congressional and Executive Branch actions are expected to expand telehealth services provided by health care providers during the emergency.E22 Specifically it is expected that the US Department of Health and Human Services will waive or modify telehealth Medicare requirements, which in practice could greatly expand telehealth services by allowing practice across state lines via relaxing the originating site requirement. Additional expansions are planned to allow provision of a follow-up visit by phone with audiovisual interaction, such as through an iPhone or android platform. This expanded telehealth coding may be limited to established patients and still require necessary documentation to support the evaluation and management code along with a telehealth place of service code, and a potential modifier if required by a commercial payer.E22, E23 Specific details of expected changes are evolving.
Shared Decision Making
Shared decision making (SDM) is a preferred alternative to physician-informed, physician-directed paternalistic decision making. This is a valued approach where there are preference-sensitive care options, defined as conditions with multiple treatment options having significant trade-offs and varying potential outcomes, with decisions reflective of personal values and preferences.E24
The emergence of the COVID-19 pandemic creates unique challenges to SDM, because societal interests may play a larger role in the doctor-patient interaction than in a nonpandemic setting. Infection control becomes critical to patients and clinicians alike, but face-to-face visits will have larger implications beyond the clinic that may not be appreciated in the moment. However, SDM can direct decision making and choices to seek face-to-face versus telehealth encounters, particularly before escalation to a “red zone” threat level (Figure 2). However, even in a “red zone” threat level, SDM will likely continue to play a role, although this will be significantly limited—for example, in decisions whether or not to postpone a course of aeroallergen immunotherapy or simply mark the course completed after 3 years of therapy. However, it must be acknowledged that in the setting of a pandemic national emergency, when faced with restrictions on ambulatory services, the clinician and patient will each have more limited access to resources that would be more available in nonemergent settings, and some decisions will be made on their behalf. What may be more challenging than limiting health care access in nonurgent situations is directing a patient with conditional health risk that exceeds the risk of contracting COVID-19 to break social distancing and seek face-to-face care. Here, the clinician must take the time to clearly explain the facts and the options, along with their potential outcomes.
Communication with Patients
The current COVID-19 pandemic has served to illuminate the best and worst impacts of living in our digital age. Information regarding this pandemic is being updated continuously across all platforms, including misinformation, incomplete information taken out of context, pseudoscientific promises of miracle “cures,” and proliferation of anecdotal reports. During such times, patients need sources of information that they can trust. Allergists/immunologists should respond to this need by extending the long-standing trust developed through years of face-to-face encounters to online resources.
There are 3 main areas where allergists/immunologists should provide information and communication with their patients online: general updates, office-specific changes to normal practice, and social media. Allergists/immunologists should discuss the need to rapidly update their existing Web site and social media channels with the personnel involved in day-to-day operations of these resources. Discussion topics should include current capabilities for updating information, decisions regarding the creation of new content and curation of existing content, and strategy regarding topics to address. Mailing letters to patients may be the preferred method of communication for some, but this does not allow for dissemination of rapidly changing updates and critical information.
Allergists/immunologists should post information on their Web site and social media channels regarding frequently asked questions surrounding COVID-19 (see Table E2 for an example template to consider). It is imperative that this messaging echoes the recommendations of vetted public health authorities such as the CDC or the World Health Organization. Patients will need to understand infection transmission (including incubation period for exposure and acute illness), symptoms, risk for specific populations, and why public health measures such as social distancing are important. Practices can either link to readily available resources on the CDC Web site or create their own content through infographics, blog posts, or new content on their Web site.
Allergists/immunologists have a responsibility to offer evidence-based information and, where evidence is lacking, use vetted resources to support opinions or discuss areas lacking in current understanding. However, it is of the utmost importance for each clinician to remember our clinical “lane” with respect to what we do and do not care for, so that we limit potential misinformation or information that may conflict with that of another clinician who is more responsible for particular care for that individual.
In addition to posting general information regarding COVID-19, allergists/immunologists should use online resources to provide information surrounding any changes to their practice setting. If done properly, this can serve as a portal for sharing timely information to large numbers of patients and reduce burden on practice resources, such as telephone calls. Information should be regularly updated and include current restrictions in regard to screening questions and emphasize that all patients should call before arrival if they have had travel to any countries currently listed as high risk or contact with someone who has known or suspected COVID-19 infection in the last 14 days, as well as fever (temperature, >100.4° F) and/or acute cough. As discussed, patients with allergic rhinitis and/or asthma who have acute symptoms may overlap significantly with those who have COVID-19. As such, allergists/immunologists should consider posting information on their Web site or social media channels regarding important differences between these conditions, as well as indications for COVID-19 testing. In addition, as new protocols are implemented regarding telehealth visits, changes to immunotherapy appointments or schedules, or contact precautions, this information should be updated as rapidly as possible online.
Finally, allergists/immunologists need to understand the influence that social media has on medical decision making.E25 Even if medical professionals are not actively using social media, they need to identify key areas of misinformation to develop anticipatory guidance during individual encounters and when posting online. Current examples include misinformation surrounding the risk of inhaled corticosteroids in patients with asthma, risk of infection/severe exacerbation among individuals with asthma, and promotion of non–evidence-based remedies or preventative treatments such as homeopathy, supplements, vitamins, and alternative/complementary medicine. Allergists/immunologists who already use social media as medical professionals should adopt a similar approach as outlined above regarding dissemination of best practice guidelines and public health measures. Specific issues pertaining to patient privacy, social media policies, and personal accounts need to be reviewed with ALL staff working in medical offices. It is imperative that no member of any medical practice post information to their personal social media accounts regarding the use of isolation/personal protective equipment in the office, patients who were tested or positive for COVID-19, or any members of the staff tested or positive for COVID-19. Such posts have high potential to induce panic among patients and their family members who may have visited the office recently or been in contact with those individuals.
Practice Implications
Practice implications of COVID-19 reduction in services include (1) imposed or voluntary 14-day physician and/or staff quarantine, (2) practice restrictions after actual physician COVID-19 infection, (3) financial reduction due to decline in consultation and follow-up assessments, immunotherapy visits, and reduction in diagnostic testing, and (4) resulting staff lay-offs or termination. These concerns are very real and valid and, understandably, there are no easy solutions to these problems.
It is critical that staff stay at home when ill. In the event of isolation, whether precautionary or after exposure or after infection, all attempts should be made to ensure that ongoing patient care coverage be arranged with other clinicians. In many situations, telehealth solutions can be provided during times of quarantine.
It is the hope that virtual care services will provide some compensation for medical assessments, although this may vary depending on jurisdiction and may be less than typical clinical services provided by the allergist. If reduction of clinical assessments and diagnostic testing is implemented, then clinics will see a significant reduction in revenue. This reduction in income may have effects on immediate financial needs and long-term financial planning and may significantly impact those who are close to retirement or just starting practice. Difficult discussions with staff who perform these assessments and diagnostic procedures may need to occur, and lay-offs may be necessary due to fiscal limitations. Early and clear communication is essential to ensure that all staff are aware of future practice implications and potential office closures and/or lay-offs. Some office insurance policies provide overhead expense coverage for scenarios that may take effect during medically necessitated quarantine or pandemic outbreaks.
Allergists/immunologists will continue to place social responsibility and professionalism ahead of personal financial expectations when making decisions about clinic closures, diagnostic reductions, and personal quarantine. At the end of the day, physicians and other health care providers must follow federal, state/provincial, and municipal regulations and imposed directions to avoid penalty/recourse.
We recognize the significant implications this viral pandemic has on both physicians and clinic staff and hope that many of these practice modifications are short-term .
Table E1.
Personal protective measures against pandemic infectionE2
|
Table E2.
COVID-19 frequently asked questions
| What is COVID-19? |
| COVID-19 is a new form of coronavirus first identified in December 2019. Coronaviruses in general are not new and are a common cause of colds and upper respiratory tract infections. We don't yet know why this new form, COVID-19, is more severe. |
| How is COVID-19 spread? |
| COVID-19 is thought to spread mainly person-to-person through respiratory droplets in coughs or sneezes. It can live on surfaces as well through these droplets. |
| What is the time period when COVID-19 can spread? |
| Unfortunately, people can spread infection to others before symptoms first appear. It can then be spread for up to 14 days after symptom onset (possibly longer). |
| What are the symptoms of COVID-19? |
| Most people experience mild illness, but severe illness and death can occur. Fever, cough, and shortness of breath are the most common symptoms. |
| How is COVID-19 treated? |
| There are no current vaccines or antiviral treatments to use when someone is acutely infected. Treatment relies on supportive care to treat symptoms when they occur. |
| When should I seek emergency care? |
| Seek immediate medical attention if you have difficulty breathing, persistent chest pain or pressure, sudden confusion, or inability to stay awake. These are not the only reasons someone may need emergency care—call your doctor for other concerns. Call any emergency department or medical provider BEFORE arrival to allow them to put precautions in place. |
| Can I get tested for COVID-19 at your office? |
| The indications for testing as well as availability for testing are constantly changing. Please refer to our Web site for current information or call our office with any questions. |
| When should I cancel my regularly scheduled allergy appointment? |
| Some nonurgent visits will likely be cancelled for you. If your visit has not been canceled, please call to discuss any specific concerns before arrival, especially if you have had recent travel to high-risk countries or contact with anyone with known/suspected COVID-19. Also call before arrival if you have had fever/cough in the past 2 wk. |
| Is it safe to come to your office? |
| Offices are taking all recommended precautions to prevent the spread of COVID-19, including reassessing what care must be done in a face-to-face manner, screening all patients and accompanying family members, regularly disinfecting examination rooms and public areas, and staying up to date with current recommendations from the local Department of Public Health. |
| I'm getting allergy shots—What should I do? |
| Please call or refer to the practice Web site for up-to-date information. Practices may need to change the way allergy shots are administered and will notify patients as soon as possible of any changes. Unless you hear differently, please continue your current schedule. However, for some patients, this may be held for the time being, and doses missed. |
| Will your office be closing? |
| Offices may need to adjust the number of appointments or types of visits depending on future spread of COVID-19. Please refer to the practice Web site for the most up-to-date information. |
| What if I have asthma? How will COVID-19 affect me? |
| We do not have a lot of information regarding risk of asthma exacerbation with COVID-19. For now, we recommend continuing all currently prescribed daily asthma medications, contacting your health care provider if you have had frequent symptoms or have needed your rescue inhaler more often, and starting your asthma treatment plan as soon as possible if symptoms occur. |
| Are steroids harmful if someone has COVID-19? |
| It does not appear that inhaled steroids or short courses of oral steroids are harmful for the treatment of asthma. Risks of stopping regular use of inhaled steroids include a loss of asthma control and possible need for treatment with oral steroids. Please do not stop any medications without discussing with your doctor. |
| I have an immune deficiency—What precautions should I take? |
| Please contact your doctor directly to discuss any necessary precautions. There are a wide range of immune deficiencies that may have different risk. All general precautions should be followed as outlined above. |
References
- 1.Del Rio C., Malani P.N. COVID-19—new insights on a rapidly changing epidemic. JAMA. 2020;323:1339–1340. doi: 10.1001/jama.2020.3072. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Coronaviurs disease 2019 (COVID-19) situation summary. https://www.cdc.gov/coronavirus/2019-ncov/index.html Available from:
- 3.Novel Coronaviurs Information Center https://www.elsevier.com/connect/coronavirus-information-center Available from:
- 4.Coronavirus COVID-19 global cases by the Center for Systems Science and Engineering at Johns Hopkins University. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Available from:
- 5.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Available from:
- 8.Proclamation on declaring a national emergency concerning the novel coronavirus disease (COVID-19) outbreak. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/ Available from:
- 9.MSNBC https://media14.s-nbcnews.com/j/MSNBC/Components/Video/202003/n_hayes_curve_200309_1920x1080.nbcnews-fp-1200-630.jpg Available from:
- 10.Report on the epidemiological features of coronavirus disease 2019 (COVID-10) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35:e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of Allergy, Asthma & Immunology Important information about COVID-19 for those with asthma from the American College of Allergy, Asthma and Immunology. https://acaai.org/news/important-information-about-covid-19-those-asthma Available from:
- 12.Marshall GD, American Academy of Allergy, Asthma, & Immunology Workforce Committee The status of US allergy/immunology physicians in the 21st century: a report from the American Academy of Allergy, Asthma & Immunology Workforce Committee. J Allergy Clin Immunol. 2007;119:802–807. doi: 10.1016/j.jaci.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Embassy & Consulates in Italy. COVID-19 information. https://it.usembassy.gov/covid-19-information/ Available from:
- 14.Spain, France take drastic measures to fight coronavirus; Georgia delays presidential primary. The Washington Post. March 14. 2020. [Google Scholar]
- 15.Elliott T., Shih J., Dinakar C., Portnoy J., Fineman S. American College of Allergy, Asthma & Immunology position paper on the use of telemedicine for allergists. Ann Allergy Asthma Immunol. 2017;119:512–517. doi: 10.1016/j.anai.2017.09.052. [DOI] [PubMed] [Google Scholar]
- 16.Portnoy J.M., Pandya A., Waller M., Elliott T. Telemedicine and emerging technologies for health care in allergy/immunology. J Allergy Clin Immunol. 2020;145:445–454. doi: 10.1016/j.jaci.2019.12.903. [DOI] [PubMed] [Google Scholar]
- 17.American Academy of Allergy, Asthma & Immunology. Telemedicine. https://www.aaaai.org/practice-resources/running-your-practice/practice-management-resources/telemedicine Available from:
- 18.Staicu M.L., Holly A.M., Conn K.M., Ramsey A. The use of telemedicine for penicillin allergy skin testing. J Allergy Clin Immunol Pract. 2018;6:2033–2040. doi: 10.1016/j.jaip.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 19.Shaker M., McWilliams S., Greenhawt M. Update on penicillin allergy delabeling. Curr Opin Pediatr. 2020;32:321–327. doi: 10.1097/MOP.0000000000000879. [DOI] [PubMed] [Google Scholar]
- 20.American Academy of Family Practice. FPM. Coronavirus (COVID-19): new telehealth rules and procedure codes for testing. https://www.aafp.org/journals/fpm/blogs/gettingpaid/entry/coronavirus_testing_telehealth.html Available from:
- 21.Center for Connected Health Policy. The National Telehealth Policy Resource Center. Billing for telehealth encounters. January 2020. https://www.cchpca.org/sites/default/files/2020-01/Billing%20Guide%20for%20Telehealth%20Encounters_FINAL.pdf Available from:
- 22.CMA Health Summit Accelerating action in health care. https://cmahealthsummit.ca/highlights/ Available from:
- 23.Portnoy J.M., Waller M., De Lurgio S., Dinakar C. Telemedicine is as effective as in-person visits for patients with asthma. Ann Allergy Asthma Immunol. 2016;117:241–245. doi: 10.1016/j.anai.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 24.American College of Allergy, Asthma & Immunology. Telehealth toolkit. https://college.acaai.org/practice-management/telehealth-toolkit Available from:
- 25.Baker J., Stanley A. Telemedicine technology: a review of services, equipment, and other aspects. Curr Allergy Asthma Rep. 2018;18:60. doi: 10.1007/s11882-018-0814-6. [DOI] [PubMed] [Google Scholar]
- 26.Shih J., Portnoy J. Tips for seeing patients via telemedicine. Curr Allergy Asthma Rep. 2018;18:50. doi: 10.1007/s11882-018-0807-5. [DOI] [PubMed] [Google Scholar]
- 27.Elliott T., Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1. doi: 10.1007/s11882-019-0837-7. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Medicare & Medicaid Services. Coverage and payment related to COVID-19 Medicare. 2020. https://www.cms.gov/files/document/03052020-medicare-covid-19-fact-sheet.pdf Available from:
- 29.Flodgren G., Rachas A., Farmer A.J., Inzitari M., Shepperd S. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2015:CD002098. doi: 10.1002/14651858.CD002098.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Global Initiative for Asthma. 2019 GINA Report, Global Strategy for Asthma Management and Prevention 2019. https://ginasthma.org Available from:
- 31.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China [published online ahead of print February 19, 2020] Allergy. [DOI] [PubMed]
- 32.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China [published online ahead of print February 28, 2020] N Engl J Med. [DOI] [PMC free article] [PubMed]
- 33.Fang L., Gao P., Bao H., Tang X., Wang B., Feng Y., et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6:421–430. doi: 10.1016/S2213-2600(18)30103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang K., Yang T., Xu J., Yang L., Zhao J., Zhang X., et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. 2019;394:407–418. doi: 10.1016/S0140-6736(19)31147-X. [DOI] [PubMed] [Google Scholar]
- 35.Zheng X.Y., Xu Y.J., Guan W.J., Lin L.F. Regional, age and respiratory-secretion-specific prevalence of respiratory viruses associated with asthma exacerbation: a literature review. Arch Virol. 2018;163:845–853. doi: 10.1007/s00705-017-3700-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Bever H.P., Chng S.Y., Goh D.Y. Childhood severe acute respiratory syndrome, coronavirus infections and asthma. Pediatr Allergy Immunol. 2004;15:206–209. doi: 10.1111/j.1399-3038.2004.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization Coronavirus disease 2019 (COVID-19) situation report. https://www.who.int/docs/default-source/coronaviruse/20200312-sitrep-52-covid-19.pdf?sfvrsn=e2bfc9c0_2 Available from: [PubMed]
- 38.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 39.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russel C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019 n-CoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Re Transmission of coronavirus by nebulizer—a serious underappreciated risk! https://www.cmaj.ca/content/re-transmission-corona-virus-nebulizer-serious-underappreciated-risk Available from: [DOI] [PMC free article] [PubMed]
- 42.van Dormalen N., Bushmaker T., Morris D., Holbrook M., Gamble A., Williamson B., et al. Aerosol and surface stability of HCoV-19 (SARS-COV-2) compared to SARS-CoV-1. https://www.medrxiv.org/content/10.1101/2020.03.09.20033217v2 Available from: [DOI] [PMC free article] [PubMed]
- 43.Desai M., Oppenheimer J., Lang D.M. Immunomodulators and biologics: beyond stepped-care therapy. Clin Chest Med. 2019;40:179–192. doi: 10.1016/j.ccm.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Denman S., Ford K., Toolan J., Mistry A., Corps C., Wood P., et al. Home self-administration of omalizumab for chronic spontaneous urticaria. Br J Dermatol. 2016;175:1405–1407. doi: 10.1111/bjd.15074. [DOI] [PubMed] [Google Scholar]
- 45.Novartis receives European Commission approval for self-administration of Xolair across all indications. https://www.novartis.com/news/media-releases/novartis-receives-european-commission-approval-self-administration-xolair-across-all-indications Available from:
- 46.Wallace D.V., Dykewicz M.S., Oppenheimer J., Portnoy J.M., Lang D.M. Pharmacologic treatment of seasonal allergic rhinitis: synopsis of guidance from the 2017 Joint Task Force on Practice Parameters. Ann Intern Med. 2017;167:876–881. doi: 10.7326/M17-2203. [DOI] [PubMed] [Google Scholar]
- 47.Cox L., Nelson H., Lockey R., Calabria C., Chacko T., Finegold I., et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127:S1–55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 48.Golden D.B.K., Bernstein D.I., Freeman T.M., Tracy J.M., Lang D.M., Nicklas R.A. AAAAI/ACAAI Joint Venom Extract Shortage Task Force Report. J Allergy Clin Immunol Pract. 2017;5:330–332. doi: 10.1016/j.jaip.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Golden D.B., Demain J., Freeman T., Graft D., Tankersley M., Tracy J., et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28–54. doi: 10.1016/j.anai.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 50.Shaker M., Briggs A., Dbouk A., Dutille E., Oppenheimer J., Greenhawt M. Estimation of health and economic benefits of clinic versus home administration of omalizumab and mepolizumab. J Allergy Clin Immunol Pract. 2020;8:565–572. doi: 10.1016/j.jaip.2019.09.037. [DOI] [PubMed] [Google Scholar]
- 51.Shaker M., Kanaoka T., Feenan L., Greenhawt M. An economic evaluation of immediate vs non-immediate activation of emergency medical services after epinephrine use for peanut-induced anaphylaxis. Ann Allergy Asthma Immunol. 2019;122:79–85. doi: 10.1016/j.anai.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 52.Bernstein J.A., Lang D.M., Khan D.A., Craig T., Dreyfus D., Hsieh F., et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270–1277. doi: 10.1016/j.jaci.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 53.Shaker M., Oppenheimer J., Wallace D., Lang D.M., Rambasek T., Dykewicz M., et al. Optimizing value in the evaluation of chronic spontaneous urticaria: a cost-effectiveness analysis [published online ahead of print November 18, 2019] J Allergy Clin Immunol Pract. [DOI] [PubMed]
- 54.Tarbox J.A., Gutta R.C., Radojicic C., Lang D.M. Utility of routine laboratory testing in management of chronic urticaria/angioedema. Ann Allergy Asthma Immunol. 2011;107:239–243. doi: 10.1016/j.anai.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez J.M., Fernandez A.P., Lang D.M. Biologic therapy in the treatment of chronic skin disorders. Immunol Allergy Clin North Am. 2017;37:315–327. doi: 10.1016/j.iac.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 56.Shaker M., Greenhawt M. Peanut allergy: burden of illness. Allergy Asthma Proc. 2019;40:290–294. doi: 10.2500/aap.2019.40.4240. [DOI] [PubMed] [Google Scholar]
- 57.Greenhawt M., Shaker M. Determining levers of cost-effectiveness for screening infants at high risk for peanut sensitization before early peanut introduction. JAMA Netw Open. 2019;2:e1918041. doi: 10.1001/jamanetworkopen.2019.18041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaker M., Stukus D., Chan E.S., Fleischer D.M., Spergel J.M., Greenhawt M. “To screen or not to screen”: comparing the health and economic benefits of early peanut introduction strategies in five countries. Allergy. 2018;73:1707–1714. doi: 10.1111/all.13446. [DOI] [PubMed] [Google Scholar]
- 59.Netting M.J., Campbell D.E., Koplin J.J., Beck K.M., McWilliam V., Dharmage S.C., et al. An Australian consensus on infant feeding guidelines to prevent food allergy: outcomes from the Australian Infant Feeding Summit. J Allergy Clin Immunol Pract. 2017;5:1617–1624. doi: 10.1016/j.jaip.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 60.Bath-Hextall F., Delamere F.M., Williams H.C. Dietary exclusions for established atopic eczema. Cochrane Database Syst Rev. 2008;1:CD005203. doi: 10.1002/14651858.CD005203.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joint statement on the current epidemics of new cornavirus SARS-CoV-2 - COVID-19 from IPOPI, ESID, INGID, APSID, ARAPID, ASID, CIS, LASID, SEAPID. https://www.ceredih.fr/uploads/COVID19_WORLDWIDE_Joint_Statement_20200311_1200CET_FINAL.pdf?utm_source=College+Insider&utm_campaign=c3588448b0-EMAIL_CAMPAIGN_2020_03_12_06_37&utm_medium=email&utm_term=0_824f79a3c1-c3588448b0-43165045 Available from:
- 62.Immune Deficiency Foundation. Coronavirus https://primaryimmune.org/coronavirus Available from:
- 63.The Plasma Protein Therapeutics Association New coronavirus (SARS-CoV-2) and plasma protein therapies. https://www.pptaglobal.org/media-and-information/ppta-statements/1055-2019-novel-coronavirus-2019-ncov-and-plasma-protein-therapies Available from:
- 64.Charles C., Gafni A., Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Soc Sci Med. 1997;44:681–692. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 65.Elwyn G., Frosch D., Thomson R., Joseph-Williams N., Lloyd A., Kinnersley P., et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361–1367. doi: 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Y.Y., Li C.M., Liang J.C., Tsai C.C. Health information obtained from the Internet and changes in medical decision making: questionnaire development and cross-sectional survey. J Med Internet Res. 2018;20:e47. doi: 10.2196/jmir.9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stukus D.R. How Dr Google is impacting parental medical decision making. Immunol Allergy Clin North Am. 2019;39:583–591. doi: 10.1016/j.iac.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 68.Centers for Disease Control and Prevention Severe acute respiratory distress syndrome. https://www.cdc.gov/sars Available from:
- 69.Centers for Disease Control and Prevention Middle East respiratory syndrome (MERS) https://www.cdc.gov/coronavirus/mers/index.html Available from:
References
- Del Rio C., Malani P.N. COVID-19—new insights on a rapidly changing epidemic. JAMA. 2020;323:1339–1340. doi: 10.1001/jama.2020.3072. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Severe acute respiratory distress syndrome. https://www.cdc.gov/sars Available from:
- Centers for Disease Control and Prevention Middle East respiratory syndrome (MERS) https://www.cdc.gov/coronavirus/mers/index.html Available from:
- Centers for Disease Control and Prevention Coronaviurs disease 2019 (COVID-19) situation summary. https://www.cdc.gov/coronavirus/2019-ncov/index.html Available from:
- World Health Organization Coronavirus disease 2019 (COVID-19) situation report. https://www.who.int/docs/default-source/coronaviruse/20200312-sitrep-52-covid-19.pdf?sfvrsn=e2bfc9c0_2 Available from: [PubMed]
- Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China [published online ahead of print February 19, 2020] Allergy. [DOI] [PubMed]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Report on the epidemiological features of coronavirus disease 2019 (COVID-10) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35:e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T., Shih J., Dinakar C., Portnoy J., Fineman S. American College of Allergy, Asthma & Immunology position paper on the use of telemedicine for allergists. Ann Allergy Asthma Immunol. 2017;119:512–517. doi: 10.1016/j.anai.2017.09.052. [DOI] [PubMed] [Google Scholar]
- Portnoy J.M., Pandya A., Waller M., Elliott T. Telemedicine and emerging technologies for health care in allergy/immunology. J Allergy Clin Immunol. 2020;145:445–454. doi: 10.1016/j.jaci.2019.12.903. [DOI] [PubMed] [Google Scholar]
- American Academy of Allergy, Asthma & Immunology. Telemedicine. 2020. https://www.aaaai.org/practice-resources/running-your-practice/practice-management-resources/telemedicine Available from:
- Staicu M.L., Holly A.M., Conn K.M., Ramsey A. The use of telemedicine for penicillin allergy skin testing. J Allergy Clin Immunol Pract. 2018;6:2033–2040. doi: 10.1016/j.jaip.2018.04.038. [DOI] [PubMed] [Google Scholar]
- Shaker M., McWilliams S., Greenhawt M. Update on penicillin allergy delabeling. Curr Opin Pediatr. 2020;32:321–327. doi: 10.1097/MOP.0000000000000879. [DOI] [PubMed] [Google Scholar]
- Portnoy J.M., Waller M., De Lurgio S., Dinakar C. Telemedicine is as effective as in-person visits for patients with asthma. Ann Allergy Asthma Immunol. 2016;117:241–245. doi: 10.1016/j.anai.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Baker J., Stanley A. Telemedicine technology: a review of services, equipment, and other aspects. Curr Allergy Asthma Rep. 2018;18:60. doi: 10.1007/s11882-018-0814-6. [DOI] [PubMed] [Google Scholar]
- Shih J., Portnoy J. Tips for seeing patients via telemedicine. Curr Allergy Asthma Rep. 2018;18:50. doi: 10.1007/s11882-018-0807-5. [DOI] [PubMed] [Google Scholar]
- Elliott T., Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1. doi: 10.1007/s11882-019-0837-7. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services Coverage and payment related to COVID-19 Medicare. 2020. https://www.cms.gov/files/document/03052020-medicare-covid-19-fact-sheet.pdf Available from:
- Flodgren G., Rachas A., Farmer A.J., Inzitari M., Shepperd S. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2015:CD002098. doi: 10.1002/14651858.CD002098.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Family Practice. Coronavirus (COVID-19): new telehealth rules and procedure codes for testing. 2020. https://www.aafp.org/journals/fpm/blogs/gettingpaid/entry/coronavirus_testing_telehealth.html Available from:
- Center for Connected Health Policy. The National Telehealth Policy Resource Center. Billing for telehealth encounters. 2020. https://www.cchpca.org/sites/default/files/2020-01/Billing%20Guide%20for%20Telehealth%20Encounters_FINAL.pdf Available from:
- Barry M.J. Shared decision making: informing and involving patients to do the right thing in health care. J Ambul Care Manage. 2012;35:90–98. doi: 10.1097/JAC.0b013e318249482f. [DOI] [PubMed] [Google Scholar]
- Stukus D.R. How Dr Google is impacting parental medical decision making. Immunol Allergy Clin North Am. 2019;39:583–591. doi: 10.1016/j.iac.2019.07.011. [DOI] [PubMed] [Google Scholar]