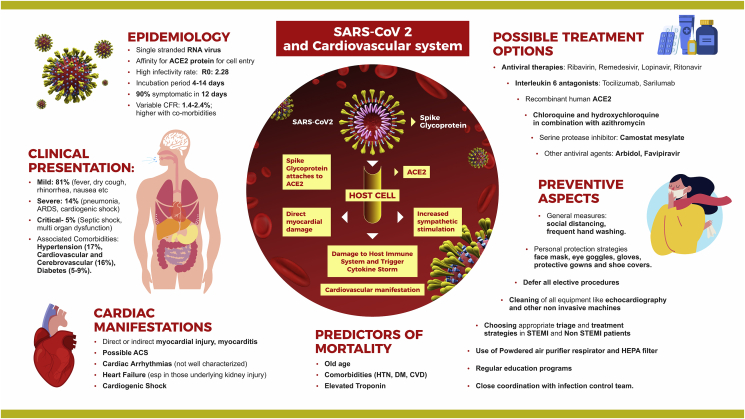
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), is a novel coronavirus first detected in Wuhan, China, in December, 2019. On March 11th, 2020, the World Health Organization declared COVID-19 as a global pandemic.1 As of March 30th, 2020, it has rapidly spread to more than 203 countries and with more than 638,146 cases worldwide and 30,039 deaths.1 In India, so far 1071 cases have been reported with 29 deaths till March 30th, 2020.2 The cases are rising at an exponential rate. Daily increase in the number of cases and associated mortality has forced lockdown in various countries to check the spread of the virus.
While having widespread effects on various systems, COVID-19 interacts with the cardiovascular system at various levels and has caused direct and indirect damage. Similarly, preexisting cardiovascular disease predisposes to serious COVID-19 infection with increased morbidity and mortality.
2. Pathophysiology
SARS-CoV-2 is a novel single-stranded enveloped positive sense RNA virus. More than 90% of its genome resembles that of a bat (now believed to be the zoonotic host for this SARS-CoV-2).3 It resembles another severe acute respiratory syndrome coronavirus (SARS-CoV) from 2012 and the Middle East respiratory syndrome coronavirus.4,5
SARS-CoV-2 belongs to β corona-virus (Co–V) and binds to zinc peptidase angiotensin-converting enzyme 2 (ACE2) protein for cell entry after activation of spike protein.6 It is well known that ACE2 is expressed majorly in the lung (that appears to be the predominant portal of entry), also in the heart, intestinal epithelium, vascular endothelium, and kidneys.7,8 ACE2 has an important role in protecting the lung. But viral binding to its receptor deregulates the protective pathway and enhances pathogenicity. Expression of ACE2 on various organs could explain the multiorgan dysfunction7,8 that has been described in some cases of COVID-19.
The estimated R0 (basic reproduction number) for SARS-CoV is about 3.28 (1.4–6.49), which exceeds the WHO estimates from 1.4 to 2.5.9 This means each infected person can approximately infect 3–4 persons in a susceptible population. While the major route of spread of infection is via respiratory droplets and fomites, the fecal–oral route of transmission is of special concern as the virus has also been detected in stool of patients.10 SARS-CoV-2 remained viable in aerosols for up to 3 h and more stable on plastic and stainless steel than on copper and cardboard, and the viable virus was detected up to 72 h after application to these surfaces.11 The median incubation period was around 5 days (1–14 days) with more than 95% experiencing symptoms within 12 days of exposure12,13,14,15.
3. Clinical presentations
Clinical presentation of COVID-19 may range from mild (81%) to severe (14%) and critical (5%)14, (Fig. 1). Typical signs and symptoms of COVID-19 include the following: fever (87.9%), dry cough (67.7%), fatigue (38.1%), sputum production (33.4%), shortness of breath (18.6%), sore throat (13.9%), headache (13.6%), myalgia or arthralgia (14.8%), chills (11.4%), nausea or vomiting (5.0%), nasal congestion (4.8%), diarrhea (3.7%), hemoptysis (0.9%), and conjunctival congestion (0.8%).15
Fig. 1.
Severe acute respiratory syndrome coronavirus-2 and the cardiovascular system.
Those presenting with severe symptoms may have pneumonia, acute respiratory distress syndrome, dyspnea with respiratory rate > 30/min and oxygen saturation ≤ 93%, and/or lung infiltrates >50% within 2 days of presentation. Critical patients had septic shock and multiorgan dysfunction. The case fatality rate (CFR) varies by location and intensity of transmission and has been reported to be 0.7–5.8%15 which is higher than that of influenza (0.1%). It is important to note that the CFR is dependent on the age at the time of presentation and associated comorbidities. The CFR is <1% for those aged <50 years, 1.3% for patients aged 50–59 years, 3.6% for patients aged 60–69 years, 8% for those aged between 70 and 79 years, and more than 14% for those aged >80 years.14 In COVID-19, the overall symptomatic secondary attack rate (the rate of transmitting the disease to close contacts) is 0.45% for close contacts and >10% for household contacts.16
In a two meta-analysis of six and eight studies from China that examined presence of comorbidities,17,18 hypertension, cardiovascular and cerebrovascular disease, and diabetes was seen in approximately 17%, 16.5%, and 5–9%, respectively, with higher incidence in those requiring intensive care. Isolated cardiovascular disease may be seen in approximately 2.5–5% of cases.19 The pathways leading to these manifestations include complex interplay of age, impaired immune system, and direct myocardial involvement via ACE2 in these patients.20 These pathways appear to be multifactorial and often bidirectional. As more international data emerge, potential relations between Cardio vascular disease (CVD) and COVID-19 will become clearer. Currently, diagnosis of COVID-19 depends on the use of real-time reverse transcriptase polymerase chain reaction using upper and lower respiratory samples.21 However, newer and faster diagnostic kits are going to be available soon. Computed tomography of chest shows abnormality in >80% of patients with often subpleural and ground glass opacification and consolidation. However, this depends on the severity of infection.22 A large number of patients (>80%) have lymphocytopenia.12
4. Cardiac manifestations
It is difficult to outline the spectrum of cardiovascular presentations of COVID-19. However, with the available evidence, it appears that the cardiovascular sequelae may range from direct or indirect myocardial injury (Fig. 1), myocarditis, possible acute coronary syndrome, cardiac arrhythmias, heart failure, and cardiogenic shock.23,24 Elevated troponin levels have been observed in many patients with COVID-19, with significant differences between those who died and those who survived.25 In a meta-analysis of 4 studies, cardiac troponin I levels were much higher in those with severe disease than in those with nonsevere disease.25 Interestingly, the median Troponin I (TnI) among survivors did not change while it rose exponentially in nonsurvivors.26 Along with rise in troponins, other inflammatory biomarkers such as D dimers, interleukin-6, and so on also showed a significant increase reflecting severe pan-inflammatory response.20 Another group of patients presented with predominant cardiac symptoms mimicking viral myocarditis or acute coronary syndrome. Two cases recently reported highlight the possible direct effect of COVID-19 on the cardiovascular system. One patient27 presented with typical chest pain and ST elevation in ECG, left ventricular (LV) dysfunction, and positive troponins but nonobstructive coronary arteries. Another patient presented with severe myocarditis and severe LV dysfunction along with respiratory infection.28 Both the cases responded well to immunoglobulins and steroids with complete normalization of all parameters.
Cardiac arrhythmias have been noted in patients with COVID-19 but have not been characterized.29 Nonspecific palpitations are seen in approximately 7% of patients and arrhythmias in 16% of the hospitalized patients.29 Whether these arrhythmias are a direct manifestation of COVID-19 on the heart or are occurring because of metabolic disturbances, hypoxic injuries, or neurohormonal imbalance is still not clear.30 Because many of the drugs used in the management of this disease prolong the QT and corrected QT interval (QTc), there is a risk of drug-induced torsades de pointes and drug-induced sudden cardiac death, especially when multiple medications are administered. Therefore, QT calculation in ECG is mandatory when drugs are used and QTc >500 should to be taken as the upper limit. Patients infected with COVID-19 are likely at increased risk of venous thromboembolism. Although there are no published case series thus far, there are reports of abnormal coagulation parameters in hospitalized patients with severe COVID-19 disease. HMG-CoA reductase inhibitors (statins) also have the potential to interact with the combination of lopinavir/ritonavir and can result in myopathy due to elevated statin levels when administered together. Similarly, heart failure has been described in almost 20% of cases, especially in those with underlying kidney disease.31 However, it is not clear whether it is because of direct myocardial injury or exacerbation of underlying LV dysfunction. Cardiogenic shock is seen as a feature in critically ill patients with COVID-19. More commonly, it is a spectrum of overlap between cardiac and primary pulmonary manifestation, thereby giving a mixed shock picture. Brain natriuretic peptide and echocardiography can help in clarifying the diagnosis.32 It is important to ascertain the type to choose the mechanical respiratory or circulatory support with extracorporeal membranous oxygenation and so on. The utility of these support systems is still not well ascertained.33 Another concern is patients of heart transplantation presenting with COVID-19. In 2 such cases described,34 both presented with fever and had ground glass opacities in the lung. They responded well to treatment after management with antivirals and glucocorticoids. In a series of 87 patients, the incidence of COVID-19 in patients with heart transplantation was not found to be higher.35 At present, it is not clear how COVID-19 is going to impact need and management of cardiac transplantation. More data are needed to draw definite conclusions in this regard. Further, if the recipient is negative for SARS-CoV-2 and has no prior exposure or symptoms in 2–4 weeks, it is currently recommended to continue heart transplantation without any change in regimens.36
5. Predictors of mortality
According to the current literature, outcomes in COVID-19 depend on various clinical and biochemical parameters (Fig. 1). The number of people aged more than 65 years, presence of comorbidities (such as hypertension and preexisting CVD) and elevated cardiac troponin I levels (signifying cardiac injury), and D dimer levels were found to be higher in nonsurvivors.24,30,31 Some of the studies showed higher levels of myoglobin, C-reactive protein, serum ferritin, and interleukin-6 in patients who died.24 This suggests the inflammatory surge in COVID-19.
6. ACE2 and practical management implications
ACE2 negatively regulates the renin angiotensin system by inactivating angiotensin II and plays a protective role. However, implications of modulation of this receptor by COVID-19 are still not clear. There have been concerns whether Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) may upregulate ACE2, thereby increasing susceptibility to the virus and hence more severe infection.37 On the contrary, there is evidence that drugs may enhance the protective effect of ACE2 on the lung.38,39 The data are currently insufficient to provide any definite recommendation regarding the same. Currently, it is advisable to continue these medications without interruption in patients.40
7. Treatment targets and strategies
Antiviral therapy is being studied for treatment of COVID-19 (Fig. 1). Ribavirin and remedesivir are antiretroviral agents that interrupt RNA replication by acting as nucleotide analog.41 Trials are underway to test their efficacy in COVID-1942,43. Similarly, lopinavir/ritonavir are a combination of protease inhibitors that inhibit RNA replication have been tried with variable success in treating COVID-1944,45. They have synergistic action with ribavirin. Other antiviral agents such as arbidol and favipiravir have been used but conclusive data are still awaited.46 In view of wide interactions of these drugs with P2Y12 inhibitors, prasugrel is the preferred choice.47 In case it cannot be used, a testing guided approach with platelet function assays is strongly recommended. In view of the cytokine storm seen in COVID-19, interleukin-6 antagonists (tocilizumab and sarilumab) are potential therapies that have been tried, and trials are underway.48,49 Chloroquine and hydroxychloroquine have been shown to block the entry of the virus into the cell.50 A small study tested the effect of hydroxychloroquine and azithromycin on respiratory viral loads in patients with COVID-19.51 Virus elimination was faster in those taking hydroxychloroquine and even faster when the combination was taken. This needs to be verified in future studies. Definitive evidence for their use in prophylaxis and management is awaited in trials.52 Other potential promising therapies include use of recombinant human ACE2 (APN01) that reduces inflammatory cytokines such as IL-6 and has potential to neutralize the virus and protect the lung53 and camostat mesylate, a serine protease inhibitor that blocks SARS-CoV-2 entry into cells.54
Lack of definitive treatment and vaccines puts preventive strategies in forefront, while managing COVID-19. It is pertinent to note that healthcare workers are at elevated risk for contracting the virus. In a study, approximately 4% of the infected individuals were healthcare providers.55 This emphasizes the need for self-protection with personal protective equipment (PPE) before caring for patients with COVID-19. As discussed, the transmission occurs most commonly via respiratory droplets, and hence self-protection with face mask, eye protection, gloves, and protective gowns are important for healthcare professionals.56
8. Specific precautions to prevent spread in cardiac patients
Cardiac patients must be informed regarding signs and symptoms of COVID-19 and should be trained in physical/social distancing along with other practices to maintain good hygiene. There is no specific guideline regarding the use of masks in cardiac patients in routine.
When a cardiac patient is presenting with fever or other symptoms of infection, a comprehensive evaluation has to be performed. At present, there is no specific guidance about COVID-19 testing in all cardiac patients. All cardiac patients must continue their treatment as prescribed including ACEI/ARBs.
9. Follow-up of cardiac patients
All cardiac patients are advised to avoid/postpone their routine follow-up visit to the hospital/doctors and to encourage teleconsultation. All routine health checkups for cardiovascular screening should to be postponed for some time.
10. Cardiac patients planned for surgery/intervention
Elective surgeries/intervention for cardiac patients can be rescheduled if possible. However, cardiologists and patients must decide, with mutual consent, after looking into the potential harms of delaying intervention/surgery.
11. Role of prophylactic therapy in cardiac patient for prevention of COVID-19
At this time, there is no evidence or published guidelines regarding the use of prophylactic antiviral therapy or hydroxychloroquine for COVID-19 in cardiac patients. Recommendation for empiric use of hydroxychloroquine for prophylaxis of SARS-CoV-2 is available at https://icmr.nic.in/
12. COVID-19 and guidelines for cardiac care providers
COVID-19 has put an enormous burden on the healthcare system and cardiac care providers, that is, doctors, nurses, technicians, and caregivers, and all other allied professionals are at increased risk for coronavirus infection as chances of acquiring infection at workplace are high. More than 10% of health workers have been infected in Italy. So, all healthcare providers should follow the guideline for PPE. In teaching hospitals, it is imperative to minimize exposure among trainees and nonessential staff (e.g. medical students) not only for their own safety and that of their patients but also for conservation of PPE. Self-reporting symptoms and isolation of healthcare providers should be performed.
13. Cardiology outdoor and indoor clinic
Because some symptoms of COVID-19 are similar to cardiac disease, exposure risk for healthcare workers in cardiology exists in outpatient settings. All cardiologists and other staff need to take proper protection as adviced by their local guidelines (including gloves, protection suits, N95 masks, work caps, goggles/protective screens, and so on). Proper screening of all the patients should be performed before they are allowed to enter the waiting area. Patients in the waiting area should maintain distance of more than one meter apart. Strict implementation of “one doctor, one patient, and one consultation room”” should be performed. Patients and their family members need to wear masks and keep a distance. The medical staff of the electrocardiogram room was also in close contact with patients when performing electrocardiogram, so they also needed proper protection. Regular cleaning of noninvasive facilities such as ECG, echocardiography, Holter monitors, and so on has been proposed to safe guard self and others.
14. Cardiac critical care unit (CCU) and cath lab
Cath lab and CCU personnel face significant issues during this time. CCUs should adopt the strict principle of single room admission. A recent article has put the things in right perspective for all the intervention doctors and staff.56 It is strongly recommended to defer all the elective cases to minimize exposure time and preserve resources. In patients with active COVID-19 presenting with ST elevation, myocardial infarction, and who are stable, fibrinolysis is a reasonable option. In case of urgent intention, for all staff directly involved in the procedure PPE should be worn, and powdered air-purifying respirators are reasonable. In patients requiring intubation, it should be ideally performed before arrival in the cath laboratory. It is recommended to use HEPA filters between tubes and bags. Further, while most of the laboratories are typically configured with positive pressure ventilation, many of such laboratories were converted to negative pressure isolation in setting of COVID-19 in China.57
Regular education programs and close coordination with infection control team are also of paramount importance. Feeling under pressure and working under extreme circumstances are likely to be experienced by medical care providers on many occasions. Stress and the panic associated with the current situation will be more evident in the near future, so managing mental health and psychosocial well-being during this time is as important as managing physical health. In addition to self-protection, we should learn to protect our families from us during the epidemic.
15. Management of family members of inpatients
Inpatients can only be accompanied by at most one family member, and the accompanying family member must complete the COVID-19 screening. They should wear masks and should go for regular screening, for COVID-19–related symptoms while in the hospital.
16. Ethical challenges
The unprecedented challenge represented by the COVID-19 pandemic has brought many new and dramatic ethical challenges to medical profession, especially in underdeveloped and developing countries. This ranges from policy issues to many clinical dilemmas. The reuse of PPE has ethical challenges even in many developed countries, but now this practice is adapted in many countries in view of severe shortage of PPE. Close interaction between patient advocates, government officials, and regulators, as well as physician groups, hospital administrators, pharmaceuticals, manufactures, and other societal leaders will be essential to solve these ethical challenges.
17. General protection steps for cardiac patients
-
•
Regular handwashing with soap and water or an alcohol-based hand rub
-
•
Cover your mouth with a tissue while coughing and sneezing, dispose the tissue, and wash hands.
-
•
Avoid touching face with unclean hands
-
•
Clean and disinfect frequently used objects (especially mobile and purse) and surfaces.
-
•
Avoid close contact with people – keep more than 1 m distance between you and others
-
•
Avoid crowded spaces, especially indoors.
-
•
Avoid contact with anyone who is ill with a cough, fever, or difficulty in breathing
-
•
Do not share objects that touch your mouth – for example, bottles, cups.
-
•
Do not shake hands.
-
•
Practice social/physical distancing
-
•
Continue all medicines and advice as given by your physician.
-
•
Refill your prescription medications and have over-the-counter medicines and supplies, for example, tissues papers and a thermometer
-
•
Make a joint plan with family, friends, and neighbors for the support you need now, or if you become unwell
-
•
Meet people in a well-ventilated room or outdoors.
-
•
Ask visitors to wash their hands properly.
-
•
Do not isolate yourself from friends and family.
18. Conclusions
The COVID-19 pandemic has devastated the health and healthcare systems globally and has far more effects that will be visible as this evolves. To suppress and control the epidemic, countries must isolate, test, treat, and trace. If this is not adopted, transmission chains can continue at a low level and then resurface once physical distancing measures are lifted. The time has come when we must think, plan, and perform professionally to fight this epidemic. Our interventions need to be self-assessed periodically whether they are relevant in the changing environment. We need not confine ourselves in providing temporary remedies but strive for ensuring sustainable and viable solution. While no definitive management is there, it is important that the countries those have not witnessed or are about to witness social transmission must learn the lessons well from countries already facing the disaster. Wisdom lies in not repeating the same mistake that others have done but learning from them.
Declaration of competing interest
There is no conflict of statement.
References
- 1.World Health Organization. Coronavirus disease (COVID-19) outbreak (https://www.who.int/).
- 2.Ministry of health & family welfare, Government of India, Noval corona virus (https://www.mohfw.gov.in/).
- 3.Zhang T.W.Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr Biol. March. 2020;13 doi: 10.1016/j.cub.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan J.W.M., Ng C.K., Chan Y.H. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003;58:686–689. doi: 10.1136/thorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. March. 2020;5 doi: 10.1016/j.cell.2020.02.052. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tikellis C., Thomas M.C. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept. 2012;2012 doi: 10.1155/2012/256294. 256294-256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H., Penninger J.M., Li Y. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. March. 2020;3 doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Ying, Gayle Albert A., Wilder-Smith Annelies, Rocklöv Joacim. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Trav Med. March 2020;27(2) doi: 10.1093/jtm/taaa021. taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holshue M.L., DeBolt C., Lindquist S. Washington state 2019-nCoV case investigation team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New Eng J Med. March. 2020;17 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan W-j, Ni Z-y, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. New Eng J Med. February. 2020;28 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauer S.A., Grantz K.H., Bi Q. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. March. 2020;10 doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization . February 28, 2020. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19)https://www.who.int/publications-detail/reportof- the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19 [Google Scholar]
- 16.Burke R.M., Midgley C.M., Dratch A. Active monitoring of persons exposed to patients with confirmed COVID-19: United States, January-February 2020. MMWR Morb Mortal Wkly Rep. 2020;69(9):245–246. doi: 10.15585/mmwr.mm6909e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li B., Yang J., Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020 Mar 11 doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J., Zheng Y., Gou X. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. March. 2020;12 doi: 10.1016/j.ijid.2020.03.017. [DOI] [Google Scholar]
- 19.BerryM, Gamieldien J., Fielding B.C. Identification of new respiratory viruses in the new millennium. Viruses. 2015;7(3):996–1019. doi: 10.3390/v7030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oudit G.Y., Kassiri Z., Jiang C. SARS coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Accessed February 22, 2020. https://www.cdc.gov/coronavirus/2019-ncov/about/symptoms.html.
- 22.Hosseiny M., Kooraki S., Gholamrezanezhad A., Reddy S., Myers L. Radiology perspective of coronavirus disease 2019 (COVID-19): lessons from severe acute respiratory syndrome and Middle East Respiratory Syndrome. AJR Am J Roentgenol. 2020;1–5:1–5. doi: 10.2214/AJR.20.22969. [DOI] [PubMed] [Google Scholar]
- 23.Murthy S, Gomersall CD, Fowler RA. Care for Critically Ill Patients with COVID-19 JAMA 2020. (online ahead of print) PMID: 32159735 DOI: 10.1001/jama.2020.3633. [DOI] [PubMed]
- 24.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 Mar 3 doi: 10.1007/s00134-020-05991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020 Mar 10 doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Y-Y S., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. March 5, 2020 doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. March. 2020;16 doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng J.H., Liu Y.X., Yuan J. First case of COVID-19 infection with fulminant myocarditis complication: case report and insights. preprints. 2020 doi: 10.1007/s15010-020-01424-5. 2020030180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV- 2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou F., Yu T., Du R. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Published:March 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Force A.D.T., Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin Definition. J Am Med Assoc. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 33.MacLaren G., Fisher D., Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. Published online February. 2020;19 doi: 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 34.Li F., Cai J., Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zong-Li Ren R.H., Wang Zhi-Wei, Zhang Min. Epidemiological and clinical characteristics of heart transplant recipients during the 2019 coronavirus outbreak in wuhan, China: a descriptive survey report. J Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guidance for cardiothoracic transplant and mechanical circulatory support centres regarding SARS CoV-2 infection and COVID-19. March 17, 2020. https://community.ishlt.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=afb06f06-5d63-13d4-c107-d152a9f6cd46 [Google Scholar]
- 37.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 Mar 4 doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imai Y., Kuba K., Rao S. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19.Accessed March 19, 2020. https://professional.heart.org/professional/ScienceNews/UCM_505836_HFSAACCAHA-statement-addresses-concerns-reusing-RAAS-antagonists-in-COVID-19.jsp. [DOI] [PMC free article] [PubMed]
- 41.Gordon C.J., Tchesnokov E.P., Feng J.Y. Thee antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. Feb. 2020;24 doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ClinicalTrialsgov . 2020. Severe 2019-nCoV remdesivir RCT. Identifier: NCT04257656.https://clinicaltrials.gov/ct2/show/NCT04257656 Last Updated Feb 24. [Google Scholar]
- 43.ClinicalTrialsgov . 2020. Study to evaluate the safety and antiviral activity of remdesivir(GS-5734™) in participants with severe coronavirus disease (COVID-19). Identifier: NCT04292899.https://clinicaltrials.gov/ct2/show/NCT04292899 Updated March 19. [Google Scholar]
- 44.Chu C.M., Cheng V.C., Hung I.F. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao B., Wang Y., Wen D. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N Engl J Med. March 18, 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsousi N., Daali Y., Fontana P. Impact of boosted antiretroviral therapy on the pharmacokinetics and efficacy of clopidogrel and prasugrel active metabolites. Clin Pharmacokinet. 2018;57:1347–1354. doi: 10.1007/s40262-018-0637-6. [DOI] [PubMed] [Google Scholar]
- 48.ClinicalTrialsgov . 2020. Tocilizumab in COVID-19 pneumonia (TOCIVID-19) (TOCIVID-19). Identifier: NCT04317092.https://www.clinicaltrials.gov/ct2/show/NCT04317092 Updated March 20. [Google Scholar]
- 49.ClinicalTrialsgov . 2020. Evaluation of the efficacy and safety of Sarilumab in hospitalized patients with COVID-19. Identifier: NCT04315298.https://www.clinicaltrials.gov/ct2/show/NCT04315298 Updated March 19. [Google Scholar]
- 50.ClinicalTrialsgov . 2020. Comparison of lopinavir/ritonavir or hydroxychloroquine in patients with mild coronavirus disease (COVID-19). Identifier: NCT04307693.https://clinicaltrials.gov/ct2/show/NCT04307693 Updated March 13. [Google Scholar]
- 51.Gautret P., Lagier J.-C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. march 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan A., Benthin C., Zeno B. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21:234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawase M., Shirato K., van der Hoek L. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol. 2012;86:6537–6545. doi: 10.1128/JVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease. 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. Published online February. 2020;24 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 56.FGP Welt, Shah P.B., Aronow H.D. Catheterization laboratory considerations during the coronavirus (COVID 19) pandemic: a joint statement from the American college of cardiology(ACC) interventional council and the society of cardiovascular angiography and intervention (SCAI) J Am Coll Cardiol. 2020 Mar 16 doi: 10.1016/j.jacc.2020.03.021. [Online ahead of print]. PMID: 32199938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chow T.T., Kwan A., Lin Z., Bai W. Conversion of operating theatre from positive to negative pressure environment. J Hosp Infect. 2006;64:371–378. doi: 10.1016/j.jhin.2006.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]