Abstract
The application of tissue engineering approaches to human induced pluripotent stem (hiPS) cells enables the development of physiologically relevant human tissue models for in vitro studies of development, regeneration and disease. The immature phenotype of hiPS derived cardiomyocytes limits their utility. We therefore developed a protocol to generate engineered cardiac tissues from hiPS cells, and electromechanically mature them towards an adult-like phenotype. This protocol also provides the optimized methods for analyzing the functionality, ultrastructure, and cellular properties of these tissues. The overall approach relies on biological adaptation of the cultured tissues subjected to biomimetic cues applied at an increasing intensity to drive accelerated maturation. Human iPS cells are differentiated into cardiomyocytes and used at an early stage, immediately after the first contractions are observed, when they still have significant developmental plasticity. This starting cell population is combined with human dermal fibroblasts, encapsulated in a fibrin hydrogel and allowed to compact under passive tension in a custom-designed bioreactor. After 7 days of tissue formation, the engineered tissues are matured for an additional 21 days, by electromechanical stimulation of an increasing intensity. Tissue properties can be evaluated by measuring contractile function, responsiveness to electrical stimuli, ultrastructure (sarcomere length, density of mitochondria, networks of transverse tubules), force-frequency and force-length relationships, calcium handling, and comprehensive responses to β-adrenergic agonists. Cell properties can be evaluated by monitoring gene and protein expression, oxidative metabolism, and electrophysiology. The overall protocol takes 4 weeks and requires experience in advanced cell culture and machining methods for bioreactor fabrication. We propose that this maturation protocol can improve modeling of cardiac diseases and testing of drugs.
Keywords: Human induced pluripotent stem cells, Cardiomyocytes, 3D Tissue, Electromechanical stimulation, Maturation, Tissue engineering
EDITORIAL SUMMARY
Cardiac tissues are derived from hiPS cells and electromechanically matured towards an adult-like phenotype. This protocol also describes optimized methods for analyses of function, ultrastructure, and cellular properties of these tissues.
COVER TEASER
Engineered adult-like human cardiac tissues
INTRODUCTION
Advances in stem cell biology and tissue engineering have led to the development of engineered tissue models or “organs-on-a-chip”, intended to serve as physiologically relevant human in vitro models of their in vivo counterparts. Cardiac tissue engineering aims to emulate the human heart, and requires methods for recapitulating the environmental signals inherent to the developing heart. In addition to repair of the damaged or diseased heart which was the original goal of cardiac tissue engineering, engineered cardiac tissues are also finding utility for in vitro modeling of heart physiology and disease [1]. The first cardiac tissues were engineered using avian cells in the early 1990s [2], and the field has made major progress since these pioneering efforts [3–11]. Current human cardiac tissue models are starting to enable humanized drug screening, mechanistic biological studies, and regenerative medicine approaches.
The immature phenotype of cardiomyocytes derived from human induced pluripotent stem (hiPS) cells limits these models from fully realizing their potential [12–14]. The immaturity results in preclinical models that are overly sensitive, causing many drugs to be incorrectly flagged for potentially dangerous side effects with subsequent removal from further testing. The immaturity is especially limiting when it comes to detecting cardiac arrhythmias at a preclinical stage, where human cell models could overcome the shortcomings in translation of animal models to the clinic [13]. Additionally, the immature hiPS derived cardiomyocytes (hiPS-CM) express the inward funny channel (If), which may cause arrhythmias when implanted into an adult heart [14]. We recently developed methods to improve maturation of hiPS-CM [6] and describe the detailed methodology required to achieve this here.
Maturation of engineered human cardiac tissues.
In recent studies, we established that adaptive engineering, where external signals are designed to drive the biological system to its limits, can mature cardiac tissues beyond the extent achieved by any of the previous approaches [3, 5, 6, 8–10, 15–19]. The components critical for the formation of adult-like cardiac tissues in vitro were: 1) the use of early hiPS-CM, at a stage of high developmental plasticity, 2) the combination of hiPS-CM and supporting human fibroblasts in a native hydrogel, 3) tissue formation around two flexible pillars enabling auxotonic contractions, and 4) electromechanical stimulation at an intensity that was gradually ramped up every day, to constantly force the cardiac tissue to adapt to the increasing workload.
The use of this protocol (Figure 1) yielded hiPS-CM derived cardiac tissues of advanced maturity, providing opportunities for cardiac tissue engineers to overcome the previous limitations of hiPS-CM immaturity. The utility of the developed mature engineered cardiac tissues in predicting human clinical responses relies on their ability to mimic the physiology, pathology, and pharmacology of the adult human heart (Figure 2). Matured engineered cardiac tissues were formed from early-stage hiPS-CM cells 10–12 days after the beginning of differentiation (Figure 2A). These tissues were able to recapitulate both the force-frequency [6] and force-length relationships of the heart (Figure 2 B–D). This is a strong indicator of their increased physiological relevance, as current preclinical small animal models and previous hiPS-CM models lack this fundamental force-frequency relationship characteristic of human cardiac physiology [20, 21]. Similarly, the mature cardiac ultrastructure attained showed increased sarcomere alignment, intercalated discs, M-line structures, dense mitochondria populations, when compared to fetal cardiac tissues (Figure 2E–F), and the development of T-tubules (Figure 2G), for the first time. The biological fidelity of the developed model was further supported by the demonstrated shift from glycolysis to fatty acid oxidation-based metabolism and switch in gene expression from fetal α-myosins to adult β-myosins [6]. The development of T-tubules enables rapid exchange of calcium through the cell membrane where the calcium elicits a calcium-induced calcium response by triggering the ryanodine receptor to release stored calcium in the sarcoplasmic reticulum. Immunostaining of matured engineered cardiac tissues revealed a colocalization of these calcium handling proteins, while functional assays demonstrated increased ryanodine function, increased calcium loading within the sarcoplasmic reticulum, and corresponding changes in gene expression that further validate an adult-like phenotype [6]. The level of maturation attained provided an opportunity to demonstrate its predictive capacity by recapitulating the well-characterized ionotropic (Figure 3A) and lusitropic (Figure 3B) responses clinically seen from the β-adrenergic agonist isoproterenol, a response notably missing from current hiPS-CM protocols [21]. This ionotropic response to isoproterenol and enhanced ultrastructure is seen in multiple cell lines when the protocol described herein is used (Figure 3C–D).
Figure 1.
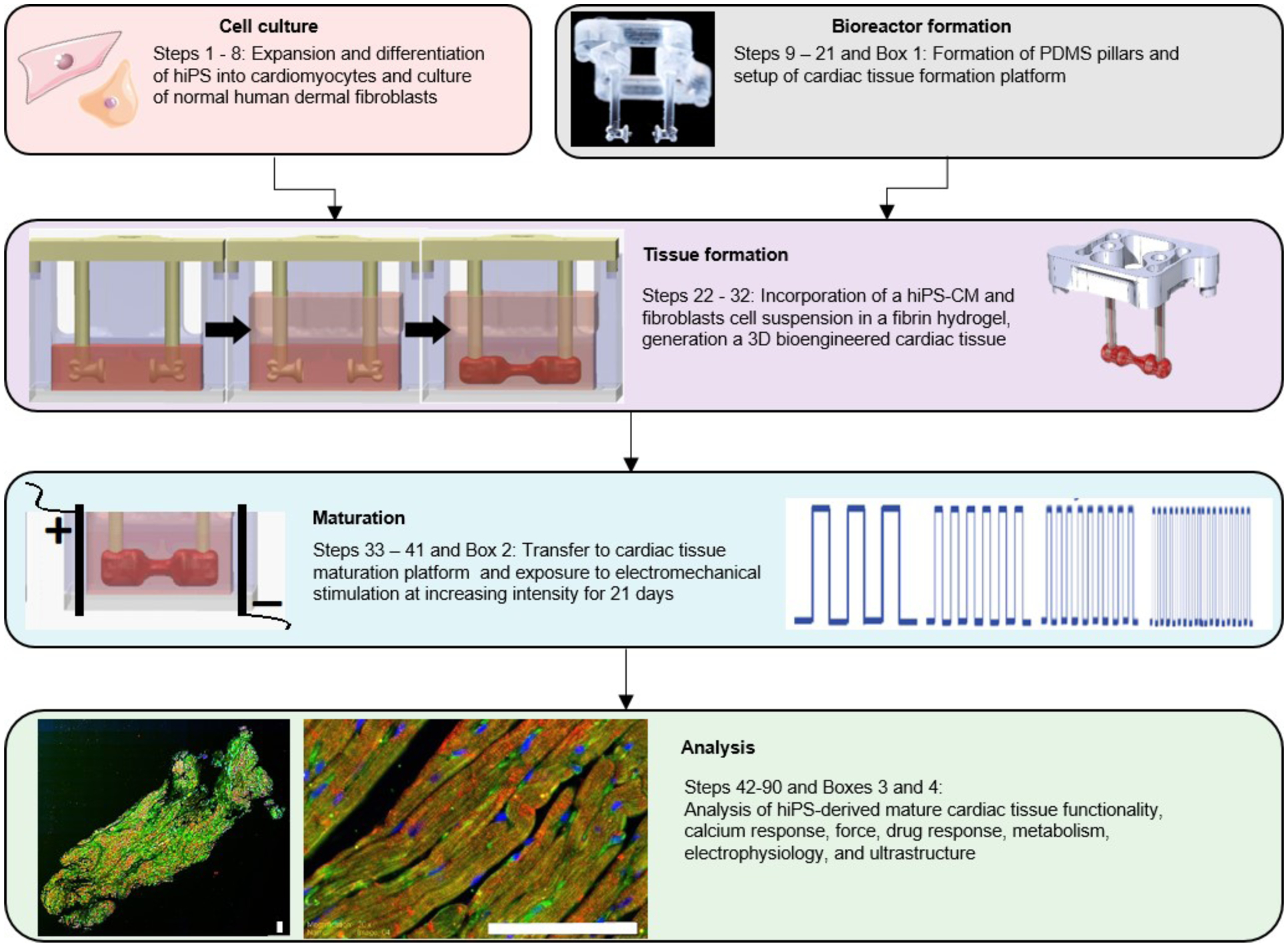
Generation of hiPS-derived cardiac tissues and analysis of their functionality, calcium, force and drug response, metabolism, electrophysiology and ultrastructure.
Figure 2. Maturation of hiPS cell derived cardiac tissues to an adult-like phenotype.
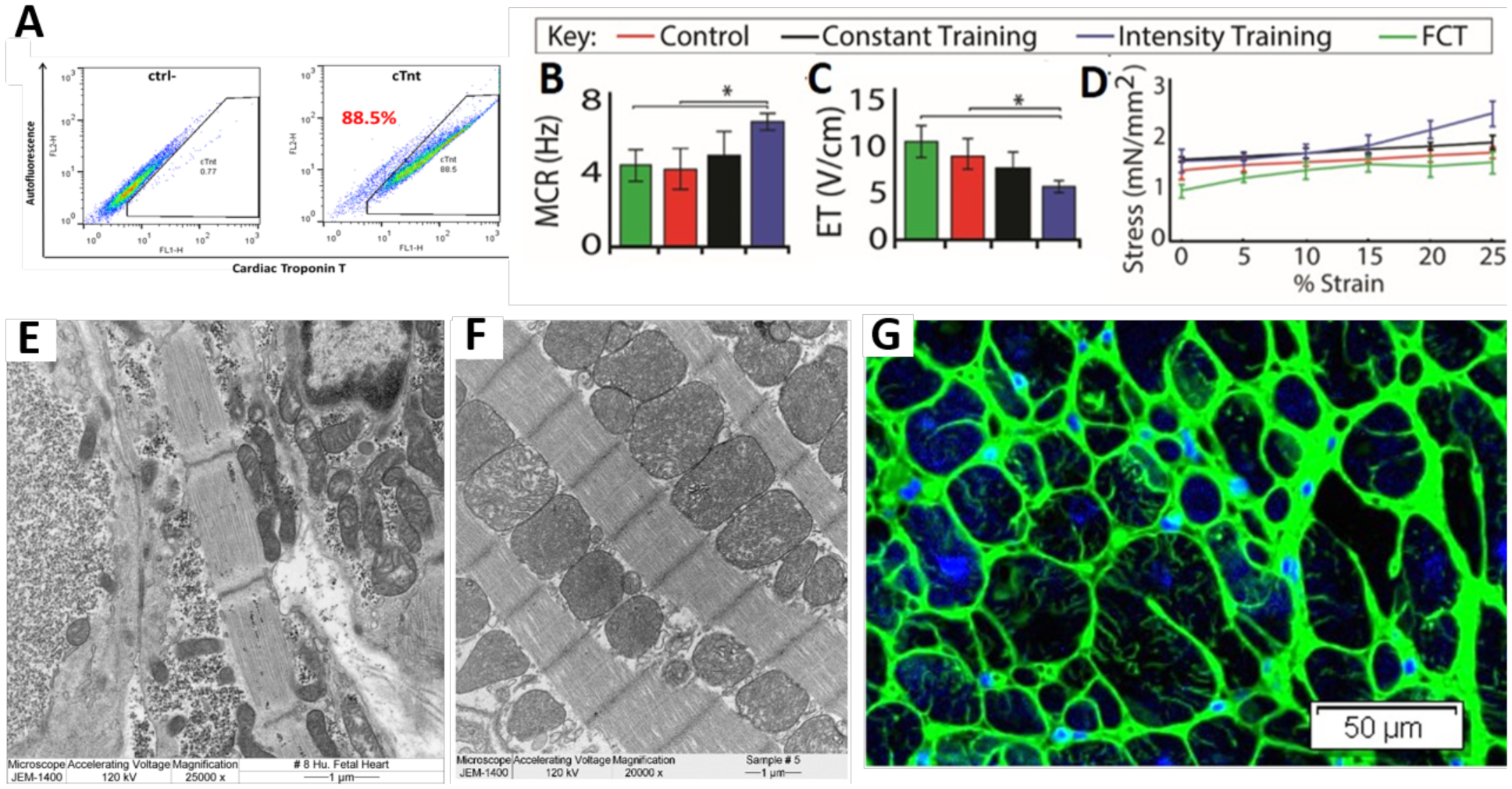
A) Flow cytometry of an early-stage differentiation containing C2A cells labelled with cardiac troponin T reveals an efficiency of 88.5 %. B-D) Maximum capture rate (MCR; B), excitation threshold (ET; C), and Frank-Starling curves (D) generated in a Muscle Strip Myograph System. Intensity trained tissues are compared to those trained at a constant frequency (2 Hz, “constant training”), unstimulated tissues (“control”) and Fetal Cardiac Tissues (FCT) (n ≥ 12 per group, mean ± 95 % CI; p<0.05; two-way ANOVA followed by Tukey’s HSD test). E-F) Transmission electron microscopy image of a representative 19 weeks fetal cardiac tissue (E) and cardiac tissue intensity trained for 4 weeks (F) show ultrastructural alignment and physiologically high fraction of mitochondria (scale bar = 1 μm) in the electromechanically matured engineered cardiac tissues. G) Cross sectional view of an intensity trained cardiac tissue after 4 weeks, detailing networks of T-tubules (Wheat Germ Agglutinin in green; ??? in blue; scale bar = 50 μm).
Figure 3. Enhanced isoproterenol response and ultrastructure within tissues formed from early-stage hiPS-CM from different cell lines.
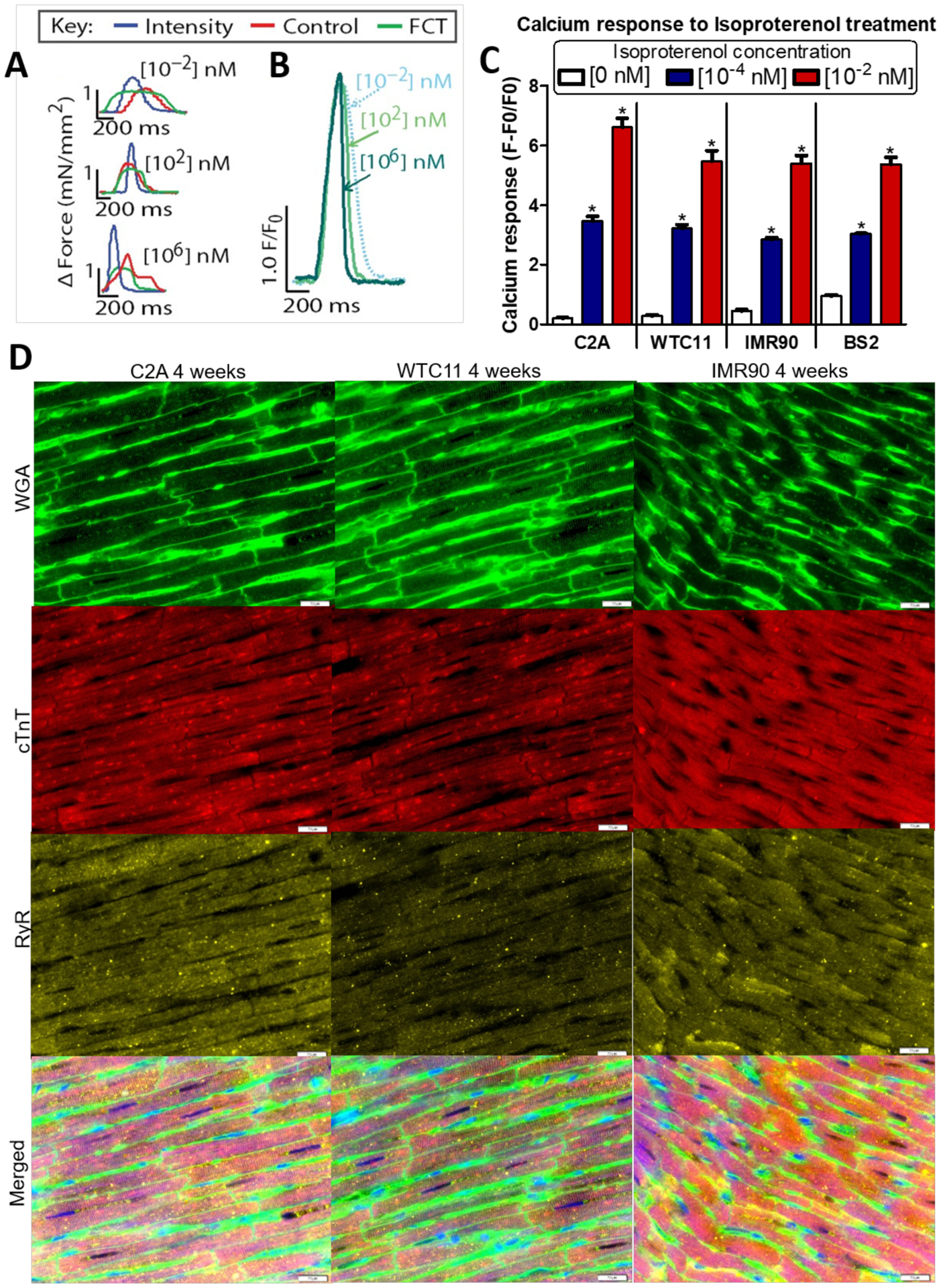
A-B) Isoproterenol induced positive ionotropic (A) and lusitropic (B) dose-dependent responses within cardiac tissues from the C2A cell line. C) Calcium transient peak heights after exposure to isoproterenol at the indicated concentration within intensity trained early-stage cardiac tissues from 4 different cell lines after 4 weeks of culture (n≥7; mean +SEM, p < 0.05 by ANOVA followed by Tukey’s Multiple Comparison Test). D) Representative immunofluorescent images from early-stage hiPS-CM from 3 different cell line after 4 weeks of maturation (Wheat germ agglutinin (WGA): green, cardiac Troponin T (cTnT): red, Ryanodine receptor (RyR): yellow, scale bar = 20 μm). A and B adapted from [6].
In addition, comparison of the transcriptome with that of matured ventricular heart tissue from the Genotype Tissue Expression Consortium (GTEx) and human fetal cardiac tissue from a recent study by Holden demonstrated closer similarities to the adult tissue (Figure 4, Supplementary Table 1). [22]
Figure 4. Gene expression of engineered heart tissues compared to fetal and adult heart tissue.
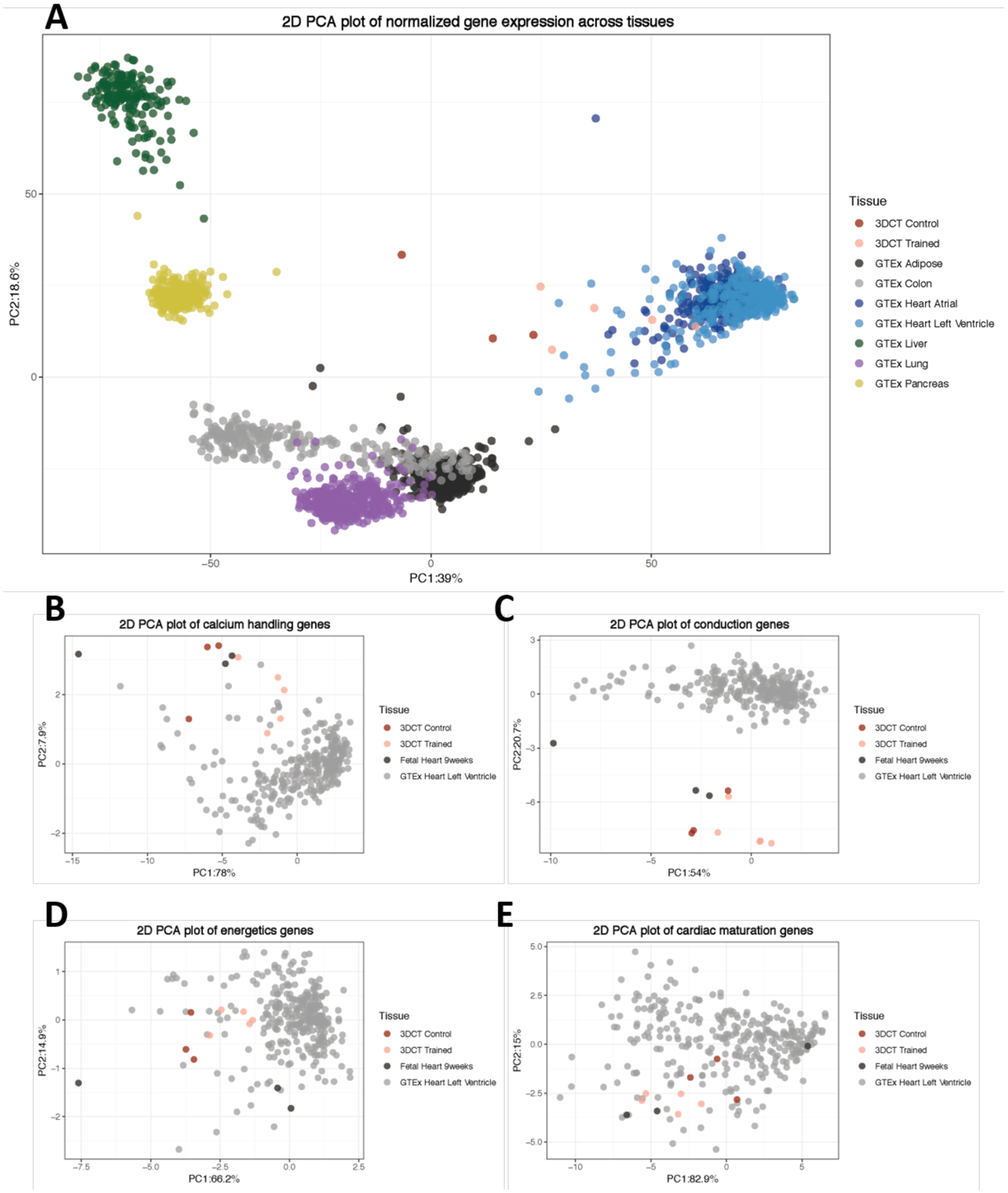
A) Principle component analysis (PCA) plot of variance stabilized gene expression of engineered heart tissues (3DCT), adult heart left ventricle tissue, and other adult tissues from the Genotype Tissue Expression (GTEx). B-E) PCA plots of variance stabilized gene expression across engineered heart tissues, adult heart, and fetal heart for cardiac-specific genes: calcium-handling genes (B; 10 genes), conduction genes (C; 10 genes), energetics genes (D; 6 genes), maturation genes (E; 4 genes).
DEVELOPMENT OF THE PROTOCOL
Cell populations.
Dissociation of hiPS-CM monolayers into a single cell suspension using cell-protective methods is key for proper tissue formation. Large cell clusters lead to heterogeneous contracting patches, while long or harsh dissociation harms the cells. The early-stage hiPS-CM (up to days 10–12 of cardiac differentiation) are easier to dissociate to a single-cell suspension as they have not yet deposited much extracellular matrix. We found that the resulting cardiac tissues were more responsive to electromechanical stimuli, presumably because these cells are still developmentally plastic. Interestingly, studies have found that hiPS-CM exhibit a bifurcation event between days 14–20 where miRNAs responsible for pluripotency are turned “off” and miRNAs associated with cardiomyocyte development and function are turned “on” [23]. Similarly, long-term culture reveals that the related miRNAs plateau by day 60 post-differentiation. The use of early-stage hiPS-CM may also coincide with their innate developmental timing, towards cardiac development. The culture of hiPS-CM in a native-like hydrogel (fibrin) containing the supporting fibroblasts is critical to the development of functional cardiac tissues. The inclusion of a fibroblast cell population facilitated tissue formation and stabilization. Without fibroblasts, cardiac tissues comprising only cardiomyocytes would beat too strongly and break apart. A ratio of 75% hiPS-CM and 25% fibroblasts was determined to yield cardiac tissues of increased robustness. A ratio of fibroblasts above 25% limited the contractile ability of the cardiac tissues while hiPS-CM ratios above 75% resulted in tissue breakage during the maturation phase.
Cardiac tissue formation.
Collagen, which has been used in the generation of cardiac tissues, can facilitate the development of a necrotic core in these tissues, possibly due to long crosslinking times and changes of pH. Instead, a combination of fibrinogen and thrombin, which is used to crosslink fibrinogen quickly, creates a fibrin hydrogel without affecting pH. Additionally, the spontaneous beating contractions seen in fibrin hydrogels may facilitate the transport of fluid into the tissue interior. Fibrin hydrogels do not compact as much as collagen hydrogels, thus requiring a smaller amount of fibrin than collagen for cardiac tissues of a similar size. We form tissues in a specialized bioreactor containing wells machined from polycarbonate and coated with a hydrophobic solution to prevent the hydrogel from attaching. The pillars were designed to control the formation of the tissue around the head of the pillar. Other methods to generate 3D cardiac tissues around flexible polydimethylsiloxane (PDMS) pillars are similar to the protocol described herein with respect to cell numbers and the steps of tissue formation [2, 10, 15]. However, they do not utilize electromechanical stimulation of an increasing intensity to achieve maturation. Currently, the method described here results in the most mature cardiac tissue phenotype to date.
Bioreactors.
For tissue formation and maturation, we developed one individual support structure for PDMS pillars and two different platforms, one for tissue formation and another for tissue maturation (Figure 5 and Box 1). The PDMS pillars are 1 mm in diameter, 9 mm in length, and spaced 6 mm center to center (Figure 5A). The free ends of the pillars flare out to a diameter of 2 mm over a distance of 1.5 mm from the center of each pillar. In the tissue attachment region, there is 3 mm of open space between the medial faces of the pillar heads.
Figure 5. Platforms for tissue formation and maturation.
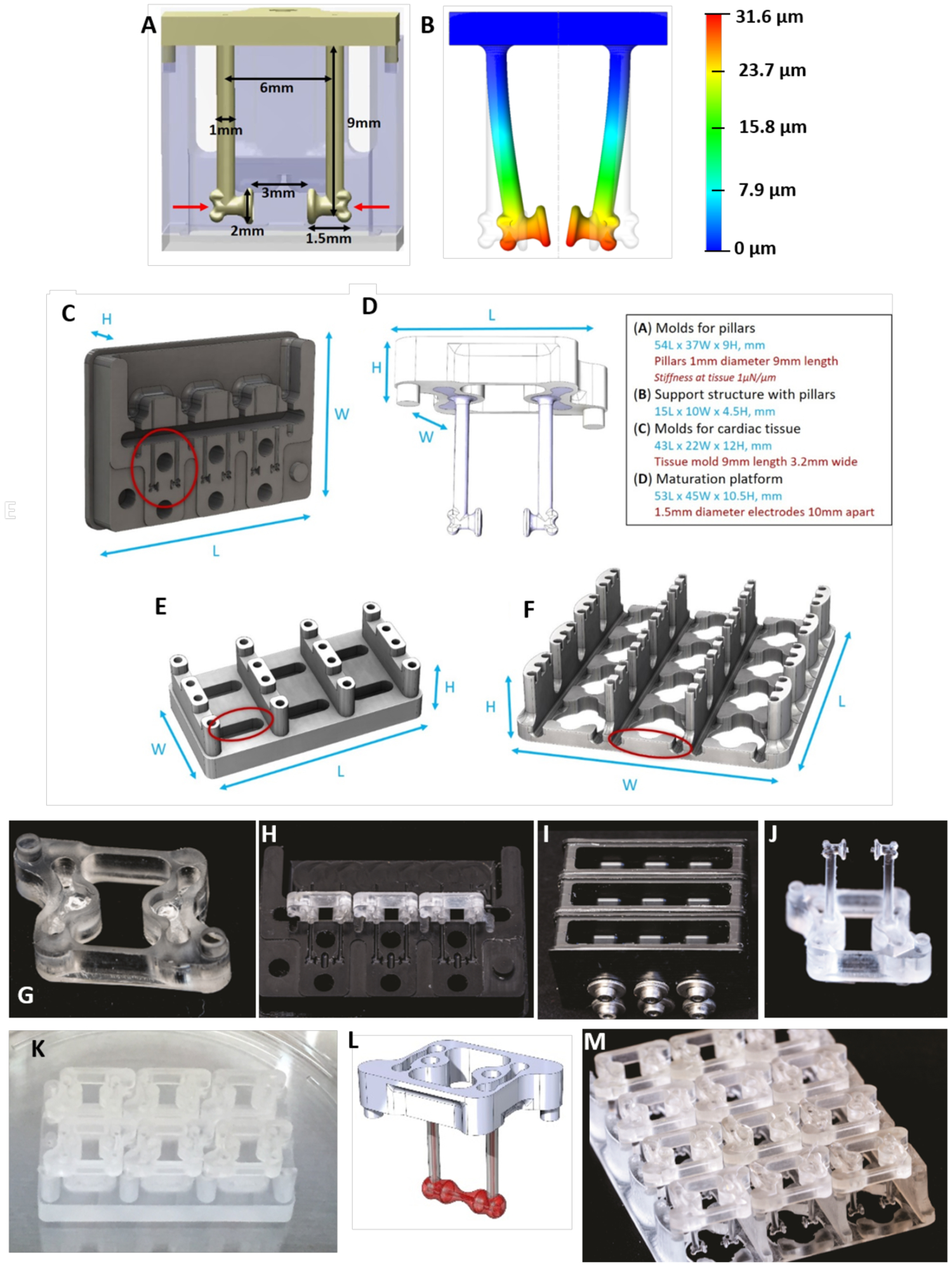
A) Dimensions of pillars and detail of a notch on the pillar’s head (indicated by red arrows). B) The design of the pillars herein enables a consistent modeling of the PDMS deflection at a set height on the pillar (i.e. where the notch is located) to facilitate on-line force readouts based on pillar deflection. C) Delrin molds for PDMS casting of pillars. D) Support structure for the pillars. E) Tissue formation platform. F) Maturation platform. G) Polycarbonate support structure for pillars with mating features. H) Three polycarbonate support structure can fit within one mold. I) Three delrin molds, containing three polycarbonate support structure each, are filled with PDMS in the top chamber and screwed tightly shut to prevent flashing. J) The polycarbonate support structure containing the cured PDMS pillars after removal from the delrin mold. K) Six polycarbonate sets of pillars with pillars can fit on one cardiac tissue formation platform. L) The polycarbonate support structure and PDMS pillars contain mating features for proper alignment in the cardiac tissue formation and maturation platforms. M) Twelve polycarbonate sets of pillars with pillars can fit on one cardiac maturation platform.
Box 1 – Bioreactor design and fabrication.
There are three main components to the cardiac platform: elastomeric pillars for tissue attachment, tissue formation well platform, and a maturation platform. Design and fabrication aspects for each component are outlined below. Depending on what facilities are accessible the platform can be made in-house, or outsourced to any machine shop with plastics experience. General recommendations for plastics machining is to avoid the use of oil coolants (air is best, water is also acceptable). We have found that immersing components in a beaker of water and running the autoclave liquid cycle (121 °C for at least 30 min using saturated steam under at least 15 psi) helps remove any unintentional surface contamination. A subsequent dry autoclaving (121 °C for at least 30 min using saturated steam under at least 15 psi, 30 min drying time) in standard packaging should be done before use.
Elastomeric pillars:
PDMS pillars are overmolded onto a polycarbonate support structure. CNC machining is utilized to fabricate molds for overmolding the pillars onto the support structure, which are themselves also machined components. The supportive structure was designed in 3D computer aided design software, we used Solidworks. There is an open window from the top perspective to provide a clear path for imaging purposes. There are embossments on the base of the structure that are utilized as alignment features. There are recessed pockets on the top and bottom sides of the structure with through-holes that allow PDMS to anchor to the support structure. Molds are machined from Delrin (acetal) resin and are recessed to allow a 2-part mold over the support structure. Molds must be burr free and incorporate through holes for shoulder screws that align the halves, and provide clamping force. These precautions minimize chances of PDMS flashing (thin film curing where there should be open space). Tolerances on components should be such that full clamping is not prevented (undersized supports and oversized mold cavities prevent this). To form pillars, support structures are inserted into the mold (3-cavity mold is pictured) and clamped together. They are mounted onto a centrifuge plate normally used for multiwell plates. PDMS is added into the mold reservoirs. Pairs of molds/centrifuge plates are carried out together for balance and should weigh within 0.5 gram difference to minimize imbalance. Prior to centrifugation (5 min at 300 g), molds are placed inside a vacuum chamber for 30 min (29 mmHg). After vacuum degassing and centrifugation, molds are transferred to an oven at 60 °C for 12 hr, cured, and opened to take out the pillars formed over their support structures that are autoclaved before use.
Cardiac tissue formation platform:
To provide consistent tissue, the pillars for initial attachment were sized to allow space for cells to attach around the pillar heads. The wells should be machined in polycarbonate and contain alignment features that interface with the support structure of the pillar component (see schematic). This ensures consistent positioning of the pillars within the mold volume. Wells are machined using standard techniques and autoclaved for use. It is helpful to assemble pillars within formation wells prior to autoclaving, to reduce manual manipulation.
Cardiac tissue maturation platform:
The maturation platform interfaces with the PDMS pillars with cardiac tissue attached. The platform contains features for aligning pillars and tissues with the carbon electrodes. This ensures a spatially consistent stimulation environment. Electrodes are placed within grooves with a slight under tolerance to provide a transition fit. Additional recesses were designed into the platform so as to maximize medium volume proximal to the tissue (see schematic). Platforms should be machined in polycarbonate using standard techniques, electrodes should be assembled with platinum wires attached and the assembly autoclaved prior to use.
We have adapted the pillar design to increase the robustness of tissue formation and precise positioning on the pillars. The head of the pillar is shaped so that the tissue aligns with the pillar head and the most force is exerted in the middle of the tissue. This is critical when using the deflection of the pillar as a measure of the force generated based on beam-bending theory [24, 25], as the bending equation depends on the height of the pillar and therefore the location of the tissue deflecting the pillar (Figure 5B).
To increase the robustness of tissue formation, we designed a single pillar support so that the tissues can be removed or separated without affecting other tissues attached to the same multi supportive structure (Figure 5C, D, G–J). This feature is different than that used in other platforms, including those we previously published, and reduces the time and reagents previously used on tissues that do not form properly [6]. A standard mold for the cardiac tissue was also developed (Figure 5E and K). With 6 wells, it allows concurrent generation of 6 individual tissues. After the tissues are formed, they can be transferred to the maturation platform (Figure 5F and M).
We found that the actual mechanical properties of the PDMS pillars can vary greatly depending on the time allowed for curing, the accuracy of mixing the base and curing agents thoroughly, the humidity in the environment, and the flux in temperature of the oven. To counteract this, we make PDMS pillars in large batches under the same conditions and ensure that the parameters are precisely maintained. To obtain accurate force readouts, we used a commercially available Muscle Strip Myograph System with a force transducer to record the force generation of the cardiac tissue. This enabled us to check for any differences that may arise from the mechanical properties of the pillars, and provided the means to precisely measure the force-length relationships.
Medium composition.
During maturation, the cardiac tissues were cultured in a larger volume of medium, that enabled crosstalk between the tissues, exchange of nutrients and secreted factors, and dilution of any reactive oxygen species resulting from electrical stimulation. To further advance maturation, cardiac tissue culture medium included fatty acids (supporting oxidative metabolism) and thyroid hormone, by adding the cell culture B-27™ Supplement [26, 27]. The combination of these environmental cues enabled the development of a biomimetic cardiac tissue that can be driven to adapt to the imposed contractile demands and yield cardiac tissue with adult-like maturity. Aprotinin was supplemented within the culture medium for the first week following cardiac tissue formation to prevent rapid enzymatic digestion of the fibrin hydrogel and allow the fibroblast population time to secrete extracellular matrix. Serum supplementation was avoided as it is not well characterized and contributes to rapid fibrin degradation.
Cardiac tissue maturation.
In order to develop an in vitro human based cardiac system capable of drug screening and disease modeling, we combined some of the best practices in the field for both generating three-dimensional (3D) cardiac tissues [3] and using electromechanical stimulation for maturation [8, 9, 28]. For mechanical stimulation, we adapted the use of auxotonic pillars to mechanically constrain the forming tissue and provide mechanical preload [2, 10, 18] (Figure 5L). To induce mechanical contractions, we applied electrical signals via an electrical field established between two carbon rods placed in parallel to the cardiac tissue and connected to an external stimulation source. This setup enabled the user to control electromechanical stimulation. Pacing the tissues at a regular rate (2 Hz) helped synchronize contractility, but it did not lead to significant cardiac maturation. To accelerate maturation, we developed an electromechanical stimulation regimen termed “intensity training”, where the cardiac tissues were forced to continuously adapt to electromechanical signals. The protocol was designed to slowly increase the stimulation frequency (0.33 Hz per day, over two weeks) so that the cardiac tissue had sufficient time to develop capacity to keep up with the increasing workload. To further accelerate the maturation process, we constantly forced the tissue to reestablish homeostasis with each increasing stimulation frequency. This regimen advanced cardiac maturation beyond the levels achieved previously [5, 8–11]. To facilitate the implementation of electromechanical stimulation, we also developed an Arduino based electrical stimulator, which is a much affordable option for broad use than the commercial units (Box 2).
Box 2 – Electromechanical stimulation of cardiac tissues - Arduino.
In order to build the electrical stimulation bioreactors described herein, tools to control and deliver the electrical stimulation regimen need to be established. In this protocol, commercial electrical stimulators were used (Grass, cat. no. S88X/CS4 Stimulator ADInstruments, DMT100273), which are expensive if not a tool used regularly in experimental studies. To overcome a potential hurdle for other labs to implement the electrical stimulation regimen described in the protocol, we have developed an alternative electrical stimulator that is based on an Arduino microcontroller and off the shelf electrical components.
Arduino’s serve as mini-computers that can be programmed to control and deliver various stimuli to cells and tissues. The Arduino platform is advantageous because of its low cost and capability to perform a multitude of functions and complex data acquisition protocols, all on the small mobile device. The Arduino programming language is based on running programs, called “sketches” which are loaded into the Arduino’s memory and can be operated without being connected to a computer. To facilitate implementation of electrical stimulation regimens, we include here details of how to use an Arduino based microcontroller and corresponding code (Supp Data 1) to create an electrical stimulator that automatically increases the stimulation intensity according to the protocol herein. It is coupled to an LCD screen which also enables the user to set custom stimulation regimens without having to change the parameters in the code manually. While the system is advantageous in cost and ease of use, it is limited by a maximum voltage output of 5V. This limitation may affect excitation threshold and maximum capture rate studies, if tissues require higher voltages to be controlled, which is not normally the case.
This program controls the electrical stimulation of cells by generating monophasic waves. The adjustable parameters are: voltage, frequency, and pulse time (the duration of time the voltage is maintained at a high level). These parameters are controlled via the LCD screen. The setup of the circuit involves the use of the Arduino Uno, the AD5206 digital potentiometer, and the TLV4110 operational amplifier. The SPI library on the arduino is used to control the digital pot via the Arduino pins 13 (SCK), 11 (MOSI), and 10 (SS) to pins 8, 7, and 5 on the AD5206 digital potentiometer respectively. Stimulation is achieved by setting the digital potentiometer to a specific resistance to achieve the desired voltage for cell stimulation. The frequency is achieved by switching the resistance on the digital potentiometer between the set level and the max resistance to obtain a monophasic wave. The pulse time is achieved by controlling when the switch occurs. The voltage can be set to a number between 0 – 5 volts, the frequency between 0 – 100 Hz, and the pulse duration time must be less than the period duration (i.e. 1 / frequency). Overall, the main parameters can be controlled through the LCD screen, with the option of either running the intensity training electrical stimulation protocol described herein (Program 1) or a custom input from the user (Program 2).
Equipment
Arduino Starterpack Kit (Arduino) or ARDUINO UNO REV3 (Arduino, A000066)
LCD Keypad Shield For Arduino Duemilanove Uno Mega 2560 Mega 1280 (SAINSMART, SKU: 101–50-104)
Computer
Digital potentiometer (AD5206, Analog Devices)
Operational amplifier (TLV4110, Texas Instruments)
Electrical wiring
Breadboard
Alligator clips
Optional: Oscilloscope
Procedure
Set up Arduino electrical stimulator:
Connect the LCD screen to the Arduino by physically inserting the LCD screen on top of the Arduino so that the corresponding pins line up (i.e. pin “A5” on the bottom right of the LCD screen will insert into pin “A5” on the Arduino board.
Plug the Digital potentiometer (AD5206) and operational amplifier (TLV4110) into a breadboard according to the PnP diagram (Figure 7A).
Connect wiring between the Arduino and the breadboard (or solder the components for a more durable connection) according to the PnP diagram (Figure 7A).
Upload adult-like cardiac maturation electrical stimulation code to Arduino:
Put the rubber bumpers on the bottom of the board or put the stimulator in a plastic case. This will protect from spills, and is essential if the table is made out of metal.
Download & install drivers (available on Arduino website: https://www.arduino.cc/en/Main/Software).
Plug Arduino into the computer (via the provided USB cable) to power up.
Double click the Arduino software icon to open up the workspace.
Configure the Arduino software for the correct chip. Look at the chip and determine the board (i.e. Arduino/Genuino Uno). Go to Tools/Microcontroller/and select your chip.
Configure the Serial Port by going to Tools/Serial Port and select the appropriate port.
Open “electrical_stimulation_cardiac_maturation sketch” (sketches are little scripts that can be sent to the Arduino to direct how to act) provided with this protocol in SUPP DATA 1.
Verify / Compile: The first step to getting a sketch ready for transfer to the Arduino is to Verify/Compile. That means checking for mistakes (editing) and translating into an application that is compatible with the Arduino hardware. To verify and compile the code, press the check mark icon in the top left of the window. If the verification and compilation steps were successful, you will see the text “Done compiling” in the bottom left of the screen.
Make sure the Arduino is plugged in, the green light is on, and the correct serial port is selected. For an NG Arduino, press the Reset Button now, just before selecting the upload menu item. Select Upload to I/O Board from the File menu.
To run the intensity electrical stimulation regimen detailed in this protocol, select “1” for Program (Figure 7B). On the following screens, input the voltage and pulse duration. We recommend a voltage of 4.5V and a pulse duration of 2ms. Press “select” using the bottom left button to start the intensity training electrical stimulation. To run a custom electrical stimulation regimen, select “2’ for the program and input the proper stimulation parameters as directed by the LCD screen prompts (Figure 7C). Press select to start stimulation.
Connect the Arduino to an oscilloscope to verify that it is outputting signals properly.
Connect custom Arduino electrical stimulator to cardiac tissue reactor and apply intensity training regimen to achieve cardiac maturation:
Using an alligator clip, connect one end to the “GND” (ground) wire of the Arduino electrical stimulator and connect the other end to one of the platinum wires in the cardiac bioreactor.
Using a separate alligator clip, connect one end to the other wire connected to pin 6 of TLV4110 (the remaining free wire) of the Arduino electrical stimulator and connect the other end to the other platinum wire in your cardiac bioreactor.
Plug the Arduino in and reset the Arduino to start the stimulation by following the instructions on the LCD screen.
CRITICAL STEP If bubbles form in the cardiac medium, disconnect the reactor and ensure none of the connections have come loose.
Analysis of mature cardiac tissues.
To measure the cardiac contractility on-line, we developed a MATLAB code for analyzing pixel movement within a predefined area in time-lapse videos of the contracting cardiac tissues (Box 3). For gene expression, protein contents, histomorphology and ultrastructure, we used tissue samples and standard methodologies [16, 19, 29, 30]. Additional methods were developed to detail the presence of T-tubules, including staining prior to permeabilization, evaluating both longitudinal and axial cross-sections, and using a virtual slide-scanner microscope for imaging. We found that immunostaining and confocal imaging of ultrastructural striations in intact tissues, rather than histological sections, is a more robust method for analyzing structural maturity. Regarding force, we used a Muscle Strip Myograph System to analyze the force generation of the cardiac tissues. For several other assays (cytometry, metabolic function using Seahorse Analyzers and, electrophysiological measurements), tissues needed to be dissociated into single cells, a process that is more difficult for mature and compact cardiac tissues. We therefore developed a papain-based dissociation to minimize cell damage and obtain a viable single cell suspension (Box 4). This enabled electrophysiological characterization, crucial for validating the function of hiPS-CM. To examine the maturity of hiPS-CM in cardiac tissues, whole-cell patch clamp recordings for action potentials and inward rectified current (IK1) current were taken. The time window for recording electrical activities in such mature hiPS-CM was narrower than in fetal cardiomyocytes, with the ideal window for mature hiPS-CM occurring within 72 hours of dissociation.
Box 3 -. Assessment of contractile motion within engineered cardiac tissues.
Methods to analyze the contractile motion of beating cardiac cells or tissues require the generation of custom data scripts or use of open-source scripts. Open-source scripts that rely on optical flow based vector mapping [32, 33] can be computationally expensive and therefore time consuming for large data files. Other approaches include analyzing the change in pixels over time in reference to a baseline frame or using edge detection methods to make a mask of the tissue and subsequently tracking the overall change in area during contracted versus relaxed states [32–34]. The pixel based approach was recently demonstrated to be comparable to contractile measurements of pillar deflection, sarcomere shortening, optical flow, and edge detection algorithms [34]. The edge detection approach defines a decrease in the area of the cardiac tissue as a contraction, while the subsequent increase in area corresponds to the cardiac tissue relaxation, to create a trace of the cardiac tissue area over time. These approaches are reliable, but can have high noise due to background movement when analyzing overall pixel motion or low detection of contractile motion when tracking the change in area overall as deciphered by the imposed mask. The limitation of these approaches is apparent when analyzing cardiac tissues with decreased contractile motion, fluctuating or uneven illumination sources during video acquisition, and low video resolution. To overcome this, our analysis utilizes both approaches to subsequently decrease the noise by only focusing on the tissue area (using edge detection techniques) and then enhance detection of the contractile motion signal by analyzing the pixel motion within this defined tissue area.
To enable analysis of the contractile motion within the cardiac tissues described herein, we provide custom Matlab code to analyze cardiac contractility based on combining both 1) cell edge detection to narrow the cardiac tissue analysis region and subsequently analyzing 2) changes in pixel motion over time, in reference to a baseline frame. Overall, the approach detailed is simple, efficient, and increases the detection of small changes in contractile motion to facilitate comparisons between tissue samples. This increase in resolution is particularly useful when analyzing the effects of drugs that reduce cardiac contractility.
To adequately assess the contractile motion at electrical stimulation frequencies of up to 6 Hz, video acquisition speeds should be set at a minimum of 100 frames per second (fps). Similarly, a minimum of 20 sec is recommended to analyze the parameters of multiple contraction cycles within each video, and capture beating abnormalities or missed beats that may arise. Because ImageJ plugins have limited available memory to run large data files, the contractility analysis script was written in Matlab to facilitate the large video files generated by high-resolution imaging at fast frame rates. The Matlab contractility code is provided in Supp Data2:
CardiacContractileMotion, analyses changes in pixel motion from a reference baseline frame within a defined region (dictated by a mask of the cardiac tissue area during a relaxed baseline state) to create a trace of pixel motion over time. The traces generated from each approach are used to calculate the resulting contractile parameters, and output them into an excel spreadsheet.
We provide our code in supplementary file Supp Data 2, with detailed instructions for use below (Supplementary Figure 1).
Equipment
Matlab (Mathworks, version 2017a used)
Computer
Videos (.tif or .nd2 formats)
Procedure
Load “CardiacContractileMotion.m” file and set matlab path to the folder containing the files in the supplementary file Supp Data2.
Within the Matlab Editor window, input the data directory where your file can be found on line 5 of the code within the single quotation marks, followed by a “\” as follows:
% input file path information
dataDirectory = ‘D:\Name of the folder containing your video\’;
Within the Matlab Editor window, input the filename of your file on line 6 of the code within the single quotation marks, followed by a “.tif” as follows:
% input file path information
scanName = ‘Name of the file with your video.tif’;
Specify the input data format by setting the “andorFlag” variable (line 9 of code) to 0 if loading tiff stack data (.tif) or set the “andorFlag” variable to 1 if loading Andor Zyla data (.nd2).
Save the file and press “Run” to run the code.
Specify the framerate (frames per second) used when acquiring the video and input into the dialog box. Press ok.
Choose the region of interest (ROI): Select “1” if there is one tissue in the field of view and using the cursor select the top left and bottom right region of interest containing the tissue.
Choose the baseline frame: Select the time where the baseline occurs (relaxed state) and enter it into the popup window. Note: this is to correct for a video being started in the middle of a contraction. An example of a proper input for the baseline frame is detailed in red below:
Choose the peak amplitude threshold by selecting a value above which there are only peaks. Note that this is to facilitate samples with high noise. An example of a proper input for the peak amplitude threshold is detailed in red below:
Troubleshooting:
if too many peaks are found/not enough peaks are found: change the “peakDistance” value (line 205 of code) to a higher value (if too many peaks are found) or a lower value (if not enough peaks are found).
Results:
The code will determine the beat frequency, contraction parameters (10 %, 50 % and 90 % contraction times indicated in green), relaxation parameters (10 %, 50 % and 90 % relaxation times indicated in red), peak width, time between beats, and number of beats and output these variables into an excel spreadsheet, images of the trace with and without contraction parameter lines, and a histogram plot of the peak to peak times.
APPLICATIONS OF THE PROTOCOL
The described methods result in a mature human cardiac tissue grown in vitro, suitable for many applications including drug screening, disease modeling, and developmental biology studies. The need for a mature cardiac phenotype is most critical when studying contractile dysfunction and diseases related to contractility. A mature cardiac phenotype also provides a more accurate representation of the adult cardiac function for drug screening. In particular, there is a need for human tissue models capable of predicting drug induced cardiac arrhythmias for drugs in the development pipeline. Mature human cardiac tissues can also be implemented to alleviate the growing burden of heart failure, through testing of potential therapeutics in a patient-specific manner. The use of hiPS-CM enables inclusion of patients with genetic mutations with known causal relationships to heart failure, such as myosin heavy chain 7 (MYH7) mutations that lead to dilated cardiomyopathy and eventual heart failure [31]. The mechanisms underlying cardiac development can also be investigated to better understand congenital heart malformations. The methods described herein provide a 3D human model for studying cardiac development, particularly during the transition from an immature to an adult-like phenotype. The mechanistic approaches may help elucidate the signals governing cardiac development and cardiac disease. Thus, a mature cardiac tissue model is necessary to capture transitions to the fetal-like phenotype and negative force-frequency responses in cardiac disease states. The cardiac maturation protocol is applicable to a number of other experimental setups. Overall, the key consideration when developing and utilizing a cardiac bioreactor is to mimic the native environment (i.e. hydrogel matrix, culture media supplements, passive tension, electromechanical stimulation).
COMPARISON WITH OTHER METHODS
Tissue engineering is becoming increasingly successful at more authentically representing the native tissue milieu [2, 17]. Instead of attempting to recapitulate the entire complexity of an organ, a reachable goal would be to replicate tissue-specific architecture and a subset of the most relevant functions, in the form of the simplest functional tissue unit, as a predictive screening platform [14]. Human cardiac muscle, engineered with the biological fidelity necessary for predictive use in high-throughput settings, would be transformative to drug testing and modeling of disease. Despite major advances [2–5], engineered tissues formed using other methods do not physiologically emulate the adult heart, largely due to the immature phenotype of human cardiomyocytes derived from hiPS cells. The method detailed here represents the only current protocol capable of recapitulating the hallmarks of adult cardiac muscle within hiPS-CM based engineered tissues: excitation-contraction (E-C) coupling (requiring networks of T-tubules), calcium homeostasis (requiring a functional sarcoplasmic reticulum), and positive force-frequency relationships [6].
The protocol described here is biomimetic in nature, but also forces the cardiomyocytes to adapt to increasing electromechanical demands, inducing biological adaption towards a more mature phenotype to sustain function. Cardiomyocytes in the heart are exposed to electrical signals from three weeks into gestation throughout life, with cascades of Ca2+ mediated events triggering the ensuing mechanical contractions. Replicating these combinatory signals in vitro transitioned hiPS-CM towards a physiologically relevant adult-like phenotype. The maturation demonstrated here via intensity training results in calcium homeostasis and EC coupling through the combined development of functional sarcoplasmic reticulum for intracellular Ca2+ storage, and enhanced ultrastructural organization for utilizing this Ca2+ storage for increased contractile efficiency. As in the native heart, the translation of synchronous cell membrane depolarization into contractile forces were recapitulated, enabling control of the rate of contraction where each electrical signal forced the cardiac muscle to exert energy when contracting against mechanical forces imposed by the elastic pillars. Cell alignment and force generation lead to physiological hypertrophy, with post-natal increases in cardiomyocytes mass and establishing a sarcomere length optimized for force production [6]. By mimicking this process in vitro at an increased level of electromechanical conditioning, and combining the best cardiac tissue engineering approaches of others in the field [2–5, 8], this protocol is able to mature the engineered cardiac tissues beyond currently achievable levels and reverse the characteristically negative force-frequency relationships seen in hiPS-CM.
ADVANTAGES AND LIMITATIONS.
The advantages of the described method are that it enables the generation of human models of advanced maturity to study cardiac tissues of health and disease (Table 1). The timeline for achieving maturity is only four weeks, which is efficient considering the maturity attained was benchmarked to be beyond that of the fetal phenotype seen during 14–19 weeks of in vivo development. However, the maturity level achieved still is not fully at the level of an adult in all parameters. Specifically, the force values generated are lower than would be expected for adult cardiomyocytes. Additionally, the method requires a high number of cells during the setup, which is a disadvantage for its utilization in high throughput screening.
Table 1.
Comparison of different cardiac models generated with cells at different time points and submitted to different environments
| Late cells | Early Cells | |||||
|---|---|---|---|---|---|---|
| Intensity stimulation |
Control | Constant stimulation |
Intensity stimulation |
|||
| CELL MORPHOLOGY | ||||||
| Cell size | + | ++ | + | ++ | +++ | +++ |
| T-tubules | - | - | - | - | ++ | +++ |
| Intercalated discs | - | - | - | + | ++ | +++ |
| Sarcomere length | ++ | - | - | + | ++ | +++ |
| Membrane potential | + | + | + | ++ | +++ | +++ |
| METABOLISM | ||||||
| Primary metabolic function | glycolysis | glycolysis | glycolysis | glycolysis | fatty acid oxidation | fatty acid oxidation |
| Mitochondria density | + | + | + | + | ++ | +++ |
| PHYSIOLOGY | ||||||
| Force- frequency relationship | - | - | - | - | + | + |
| Calcium- induced response |
+ | + | + | ++ | +++ | +++ |
| Isoproterenol response | lonotropic, lusitropic chonotropic |
lonotropic, lusitropic chonotropic |
||||
| GENE EXPRESSION | ||||||
| Gene Expression Profile |
Fetal-like | Fetal-like | Fetal-like | Fetal-like | Adult-like | Adult |
EXPERIMENTAL DESIGN
This protocol, outlined in Figure 1, describes a step-by-step process for cell preparation, engineered cardiac tissue formation, fabrication of bioreactors used to generate and mature the cardiac tissues, the electromechanical stimulation parameters for accelerated tissue maturation, and the analysis techniques that were optimized specifically for mature engineered cardiac tissues.
The first steps involve expanding, differentiating and culturing the cells needed to make the cardiac tissues (cardiomyocytes and fibroblasts). There are multiple cardiac differentiation techniques that relatively easily produce cardiac troponin-T positive (cTnT+) cells from hiPS cells, by mimicking the embryonic development signals that induce mesoderm and cardiac specification. In our previous work, we differentiated hiPS cells into cardiomyocytes using multiple methods, before adopting the GiAB protocol developed in Sean P. Palecek’s lab where directed cell differentiation is achieved using glycogen synthase kinase (Gsk) 3 inhibitor, Activin A, and bone morphogenic protein 4 (BMP4) [7]. During differentiation, cells were cultured in RPMI 1640 medium supplemented with B27™ without insulin, ascorbic acid or antibiotics. Over the first 24 hours (hr), this base medium was supplemented with activin A and BMP4. From 24 to 72 hr, it was supplemented with vascular endothelial growth factor (VEGF165). Beyond 72 hr, supplements were not added. In recent studies, we routinely differentiate hiPS cells using the chemically defined protocol developed in Joseph C Wu’s lab [4]. This protocol needs fewer medium components to successfully differentiate hiPS cells and does not require the strict 24 hr time point characteristic of the GiAB protocol. The chemically defined medium described by Wu’s article (CDM3) consists of RPMI 1640 medium supplemented with human recombinant albumin and ascorbic acid. In the first 48 hr, this base medium is supplemented with CHIR 99021, a potent and highly selective inhibitor of Gsk 3, to activate the Wnt/beta-catenin signaling pathway. From 48 to 96 hr, the medium is changed to CDM3 supplemented with Wnt-C59, a Wnt/beta-catenin pathway inhibitor. Beyond this time point, cells were cultured in the base differentiation medium. This protocol provided us with a reproducible and scalable method for generating cardiomyocytes and is the protocol detailed herein. Overall, any cardiac differentiation that results in at least 85% of hiPS-CM efficiency can be utilized for the tissue engineering protocol proposed here. We found that the cardiac differentiation protocols that use cell monolayers are more reproducible than those using embryoid bodies (EB), and that dissociation of cell monolayers is more robust and less damaging to the cells. Cardiomyocytes are harvested at an early-stage (day 10–12) of cardiac differentiation. At this stage, the collagenase digestion times needed to obtain a single-cell suspension are relatively short compared to later cell differentiation stages, and the cells are highly responsive to external stimuli. This timing is critical for applying electromechanical stimulation protocols of an increasing intensity, as required for cardiac maturation. Cardiac tissues formed from hiPS-CM beyond day 28 were not as responsive to the electromechanical stimuli, and lacked the adaptive ability to mature in response to the stimuli as demonstrated for early-stage hiPS-CM.
To form cardiac tissues, three bioreactor platforms were developed: elastomeric pillars for tissue attachment, a cardiac tissue formation platform, and a cardiac maturation platform (Figure 5). All bioreactor components were machined in house, but can also be outsourced to a machine shop for fabrication if machining expertise or equipment are limited. Elastomeric pillars for tissue attachment are formed by centrifugal casting of PDMS around a polycarbonate supportive structure that the resulting pillars are later attached to. The polycarbonate supportive structure was designed with mating features to facilitate proper alignment of the pillars within the bioreactor chambers. A two-part delrin mold contains the negative imprint of the pillar structure on either side so that both sides of the delrin mold can be closed around the polycarbonate supportive structure to leave a negative area in the shape of the elastomeric pillars where PDMS is inserted (Figure 5C). This mold enables easy removal of the cured PDMS and allows repeated exposure to high temperatures without deforming. The molds are tightened with screws to prevent flashing of PDMS around the pillars (Figure 5I). The mold contains a reservoir at the top to facilitate loading of PDMS into the mold. Injection molding of PDMS requires high pressure to push the polymer into the narrow channels of the mold. Centrifugal force was chosen to push the PDMS into the delrin mold, because it’s an easy and accessible method that utilizes a common piece of lab equipment in cell culture-based labs.
To produce fibrin gels consistently around the elastomeric pillars, we developed a cardiac tissue formation platform, machined out of biocompatible polycarbonate and autoclave-sterilized to facilitate ease of use (Figure 5E). The platform contains two rows of three cylindrical wells that each hold a desired amount of hydrogel for tissue formation. Above the wells are shelves with mating features that match those of the pillar polycarbonate supportive structure to facilitate proper positioning of the pillars in each well. The body of the bioreactor is coated with a hydrophobic solution to prevent the hydrogel from attaching to the sides of the wells (Figure 6A). The PDMS pillars are inserted after this step so that they are not coated (Figure 6B).
Figure 6. Overview of cardiac tissue formation.
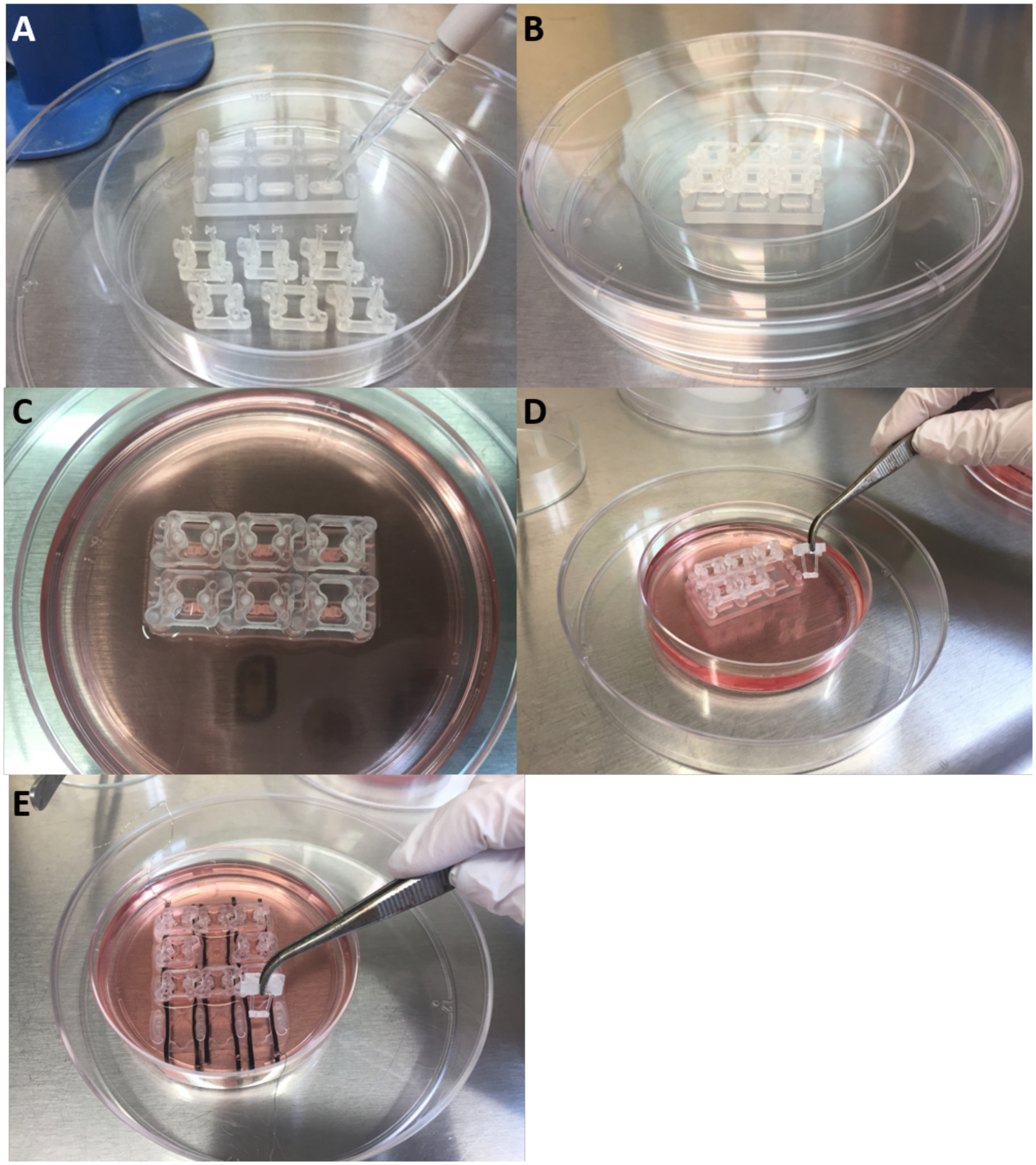
A) Hydrophobic coating of tissue formation chamber platform. B) PDMS pillars are placed back into tissue formation reactor after coating. C) The cardiac tissue is formed around the pillars and let compact over 7 days. D-E) The cardiac tissues are transferred to the cardiac maturation platform.
The dissociated hiPS-CM and fibroblasts are combined at a 75:25 ratio in a fibrinogen solution. Half of the overall amount of thrombin needed per tissue is added to the bottom of each tissue formation bioreactor well. The cell suspension in fibrinogen is immediately added to each well and subsequently crosslinked by adding the remaining half of the thrombin. Once the cardiac constructs have fully crosslinked, cardiac medium is added and supplemented with aprotinin to slow the degradation of the fibrin hydrogel (Figure 6C).
The hydrogel compacts over 7 days to form a cardiac construct stretched between the pillars. It is then transferred into the cardiac maturation bioreactor where it is maintained in a larger amount of culture medium and electromechanically stimulated at an increasing intensity (Figure 6D and E). The bioreactor is placed into a 100 mm diameter petri dish and enclosed (for sterility during transfer to and from the incubator) in a covered 150 mm diameter petri dish.
To mature the cardiac tissues, expose to electromechanical stimulation at an intensity that increases from 2 Hz by 0.33 Hz per day until the frequency of 6 Hz is reached. Following this regimen, the stimulation frequency is set back to 2 Hz and the tissues are stimulated for an additional 7 days. The use of this regimen was largely influenced by the pioneering work of the Radisic lab, in which they used electrical stimulation at rates that increased up to either 3 or 6 Hz to mature cardiac biowires [8]. As the best results were achieved for the 6 Hz group, we adopted this frequency as the high end for electromechanical stimulation.
The resulting cardiac tissue maturity can then be assessed for functionality (video-based analysis of contractile motion, response to electrical pacing and voltage threshold, force-frequency and force-length measurements), calcium handling (calcium transients, drug response) and ultrastructure (paraffin embedded immunohistochemistry and slide-scanner imaging of tissue sections, immunostaining and confocal imaging of whole tissue constructs). To enable analysis of metabolic functionality, electrophysiology, and cell populations the cardiac tissues should be dissociated into single-cells using a papain dissociation protocol. This dissociation protocol was developed specifically for mature cardiac tissues and optimized to reduce the time required for dissociated and minimize damage to the cells. Here we describe detailed protocols for deriving, maturing and analyzing the engineered cardiac tissues at cellular and tissue levels, on-line and in end-point assays.
MATERIALS
Cells
Human induced pluripotent stem (hiPS) cells:
We have successfully used C2A, WTC-11, IMR90 and BS2 cell lines obtained through material transfer agreements from S. Duncan, University of Wisconsin (C2A line), B. Conklin, Gladstone Institute (WTC-11 line) and M.Y., Columbia University (IMR90 line). BS2 was derived at Columbia University. With informed consent, one vial of peripheral blood was taken from a healthy volunteer. The Columbia University’s Stem Cell Core Facility reprogrammed cells and characterized the final hiPS cells. The protocol for the BS2 cell line was approved by a Columbia University Institutional Review Board. CAUTION The cell lines should be regularly tested for mycoplasma, karyotyped and assessed for expression of pluripotency markers. CAUTION When handling cells and tissues, take the necessary precautions, by wearing personal protective equipment and performing the work within a class II biosafety cabinet.
Normal Human Dermal Fibroblasts (NHDF, Lonza, cat. no. CC-2509)
CAUTION The cells should be regularly tested for mycoplasma, karyotyped and assessed for expression of fibroblasts markers. CAUTION When handling cells and tissues, take the necessary precautions, by wearing personal protective equipment and performing the work within a class II biosafety cabinet.
Reagents
CAUTION When handling reagents, use proper personal protective equipment and refer to specific reagent material safety data sheets for additional information.
CAUTION Room temperature, when mentioned, is defined as 20 – 25 °C.
CRITICAL We have not tested medium, supplements or small molecules from other vendors.
mTeSR™1 medium (StemCell Technologies, cat. no. 85850)
Penicillin-streptomycin (Gibco by Life Technologies, cat. no. 15070063)
Y-27632 dihydrochloride (Tocris, cat. no. 1254)
Matrigel® Growth Factor Reduced Basement Membrane Matrix (Corning, cat. no. 354230)
100% Ethanol (Decon laboratories, cat. no. 2701)
Phosphate-Buffered Saline (PBS; Corning, cat. no. 21–040-CV)
0.5 M EDTA (Thermo Fisher Scientific, cat. no. 15575020)
RPMI 1640 Medium (Gibco by Life Technologies, cat. no. 11875093)
L-Ascorbic acid 2-phosphate sesquimagnesium salt hydrate (Sigma-Aldrich, cat. no. A8960)
Recombinant human albumin (Sigma-Aldrich, cat. no. A9731)
CHIR 99021 (Tocris, cat. no. 4423)
Wnt-C59 (Tocris, cat. no. 5148)
DMEM, high glucose (Thermo Fisher Scientific, cat. no. 12430–054)
Fetal Bovine Serum (Atlanta Biologicals, cat. no. S11150)
Trypsin-EDTA (Gibco by Life Technologies, cat. no. 25200072)
Sylgard® 184 Silicone Elastomer Kit (Krayden, cat. no. DC2065622)
Collagenase, Type 2 (Worthington, cat. no. LS004176)
HBSS, no calcium, no magnesium (Gibco by Life Technologies, cat. no. 14170112)
Fibrinogen from human plasma (Sigma-Aldrich, cat. no. F3879)
Thrombin from human plasma (Sigma-Aldrich, cat. no. T6884)
PLL grafted with PEG (PLL-g-PEG, Surface Solutions, cat. No. PLL(20)-g[3.5]- PEG(2))
Aprotinin from bovine lung (Sigma-Aldrich, cat. no. A3428)
Tyrode’s solution (Sigma, cat.no. T2145)
B-27™ Supplement (50X), serum free (Gibco by Life Technologies, cat. no. 17504044)
Fluo-4 Direct Calcium Assay Kit (ThermoFisher, cat. no. F10471)
(−)-Blebbistatin (Sigma-Aldrich, cat. no. B0560)
Papain Dissociation System (Worthington-Biochem, cat. no. LK003150)
20 unit/ml papain (Sigma-Aldrich, cat. no. 76220)
Barium chloride (BaCl2, Sigma-Aldrich, cat. no. 342920)
Nisoldipine (Sigma-Aldrich, cat. no. N0165)
E-4031 dihydrochloride (TOCRIS, cat. no. 1808)
Potassium D-gluconate (Sigma-Aldrich, cat. no. G4500)
Adenosine 5’-triphosphate magnesium salt (Sigma-Aldrich, cat. no. A9187)
Guanosine 5’-triphosphate sodium salt (Sigma-Aldrich, cat. no. G8877)
Phosphocreatine disodium salt hydrate (Sigma-Aldrich, cat. no. P7936)
Ethyline glycol-bis(2-aminoethylether)-N,N,N’,N’-tetraacetic acid (EGTA, Sigma-Aldrich, cat. no. E3889)
Calcium chloride (CaCl2, Sigma-Aldrich, cat. no. 21115)
Sodium chloride (NaCl, Sigma-Aldrich, cat. no. S7653)
Potassium chloride (KCl, Sigma-Aldrich, cat. no. P9333)
Magnesium chloride (MgCl2, Sigma-Aldrich, cat. no. M2670)
D-(+)-glucose (Sigma-Aldrich, cat. no. G7021)
MEM Non-essential amino acid (Thermo Fisher, cat. no. 11140050)
N-Methyl-D-glucamine (Sigma-Aldrich, cat. no. 66930)
2-Mercaptoethanol (Gibco by Life Technologies, cat no. 21985–023)
L-Cystein Hydrochloride monohydrate (Sigma-Aldrich, cat. no. C7880)
Earle’s Balanced Salt Solution (EBSS, Thermo Fisher Scientific, cat. no. 24010043)
Paraformaldehyde Solution 4% in PBS (Santa Cruz Biotechnology, cat. no. sc-281692)
HEPES Buffer (Corning, cat. no. 25–060-CI)
UltraPure™ DNase/RNase-Free Distilled Water (ThermoFisher Scientific, cat. no. 10977–015)
Bovine Serum Albumin (BSA; Millipore Sigma, cat. no. 820451)
Formalin (Fisher Scientific, cat. no. SF100–4)
Citrisolv (Decon Laboratories Inc, cat. no. 1601)
Dako target retrievel solution (Agilent, cat. no. S2368)
Triton™ X-100 (Sigma-Aldrich, cat. no. X100)
Vectashield (Vectorlabs, cat. No. H-1200–10)
Tween® 20 (Sigma-Aldrich, cat. no. P1379)
Dimethyl Sulfoxide (DMSO, Corning, cat. no. MT-25950CQC)
BD Cytofix/Cytoperm Kit (BD Biosciences, 554714)
Geltrex (Thermo Fisher, cat. no. A1413302)
DMEM/F12-GlutaMax Supplement (Thermo Fisher, cat. no. 10565018)
Goat serum (Thermo Fisher, cat. no. 16210072)
Antibodies
Anti-sarcomeric α-actinin (1:200, Abcam, cat. no. ab9465)
Anti–cardiac troponin T (cTnT, 1:100, Thermo Scientific, cat. no. MS-295-P1)
Anti-ryanodine receptor 2 (1:100, Abcam, cat. no. ab2827)
Anti-CACNA1C (1:200, Abcam, cat. no. ab58552)
Anti-BIN1 (1:100, Abcam, cat. no. ab137459)
Anti-mitochondria (1:50, Abcam, cat. no. ab3298)
Anti-OXPHOS (1:100, Acris, cat. no. MS601–720)
Alexa Fluor™ 350 Phalloidin (1:40, when dissolved in 1.5 mL methanol according to
manufacturer protocol, Thermo Fisher, cat. no. A22281)
NucBlue™ Fixed Cell ReadyProbes™ Reagent (Molecular Probes, cat. no. R37606)
Wheat Germ Agglutinin (WGA), Alexa Fluor™ 488 Conjugate (1 ug/mL, Molecular Probes, cat. no. W11261)
Di-8-ANEPPS (1:100, Life Technologies, cat. no. D-3167)
Donkey anti-mouse, Alexa Fluor 488 (1:400, Invitrogen, cat. no. A21202)
Goat anti-rabbit, Alexa Fluor 568 (1:400, Invitrogen, cat. no. 81–6114)
Goat anti-mouse, Alexa Fluor 635 (1:400, Invitrogen, cat. no. A31574)
Anti-vimentin (1:5000, Abcam, cat. no. ab195878, clone V9, Alexa Fluor 647)
Anti-Cardiac Troponin T antibody (1:50, Abcam, cat. no. ab105439, clone 1C11, FITC)
Anti-Human CD42b (1:200, BD Pharmingen, cat. no. 551061, clone HIP1, APC)
Anti-MLC2a 1:100, BD Pharmingen, cat. no. 565496, Clone S58–205, RUO)
Anti-MLC2v (1:100, Miltenyi Biotec, cat. no. 130–106-184, Clone REA401)
Alexa Fluor® 488 goat anti-mouse (1:2000, Abcam, cat. no. ab150113, IgG (H&L))
For digestion to single cells
Cardiac tissue dissociation solution
Glass-bottom dish coating solution
Post-papain digestion cardiomyocyte culture medium
Equipment
Water bath (Fisher Scientific, 2302)
Liquid nitrogen tank (Thermo Fisher, CY509106)
Biological safety cabinet (Labconco, 3621304)
15 mL Centrifuge tube (Corning, cat. no. 352097)
50 mL Centrifuge tube (Corning, cat. no. 352098)
6 Well clear flat bottom TC-treated multiwell cell culture plate (Corning, cat. no. 353046)
T75 Tissue culture treated flask (Fisher Scientific, cat. no. 353136)
T150 Tissue culture treated flask (Fisher Scientific, cat. no. 355001)
35 mm glass-bottom dish (MatTek, cat. no. P35G-1.5–7-C)
Automated Cell Counter (Thermo Fisher, cat. no. C10281)
Metallized Hemocytometer (Hausser Scientific, cat. no. 3200)
Centrifuge (Eppendorf, cat. no. B1–022628092)
Incubator (Thermo Fisher, HeRAcell® 150i)
Inverted Microscope (Nikon, TMS and Olympus, IX81))
Single-Channel Pipettors (Thermo Fisher, cat. no. 07–764-701, 07–764-702, 07–764-703, 07–764-705)
Pipettors Barrier Tips (Denville Scientific, cat. no. P1126, P1122, P1096-FR, P1121)
Pipet Controller (Thermo Fisher, cat. no. 07–202-350)
Serologic Pipets (Thermo Fisher, cat. no. 357558, 357551, 357543, 357525)
50 mL 0.22 μm Steriflip filter (Milipore Sigma, cat. no. SCGP00525)
500 mL Vacuum filter/Storage Bottle System (Corning, cat. no. 431097)
CNC milling machine using uncoated carbide endmills (Haas Automation, OM2 3 axis)
Polycarbonate (McMaster Carr, cat. no. 8574K321)
Delrin Acetal resin (McMaster Carr, cat. no. 8575K142)
Vacuum chamber (Corning, cat. no. 3121–150)
Lab oven (Thermo Fisher, cat. no. 51028112)
Autoclave-Stem Sterilizer (Tuttnauer, cat. no. 2540E)
1.5 mm Carbon rods (Graphitestore, cat. no. BL001601)
Cell Culture Dish (Corning, cat. no. 430599, 430591)
Stimulator (Grass, cat. no. S88X and ADInstruments, cat. no. DMT100273)
Platinum wire (Ladd Research, cat. no. PW3N5)
Forces (Fine Science Tools, cat. no. 11274–20, 1100–12)
Alligator test leads (uxcell, cat. no. 0700955248310)
Camera (Andor, cat. no. Zyla 5.5 sCMOS)
Muscle Strip Myograph System (DMT, cat. no. 840MD)
Fluo-4-Direct™ Calcium Assay Kit (ThermoFisher, cat. no. F10471)
Hydrophobic barrier pen (Vector Laboratories, cat. no. H-4000)
Slide Scanner microscope (Virtual Slide Microscope VS120, Olympus)
Confocal microscope (Leica TCS SP5 Confocal and Multiphoton Microscope and
Olympus Fluoview FV1000 Confocal Microscope were used)
Sylgard® 184 Silicone Elastomer Kit (Krayden, cat. no. DC2065622)
Myograph chamber stimulation lids (ADInstruments, cat. no. DMT100238)
PowerLab 4/35 (ADInstruments, cat. no. PL3504)
Patch-clamp amplifier (Molecular Devices, MultiClamp 700B and Digidata 1440)
Inverted microscope (Nikon, Ti-U)
EMCCD camera (Photometrics Evolve Intelligent, 512X512 EMCCD Digital Monochrome, cat. no. EVO-512-M-FW-16-AC)
Micropipette puller (Sutter Instrument, Model P-97)
Borosilicate glass (Sutter Instrument, cat. no. BF150–110-10)
Heating system (Warner instrument, cat. no.TC324C)
Quick exchange platform for 35–40 mm dish (Warner instrument, cat. no. QE-1)
Flow cytometry tubes (Corning, cat. no. 352235)
Flow cytometer (FACSCanto II; BD Bioscience)
Arduino (Mega 2560 Rev3, Genuino)
Software
FlowJo (FlowJo, LLC)
ImageJ (National Institute of Health)
MATLAB (MathWorks)
pClamp (Molecular Devices)
MetaMorph Microscopy Automation and Image Analysis Software (Molecular Devices)
LabChart (ADInstruments)
Arduino IDE (Genuino)
SolidWorks (Dassault Systèmes)
REAGENTS
hiPS medium
Add together mTeSR™1 basal medium, supplemented with 5X mTeSR™1 and 1 % penicillin-streptomycin. Filter-sterilize the medium by passing it through a 0.22 μm filter. Store at 4 °C for up to 1 week. Pre-warm to 37 °C before use.
Y-27632 dihydrochloride stock
Dissolve at 5 mM in UltraPure™ DNase/RNase-Free Distilled Water. Make 150 μL aliquots and store them at −20 °C for up to 1 month. Avoid thaw/freeze cycles.
Matrigel® growth factor reduced basement membrane matrix stock
Thaw matrigel at 4 °C. Make 600 μL aliquots and store them at −20 °C. Avoid thaw/freeze cycles and work with it on ice. CRITICAL Matrigel will rapidly begin to solidify near room temperature (20–25 °C), so it is vital to work quickly, on ice and with cold tips.
0.5 mM EDTA solution
Add 0.5 M EDTA to PBS to make a 0.5 mM solution. Filter-sterilize by passing through a 0.22 μm filter and store at 4 °C. Store at 4 °C for up to 6 months. Pre-warm to 37 °C before use.
Cardiac differentiation medium (CDM)
Add together RPMI 1640 medium, with 213 μg/mL LAscorbic acid 2-phosphate, 500 μg/mL recombinant human albumin and 1 % penicillin-streptomycin. Filter-sterilize medium by passing it through a 0.22 μm filter. Store at 4 °C for up to 1 week. Pre-warm to 37 °C before each medium change.
L-Ascorbic acid stock
Dissolve at 21.3 mg/mL in UltraPure™ DNase/RNase-Free Distilled Water. Filter-sterilize by passing it through a 0.22 μm filter. Make 5 mL aliquots and store at −20 °C for up to 1 month. Avoid thaw/freeze cycles.
Recombinant human albumin stock
Dissolve at 50 mg/mL in UltraPure™ DNase/RNase-Free Distilled Water. Filter-sterilize it by passing it through a 0.22 μm filter. Make 5 mL aliquots and store at −20 °C for up to 6 months. Avoid thaw/freeze cycles.
CHIR 99021 stock
Dissolve at 3 mM in DMSO. Make 150 μL aliquots and store at −20 °C for up to 1 month. Avoid thaw/freeze cycles.
Wnt-C59 stock
Dissolve at 2 mM in DMSO. Make 150 μL aliquots and store at −20 °C for up to 1 month. Avoid thaw/freeze cycles.
Fibroblast medium
Add together high glucose DMEM, 5 % FBS and 1 % penicillin-streptomycin. Filter-sterilize medium by passing it through a 0.22 μm filter. Store at 4 °C for up to 1 week. Pre-warm before each medium change.
Collagenase II solution
Dissolve at 2 mg/mL in HBSS. Mix thoroughly. Filter-sterilize by passing through a 0.22 μm filter. Make fresh each time and pre-warm to 37 °C before use.
Fibrinogen stock
Dissolve at 33 mg/mL in 20 mM HEPES buffer in 0.9 % saline, over several hours at 37 °C. Filter-sterilize by passing through a 0.22 μm filter and store at 4 °C. Make 1 mL aliquots and store at −20 °C for up to 2 months. Avoid thaw/freeze cycles.
Thrombin stock
Dissolve at 25 U/mL in 0.1 % BSA in PBS. Mix thoroughly. Make 100 μL aliquots and store at −80 °C for up to 1 year. Avoid thaw/freeze cycles. CRITICAL Thrombin solution absorbs to glass, so it is recommended to aliquot solutions in plastic tubes.
PLL-g-PEG
Dissolve at 1 mg/mL in HEPES buffer. Mix thoroughly. Filter-sterilize by passing it through a 0.22 μm filter and store at 4 °C for up to 1 month.
Aprotinin stock
Dissolve at 33 mg/mL in diH2O. Mix thoroughly. Filter-sterilize it by passing through a 0.22 μm filter. Make 50 μL aliquots and store at −20 °C for up to 1 year. Avoid thaw/freeze cycles
Cardiac medium
Combine RPMI 1640 medium, 213 μg/mL L-Ascorbic acid 2-phosphate, 2 % B-27™ Supplement and 1 % penicillin-streptomycin. Filter-sterilize medium by passing through a 0.22 μm filter. Store at 4 °C for up to 1 week. Pre-warm to 37 °C before each medium change.
Blebbistatin Solution
Mix 10 μL of blebbistation into 7 mL of pre-warmed (44 °C), vigorously agitated, oxygenated Tyrode’s Solution. Add extra Tyrode’s Solution until a final volume of 17 mL is reached, at a final concentration of 10 μM. Make fresh each time.
250 mM Stock Probenecid Solution
Add 1 mL of Fluo-4 Direct calcium assay buffer to a 77 mg vial of water-soluble probenecid. Vortex until dissolved. Store at 20 °C for up to 6 months.
2X Fluo-4 Direct™ Calcium Reagent Loading Solution
Add 10 mL Fluo-4 Direct™ calcium assay buffer and 200 μL of 250 mM Stock Probenecid Solution (prepared above) to one bottle of Fluo-4 Direct™ calcium reagent. Vortex briefly and allow the prepared solution to sit for 5 min to ensure it is completely dissolved and then vortex again before proceeding. Make fresh each time.
Glass dish coating solution
Dissolve Geltrex in DMEM/F12-GlutaMax-I at a 1/1000 dilution. Prepare fresh each time.
Modified Tyrode’s Solution
Add together 129 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 30 mM Glucose and 25 mM HEPES Buffer. Check that the pH is 7.4 and adjust accordingly. Add 2 % B-27™ Supplement. Prepare fresh each time.
Quenching solution
Add together 0.5M NH4CL, PBS and 0.1% BSA. Prepare fresh each time.
Blocking solution
Add together PBS, 0.1 % BSA and 10 % goat serum and store aliquots at −20 °C for up to 1 year.
Cardiac tissue dissociation solution
Mix together 20 unit/ml papain, 1.1 mM EDTA, 67 μM 2-Mercaptoethanol, 5.5 mM L-Cystein HCl, and 1x EBSS. Sterile filter the medium by passing through a 0.22 μm filter. Prepare fresh each time and use immediately.
Post-papain digestion cardiomyocyte culture medium
Mix together 500 mL DMEM/F12-GlutaMax- I, 56 mL Fetal bovine serum, 5.6 mL Penicillin/Streptomycin, 5.6 mL MEM Non-Essential Amino Acid, 3.5 μl 2-Mercaptoethanol. Sterile filter the medium by passing through a 0.22 μm filter. Store at 4 °C for up to 1 week. Pre-warm before each medium change.
External action potential recording solution
Mix together 140 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 10 mM D-glucose, 1.8 mM CaCl2, 10 mM HEPES. Adjust pH to 7.4 using NaOH at 25 °C. Solution can be stored at 4 °C for up to 6 months.
Internal (pipette) action potential recording solution
Mix together 120 mM K D-gluconate, 25 mM KCl, 4 mM MgATP, 2 mM NaGTP, 4 mM Na2-phospho-creatin, 10 mM EGTA, 1 mM CaCl2, and 10 mM HEPES. Adjust pH to 7.4 using KOH at 25 °C. Solution can be stored at −80 °C for up to 2 years or at 4 °C for up to 7 days.
External IK1 recording solution
Mix together160 mM NMDG, 5.4 mM KCl, 2 mM MgCl2, 10 mM D-glucose, 10 μM nisoldipine, 1 μM E-4031, and 10 mM HEPES. Adjust pH to 7.2 using HCl at 25 °C. Solution can be stored at 4 °C for up to 6 months.
Internal IK1 recording solution
Mix together 150 mM K-gluconatem, 5 mM EGTA, 1 mM MgATP, and 10 mM HEPES. Adjust pH to 7.2 using KOH at 25 °C. Solution can be stored at −80 °C for up to 2 years or at 4 °C for up to 7 days.
XF Assay Medium
For 100 mL of XF medium add to 100 mL XF Base Medium 400 μL 2.5 M glucose, 1 mL 100 mM sodium pyruvate, and 1 mL 200 mM L-glutamine (yielding a final concentration of 1 mM pyruvate and 2 mM glutamine). Warm the medium and subsequently adjust the pH to 7.4 with pH meter by adding 1N NaOH in 2 μL increments. Sterile filter the medium by passing through a 0.22 μm filter prior to use. Store at 4 °C for up to 18 months.
Oligomycin stock
Dissolve oligomycin in DMSO to yield a final concentration of 100 μM and store aliquots at −20 °C for up to 3 months.
FCCP stock
Dissolve FCCP in DMSO to yield a final concentration of 100 μM and store aliquots at −20 °C for up to 1 month.
Rotenone/Antimycin A stock
Dissolve Rotenone and Antimycin A in DMSO to yield a final concentration of 50 μM and store aliquots at −20 °C for up to 1 month.
Oligomycin
Add 450 μL of Oligomycin stock to 2350 μL XF Assay medium to yield 3 mL of Oligomycin at a final concentration of 1.5 μM. Prepare fresh each time.
FCCP
Add 700 μL stock in 2800 μL assay medium to yield 3 mL of FCCP a final concentration of 1.5 μM. Prepare fresh each time.
Rotenone/Antimycin A
Add 300 μL Rotenone/Antimycin A stock to 2800 μL XF Assay medium to yield 3 mL of FCCP at a final concentration of 1.5 μM. Prepare fresh each time.
Perm Wash buffer
Dilute Cytoperm reagent 1:10 with water to make working Perm Wash buffer. Prepare fresh each time.
70 % ethanol
Dilute 100% ethanol 7:10 with water to make working 70 % ethanol solution. Can be stored at room temperature for up to 1 year.
COATING PROTOCOLS
Matrigel coating of cell culture plates
Thaw Matrigel growth factor reduced basement membrane matrix at 4 °C. Dilute 1:60 in ice-cold RPMI 1640. Coat the growth surface of the plates using 1 mL per 9.5 cm2. Incubate plates at 37 °C for 30 min, or at room temperature for 60 min. After coating, the plates can be stored at 4 °C for a couple of days before being used. Aspirate excess Matrigel right before use. CRITICAL Matrigel will rapidly begin to solidify near room temperature, so it is vital to work quickly, on ice and with cold tips.
PLL-g-PEG coating of cardiac tissue formation platform
After autoclaving bioreactors (dry cycle, 121° C for at least 30 min using saturated steam under at least 15 psi of pressure, 30 min drying time), remove pillars from the platform and add 100 μL of PLL-g-PEG to each well of the bioreactor platform. Incubate at room temperature for 1 hr. Wash the wells with 100 μL of PBS twice. Insert the formed pillars back into the platform. Platforms should be prepared fresh each time.
Geltrex coating of glass bottom dishes
Coat the surface of the glass dishes using 1 mL per 10 cm2. Incubate plates at 37 °C for 30 min. After coating, they can be stored at 4 °C for up to 2 weeks before being used. Aspirate any excess fluid right before use.
PROCEDURE
THAWING AND EXPANSION OF CELLS
Thaw and expand both hiPS cells and NHDF as described in option A and option B, respectively.
A) THAWING AND EXPANSION OF hiPS CELLS
TIMING 10 min plus 3 days for cell expansion before cardiac differentiation
Equilibrate an aliquot of hiPS medium of a desired volume supplemented with 5 μM Y-27632, in a 37°C water bath before thawing cells.
Remove the cryovial with cells from the liquid nitrogen storage tank.
PAUSEPOINT If needed, place cryovial on dry ice for up to 10 min before thawing.
iii)Immerse the cryovial in a 37 °C water bath, avoiding submerging the cap and keeping the tube stationary.
CRITICAL STEP Use of a floating microcentrifuge tube rack is recommended.
Remove the cryovial from the water bath, spray with 70 % ethanol, and place in a biological safety cabinet.
Gently transfer the cryovial content into the tube containing the hiPS medium at 37 °C supplemented with Y-27632.
CRITICAL STEP Avoid repeated pipetting of the cell suspension.
Rinse the empty cryovial with 1 mL of 37 °C hiPS medium to recover any residual cells and transfer the cryovial contents to a 15 mL centrifuge tube containing the hiPS cell suspension. Invert the tube slowly to mix the cell suspension.
Remove a sample of cells to confirm viability and count cells using a hemocytometer or an automated cell counter.
Dispense the cell suspension into Matrigel pre-coated 6-wells plate. Distribute the cells by moving it in short side-to-side and back-to-forth motions.
ix)Culture hiPS in an incubator set to 37 °C, 5 % CO2 and 90 % humidity.
Feed the hiPS daily with 2 mL of pre-warmed hiPS medium per 9.5cm2 growth surface. Passage them when they reach 60 % confluence (as described in step 2 option A) and start cardiac differentiation (step 3 onward) when they are 85–90 % confluent.
TROUBLESHOOTING
B) THAWING AND EXPANSION OF NHDF
TIMING 10 min plus 3 days for expansion
Equilibrate an aliquot of fibroblast medium of desired volume, in a 37 °C water bath before thawing cells.
Remove a cryovial with cells from the liquid nitrogen storage tank.
PAUSEPOINT If needed, place cryovial on dry ice for up to 10 min before thawing.
Immerse the cryovial in a 37 °C water bath, avoiding submerging the cap and keeping the tube stationary.
CRITICAL STEP Use of a floating microcentrifuge tube rack is recommended.
Remove the cryovial from the water bath, spray with 70 % ethanol, and place into a biological safety cabinet.
Gently transfer the cryovial content into the tube containing the 37 °C fibroblast medium. CRITICAL STEP Avoid repeated pipetting of the cell suspension.
Rinse the empty cryovial with 1 mL of 37 °C fibroblast medium to recover any residual cells and transfer the cryovial contents into the 15 mL centrifuge tube containing the fibroblasts cell suspension. Invert the tube slowly to mix the cell suspension.
Remove a sample of cells to confirm viability and count cells using a hemocytometer or an automated cell counter.
Dispense the cell suspension into cell culture flasks. Distribute the cells by moving it with short side-to-side and back-to-forth motions.
Culture NHDF in an incubator set to 37 °C, 5 % CO2 and 90 % humidity.
Feed the cells daily with 2 mL of pre-warmed fibroblast medium per 9.5 cm2 growth surface. Passage them when they reach 80 % confluence.
PASSAGING OF CELLS
Passage hiPS cells as described in option A and NHDF as described in option B.
A) PASSAGING OF hiPS CELLS
TIMING 10 min
When cells are 60 % confluent, select plates to be passaged. Wash using room temperature PBS.
Incubate the cells with 1 mL of warm 0.5 mM EDTA per 9.5 cm2 for 5 min at room temperature. CRITICAL STEP Monitor the cells under a microscope.
Remove the EDTA.
Vigorously pipette up and down the surface of the wells with 1 mL of hiPS medium supplemented with Y-27632 to detach cells.
Transfer the cell suspension to a centrifuge tube containing hiPS medium supplemented with Y-26732.
Plate the cell suspension in Matrigel pre-coated plates at ratios of 1: 6 to 1:8 and incubate cells in the tissue culture incubator.
TROUBLESHOOTING
B) PASSAGING OF NHDF
TIMING 10 min
At 80 % confluence, select flasks to be passaged and transfer them into a biological safety cabinet.
Aspirate the medium from the flasks and add 7 mL of room temperature PBS to wash the cells. Rotate the flask back and forth to fully wash the cells with PBS.
CRITICAL STEP Completely wash the surface of the flask with PBS to remove residual FBS from the culture medium, as this will deactivate the trypsin treatment.
Aspirate the PBS and add 1 mL of warm 0.25 % EDTA/Trypsin per 9.5 cm2. Incubate the cells for 5 min at 37 °C.
CRITICAL STEP Monitor the cells under a microscope to observe cell dissociation has resulted in a detached single cell suspension.
In the biological safety cabinet, gently transfer the cell suspension from the flask into a centrifuge tube with an equal amount of 37°C fibroblast medium.
Rinse the empty flask with fibroblast medium to recover any residual cells. Transfer the contents to the centrifuge tube containing the cell suspension. Invert the tube slowly to mix the cell suspension.
Centrifuge the cell suspension at 200 g for 3 min at room temperature.
Carefully aspirate the supernatant, taking care not to disturb the cell pellet, and resuspend the cells in a desired volume of fibroblast medium.
Plate the cell suspension in new cell culture flasks at ratios of 1:3 to 1:16.
TROUBLESHOOTING
CARDIAC DIFFERENTIATION
TIMING 12 days
CRITICAL Culture cells in an incubator set to 37 °C, 5 % CO2 and 90 % humidity throughout the differentiation.
When hiPS cells reach 85–90 % confluence, start cardiac differentiation of selected plates by washing hiPS cells using PBS at room temperature.
Change medium to CDM medium freshly supplemented with 3 μM of CHIR99021. This is day 0 of differentiation.
On day 2 of differentiation, change medium to CDM medium freshly supplemented with 2 μM of Wnt-C59.
On day 4, change medium to CDM medium with no additional supplements.
Continue to change medium every 2 days, replacing medium with 2 mL of pre-warmed CDM medium per 9.5 cm2 growth surface.
Beating cells should begin to appear at day 10; check that this occurs. On day 12 of differentiation, hiPS-CM are ready for use to form cardiac tissues. Proceed to the next section.
TROUBLESHOOTING
FABRICATION OF PILLARS
TIMING 16 hr
Manufacture the pillar’s supportive structure according to Box 1.
Clean the pillar’s supportive structures in a 70 % ethanol bath for 30 min followed by a wet autoclave cycle where they are immersed in a beaker of water and exposed to the autoclave liquid cycle (121 °C for at least 30 min using saturated steam under at least 15 psi of pressure) to remove any unintentional surface contamination.
Prepare PDMS by mixing the silicone elastomer base with the curing agent at a 10:1 ratio. Leave in the vacuum chamber for 20 min.
CRITICAL STEP Ensure PDMS is fully mixed before using by stirring at least 50 times. For ease of use, a 1 mL glass aspiration tip can be used to stir the PMDS and subsequently disposed of in the biological sharps’ container.
Assemble the pillar molds with the supportive structures and pour in the PDMS. Leave in the vacuum chamber for 20 min.
Centrifuge the molds at 300 g for 5 min.
CRITICAL STEP Ensure PDMS is free of bubbles before putting in the oven. If needed, centrifuge one more time or increase the time in the vacuum chamber.
Cure in oven overnight at 60–70 °C.
CRITICAL STEP Ensure PDMS is totally cured before removing from the oven by touching the exposed PDMS to ensure it is no longer sticky.
Open the molds, trim and clean the pillars and their supportive structures.
TROUBLESHOOTING
Wet autoclave the PDMS pillars by immersing them in a beaker of water and running the autoclave liquid cycle (121 °C for at least 30 min using saturated steam under at least 15 psi of pressure) to remove any unintentional surface contamination.
Assemble the cardiac tissue formation platform by placing the pillars into the bioreactor wells, using the mating features for alignment.
Autoclave all components (121 °C for at least 30 min using saturated steam under at least 15 psi of pressure, 30 min drying time).
GENERATION OF HUMAN CARDIAC TISSUES
TIMING 2–4 hr
Select and prepare cells to be used depending on the number of tissues being made. Option A and B describe how to prepare NHDF and hiPS cells respectively. For each tissue, plan to use one well of a 6-well plate of hiPS-CM at 85–90% confluency.
CRITICAL STEP Test the efficiency of your cardiac differentiations by flow cytometry by counting cardiac troponin positive cells (Step 48, option C) from the dissociated monolayer. If the cardiac differentiation is 85 % efficient, the remaining population can be considered a part of the 25 % supporting cell population. Therefore, to reach a final cell concentration containing 25 % supporting cells from a cardiac differentiation with an efficiency of 85 %, 15 % NHDF needs to be added. For a cardiac differentiation efficiency of 75 %, the NHDFs would not be added. We recommend a minimum cardiac differentiation efficiency of 80 %, and therefore a minimum NHDF cell population of 5 %.
A) Preparation of a NHDF cell suspension
After selecting the number of NHDF cells needed, transfer the flasks to the biological safety cabinet and aspirate the medium from the flasks. Add 7 mL of room temperature PBS to wash the cells. Rotate the flask back and forth to fully wash the cells with PBS.
CRITICAL STEP Completely wash the surface of the flask with PBS to remove residual FBS from the culture medium, as this will deactivate the trypsin treatment.
Aspirate the PBS and add 1 mL of warm 0.25 % EDTA/Trypsin per 9.5 cm2. Incubate the cells for 5 min at 37 °C.
CRITICAL STEP Monitor the cells under a microscope to observe cell dissociation has resulted in a detached single cell suspension.
In the biological safety cabinet, gently transfer the cell suspension from the flask into a centrifuge tube with an equal amount of 37 °C fibroblast medium.
Rinse the empty flask with fibroblast medium to recover any residual cells. Transfer the contents to the centrifuge tube containing the cell suspension. Invert the tube slowly to mix the cell suspension.
Centrifuge the cell suspension at 200 g for 5 min at room temperature.
Carefully aspirate the supernatant, taking care not to disturb the cell pellet, and resuspend the cells in 1 mL of fibroblast medium.
Repeat steps v and vi once.
Remove a sample of cells to confirm viability and count cells using a hemocytometer or an automated cell counter. Keep cell suspension on ice until use for tissue formation.
PAUSEPOINT Cells can be placed on ice for up to 30 min.
B) Preparation of a hiPS cell suspension
Incubate the plates of hiPS-CM with 1 mL of warm fresh collagenase II solution per 9.5 cm2 for 15 min at 37 °C.
CRITICAL STEP Monitor microscopically the progress of cell dissociation.
Vigorously pipette up and down the surface of the wells and add 1 mL of fresh collagenase II solution. Incubate for another 15 min at 37 °C.
Gently transfer the cell suspension from the culture wells into a centrifuge tube with cardiac medium supplemented with 10 % FBS at 37 °C.
Rinse the empty flask with Cardiac Medium supplemented with 10 % FBS to recover any residual cells. Transfer to the centrifuge tube containing the cell suspension. Invert the tube slowly to mix the cell suspension.
Centrifuge the cell suspension at 200 g for 3 min at room temperature.
Carefully aspirate the supernatant, taking care not to disturb the cell pellet, and resuspend the cells in cardiac medium supplemented with 10 % FBS.
CRITICAL STEP Ensure that the supernatant is fully aspirated from the cell pellet prior to adding Fibrinogen hydrogel as residual supernatant will dilute the final hydrogel concentration.
Repeat steps v and vi.
Take a sample of cells to confirm viability, and count cells using a hemocytometer or an automated cell counter. Put cell suspension on ice until tissue formation.
PAUSEPOINT Cells can be kept on ice for a maximum of 30 min.
After being autoclaved, according to section “Fabrication of pillars, steps 9 – 18, put one cardiac tissue formation platform inside a cell culture dish. Remove autoclaved pillars from the platform and add 200 μL of PLL-g-PEG to each well. Incubate at room temperature for 1 hr. Wash the wells with 200 μL of PBS twice. Place the pillars back into the platform.
Mix the required amount of the hiPS-CM and NHDF cell suspensions to create an overall cell number equal to 1 million cells per tissue being formed (include calculations for the cells and hydrogel of an extra cardiac tissue to account for pipetting losses). Ensure a ratio of 75 % hiPS-CM and 25 % NHDF (adjusted according to the % of cardiac troponin positive cells, as discussed in step 19).
Centrifuge at 200 g for 5 min at room temperature.
Add 8 μL of thrombin to the bottom of each cardiac tissue formation well.
Resuspend cells in 84 μL of fibrinogen per tissue by gently pipetting the fibrinogen and swirling the pipette tip to thoroughly mix the cells and fibrinogen.
CRITICAL STEP Before resuspending the cells, test the fibrinogen and thrombin by making hydrogel without cells.
CRITICAL STEP Fibrinogen should be used at room temperature.
CRITICAL STEP Calculate the extra volume of one cardiac tissue to compensate for hydrogel loss during pipetting.
Add 84 μL of the hydrogel to each well, making sure to change pipette tips after each addition.
Add 8 μL of thrombin to the top of each well.
CRITICAL STEP Avoid air bubble formation when pipetting. If formed, use sterile needle to pop them or gently aspirate using a small pipette tip.
CRITICAL STEP Change tips every time when thrombin is used.
Place culture plates in a 37 °C, 5 % CO2 and 90 % humidity incubator for 20 min.
Add 25 mL of Cardiac Medium supplemented with 0.02 mg/mL of aprotinin to the wells.
Keep culture plates in a 37 °C, 5 % CO2 and 90 % humidity incubator and change cardiac medium supplemented with 0.02 mg/mL aprotinin every two days for seven days.
TROUBLESHOOTING
MATURATION OF HUMAN CARDIAC TISSUES
TIMING 21 days
On day 7, check to see that the cardiac tissues have compacted within the cardiac tissue formation platform. Use a sterile needle to remove any cardiac tissue that is attached to the side of the tissue formation platform wells.
Using sterile forceps, gently remove the cardiac tissues from the tissue formation platform by gently wiggling the pillars’ supportive structure until the tissue is easy to remove via handling of the pillar’s supportive structure. Transfer the cardiac tissues to the cardiac maturation platform, designed with mating features to enable the alignment of 12 tissues per maturation platform.
CRITICAL STEP A second forceps can be used to stabilize the platforms during tissue removal and transfer.
Connect one alligator test lead to one platinum wire of the cardiac maturation platform and another alligator test lead to the other platinum wire of the cardiac maturation platform.
CRITICAL STEP Secure the test leads with tape outside the incubator so they do not move when the incubator door is open and closed.
Connect both alligator test leads to an electrical stimulator (see Box 2). CRITICAL STEP Make sure the stimulator is turned off when performing this step.
Start stimulation with 2 ms duration monophasic square repeating pulses, delivered at 2 Hz and amplitude of 4.5 V/cm. Place platform and stimulator in the tissue culture incubator.
Increase the stimulation frequency daily 0.33 Hz from 2 Hz (day 0 of stimulation) to 6 Hz on day 12. Culture cardiac tissues in a 37 °C, 5 % CO2 and 90 % humidity incubator and replace 50 % of the culture medium every two days.
From day 12 to day 15 of stimulation, keep the stimulation at 6 Hz.
At day 15, drop the stimulation to 2 Hz and keep it at that level until day 21 of stimulation.
During the entire 21 days of stimulation, culture cardiac tissues in a 37 °C, 5 % CO2 and 90 % humidity incubator and replace 50 % of the culture medium every two days.
TROUBLESHOOTING
CHARACTERIZATION OF HUMAN ADULT-LIKE CARDIAC TISSUES
From day 22 of culture onward, cells can be characterized. Follow option A for video analysis of contractility, option B to assess the excitation threshold of the tissues, option C to assess the maximum capture rate of the tissues, option D to measure the contractile force within a muscle strip myograph, option E for calcium imaging, option F for histological analysis and option G for immunostaining of tissue.
A. VIDEO ANALYSIS OF CONTRACTILITY
TIMING 1 min per tissue
Assess contractility by taking 20 sec videos at 100 frames per sec of the cardiac tissue beating spontaneously. Process results as described in Box 3.
B. EXCITATION THRESHOLD ASSESSMENT
TIMING 5 min per tissue
To assess excitation threshold (ET), transfer cardiac tissues in the cardiac maturation platform to a microscope and initiate electrical stimulation at 1.0 Hz, 2 ms pulse duration, 4.5 V/cm voltage. Wait 1 minute to allow tissue to equilibrate to applied electrical stimulation parameters. If the tissue beats synchronously with electrical stimulus of 1 Hz go to step ii. If the tissue is not beating synchronously with electrical stimulus of 1 Hz, go to step iii.
Decrease the stimulation voltage by 0.1 V until the tissue stops beating in sync with the applied electrical stimuli. Note this value as the ET.
Increase the stimulation voltage by 0.1 V until the tissue starts to beat in sync with the applied electrical stimuli. Note this value as the ET.
CRITICAL STEP If ET cannot be determined at 1 Hz due to the presence of spontaneous beating, increase the stimulation rate to 1.5 Hz or 2 Hz and repeat process at this higher stimulation rate.
C. MAXIMUM CAPTURE RATE ASSESSMENT
TIMING 5 min per tissue
Set the voltage of the electrical stimulator to twice that of the ET determined in section B of step 42.
Increase the rate of electrical stimulation by 0.1 Hz every 2 sec until the tissue can no longer synchronously beat in response to the applied stimulus frequency. Record this value as the maximum capture rate (MCR).
CRITICAL STEP The tissue should continue to increase in beat frequency until the MCR where it may miss every other beat or show a more sporadic beating pattern where the tissue is only responding to some of the applied stimuli.
D. MUSCLE STRIP MYOGRAPH MEASUREMENT OF CONTRACTILE FORCE
TIMING 2–4 hr
Set up the Muscle Strip Myograph System 1 hr before transferring cardiac tissues to the instrument, by opening the controlling software (LabChart 8) and filling each chamber of the instrument with 6 mL of modified Tyrode solution, setting to 37 °C.
On the instrument, follow the directions to calibrate each chamber using the 2 g weight. CRITICAL STEP Ensure the chambers have stabilized at 37 °C before moving to this step.
On the computer, also calibrate the corresponding chambers force by selecting the trace containing the force measurement from Step ii. On the top right of each channel, select the drop-down menu and select unit calibration. Select the baseline part of the force trace and set this to “0”. Select the part of the trace after the 2 g weight as added and set this to “9.81”. Set the unit to “mN”
Turn on gas supply (95 % O2 and 5 % CO2) and open the bubbler so a slow bubbling of the gas can be seen within each chamber. Let equilibrate for 15 min.
Load engineered cardiac tissues into organ bath chamber by moving the holder closer to the force transducer and by turning the dial attached to each organ bath chamber until the attachment hooks are spaced to align with the inside edge of the PDMS pillar within the cardiac tissue (Supplementary Figure 2A).
Place the tissue in the chamber and using forceps to hold the pillars’ supportive structure of the cardiac tissue, gently move the tissue towards the hooks until the tissue is pierced fully on each inside edge of the PDMS pillar (Supplementary Figure 2B).
Using another set of forceps, hold the cardiac tissue in place by gently applying pressure from the forceps on the area of the tissue in between the two hooks, while using the other forceps already holding the cardiac tissue pillar’s supportive structure to pull the PDMS pillars away from the tissue (Supplementary Figure 2C).
Ensure the cardiac tissue is fully mounted on both hooks, and use forceps to push the tissue further onto each hook as needed (Supplementary Figure 2D). Let the tissue equilibrate for 15 min.
CRITICAL STEP Use caution to not touch the force transducer while doing this.
Turn the stimulator on to a frequency of 2 Hz, 5 ms pulse duration, 80–100 mA and let the tissue equilibrate for another 15 min.
Optimize the length for maximal force generation (Frank-Starling Response) by adjusting the length of the tissue at stepwise increments (in Step 104) to determine the maximal length at which the tissue exerts the maximal force (Supplementary Figure 2E-F). Record the initial length of the tissue.
xi)Turn the dial that controls the position of the tissue holder 0.1 mm and let the tissue force equilibrate for a minimum of 1 min. Take note of the new length.
Repeat Step xi until subsequent increases in length no longer increase the force generated. At this point, stop increasing the length and leave the tissue at this maximal length for the remainder of the experiment. Record the final length of the tissue used to perform the remainder of the steps.
Determine the calcium-induced calcium response (CICR) by continuously adding 0.2 or 0.4 mM increments of CaCl into the chamber until a maximum of 2.8 mM is reached.
CRITICAL STEP Use the note function within the LabChart 8 software to make notes of each calcium concentration upon adding more CaCl to the chamber.
Obtain the force-frequency response (FFR) by increasing the frequency of stimulation by 1 Hz every 30–60 sec until 6 Hz is reached.
TROUBLESHOOTING
After reaching 6 Hz following the FFR, turn the stimulation off and wait an additional 10, 30, or 60 sec. After these times, turn the stimulation back on to a frequency of 1 Hz. This first beat will detail the post-rest potentiation (PRP).
To assess the response to cardiac drugs, record the baseline force for 1 min by making a note in the LabChart 8 software and continuing to record the experiment.
Use a concentrated stock solution of the drug to sequentially add the drug directly to the tissue bath using a pipette. Record the addition of the drug and the concentration in the LabChart 8 software.
Wait 5–15 min between drug additions, depending on the time needed to elicit a response, to record the full response before adding the next dose. Record each serial addition of the drug and the new concentration in the LabChart 8 software.
Upon conclusion of experiment, measure cross-sectional area (width and thickness) in the middle of the tissue using calipers.
CRITICAL STEP Record these measurements for use in determining the cross-sectional area when analyzing the force data.
Calculate twitch forces using LabChart 8 software. Twitch forces are the average of the difference between cyclic peak maximum and minimum force normalized to the cross-sectional area.
E. CALCIUM IMAGING
TIMING 1–3 hr
Prepare 1X Fluo-4 Direct™ calcium assay reagent solution by mixing an equal amount of 2X Fluo-4 Direct™ calcium assay reagent and cardiac medium.
Aspirate medium from cardiac tissues within the maturation platform and add 25 mL of the 1X Fluo-4 Direct™ calcium assay reagent.
Incubate plates at 37 °C for 35 min.
Wash out the 1X Fluo-4 Direct™ calcium assay reagent solution and replace it with 25 mL of prewarmed Blebbistatin Solution.
Transfer the tissue to a fluorescence microscope with filters appropriate for excitation at 494 nm and emission at 516 nm.
Connect tissue maturation platform to an electrical stimulator and turn it on to a frequency of 1 Hz, 2 ms pulse duration and 3 V voltage.
Capture the calcium baseline transient by recording a 20 sec video at 100 frames/second.
After recording the baseline calcium measurements, perform the intended dose-response study by adding increasing drug dosages via a syringe connected to sterile tubing that is inserted into the medium of the cardiac maturation platform. Take a video immediately after adding the drug and again 10 min after each drug addition.
CRITICAL STEP Electrical pacing is critical when analyzing the changes in calcium handling in response to drugs that may also affect beat frequency, since the beating frequency directly influences calcium dynamics. Thus, for drugs such as isoproterenol, electrical pacing should be applied throughout the dose-response study.
F) IMAGING OF HISTOLOGICAL SECTIONS OF ADULT-LIKE CARDIAC TISSUES
TIMING 48 hr
Fix mature cardiac tissues using 4 % paraformaldehyde or 10 % neutral buffered formalin overnight with gentle tumbling at 4 °C.
Wash in PBS three times for 15 min each.
PAUSE POINT Samples can be left in PBS at 4 °C, up to 48 hr, until they are sent for histological processing.
Outsource paraffin embedding and sectioning to appropriate specialized core/s.
CRITICAL STEP To ensure tissues stay intact during sectioning, request 10 μm thick sections. CRITICAL STEP To evaluate the presence of T-tubules, axial and longitudinal cross-sections should be evaluated.
Dewax sectioned paraffin embedded cardiac tissues by immersing the slide in fresh Citrisolv twice for 10 min each time with agitation about once a minute.
Rehydrate the slides with a serial exposure to solutions with smaller concentration of ethanol (100%, 90%, 70%, water). Wash twice with each solution for 3 min and agitate every 10–20 sec.
Antigen retrieve by transferring slides to Dako target retrieval solution in a container in a 95 °C water bath for 30 min.
Let slides cool at room temperature for 20 min and then place under running cold water for 5 min.
Wash slides three times with PBS for 5 min each time.
PAUSE POINT Samples can be left in PBS for 24 hr at 4 °C.
When staining for t-tubules, add WGA at a concentration of 1 μg/ml in PBS covering the entire slide at 4 °C for 15 min. Skip this step and proceed directly to next step if you are not staining for t-tubules. Gently wash tissues three times for 15 min each with PBS.
Permeabilize with 0.25 % Triton-X-100 in PBS for 15 min at room temperature.
Wash slides three times with PBS for 5 min each time.
Remove slides from rack, wipe around the section of the tissue to create an “island” and pipette on 100–200 μL of PBS onto section to prevent from drying out.
Use a hydrophobic pen to draw a ring/rectangle around each tissue and shake off PBS.
Wash three times with PBS for 5 min each.
Add 100–200 μL of Blocking buffer to the tissues and incubate for 60 min at room temperature.
Incubate with primary antibody diluted in Blocking buffer for 60 min at room temperature or overnight at 4°C.
Shake off primary antibody and wash three times with 100–200 μL of PBS for 5 min.
Incubate with 100–200 μL secondary antibody diluted to 2 μg/mL in Blocking buffer for 60 min.
Shake off secondary antibody and wash 3 times with 100–200 μL of PBS for 5 min.
CRITICAL STEP Check under a fluorescence microscope if the staining worked. If expected staining is not seen, repeat antibody incubation for longer time periods.
Place one drop of Vectashield with DAPI on the short end of the glass slide and using forceps slowly lower a coverslip at an angle starting from the side of the coverslip with the vectashield and working towards the other end.
CRITICAL STEP Avoid the formation of air bubbles. If an air bubble is seen, try to reorient the slide without lifting the coverslip up so that the air bubble is not located over the tissue region. PAUSEPOINT Store in fridge for up to 2 weeks. Slides can be stored at −20 °C for longer.
Image tissues using a Virtual Slide Scanner, or according to the microscope’s user manual. CRITICAL STEP To evaluate t-tubules, the use of a Virtual Slide Scanner provides ideal resolution to image these structures. We found that imaging at either 20X or 40X was sufficient. Additionally, a virtual z-stack should be acquired, and combined into a flattened image afterwards, to encompass the entire tissue section.
TROUBLESHOOTING
G) IMMUNOSTAINING OF WHOLE ADULT-LIKE CARDIAC TISSUE
TIMING 62–116 hr
Fix tissues with 4 % PFA.
Gently wash fixed tissues three times for 5 min each with PBS.
When staining for t-tubules, add WGA at a concentration of 1 μg/ml in PBS covering the entire tissue and placing on a shaker under low agitation at 4 °C for 30 min. Skip this step and proceed directly to Step iv) if you are not staining for t-tubules. Gently wash tissues three times for 15 min each with PBS.
If staining structural proteins, add 0.25 % Triton-X-100, covering the tissues completely and place them on the shaker under low agitation at 4 °C for 15 min. Otherwise proceed to next step.
Add 0.5 % Triton-X-100 so that it completely covers the tissues and place them on the shaker under low agitation at 4 °C for 15 min.
Dip the tissue in 1 % Tween-20 so that it completely covers the tissues at room temperature five times for 15 sec each.
Wash tissue immediately by dipping in PBS five times for 5 sec each.
For surface marker stains/gap junctions, dip the tissue in 1 % Tween-20 so that it completely covers the tissues at 4 °C for 15 min. Otherwise proceed to the next step.
Wash tissue immediately by dipping in PBS five times for 5 sec each.
Gently wash tissues three times on the shaker under low agitation at 4 °C for 5 min each with PBS.
Add Quenching solution so that it completely covers the tissues and place them on the shaker under low agitation at 4 °C for 30 min.
Add Blocking buffer so that it completely covers the tissues and place them on the shaker under low agitation at 4 °C for 2 hr.
PAUSE POINT Can leave overnight on the shaker at low agitation at 4 °C.
Add primary antibodies at the appropriate dilution in 0.2 % BSA so that the tissue is completely covered. Place tissues on the shaker under low agitation at 4 °C for 12–48 hr. For a list of standardized antibody dilutions see Reagents.
CRITICAL STEP If using multiple primary antibodies, ensure they are from different species and that there is no cross-reactivity.
CRITICAL STEP 0.1% Tween-20 can be used during primary antibody incubation to increase binding for structural proteins.
Gently wash tissues three times for 5 min each with PBS.
Continue to wash tissues in PBS with 0.2 % BSA on the shaker under low agitation at 4 °C for 6–12 hr.
Add secondary antibodies at the appropriate dilution in 0.2 % BSA so that it completely covers the tissues and place them on the shaker under low agitation at 4 °C for 12–48 hr.
CRITICAL STEP If using multiple secondary antibodies, ensure the species reactivity corresponds with the intended primary antibody and that there is no cross-reactivity.
Gently wash tissues three times for 5 min each with PBS.
Continue to wash tissues in PBS with 0.2 % BSA on the shaker under low agitation at 4 °C for 6–12 hr.
Add DAPI antibody at a dilution of 1/1000 in PBS so that it completely covers the tissues and place them on the shaker under low agitation at 4 °C for 30 min.
Gently wash tissues three times for 15 min each with PBS.
Prepare tissues for confocal imaging (Supplementary Figure 5) and take images according to the confocal user guide.
Papain digestion of mature engineered cardiac tissues for single cell analysis
TIMING 1 hr
CRITICAL STEP Single cells are needed to perform certain analyses, such as electrophysiology studies, single cell sequencing, or metabolism studies using the SeaHorse assays. We found that common methods to digest cells (i.e. collagenase II or trypsin/EDTA solutions) were too harsh, and did not produce a completely dissociated, single cell suspension when applied to adult-like cardiac tissues. We therefore developed a papain-based dissociation solution capable of dissociating the adult-like tissues discussed in this protocol into a single cell suspension.
Prepare the papain-based cardiac tissue dissociation solution. Incubate the dissociation solution in 37°C water bath for 30 min to activate papain. Add DNAse solution (Worthington, #LS002145, 25 unit/mL) into the papain solution before use.
Wash cardiac tissues with PBS twice. Next, incubate the tissues in the papain-based cardiac tissue dissociation solution containing DNase for 15 min at 37 °C (one cardiac tissue in 10 ml of the papain solution in a 15 ml tube). Gently shake the tube every 2 min. After 15 min incubation, gently pipette the tissues with 10 ml serological plastic pipette 10 times.
CRITICAL STEP Fifteen min incubation of the cardiac tissue in papain solution was used to achieve a mild dissociation of the tissue and enhance the recovery of the cells after the dissociation. The tissue might not be completely dissociated into single cells under this condition.
CRITICAL STEP It may also be necessary to undertake preparations for downstream assays during this step. Consult the procedure for the downstream assay you plan to complete next for details (these can be found in step 45).
Terminate the papain activity using serum-containing post-papain digestion cardiomyocyte culture medium (5 ml DMEM supplemented with 10 % FBS for 10 ml papain solution).
Centrifuge the cell suspension at 300 g for 5 min, aspirate all of the solution, wash one more time with cardiac medium and add fresh culture medium into the tube.
Count the cells and plate them for the desired single cell experiment (see step 45 for some possible assays).
CHARACTERIZATION OF CELLS USING SINGLE CELL TECHNIQUES.
If you have followed steps 40 – 44, you can proceed to electrophysiological analysis (option A), metabolic flux assay (option B), flow cytometry (option C), histology of sections (option D) and immunostaining (option E).
A) ELECTROPHYSIOLOGICAL ANALYSIS OF SINGLE CELL CARDIOMYOCYTES FROM ADULT-LIKE CARDIAC TISSUES
TIMING 3 days
During dissociation (step 40 to 44), incubate the center glass part of the 35 mm glass-bottom dishes with 70 μl Geltrex coating solution/dish. Incubate for one hour at 37 °C and one hour at room temperature. Aspirate the solution immediately prior to plating cardiomyocytes.
Resuspend the dissociated cells with the post-papain digestion cardiomyocyte culture medium (2 ml to 6 ml medium per a tissue depending on the original size). Plate the dissociated single cardiomyocyte on the center glass part of the Geltrex-coated dishes (7 mm diameter, the center part can hold ~70 μl solution).
CRITICAL STEP Low cell confluency (~2–5 %) is required to efficiently use multiple electrophysiological recording conditions.
Put the dishes into a cell culture incubator (37 °C, 5 % CO2, 90% humidity) for the dissociated cells to recover. A dish containing sterile water without a cover can be placed next to the two dishes containing cells in a 100 mm dish to keep humidity and prevent the medium from drying out.
Twenty-four hours after cardiomyocyte plating, add 2 ml post-papain digestion cardiomyocyte culture medium into the dishes.
Two or three days after cardiomyocyte plating, wash the dishes using Patch clamp external recording solution, at 37 °C and add the recording solution (~2 ml/dish at 37 °C).
CRITICAL STEP On the first day after dissociation, the dissociated myocytes are not fully recovered. More than four days after myocyte dissociation, the cells (without any spontaneous contraction) will no longer be healthy enough for electrophysiological recordings. Therefore, the whole-cell patch clamp recordings should be completed in 2–3 days after the cell dissociation and plating.
Transfer dish to the electrophysiological recording setup (Supplementary Figure 3) and use the microscope to find single cardiomyocytes with no spontaneous beating in the dish.
Fill the internal solution (use the Internal/pipette action potential recording solution reagent to obtain action potential recordings) into a glass pipette, and install the pipette into the electrode connected with the manipulator holder. Move the pipette in the external solution using the manipulator controller (speed 0 or 1).
Examine pipette resistance (smaller, ~8 MΩ, is better)
CRITICAL STEP Using a larger pipette with 2–6 MΩ resistance often results in a failure to seal and breaking through and/or leakage from the cardiomyocytes.
Move the pipette tip on the top of the target cell slowly (speed 4).
CRITICAL STEP Confirm that there is no bubble inside the glass pipette. If any bubbles or cell debris are observed, use a 10 ml syringe to remove bubbles and debris after keeping the pipette far from the target cell.
Move the pipette down slowly (speed 6, down to ~30 μm above the cell) and increase the magnitude of the microscope objectives.
Seal the cell using a glass pipette (gentle pressure).
Seal thicker and elastic region of the target cell (but not close to the nucleus). Hold potential at −60 mV after sealing is successful (>3 GΩ).
Apply a small amount of pressure multiple times using mouth suction (i.e. sharp pulses) to the electrode/pipette in order to break through the plasma membrane of the sealed cardiomyocyte. Immediately after breaking through, the cell capacitance should be measured using the whole cell clamp mode. Then, switch the mode to current-clamp to make sure that there are no spontaneous action potentials in the patched cardiomyocytes.
xiv)Start action potential recordings. For IK1 current recording in human pluripotent stem cell-derived cardiomyocytes, the External IK1 recording solution reagent and Internal IK1 recording solution reagent are used as the external and internal solutions, respectively. BaCl2 (0.5 mM) is used for measuring Ba2+-sensitive current to dissect IK1 current in the cardiomyocytes. Use 2 sec long voltage-clamp applied from −130 to +10 mV (holding at −40 mV, 0.1 Hz 2 sec voltage pulse). The IK1 reversal potential (Ba2+-sensitive current) had a negative slope conductance consistent with inward rectification. The current-voltage plot can be analyzed before and after the addition of 0.5 mM BaCl2 for 2 min.
B) METABOLIC FUNCTIONALITY USING SEAHORSE XF96 ASSAY
TIMING 3 days
Prepare Seahorse XF96 Cell Culture Microplates for cell attachment by coating each well with 200 μL matrigel diluted 1:40 in cold RPMI 1640 medium (i.e. 30 μL matrigel in 1.2 mL cold RPMI 1640 medium). Place matrigel coated plate into a 37 °C incubator for 1 hr. CRITICAL STEP This must be carried out at least 1 day before running Seahorse assay.
After dissociation of the cardiac tissues (steps 40 to 44), transfer cells to a 15 mL Falcon centrifuge tube and spin down at 1000 g for 5 min.
Wash cells by resuspending in 10 mL post-papain digestion cardiomyocyte culture medium and spin down at 1000 g for 5 min.
Resuspend cells in 15 mL post-papain digestion cardiomyocyte culture medium and add cells to a 150 cm2 tissue-culture treated flask to enrich the cell population for mature hiPS-CMs. Place cells into a 37 °C incubator for 30 min.
Check the flask containing the dissociated cells under a brightfield microscope to see that fibroblasts have attached to the bottom. If no cell attachment is seen, culture cells for an additional 15 min.
CRITICAL STEP: Do not exceed 1 hr of culture in the tissue-treated flask total.
Remove the supernatant from the tissue culture flask, which will now be enriched for mature hiPS-CMs. Spin cells down at 100 g for 5 min.
Resuspend cells in 1 mL post-papain digestion cardiomyocyte culture medium and count cells using a hemocytometer.
CRITICAL STEP: Single cells are needed to promote cell seeding of evenly cultured monolayers that do not contain cell clumps, which interfere with the proper data acquisition.
Dilute cells with post-papain digestion cardiomyocyte culture medium to achieve a final concentration of 225,000 cells/mL.
Remove the matrigel coated XF96 cell culture microplate from the incubator and aspirate excess media from each well.
Seed 200 μL of the cell-containing medium (Step viii) to plate 45,000 hiPS-CMs into each well.
Tilt the plate to the left and right, front and back, and towards each corner to ensure even seeding of the cells within the well plate.
Place XF96 cell culture microplate containing cells into the incubator and allow cells to attach for 24 hr.
To hydrate Seahorse XFe96 Sensor Cartridge, open the Seahorse XFe96 Extracellular Flux Assay Kit in a sterile biosafety hood and remove contents. Take the sensor cartridge off and place it upside down next to utility plate. Add 200 μL of Seahorse XF calibrant to each well of the utility plate, regardless of whether or not sample will be run in that well. Replace the lid and seal the plate by wrapping it with parafilm around the edges. Place the plate in a non-CO2 37 °C incubator overnight for at least 1 day before running Seahorse assay.
CRITICAL STEP: Add Seahorse XF calibrant to every well of the plate regardless of whether or not sample will be run in that well.
CRITICAL STEP: A non-CO2 37 °C incubator must be used.
On the day of planned Seahorse assay, prepare the XF Assay medium.
Look at the cells plated in the XF96 cell culture plate under a brightfield microscope to confirm that the cells are adhered to the bottom of the well and that they look healthy.
Remove 180 μL from each well containing cells (leaving 20 μL remaining in the well) and rinse with 200 μL XF Assay medium.
Remove all media from each well containing cells and replace with 175 μL XF Assay medium. Place the plate in a 37 °C incubator without CO2 for 1 hr prior to the assay.
CRITICAL STEP: A non-CO2 37 °C incubator must be used.
Retrieve the sensor cartridge from the 37 °C incubator without CO2 and load the XFe96 Sensor Cartridge by adding 20 μL of Oligomycin to port A, 22 μL of FCCP to port B, and 25 μL of Rotenone/Antimycin A to port C.
Run the assay according to the Seahorse instruction manual, choosing the following options in the Seahorse machine: in group definitions: injection strategy 1 and assay medium 1; in the instrument protocol: three injections; and in the plate map: add groups according to experimental design.
C) FLOW CYTOMETRY OF SINGLE CELLS ISOLATED FROM MATURE CARDIAC TISSUES
TIMING 1–3 hr
Spin papain dissociated cells down into a pellet in small 1.5 mL eppendorf tubes at 300 g for 5 min.
Resuspend cells in 250 μL of BD Cytofix reagent; mix well, and keep at 4 °C for 15 min.
Wash cells twice with 1:10 Perm Wash reagent (diluted from 10x Cytoperm stock in DI water).
Use a centrifuge to spin cells down again at 300 g for 5 min.
Resuspend cells in 100 μL of the Perm Wash solution.
Add the appropriate amount of primary antibody.
Incubate 20 min at 4 °C in the dark.
Wash twice with 1 mL of Perm Wash.
If conducting double staining, repeat steps vi-viii with secondary antibody.
Resuspend in 500 μL BD Stain buffer (0.1% BSA).
Pass cells through a filter cap flow tube and proceed to flow cytometry for analysis. We undertook Flow cytometry and analyzed using a BD FACS CANTOII. A minimum of 10,000 events should be collected for each run. We analyzed data in FlowJo (Ashland, OR), with every stained sample being compared with an unstained control that otherwise went through the same fix/perm process. For example gating and flow cytometry results of early-stage hiPS-CMs, see Supplementary Figure 4.
CRITICAL STEP The use of a flow cytometry expert or core center is recommended. Proper training and equipment are required.
TROUBLESHOOTING
Troubleshooting advice can be found in Table 2.
Table 2 –
Troubleshooting table
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| Step 1: Thawing and expansion of hiPSC and NHDF | Poor attachment of hiPSC | hiPSC medium wasn’t supplemented with Y-27632 | Supplement medium with Y-27632 |
| Imperfect coating | Plate cells in appropriately coated plates | ||
| Spontaneous differentiation of hiPSC | Cultures were too sparse or too dense | Adjust passage ratio | |
| hiPSC are proliferating very slowly | hiPSC will only show their normal growth behavior after being passaged several times | Wait for some passages to occur | |
| NHDFare proliferating very slowly | Cultures were too sparse | Adjust passage ratio | |
| Step 2Avi: Passaging of hiPSC | Many dead cells | EDTA incubation lasted too long | Monitor the cells under a microscope and stop dissociation at the right time |
| Step 8: Cardiac differentiation | No spontaneously contracting cells on day 12 | Poor quality hiPSC culture | See above problems as the problem, and hence solution are likely to be a consequence of problems during the expansion of hiPSC |
| Small molecules were not added or wrongly added in the CDM medium | Supplement CDM medium with the right molecules at the right time | ||
| Small molecules might be old | Prepare new stocks of small molecules | ||
| Step 18: Fabrication of pillars | Bubbles in PDMS pillars | PDMS was left to sit too long before pouring into mold and therefore was not as viscous; vacuum pump not used to pull bubbles out; centrifuge was not set high enough to push bubbles out. | Pour PDMS immediately after mixing, immediately transfer mold with PDMS to de-bubble in the vacuum chamber (can increase vacuuming time to 30 min); centrifuge PDMS into Pillar formation molds at 400 g for 5 min. |
| Step 92: Generation of cardiac tissues | Tissues did not form | Fibrinogen, thrombin, or aprotinin stocks may not be fresh. If the tissue did not gel properly from the beginning, it is likely the fibrinogen or thrombin. If the tissue did gel properly but subsequently fell apart, it is likely the aprotinin. | Remake the fibrinogen and thrombin fresh, and test that the two gel when mixed together by combining them in a 5:1 ratio in a petri dish, and confirming that they gel by touching hydrogel with pipette tip. If it is the aprotinin, make a fresh stock. If this does not solve the problem, increase the aprotinin concentration to 6.6 mg/mL or decrease the amount of fibroblasts added to the coculture (be cognizant of the fibroblasts already present in the hiPS-CM differentiation culture). |
| Tissues are not; beating well | Dt Cardiac differentiation efficiency may have been too low and/or the cell digestion was too harsh. | Only use hiPS-CM differentiations with an efficiency ^ 80 %. Limit the time in collagenase during digestion. If cells are difficult to dissociate, use the cells a day or two early when they will be easier to digest. | |
| Medium turninig yellow quickly | Tissue is too metabolically active to be supported by surrounding medium | Ensure tissues are cultured in a large bath of medium. Ensure medium has fatty acids (B-27 supplement) and buffer (bicarbonate) | |
| Step 39 Dxiv: Muscle Strip Myograph System measurements of contractile force | Force not increasing at higher stimulation frequencies | Tissue may not be stretched to allow optimal force generation. Or, organ bath solution may not be being refreshed and thereby has become toxic to the tissue. | Use the Frank-Starling force-length relationship to continuously increase tissue length within the Muscle Strip Myograph System until the force output is stable. Then retry force-frequency response. If the organ-bath solution is bubbling or has not been exchanged recently, replace with fresh solution and ensure it is bubbled properly. |
| Step 39Fxxi: Imaging of histological sections of adult-like cardiac tissues | High background in immunostained samples | Inadequate wash steps | Increase the time of wash steps (can do 24 hr washes on a shaker at 4 °C) and add gentle detergent (0.01 % Triton-X) to facilitate removal of excess antibody. |
ANTICIPATED RESULTS
In our protocol, we demonstrate the ability to generate cardiac tissues with electrophysiological activity, cell structure and mechanical activity closer to that seen in the adult myocardium than the fetal myocardium[6]. Similar results should be obtained it the cells are used during the period of high plasticity, directly following cardiac differentiation, and tissues are properly formed and subsequently exposed to electromechanical stimulation at a continuously increasing intensity for a period of 2 weeks. This protocol facilitates the development of cardiomyocytes of enhanced maturity, enabling more physiological results from standard assays (immunohistochemistry, electrophysiology, metabolic function, calcium handling, contractility) and new assays that were previously not possible to carry out on hiPS-CMs due to their immaturity (force frequency relationship, chronotropic, lusitropic, and ionotropic drug responses) (Supplementary Figure 6). Different types of evaluations can also be carried out on tissues obtained during this protocol, including quantification of the tissue’s developmental stage (maturity), and recapitulation of disease phenotypes (i.e. the effects of genetic mutations that can only be modeled if healthy controls display positive force-frequency relationship).
Supplementary Material
Figure 7. Setup of the intensity stimulation program in an arduino electrical stimulator intensity program setup.
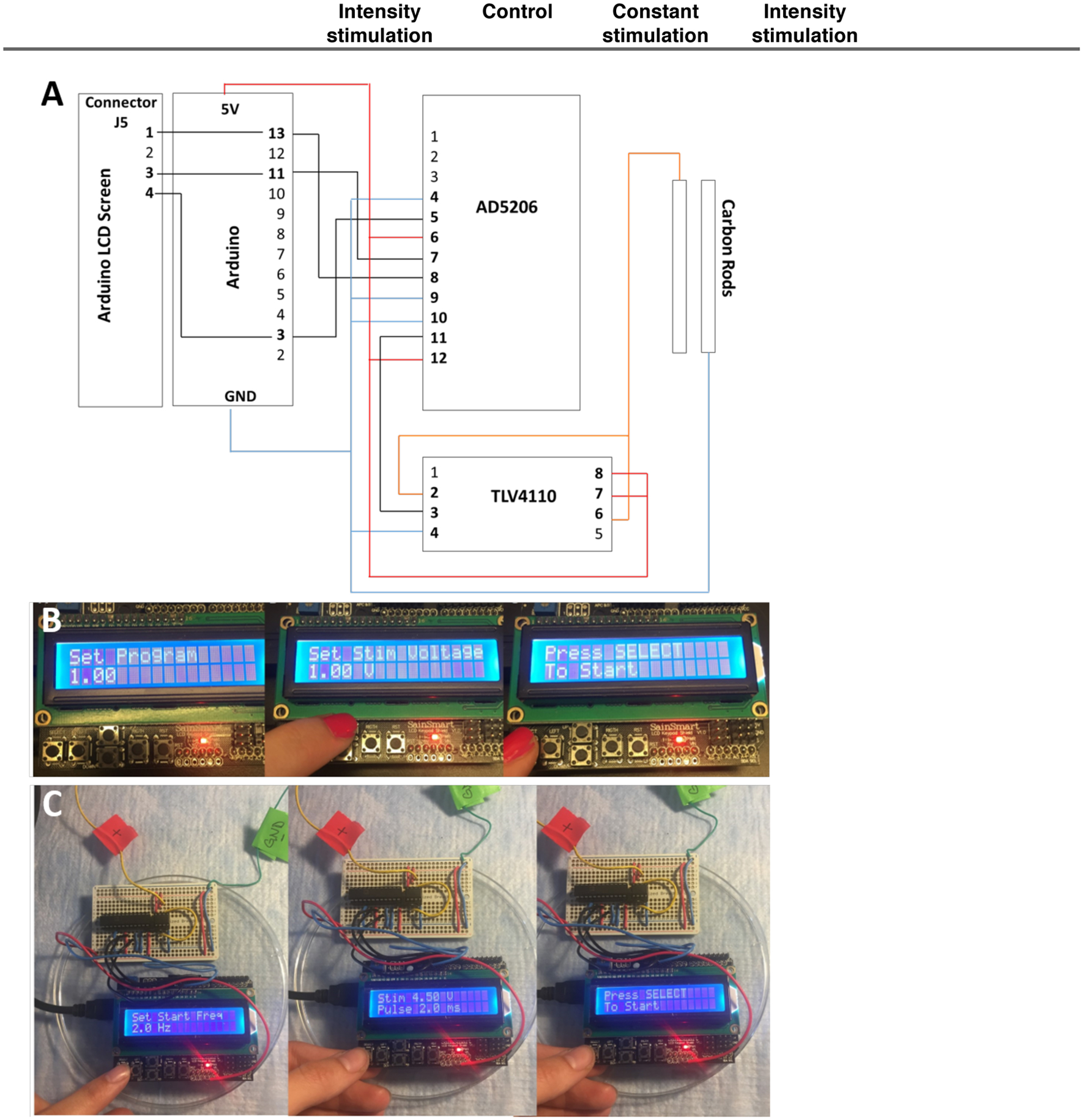
A) PnP setup up of the Arduino. B) Select Program “1” for the electrical intensity stimulation intensity regimen. Use the up and down buttons on the bottom left to select the stimulation parameters and then press “select” at the bottom left. Upon selecting the parameters, press “select” to begin the program. C) For setting up of a custom program, input the desired frequency by using the up and down buttons on the bottom left and then press “select” at the bottom left. Then press the “select” button to start the program.
ACKNOWLEDGMENTS
The authors acknowledge funding support from the NIBIB and NCATS grant EB17103 (G.V.-N.); NIBIB, NCATS, NIAMS, NIDCR and NIEHS grant EB025765 (G.V.-N.); NHLBI grants HL076485 (G.V.-N.) and HL138486 (M.Y.); University of Minho MD/PhD program (D.T.); Japan Society for the Promotion of Science fellowship (K.M.); and Columbia University Stem Cell Initiative (L.S., M.Y.). The authors also acknowledge all co-authors of the original manuscript describing the methodology for bioengineering adult-like cardiac tissues for their initial support and contribution, S. Duncan and B. Conklin for providing human iPSC, M. B. Bouchard for assistance with image and video analysis, D. Sirabella at the Columbia Stem Cell Core for assistance with cell derivation, and A. Vasciaveo and A. Califano for assistance with gene expression analysis.
Footnotes
COMPETING INTERESTS
G.V.-N. and K.R.-B. are co-founders of TARA Biosystems, a Columbia University spin-off that is commercializing the use of bioengineered human cardiac tissues for drug development.
DATA AVAILABILITY
Source data for quantitative data shown in all figure panels are available without restrictions and can be accessed at 10.6084/m9.figshare.7770947. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS. The data used for the analyses described in this manuscript were obtained from the GTEx Portal at the following dbGaP accession number phs000424.v7.p2.
REFERENCES
- 1.Ronaldson-Bouchard K and Vunjak-Novakovic G, Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell, 2018. 22(3): p. 310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmermann WH, et al. , Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol Bioeng, 2000. 68(1): p. 106–14. [PubMed] [Google Scholar]
- 3.Au - Ye KY, Au - Sullivan KE, and Au - Black LD, Encapsulation of Cardiomyocytes in a Fibrin Hydrogel for Cardiac Tissue Engineering. JoVE, 2011(55): p. e3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burridge PW, et al. , Chemically Defined and Small Molecule-Based Generation of Human Cardiomyocytes. Nature methods, 2014. 11(8): p. 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen A, et al. , Development of a drug screening platform based on engineered heart tissue. Circ Res, 2010. 107(1): p. 35–44. [DOI] [PubMed] [Google Scholar]
- 6.Ronaldson-Bouchard K, Ma SP., Yeager K, Chen T, Song LJ, Sirabella D, Morikawa K, Teles D, Yazawa M and Vunjak-Novakovic G Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature, 2018. 556(7700), p. 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lian X, et al. , Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nature protocols, 2013. 8(1): p. 162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nunes SS, et al. , Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods, 2013. 10(8): p. 781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruan JL, et al. , Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation, 2016. 134(20): p. 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaaf S, et al. , Generation of strip-format fibrin-based engineered heart tissue (EHT) Methods Mol Biol, 2014. 1181: p. 121–9. [DOI] [PubMed] [Google Scholar]
- 11.Turnbull IC, et al. , Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. Faseb j, 2014. 28(2): p. 644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mordwinkin NM, Burridge PW, and Wu JC, A review of human pluripotent stem cell-derived cardiomyocytes for high-throughput drug discovery, cardiotoxicity screening, and publication standards. J Cardiovasc Transl Res, 2013. 6(1): p. 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piccini JP, et al. , Current challenges in the evaluation of cardiac safety during drug development: translational medicine meets the Critical Path Initiative. Am Heart J, 2009. 158(3): p. 317–26. [DOI] [PubMed] [Google Scholar]
- 14.Robertson C, Tran DD, and George SC, Concise Review: Maturation Phases of Human Pluripotent Stem Cell-Derived Cardiomyocytes. Stem cells (Dayton, Ohio), 2013. 31(5): p. 10.1002/stem.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breckwoldt K, et al. , Differentiation of cardiomyocytes and generation of human engineered heart tissue. Nat Protoc, 2017. 12(6): p. 1177–1197. [DOI] [PubMed] [Google Scholar]
- 16.Eng G, et al. , Autonomous beating rate adaptation in human stem cell-derived cardiomyocytes. Nature Communications, 2016. 7: 10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radisic M, et al. , Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proceedings of the National Academy of Sciences, 2004. 101(52): p. 18129–18134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boudou TWRL, Anbin Mu, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB, Chen CS, A Microfabricated Platform to Measure and Manipulate the Mechanics of Engineered Cardiac Microtissues. Tissue Eng Part A, 2012. May(18): p. 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmermann WH, et al. , Tissue engineering of a differentiated cardiac muscle construct. Circ Res, 2002. 90(2): p. 223–30. [DOI] [PubMed] [Google Scholar]
- 20.Robertson C, Tran DD, and George SC, Concise Review: Maturation Phases of Human Pluripotent Stem Cell-Derived Cardiomyocytes. STEM CELLS, 2013. 31(5): p. 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feric NT and Radisic M, Maturing human pluripotent stem cell-derived cardiomyocytes in human engineered cardiac tissues. Advanced Drug Delivery Reviews, 2016. 96: p. 110–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pervolaraki Eleftheria, Dachtler James, Anderson Richard A., Holden Arun V.. The development transcriptome of the human heart. Scientific Reports, 2018. 8:15362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White MC, Pang L, and Yang X, MicroRNA-mediated maturation of human pluripotent stem cell-derived cardiomyocytes: Towards a better model for cardiotoxicity? Food and Chemical Toxicology, 2016. 98: p. 17–24. [DOI] [PubMed] [Google Scholar]
- 24.Vandenburgh H, et al. , Automated drug screening with contractile muscle tissue engineered from dystrophic myoblasts. Faseb j, 2009. 23(10): p. 3325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polacheck WJ and Chen CS, Measuring cell-generated forces: a guide to the available tools. Nat Methods, 2016. 13(5): p. 415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chattergoon NN, et al. , Thyroid hormone drives fetal cardiomyocyte maturation. The FASEB Journal, 2012. 26(1): p. 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parikh Shan S., et al. , Thyroid and Glucocorticoid Hormones Promote Functional T-Tubule Development in Human-Induced Pluripotent Stem Cell–Derived Cardiomyocytes. Circulation Research, 2017. 121:1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tandon N, et al. , Electrical stimulation systems for cardiac tissue engineering. Nature protocols, 2009. 4(2): p. 155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petriccione M, et al. , Reference gene selection for normalization of RT-qPCR gene expression data from Actinidia deliciosa leaves infected with Pseudomonas syringae pv. actinidiae. Scientific Reports, 2015. 5: p. 16961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godier-Furnemont AF, et al. , Physiologic force-frequency response in engineered heart muscle by electromechanical stimulation. Biomaterials, 2015. 60: p. 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burke MA, et al. , Clinical and Mechanistic Insights Into the Genetics of Cardiomyopathy. J Am Coll Cardiol, 2016. 68(25): p. 2871–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayakawa T, et al. , Noninvasive evaluation of contractile behavior of cardiomyocyte monolayers based on motion vector analysis. Tissue Eng Part C Methods, 2012. 18(1): p. 21–32. [DOI] [PubMed] [Google Scholar]
- 33.Huebsch N, et al. , Automated Video-Based Analysis of Contractility and Calcium Flux in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Cultured over Different Spatial Scales. Tissue Eng Part C Methods, 2015. 21(5): p. 467–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sala L, et al. , MUSCLEMOTION: A Versatile Open Software Tool to Quantify Cardiomyocyte and Cardiac Muscle Contraction In Vitro and In Vivo. Circulation Research, 2018. 122(3): p. e5–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Key reference(s) using this protocol
- Radisic M, et al. PNAS 101(52),18129–18134 (2004). DOI: 10.1073/pnas.0407817101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson - Bouchard K, et al. Nature, 556(7700), 239–243 (2018) DOI: 10.1038/s41586-018-0016-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, et al. Cell, 176(4), 913–927 (2019) DOI: 10.1016/j.cell.2018.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


