Abstract
Background:
Diastolic dysfunction (DD) is common and occurs at an earlier age among human immunodeficiency virus -infected (HIV+) individuals, but the mechanisms and consequences of DD among HIV+ individuals are unclear.
Methods and Results:
The Characterization of Heart Function on Antiretroviral Therapy (CHART) study was a multi-center cross-sectional case-control study of treated and virally suppressed HIV+ individuals with (DD+) and without DD (DD−). All patients had normal ejection fraction (>50%), no significant valvular disease, and no history of coronary revascularization or persistent atrial fibrillation. Overall, 94 DD+ and 101 DD− patients were included. DD+ patients were older with higher body mass index and more likely to have hypertension, renal dysfunction, and dyslipidemia. Groups were similar with respect to sex, race, CD4 count, and HIV RNA copies. NT-pro-B-type natriuretic peptide levels (median 36 [23, 85] vs. 26 [12, 49] pg/mL, p<0.01) and high-sensitivity troponin I (3.6 [2.6, 5.1] vs. 2.5 [1.8, 3.5] pg/mL, p<0.01) were higher among DD+ patients. The latter had similar left atrial size, but increased stiffness (conduit strain, 23.5 [17.5, 36.9] vs. 30.0 [22.9, 37.0], p<0.01) and impaired relaxation (reservoir strain, 39.7 [32.0, 58.0] vs. 45.9 [37.0, 60.6], p=0.04). On CMR, the prevalence of focal fibrosis was higher among DD+ patients (19.0% vs. 5.3%, p<0.01). DD+ patients demonstrated higher levels of carboxyl-terminal telopeptide of collagen type I (p=0.04), and trends towards higher IL-6 and oxidized low-density lipoprotein levels (p≤0.08). KCCQ physical limitation (87.1±21.4 vs. 93.1±18.1, p=0.01) and symptom frequency scores were lower among DD+ patients (86.0±21.5 vs. 92.5±16.8, p=0.01).
Conclusions:
In this contemporary HIV+ population receiving antiretroviral therapy, DD was associated with multiple alterations in cardiac structure and function, including myocardial fibrosis and left atrial abnormalities, and worse quality of life. Further studies are needed to assess longitudinal changes in these parameters and their potential as therapeutic targets to prevent progressive cardiac remodeling and dysfunction in HIV.
Keywords: human immunodeficiency virus, antiretroviral therapy, diastolic dysfunction, myocardial fibrosis
INTRODUCTION
With the development and widespread use of modern antiretroviral therapy (ART), the natural history of human immunodeficiency virus (HIV) infection has changed from an infection with high mortality to a treatable chronic condition with near-normal life expectancy.1 Likewise, there has been a shift in the epidemiology of HIV-associated cardiomyopathy from a condition of predominantly acute left ventricular (LV) systolic dysfunction, sometimes referred to as “HIV myocarditis,” to largely one of chronic LV diastolic dysfunction (DD).2, 3 Contemporary studies have reported DD in up to 50% of HIV-infected (HIV+) individuals and with earlier age of onset compared with that occurring in the general population.4–7 The increasing prevalence of early onset DD among HIV+ patients and the associated risks of heart failure (HF) (especially HF with preserved ejection fraction [HFpEF]), atrial fibrillation, and mortality represent significant cardiovascular health concerns for these patients.8, 9
Limited data are available regarding clinical characteristics and mechanisms underlying the heightened burden of DD among HIV+ patients receiving modern ART. Chronic systemic inflammation has been proposed as a key mediator of DD and HFpEF in the general population, and may also be linked to a state of persistent immune activation and inflammation in HIV despite ART and viral suppression.10, 11 Other studies suggest higher rates of DD in HIV+ populations may be related to higher prevalence of cardio-metabolic risk factors (e.g., impaired glucose tolerance, hyperlipidemia).12, 13 Cardiac magnetic resonance (CMR) and serum biomarker studies among HIV+ populations demonstrate several derangements in cardiac structure and function, including accelerated development of focal and diffuse myocardial fibrosis and increased myocardial lipid content.14–16
As the prevalence of DD and HFpEF among HIV+ patients is expected to continue to rise, there remains a need for comprehensive understanding of the biologic processes related to DD in these patients. This Characterization of Heart Function on Antiretroviral Therapy (CHART) study was designed to characterize systematically the biologic determinants, mechanisms, and consequences of DD among HIV+ patients receiving contemporary ART. The primary study hypotheses were that, despite similar ART and viral suppression, HIV+ patients with DD demonstrate a higher degree of systemic inflammation, myocardial fibrosis, and left atrial dysfunction than HIV+ patients without DD.
METHODS
CHART was a prospective case-control study comparing ART-treated virally suppressed HIV+ individuals with DD (HIV+/DD+) versus without DD (HIV+/DD−). The design of the CHART study has been previously described.17 Institutional review board or ethics committee approval was obtained at each study site. All subjects provided written informed consent.
Study Patients
The inclusion and exclusion criteria of the CHART study have been previously published.17 Briefly, eligible HIV+ patients age >40 years receiving ART for >6 months and with HIV RNA level <200 copies/ml were eligible. All patients were required to have an LV ejection fraction (EF) >50% as determined by a screening 2D echocardiographic examination. Notable exclusion criteria included a history of moderate or severe valve disease or history of valve repair/replacement, prior EF <50%, history of prior coronary revascularization or active angina, persistent atrial fibrillation, uncontrolled hypertension (systolic >160 mmHg or diastolic >100 mmHg), comorbid inflammatory disease (e.g., rheumatoid arthritis, systemic lupus erythematosus), active cancer or cancer chemotherapy in past year, and chronic use of steroid or anti-inflammatory medication. A history of pericardial disease was not a specific exclusion criteria independent of the above noted exclusions.
Study Design
Patients meeting eligibility criteria were stratified by presence or absence of DD (i.e., DD+ versus DD−), as defined by pre-specified criteria.17 The presence of DD was defined using 2d echocardiography as 1) septal e’ velocity <7 cm/s or lateral wall e’ velocity <10 cm/s, and 2) evidence of chronically elevated LV filling pressure or LV hypertrophy, including left atrial volume index >28 mL/m2, LV mass index >95/m2 in women or >115 g/m2 in men, or relative wall thickness >0.42.
Data collection for this cross-sectional study occurred at time of study baseline. Protocol pre-specified assessments and data collection included the following: i) history, physical examination, laboratory tests, and medications; ii) electrocardiogram; iii) patient-reported quality of life (QOL) measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ); iv) comprehensive transthoracic echocardiographic assessment including speckle tracking to evaluate ventricular and atrial mechanics (blinded interpretation by the Northwestern University Cardiovascular Imaging Core Laboratory, Chicago, IL); v) cardiac magnetic resonance (CMR) assessment including delayed-enhancement imaging (blinded interpretation by the Duke University Cardiac Magnetic Resonance Core Laboratory, Durham, NC); vi) and multiple domains of biomarkers including markers of inflammation, oxidative stress, and immune activation (all samples stored at −80° C in the core biobank at University of Vermont, Burlington, VT).
Study Endpoints
Given the descriptive and mechanistic nature of CHART, all endpoints were pre-specified as exploratory. Endpoints included specific measures of ventricular and atrial structure and function as measured by 2D echocardiography and CMR, serum biomarker panels, and patient-reported QOL as measured by the KCCQ.
Statistical Analysis
Sample size requirement was estimated based on differences in log-transformed IL-6 levels between DD+ and DD− groups assuming a standard deviation (SD) of approximately 0.75. CHART was intended to enroll approximately 200 patients, based on a sample size of 100 patients in each group providing 80% and 90% power to detect differences of 0.30 and 0.35, respectively, in a 2-sample t test with 2-sided error type 1 error of 0.05. Comparisons of continuous variables between the DD+ and DD− groups were estimated to have 80% and 90% power to detect differences of 0.40 and 0.46, respectively. Given the single visit nature of CHART, statistical models were univariate and were designed as non-parametric rank-based models. Unadjusted comparisons between DD+ and DD− groups were performed. P values for continuous variables and ordinal categorical variables were calculated using Wilcoxon rank-sum tests, and P values for remaining categorical variables were calculated using Likelihood Ratio Chi-Square or Fisher’s Exact Test. Data are presented as n (%), mean ± SD, and median (25th–75th), as appropriate. To examine the impact of age on the relationship between DD and study endpoints, post-hoc sensitivity analyses of patient characteristics, imaging parameters, and serum biomarkers stratified by age ≥55 years/ <55 years were performed. Given the exploratory and hypothesis-generating nature of the CHART study, adjustment for multiple comparisons was not performed. For all analyses, a P value of <0.05 was considered statistically significant; P values between 0.05 and 0.10 were considered to be trends. In sensitivity analyses, all analyses were repeated after excluding patients with a history of heart failure. All analyses were conducted with SAS, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Patients were screened at 12 sites across the United States, with 11 sites enrolling ≥1 patient.
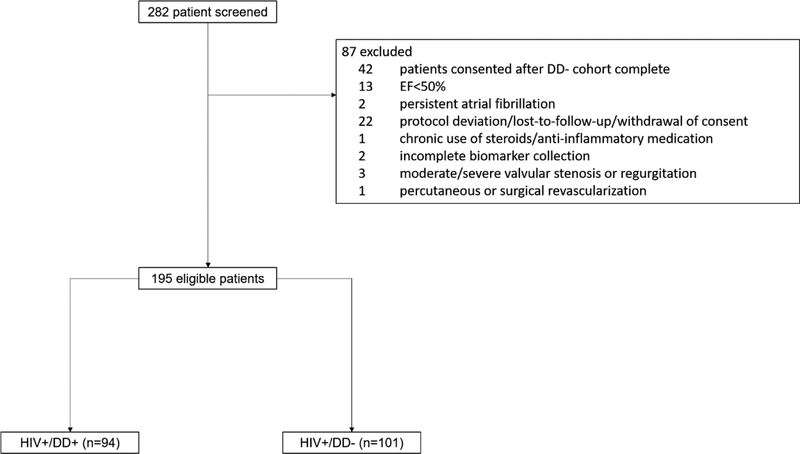
Of 282 patients screened, 195 patients met eligibility criteria (Figure 1). The DD+ group included 94 patients and the DD− group included 101 patients. Table 1 displays baseline demographic, clinical, and laboratory data for DD+ and DD− patients. HIV status was similar and well-controlled in both groups with median HIV duration 16.4 (9.4–22.3) years and median CD4 count 674 (435–838) cells/mm3. In total, 160 (82%) patients had an undetectable HIV RNA viral load. DD+ patients were older with higher blood pressure, heart rate, and body mass index and longer QRS duration (all p≤0.04). Laboratory results were similar between the groups, with exception of higher creatinine and CD8 count among DD+ patients (all p≤0.02). DD+ patients were more likely to have many non-HIV related comorbidities including hypertension, hyperlipidemia, and chronic renal dysfunction (p<0.01). Rates of prior HF diagnosis were 9% and 2% among DD+ and DD− groups, respectively (p=0.05). Rates of prior HIV related infections were similar between DD groups (p≥0.08).
Figure 1. Selection of the study cohorts.
DD, diastolic dysfunction; EF, ejection fraction; HIV, human immunodeficiency virus
Table 1.
Baseline Patient Characteristics by Diastolic Dysfunction Status
| Diastolic Dysfunction Absent (N=101) | Diastolic Dysfunction Present (N=94) | P Value | |
|---|---|---|---|
| Age | 52.5 ± 5.7 | 58.0 ± 8.1 | <0.01 |
| Women | 29 (29) | 27 (29) | 0.99 |
| Race | 0.46 | ||
| White | 42 (42) | 44 (47) | |
| Black | 59 (58) | 50 (53) | |
| Other | 0 (0) | 0 (0) | |
| Hispanic ethnicity | 4 (4) | 4 (4) | 1.00 |
| Vital sign and exam findings | |||
| Systolic blood pressure (mmHg) | 124 (116, 134) | 130 (122, 143) | <0.01 |
| Diastolic blood pressure (mmHg) | 80 (71, 84) | 82 (75, 88) | <0.01 |
| Heart rate (bpm) | 73 (65, 81) | 75 (66, 84) | 0.04 |
| Weight (lbs) | 179 (152, 198) | 195 (168, 227) | <0.01 |
| BMI (kg/m2) | 26.6 (24.3, 30.4) | 28.4 (25.5, 34.2) | 0.01 |
| Jugular venous distention | 1 (1) | 2 (2) | 0.61 |
| Peripheral edema | 0.10 | ||
| None | 95 (97) | 83 (91) | |
| Trace | 1 (1) | 4 (4) | |
| Mild (1+) | 1 (1) | 3 (3) | |
| Moderate (2+/3+) | 1 (1) | 1 (1) | |
| Severe (4+) | 0 (0) | 0 (0) | |
| Rhythm on ECG | 0.54 | ||
| Sinus rhythm | 90 (90) | 82 (87) | |
| Atrial fibrillation/flutter | 0 (0) | 0 (0) | |
| Other | 10 (10) | 12 (13) | |
| QRS Duration | 86 (80, 96) | 90 (82, 97) | 0.04 |
| Non-HIV Related Laboratories | |||
| Sodium (mEq/L) | 139 (138, 141) | 140 (138, 142) | 0.18 |
| BUN (mg/dL) | 13 (11, 18) | 15 (13, 19) | 0.09 |
| Creatinine (mg/dL) | 1.0 (0.8, 1.2) | 1.1 (0.9, 1.2) | <0.01 |
| Albumin (g/dL) | 4.3 (4.0, 4.5) | 4.2 (4.0, 4.4) | 0.57 |
| ALT (IU/L) | 23 (15, 33) | 22 (16, 32) | 0.91 |
| Total Bilirubin (mg/dL) | 0.4 (0.3, 0.6) | 0.5 (0.3, 0.7) | 0.51 |
| HbA1c (%) | 5.5 (5.1, 6.7) | 5.7 (5.4, 6.3) | 0.22 |
| Hemoglobin (g/dL) | 13.8 (13.2, 15.0) | 13.8 (13.0, 15.2) | 0.76 |
| LDL (mg/dL) | 105 (88, 120) | 104 (82, 119) | 0.95 |
| HDL (mg/dL) | 49 (41, 60) | 46 (38, 57) | 0.13 |
| Triglycerides (mg/dL) | 124 (82, 154) | 121 (97, 198) | 0.19 |
| HIV Related Laboratories | |||
| CD4 Count (cells/mm3) | 680 (469, 838) | 668 (416, 836) | 0.84 |
| Nadir CD4 Count (cells/mm3) | 319 (184, 447) | 269 (80, 450) | 0.48 |
| CD8 Count (cells/mm3) | 710 (416, 936) | 838 (568, 1158) | 0.02 |
| CD4/CD8 Ratio | 1.0 (0.7–1.4) | 0.8 (0.5, 1.2) | 0.14 |
| HIV RNA (copies/mL)* | 27.3 ± 12.8 | 28.1 ± 16.3 | 0.74 |
| Non-HIV Related Medical History | |||
| Heart failure diagnosis | 2 (2) | 8 (9) | 0.05 |
| Prior MI | 1 (1) | 2 (2) | 0.61 |
| Hypertension | 38 (38) | 59 (63) | <0.01 |
| Hyperlipidemia | 21 (21) | 41 (44) | <0.01 |
| Stroke | 2 (2) | 7 (7) | 0.09 |
| Diabetes | 11 (11) | 19 (20) | 0.07 |
| Chronic renal insufficiency | 2 (2) | 14 (15) | <0.01 |
| Cancer (non-AIDS associated) | 6 (6) | 12 (11) | 0.13 |
| Illicit drug use | 39 (39) | 31 (34) | 0.41 |
| Smoking history | 64 (63) | 49 (53) | 0.13 |
| HIV Related Medical History | |||
| Duration of HIV Diagnosis (years) | 15.2 (9.9, 22.2) | 16.5 (9.4, 22.4) | 0.44 |
| Hepatitis B | 4 (4) | 9 (10) | 0.11 |
| Hepatitis C | 14 (14) | 13 (14) | 0.98 |
| HIV-associated Cancer | 1 (1) | 3 (3) | 0.35 |
| Pneumocystis Jiroveci | 11 (11) | 4 (4) | 0.08 |
| Candida Esophagitis | 5 (5) | 11 (10) | 0.14 |
| CMV Infection Diagnosed by | 5 (5) | 2 (2) | 0.45 |
| Histopathology | |||
| Pulmonary Tuberculosis | 4 (4) | 2 (2) | 0.68 |
| Extrapulmonary Cryptococcal Infection | 2 (2) | 2 (2) | 1.00 |
Data presented as n (%), mean ± standard deviation, or median (25th, 75th).
ALT, alanine aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; CMV, cytomegalovirus; ECG, electrocardiogram; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; HBA1c, hemoglobin A1c; LDL, low-density lipoprotein; MI, myocardial infarction; RNA, ribonucleic acid.
Mean viral load data only among patients with detectable values (DD+, n=20; DD−, n=15). Overall, the assay level of detection was available for 160 total patients. Overall, 118 patients were tested with an assay detection threshold of 20 copies/mL, 1 patient with an assay detection threshold of 30 copies/mL, and 41 patients with an assay detection threshold of 40 copies/mL. All patients in CHART had a viral load <200 copies/mL.
Active non-HIV medical therapy was similar between groups, with exception of more frequent use of antiplatelet and lipid lowering therapies among DD+ patients (Table 2). Regarding HIV medications, a total of 121 (62%) and 76 (39%) patients were receiving multiclass combination and nucleoside reverse transcriptase inhibitor (NRTI)/combination NRTI therapy at study baseline, respectively. DD+ patients were more likely to have received NRTI or combination NRTI therapy in the past (p<0.01), but there were no significant differences in active HIV therapy.
Table 2.
Background Medical Therapy by Diastolic Dysfunction Status
| Diastolic Dysfunction Absent (N=101) | Diastolic Dysfunction Present (N=94) | P Value | |
|---|---|---|---|
| Active Non-HIV Related Medications | |||
| Any diuretic | 13 (13) | 17 (18) | 0.31 |
| ACEI | 16 (16) | 23 (24) | 0.13 |
| ARB | 2 (2) | 7 (7) | 0.09 |
| Beta-blocker | 8 (8) | 13 (14) | 0.18 |
| Any antiplatelet | 9 (9) | 19 (18) | 0.04 |
| Lipid lowering therapy | 31 (32) | 45 (49) | 0.02 |
| Insulin | 5 (5) | 5 (5) | 1.00 |
| Oral glucose lowering therapy | 9 (9) | 17 (18) | 0.06 |
| Active HIV Medications | |||
| ≥2 class ART regimen | 58 (57) | 63 (67) | 0.17 |
| NRTI or multiple NRTI | 42 (42) | 34 (37) | 0.47 |
| NNRTI | 5 (5) | 8 (9) | 0.32 |
| Protease inhibitor | 25 (25) | 17 (18) | 0.27 |
| Integrase Inhibitor | 25 (25) | 22 (24) | 0.86 |
| Prior HIV Medications* | |||
| ≥2 class ART regimen | 34 (35) | 39 (43) | 0.25 |
| NRTI or multiple NRTIb | 46 (46) | 64 (70) | 0.01 |
| NNRTI | 15 (15) | 16 (18) | 0.70 |
| Protease inhibitor | 38 (38) | 45 (50) | 0.11 |
| Integrase Inhibitor | 19 (20) | 17 (19) | 0.90 |
Data presented as n (%)
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor.
Describes medications patients received in the past but no longer receiving at time of study baseline.
Overall, 10 (5.1%) patients had a history of HF at baseline (DD+, n=8 [9%]; DD−, n=2 [2%]; p=0.05). Characteristics among patients without a history of HF were generally similar to the overall study cohort (Supplemental Tables 1 and 2).
Cardiac Structure and Function
2D echocardiographic and CMR data are displayed in Table 3. Median mitral E/A ratio and E/e’ ratio were 0.9 (0.8, 1.0) and 9.1 (7.6, 10.9) among DD+ patients, and 1.1 (0.9, 1.3) and 6.9 (6.0, 7.9) among DD− patients (all p<0.01). Median septal and lateral e’ velocities were 6.8 (6.0, 7.7) and 8.8 (7.5, 9.6) cm/s for DD+ patients, as compared with 9.9 ±1.9 and 12.9 ± 2.1 for DD− patients (all p<0.01). By echocardiography, DD+ patients had greater relative wall thickness and greater LV mass index (all p<0.01). Compared with DD− patients, DD+ patients had lower LV end-diastolic volume index (p=0.01), but similar LA volume index (p=0.45). Global longitudinal strain (GLS) was reduced among DD+ patients (p<0.01), as were all measures of LA strain (all p<0.04) except LA booster strain (p=0.19). The relationships between DD and echocardiographic parameters were similar among patients without baseline HF (Supplemental Table 3), with exception that differences in LA reservoir strain were not statistically significant (p=0.11).
Table 3.
Cardiac Structure and Function Characteristics
| Diastolic Dysfunction Absent (N=101) | Diastolic Dysfunction Present (N=94) | P Value | |
|---|---|---|---|
| 2D Echocardiography | |||
| LV mass index (g/m2) | 83.3 (72.8, 96.7) | 93.0 (80.4, 109.0) | <0.01 |
| Relative wall thickness | 0.4 (0.4, 0.5) | 0.5 (0.4, 0.5) | <0.01 |
| LV end-diastolic dimension (cm) | 4.5 (4.3, 4.9) | 4.5 (4.1, 4.9) | 0.40 |
| LV end-systolic dimension (cm) | 3.0 (2.7, 3.3) | 3.0 (2.8, 3.3) | 0.63 |
| LV end-diastolic volume index (mL/m2) | 45.8 (40.6, 51.8) | 42.7 (36.5, 48.5) | 0.01 |
| LV end-systolic volume index (mL/m2) | 18.4 (15.0, 21.7) | 17.3 (13.8, 20.5) | 0.06 |
| LV ejection fraction (%) | 61 (57, 63) | 60 (57, 63) | 0.82 |
| LA volume index (mL/m2) | 26.3 (21.4, 30.3) | 26.5 (21.5, 32.2) | 0.45 |
| Mitral E/A ratio | 1.1 (0.9, 1.3) | 0.9 (0.8–1.0) | <0.01 |
| E/e’ ratio | 6.9 (6.0, 7.9) | 9.1 (7.6, 10.9) | <0.01 |
| Septal e’ velocity (cm/s) | 9.6 (8.6, 10.8) | 6.8 (6.0, 7.7) | <0.01 |
| Lateral e’ velocity (cm/s) | 12.8 (11.2, 14.3) | 8.8 (7.5, 9.6) | <0.01 |
| Global longitudinal strain (%) | 19.6 (17.4, 21.3) | 18.2 (15.3, 20.1) | <0.01 |
| LA reservoir strain (%) | 45.9 (37.0, 60.6) | 39.7 (32.0, 58.0) | 0.04 |
| LA conduit strain (%) | 30.0 (22.9, 37.0) | 23.5 (17.5, 36.9) | <0.01 |
| LA booster strain (%) | 18.4 (13.1, 25.1) | 18.8 (14.5, 26.9) | 0.32 |
| Cardiac Magnetic Resonance | |||
| Focal Fibrosis by delayed-enhancement | 5 (5) | 16 (19) | <0.01 |
| Focal Fibrosis as % LV myocardial mass (%) | 0.1 ± 0.4 | 0.5 ± 1.8 | <0.01 |
| Focal fibrosis as % LV myocardial mass among patients with delayed-enhancement (%) | 1.5 ± 0.9 | 2.6 ± 3.6 | 0.93 |
| Myocardial T1 value pre-contrast* | 956 (914, 983) | 940 (900, 968) | 0.26 |
| Myocardial extracellular volume (%)^ | 28.0 (25.7, 30.5) | 25.2 (23.5, 29.2) | 0.06 |
| LV end-diastolic volume (mL) | 123.6 (99.8, 142.1) | 120.3 (100.5, 138.9) | 0.55 |
| LV end-systolic volume (mL) | 40.7 (31.9, 51.4) | 42.2 (32.9, 51.8) | 0.56 |
| LV myocardial mass (g) | 118.6 (95.1, 138.5) | 141.3 (115.2, 161.1) | <0.01 |
| LV ejection fraction (%) | 66.2 (64.0, 68.8) | 64.4 (60.6, 67.1) | <0.01 |
| LV cardiac output (L/min) | 5.3 (4.2, 6.2) | 4.9 (4.1, 5.6) | 0.03 |
| RV end-diastolic volume (mL) | 124.5 (105.3, 147.0) | 125.8 (108.1, 148.8) | 0.96 |
| RV ejection fraction (%) | 59.3 (55.7, 64.2) | 58.7 (54.4, 63.1) | 0.17 |
Data presented as n (%), median (25th, 75th), or mean ± standard deviation.
LA, left atrial; LV, left ventricular; RV, right ventricular.
Data available for 53 patients in the DD− group and 30 patients in the DD+ group.
Data available for 52 patients in the DD− group and 29 patients in the DD+ group.
By CMR, DD+ patients had lower LV EF and cardiac output and greater LV mass (all p≤0.032). Focal myocardial fibrosis was more common among DD+ patients (19.0%) among DD+ as compared with DD− patients (5.3%) (p<0.01). The relative extent of LV fibrosis was greater among DD+ patients (p<0.01), but the extent of fibrosis among those with any fibrosis present was similar between DD+ and DD− patients (p=0.93). There were no significant differences between DD+ and DD− patients in LV or RV volumes, or RV ejection fraction. For all left ventricular segments, wall motion was normal in ≥95% of patients. There were no significant differences in wall motion score for any segment between DD+ and DD− groups (all p≥0.13), with exception of a trend for greater frequency of abnormal apical anterior wall motion score among DD+ patients (p=0.07). CMR findings for DD+ and DD− groups were consistent after excluding patients with a history of HF.
Serum Biomarkers
Both DD+ and DD− groups had median NT-proBNP levels within the normal range (e.g., ≤100 pg/mL), but elevated levels were more common among DD+ patients (p=0.01) (Table 4). DD+ patients had a higher degree of myocardial injury, as measured by high-sensitivity troponin I (p<0.01). There were trends towards higher levels of inflammatory markers among DD+ as measured by IL-6 (p=0.08) and oxidized low-density lipoprotein (LDL) (p=0.07), but levels of high sensitivity C-reactive protein (hs-CRP) were similar. Among markers of cardiac fibrosis and remodeling, compared with DD− patients, DD+ patients showed increased levels of carboxy-terminal telopeptide of collagen type 1 (CITP) (p=0.04), a trend towards increased levels of GDF-15 (p=0.07), and similar levels of soluble ST-2 and galectin-3. Markers of immune activity were generally similar between DD+ and DD− groups, with exception of higher percentages of CD4+/IFNg+ and CD8+/HLADR+ cells among DD+ patients (all p≤0.03). Serum biomarker findings were generally consistent after excluding patients with a history of HF (Supplemental Table 4).
Table 4.
Serum Biomarker Profile by Diastolic Dysfunction Status
| Biomarker Domain | Diastolic Dysfunction Absent (N=101) | Diastolic Dysfunction Present (N=94) | P Value |
|---|---|---|---|
| Ventricular wall stress | |||
| NT-proBNP (pg/mL) | 26.2 (12.0, 46.8) | 36.1 (22.7, 85.3) | <0.01 |
| NT-proBNP >100 pg/mL | 9 (9) | 20 (21) | 0.01 |
| Myocardial injury | |||
| High-sensitivity troponin I (pg/mL) | 2.5 (1.8, 3.5) | 3.6 (2.6, 5.1) | <0.01 |
| High-sensitivity troponin I >3.0 pg/mL | 37 (37) | 56 (61) | <0.01 |
| Inflammation | |||
| hs-CRP (ug/mL) | 1.6 (0.8, 4.0) | 2.4 (1.1, 5.7) | 0.11 |
| IL-6 (pg/mL) | 0.9 (0.5, 1.3) | 1.0 (0.7, 1.6) | 0.08 |
| Oxidized LDL (U/L) | 55.3 (44.6, 62.1) | 57.0 (50.4, 69.1) | 0.07 |
| Cardiac fibrosis and remodeling | |||
| CITP (ug/L) | 3.7 (2.9, 4.8) | 4.1 (3.2, 5.7) | 0.04 |
| ST-2 (ng/mL) | 22.4 (18.7, 27.0) | 23.1 (19.4, 27.0) | 0.54 |
| Galactin-3 (ng/mL) | 9.7 (8.0, 12.2) | 10.6 (8.6, 13.4) | 0.15 |
| GDF-15 (pg/mL) | 734.8 (569.5, 1078.4) | 867.2 (627.5, 1385.2) | 0.07 |
| GDF-15 >1000pg/mL | 30 (30) | 38 (40) | 0.12 |
| MMP-1 (pg/mL) | 6463.0 (4021.0, 9909.0) | 6068.4 (3803.2, 9525.0) | 0.62 |
| Oxidative stress | |||
| Myeloperoxidase (ng/mL) | 19.4 (14.9, 27.0) | 20.8 (14.2, 32.7) | 0.90 |
| Immune activation | |||
| Classic monocytes (%) | 58.6 (46.1, 68.1) | 59.1 (51.4, 69.5) | 0.66 |
| Intermediate monocytes (%) | 4.6 (3.1, 6.7) | 5.1 (3.2, 6.9) | 0.67 |
| Non-classic monocytes (%) | 4.8 (2.8, 7.5) | 4.5 (2.8, 8.0) | 0.72 |
| sCD163 ng/mL | 553.1 (430.1, 787.7) | 602.7 (445.8, 816.1) | 0.24 |
| sCD14 (ng/mL | 2003.1 (1702.9, 2301.6) | 1942.7 (1688.4, 2220.9) | 0.49 |
| CD4+/CD38+ cells (%) | 37.1 (23.4, 51.4) | 33.4 (24.9, 40.7) | 0.07 |
| CD4+/HLADR+ cells (%) | 15.5 (11.4, 19.3) | 15.5 (12.4, 19.1) | 0.50 |
| CD4+/IFNg+ cells (%) | 16.8 (12.8, 22.4) | 20.5 (14.9, 27.2) | <0.01 |
| CD4+/IL-4+ cells (%) | 3.2 (2.1, 4.7) | 3.1 (2.1, 4.4) | 0.95 |
| CD4+/IL-17+ cells (%) | 2.0 (1.4, 3.4) | 2.2 (1.5, 2.9) | 0.64 |
| CD8+/CD38+ cells (%) | 13.7 (9.6, 20.9) | 13.5 (9.2, 17.6) | 0.50 |
| CD8+/HLADR+ cells (%) | 17.7 (13.0, 23.3) | 21.4 (14.4, 29.9) | 0.03 |
| CD8+/IFNg+ cells (%) | 42.5 (31.2, 51.9) | 47.6 (35.9, 58.0) | 0.06 |
| CD8+/IL-4+ cells (%) | 2.8 (1.9, 3.7) | 2.6 (2.0, 3.3) | 0.85 |
| CD8+/IL-17+ cells (%) | 1.7 (1.0, 2.5) | 1.5 (0.8, 2.5) | 0.35 |
Data presented as n (%) or median (25th, 75th).
CITP, C-terminal telopeptide of collagen I; GDF-15, growth differentiation factor 15; hs-CRP, high sensitivity C-reactive protein; IL, interleukin; LDL, low density lipoprotein; MMP, matrix metalloproteinases; NT-proBNP, N-terminal pro-B-type natriuretic peptide; ST-2, suppression of tumorigenicity-2
Patent-reported Quality of Life
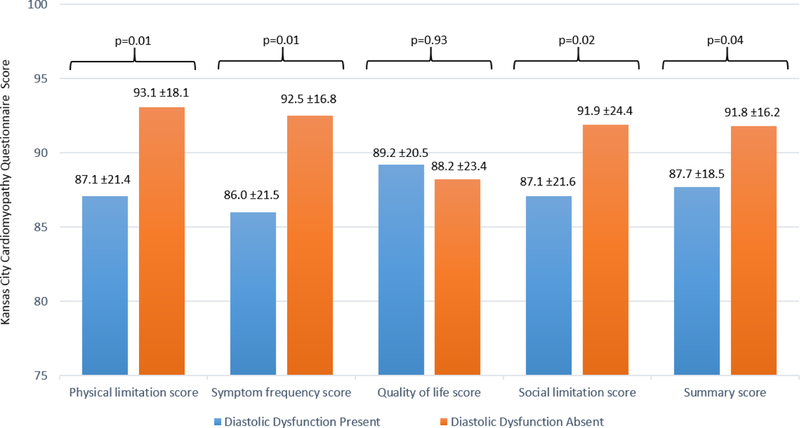
KCCQ scores are displayed in Figure 2. Across all measures, baseline QOL was generally favorable with all scores for both study groups >85. However, compared with DD− patients, DD+ patients reported symptomatic limitation with lower scores for the physical limitation, symptom frequency, social limitation, and summary scores (all p≤0.04). Among these scores, magnitude of difference was largest for the symptom frequency score (difference of means, 6.5), followed by the physical limitation score (difference of means, 6.0). After excluding patients with baseline HF, absolute differences between DD+ and DD− were attenuated but remained statistically significant for the physical limitation (difference of means, 4.6; p=0.04) and symptom frequency scores (difference of means, 4.6; p=0.04) (Supplemental Figure 1).
Figure 2. Patient-Reported Quality of Life As Measured by the Kansas City Cardiomyopathy Questionnaire by Diastolic Dysfunction Status.
Scores range from 0–100 with higher scores reflecting a more favorable response. Data presented as mean ± standard deviation
Patient Characteristics and Study Endpoints Stratified by Age
Post-hoc sensitivity analyses of patient characteristics and study endpoints stratified by age ≥55 years/ <55 years are presented in Supplemental Tables 5–8. In general, although absolute values varied by age in some instances, relationships between DD and variables were consistent among both older patients and younger patients. Notable exceptions included weight and BMI, whereby DD was associated with significantly higher weight and BMI among patients age <55 years, as compared with no significant differences in weight and BMI for older DD+ versus DD− patients (Supplemental Table 5). In addition, global longitudinal strain was significantly impaired among younger DD+ patients as compared with younger DD− patients, whereas there was no significant association between DD and global longitudinal strain among older patients (Supplemental Table 7).
DISCUSSION
This cross-sectional CHART study was designed to investigate the mechanisms and consequences of DD among treated and suppressed HIV+ patients without overt systolic dysfunction or valvular heart disease. Despite generally mild DD and similar LA size, compared with DD− patients, HIV+ patients with DD had evidence of LA and LV dysfunction by speckle tracking echocardiography and strain measurements. CMR and biomarker findings suggest that these functional differences may reflect an increased burden of myocardial fibrosis and injury among DD+ patients. In addition, despite nearly all study patients reporting no prior history of HF and having NT-proBNP levels within the normal range, mild DD and the attendant subclinical alterations in cardiac structure and function were associated with statistically significant and clinically relevant impairments in QOL scores. Relationships between DD and the various endpoint domains remained generally similar after excluding the small number of patients with prior history of HF. Overall, these results are consistent with the pre-specified study hypothesis that despite similar ART and viral suppression, HIV+ patients with DD demonstrate a higher degree of myocardial fibrosis and left atrial dysfunction.
Despite CHART eligibility criteria requiring absence of overt structural heart disease, rigorous cardiac assessment using CMR and speckle-tracking echocardiography detected several key differences between patients with and without DD. Prior studies have found HIV+ patients to demonstrate greater myocardial fibrosis.14, 15, 18, 19 In CHART, CMR with late gadolinium enhancement demonstrated an increased prevalence of focal fibrosis among HIV+ patients with DD, findings further supported by concurrently higher levels of CITP, a marker of collagen degradation and turnover. Likewise, despite LA size generally within the normal range and similar to patients without DD, DD+ patients demonstrated impaired LA function as reflected by LA conduit strain (i.e., increased LA stiffness) and LA reservoir strain (i.e., impaired LA relaxation). Similar findings were seen in the ventricle where DD+ patients had reduced systolic function as measured by GLS despite a preserved EF. The fibrosis and strain findings suggest that HIV+ patients with DD demonstrate clear subclinical alterations in cardiac structure and function that may go undetected in routine practice. These changes are particularly noteworthy as CHART patients generally had mild DD (i.e., mean E/A ratio 0.9, E/e’ ratio 9.6, LA volume index 27.2 mL/m2). Further research is needed to clarify the natural history of these structural and functional alterations, including the risk of subsequent symptomatic cardiovascular disease, the pace at which progression may occur, and whether the clinical course can be modified by specific therapies. The current data support the possibility of a prevention window, a period during which a therapy could possibly attenuate or halt disease progression and prevent clinical deterioration. This rationale underlies current guideline recommendations regarding Stage B HF (i.e., structural heart disease without signs and symptoms of HF), with the present data extending this approach to the HIV population.20
CHART is among the first multi-center investigations of potential mechanisms of DD among virally suppressed HIV+ patients effectively treated with contemporary ART. Prior research supports a systemic proinflammatory state as the potential cause of structural and functional cardiac abnormalities, incident DD, and eventual symptomatic HFpEF.10 Comorbidities common to patients with DD, such as hypertension, obesity, and metabolic syndrome, are associated with elevated levels of pro-inflammatory cytokines and endothelial dysfunction in the general population.10 Among virally suppressed HIV+ patients, the degree of systemic inflammation can vary and prior work has shown higher inflammatory levels associated with higher risk of cardiovascular morbidity and mortality.21–23 Although prior work has focused near uniformly on atherosclerotic cardiovascular disease and symptomatic HF, the present study assessed whether such associations with inflammatory markers may extend to earlier stages of development of cardiac abnormalities. Although failing to reach formal statistical significance and in need of future confirmation, the observed trends towards higher levels of IL-6 and oxidized LDL among DD+ patients are compatible with a potential inflammatory mechanism of DD within the HIV+ population. hs-CRP levels were comparable between DD+ and DD− patients, but hs-CRP may be a less reliable inflammatory marker among patients with HIV and particularly impacted by co-infections.24 However, the lack of robust association in this study also highlights the importance of investigating alternate pathways that might mediate the earlier stages of pathophysiology. Further studies assessing proteomic and metabolomics profiling in these patients may provide further insight in this respect.
Aside from biomarker and imaging findings, associations between DD and patient-reported QOL warrant mention. Overall, 4 of 5 KCCQ scores demonstrated statistically significant symptomatic limitation among DD+ patients. In the setting of prior data from HF populations supporting a score difference ≥5 as clinically relevant, the magnitudes of difference between DD+ and DD− patients approached or surpassed this threshold.25 Despite >90% of DD+ patients having no formal diagnosis of HF and NT-proBNP levels generally within the normal range, it is possible that these findings reflect early stages of clinical progression towards symptomatic HF. Alternatively, while HIV-specific characteristics were relatively similar between groups, it is possible that worse QOL among DD+ patients was driven by a modestly greater burden of cardiometabolic comorbidity (e.g., hypertension, hyperlipidemia), including higher baseline systolic blood pressure and body mass index. Studies with larger sample size and serial assessments are required to validate these findings and confirm potential links between mild DD, myocardial fibrosis, impaired LA and LV strain, and patient-reported outcomes.
Limitations of this study includes the cross-sectional design that cannot prove causality or the directionality of relationships between DD and any of the parameters tested. Given the single visit nature of CHART, statistical models were univariate and designed as non-parametric rank-based models. Multivariable models required a switch to a parametric-based approach and none of the tested models were able to meet minimum assumptions for validity (e.g., normality of the model residuals). Thus, multivariable modeling was not performed. In addition, in keeping with the hypothesis-generating nature of this early phase study, correction for multiple comparisons was not performed. Likewise, sample size was modest and may have limited power for detecting significant relationships. Although CMR assessments were performed in nearly all patients, only a subset of patients had available data for the myocardial T1 value pre-contrast and extracellular volume fraction. Larger studies with longitudinal assessments of myocardial structure and function are needed to confirm the hypotheses derived from the current study.
In conclusion, in this contemporary experience of virally suppressed HIV+ patients receiving ART, DD was associated with multiple alterations in cardiac structure and function not evident on routine imaging. These abnormalities corresponded with biomarker differences and impaired QOL scores. Collectively, these findings support the clinical relevance of even mild DD among well-treated HIV+ patients and suggest a pathophysiologic role for myocardial fibrosis and potentially systemic inflammation. Further studies are needed to assess changes in fibrosis and inflammatory parameters over time, their abilities to predict onset of symptomatic HF, and their potential as therapeutic targets to attenuate or halt progressive cardiac remodeling and dysfunction.
Supplementary Material
ACKNOWLEDGEMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
SOURCES OF FUNDING
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers U10 HL084904, U10 HL110297, U10 HL110342, U10 HL110309, U10 HL110262, U10 HL110338, U10 HL110312, U10 HL110302, U10 HL110336, and U10 HL110337.
DISCLOSURES
J.B. has received research support from the National Institutes of Health, Patient Centered Outcomes Research and the European Union. He serves on the speaker bureau for Novartis, Janssen, and NovoNordisk. He serves as a consultant and/or serves on steering committee, clinical events committee, or data safety monitoring boards for Abbott, Adrenomed, Amgen, Array, Astra Zeneca, Bayer, BerlinCures, Boehringer Ingelheim, Bristol Myers Squib, Cardiocell, CVRx, G3 Pharmaceutical, Innolife, Janssen, Lantheus, LinaNova, Luitpold, Medscape, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, StealthPeptide, SC Pharma, V-Wave Limited, Vifor, and ZS Pharma. S.J.G. has received support from a Heart Failure Society of America/ Emergency Medicine Foundation Acute Heart Failure Young Investigator Award funded by Novartis; has received research support from Amgen, Bristol-Myers Squibb, and Novartis; and serves on an advisory board for Amgen. S.H.S. has received support from the National Institutes of Health and the American Heart Association; holds a patent on an unrelated finding. S.J.S. has received research support from the National Institutes of Health (R01 HL127028 and R01 HL107577) and the American Heart Association, Actelion, Astra Zeneca, Bayer, and Novartis; consultant to Actelion, Amgen, Astra Zeneca, Bayer, Boehringer-Ingelheim, Cardiora, Eisai, Gilead, Ironwood, Merck, Myokardia, Novartis, Sanofi, and United Therapeutics. R.J.K. has received research support from Bayer, Stealth Biotherapeutics, and Astellas. A.P.K. has received research support from the American Heart Association; and has been a consultant to Roche Diagnostics. E.J.V. has received research support from National Institutes of Health, Novartis, Amgen, Pfizer, Alnylam, Philips, GE, and Bay Labs; honoraria from Novartis, Amgen, Merck, Pfizer, Expert Exchange, and Abiomed. A.F.H. reports consulting fees from AstraZeneca, Bayer, Boston Scientific, Merck, Novartis, Sanofi, and research support from AstraZeneca, GlaxoSmithKline, Luitpold, Merck, Novartis. P.Y.H. has received research support from the National Institutes of Health and Pfizer; honoraria received from Gilead. E.B. has received grant support from Duke University as Chair of the Heart Failure Network of the National Heart, Lung, and Blood Institute Heart Failure Network and from Merck and Company, Astra Zeneca, Novartis, Daiichi Sankyo, and Glaxo Smith Kline; and consulting fees from The Medicines Company and Theravance; personal fees for lectures from Medscape and Menarini International. He was also uncompensated for consultancies and lectures from Merck and Novartis. All other authors report no conflicts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT02860156
REFERENCES
- 1.Wandeler G, Johnson LF and Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS. 2016;11:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerrato E, D’Ascenzo F, Biondi-Zoccai G, Calcagno A, Frea S, Grosso Marra W, Castagno D, Omedè P, Quadri G, Sciuto F, Presutti D, Frati G, Bonora S, Moretti C and Gaita F. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J. 2013;34:1432–6. [DOI] [PubMed] [Google Scholar]
- 3.Lumsden RH and Bloomfield GS. The Causes of HIV-Associated Cardiomyopathy: A Tale of Two Worlds. Biomed Res Int. 2016;2016:8196560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontes-Carvalho R, Mancio J, Marcos A, Sampaio F, Mota M, Rocha Gonçalves F, Gama V, Azevedo A and Leite-Moreira A. HIV patients have impaired diastolic function that is not aggravated by anti-retroviral treatment. Cardiovasc Drugs Ther. 2015;29:31–9. [DOI] [PubMed] [Google Scholar]
- 5.Hsue PY, Hunt PW, Ho JE, Farah HH, Schnell A, Hoh R, Martin JN, Deeks SG and Bolger AF. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail. 2010;3:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo L, Zeng Y, Li T, Lv W, Wang H, Guo F, Han Y, Xie J, Qiu Z, Li Y, Song X, Zhu T, Zhang X, Li L, Ye Y, He Y, Lu H, Huang A, Tang X, Wang H, Zhang T, Gao G, Lei J, Wu X, Sun Y, Bai J, Li K and China AIDS Clinical Trial 0810 Group. Prospective echocardiographic assessment of cardiac structure and function in Chinese persons living with HIV. Clin Infect Dis. 2014;58:1459–66. [DOI] [PubMed] [Google Scholar]
- 7.Schuster I, Thöni GJ, Edérhy S, Walther G, Nottin S, Vinet A, Boccara F, Khireddine M, Girard P-M, Mauboussin J-M, Rouanet I, Dauzat M, Cohen A, Messner-Pellenc P and Obert P. Subclinical cardiac abnormalities in human immunodeficiency virus-infected men receiving antiretroviral therapy. Am J Cardiol. 2008;101:1213–7. [DOI] [PubMed] [Google Scholar]
- 8.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jacobsen SJ and Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halley CM, Houghtaling PL, Khalil MK, Thomas JD and Jaber WA. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171:1082–1087. [DOI] [PubMed] [Google Scholar]
- 10.Paulus WJ and Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen KK, Pedersen M, Gaardbo JC, Ronit A, Hartling HJ, Bruunsgaard H, Gerstoft J, Ullum H and Nielsen SD. Persisting inflammation and chronic immune activation but intact cognitive function in HIV-infected patients after long-term treatment with combination antiretroviral therapy. J Acquir Immune Defic Syndr. 2013;63:272–9. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen KA, Peer N, Mills EJ and Kengne AP. A Meta-Analysis of the Metabolic Syndrome Prevalence in the Global HIV-Infected Population. PLoS ONE. 2016;11:e0150970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingery JR, Alfred Y, Smart LR, Nash E, Todd J, Naguib MR, Downs JA, Kalluvya S, Kataraihya JB and Peck RN. Short-term and long-term cardiovascular risk, metabolic syndrome and HIV in Tanzania. Heart. 2016;102:1200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiara DK, Liu CY, Raman F, Mangat S, Purdy JB, Duarte HA, Schmidt N, Hur J, Sibley CT, Bluemke DA and Hadigan C. Abnormal Myocardial Function Is Related to Myocardial Steatosis and Diffuse Myocardial Fibrosis in HIV-Infected Adults. J Infect Dis. 2015;212:1544–1551-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, Hancock G, Beak P, Tajar A, Piechnik SK, Schneider JE, Angus B, Clarke K, Dorrell L and Neubauer S. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128:814–22. [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Zamudio M, Dey D, LaBounty T, Nelson M, Fan Z, Szczepaniak LS, Hsieh BP, Rajani R, Berman D, Li D, Dharmakumar R, Hardy WD and Conte AH. Increased pericardial fat accumulation is associated with increased intramyocardial lipid content and duration of highly active antiretroviral therapy exposure in patients infected with human immunodeficiency virus: a 3T cardiovascular magnetic resonance feasibility study. J Cardiovasc Magn Reson. 2015;17:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butler J, Kalogeropoulos AP, Anstrom KJ, Hsue PY, Kim RJ, Scherzer R, Shah SJ, Shah SH, Velazquez EJ, Hernandez AF, Desvigne-Nickens P and Braunwald E. Diastolic Dysfunction in Individuals With Human Immunodeficiency Virus Infection: Literature Review, Rationale and Design of the Characterizing Heart Function on Antiretroviral Therapy (CHART) Study. Journal Card Fail. 2018;24:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ntusi N, O’Dwyer E, Dorrell L, Wainwright E, Piechnik S, Clutton G, Hancock G, Ferreira V, Cox P, Badri M, Karamitsos T, Emmanuel S, Clarke K, Neubauer S and Holloway C. HIV-1-Related Cardiovascular Disease Is Associated With Chronic Inflammation, Frequent Pericardial Effusions, and Probable Myocardial Edema. Circ Cardiovasc Imaging. 2016;9:e004430. [DOI] [PubMed] [Google Scholar]
- 19.Luetkens JA, Doerner J, Schwarze-Zander C, Wasmuth J-C, Boesecke C, Sprinkart AM, Schmeel FC, Homsi R, Gieseke J, Schild HH, Rockstroh JK and Naehle CP. Cardiac Magnetic Resonance Reveals Signs of Subclinical Myocardial Inflammation in Asymptomatic HIV-Infected Patients. Circ Cardiovasc Imaging. 2016;9:e004091. [DOI] [PubMed] [Google Scholar]
- 20.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ and Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–52. [DOI] [PubMed] [Google Scholar]
- 21.Kuller LH, Tracy R, Belloso W, De Wit S, Drummed F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaon JD and Group ISS. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Nixon D, Paton NI, Prineas RJ, Neaton JD and Group ISS. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS ONE. 2012;7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grund B, Baker JV, Deeks SG, Wolfson J, Wentworth D, Cozzi-Lepri A, Cohen CJ, Phillips A, Lundgren JD, Neaton JD and Group ISESS. Relevance of Interleukin-6 and D-Dimer for Serious Non-AIDS Morbidity and Death among HIV-Positive Adults on Suppressive Antiretroviral Therapy. PLoS ONE. 2016;11:e0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reingold J, Wanke C, Kotler D, Lewis C, Tracy R, Heymsfield S, Tien P, Bacchetti P, Scherzer R, Grunfeld C and Shlipak M. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr. 2008;48:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS and Cardiovascular Outcomes Research C. Monitoring clinical changes in patients with heart failure: a comparison of methods. American Heart J. 2005;150:707–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.