Abstract
The use of multiple medications is growing at an alarming rate with some reports documenting an average of 12 to 22 prescriptions being used by individuals ≥50 years of age. The indirect consequences of polypharmacy include exacerbation of drug-drug interactions, adverse drug reactions, increased likelihood of prescribing cascades, chronic dependence, and hospitalizations - all of which have significant health and economic burden. While many practical solutions for reducing polypharmacy have been proposed, they have been met with limited efficacy. This highlights the need for a new systematic approach for fine-tuning dispensing of medications. Pharmacogenetic testing provides an empirical and scientifically rigorous approach for guiding appropriate selection of medicines, with the potential to reduce unnecessary polypharmacy while improving clinical outcomes. The goal of this review article is to provide healthcare providers with an understanding of polypharmacy, its adverse effects on the healthcare system and highlight how pharmacogenetic information can be used to avoid polypharmacy in patients.
Keywords: polypharmacy, pharmacogenetics, PGx testing
Introduction
The use of multiple medications, polypharmacy, is growing at an alarming rate, with some reports documenting a range of 12 to 22 prescriptions prescribed to individuals ≥50 years of age [1]. From a clinical perspective, polypharmacy is often necessary to treat multiple illnesses and comorbidities. Prescribing multiple medications creates a conundrum. While the goal of polypharmacy is to improve patient quality of life and longevity, excessive use of medications has adverse health effects and creates substantial financial burden for patients and the healthcare system. Patients with polypharmacy are more likely to experience drug-drug interactions (DDIs) and adverse drug reactions (ADRs), associated with increased morbidity and mortality and require more hospital admissions [2]. Few strategies exist for managing polypharmacy and lack of their utility is shown by the ever-rising incidence of this condition [3–5]. In this review we focus on evidence that supports the notion that unnecessary polypharmacy is a national healthcare problem, and how implementing pharmacogenetic (PGx)-testing into everyday practice could provide a sustainable solution. We start by reviewing the definition of polypharmacy, root causes and its adverse consequences. We then review the fundamental basics of PGx and PGx-testing. We then focus on evidence that supports the implementation of PGx testing as a solution to the polypharmacy conundrum, and the needs to incorporate the use of pharmacogenetics information into everyday prescribing practice.
Part 1: Polypharmacy as a Healthcare Problem
Defining Polypharmacy
Polypharmacy is commonly defined as the simultaneous use of multiple medications, but polypharmacy studies often have their own criteria for what classifies as “polypharmacy”, making it difficult to interpret and integrate findings from different groups. In general, there are two working definitions of polypharmacy. One definition is based solely on the number of medications taken by an individual, referred to as the “quantitative definition”. This definition does not take into consideration adverse effects that arise with excessive drug use, nor does it settle on a “numerical threshold” for what classifies as polypharmacy. Hajjar et al. analyzed 21 polypharmacy studies, and indicated there was no consensus on the number of drugs used to define the term with a range of 2–9 drugs per person per day [6]. The definition of polypharmacy is further complicated by the time frame for which polypharmacy is defined (e.g., the number of drugs taken per day, per month, etc.) [6].
Polypharmacy can also refer to the administration of more medications than clinically indicated [6]. This is a functional definition that separates “unnecessary” or “excessive” polypharmacy from “necessary” polypharmacy, and accounts for improper use of drugs. Using this definition, Lipton et al. showed that 60% of patients in their study were taking medications that were either suboptimal for the patient’s clinical condition, or lacked an indication altogether [7]. Furthermore, 16% of people in this study had therapeutic duplication in their medication list. Regardless of the definition one chooses, polypharmacy is an iatrogenic condition causing harm to patients and placing economic burden on the healthcare system. In order to understand polypharmacy as a health problem, we must first recognize the inherent drivers of this condition.
Drivers of Polypharmacy
There are many drivers of polypharmacy. Here we focus on the influence of aging and the influence of a market-driven approach to healthcare [2,6,8].
Effects of aging
As of 2017, the overall age-independent prevalence of polypharmacy in the United States was 10%, but the rate of polypharmacy in people ≥65 years old is 30–40% [6,9]. This three-fold increase of polypharmacy in aged patients may be attributed to the general trend that with aging, people are more likely to develop multiple health conditions. These comorbid conditions include cardiovascular disease, chronic kidney disease, cancer and metabolic diseases (Figure 1) [10,11]. Not surprisingly, common classes of drugs prescribed to patients ≥65 years of age correspond well to the comorbidities frequently encountered in this cohort. These medications include cardiovascular/cholesterol-lowering agents, diuretics, anti-depressants, and opioids [6]. The effects of these drugs are highly variable due to aging physiology, and when combined with other medications, often induce unwanted effects through drug-drug interactions [2,6]. Within this cohort, depression is a prominent comorbidity. A study conducted by the Veterans Administration Healthcare System reported that the incidence of polypharmacy was high in subjects taking antidepressants, and patients ≥ 60 years old on antidepressants had the highest incidence of polypharmacy [12].
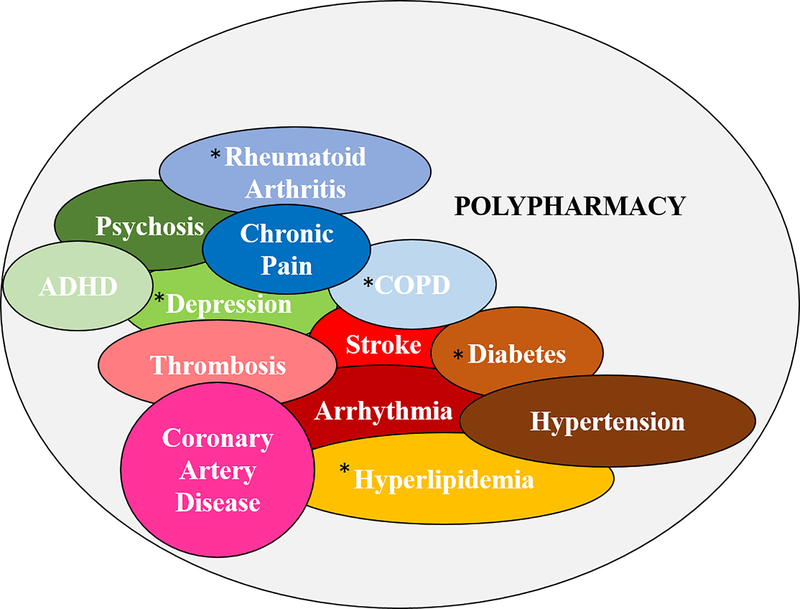
Figure 1.
Common comorbidities associated with polypharmacy. COPD, rheumatoid arthritis, depression, diabetes, and hyperlipidemia are the most common comorbidities in the elderly.
Elderly patients also take over-the-counter (OTC) drugs, producing another source of polypharmacy that may go largely unnoticed by healthcare providers [6]. A misconception is that, because these drugs do not require a prescription, they are safe to use without consequence. Many of these OTC drugs are dietary supplements, which are not regulated by the FDA for safety and efficacy. Contraindications of OTC drugs are prominent, especially in combination with prescription drugs. As example, GB is an OTC extract that has several health benefits, including enhancement of memory in patients with dementia or Alzheimer’s disease, treatment of vertigo, and other indications in stroke and claudication [13]. However, the combination of GB with warfarin can increase the risk of bleeding events because GB itself causes decreased platelet aggregation [13,14]. Using a large database associated with VA hospitals across the US, Stoddard et al. identified 807,399 patients taking warfarin with 11,003 taking warfarin with GB. Eighteen percent of patients using warfarin alone had an adverse bleeding event during this longitudinal six-year study and the number of bleeding events increased to 24% among patients taking warfarin and GB concomitantly [14].
Typically, most studies on polypharmacy are done in aged populations, since the prevalence of polypharmacy is higher. However, polypharmacy is becoming more prominent in pediatric populations, although these studies are fewer in number and focus on the use of psychotropic drugs. In this specific population, polypharmacy is likely driven by the number of healthcare providers a child sees and the severity of the disease [15]. In a meta-analysis by Baker et al., the prevalence of polypharmacy varied greatly (22%−54%) depending on disease severity, methodology used to conduct the study, and the definition of polypharmacy used [16]. This report indicated that the prevalence of pediatric polypharmacy was 34% during 2011–2017 in the United States [16]. An earlier meta-analysis from 1987–1996 by Zonfrillo et al. also showed a wide variation in prevalence of polypharmacy, ranging from 0.7%−42% [17–19]. The discrepancy between these two rates can be attributed to the wide variation in the type of information collected to infer rates of polypharmacy, the number of pediatric patients included in each study, and the definition of polypharmacy used [17].
Other populations at risk for polypharmacy include cancer patients and those with HIV/AIDS - all demonstrating a high level of polypharmacy [20–22]. Prevalence of polypharmacy in AIDS patients is estimated to be between 30–70%, and HIV infection independently accounts for a higher prevalence of polypharmacy when compared to HIV-negative patients [23]. Polypharmacy in oncology patients can be as high as 75%, and the rate is doubled in cancer survivors compared to age-matched, non-cancer patients [20–22,24].
Market-driven Healthcare
Direct-to-consumer (DTC) marketing and pressure from patients on their healthcare providers are two additional drivers of polypharmacy. Both drivers stem from commercial efforts by the pharmaceutical industry to advertise their products.
In 1997, the Food and Drug Administration made it easier for companies to legally broadcast prescription drug advertisements [25]. According to one estimate, after this FDA policy change, televised advertisement spending by pharmaceutical companies increased, peaking at 3.3 billion dollars in 2006 (16). Furthermore, DTC advertisements comprised approximately 13% of total promotional spending in 2012[26,27]. This increase in spending was met with an increase in prescription drug sales [28].
A study by McKinlay and summarized by Fain and Alexander provides evidence that patient requests that stem from DTC advertisements affect physician prescription practices [29–32]. A randomized cohort of 192 primary care physicians from six states were presented with clinical scenarios depicting patients with symptoms of sciatica or chronic osteoarthritis. Each scenario consisted of two patient requests - either an active request for a specific medication or passive request for pain relief. One in five physicians (20%) reported they would prescribe oxycodone for the sciatic patient actively requesting the drug, compared to just 1% of physicians whose patients made a passive request for pain relief. More than half (53%), of physicians reported they would prescribe Celebrex to patients with diagnosed osteoarthritis requesting the drug, while only 25% reported they would prescribe the drug for patients making a passive request for pain relief [30,31]. The effects of patients’ active requests for oxycodone is particularly noteworthy, given the reported epidemic of opioid-related morbidity and mortality in the United States, as well as the common prescribing of opioids in the elderly population and to patients that have polypharmacy [30,33]. Kravitz et al. also reported that patient requests for antidepressants based on DTC advertisement had a profound effect on physician prescribing [34]. In the context of polypharmacy, prescription of a drug due to request rather than taking the time to determine the best drug for the patient may mean the drug is less efficacious and may require further prescription of other medications to improve efficacy. Furthermore, the patient may be at risk for adverse side effects due to alterations in pharmacokinetics and drug response that are not taken into consideration before the drug is prescribed, leading to increased risk for drug-drug interactions or adverse drug reactions.
In conclusion, several drivers are responsible for polypharmacy. Some of these drivers can be controlled, especially the influence of DTC advertising and patient pressure on physicians to prescribe specific drugs. Aging and associated comorbidities are unavoidable and require different management approaches. Regardless of the driver, polypharmacy puts patients at risk for severe clinical consequences.
Clinical Consequences of Polypharmacy
Polypharmacy affects patient quality of life by increasing the risk of adverse drug reactions (ADRs), drug-drug interaction (DDIs), and initiation of prescribing cascades (prescribing other medications to treat a side effect of another drug). Having an ADR or a DDI can increase the risk of future events such as frequent hospitalizations, emergency room visits, and need for outpatient consultations, as well as increased risk of morbidity and mortality.
Adverse Drug Reactions (ADRs)
ADRs are defined as unfavorable medical events that are the direct result of either the correct or incorrect use of a medication [2,35,36]. ADRs account for 3–23% of all hospital admissions, and identification of risk factors may be a strategy to reduce the number of ADRs [37,38]. Classification of patients as “high-risk” for an ADR would mean that extra attention would be paid to all drugs prescribed [38]. Proposed risk factors for ADRs include age, gender, polypharmacy, renal and hepatic insufficiency, alcoholism, and alteration in drug metabolism [37,38]. Indeed, several analyses of risk factors have implied connections between polypharmacy and known pharmacogenetic alterations in drug metabolism [37–41]. Genetic polymorphisms are known to increase risk of adverse events when other risk factors are present - especially with use of multiple medications [37,42]. Polypharmacy is an independent risk factor for ADRs. Nguyen et al. found that there was a positive correlation between the use of ≥9 medications and risk of having an ADR even when other factors such as age and gender were eliminated between the control (<9 medications) and case groups [41].
Although ADRs are a common reason for hospital admission, they are not often explored as a cause-of-visit when a patient arrives for emergency care. The failure to screen for ADRs can increase the risk for a patient to be prescribed additional drugs while hospitalized that may cause additional ADRs, exacerbate the patient’s current ADR, or lead to the initiation of a prescribing cascade [2]. As a result, ADRs often prolong hospital stays and are associated with increased morbidity and mortality [40]. Carmona-Huerta et al. showed that polypharmacy was directly related to the length of the hospital stay for psychiatric patients, resulting in an average of 6.56 days in the hospital for each additional prescription medication [43]. Patients with polypharmacy (≥5 medications) who underwent elective surgeries had a 3% 90-day mortality rate, and this was significantly increased compared to patients who did not meet criteria for polypharmacy even after multilevel, multivariable adjustment [44]. Additionally, polypharmacy was also associated with increased number of readmissions and increased cost of stay which could have been further attributed to the increased likelihood of an in-hospital complication [44].
One approach to reducing risk associated with ADRs is by implementing better screening techniques. Hohl et al. defined an ADR as unwanted effects of a drug, adverse event associated with drug noncompliance, and drug withdrawal reactions [40]. In a cohort of 282 patients ≥65 years of age who presented to the ER, 90.8% were taking one or more prescribed or OTC medications upon arrival to the ER, with an average of 4.6 medications per person [40]. Approximately 10% of these patients were classified as having an ADR, which were most frequently caused by NSAIDS, antibiotics, diuretics, and anticoagulants- all medications commonly seen in cases of polypharmacy. None of the patients taking only one medication experienced an ADR - further drawing the link between polypharmacy and ADRs [40].
Drug-Drug Interactions (DDIs)
In order to have a DDI, a patient must have polypharmacy as a drug-drug-interaction (DDI) is defined as a change in a drug’s effect, absorption, or metabolism when two or more drugs are administered simultaneously [2,45,46]. Like polypharmacy, the true prevalence of DDIs is not well-established and ranges from 9–70% depending on the population being studied and the methods used to identify DDIs [47]. In most circumstances, these interactions are associated with increased risk of toxicity and substantial ADRs. Approximately 3% of all hospitalizations are attributed to a DDI, resulting in over a billion dollars in added healthcare cost for a year [48]. In some instances, interaction between drugs can also enhance a drug’s effect and improve efficacy. In psychiatry, for example, DDIs are necessary as they are reported to improve brain functioning, boost the desired effect of an anti-psychotic, or speed up the onset of the desired drug effect [12].
The presence of polypharmacy correlates to increased risk of developing a DDI. In a study by Guthrie et al., data on age, gender, socioeconomic status, residence, and number of medications (including classes of medication) were collected from 300,000 people from the Tayside region of Scotland in 1995 and again in 2010 [49]. The main predictor for a DDI was the number of drugs prescribed - as the number of drugs increased, so did the risk of having a DDI. A large portion of people who received ≥15 medications had a potential DDI (80.8%), while only 10.4% of those taking 2–4 drugs had a potential DDI. This study corroborates with data from Haider et al. that indicated the prevalence of polypharmacy in Sweden increased from 1992–2002, and coincided with a greater exposure to a potentially serious DDIs [46].
In general, identifying DDIs is difficult. Although software programs can identify some DDIs, the quality of evidence for clinical utility of an electronic approach is low [48]. Another strategy implements medication review and screening by pharmacists for DDIs. However, this approach largely depends on memorization of DDIs [48]. More recently the idea that individual differences in drug-metabolizing enzymes can not only explain DDIs, but can also be used as a way to identify potential drug-interactions has gained momentum [48]. While the interaction between two or more medications may increase or decrease the efficacy of a medication of interest, unrecognized genetic polymorphisms - described later in this article - can affect the magnitude by which a drug interaction occurs [48]. Identifying DDIs using information based on drug-metabolizing status requires pharmacogenetic (PGx) testing, and presents a potential area for improvement in management of DDIs.
Prescribing Cascades
Prescribing cascades occur when a drug’s side-effect or an ADR is identified as a new health condition for which additional medications are prescribed, creating a cycle for increasing the risk for an additional ADR, DDI, and further driving polypharmacy. This process is exacerbated when the goal is to treat symptoms rather than the underlying disease, which is often the case for psychological disorders where the pathophysiology is poorly understood [12]. Many of the same drugs associated with ADRs are commonly found in prescribing cascades, including drugs for dementia, antihypertensives, sedatives, opioids, NSAIDs, seizure medications, and nausea medications [50]. Although the risk factors for prescribing cascades are not clearly defined, any risk factor for ADRs are likely to promote prescribing cascades, including age and polypharmacy [50].
The phenomenon of the prescribing cascade is clearly demonstrated by Nguyen et al., who reported on a 71 year-old female with a history of high blood pressure, asthma, hypothyroidism, depression, osteoarthritis, and Meniere’s disease who presented to the hospital after a fall leading to a fracture [51]. Four months before her hospital admission, the patient had been prescribed amlodipine to lower her blood pressure by her cardiologist. She developed edema as an ADR associated with amlodipine, but edema was subsequently misinterpreted as a new condition contributed to heart failure. For the edema, an urologist prescribed furosemide and another diuretic. Urinary incontinence then developed, and an antimuscarinic agent was prescribed by a third doctor. The antimuscarinic effect caused dry mouth that was further exacerbated by furosemide. Finally, anetholtrithion was given to the patient to treat her dry mouth. After admission to the hospital, a clinical pharmacist was able to identify the prescribing cascade, and even attributed the patient’s fall to the combination of drugs [51]. Upon identification of the prescribing cascade most of the patient’s medications were discontinued. A week after discontinuation, the patient no longer suffered from edema, urinary incontinence, or dry mouth [51]. This case study highlights how polypharmacy can initiate a prescribing cascade, and how this phenomenon can cause unnecessary polypharmacy.
A more general example of the prescribing cascade occurs in patients who take cholinesterase inhibitors for dementia, as they often develop the side effect of urge incontinence that requires additional medications for treatment [52]. A population-based retrospective study in Canada followed 44,884 adults with dementia over a four year period [52]. In this group, approximately 21,000 people received a cholinesterase inhibitor for dementia. Over the course of the four year study 1,662 patients (8%) were prescribed anticholinergic drugs to alleviate their incontinence [52]. Anticholinergic drugs themselves have severe ADRs including cognitive decline and delirium, which can be further exacerbated in patients with dementia. Thus, prescription of anticholinergics is contraindicated for use with cholinesterase inhibitors [52]. This high-risk prescribing cascade in a large population emphasizes the severity of the prescribing cascade problem, and how it can lead to unnecessary polypharmacy.
Economic Burden Associated with Polypharmacy
The cost of therapeutic drugs comprise a large portion of the total health expenditure in many countries [53]. Increases in drug expenditure are often explained by the increase in cost of drugs, the volume of drugs on the market, and the variety of drugs available. An additional explanation is polypharmacy, resulting in unnecessary healthcare expenditures associated with increased cost from the number of prescribed medications and costs associated with hospitalizations for ADRs, DDIs, and prescribing cascades [6,36,53].
Drug expenditures in countries that are part of the Organization for Economic Cooperation and Development (OECD) have increased 50% in the past ten years [53]. Approximately 25% of the population in Sweden have polypharmacy (≥5 prescribed drugs), and this group of people contribute to 80% of the total acquisition costs for prescription medications - a major component of the total prescription expenditure [53,54]. To analyze the total impact of polypharmacy on Sweden’s costs for medications, Hovstadius et al. utilized a registry of the entire population to determine the prevalence of polypharmacy and its associated cost. In general, prescription drug expenditure increased 4.8% over a five-year period, and this increase in was higher in the subset of patients with polypharmacy (6.2% increase) [53]. During this same time frame, the prevalence of polypharmacy increased 1.6% and the proportion of the prescription drug expenditure related directly to polypharmacy (acquisition costs) also increased [53]. These data indicate that as the prevalence of polypharmacy continues to increase, so will its associated costs and contribution to total healthcare expenditure.
In the US, the highest prevalence of polypharmacy is seen in the long-term care setting, with reports indicating polypharmacy in approximately 40% of these patients [55]. As such this population is commonly used for polypharmacy studies, especially those relating to economic outcomes [36,55]. Kojima et al. screened 70 long-term care residents with polypharmacy (≥9 medications) for potential high-risk medications using Beers Criteria and the Epocrates online DDI program. For each resident, recommendations were made to either continue or discontinue high-risk medications, or medications with potential DDIs. An average of three recommendations were made per resident, and these recommendations resulted in an overall decrease in the number of medications. In a secondary analysis, cost savings from these recommendations were calculated. A total savings of $6300 a month was recorded, averaging $90 per resident [36,55]. This study demonstrates that reducing the number of medications has a direct impact on reducing costs associated with polypharmacy. While this is only one component of total drug-related costs, it is one area that can be controlled simply by reducing the number of prescriptions.
Potential Solutions for Reducing Polypharmacy
Significant economic burden and poor patient outcomes resulting from polypharmacy highlight the need for a solution to this ever-increasing health problem. Traditional solutions have focused on: (1) improving communication between the patient and their healthcare provider, and (2) reviewing prescribed medication lists for medical necessity, potential high-risk medications, and other contraindications. The goal of these approaches is to assist clinicians in determining prescription appropriateness for their patients.
Improving Patient-Physician Communication
A proposed solution towards reducing polypharmacy is to improve prescriber and patient communication [56]. This has the potential to reduce the number of medications a patient is prescribed by eliminating drugs that have similar functions or drugs that show poor efficacy, while at the same time decreasing risk of an adverse outcome associated with potential DDIs. This approach can be particularly useful when patients are taking OTC medications, which may not be recorded in the patient’s medical record [56,57]. Furthermore, the prescriber may not be informed of medications that have been prescribed by other specialists and rely on the patient to provide that information. This is important because the number of prescribers a patient has seems to correlate to an increase in the number of medications a patient takes [12]. Despite the advantages of this approach, patient-physician communication may not always include conversations related to medications. Stevenson et al. recorded 62 physician/patient conversations regarding medication use, and found that only 19 patients volunteered information to their physician about their OTC medication or herbal remedy use, and five patients did not report their OTC use at all, even though they were using at least one OTC drug [56].
In the context of polypharmacy, Schäfer et al. conducted a year-long study to determine if a narrative-based approach could reduce polypharmacy in patients from 55 care facilities in Germany and ultimately improve quality of life [58]. Before the study started, patients were taking seven medications and had three chronic conditions on average. The intervention consisted of 30 minute sessions prompted by general practitioners to understand the patients’ personal prescription needs. At the conclusion of the study, the average number of medications did not change, and quality of life was nor improved [58]. The studies highlighted here show the limited efficacy of a narrative-based approach for addressing polypharmacy.
Review of Patient Medication Lists
Beers Criteria have been used to avoid prescribing medications where harm outweighs the potential benefits. These criteria are used only for patients ≥65 years old in the ambulatory, acute, and institutionalized care settings. The first iterations of Beers Criteria, focused on drugs that were considered high-risk drugs due to changes that occur with physiological aging. Over time, this has been expanded and in 2015, categories were added for potentially harmful DDIs, as well as drugs with severe ADRs associated with particular diseases [59]. While the use of Beers Criteria may be a good starting point for physicians to identify “high-risk” medications, their clinical utility is limited [59] [60]. For one, Beers Criteria uses a population-risk assessment approach, and cannot account for individual differences in drug metabolism and effect which may put certain patients at higher risk or lower risk of adverse outcomes with respect to the drug of interest. Secondly, Beers Criteria do not provide a comprehensive list of medications that may be contraindicated in the elderly population [60]. As drugs are added to the list, others may be removed depending on whether or not the risk is unique to the geriatric population [61]. Despite their limitations, Beers Criteria are commonly used in polypharmacy studies to review medication lists and provide a guide for reducing harmful polypharmacy.
Tamura et al. conducted a study in a nursing home facility to determine if medication review could decrease the number of medications [5]. Data on 74 patients with polypharmacy (≥9 medications) were collected for medication start date, frequency of administration, and therapeutic indication. Records were then reviewed for high-risk medications as described by Beers Criteria, as well as potential DDIs [5]. Medical personnel then consulted with the primary care physician and made recommendations to continue, discontinue, or taper medications for each patient. Seventy-one percent of patients had therapeutic regimens altered after medication review [5]. According to the published study there was a statistically significant change (although small) from 16.64 to 15.50 drugs/person on average, but risk reduction associated with polypharmacy was not examined in this study [5].
Other studies using physician review of medication lists have shown mixed results with regards to decreasing polypharmacy, and few examine actual risk reduction. In Sweden, a study focused on identification of unnecessary medications through medication review indicated there was no change in the total number of medications compared to patients who did not receive medication review [4]. In an Australian nursing home setting, the number of medications trended downward after patient medication lists were discussed at interdisciplinary case conferences, but this trend was not significant [62]. Hanlon et al. also reported a non-significant decrease in the total number of medications taken by patients when a clinical pharmacist reviewed medication lists and provided recommendations to the primary care physician over a three month period [3].
In summary, improvement of patient-physician communication, as well as review of patient medication lists by qualified healthcare professionals are practical solutions, but are limited in their efficacy for decreasing number of medications, and studies on risk prevention by identification of ADRs, DDIs, and prescribing cascades are limited [3,56,62]. This highlights the need for a new, systematic solution that may establish more rigorous and defined criteria for which medications may be contraindicated, are likely to precipitate ADR, or have little or none clinical benefit. This need has the potential to be addressed by fine-tuning prescribing using a pharmacogenetics approach.
Part 2: Pharmacogenetics as a Potential Solution to Polypharmacy
Genomic medicine, the concept of using genetic information to diagnose and guide therapy, has multiple applications. Of relevance to polypharmacy are the principles of pharmacogenetics which incorporate the concepts of precision and personalized medicine to improve therapeutic efficacy. Using pharmacogenetics to target appropriate medications has the potential to decrease the number of prescriptions and reduce risks associated with polypharmacy.
Individualizing Therapy
For most patients with disease, appropriate treatment is largely based on disease-specific guidelines. However, these guidelines are not efficacious when patients have multiple comorbidities or polypharmacy, and in some cases, following the guidelines may actually induce more harm than good [63]. As a result, guidelines have been developed to aid in treating this more complex population. In a meta-analysis of polypharmacy-related guidelines, individualization of therapy was one of the most common recommendations made [63]. In particular, these recommendations called for choosing strategies that optimized patient benefit, minimized harm, and enhanced quality of life [63]. This goal aligns with the goals of pharmacogenetics- to improve efficacy of medications while minimizing toxicity.
Deficits in individualizing therapy to maintain ideal therapeutic efficacy while decreasing risk of adverse outcomes can be seen even before drugs are available on the market. The efficacy and toxicity of a drug are determined by large clinical trials, where the recruited population may not be representative of the individual who ultimately is prescribed the medicine. Most people recruited for clinical trials are relatively healthy, having only the condition of interest that is being studied in the trial, and are likely to be on few, if any, other medications [12]. This is in stark contrast to the “normal” patient seen in the clinic, who often has multiple comorbidities and polypharmacy. Thus, efficacy and toxicity findings from clinical trials for a given medication may not translate well into everyday practice. As example, the highest-grossing drugs prescribed today are reported to only be effective in approximately 1-in-4 to 1-in-25 of the patients that take them (Table 1)[64]. As a result, “N-of-1” trials have been proposed as an alternative to traditional clinical trials “N-of-1” trials have been commonly practiced in a basic sense - many physicians will place a patient on a drug regimen and monitor their response before ultimately making a decision to keep the patient on the current drug or switch to an alternative [64]. However, this approach is based on trial and error, putting the patient at risk for toxicity or other adverse effects.
Table 1:
| Highest Grossing Drugs [64] | Effectiveness | Is there a pharmacogenetic contraindication? | Recommendation [103] |
|---|---|---|---|
| Abilify | 1 in 5 people | Yes | CYP2D6 poor metabolizers should have their dose decreased 33% |
| Nexium | 1 in 25 people | Yes | CYP2C19 ultra-metabolizers require a 50–100% increase in dose for the treatment of H. Pylori |
| Humira | 1 in 4 people | Not yet determined | N/A |
| Crestor | 1 in 20 people | Yes | SLC01B1 alterations may affect metabolism |
| Cymbalta | 1 in 9 people | Not yet determined | N/A |
| Advair Diskus | 1 in 20 people | Not yet determined | N/A |
| Enbrel | 1 in 4 people | Not yet determined | N/A |
| Remicade | 1 in 4 people | Not yet determined | N/A |
| Copaxone | 1 in 16 people | Not yet determined | N/A |
| Neulasta | 1 in 13 people | Not yet determined | N/A |
Application of pharmacogenetics to this concept could prove effective in individualizing therapy for the everyday patient. This is exemplified by the fact that several of the highest-grossing, yet most ineffective drugs are associated with genetic polymorphisms (Table 1). To appreciate pharmacogenetics and its role in individualizing therapy, it is important to understand basic principles of pharmacology, and how genetic changes can alter these principles.
Basic Principles of Pharmacology and Pharmacogenetics
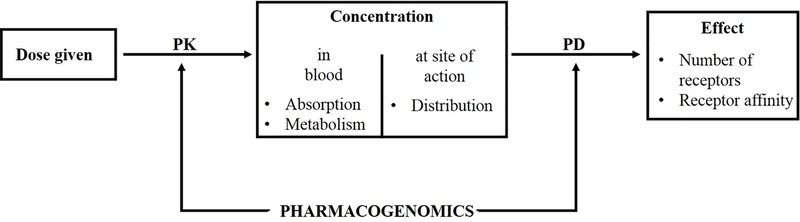
Pharmacogenetics is the study of how alterations in genes can affect the metabolism of a drug (pharmacokinetics) and ultimately the patient’s response to the drug of interest (pharmacodynamics). Pharmacokinetics (PK) describes the processing and transport of drugs by a living organism, including the rates of absorption, distribution, metabolism, and excretion- commonly referred by the acronym ADME [65]. Pharmacodynamics (PD) is the effect of the drug on the body and is based on principles of the receptor occupancy theory.
Pharmacogenetics can be used to account for differences in both PK and PD via control of their respective proteins (Figure 2). The functionality and amounts of PK and PD-associated proteins are affected by genetic variants, referred to as polymorphisms. A genetic polymorphism occurs as a structural change to the gene, and includes nucleotide substitution, complete gene deletion, gene duplication, and genetic translocation (portions of similar genes are combined creating a new gene hybrid) [66]. The most common genetic polymorphism is a single nucleotide polymorphism (SNP), in which the nucleotide sequence at one specific position is changed by substitution, translocation, insertion, or deletion [67]. Each change in the gene structure introduces a variant form of the gene into the population, and is designated as a variant allele of the original gene [67]. Two copies of the same allele yield a homozygous genotype and any combination of two different alleles yields a heterozygous genotype [66].
Figure 2.
Relationship between PK, PD, and pharmacogenetics. Once a drug is administered, PK affects the concentration of that drug that reaches the bloodstream. The amount of drug that ends up at the desired site of action is largely dependent on the distribution component of PD. Both PK and PD can be altered by pharmacogenetics variants, ultimately resulting in changes in concentration and desired effect. Modified from [105].
Genetic polymorphisms are classified by their resulting influence on protein expression and phenotype. Polymorphisms resulting from gene deletion or translocation invariably lead to partial or complete loss of protein function. In contrast, gene duplication leads to increased expression of the protein and a hyperactivity phenotype [67]. The location of SNPs can also result in various changes in protein function depending on where the polymorphism occurs in the gene [67]. SNPs in the coding exons only influence function if there is a resulting amino acid change that alters the protein’s function [67]. On the other hand, SNPs within the intron regions are typically silent unless the SNP alters a nucleotide critical for splicing of the RNA during maturation, leading to loss or decrease in protein function [67].
Pharmacokinetics (Metabolic and Transport Proteins)
PK is defined as the “metabolism of the drug”, or “what the body does to the drug”. Focusing on drug metabolism, PK is further divided into Phase I and Phase II metabolism. Although these numerical categories suggest that Phase I metabolism occurs prior to Phase II metabolism, Phase II metabolism can occur on its own [68]. Phase I drug metabolism involves cytochrome P450 (CYP) enzymes. In terms of pharmacogenetics, the main polymorphism type associated with CYPs are SNPs.
CYPs are a superfamily of heme-containing, drug-metabolizing enzymes that catalyze the conversion of lipophilic substances into hydrophilic molecules that can be excreted by the kidneys and removed from the body [69]. These enzymes metabolize both endogenous and exogenous substrates through various reactions, including epoxidation, N-dealkylation, O-dealkylation, S-oxidation, and hydroxylation [70]. Importantly, more than half of all drugs are cleared by the CYP system [69]. Of the CYP enzymes, CYP2D6, 2C9, and 2C19 enzymes play a role in the metabolism of more than 40% of all prescription drugs, and pharmacogenetic (PGx) testing of these CYPs has clinical application (Table 2) (47, 49, [71]). CYP2D6 metabolizes many of the drugs associated with polypharmacy, including medications for depression, cardiovascular disease, schizophrenia, attention deficit hyperactivity disorder (ADHD), prevention of nausea and vomiting for patients undergoing cancer chemotherapy, prevention of symptoms associated with allergies, and even the simple cold (1, 25, 26, 49).
Table 2:
Cytochrome P-450 (CYPs) and drug metabolism
| Gene | Role | Drug(s) Affected |
|---|---|---|
| CYP2D6 | Metabolism of 25% of all drugs | Antidepressants, antipsychotics, beta blockers, opioids |
| CYP2C9 | Metabolism of 10% of all drugs | Warfarin, NSAIDs, sulfonylureas, phenytoin |
| CYP2C19 | Metabolism of 10–15% of all drugs | Clopidogrel, proton pump inhibitors, citalopram, diazepam |
| CYP1A2 | Metabolism of 9% of all drugs | Clozapine, olanzapine, theophylline, caffeine |
| CYP3A4/3A5 | Metabolism of 50% of all drugs | Statins, benzodiazepenes, antibiotics, antipsychotics |
| SLC6A4 | Serotonin transporter | SSRIs |
| OPRM1 | Mu opioid receptor | Hydromorphone, morphine, oxymorphone, other opioid agonists |
| VKORC1 | Vitamin K clotting cascade | Warfarin (sensitivity) |
| SLCO1B1 | Transport of statins in liver | Statins |
| Factor V (Leiden) | Clotting factor V | Oral contraceptives |
| COMT | Dopamine and norepinephrine degradation | Stimulatory therapies |
SNP variations in CYPs result in four metabolizing phenotypes: (1) ultra-rapid metabolizers (UMs), (2) normal metabolizers (NMs), (3) intermediate metabolizers (IMs), and (4) poor metabolizers (PM) (Table 3). As a caveat, the term “extensive” metabolizer has been routinely used to indicate “normal” metabolism, but the Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines have largely eliminated “extensive metabolizers” as a phenotypic category [72].
Table 3:
Phenotypic categories as determined by PGx testing for CYP enzymes
| Metabolism Status | Effect |
|---|---|
| Poor metabolizers (PM) | Individuals have a deficiency in drug metabolism. Individuals are at risk for adverse effects due to diminished drug elimination, or lack of therapeutic effect resulting from failure to generate the active form of the drug. |
| Intermediate metabolizers (IM) | Individuals may require lower than average drug dosages for optimal therapeutic response. |
| Normal metabolizers (NM) | Individuals have two active genes producing the drug-metabolizing enzyme and therefore can metabolize the drug normally. Individuals with an NM phenotype can be dosed using standard practices. |
| Ultra-rapid metabolizers (UM) | Individuals with the UM phenotype have three or more active genes producing the drug- metabolizing enzyme and have increased metabolic capacity. Individuals may require an increased dosage because of higher than normal rates of drug metabolism. |
Genetic polymorphisms and resulting phenotypes in CYP2D6 can be exemplified by codeine and nortriptyline metabolism. Codeine is a prodrug that requires CYP2D6 activity for its conversion to morphine - a powerful analgesic (5). People who are classified as CYP2D6 NMs are typically able to convert 10% of codeine to morphine, which is sufficient for a therapeutic effect. PMs will not be able to achieve a therapeutic response if given the same dose of codeine as a NM. Even more concerning is the safety of using codeine in UMs - a higher conversion of codeine to morphine can lead to unintentional overdose and death (5). In contrast, nortriptyline is a widely used tricyclic antidepressant and unlike codeine, nortriptyline is inactivated by CYP2D6. Thus, PMs will accumulate nortriptyline leading to anticholinergic toxicity and the potential for a life-threatening ADR. UMs will often require a higher dose of nortriptyline for proper therapeutic efficacy (5).
Phase II metabolism utilizes enzymes to conjugate drugs with acyl, sulfate, or glucuronyl groups [68]. Similar to Phase I metabolism, the outcome of conjugation is readying drugs for elimination from the body. Although genetic polymorphisms are less commonly described in Phase II metabolism, those that do occur have an obvious effect on drug metabolism. Thiopurine S-methyltransferase (TPMT) is involved in the inactivation of 6-mercaptopurine (6-MP) [68]. 6-MP and azathioprine (a prodrug metabolized to 6-MP) are commonly used for the treatment of acute lymphoblastic leukemia. In hematopoietic tissue, TPMT is the only viable inactivation pathway for these drugs. Thus, genetic alterations in TPMT can put patients at risk for severe toxicity [68].
In addition to metabolic enzymes, proteins for drug transport across membranes can also be affected by genetic polymorphisms. These proteins play a role in both the distribution of drugs and drug availability. An example of a transport protein affected by genetic polymorphism is OATP1B1, an organic anion transport protein that is involved in the uptake of statins into the liver. OATP1B1 is encoded by the SLC01B1 gene, and variations in this gene can affect the therapeutic efficacy of statins, as well as risk of toxicity [73,74]. Data indicate that the rs4363657 C allele is associated with an elevated statin concentration due to increased uptake of simvastatin into the liver compared to the G allele. Thus, people with a C allele have an increased risk of developing statin-associated myopathy [73]. Other studies have found that other genetic variants of SLC01B1 are associated with increased LDL cholesterol levels due to decreased efficacy of statins [74].
Genetics and Pharmacodynamics (Receptors)
PD describes the effect of the drug on the body and is based on the receptor occupancy theory, which is central to understanding drug action [65]. PD involves the ability of a receptor on the cell surface or within the cell to recognize the drug of interest. Genetic variants (i.e., polymorphisms) in the promoter region of the receptor gene may reduce transcription of that gene, and ultimately the number of receptors. Genetic variants in the coding region may affect the structure of the protein, altering the affinity of the receptor for the drug [69]. The overall effect of a drug is influenced by the number and affinity of the receptors, as well as the drug concentration at the receptor site.
Of the 94 dosing guidelines by the Pharmacogenomics Knowledgebase (PharmGKB), only 14% are related to the genotype of a PD-associated gene [75]. Glucose-6-phosphate dehydrogenase (G6PD) deficiency has been indicated as one of the major PD genes of interest for toxicity. In particular rasburicase, a common drug used for treatment of hyperuricemia, is contraindicated in patients with G6PD deficiency as it increases susceptibility of organ damage due to ineffective protection against reactive oxygen species [75].
Application of Pharmacogenetic Testing is Fundamental to Pharmacotherapeutics
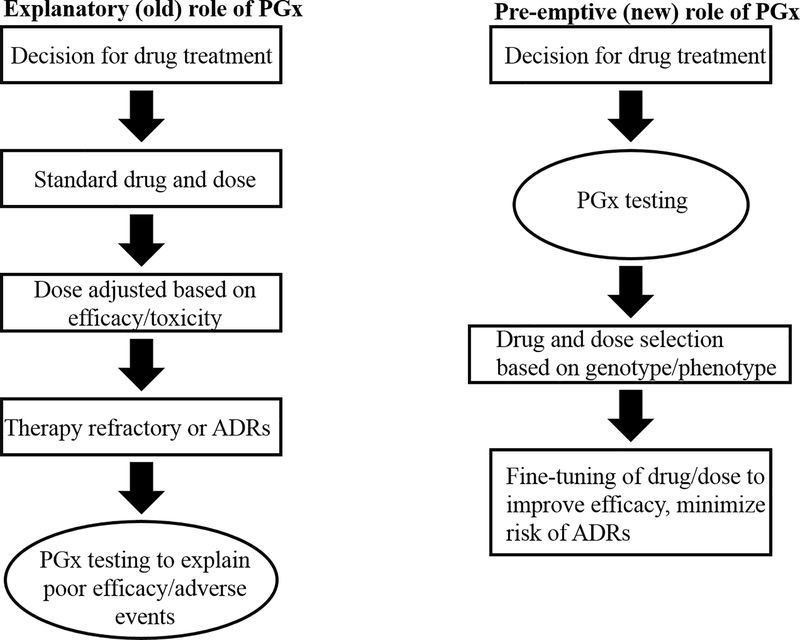
Information on how genetic polymorphisms can alter drug metabolism and drug effect can be applied to pharmacotherapeutics through PGx testing. PGx testing allows effective selection of appropriate medications as well as the optimal dosing for an individual. The goals of being able to predict therapeutic dosing and drug response are to minimize potential ADRs/toxicity, reducing the number of adjustments made to dosing, optimize drug efficacy, and avoid DDIs - all of which can help address the adverse risk of polypharmacy [76,77]. Based on these goals, the best time to incorporate PGx testing would be before any drugs are prescribed. This approach is referred to as the pre-emptive approach, and is contrasted to the reactive approach which relies on PGx information to explain an adverse event rather than predict it (Figure 3)) [78].
Figure 3.
.Pre-emptive versus explanatory approach to PGx testing. In the past, testing was performed after a drug had been given. With the new, pre-emptive approach PGx testing is performed first, and provides guidance for drug and dosage selection. Modified from [78].
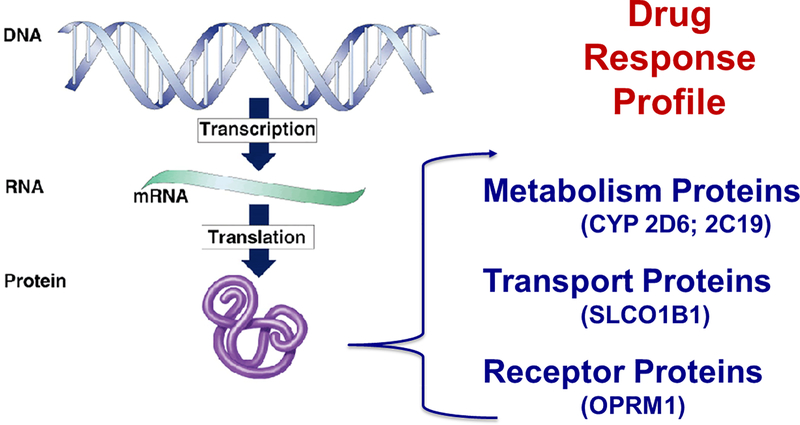
The pre-emptive approach can be used to incorporate PGx information into a “drug response profile” for each individual patient, which is then used to guide initial selection and dosing of a medication (Figure 4) [65,79]. It is through this approach that many of the adverse effects of polypharmacy could be avoided.
Figure 4.
Drug response profile. A drug response profile consists of information on the status of metabolic, transport, and receptor proteins that are responsible for drug metabolism, uptake, and activity. The genotype for these proteins is an important aspect of the drug response profile that can be obtained through PGx testing.
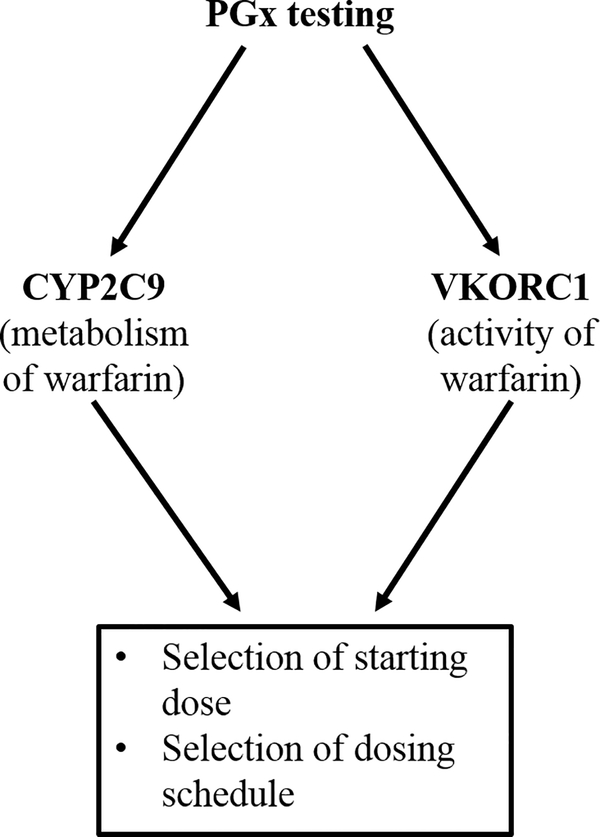
For warfarin, there is a well-defined set of genetic variants for both the metabolizing enzyme (CYP2C9) and warfarin’s target for inhibition (VKORC1) (Figure 5), making it an ideal candidate for a drug response profile. When combined into a functional algorithm, this information can be used to select the starting dose and the optimal schedule for maintaining steady-state, thus preventing unwanted swings in the International Normalized Ratio (INR), a measure of warfarin efficacy. Using a PGx-based algorithm for VKORC1 and CYP2C9, Reynolds et al. demonstrated a decrease in time to reach steady-state INR and a reduction in the number of changes to the maintenance dosing regimen [80]. Ultimately, this would mean that the patient would have decreased risk of warfarin toxicity and improved warfarin therapeutic effect. Several other studies have shown similar advantages of using both PK and PD genetic information-based algorithms, resulting in an increased amount of time spent in the therapeutic range for warfarin, and a decrease in the number of patients who were exposed to initial warfarin over-dose [81,82].
Figure 5.
.PGx testing for warfarin treatment. For warfarin, a pre-emptive PGx testing approach is used to develop a drug response profile, focusing on CYP2C9 and VKORC1.
In contrast, some reports using PGx status to guide warfarin therapy do not indicate an advantage to using this approach. In the Clarification of Optimal Anticoagulation through Genetics (COAG) trial, there was no difference in the amount of time spent in the therapeutic target range between individuals who had been genotyped for VKORC1 and CYP2C9 versus those whose dosing was based solely on a developed clinical algorithm for warfarin dosing [83]. Differences in the COAG clinical trial findings compared to other PGx-based warfarin studies could be attributed to the fact that most studies compare PGx-guided dosing to standard clinical practice, rather than a developed clinical algorithm. In addition, only 55% of patients had their PGx information available prior to the first dose of warfarin in the COAG study, thereby making it difficult to draw conclusions on overall benefit from warfarin therapy as PGx testing for warfarin is usually most useful in guiding the initial dose of the drug [83,84].
Although warfarin may be the “poster-child” for including PGx testing in clinical decision-making for medication use, several other drugs and their respective diseases show benefit from incorporating PGx testing as well. Clopidogrel is an antiplatelet therapy widely prescribed to prevent stroke or transient ischemic attack (TIA), especially after a patient has already sustained a prior stroke/TIA [85,86]. Clopidogrel is a prodrug metabolized to its active form by CYP2C19. For people with CYP2C19 PM Status, standard dosing of clopidogrel may not be efficacious, putting them at higher risk for having a stroke/TIA. One of the major drawbacks in assessing the value of CYP2C19 genetic testing for determining proper clopidogrel treatment is that the efficacy of clopidogrel itself is not well-defined. The Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE) trial found that alone, clopidogrel had no significant risk reduction for stroke/TIA of 7.3%. When clopidogrel was combined with aspirin in the Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trial, total risk reduction for an ischemic event was 22% [85].
Another potential limitation of PGx testing for clopidogrel is that application may be population-dependent. East Asians have an increased rate of stroke/TIA and increased prevalence of CYP2C19 PM status compared to the USA or European populations [85,86]. Thirty percent of Caucasians have at least one loss-of-function allele for CYP2C19, and only 2–3% are classified as PMs for clopidogrel [85]. However, the frequency of a loss-of-function allele in East Asian populations is closer to 50%, with 10–12% being classified as PMs [85]. In order to account for these differences and determine both the efficacy of clopidogrel and the utility of CYP2C19 genetic testing, Pan et al. conducted a meta-analysis and systematic review of 15 publications, encompassing 4,762 test subjects who had a prior stroke/TIA that were being treated with clopidogrel [86]. Of the studies included in the meta-analysis, 12 studies were conducted on East Asian populations, two on US populations, and one conducted worldwide. After carefully reviewing all studies, Pan et al. indicated that genetic testing for CYP2C19 metabolizing status was clinically valuable [87]. People who had at least one loss-of-function allele had an increased risk of stroke/TIA when taking clopidogrel compared to those who had normal CYP2C19 function. Although more people were of Asian descent in this meta-analysis, Pan et al. concluded this effect was comparable in Asian and Caucasian populations, regardless of the increased prevalence of loss-of-function alleles [86]. Thus, CYP2C19 loss-of-function alleles were associated with decreased response to clopidogrel, and genetic testing to determine metabolizing status was advisable to personalize antiplatelet therapy [85,86]. As such, clopidogrel is one of the few drugs on the market for which the FDA label explicitly states that the effectiveness of the drug depends on phenotype status of CYP2C19, and references the utility of pharmacogenetic testing to aid in determining optimal therapeutic strategy.
Part 3: Application of Pharmacogenetic Testing to Polypharmacy
PGx and Adverse Patient Outcomes
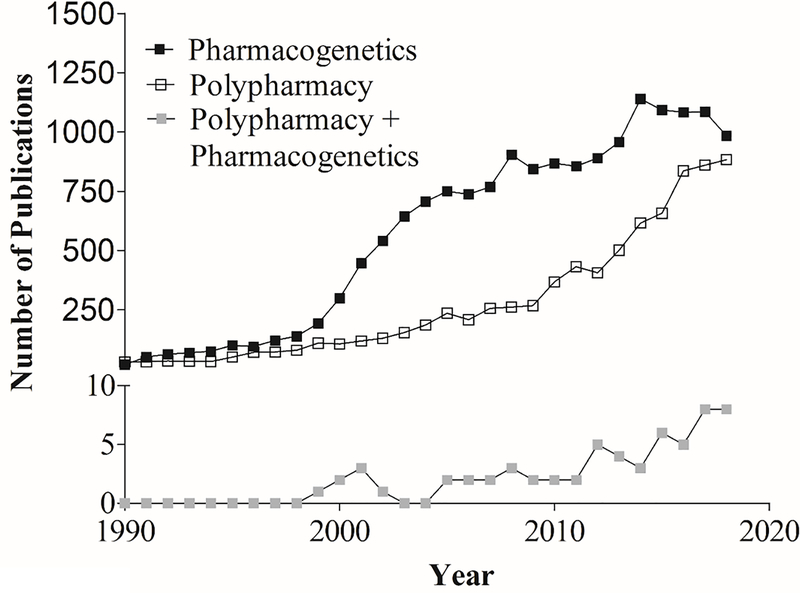
The application of PGx testing to polypharmacy is still not well established in the literature, although available data on both polypharmacy and PGx separately have steadily increased since 1990 (Figure 6). Polypharmacy is multi-factorial in its causes and outcomes, making it unclear what the best approach may be for addressing this condition. As discussed, aging is a risk factor for polypharmacy, and many medications that are contraindicated in the elderly per Beers Criteria also have PGx recommendations (Table 4).
Figure 6.
Publications for polypharmacy and Pharmacogenetics (data obtained from PubMed; 1990–2018).
Table 4:
High risk medications in elderly patients per Beers Criteria with an associated pharmacogenetic contraindication [59,103]
| Drug | Rationale for inclusion in Beers Criteria [59] | Gene(s) associated with altered phenotype [103] |
|---|---|---|
| Dabigatran | Increased risk of gastrointestinal bleeding | ABCB1, CES1 |
| Rivaroxaban | Increased risk of gastrointestinal bleeding | F5, ABCB1 |
| Prasugrel | Increased risk of bleeding | CYP2C19, CYP2B6, CYP2C9, CYP3A5, PEAR1 |
| Carbamazepine | May cause/exacerbate hyponatremia or SIADH | HLA-A, HLA-B |
| Mirtazapine | May cause/exacerbate hyponatremia or SIADH | CYP2D6, CYP2C19 |
| Oxcarbazepine | May cause/exacerbate hyponatremia or SIADH | HLA-B |
| Tramadol | May cause/exacerbate hyponatremia or SIADH | CYP2D6, SULT1A3 |
| Dextromethorphan/quinidine | Increased risk of fall; concerns for drug interactions | CYP2D6 |
| Trimethoprimsulfamethoxazole | Increased risk of hyperkalemia when used with ACE inhibitors | G6PD, NAT2 |
Examining just the aged population, there is considerable difference in hospitalization rates, even when disease severity is similar among all patients [88]. Finkelstein et al. hypothesized that variation in ADR- related hospitalization rates in a seemingly homogeneous population of patients with polypharmacy could be explained by differences in genetic polymorphisms[88]. This nested, case-control study included patients ≥65 years old who were taking ≥5 medications and to control for disease, only patients with cardiovascular disease were enrolled. The cohort was then separated into a case group (those with a high rate of hospitalization defined by ≥3 admittances to the hospital within the past two years), and a control group (patients with ˂3 hospitalizations in two years). Due to the strict parameters put on evenly distributing age, gender, and comorbidities between the case and control groups, only 12 patients were included in this pilot study. All 12 patients were subjected to PGx testing for detection of variant alleles for CYP2C19, CYP2C9, VKORC1, CYP2D6, and CYP3A4/3A5. Finkelstein et al. reported an average of 2.8 gene-drug interactions in the case group, and each case had at least one polymorphism that resulted in a PM or UM status [88]. This coincided with an average of 4.8 hospitalizations per person per year. In comparison, no gene-drug interactions were reported in the control group. This study, although limited in cohort size, documents a retrospective correlation between drug-based genetic polymorphisms and polypharmacy-based hospitalizations, providing evidence for the need to incorporate PGx testing into clinical practice to address polypharmacy.
The major limitation of the study by Finkelstein et al., aside from cohort size, was that it demonstrated a correlative rather than causative effect, demonstrating the need for large-scale, prospective research to show that application of PGx testing can directly impact polypharmacy and its associated risks.
In a case study by Isidoro-Garcia et al., a 74-year-old female with congestive heart failure and chronic renal failure developed psoriasis after being prescribed a set of drugs for her multiple illnesses. For the psoriasis, the patient was prescribed prednisone and hydrocortisone, which caused euphoria and disorientation and initiated a prescribing cascade [89]. To combat these adverse effects, the patient was further prescribed alprazolam, which caused extreme drowsiness. As the patient’s psoriasis worsened, she was given methotrexate and folic acid further worsened her condition [89]. Isidoro-Garcia et al. then utilized PGx testing to better understand why the patient was suffering severe toxicity with minimal efficacy for drugs prescribed. PGx test results indicated deficient CYP3A4 and CYP3A5 metabolizing capacity. Both of these CYPs are heavily involved in metabolism of prednisone and hydrocortisone. Numerous DDIs based on the patient’s specific drug-gene related polymorphisms were also identified [89]. Adjusting the dose and medications prescribed based on PGx findings, the patient experienced remission from psoriasis, amelioration of digitalis toxicity, and a decrease in liver transaminase levels [89]. This study shows how PGx information can be used to understand an individual’s PK for medications, but also shows the complexity of the interplay between gene-drug interactions and DDIs. Finding better ways to incorporate and interpret this kind of data is one of the major limitations for implementation of PGx testing into everyday practice, and will be discussed later.
The impact of PGx testing on reducing risks associated with polypharmacy was demonstrated on a larger scale by Saldivar et al. In a long-term care facility, 132 male and females ≥45 years of age were subjected to PGx testing. Polypharmacy was defined as patients taking ≥5 medications a month, and to control for potential comorbidities, people with chronic renal dysfunction, abnormal hepatic function, and malabsorption syndrome were excluded [90]. In total, 17 genetic polymorphisms related to drug metabolism were assessed, and interpretation of the PGx data was performed using a proprietary algorithm. This algorithm provided recommendations for how to alter participants’ current medications to decrease the potential for drug-gene interactions, DDIs, and unnecessary medications. These recommendations were then assessed by a pharmacist [90]. One-half of the patients enrolled in the study had replacement or consolidation of 1–3 medications, and most recommendations to alter medications was based on gene-drug interactions [90]. While there is still much to be learned, preliminary evidence supports using PGx data to reduce use of unnecessary medications, reduce risk of adverse events, and improve patient outcomes.
PGx and Economic Burden
One of the arguments made against the wide use of PGx testing is that the potential clinical utility does not offset the cost of testing, especially in a healthcare system where financial resources are already limited. As with other tests, PGx testing is only favourable to common practice if it demonstrates cost-effectiveness. Many of the economic studies related to using PGx testing focus specifically on an individual drug, rather than the use of multiple medications. However, an abstract by Mayhew et al. indicated that application of PGx testing reduced the number of medications used by psychiatric patients, and this was a driver for medication-related cost savings of $4,114 per patient per year [91].
Verbelen et al. conducted a literature review to determine the value of using PGx information and included studies that utilized several different cost evaluation techniques [92]. A total of 44 articles were selected, covering a wide variety of drugs related to oncology, infectious diseases, psychiatry, and cardiovascular disease- many of which are indicated as common medications associated with polypharmacy [92]. “Cost-effectiveness” was defined as the PGx testing being more effective compared to the standard treatment regimen, but with extra cost added. “Cost-saving” was defined as PGx testing being more effective and costing less than the standard treatment. Twenty two publications were economically favourable for PGx testing, with 27% being defined as “cost-saving” and 30% as “cost-effective” [92]. PGx testing pertaining to metabolism of azathioprine, abacavir, clopidogrel, and irinotecan was the most economically favourable overall. Verbelen et al. concluded that if testing was available at negligible cost, 75% of the studies in the literature review would favor using PGx information over the standard treatment option [92]. However, this study did not provide a specific target cost that would need to be met for this testing to be preferred over the standard treatment option for any drug prescribed.
Several studies have highlighted that PGx testing can improve the economic burden associated with ADRs that may result from polypharmacy. Johnson et al. utilized a theoretical cohort of 1,000 patients with acute coronary syndrome (ACS) to estimate the financial impact of using CYP2C19 genotyping to determine metabolizing status of clopidogrel [93]. Patients with ACS who have an IM or PM status for clopidogrel are at higher risk for nonfatal myocardial infarction, stroke, and bleeding. These adverse outcomes result in increased use of already limited healthcare resources and more frequent and extended hospital stays - all of which are associated with increasing cost of healthcare [93,94]. Johnson et al. found that theoretical genotyping of all 1,000 patients for CYP2C19 status would result in a savings of $444,426 annually, based on corresponding USA-based Medicare reimbursement rates for diagnosis-related group codes associated with ACS [93]. It was estimated that the average cost for PGx testing was only $315 per person, and the cost of this testing was completely offset by the estimated total savings accrued from adverse events related to ACS.
In the context of polypharmacy, Saldivar et al. indicated a reduction in the number of drugs per patient and decreased risk of adverse events using PGx testing results to make recommendations about appropriate drug use [90]. These recommendations resulted in a yearly savings of $671 per enrolee [90]. Brixner et al. hypothesized that better health resource utilization and lower costs could be achieved by calculating the sum effect of DDIs, drug-gene, drug-drug-gene interactions, and modifying patient’s drug regimens accordingly [95]. In this observational study, 205 patients received PGx testing and data was analyzed using a clinical decision support tool that performed analysis of a patient’s medication record and their genetic profiling using a proprietary algorithm to predict changes in dosing [95]. Patients receiving PGx testing were compared to an age-matched cohort of patients who were not tested, but also met the definition of polypharmacy (≥3 prescribed medications). The primary goal of this study was to determine the resource utilization from hospitalization, number of ER visits, and outpatient care between the two cohorts [95]. The overall total utilization improved for patients that were tested, meaning that healthcare resources were being more efficiently utilized. A cost-analysis revealed an average savings of $1,132 per patient in the test group, which was enough to fully cover the cost of the PGx testing and analysis with a net savings of $218. These studies indicate that PGx testing, when used to address polypharmacy, not only decreases adverse patient outcomes but decreases associated economic burden as well.
Part 4: Current limitations for implementing PGx testing to address polypharmacy
Despite evidence that shows PGx testing may reduce the risks associated with polypharmacy, as well as some of the costs, PGx testing is not commonly used in clinical practice. The limitations for pharmacogenetics in practice have been detailed elsewhere, but here we focus on the lack of ways to use and interpret pharmacogenetic information in a clinically-applicable manner [96,97]
At the forefront of pharmacogenetic testing is the clinical laboratory- the vehicle for providing PGx-related services to the medical community. These services include access to testing, selecting appropriate testing profiles and, among other responsibilities, providing the evidence required to formulate decisions based on medical applications often through the use of algorithms (37). While evidence suggests that a targeted application of pharmacogenetics could aid in addressing polypharmacy, this area remains early in its development (37). However, the number of clinical laboratories providing PGx testing services have blossomed in recent years. According to the 2018 survey from the College of American Pathologists, 254 labs participated in the PGX-B mailing list alone (46). Routinely, large reference laboratories perform PGx testing. These laboratories provide test panels focused on the most prominent genes known to have a clear indication in altered drug metabolism or response.
Despite a growing number of laboratories available to perform PGx testing, there is still a lack of implementation into everyday clinical practice. This delay can be attributed to lack of formal clinical practice guidelines regarding the utility of these tests and that practitioners often lack knowledge and tools for interpreting and utilizing PGx information that could be incorporated into every day practice [97]. This highlights the need for informatics-based clinical decision support systems (CDSS) to advance the use of genomic information in the context of drug prescribing. CDSS combine technology and healthcare knowledge to provide physicians with a tool to aid in clinical decision-making. A survey of physicians in Sweden highlighted some areas for improvement for developing a CDSS in prescribing medications [98]. Physicians agreed that in order for this type of CDSS to be useful it would have to: (1) indicate whether a drug of interest was appropriate for a patient in terms of efficacy and safety, (2) prevent prescribing mistakes, and (3) provide up-to-date drug and dosing recommendations as new drugs enter the market [98]. Incorporating pharmacogenetic information could address many of these points. Pharmacogenetic information would provide a first-step guide to ruling out medications that would not be efficacious based on a patient’s metabolizing status for the drug of interest, as well as flag medications that would put the patient at risk for serious adverse effects. Furthermore, as the field of pharmacogenetics continues to expand, new information added to the CDSS could remain current as new recommendations and drugs are introduced.
A recent study by van der Wouden et al. showed that pre-emptive PGx testing and incorporation of this information into a CDSS was feasible and had an actionable impact on future medications prescribed [99]. PGx testing was performed on patients already taking medications, but the PGx information was then added to patient’s medical record so that it would be available to guide future prescribing practices in conjunction with the Dutch Pharmacogenetics Working Group (DPWG) clinical guidelines. Overall, 24.2% of newly initiated prescriptions had actionable drug-gene interactions, and resulted in pharmacotherapy adjustment for patients [99]. One of the limitations of this study was that DPGW guidelines do not apply to all medications, and so only 41 medications were examined in this study. To aid in the development of useful CDSS for polypharmacy, a good starting point would be to develop an easily accessible database that combines pharmacogenetic information, information on DDIs, and information on medications deemed “high-risk” based on age, sex, or comorbidities. Not only would a database of this kind help clinicians make informed prescribing decisions, but the combination of this information would go a long way in reducing a patient’s exposure to adverse effects associated with polypharmacy. Although a database of this caliber does not yet exist, the basic components are in place. Beers Criteria, although not comprehensive, provide a good starting point for assessing high-risk medications, specifically in elderly patients [59,60]. DDI databases are readily available online and provide drug interaction checkers where a patient/healthcare provider can enter a full medication list and all pertinent DDIs will be displayed and organized based on severity of the DDI. However, information in the databases these websites use may not cover all possible DDIs, and many of the sites make a disclaimer that they are to be used for informational purposes only and should not be used to make clinical decisions.
Databases for pharmacogenetic information are also available, but are not as user-friendly.Often the information provided requires further interpretation, and may only provide information on genetic variants, but not the effect these variations have on drug handling. The Pharmacogenetics of Membrane Transporters (PMT) database and the Transporter (TP) database provide information on sequence variations in transporter genes, but no information on resulting phenotype [100]. The PharmaADME database provides a compilation of genes associated with the PK of drugs, but this information is aimed at pharmaceutical companies involved in clinical trials and drug development to provide a way to screen for potential variability in the safety and/or efficacy of drugs being developed [100,101]. The Drug Gene Interaction database (DGIdb) allows users to type in either drugs or genes of interest to find all potential drug-gene interactions [102]. However, this database is more for research purposes rather than clinical practice. The most comprehensive pharmacogenomic database available is PharmGKB [103]. This database provides clinical annotations for many genetic variants, dosage suggestion guidelines when available, publications on drug-gene interactions, and PGx-related FDA warnings for drugs [103].
One of the areas that could benefit the most from CDSS tools incorporating PGx testing profiles, DDIs, and comorbidities is the treatment of major depressive disorder (MDD) [12]. There are many anti-depressants available, all with differing mechanisms of action that are not well understood, making it difficult to proactively select appropriate therapy for individual patients. Instead, this practice relies heavily on trial and error. Approximately one-third of patients will not respond to treatment even after multiple, dissimilar-acting drugs are tried [104]. The variability in response to anti-depressants can be attributed to a number of factors including age, gender, renal/hepatic function, and adherence to the therapeutic regimen [104]. Recent reports suggest that 42% of the variance in response to anti-depressant therapy can be explained by polymorphisms in genes responsible for anti-depressant PK/PD [104]. To determine the efficacy of PGx-testing for guiding MDD treatment selection, Rosenblat et al. reviewed current literature focusing on using PGx information in patients with mild to severe MDD [104]. In total, only five studies were identified and analyzed for utility. Three used the same commercially available PGx profiles that assessed both PK and PD-associated genotypes. In one study, a small, non-randomized, non-blinded cohort of people were subjected to PGx testing [104]. After testing was done, patients were divided into “unguided” (physicians did not receive PGx test results), and “guided” (physicians received results and made recommendations on treatment based on these results) groups. Patients in the guided group has a 30.8% decrease rating on the HDRS-17 scale- a scale commonly used to diagnose the severity/presence of MDD [104]. Patients in the unguided group had only an 18.2% improvement [104]. While this study suggested that PGx data helped improve clinical outcomes, it was hindered by a small sample size, and the study was not random or blinded. To improve on this, a follow-up study was conducted with a larger sample size. This study confirmed findings of the smaller pilot study- 46.9% of patients in the guided group showed an improved HDS-17 score compared to only 29.9% in the unguided group [104].
Polypharmacy is the rule rather than the exception in the treatment of depressive disorders [12]. While PGx testing may provide a basis to identify individual drugs that are likely to work for an individual patient, interpreting this information while patients are using other medications may be difficult. In this regard, a multidimensional CDSS integrating PGx data on toxicity and efficacy, potential DDIs, and other high-risk medications could provide a systematic approach for understanding what drugs will work and which ones should be avoided[12]. Although this approach may not necessarily decrease the number of medications taken by the patient, and some may argue that polypharmacy may become more common with this approach, it does mean that there will be less “unnecessary” or inappropriate prescribing, resulting in more effective therapy with minimized risks of toxicity [12].
Conclusions
Polypharmacy is a growing, iatrogenic condition precipitated by both medical necessity and external pressures. While the intention is to improve patient quality of life and longevity, there are potential adverse effects to unnecessary and inappropriate use of medicines. Patients experiencing polypharmacy are more likely to have an ADR or DDI events requiring hospital admission, increased length of stay and increased mortality/morbidity. These adverse outcomes place economic burden on patients and hospitals alike resulting in underutilization of already limited hospital resources, thus highlighting the need for an effective solution. Improving communication between patients and physicians and medication list reviews by clinical pharmacists present two practical approaches for preventing polypharmacy. However, these approaches have shown mixed or at best modest efficacy in minimizing the use of medications or decreasing the risk associated with polypharmacy. Use of Pharmacogenetics-based testing presents a more logical, empirical approach to polypharmacy with the potential to alleviate both economic burden and adverse effects. The limiting factor for implementation of this approach in clinical practice is not the lack of availability of PGx testing, but rather the need for a CDSS that incorporates PGx information with other important clinical factors.
Acknowledgments
Funding Details: This work was in part supported by the NIH NHLBI under grant 1R43HL136220-01A1 (RV).
Footnotes
Disclosure statement: None of the authors have conflicts to disclose
References
- 1.Barto TG. Which Drugs Should Be Deprescribed in the Elderly? MedScape 2015. [cited 2019 April 2]. Available from: https://www.medscape.com/viewarticle/847187_2
- 2.Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014. Jan;13(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996. Apr;100(4):428–37. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt I, Claesson CB, Westerholm B, et al. The impact of regular multidisciplinary team interventions on psychotropic prescribing in Swedish nursing homes. J Am Geriatr Soc. 1998. Jan;46(1):77–82. [DOI] [PubMed] [Google Scholar]
- 5.Tamura BK, Bell CL, Lubimir K, et al. Physician intervention for medication reduction in a nursing home: the polypharmacy outcomes project. J Am Med Dir Assoc. 2011. Jun;12(5):326–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007. Dec;5(4):345–51. [DOI] [PubMed] [Google Scholar]
- 7.Lipton HL, Bird JA. The impact of clinical pharmacists’ consultations on geriatric patients’ compliance and medical care use: a randomized controlled trial. Gerontologist. 1994. Jun;34(3):307–15. [DOI] [PubMed] [Google Scholar]
- 8.Swinglehurst D, Fudge N. The polypharmacy challenge: time for a new script? Br J Gen Pract. 2017. Sep;67(662):388–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn KJ, Shah NH. A dataset quantifying polypharmacy in the United States. Sci Data. 2017. Oct 31;4:170167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vu T, Finch CF, Day L. Patterns of comorbidity in community-dwelling older people hospitalised for fall-related injury: a cluster analysis. BMC Geriatr. 2011. Aug 18;11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fillenbaum GG, Pieper CF, Cohen HJ, et al. Comorbidity of five chronic health conditions in elderly community residents: determinants and impact on mortality. J Gerontol A Biol Sci Med Sci. 2000. Feb;55(2):M84–9. [DOI] [PubMed] [Google Scholar]
- 12.Preskorn SH. Pharmacogenomics, informatics, and individual drug therapy in psychiatry: past, present and future. J Psychopharmacol. 2006. Jul;20(4 Suppl):85–94. [DOI] [PubMed] [Google Scholar]
- 13.Saper RB. Clinical Use of Gingko Biloba 2018 [updated 7/28/2018; cited 2019 2/18/2019]. [Google Scholar]
- 14.Stoddard GJ, Archer M, Shane-McWhorter L, et al. Ginkgo and Warfarin Interaction in a Large Veterans Administration Population. AMIA Annu Symp Proc. 2015;2015:1174–83. [PMC free article] [PubMed] [Google Scholar]
- 15.Medhekar R, Fujimoto K, Aparasu RR, et al. Physician Care Coordination and the Use of Psychotropic Polypharmacy in the Management of Pediatric Mental Disorders. J Manag Care Spec Pharm. 2019. Jan;25(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker C, Feinstein JA, Ma X, et al. Variation of the prevalence of pediatric polypharmacy: A scoping review. Pharmacoepidemiol Drug Saf. 2019. Mar;28(3):275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zonfrillo MR, Penn JV, Leonard HL. Pediatric psychotropic polypharmacy. Psychiatry (Edgmont). 2005. Aug;2(8):14–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Zito JM, Safer DJ, DosReis S, et al. Psychotropic practice patterns for youth: a 10-year perspective. Arch Pediatr Adolesc Med. 2003. Jan;157(1):17–25. [DOI] [PubMed] [Google Scholar]
- 19.Martin A, Van Hoof T, Stubbe D, et al. Multiple psychotropic pharmacotherapy among child and adolescent enrollees in Connecticut Medicaid managed care. Psychiatr Serv. 2003. Jan;54(1):72–7. [DOI] [PubMed] [Google Scholar]
- 20.Deza EG, Morgenfeld EL, Rivarola EGJ, et al. Polypharmacy in oncology patients. Journal of Clinical Oncology. 2014;32(15_suppl):e17582–e17582. [Google Scholar]
- 21.Molas E, Luque S, Retamero A, et al. Frequency and severity of potential drug interactions in a cohort of HIV-infected patients Identified through a Multidisciplinary team. HIV Clin Trials. 2018. Feb;19(1):1–7. [DOI] [PubMed] [Google Scholar]
- 22.Krentz HB, Gill MJ. The Impact of Non-Antiretroviral Polypharmacy on the Continuity of Antiretroviral Therapy (ART) Among HIV Patients. AIDS Patient Care STDS. 2016. Jan;30(1):11–7. [DOI] [PubMed] [Google Scholar]
- 23.Ware D, Palella FJ Jr., Chew KW, et al. Prevalence and trends of polypharmacy among HIV-positive and -negative men in the Multicenter AIDS Cohort Study from 2004 to 2016. PLoS One. 2018;13(9):e0203890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy CC, Fullington HM, Alvarez CA, et al. Polypharmacy and patterns of prescription medication use among cancer survivors. Cancer. 2018. Jul 1;124(13):2850–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.FDA. Guidance for Industry: Consumer-Directed Broadcast Advertisements. 1999.
- 26.Kornfield R, Donohue J, Berndt ER, et al. Promotion of prescription drugs to consumers and providers, 2001–2010. PLoS One. 2013;8(3):e55504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trusts PC. Persuading the Prescribers: Pharmaceutical Industry Marketing and its Influence on Physicians and Patients 2013. [cited 2014 February 8]. Available from: https://www.pewtrusts.org/en/research-and-analysis/fact-sheets/2013/11/11/persuading-the-prescribers-pharmaceutical-industry-marketing-and-its-influence-on-physicians-and-patients
- 28.Gilbody S, Wilson P, Watt I. Benefits and harms of direct to consumer advertising: a systematic review. Qual Saf Health Care. 2005. Aug;14(4):246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.FDA. Office of Prescription Drug Promotion (OPDP) Research [cited 2014 February 8]. Available from: https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm090276.htm
- 30.Alexander GC, Kruszewski SP, Webster DW. Rethinking opioid prescribing to protect patient safety and public health. JAMA. 2012. Nov 14;308(18):1865–6. [DOI] [PubMed] [Google Scholar]
- 31.McKinlay JB, Trachtenberg F, Marceau LD, et al. Effects of patient medication requests on physician prescribing behavior: results of a factorial experiment. Med Care. 2014. Apr;52(4):294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fain KM, Alexander GC. Mind the gap: understanding the effects of pharmaceutical direct-to-consumer advertising. Med Care. 2014. Apr;52(4):291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frosch DL, Grande D, Tarn DM, et al. A decade of controversy: balancing policy with evidence in the regulation of prescription drug advertising. Am J Public Health. 2010. Jan;100(1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kravitz RL, Epstein RM, Feldman MD, et al. Influence of patients’ requests for direct-to-consumer advertised antidepressants: a randomized controlled trial. JAMA. 2005. Apr 27;293(16):1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies EC, Green CF, Mottram DR, et al. Adverse drug reactions in hospital in-patients: a pilot study. J Clin Pharm Ther. 2006. Aug;31(4):335–41. [DOI] [PubMed] [Google Scholar]
- 36.Kojima T, Akishita M, Kameyama Y, et al. High risk of adverse drug reactions in elderly patients taking six or more drugs: analysis of inpatient database. Geriatr Gerontol Int. 2012. Oct;12(4):761–2. [DOI] [PubMed] [Google Scholar]
- 37.Meyer UA. Pharmacogenetics and adverse drug reactions. Lancet. 2000. Nov 11;356(9242):1667–71. [DOI] [PubMed] [Google Scholar]
- 38.Bates DW, Miller EB, Cullen DJ, et al. Patient risk factors for adverse drug events in hospitalized patients. ADE Prevention Study Group. Arch Intern Med. 1999. Nov 22;159(21):2553–60. [DOI] [PubMed] [Google Scholar]
- 39.Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012. May;28(2):173–86. [DOI] [PubMed] [Google Scholar]
- 40.Hohl CM, Dankoff J, Colacone A, et al. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med. 2001. Dec;38(6):666–71. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen JK, Fouts MM, Kotabe SE, et al. Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. The American Journal of Geriatric Pharmacotherapy 2006. p. 36–41. [DOI] [PubMed] [Google Scholar]
- 42.Pirmohamed M, Breckenridge AM, Kitteringham NR, et al. Adverse drug reactions. BMJ. 1998. Apr 25;316(7140):1295–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carmona-Huerta J, Castiello-de Obeso S, Ramirez-Palomino J, et al. Polypharmacy in a hospitalized psychiatric population: risk estimation and damage quantification. BMC Psychiatry. 2019. Feb 21;19(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McIsaac DI, Wong CA, Bryson GL, et al. Association of Polypharmacy with Survival, Complications, and Healthcare Resource Use after Elective Noncardiac Surgery: A Population-based Cohort Study. Anesthesiology. 2018. Jun;128(6):1140–1150. [DOI] [PubMed] [Google Scholar]
- 45.Sikka R, Magauran B, Ulrich A, et al. Bench to bedside: Pharmacogenomics, adverse drug interactions, and the cytochrome P450 system. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2005. Dec;12(12):1227–35. [DOI] [PubMed] [Google Scholar]
- 46.Haider SI, Johnell K, Thorslund M, et al. Trends in polypharmacy and potential drug-drug interactions across educational groups in elderly patients in Sweden for the period 1992 – 2002. Int J Clin Pharmacol Ther. 2007. Dec;45(12):643–53. [DOI] [PubMed] [Google Scholar]
- 47.Malone DC, Hutchins DS, Haupert H, et al. Assessment of potential drug-drug interactions with a prescription claims database. Am J Health Syst Pharm. 2005. Oct 1;62(19):1983–91. [DOI] [PubMed] [Google Scholar]
- 48.Carpenter M, Berry H, Pelletier AL. Clinically Relevant Drug-Drug Interactions in Primary Care. Am Fam Physician. 2019. May 1;99(9):558–564. [PubMed] [Google Scholar]
- 49.Guthrie B, Makubate B, Hernandez-Santiago V, et al. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995–2010. BMC Med. 2015. Apr 7;13:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalisch LM CG, Roughead EE, Gilbert AL. The prescribing cascade. Australian Prescriber. 2011;34:162–166. [Google Scholar]
- 51.Nguyen PV, Spinelli C. Prescribing cascade in an elderly woman. Can Pharm J (Ott). 2016. May;149(3):122–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gill SS, Mamdani M, Naglie G, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005. Apr 11;165(7):808–13. [DOI] [PubMed] [Google Scholar]
- 53.Hovstadius B, Petersson G. The impact of increasing polypharmacy on prescribed drug expenditure-a register-based study in Sweden 2005–2009. Health Policy. 2013. Feb;109(2):166–74. [DOI] [PubMed] [Google Scholar]
- 54.Hovstadius B, Astrand B, Persson U, et al. Acquisition cost of dispensed drugs in individuals with multiple medications--a register-based study in Sweden. Health Policy. 2011. Jul;101(2):153–61. [DOI] [PubMed] [Google Scholar]
- 55.Kojima G, Bell C, Tamura B, et al. Reducing cost by reducing polypharmacy: the polypharmacy outcomes project. J Am Med Dir Assoc. 2012. Nov;13(9):818 e11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevenson FA, Barry CA, Britten N, et al. Doctor-patient communication about drugs: the evidence for shared decision making. Soc Sci Med. 2000. Mar;50(6):829–40. [DOI] [PubMed] [Google Scholar]
- 57.Sihvo S, Klaukka T, Martikainen J, et al. Frequency of daily over-the-counter drug use and potential clinically significant over-the-counter-prescription drug interactions in the Finnish adult population. Eur J Clin Pharmacol. 2000. Sep;56(6–7):495–9. [DOI] [PubMed] [Google Scholar]
- 58.Schafer I, Kaduszkiewicz H, Mellert C, et al. Narrative medicine-based intervention in primary care to reduce polypharmacy: results from the cluster-randomised controlled trial MultiCare AGENDA. BMJ Open. 2018. Jan 23;8(1):e017653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radcliff S, Yue JR, Rocco G, et al. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society. 2015. Nov;63(11):2227–2246. [DOI] [PubMed] [Google Scholar]
- 60.Steinman MA, Fick DM. Using Wisely: A Reminder on the Proper Use of the American Geriatrics Society Beers Criteria (R). Journal of the American Geriatrics Society. 2019. Apr;67(4):644–646. [DOI] [PubMed] [Google Scholar]
- 61.By the American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019. Apr;67(4):674–694. [DOI] [PubMed] [Google Scholar]
- 62.King MA, Roberts MS. Multidisciplinary case conference reviews: improving outcomes for nursing home residents, carers and health professionals. Pharm World Sci. 2001. Apr;23(2):41–5. [DOI] [PubMed] [Google Scholar]
- 63.Muth C, Blom JW, Smith SM, et al. Evidence supporting the best clinical management of patients with multimorbidity and polypharmacy: a systematic guideline review and expert consensus. J Intern Med. 2019. Mar;285(3):272–288. [DOI] [PubMed] [Google Scholar]
- 64.Schork NJ. Personalized medicine: Time for one-person trials. Nature. 2015. Apr 30;520(7549):609–11. [DOI] [PubMed] [Google Scholar]
- 65.Linder MW, Valdes R Jr. Fundamentals of pharmacogenetics. AACC Press; 2006. (Wong SHYLM, Valdes R Jr, editor. Pharmacogenomics and proteomics: enabling the practice of personalized medicine). [Google Scholar]
- 66.Syvanen AC. Accessing genetic variation: Genotyping single nucleotide polymorphisms. Nature Reviews Genetics. 2001. Dec;2(12):930–942. [DOI] [PubMed] [Google Scholar]
- 67.Linder MW, Valdes R Jr. Genetic Mechanisms for Variability in Drug Response and Toxicity. Journal of Analytical Toxicology. 2001. July 1, 2001;25(5):405–413. [DOI] [PubMed] [Google Scholar]
- 68.Burtis CA, Bruns DE. Pharmacogenetics. In: Sawyer BG, editor. Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics: Elsevier; 2015. p. 885–898. [Google Scholar]
- 69.Valdes R Jr, Payne DA, Linder MW. Laboratory analysis and application of pharmacogenetics to clinical practice: National Academy of Clinical Biochemistry; 2010. Available from: https://www.aacc.org/~/media/practice-guidelines/pharmacogenetics/pgx_guidelines.pdf?la=en [Google Scholar]
- 70.Wilkinson GR. Drug Metabolism and Variability among Patients in Drug Response. New England Journal of Medicine. 2005;352(21):2211–2221. [DOI] [PubMed] [Google Scholar]
- 71.Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Therapeut. 2013. Apr;138(1):103–141. [DOI] [PubMed] [Google Scholar]
- 72.Caudle KE, Dunnenberger HM, Freimuth RR, et al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet Med. 2017. Feb;19(2):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Group SC, Link E, Parish S, et al. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med. 2008. Aug 21;359(8):789–99. [DOI] [PubMed] [Google Scholar]
- 74.Li JH, Suchindran S, Shah SH, et al. SLCO1B1 genetic variants, long-term low-density lipoprotein cholesterol levels and clinical events in patients following cardiac catheterization. Pharmacogenomics. 2015;16(5):449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Picard N, Bergan S, Marquet P, et al. Pharmacogenetic Biomarkers Predictive of the Pharmacokinetics and Pharmacodynamics of Immunosuppressive Drugs. Ther Drug Monit. 2016. Apr;38 Suppl 1:S57–69. [DOI] [PubMed] [Google Scholar]
- 76.Daughton CG, Ruhoy IS. Lower-dose prescribing: Minimizing “side effects” of pharmaceuticals on society and the environment. Science of The Total Environment. 2013. 1/15/;443:324–337. [DOI] [PubMed] [Google Scholar]
- 77.Murray M Role of CYP pharmacogenetics and drug-drug interactions in the efficacy and safety of atypical and other antipsychotic agents. The Journal of pharmacy and pharmacology. 2006. Jul;58(7):871–85. [DOI] [PubMed] [Google Scholar]
- 78.Brockmoller J, Kirchheiner J, Meisel C, et al. Pharmacogenetic diagnostics of cytochrome P450 polymorphisms in clinical drug development and in drug treatment. Pharmacogenomics. 2000. May;1(2):125–51. [DOI] [PubMed] [Google Scholar]
- 79.Linder MW, Valdes R Jr. Pharmacogenetics in the practice of laboratory medicine. Molecular diagnosis : a journal devoted to the understanding of human disease through the clinical application of molecular biology. 1999. Dec;4(4):365–79. [DOI] [PubMed] [Google Scholar]
- 80.Reynolds KK, Valdes R Jr., Hartung BR, et al. Individualizing warfarin therapy. Per Med. 2007. Feb;4(1):11–31. [DOI] [PubMed] [Google Scholar]
- 81.Gage BF, Eby C, Milligan PE, et al. Use of pharmacogenetics and clinical factors to predict the maintenance dose of warfarin. Thromb Haemost. 2004. Jan;91(1):87–94. [DOI] [PubMed] [Google Scholar]
- 82.Pirmohamed M, Burnside G, Eriksson N, et al. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med. 2013. Dec 12;369(24):2294–303. [DOI] [PubMed] [Google Scholar]
- 83.Johnson JA, Cavallari LH. Warfarin pharmacogenetics. Trends Cardiovasc Med. 2015. Jan;25(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rouse M, Cristiani C, Teng KA. Q: Should we use pharmacogenetic testing when prescribing warfarin? Cleve Clin J Med. 2013. Aug;80(8):483–6. [DOI] [PubMed] [Google Scholar]
- 85.Simon T, Danchin N. Clinical Impact of Pharmacogenomics of Clopidogrel in Stroke. Circulation. 2017. Jan 3;135(1):34–37. [DOI] [PubMed] [Google Scholar]
- 86.Pan Y, Chen W, Xu Y, et al. Genetic Polymorphisms and Clopidogrel Efficacy for Acute Ischemic Stroke or Transient Ischemic Attack: A Systematic Review and Meta-Analysis. Circulation. 2017. Jan 3;135(1):21–33. [DOI] [PubMed] [Google Scholar]
- 87.Hiemke C, Baumann P, Bergemann N, et al. AGNP Consensus Guidelines for Therapeutic Drug Monitoring in Psychiatry: Update 2011. Pharmacopsychiatry. 2011. Sep;44(6):195–235. [DOI] [PubMed] [Google Scholar]
- 88.Finkelstein J, Friedman C, Hripcsak G, et al. Pharmacogenetic polymorphism as an independent risk factor for frequent hospitalizations in older adults with polypharmacy: a pilot study. Pharmgenomics Pers Med. 2016;9:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Isidoro-Garcia M, Sanchez-Martin A, Garcia-Berrocal B, et al. Primun non nocere, polypharmacy and pharmacogenetics. Pharmacogenomics. 2015. Nov;16(17):1903–5. [DOI] [PubMed] [Google Scholar]
- 90.Saldivar JS, Taylor D, Sugarman EA, et al. Initial assessment of the benefits of implementing pharmacogenetics into the medical management of patients in a long-term care facility. Pharmgenomics Pers Med. 2016;9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mayhew M, Jablonski M, Li J, et al. Combinatorial Pharmacogenomics Reduces Polypharmacy and Medication Cost in Elderly Patients with Anxiety and Depression. AAGP Annual Meeting2017. [Google Scholar]
- 92.Verbelen M, Weale ME, Lewis CM. Cost-effectiveness of pharmacogenetic-guided treatment: are we there yet? Pharmacogenomics J. 2017. Oct;17(5):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnson SG, Gruntowicz D, Chua T, et al. Financial Analysis of CYP2C19 Genotyping in Patients Receiving Dual Antiplatelet Therapy Following Acute Coronary Syndrome and Percutaneous Coronary Intervention. J Manag Care Spec Pharm. 2015. Jul;21(7):552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johnson JA, Humma LM. Pharmacogenetics of cardiovascular drugs. Briefings in functional genomics and proteomics. 2002;1(1):66–79. [DOI] [PubMed] [Google Scholar]
- 95.Brixner D, Biltaji E, Bress A, et al. The effect of pharmacogenetic profiling with a clinical decision support tool on healthcare resource utilization and estimated costs in the elderly exposed to polypharmacy. J Med Econ. 2016;19(3):213–28. [DOI] [PubMed] [Google Scholar]
- 96.Haga SB, Allen LaPointe NM, Moaddeb J. Challenges to integrating pharmacogenetic testing into medication therapy management. J Manag Care Spec Pharm. 2015. Apr;21(4):346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Leon J, Spina E. What is needed to incorporate clinical pharmacogenetic tests into the practice of psychopharmacotherapy? Expert Rev Clin Pharmacol. 2016;9(3):351–4. [DOI] [PubMed] [Google Scholar]
- 98.Rahmner PB, Eiermann B, Korkmaz S, et al. Physicians’ reported needs of drug information at point of care in Sweden. Br J Clin Pharmacol. 2012. Jan;73(1):115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van der Wouden CH, Bank PCD, Ozokcu K, et al. Pharmacist-Initiated Pre-Emptive Pharmacogenetic Panel Testing with Clinical Decision Support in Primary Care: Record of PGx Results and Real-World Impact. Genes (Basel). 2019. May 29;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sim SC, Altman RB, Ingelman-Sundberg M. Databases in the area of pharmacogenetics. Hum Mutat. 2011. May;32(5):526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.PharmaADME [Internet]. [cited 5/23/2019]. Available from: http://www.pharmaadme.org/joomla/.
- 102.The Drug Gene Interaction Database [Internet]. [cited 5/23/2019]. Available from: http://www.dgidb.org/.
- 103.Whirl-Carrillo M, McDonagh EM, Hebert JM, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012. Oct;92(4):414–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosenblat JD, Lee Y, McIntyre RS. Does Pharmacogenomic Testing Improve Clinical Outcomes for Major Depressive Disorder? A Systematic Review of Clinical Trials and Cost-Effectiveness Studies. J Clin Psychiatry. 2017. Jun;78(6):720–729. [DOI] [PubMed] [Google Scholar]
- 105.Milone MC, Shaw LM. Therapeutic Drugs and Their Management. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics: Elsevier; 2006. [Google Scholar]