Abstract
Objective:
Lung ischemia-reperfusion injury (IRI) is a common complication after lung transplantation, and immune cells have been implicated in modulating outcomes. We hypothesized that a newly described subset of αβ T cell receptor positive cells: CD4−CD8− (double-negative; DN) T cells, are found in lungs and can protect against lung ischemia reperfusion injury (IRI).
Methods:
Ischemia was induced in C57BL/6 mice by left pulmonary artery and vein occlusion for 30 minutes followed by 180 minutes of reperfusion. These mice were paired with sham hilar dissected surgical controls. In mice undergoing IRI, adoptive transfer of DN T cells or conventional T cells was performed 12 hours before occlusion. Flow cytometry was used to quantify T cells and inflammatory cytokines, and apoptotic signaling pathways were evaluated with immunoblotting. Lung injury was assessed with Evans Blue dye extravasation.
Results:
DN T cells were significantly higher (5.29% ± 1% vs 2.21% ± 3%,, p< 0.01) in IRI lungs and secreted higher levels of IL-10 (30% ± 5% vs 6% ± 1%, p<0.01) compared with surgical sham controls. Immunoblotting, H&E staining and Evans Blue Dye demonstrated that adoptive transfer of DN T cells significantly decreased interstitial edema (p<0.01) and attenuated apoptosis/cleaved caspase-3 expression in the lungs following lung IRI (p<0.01).
Conclusion:
Double negative T cells traffic into lungs during IRI, and have tissue protective functions regulating inflammation and apoptosis. We propose a potential novel immunoregulatory function of DN T cells during lung IRI.
Introduction
Lung ischemia-reperfusion injury (IRI) remains a major complication after lung transplantations1. IRI is characterized by a robust inflammatory response mediated by a multitude of immune and non-immune cells—where they function to balance both pro- and anti-inflammatory properties in the event of cellular injury2. Following lung transplantation, IRI leads to primary graft dysfunction (PGD) in 10-25% of lung recipients, which is the predominant risk factor for early mortality as well as chronic rejection3–6.
Previous studies have demonstrated lymphocytes such as CD4+ T cells and CCR2+ classical monocytes mediate lung IRI7, 8. These investigations have elucidated the molecular mechanism by which these cells stimulate the release of specific cytokines and chemokines (IL-1B, TNF-a, CXCL1, CCL3) within the injured area—recruiting other cells to mediate lung injury. While immune cells from both the innate and adaptive response have been investigated in lung IRI, studies on the role of nonconventional T cells in lung IRI have been limited9–11. Current efforts to comprehend the role of nonconventional T cells in extrapulmonary IRI has been growing12. TCR+CD4−CD8− (double negative;DN) T cells have been identified in kidney and intestine13, 14. These DN αβ+ T cells were found to be early responders to acute kidney injury (AKI) and possessed anti-inflammatory properties—secreting IL-10 to attenuate kidney IRI15. Furthermore, these cells were also found to work in conjunction with regulatory (Treg) T cells in modulating Graft-Versus-Host Disease (GVHD), stimulating immune tolerance to prevent graft rejection16. A DN T cell population in the lungs has also been reported17. Upon infection with influenza A virus, DN T cells were found to expand rapidly in the pulmonary extravascular space. This data illustrates a potential immunoregulatory role of lung DN T cells as found in kidney and skin. However, DN T cells trafficking and their functions during lung IRI are not known.
In the current study, we sought to characterize murine lung DN T cells in the steady-state and in response to lung IRI. We also aimed to elucidate the molecular changes of lung DN T cells and investigate the potential of DN T cells to attenuate lung IRI.
Methods and Methods
Animals
Male C57BL/6J WT and gld mice (Jackson Laboratory, Bar Harbor ME) were bred under pathogen-free conditions at the central animal facility of the Johns Hopkins University School of Medicine. For all experiments, 8-10 week-old mice were utilized in accordance with the Animal Care and Use Committee guidelines.
Murine Lung Ischemia Model
Mice were anesthetized by intraperitoneal injection of ketamine (130mg/kg) and xylazine (7mg/kg). Buprenorphine (5mg/kg) was given subcutaneously before skin incision. Mice were orotracheally intubated and connected to a rodent ventilator (Harvard Apparatus, model 845) with tidal air volumes of 150 uL/g and respiratory rate set at 150 breaths per minute. A left dorsolateral thoracotomy was performed in the fourth intercostal space and a microvascular clamp (Roboz Surgical Instrument, Gaithersburg, MD) was applied across the pulmonary artery and vein to occlude the vasculature. The left lung was occluded for 30 minutes, and the microvascular clamp was removed. The thoracotomy was closed and the mouse was removed from the ventilator and allowed to recover for the 3-hour reperfusion period.
Isolation of Lung T cells
The protocol for lung and peripheral T cell isolation was modified from previous established protocol13. Mice were anesthetized with intraperitoneal ketamine and xylazine, underwent midline abdominal incisions, were exsanguinated, and had their lung lobes dissected and removed. Tissue was cut into small pieces (1-2mm in dimension) and was incubated with Collagenase D solution. Cells were processed and lymphocytes were isolated with usually >90% purity. The absolute number of lymphocytes was calculated by multiplying the total number of CD45+ cells by the percentage of each T cell subset (determined by flow cytometry).
Antibodies and Reagents
All reagents used for media, PBS, and Percoll were obtained from Sigma-Aldrich and BD Bioscience. The primary antibodies used in these studies included rabbit monoclonal antibodies against phospo-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (Cell Signaling #9101, Danvers, MA, USA), phospho-SAPK/JNK (Thre183/Tyr185) (Cell Signaling #4668), phospho-p38 MAPK (Thr180/Tyr182) (Cell Signaling #9221), phospho-NF-κB p65 (Ser436) (93H1) (Cell Signaling #3033), Cleaved Caspase-3 (Asp 175) (Cell Signaling #9661, Danvers, MA, USA), Bcl-2 (D17C4) (Cell Signaling #3498, Danvers, MA, USA), β-Actin (13E5) (Cell Signaling #4970, Danvers, MA, USA). Secondary antibodies included ones conjugated to Alexa Flour 488 (Abcam, Cambridge, UK), Alexa Flour 647 (Abcam, Cambridge, UK) Alexa Flour 594 (Abcam, Cambridge, UK) for horseradish peroxidase (HRP)-conjugated anti-rabbit antibodies (Sigma-Aldrich, St. Louis, MO, USA) for 37 chemilluminescence immunoblotting. Antibodies for flow cytometry included APC/fire 750 anti-mouse CD8a (BioLegend #100766), BUV395 Rat Anti-mouse Cd45 (BD Horizon #564279), Purified Rat Anti-Mouse CD16/CD32 Mouse Fc Block (BD Pharmingen #553142), PerCP-Cy Rat Anti-mouse CD4 (BD Pharmingen #550394), and Anti-Mouse FITC TCR beta (Invitrogen #1939141)
All antibodies were used according to the manufacture’s recommendation.
Intracellular Cytokine Staining
Preparations of lymphocytes were prepared and stimulated with 5ng/ml phorbol 12-myristate 13-acetate and 500 ng/ml ionomycin (Sigma-Aldrich) for 4 hours at 37C in a 5% CO2 humidified incubator in the presence of Golgi Plug (BD Biosciences). Surface staining of stimulated lymphocytes was performed with mAb anti-CD45, anti-CD8, anti-CD4, and anti-abTCR for 30 minutes at 4C. Cells were then permeabilized with perm/wash solution followed by an additional 30 minutes of incubation with fluorochrome-conjugated monoclonal antibodies IL-10, TNF-a, IFN-g, and analyzed by flow cytometry.
Flow Cytometry Analysis
Lymphocytes were incubated with anti-CD45 Fc-R blocker for 20 minutes to minimize nonspecific antibody binding. Cells were then incubated with different combinations of mAb for 30 minutes at 4C, washed with FACS buffer, and analyzed. Six-color immunofluorescence staining was analyzed with LSR II using FACS Diva software (BD Biosciences) and analyzed using FlowJo V10 software (Tristar Software). The lymphocytes were gated using forward and side-scatter to exclude debris and dead cells, and at least 200,000 cell sorting events were acquired.
Adoptive Transfer
For T cell adoptive transfer, highly purified (>97%) total T cells were isolated from gld mice lymph nodes as reported recently18. These mice have high numbers of DN T cells needed to perform adoptive transfer studies. We transferred (2.0 X 106) of either DN T cells or all conventional TCR+ cells without DN T cells through intraperitoneal injection into separate groups of 8-week-old C57BL/6 male mice (n=5 per group), 12 hours before lung IRI.
Lung Wet/Dry Weight Ratio
In separate groups the lungs were harvested after a sham procedure or after IRI. The lung tissues were weighted, and then placed into a vacuum oven at (58C) until a stable dry weight was achieved. The ratio of lung wet to dry weight was then calculated.
Western blotting and Image Quantification
Cell protein lysates for immunoblotting were prepared in urea sample buffer (8 M deionized urea, 0.5% SDS, 30 mM Tris, pH 6.8, 5% glycerol and 5% β-mercaptoethanol) after collecting whole cell lysates from cultured cells. All samples were sheared using progressively finger-gauged needles (22 1/2, 25 1/2, and 26 ½) and subjected to a Bradford assay and processed. All samples were loaded at 10 μg of protein per lane for immunoblotting. Samples were separated using either 10% or 12.5% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis and transferred to 0.45μm nitrocellulose membranes (Bio-Rad, Hercules, CA, USA) via a trans-blot turbo transfer system (Bio-Rad). Blots were blocked in 5% milk in TBST-T (w/v) 38 for >1hr at room temperature (21°C) and subsequently incubated overnight at 4°C in their appropriate antibodies. Secondary antibodies were applied for 1hr at room temperature (21°C). Blots were developed using either ECL Select developing solution (GE Healthcare, Pittsburgh, PA, USA) or SuperSignal West Pico chemiluminescent substrate (Thermo Scientific, Waltham, MA, USA) and imaged using a FluorChem Q system (ProteinSimple, San Jose, CA, USA). Signal quantification for western blots were conducted using ImageJ software. All densitometry quantifications were calculated by dividing the band intensity for the target of interest by the loading control, and then averaging all the biological replicates across the same time point.
Statistical Analysis
Data were expressed as mean ± SD or mean ± SEM using Prism 7 (GraphPad Software); n indicates the numbers of animals per group. Comparisons were drawn using a two-tailed t test. Comparisons between multiple groups were performed by a one-way ANOVA test followed by the Student-Newman-Keuls test where appropriate. Statistical significance was determined as P<0.05.
Results
Rapid expansion of DN T cells following lung IRI
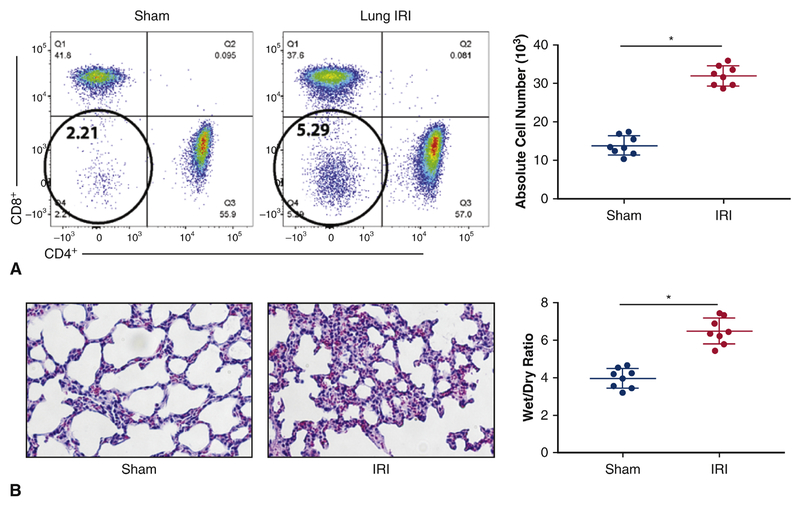
Lung tissues were obtained after IRI surgery or sham controls to assess T cell profiles. In lungs subjected to IRI, DN T cells expanded (5.29% ± 1%, p< 0.01) at 3 hrs post IRI when compared with surgical sham controls (2.21% ± 3%, p<0.01) (Figure 2A). Other subsets of αβ+ T cells such as CD4+ and CD8+ cells did not exhibit any significant changes at 3 hrs when subjected to IRI. Absolute cell number of DN T cells were quantified and there were significantly higher DN T cells after IRI when compared with the sham (30 x 103 vs. 15 x 103, p<0.05). Analysis with hematoxylin and eosin staining demonstrated thickened interstitium and neutrophil infiltration following IRI, which correlated with a higher Wet/Dry ratio (7.6) than sham surgical controls (4.1) (p<0.05) (Figure 2B).
Figure 2.
Rapid Expansion of DN T cells following lung IRI. A left dorsolateral thoracotomy was performed in the fourth intercostal space and a microvascular clamp was applied across the pulmonary artery and vein to occlude the vasculature. The left lung was occluded for 30 minutes, and the microvascular clamp was removed. The thoracotomy was closed and the mouse was removed from the ventilator and allowed to recover for the 3-hour reperfusion period. Mice were sacrificed, underwent midline abdominal incisions, were exsanguinated, and had their lung lobes removed for lymphocyte collection. (A) Mice that underwent lung IRI demonstrated an approximately 2-fold rise in DN T cells in the lungs (5.29% ± 1%, p< 0.01) when compared with surgical sham controls (2.21% ± 3%, p<0.01). (C) Illustrates absolute number of DN T cells and it is a cumulative data on eight independent experiments (n=8). (B) Hematoxylin and eosin staining showed mice that underwent lung IRI exhibited thickened interstitium and neutrophil infiltration when compared to the sham controls. (D) Shows Wet/Dry ratio, with lungs that underwent IRI exhibiting higher W/D ratio.
Lung IRI induces increased DN T cell IL-10/IFN-γ production and IL-10 dependent p38 MAPK Signaling
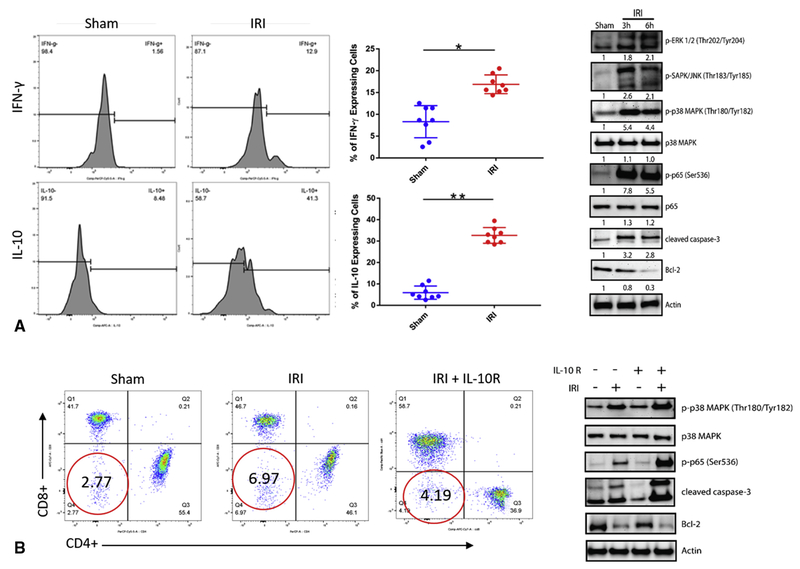
DN T cells cytokine profiles were compared between the sham and lung IRI. Histogram analysis revealed a significant fold induction of IL-10 (30% ± 5%, p<0.01) and IFN-γ (20% ± 2%, p<0.01) when compared to the sham (6% ± 1%, p<0.01) and (9.3% ± 2.8%, p<0.01), respectively (Figure 3A). Immunoblotting was carried out to investigate the p38 MAPK signaling pathway under IRI conditions. Analysis revealed that mice who underwent IRI had significant activation of signaling proteins in the p38 MAPK pathway, and induced high levels of apoptotic markers, such as cleaved caspase-3 and Bcl-2 (Figure 3B). Intraperitoneal administration of anti-IL-10 receptor neutralizing antibody (anti-10R mAb) was given prior to lung IRI. Mice who received anti-10R mAb preoperatively exhibited lower levels of DN T cells in the lungs following IRI (4.1 % ± 1.5% vs. 6.9% ± 2.4%, p<0.01). Immunoblotting analysis also revealed anti-10R mAb treatment followed by subsequent IRI showed higher expression of phospho-p38 MAPK as well as cleaved caspase-3 and Bcl-2 compared with mice who underwent IRI without preoperative anti-10R mAb treatment (Figure 3C).
Figure 3.
Lung IRI induces lung DN T cell IL-10 and interferon production, and lung IL-10 dependent p38 MAPK Signaling. Preparations of lymphocytes were prepared and stimulated to secrete cytokines and interferons. Surface staining of stimulated lymphocytes was performed. Cells were then permeabilized with perm/wash solution followed by an additional 30 minutes of incubation with fluorochrome-conjugated monoclonal antibodies. For protein expression, immunoblotting was performed by detecting specific signaling molecules in the lungs following IRI. (A) Expansion of DN T cells in the lung following IRI resulted in significant higher secretions of IL-10 (30% ± 5%, p<0.01) and IFN-γ (20% ± 2%, p<0.01) when compared to the sham (6% ± 1%, p<0.01). (B) Immunoblotting analysis revealed lungs that underwent IRI had significant activation of signaling proteins in the p38 MAPK pathway, and induced robust levels of apoptotic markers such as cleaved caspase-3 and Bcl-2. (C) Mice preoperatively treated with IL-10R neutralizing antibody exhibited lower levels of DN T cell following IRI (4.1 % ± 1.5% v 6.9% ± 2.4%, p<0.01). Immunoblotting analysis also revealed IL-10R treatment followed by subsequent IRI showed higher expression of phospho-p38 MAPK as well as cleaved caspase-3 and Bcl-2 compared to the lungs that underwent IRI without preoperative IL-10R treatment.
Left and Right Lung differences following IRI at different Reperfusion Times
To determine if there were any differences in DN T cell expansion between the left and right lung, various reperfusion times were performed to identify notable changes. Between 0.5, 1, 3 and 6 hours of reperfusion, only at the one hour time point did the data exhibit a significant difference in DN T cell expansion between the left and right lung following IRI (2.6 x 104 vs. 2.0 x 104 , respectively, p<0.05) (Figure 4A). For the Wet/Dry ratio, significant differences seen between the sham and IRI were seen following 3 and 6 hour reperfusion (Figure 4B). The cytokine profile also exhibited similar kinetics with the Wet/Dry ratio, with robust secretion of IFN-γ and IL-10 from DN T cells following 3 and 6 hour reperfusion (Figures 4C and D).
Figure 4.
Left and Right Lung differences following IRI at different Reperfusion Times. (A) Significant DN T cell expansion between the right and left lung following IRI was only seen at one-hour reperfusion. (B) Wet/Dry ratio differences were only significant at 3 and 6 hours of reperfusion in respective to the sham. (C) Changes in IFN-γ cytokines secreted from DN T cells were only seen following 3 and 6 hour reperfusion. (D) Changes in IL-10 cytokines secreted from DN T cells were only seen following 3 and 6 hour reperfusion.
DN T cell transfer attenuates lung IRI
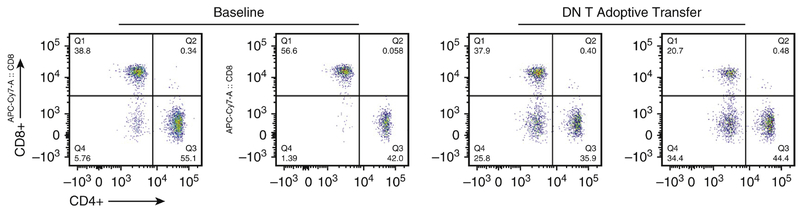
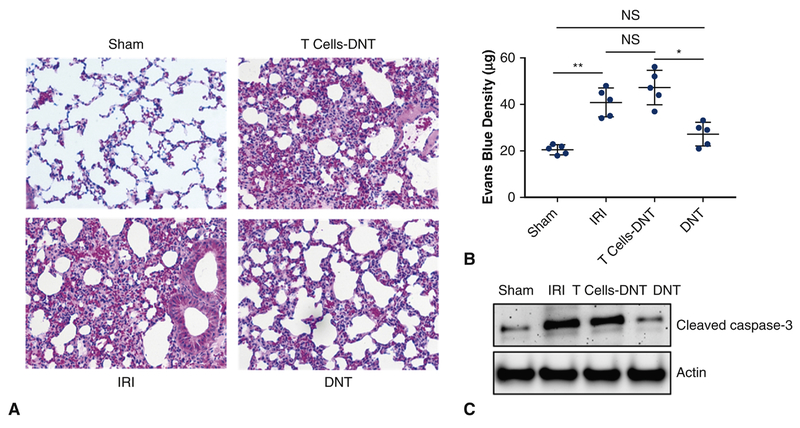
Given the rapid expansion of DN T cells in the lungs and its robust secretion of anti-inflammatory cytokines following IRI, we next determined whether DN T cells could protect against IRI-induced lung injury. For these experiments, we utilized DN T cells isolated from lymph nodes of gld donors because they possessed large number of DN T cells needed for successful adoptive transfer studies as previously described15. Confirmatory experiments of successful transfer of DN T cells that trafficked and remained in the lungs after 12 hours were performed and were consistent with robust numbers DN T cells, compared to sham, still within the lungs at this timepoint. (Figure 5). For controls, all TCR+ T cells except DN T cells were adoptively transferred. Mice preoperatively treated with DN T cells 12 hours before surgery exhibited significant protection from lung IRI, as evident by differences in H&E staining that revealed diminished thickened lung interstitium (Figure 6A). A significant decrease in Evans Blue Dye extravasation was also evident between standard IRI compared to IRI preoperatively treated with DN T cells (39.5 μg vs. 28 μg, p<0.05) (Figure 6B). Adoptive transfer with conventional T cells did not provide significant protection from lung IRI. There was no significant difference in Evans Blue Dye between standard IRI and IRI preoperatively treated with conventional T cells (39.5 μg v. 44.7 μg, p>0.05). Immunoblotting analysis revealed that adoptive transfer with DN T cells attenuated levels of cleaved caspase-3. (Figure 6C) Adoptive transfer with conventional T cells did not result in significant cleaved caspase-3 attenuation.
Figure 5.
Flow Cytometry of DN T cell transfer. Lymphocytes were isolated from the lungs 12 hours following adoptive transfer with DN T cells. Flow cytometric data exhibit robust levels of DN T cells (25.8%, 34.4%) when compared to the baseline (5.76%, 1.39%).
Figure 6.
DN T cell transfer attenuates lung IRI. For T cell adoptive transfer, highly purified (>97%) total T cells were isolated from lymph nodes of gld mice. 2.0 X 106 of either unconventional DN T cells or conventional CD4+ and CD8+ T cells were transferred into separate groups of mice (n=5 per group), 12 hours before lung IRI. (A) Mice preoperatively treated with DN T cells and then underwent lung IRI, exhibited a significant reduction in interstitial edema and Evans blue dye permeability when compared to mice who only received conventional T cells. (C) Immunoblotting analysis also showed a significant decrease in cleaved caspase-3 in mice who received DN T cells. There was no significant difference between mice that only underwent IRI and mice who received conventional T cells prior to IRI.
Discussion
Our study used a murine model with significant lung IRI occurring at 30 minutes of vascular occlusion followed by three hours of reperfusion. Lung IRI was characterized by thickened interstitium, neutrophil infiltration, and microvascular leak. We demonstrated lung IRI significantly expanded a subset of unconventional αβ+ T cells, DN T cells. However, lung IRI did not significantly alter the levels of CD4+ and CD8+ cells in the lungs at the 3 hour point. Further analysis also demonstrated a significant difference in the local DN T cell population following one hour of reperfusion but not at three hours when compared to the contralateral non-ischemic lung. Intracellular cytokine staining revealed that the expansion of DN T cells in the lungs was also accompanied with a robust secretion of IL-10 and IFN-γ. Immunoblotting analysis revealed that levels of phosphor-p38 MAPK, phospho-ERK 1/2, phospho-SAPK/JNK, cleaved caspase 3 and Bcl-2 were elevated following lung IRI. Preoperative treatment with anti-10R mAb attenuated DN T cell expansion in the lungs and cell death. We also demonstrated that adoptive transfer with DN T cells into mice 12 hours prior to lung IRI significantly attenuated lung injury compared to adoptive transfer of conventional T cells only, evidenced by histological, immunoblotting, and flow cytometric analysis (Figure 6).
Previous studies have focused on neutrophil infiltration, natural killer (NK) cells and conventional CD4 and CD8 T cells in the pathogenesis of lung IRI19–24. These studies have shown that neutrophils mediate pulmonary tissue destruction during IRI by inducing the onset of pro-inflammatory responses, oxidative stress, and apoptotic factors. These groups have also shown that T cells play a pivotal role in lung IRI. In several studies, T cells were found to be activated during IRI and thereby infiltrate into the lungs during reperfusion. Thus, inhibiting T cells prior to reperfusion has been demonstrated to attenuate inflammation and lung injury25, 26.
Recently, several groups have identified and investigated a subset of unconventional αβ+ T cells, DN T cells. DN T cells were found in the kidneys at a steady state and were seen to proliferate when both kidneys were subjected to bilateral renal IRI15. The expanded DN T cells in the kidneys produced higher levels of IL-10 and IL-27. Adoptive transfer with DN T cells 24 hours prior to bilateral renal IRI demonstrated significant protection from acute kidney injury (AKI). DN T cells have been reported to reside in the lung parenchyma and rapidly expand in response to an Influenza A virus infection in mice17. Our study builds upon these data and others by demonstrating that DN T cells expand upon lung IRI. We showed that lung DN T cells secrete high levels of IL-10 and IFN-γ. We also show the protective effects of DN T cells in the lungs by performing an adoptive transfer of both DN T cells and unconventional T cells.
It is important to state that although DN T cells were seen to rapidly expand in response to lung IRI or Influenza A virus infection, the precise origin of these cells remains unclear. Since DN T cells are early T cell precursors that arise in the thymus, it is reasonable to hypothesize that these cells migrated from the thymus to the lungs following lung IRI. However, Neyt and colleagues have demonstrated that athymic mice exhibited similar levels of DN T cells in the lungs following Influenza A infection17. This may suggest that these cells may arise extra-thymically. Further characterization of DN T cell expansion in nephrectomized, thymectomized and splenectomized mice is warranted to understand the origin of these unconventional cells.
To further investigate the molecular mechanisms of our lung IRI model, we investigated a canonical stress signaling pathway, p38-MAPK. Previously studies demonstrated that levels of phosphor-p38 MAPK, phospho-ERK, phospho-JNK, and phospho-NF-kB increased in the lungs following IRI. This showed that lung IRI was able to induce DAMP-associated signaling and induce inflammation through NF-kB. Here, we also identified similar results in our murine lung IRI model. Immunoblotting analysis revealed that the levels of phospho-p38 MAPK, phospho-ERK 1/2, and phospho-SAPK/JNK were significantly elevated in the lungs following IRI. However, total protein levels of p38-MAPK did not change. Apoptotic markers such as cleaved caspase-3 and Bcl-2 were also elevated following lung IRI, demonstrating cellular death. These results illustrated that lung IRI induced the phosphorylation of p38-MAPK associated signaling proteins, activating downstream effects such as programmed cell death. Since lung IRI was an acute injury on the lungs, total p38-MAPK protein levels did not change. Due to the robust IL-10 secretion by DN T cells, we treated mice with either anti-10R mAb or a control isotype antibody prior to surgery. Flow cytometric analysis revealed that mice preoperatively treated with anti-10R mAb exhibited a lower DN T cell expansion when compared with normal IRI conditions. Furthermore, immunoblotting analysis showed treatment with anti-10R mAb prior to lung IRI exacerbated activation of the p38-MAPK signaling pathway when compared with our standard IRI without preoperative treatment. In addition, anti-10R mAb treatment also increased the levels of cleaved caspase-3 and reduced the levels of Bcl-2. Taken together, the data revealed that DN T cell expansion was dependent on IL-10, which may also possess its anti-apoptotic capabilities in the lungs following IRI. However, there could be other sources of IL-10 besides DN T cells that were blocked with the antibody. The exact mechanism of IL-10 and its effect on apoptosis and DN T cell expansion remains unclear. Additional in vitro experiments will be conducted to dissect the function of IL-10.
While most efforts to understand the roles of T cells in lung IRI have focused on CD4+, CD8+, and Tregs, this study has demonstrated for the first time that lung DN αβ+ T cells can function as a major modulator of the immune system in response to IRI. Future in vitro studies are required to elucidate the molecular mechanisms of how DN T cells communicate with other immune cells under IRI conditions, as well as to determine trafficking mechanisms in DN T cells. We also plan to investigate the immune profile of human lung tissues derived from lung transplant patients to see if our murine findings extend to humans.
Conclusion
This is the first description of a protective role for DN T cells in experimental lung IRI. We observed significant protection from lung IRI in mice that received DN T cell adoptive transfer compared to those receiving conventional T cells. p38-MAPK and apoptotic signaling pathways were seen to be robustly elevated under the setting of lung IRI. Attenuation of IL-10, a significant anti-inflammatory cytokine secreted by DN T cells, was seen to exacerbate the expression of p38-MAPK and apoptotic proteins. We propose a potential novel immunoregulatory function of DN T cells during lung IRI. Future studies are needed to elucidate the cellular and molecular mechanisms underlying DN T cells responses and its potential therapeutic role in lung IRI during transplantation.
Limitations
It is important to state that our experiment had several limitations. A warm model of lung IRI was used because it is a simple technique to induce ischemia and reperfusion to the left lung. It represents a severe model of ischemia void of the cryoprotective effects of cold ischemia. Metabolic demands are higher in the warm model, leading to more extensive damage to the lung, allowing us to capture greater changes in DN T cell levels, and better measure the effects of IL-10. There are many limitations to the warm ischemia model. For example, our model does not directly represent the clinical practice of lung transplantation as organs are cooled down during organ recovery until the time of implantation. Our model also does not take into consideration the epigenetic changes that may occur due to temperature alterations. Furthermore, DN T cells lack specific molecular surface markers, which hampers localization studies. We are currently working with other investigators in establishing a new immunofluorescence protocol to successfully visualize DN T cell localization.
Figure 1.
Flow Cytometry gating for DN T cells. Cells isolated from murine lungs were cultured in 5% fetal bovine serum in RPMI medium with 100 mg of Collagenase D for 30 minutes. Cells were strained and lymphocytes were isolated with percoll extraction. Lymphocytes were stained with CD45 (APC-Cy7), TCR αβ (BV421), CD4+ (APC), CD8+ (FITC) antibodies and analyzed through LSR-II flow cytometry. The lymphocyte population was isolated from SSC-A and FSC-A. CD45+ TCR+ subpopulation was selected to visualize CD4+, CD8+, Double-Positive (CD4+CD8+) and Double-Negative (CD4−CD8−) T cells. Double-Negative T cells are represented as the population within the red circle.
Figure 7.
Adoptive transfer with Double Negative αβ T cells attenuated lung damage. 8-10 week-old gld male mice were utilized to isolate highly purified (>97%) total T cells from lymph nodes and then adoptively transfer the enriched T cells by intraperitoneal (IP) injection into C57BL/6J WT mice 12 hours before onset of lung ischemia-reperfusion injury (IRI). After 30 minutes of left lung hilar ischemic vascular occlusion followed by 180 minutes of reperfusion, animals treated with DN T cell adoptive transfer sustained significantly less lung injury compared to adoptive transfer of conventional T cell controls; evidenced by decreased interstitial thickening on H & E staining, attenuated apoptosis/cleaved caspase-3 expression by immunoblotting, and higher frequency of DN T cells and levels of anti-inflammatory cytokine expression by flow cytometry.
Central Message:
Adoptive transfer with Double-negative αβ T cells attenuated lung ischemia reperfusion injury. This discovery improves our understanding of unconventional T cells and their immunoregulatory roles.
Perspective Statement:
The discovery of unconventional αβ DN T cells and their protective effects in lung ischemia reperfusion injury increases our understanding of the pathogenesis of primary graft dysfunction during lung transplantation, and enables the development of new therapeutic approaches for this serious condition
Biographies
A. Sasha Krupnick

Daniel Kreisel

Joshua Hsu

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conference Affiliation: The abstract associated with this manuscript was presented at the 99th annual American Association for Thoracic Surgery in Toronto, Canada, May 4th-7th, 2019 free from embargo. All authors have nothing to disclose.
References
- 1.Granton J Update of early respiratory failure in the lung transplant recipient. Curr Opin Crit Care. 2006;12:19–24. [DOI] [PubMed] [Google Scholar]
- 2.Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov 2014; 13: 852–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porteous MK, Diamond JM, Christie JD. Primary graft dysfunction: lessons learned about the first 72h after lung transplantation. Curr Opin Organ Transplant. 2015;20(5):506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christie JD, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24(10):1454–1459. [DOI] [PubMed] [Google Scholar]
- 5.Bharat A, et al. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008;86(1): 189–195; discussion 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daud SA, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175(5):507–513. [DOI] [PubMed] [Google Scholar]
- 7.Hsiao HM, Fernandez R, Tanaka S, et al. Spleen-derived classical monocytes mediate lung ischemia-reperfusion injury through IL-1β. J Clin Invest. 2018;128(7):2833–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z, Sharma AK, Linden J, et al. CD4+ T lymphocytes mediate acute pulmonary ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2009;137(3):695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burne MJ, Daniels F, El Ghandour A, et al. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest. 2001;108: 1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandolfo MT, Jang HR, Bagnasco SM, et al. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009;76: 717–729. [DOI] [PubMed] [Google Scholar]
- 11.Kinsey GR, Sharma R, Huang L, Li L, et al. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20: 1744–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Huang L, Sung SS, et al. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 2007;178: 5899–5911. [DOI] [PubMed] [Google Scholar]
- 13.Ascon DB, Ascon M, Satpute S, et al. Normal mouse kidneys contain activated and CD3+CD4−CD8− double-negative T lymphocytes with a distinct TCR repertoire. J Leukoc Biol. 2008;84(6): 1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martina MN, Noel S, Saxena A, et al. Double Negative (DN) αβ T Cells: misperception and overdue recognition. Immunol Cell Biol. 2015;93(3):305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martina MN, Noel S, Saxena A, et al. Double-Negative αβ T cells Are Early Responders to AKI and Are Found in the Human Kidney. J AM Soc Nephrol. 2016;(4): 1113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyagawa F, Okiyama N, Villarroel V, Katz SI. Identification of CD3+CD4−CD8− T cells as potential regulatory cells in an experimental murine model of graft-versus-host skin disease (GVHD). J Invest Dermatol. 2013;133(11):2538–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neyt K, GeurtsvanKessel CH, Lambrecht BN. Double-negative T resident memory cells of the lung react to influenza virus infection via CD11chi dendritic cells. Mucosal Immunol. 2016;(4):999–1014. [DOI] [PubMed] [Google Scholar]
- 18.Hamad AR, Mohamood AS, Trujillo CJ, Huang CT, Yuan E, Schneck JP. B220+ double-negative T cells suppress polyclonal T cell activation by a Fas-independent mechanism that involves inhibition of IL-2 production. J Immunol. 2003; 171: 2421–2426. [DOI] [PubMed] [Google Scholar]
- 19.Granton J Update of early respiratory failure in the lung transplant recipient. Curr Opin Crit Care. 2006;12:19–24. [DOI] [PubMed] [Google Scholar]
- 20.Trulock EP, Edwards LB, Taylor DO, Boucek MM, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-third official adult lung and heart-lung transplantation report—2006. J Heart Lung Transplant. 2006;25:880–92. [DOI] [PubMed] [Google Scholar]
- 21.Fiser SM, Tribble CG, Long SM, Kaza AK, Cope JT, Laubach VE, et al. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J Thorac Cardiovasc Surg. 2001;121:1069–75. [DOI] [PubMed] [Google Scholar]
- 22.Gazoni LM, Laubach VE, Mulloy DP, Bellizzi A, Unger EB, Linden J, et al. Additive protection against lung ischemia-reperfusion injury by adenosine A2A receptor activation before procurement and during reperfusion. J Thorac Cardiovasc Surg. 2008;135:156–65. [DOI] [PubMed] [Google Scholar]
- 23.Sharma AK, LaPar DJ, Zhao Y, et al. Natural killer T cell-derived IL-10 mediates lung ischemia-reperfusion injury. Am J Respir Crit Care Med. 2011; 183(11): 1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma AK, LaPar DJ, Stone ML, et al. NOX2 Activation of Natural Killer Cells Is Blocked by the Adenosine A2A Receptor to Inhibit Lung Ischemia-ReperfUsion Injury. Am J Respir Crit Care Med. 2016;193(9):988–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross SD, Tribble CG, Gaughen JR Jr, Shockey KS, Parrino PE, Kron IL. Reduced neutrophil infiltration protects against lung reperfusion injury after transplantation. Ann Thorac Surg. 1999;67:1428–34. [DOI] [PubMed] [Google Scholar]
- 26.Krishnadasan B, Naidu B, Rosengart M, Farr AL, Barnes A, Verrier ED, et al. Decreased lung ischemia-reperfusion injury in rats after preoperative administration of cyclosporine and tacrolimus. J Thorac Cardiovasc Surg. 2002; 123:756–67. [DOI] [PubMed] [Google Scholar]