Abstract
Cell preservation is an enabling technology for widespread distribution and applications of mammalian cells. Traditional cryopreservation via slow-freezing or vitrification provides long-term storage but requires cytotoxic cryoprotectants (CPA) and tedious CPA loading, cooling, and recovering procedures. Hypothermic storage around 0 – 4 °C is an alternative method but only works for a short period due to its high storage temperature. Here, we report on the deep-supercooling (DSC) preservation of human adipose-derived stem cells at deep subzero temperatures without freezing for extended storage. Enabled by surface sealing with an immiscible oil phase, cell suspension can be preserved in a liquid state at −13 °C and −16 °C for 7 days with high cell viability; and retention of stemness, attachment, and multilineage differentiation capacities. These results demonstrate that DSC is an improved short-term preservation approach to provide off-the-shelf cell sources for booming cell-based medicine and bioengineering.
1. Introduction
Preservation of mammalian biospecimens, such as cells, tissues, and organs, is a practical strategy to maintain and extend their life and functions outside their native conditions. It is an essential technology for assisted reproduction [9], tissue/organ transplantation (including blood transfusion) [10; 12], cell therapeutics [45; 46; 59], and tissue regeneration and repairing [25; 48]. Conventional long-term preservation (cryopreservation) is achieved by cooling biospecimens to deep subzero temperatures (e.g. −196 °C), storing them in a state of suspended animation, and then warming them back to normothermic temperature (e.g. 37 °C) on demand as necessary. There are two methods for cryopreservation, slow-freezing and vitrification [18]. The former is to cool biospecimens at a low cooling rate (e.g. 1 °C/min) to gradually dehydrate cells and minimize intracellular ice formation, but it can cause osmotic shock and extensive dehydration and deformation [18; 27; 31]. The latter is to cool the biospecimens at a high cooling rate without ice formation, but it requires a high concentration of cryoprotectant (CPA) and/or limits the sample volume within the order of 100 μl [18; 19]. Both of these methods require cell membrane-permeable CPAs (e.g. dimethyl sulfoxide) to minimize cryoinjuries. The presence of cytotoxic CPAs not only requires rigorous removal before further applications via tedious washing and centrifugation [8; 23], but also causes spontaneous differentiations [5], intravascular hemolysis[33], and cell loss[43]. Thus, these traditional cryopreservation approaches, while critical for theoretically infinite storage time, have shown a series of inadequacies and bottlenecks which currently hinder some of their promises.
Mesenchymal stem cells (MSCs) recently have attracted great interest for scientific research and clinical applications [51]. They are adult stem cells that can be found in many organs and tissues, such as bone marrow, adipose tissue, and amniotic fluid [52]. Due to their self-renewal capacity, multilineage differentiation ability, and extraordinary potential of paracrine secretions, MSCs are widely used as cell therapeutic agents for immunoregulation, antimicrobial medicine, tissue regeneration and repair [32; 44]. Adipose-derived stem cells (ADSCs) are MSCs derived from adipose tissues, which are abundant, accessible, and reliable sources of stem cells [4]. Their easy isolation procedure and high isolation yield make them a perfect candidate for cell-based therapies [35]. Therefore, an effective and efficient biopreservation method of ADSCs would have a significant impact on their widespread dissemination for research and clinical applications [24].
Hypothermic storage below normothermic temperature (37 °C) is an alternative approach for short-term biopreservation. In this method, biospecimens are usually stored above freezing temperatures so that phase transition will not occur, cytotoxic CPA will not be required, and thus, cryoinjuries (such as osmotic shock, intracellular and extracellular ice formation, and freezing concentration) associated with cryopreservation can be avoided. It has been used to preserve various mammalian cell (e.g. primary human hepatocytes [13; 37], cardiomyocytes [47], multipotent stromal cells [41], and blood cells [26; 38; 54]) and cell-biomaterial constructs (e.g. two-dimensional (2D) cell monolayers [7], three-dimensional (3D) cell aggregates [7], and cell/tissue/organ-on-a-chip [14; 57]). Since there is no ice formation, hypothermic storage is preferred for preserving large-volume tissues and organs with complex and delicate structures (e.g. microcapillaries) that are highly susceptible to ice crystal formation [12]. Therefore, it was utilized to preserve livers [1; 15], kidneys [1; 56], and other organs [16] for in vitro transportation and transplantation. However, due to relatively high storage temperatures (usually above 0 °C), biospecimens in hypothermic storage still undergo significant metabolic activities, and thus, they gradually decay and deteriorate as storage proceeds. Depending on physicochemical properties and characteristics of biospecimens, the storage time is usually short, varying from several hours (e.g. 4–6 hours for hearts and lungs) to several days (e.g. 3 days for kidneys).
Extending the storage time for hypothermic preservation requires the decrease of storage temperature to reduce metabolic and deteriorating rates. The gold standard of cold storage is to store biospecimens just above their equilibrium freezing points. Further decrease of the storage temperature below 0 °C will subjugate biospecimens to spontaneous ice nucleation, subsequent sample freezing, and resultant cryoinjuries. Although supercooling, a metastable liquidus state below equilibrium freezing temperatures without freezing, was used to temporarily preserve biospecimens before at high subzero temperatures (≥ – 6 °C) [2; 53], the fate of biospecimens are constantly threatened by stochastic ice formation. To address this challenge, we recently developed a novel method that can eliminate the primary mechanisms of ice crystallization and thus, achieve stable storage of large-volume water and red blood cell suspensions at deep subzero temperatures (< −10 °C) without freezing [22].
In this study, we applied the deep-supercooling (DSC) approach to preserve human adipose-derived stem cells (hADSCs). We achieved stable DSC of hADSC suspensions at temperatures as low as – 16 °C for 7 days with surface sealing by an immiscible oil phase. With the optimization of storage solution, hADSCs were recovered with high viability, surface attachment capability, stemness, and multilineage differentiation capacities. Overall, our results demonstrate that DSC is an innovative and feasible approach for short-term preservation of stem cells.
2. Materials and methods
2.1. Materials.
Fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Invitrogen (Carlsbad, CA, USA). The hADSCs and their culture media were purchased from Lonza (Allendale, NJ, USA), and their differentiation media and staining assay were purchased from Stemcell Technologies (Cambridge, MA, USA). The antibodies of immunofluorescence staining were purchase from ThermoFisher Scientific (Waltham, MA, USA). The primary hypothermic storage solution, University of Wisconsin solution (UW), was purchased from Bridge to Life (Columbia, SC, USA). All other chemicals were purchased from Sigma (St. Louis, MO, USA) unless specifically noted otherwise.
2.2. Cell culture.
hADSCs were cultured in their basal media supplemented with 10% FBS, 1% L-glutamine, 0.1% gentamicin-amphotericin, and 1% penicillin and streptomycin at 37 °C in a humidified atmosphere with 5% CO2. The media was changed every other day until ~ 80% confluence. Cells (less than passage 5) were detached for passage and/or experiments by incubating with 1% trypsin for 5 mins. Before experiments, cell suspensions in culture media were centrifuged at 300 g for 5 mins at 4 °C, and the resulting pellet was resuspended in hypothermic storage solution.
2.3. Deep-supercooling for cell suspensions.
The DSC protocol for hADSC cell suspension was adapted from the protocol we developed for water and red blood cells [22]. Briefly, 1 ml UW solutions, hADSC suspensions in UW (UW+ cell), and hADSC suspensions in UW supplemented with 5% (w/v) 35 Kd polyethylene glycol (PEG) and 0.2 M 3-O-methyl glucose (3-OMG) (UW + cell+ PO), were aliquoted into 5-ml round-bottomed polystyrene tubes purchased from ThermoFisher Scientific (Waltham, MA, USA). Cell concentration in hADSC suspension is 1 million/ml. Then, 0.5 ml surface sealing agent (paraffin oil) was gently added onto the surface of solutions or suspensions using serological pipets, trickling down along the wall of tubes to avoid splashing or trapping air bubbles at the interface. The sample-laden tubes were transferred into portable temperature-controlled freezers (Engel MHD-13, Engel, Jupiter, FL, USA) that were placed in 4 °C cold room to minimize temperature fluctuations. In addition, temperatures inside these freezers were verified by Toluene-filled low-temperature thermometer. The freezers were set at different temperatures (from 4 °C to −16 °C) to preserve hADSC suspensions at different temperatures. We refer to all the samples stored in this temperature range without freezing, as hypothermic or cold storage in general. Samples sealed by immiscible oils so that they can be stored below −10 °C without freezing are denoted as DSC storage. The freezing probability of supercooled samples was quantified by cumulative freezing frequency (ff = number of frozen samples/total number of initial samples), which would increase with time as only more freezing events might occur during supercooling.
2.4. Cell recovery.
After 7-day hypothermic storage, cell suspensions were taken out of the freezers and warmed in 4 °C cold room for 10 mins. Then the oil for surface sealing in DSC tubes was removed by careful aspiration, and 3 ml cell culture media at room temperature was added into each tube. After gentle mixing with the newly added warm media, cell suspensions were transferred to 15-ml centrifuge tubes for centrifugation at 300 g for 5 mins. Finally, hADSC pellets were resuspended in warm culture media, transferred into cell culture flasks or plates, and taken into 37 °C incubator for cell recovery and culture.
2.5. Cell viability and attachment tests.
After recovering and culturing the hADSCs for 1 hour, the cell viability post hypothermic storage was evaluated using standard live/dead assay kit (Invitrogen, Carlsbad, CA, USA). Calcein AM was used to identify cell metabolic activity and ethidium homodimer to check cell membrane integrity via fluorescence imaging. 2 μM calcein AM and 4 μM ethidium homodimer were added into cell suspensions and incubated for 10 min at 37 °C. Cell viability was determined via fluorescence and phase contrast imaging by an EVOSFL microscope (ThermoFisher Scientific, Waltham, MA, USA). The total cell number under each field was determined using phase images. Cells that excluded ethidium homodimer (red) and maintained calcein (green) were counted as viable while cells stained with ethidium homodimer (red) were counted as dead. At least ten randomly selected fields were used for each sample. The immediate cell viability was calculated as the ratio of the number of viable cells to the total number of cells per field (10×). To quantify cell attachment efficiency, 0.25 million fresh or preserved hADSCs in UW+PEG+3-OMG were seeded in 6-well plate. After 1-day culturing at 37 °C, the media was removed and cells washed by PBS twice. Then 1 ml culture media was added to each well, and at least 20 random phase contrast images were taken for each scenario to quantify cell number per field (4X). The attachment efficiency was calculated as the percentage of average cell number per field in preserved samples, relative to that in the fresh control sample.
2.6. Stemness tests for hADSCs.
To evaluate gene expression levels, quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed for fresh control and preserved hADSCs. Four typical stem cells genes of hADSC, OCT4, SOX2, KLF4, and NANOG, have been examined [43], and their corresponding primers are listed in Table S1. For immunofluorescence staining of positive (CD 44+) and negative (CD 31−) surface receptors, 0.1 million hADSCs were seeded into 6-well plate and incubated overnight for attachment. Then, the samples were washed with PBS, fixed with 4% paraformaldehyde, incubated in 3% BSA in PBST at room temperature for 1 hour to block nonspecific bindings, and incubated overnight at 4 °C with primary antibody of CD44 or CD31 according to the manufacturer’s instructions. Afterwards, the samples were washed three times with PBS and incubated in dark at room temperature for 1 hour with secondary antibodies diluted in 3% BSA in PBST (1:50 dilution). The nuclei of the cells were stained by 5 μM Hoechst 33342. The immunofluorescence staining of these surface makers and nuclei were imaged by EVOS fluorescence microscope.
2.7. Multilineage differentiation capacities.
hADSCs possess distinctive differentiation abilities into multiple mesodermal lineages, and the procedures of its adipogenic, osteogenic, and chondrogenic differentiations had been documented in detail [21]. Briefly, for adipogenic differentiation, 0.2 million cells were seeded in each well of 6-well plate, and after confluence, the cell culture media was replaced by adipogenic differentiation media. The media was changed every 3 days until day-21. The adipogenic differentiation would produce oil lipids, which were stained by oil red O. For osteogenic differentiation, 0.2 million hADSCs were seeded in each well of 6-well plate. After culturing for 24 hours, the culture media was replaced with osteogenic differentiation media and was changed every 3 days until day-21. The calcium deposit of osteogenic differentiation was stained by alizarin red S. For chondrogenic differentiation, 0.3 million hADSCs were centrifuged into a pellet and cultured in chondrogenic differentiation media in 15 ml polypropylene culture tubes. Caps of the tubes were loosened for gas exchange. The pellets were cultured in 37 °C incubator for 21 days with a media change every 3 days. Afterwards, the pellets were harvested, fixed with 4% paraformaldehyde, embedded with paraffin, and stained with Alcian blue to determine acidic polysaccharide (acidic polysaccharide is abundant in cartilage after chondrogenic differentiation). All the stained samples were imaged Nikon 80i microscope equipped with a Nikon D5100 digital camera. The percentages of area stained by Oil red O, Alizarin red S, and Alcian blue for hADSCs’ adipogenic, osteogenic, and chondrogenic differentiations, respectively, were calculated using the software, Matlab (Mathworks, Natick, MA, USA).
2.8. Statistical analysis.
All data were reported as the mean ± standard deviation from at least three independent runs. The statistical significance in mean values between two groups was determined using Microsoft Excel based on Student’s two-tailed t-test, assuming equal variance. A p-value of < 0.05 was taken as statistically significant.
3. Results
3.1. Deep-supercooling of ADSC suspensions
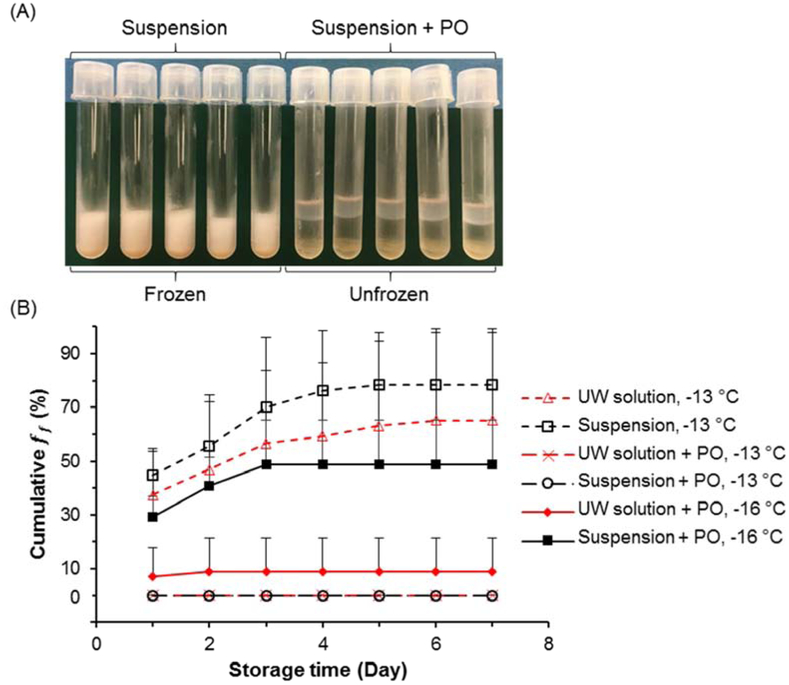
Stable deep-supercooling, a liquidus state far below the equilibrium freezing temperature (e.g.−10 °C) but without freezing, was achieved via surface sealing with paraffin oil (PO). The choice of PO rather than other water-immiscible oils is based on its high efficacy of nucleation inhibition, biocompatibility, and low cost [22]. The base solution for hADSCs supercooling storage used was standard UW solution as it has proven effective for supercooling of a wide spectrum of mammalian cells and tissues [30]. Figure 1 shows that suspensions of hADSCs can be successfully supercooled at −13 °C for one week. Specifically, all suspensions without PO sealing reached a ff higher than 50% in the first 3 days at −13 °Cand almost all samples froze by the 4th day. In contrast, suspensions with PO sealing did not present a single freezing event over 7-day storage at the same temperature. When the temperature is further decreased to −16 °C, the freezing probability of PO-sealed UW and cell suspensions increased, but were still relatively low (9% and 48.6%, respectively), particularly for UW solution. These results indicate that with the aid of PO sealing, we can obtain deep supercooled hADSCs at −13 °C and −16 °C for at least 7 days. As a result, this method allows us to investigate cell responses post DSC treatment and pursue extended preservation at previously unachievable temperatures, without considering the interruption and complication of phase transition.
Figure 1.
Deep-supercooling (DSC) of solutions and cell suspensions via surface sealing by paraffin oil (PO). (A) Representative image of cell suspensions without (Suspension) or with (Suspension + PO) surface sealing at −13 °C. 1 ml cell suspension of 1 million human adipose-derived stem cells (hADSCs) was stored in each polystyrene tube for 7 days. The storage solution is the basic University of Wisconsin solution (UW solution). (B) Cumulative freezing frequency (ff = number of frozen samples/total number of initial samples) for 1 ml UW solution or cell suspensions with or without surface sealing over 7 days. Number of independent experiments n = 5, number of tested samples for each case N = 50. Error bars represent standard deviations.
3.2. Short-term cell viability and attachment post DSC
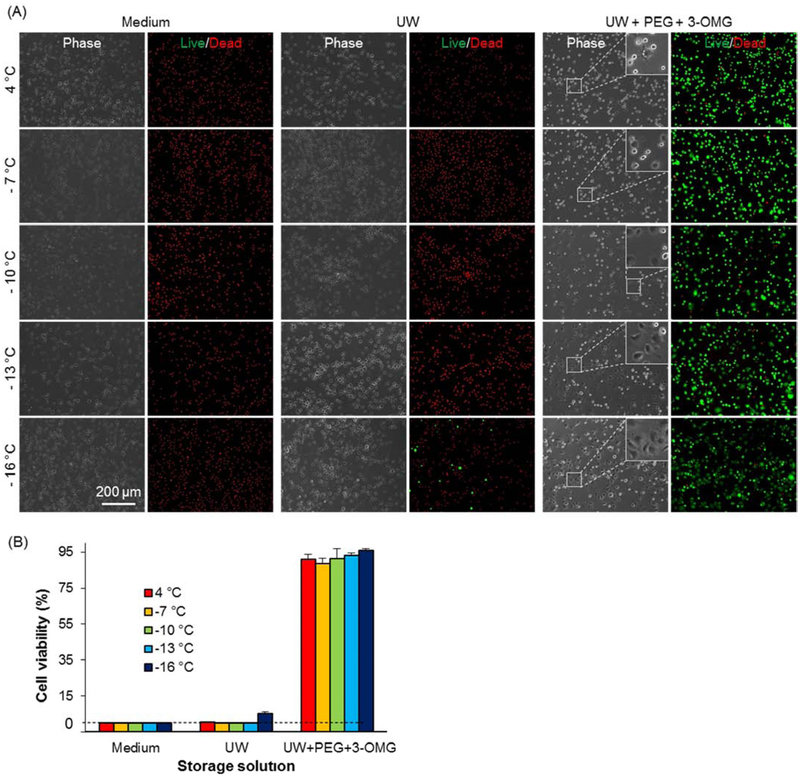
To identify suitable storage solution for hADSCs, we tested three solutions, cell culture media, UW, and UW supplemented with 35 kD polyethylene glycol (PEG, 5%(w/v)) and 3-O-methyl glucose (3-OMG, 0.2 M). The inclusion of 35 kD PEG, which cannot penetrate the cell membrane, is due to its protective effects as an extracellular cell membrane stabilizer [36], and cell membrane penetrating but non-cytotoxic non-metabolizable 3-OMG as an intracellular cytoprotectant [50]. In addition, we screened five different storage temperatures, 4 °C, −7 °C, −10 °C, −13 °C, and −16 °C. The results show that both the formulation of the storage solution and the temperature have a significant impact on immediate cell viability post 7-day cold storage (Figure 2). When cell culture media or UW is used as a storage solution, very few cells can survive storage at any of the tested temperatures, except for UW at −16 °C that can render a viability of 5.3%. However, when UW+PEG+3-OMG is used as the storage solution, the immediate cell viability reaches 90% or higher, which fully demonstrates the significant impact of storage solutions on preservation. In addition, if we carefully examine the cell morphology of the preserved cells post 1-hour of culturing at 37 °C, we will find that the lower the storage temperature, the more cells would attach and spread (as shown bythe insets in Figure 2(A)).
Figure 2.
DSC preservation of hADSCs enabled by oil sealing. (A) Phase and fluorescence micrographs of cell viability staining post 7 - day storage at various temperatures. Three typical storage solutions, ADSC media, UW, and UW supplemented with 5% (w/v) 35 Kd polyethylene glycol (PEG) and 0.2 M 3-O-methyl glucose (3-OMG) (UW + PEG + 3-OMG), were tested. Live cells were stained green by calcein AM while dead cells were red by ethidium homodimer. (B) Quantitative cell viabilities post 7 - day storage under various scenarios. Number of independent experiments n = 3.
This suggests that the storage temperature can affect the surface attachment ability of the cells. Because cell attachment largely depends on the activation of transmembrane proteins called integrins and/or adhesion molecules [29], the difference in cell attachment found indicate different levels of energy remaining and cellular activity post storage at different temperatures. Overall, both the inclusion of proper extracellular and intracellular protectants, and the decrease of storage temperature are beneficial to the maintenance of cell viability and activity for hypothermic preservation.
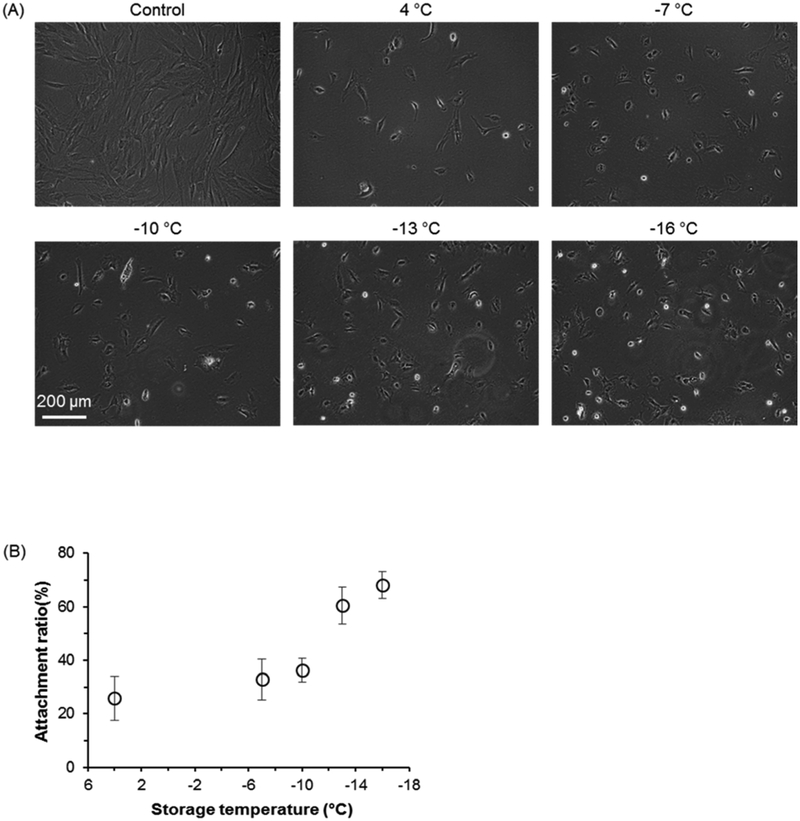
Next, we examined cell attachment post hypothermic preservation, rewarming, and 1-day cell culture in tissue culture plates. As indicated by Figure 2, only when UW+PEG+3-OMG is used as a storage solution, a significant portion of cells can survive. In addition, as the storage temperature decreases, the retention of cell attachment ability increases (Figure 3(A)). Therefore, the relative percentage of attached cells (compared to fresh control cells) post hypothermic storage increases as storage temperature decreases (Figure 3(B)).
Figure 3.
Attachment of hADSCs post 7-day storage in UW + PEG + 3-OMG solution at different temperatures. (A) Representative phase micrographs of hADSCs attachment after 1-day culture at 37 °C incubator. Control is the fresh cells of the same seeding density. (B) Cell attachment ratio of preserved hADSCs. Attachment ratio was calculated as the percentage of the number of attached live cells after one day culture of post-storage cells out of the number of fresh cells seeded and cultured in the same way. All storage groups were sealed with PO to prevent potential ice formation. Number of independent experiments n = 3.
3.3. Maintenance of cell stemness post DSC
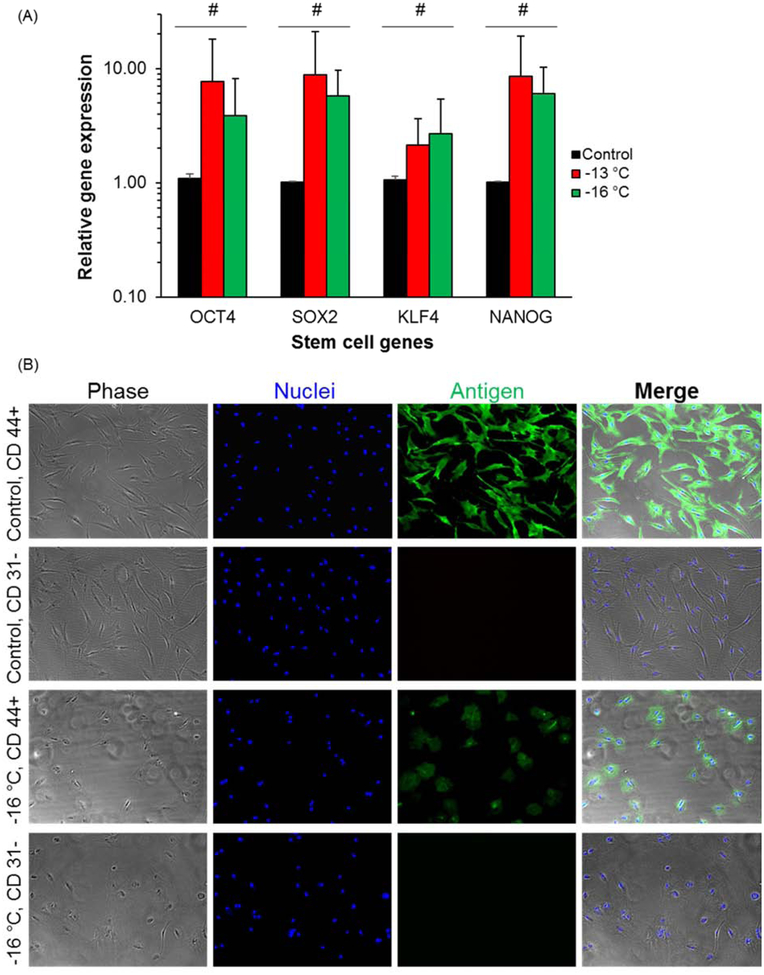
To evaluate the stemness of hADSCs, we performed gene expression analysis via reverse transcription polymerase chain reaction (RT-PCR) for four typical genes OCT4, SOX2, KLF4, and NANOG that indicate stemness. The results show there is no statistically significant difference (p > 0.1) between fresh control cells and DSC cells (stored at −13 °C or −16 °C) in the expression of these four genes (Figure 4(A)).
Figure 4.
Stem cell genes expressions and immunofluorescence staining of surface protein markers. (A) Gene expression levels examined by RT-PCR. Four stem cell genes OCT 4, SOX2, KLF4 and NANOG for ADSCs were examined. Number of independent experiments n = 3, #: p > 0.1. (B) Immunofluorescence staining for stem cell surface markers. Both positive (CD 44+) and negative (CD 31 −) surface markers were examined for fresh control and DSC preserved cells at −16 °C for 7 days.
To further understand the retention of hADSC phenotype post preservation, we examined two typical markers of hADSCs. The first one is a positive surface marker (CD 44+) and the other one is a negative surface marker (CD 31−) [34]. Through immunofluorescence staining, we observed that the DSC-preserved hADSCs maintain the expression of these surface markers at similar levels with fresh control cells (Figure 4(B)). The seemingly lower positive staining area (CD 44+) for DSC preserved cells is due to relatively lower cell attachment and spreading compared to fresh cells, as shown in Figure 3. For negative surface marker CD 31−, neither control nor DSC cells show any signals of immunofluorescence staining as expected. Therefore, we conclude that phenotype of hADSCs, through stemness and surface marker expression, is adequately preserved during extended DSC storage over 7 days at −16°C.
3.4. Long-term stem cell differentiation ability
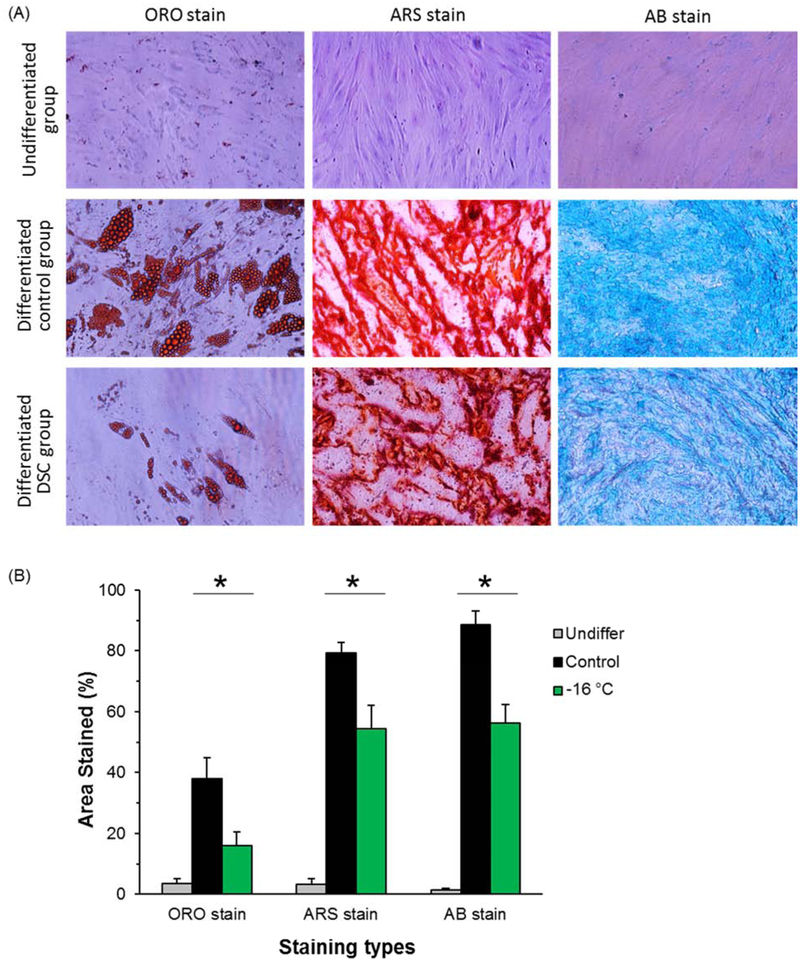
ADSCs have the capacity to differentiate into multiple mesenchymal lineages through adipogenesis, osteogenesis, and chondrogenesis [49]. Accordingly, we performed these three differentiation assays for fresh control and DSC preserved hADSCs for 21 days to assess the retention of differentiation capacity post preservation. The results show that before differentiations (negative control), the staining markers for lipids in adipogenic differentiation, calcium deposit for osteogenic differentiation, and acidic polysaccharide in cartilage for chondrogenic differentiation are minimally visible (first row in Figure 5(A)). For fresh cells differentiated into the three different lineages (positive control), these markers demonstrate abundant lipids, calcium deposition, or acidic polysaccharide (second row in Figure 5(A)). The DSC preserved cells at −16°C for 7 days (experimental group), also demonstrate multilineage differentiation ability, but the degrees of differentiations have been compromised as shown by their lower areas of staining compared to positive control groups (third row in Figure 5(A)). The quantitative results of staining areas are presented in Figure 5(B), which also indicates a statistically significant decrease of hADSC differentiation post DSC preservation. However, given the lower cell attachment number, smaller cell spreading area, and reduced cell metabolism level after extended DSC storage, this quantitative decrease in differentiation markers and staining area is expected. It’s important to note that even though expression levels of stem cell genes are increased as shown in Figure 4(A), it does not directly enhance differentiation capability [6; 40]. This is because the processes and degree of stem cell differentiation are also regulated by other factors such as oxygen tension level [6] and culture microenvironment [17].
Figure 5.
Multilineage differentiations of hADSCs post DSC storage. (A) Qualitative staining images for three differentiations, lipid staining by Oil red O (ORO) for adipogenic differentiation, calcium deposit staining by Alizarin red S(ARS) for osteogenic differentiation, and acidic polysaccharide (abundant in cartilage) staining by Alcian blue (AB) for chondrogenic differentiation. (B) Quantitative analysis for the relative staining areas in whole imaging regions. Number of independent experiments n = 3, *: p < 0.05
4. Discussion and conclusion
Although deep-supercooling of water and simple red blood cells (without nuclei and other organelles) has been reported before [22], there is still a significant curiosity about its applicability to other biological samples. Our results, here, demonstrate stable DSC of hADSC suspensions without freezing at −13 °C and even −16 °C. These results indicate that DSC is also applicable to nucleated mammalian cells, and the intracellular components such as the lipid membrane and mitochondrion, and the macromolecules such as nucleotides, proteins, and carbohydrates, are not catalyzers for ice crystallization. Their presences in deep supercooled aqueous solutions do not necessarily cause successful ice nucleation and freezing. Therefore, DSC is a viable approach to preserve various biospecimens including both non-nucleated (red blood cells as in our previous work) [22] and nucleated cells (as in the hADSCs in this work).
In our experiments, we generally observed that post hypothermic preservation, cells have higher abilities of attachment and proliferation under lower storage temperatures. Although exact molecular and cellular mechanisms are still unknown, this is probably due to slower metabolic activity and ATP depletion rate at lower temperatures, which results in higher ATP remaining level, higher degree of metabolic recovery, and more integrin/adhesion molecule activation post storage [29; 53]. Therefore, more cells can attach to the culture plate for consequent proliferation. In addition, it is not uncommon to observe averagely higher gene expression post DSC storage in our experiments. Multiple previous studies have also demonstrated increased levels of gene expression post stem cell preservation [55; 58], which is consistent with our results. Nevertheless, the mechanisms for this gene overexpression are yet to be explored.
It is generally believed that the critical event that initiates water freezing is ice nucleation. Once there is a successful ice nucleation event, crystallization will rapidly propagate throughout the whole aqueous system freezing it. Therefore, to study ice formation in supercooling or deep-supercooling, it will be a great approach if the ice embryos or tiny crystals could be observed and monitored by high resolution imaging. Although the process of ice growth has been recorded by high-speed microscopy in cells or supercooled microdroplets [11; 20; 28], it has not been reported in bulk aqueous solutions as there are too many uncertain ice nucleation sites. Moreover, to the best of our knowledge, the ice embryo required for successful ice nucleation only includes hundreds of water molecules with critical nucleus size in the magnitude of 10−9 meter [39; 42]. As a result, it would be very difficult, if not impossible, to detect those ice crystals by using optical microscopes due to their resolution limit (~ 10−7 meter). Electron microscopy might have this resolution, but it does not work properly for these aqueous samples until now [3]. Therefore, breakthroughs in spatiotemporally high-resolution microscopy or in other alternative methods are urgently demanded to enable non-invasive observation, recording, and characterization of nanoscopic ice crystals in supercooled samples. In addition, these advances will provide powerful tools to investigate the mechanisms and dynamics of ice nucleation, vapor nucleation, and solute precipitation.
The DSC preservation approach does not require cytotoxic CPAs such as dimethyl-sulphoxide (DMSO) or 1,2-propanediol (PROH). As a result, the tedious multistep processes of CPA loading before cryopreservation and unloading post cryopreservation by washing and centrifugation can be avoided [18], preventing cell loss and culture media waste. Furthermore, in conventional cryopreservation methods (either slow-freezing or vitrification), there is a dramatic phase transition (warming and melting) and long recovery time before further use of the cells. On the contrary, the transition from suboptimal DSC conditions to the physiological state is very gentle and mild, which can significantly shorten cell recovery time (including warming and culturing). This is particularly important for clinical applications with shortened post-processing and overall procedure time. Therefore, DSC preservation for cell suspension can provide a convenient off-the-shelf cell source for experimental research and clinical transfusion.
Overall, stable DSC preservation of hADSCs has been achieved for unprecedented 7 days at record −13 °C and −16 °C. Through surface sealing by paraffin oil, primary sites of heterogeneous nucleation in cell suspensions can be effectively eliminated to obtain stable supercooling. DSC at deep subzero temperatures can significantly decrease cell metabolic and deterioration rates, and thus significantly extend in vitro storage time. With the optimization of the storage solution, we demonstrated that this approach can enable preservation of hADSCs for 7 days with high viability, uncompromised stemness, and retaining capacities of attachment and multilineage differentiations. Further optimization of storage solution and conditions are warranted to improve cell activity and functionality post DSC storage. We thus conclude that, by providing convenient, high-quality, feasible, and reliable off-the-shelf cell sources, DSC preservation can significantly improve cell availability for a wide variety of uses in cell and tissue engineering, cell-based therapies, and tissue regeneration.
Supplementary Material
Acknowledgments
We would also like to thank the NIH for funding this work through grants no. 5P41EB002503 (BioMEMS Resource Center), 1R21AI142415, 5R01EB023812. We also thank the Shriners Hospitals for Children for supporting this work through core facilities including the “Genomics and Proteomics Shared Facility”, “Research Computer Shared Facility”, and “Morphology and Imaging Shared Facility” at the Boston site.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Berendsen TA, Bruinsma BG, Puts CF, Saeidi N, Usta OB, Uygun BE, Izamis M-L, Toner M, Yarmush ML, and Uygun K, Successful Supercooled Liver Storage for 4 Days. Nature medicine 20 (2014) 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Berendsen TA, Bruinsma BG, Puts CF, Saeidi N, Usta OB, Uygun BE, Izamis M-L, Toner M, Yarmush ML, and Uygun K, Supercooling enables long-term transplantation survival following 4 days of liver preservation. Nature medicine 20 (2014) 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bogner A, Jouneau P-H, Thollet G, Basset D, and Gauthier C, A history of scanning electron microscopy developments: towards “wet-STEM” imaging. Micron 38 (2007) 390–401. [DOI] [PubMed] [Google Scholar]
- [4].Bunnell BA, Flaat M, Gagliardi C, Patel B, and Ripoll C, Adipose-derived stem cells: isolation, expansion and differentiation. Methods 45 (2008) 115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chetty S, Pagliuca FW, Honore C, Kweudjeu A, Rezania A, and Melton DA, A simple tool to improve pluripotent stem cell differentiation. Nat Methods 10 (2013) 553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Choi JR, Pingguan-Murphy B, Wan Abas WA, Noor Azmi MA, Omar SZ, Chua KH, and Wan Safwani WK, Impact of low oxygen tension on stemness, proliferation and differentiation potential of human adipose-derived stem cells. Biochem Biophys Res Commun 448 (2014) 218–24. [DOI] [PubMed] [Google Scholar]
- [7].Correia C, Koshkin A, Carido M, Espinha N, Saric T, Lima PA, Serra M, and Alves PM, Effective Hypothermic Storage of Human Pluripotent Stem Cell-Derived Cardiomyocytes Compatible With Global Distribution of Cells for Clinical Applications and Toxicology Testing. Stem Cells Transl Med 5 (2016) 658–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Davis JM, Rowley SD, Braine HG, Piantadosi S, and Santos GW, Clinical toxicity of cryopreserved bone marrow graft infusion. Blood 75 (1990) 781–6. [PubMed] [Google Scholar]
- [9].De Vos M, Smitz J, and Woodruff TK, Fertility preservation in women with cancer. Lancet 384 (2014) 1302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Deller RC, Vatish M, Mitchell DA, and Gibson MI, Synthetic polymers enable non-vitreous cellular cryopreservation by reducing ice crystal growth during thawing. Nat Commun 5 (2014) 3244. [DOI] [PubMed] [Google Scholar]
- [11].Edd JF, Humphry KJ, Irimia D, Weitz DA, and Toner M, Nucleation and solidification in static arrays of monodisperse drops. Lab Chip 9 (2009) 1859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Giwa S, Lewis JK, Alvarez L, Langer R, Roth AE, Church GM, Markmann JF, Sachs DH, Chandraker A, Wertheim JA, Rothblatt M, Boyden ES, Eidbo E, Lee WPA Pomahac B, Brandacher G, Weinstock DM, Elliott G, Nelson D, Acker JP, Uygun K, Schmalz B, Weegman BP, Tocchio A, Fahy GM, Storey KB, Rubinsky B, Bischof J, Elliott JAW, Woodruff TK, Morris GJ, Demirci U, Brockbank KGM, Woods EJ, Ben RN, Baust JG, Gao D, Fuller B, Rabin Y, Kravitz DC, Taylor MJ, and Toner M, The promise of organ and tissue preservation to transform medicine. Nat Biotechnol 35 (2017) 530–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gramignoli R, Dorko K, Tahan V, Skvorak KJ, Ellis E, Jorns C, Ericzon BG, Fox IJ, and Strom SC, Hypothermic storage of human hepatocytes for transplantation. Cell Transplant 23 (2014) 1143–51. [DOI] [PubMed] [Google Scholar]
- [14].Groger M, Dinger J, Kiehntopf M, Peters FT, Rauen U, and Mosig AS, Preservation of Cell Structure, Metabolism, and Biotransformation Activity of Liver-On-Chip Organ Models by Hypothermic Storage. Adv Healthc Mater 7 (2018). [DOI] [PubMed] [Google Scholar]
- [15].Guarrera JV, Henry SD, Samstein B, Odeh-Ramadan R, Kinkhabwala M, Goldstein MJ, Ratner LE, Renz JF, Lee HT, Brown RS Jr., and Emond JC, Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant 10 (2010) 372–81. [DOI] [PubMed] [Google Scholar]
- [16].Guarrera JV, Henry SD, Samstein B, Reznik E, Musat C, Lukose TI, Ratner LE, Brown RS Jr., Kato T, and Emond JC , Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am J Transplant 15 (2015) 161–9. [DOI] [PubMed] [Google Scholar]
- [17].Han S, Zhao Y, Xiao Z, Han J, Chen B, Chen L, and Dai J, The three-dimensional collagen scaffold improves the stemness of rat bone marrow mesenchymal stem cells. J Genet Genomics 39 (2012) 633–41. [DOI] [PubMed] [Google Scholar]
- [18].He X, Thermostability of biological systems: fundamentals, challenges, and quantification. The open biomedical engineering journal 5 (2011) 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].He X, Park EY, Fowler A, Yarmush ML, and Toner M, Vitrification by ultra-fast cooling at a low concentration of cryoprotectants in a quartz micro-capillary: a study using murine embryonic stem cells. Cryobiology 56 (2008) 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Holden MA, Whale TF, Tarn MD, O’Sullivan D, Walshaw RD, Murray BJ, Meldrum FC, and Christenson HK, High-speed imaging of ice nucleation in water proves the existence of active sites. Sci Adv 5 (2019) eaav4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Huang H, Choi JK, Rao W, Zhao S, Agarwal P, Zhao G, and He X, Alginate Hydrogel Microencapsulation Inhibits Devitrification and Enables Large-Volume Low-CPA Cell Vitrification. Adv Funct Mater 25 (2015) 6939–6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Huang H, Yarmush ML, and Usta OB, Long-term deep-supercooling of large-volume water and red cell suspensions via surface sealing with immiscible liquids. Nat Commun 9 (2018) 3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huang H, Zhao G, Zhang Y, Xu J, Toth TL, and He X, Predehydration and Ice Seeding in the Presence of Trehalose Enable Cell Cryopreservation. ACS Biomater Sci Eng 3 (2017) 1758–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hunt CJ, Cryopreservation of Human Stem Cells for Clinical Application: A Review. Transfus Med Hemother 38 (2011) 107–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kaita Y, Tarui T, Yoshino H, Matsuda T, Yamaguchi Y, Nakagawa T, Asahi M, and Ii M, Sufficient therapeutic effect of cryopreserved frozen adipose-derived regenerative cells on burn wounds. Regenerative therapy 10 (2019) 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kao GS, Kim HT, Daley H, Ritz J, Burger SR, Kelley L, Vierra-Green C, Flesch S, Spellman S, Miller J, and Confer D, Validation of short-term handling and storage conditions for marrow and peripheral blood stem cell products. Transfusion 51 (2011) 137–47. [DOI] [PubMed] [Google Scholar]
- [27].Karlsson J, Cravalho E, and Toner M, A model of diffusion‐limited ice growth inside biological cells during freezing. Journal of Applied Physics 75 (1994) 4442–4455. [Google Scholar]
- [28].Karlsson JO, Measurement of intracellular ice formation kinetics by high-speed video cryomicroscopy. Methods Mol Biol 1257 (2015) 181–227. [DOI] [PubMed] [Google Scholar]
- [29].Khalili AA, and Ahmad MR, A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int J Mol Sci 16 (2015) 18149–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mangus RS, Fridell JA, Vianna RM, Milgrom MA, Chestovich P, Chihara RK, and Tector AJ, Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution in extended criteria liver donors. Liver Transplantation 14 (2008) 365–373. [DOI] [PubMed] [Google Scholar]
- [31].Mazur P, Freezing of living cells: mechanisms and implications. American journal of physiology-cell physiology 247 (1984) C125–C142. [DOI] [PubMed] [Google Scholar]
- [32].Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, and Frenette PS, Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466 (2010) 829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Meryman H, and Hornblower M, A method for freezing and washing red blood cells using a high glycerol concentration. Transfusion 12 (1972) 145–156. [DOI] [PubMed] [Google Scholar]
- [34].Mildmay-White A, and Khan W, Cell Surface Markers on Adipose-Derived Stem Cells: A Systematic Review. Curr Stem Cell Res Ther 12 (2017) 484–492. [DOI] [PubMed] [Google Scholar]
- [35].Naderi N, Combellack EJ, Griffin M, Sedaghati T, Javed M, Findlay MW, Wallace CG, Mosahebi A, Butler PE, Seifalian AM, and Whitaker IS, The regenerative role of adipose-derived stem cells (ADSC) in plastic and reconstructive surgery. Int Wound J 14 (2017) 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Oltean M, Joshi M, Bjorkman E, Oltean S, Casselbrant A, Herlenius G, and Olausson M, Intraluminal polyethylene glycol stabilizes tight junctions and improves intestinal preservation in the rat. Am J Transplant 12 (2012) 2044–51. [DOI] [PubMed] [Google Scholar]
- [37].Ostrowska A, Gu K, Bode DC, and Van Buskirk RG, Hypothermic storage of isolated human hepatocytes: a comparison between University of Wisconsin solution and a hypothermosol platform. Arch Toxicol 83 (2009) 493–502. [DOI] [PubMed] [Google Scholar]
- [38].Pamphilon D, and Mijovic A, Storage of hemopoietic stem cells. Asian journal of transfusion science 1 (2007) 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pereyra RG, Szleifer I, and Carignano MA, Temperature dependence of ice critical nucleus size. J Chem Phys 135 (2011) 034508. [DOI] [PubMed] [Google Scholar]
- [40].Pierantozzi E, Gava B, Manini I, Roviello F, Marotta G, Chiavarelli M, and Sorrentino V, Pluripotency regulators in human mesenchymal stem cells: expression of NANOG but not of OCT-4 and SOX-2. Stem Cells Dev 20 (2011) 915–23. [DOI] [PubMed] [Google Scholar]
- [41].Pogozhykh D, Prokopyuk V, Pogozhykh O, Mueller T, and Prokopyuk O, Influence of Factors of Cryopreservation and Hypothermic Storage on Survival and Functional Parameters of Multipotent Stromal Cells of Placental Origin. PLoS One 10 (2015) e0139834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Qiu Y, Odendahl N, Hudait A, Mason R, Bertram AK, Paesani F, DeMott PJ, and Molinero V, Ice nucleation efficiency of hydroxylated organic surfaces is controlled by their structural fluctuations and mismatch to ice. Journal of the American Chemical Society 139 (2017) 3052–3064. [DOI] [PubMed] [Google Scholar]
- [43].Rao W, Huang H, Wang H, Zhao S, Dumbleton J, Zhao G, and He X, Nanoparticle-mediated intracellular delivery enables cryopreservation of human adipose-derived stem cells using trehalose as the sole cryoprotectant. ACS Appl Mater Interfaces 7 (2015) 5017–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, and Riminucci M, Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131 (2007) 324–336. [DOI] [PubMed] [Google Scholar]
- [45].Schäffler A, and Büchler C, Concise review: adipose tissue-derived stromal cells—basicand clinical implications for novel cell-based therapies. Stem cells 25 (2007) 818–827. [DOI] [PubMed] [Google Scholar]
- [46].Schmidt D, Achermann J, Odermatt B, Genoni M, Zund G, and Hoerstrup SP, Cryopreserved amniotic fluid-derived cells: a lifelong autologous fetal stem cell source for heart valve tissue engineering. The Journal of heart valve disease 17 (2008) 446–55; discussion 455. [PubMed] [Google Scholar]
- [47].Snyder KK, Baust JM, Van Buskirk RG, and Baust JG, Enhanced hypothermic storage of neonatal cardiomyocytes. Cell Preservation Technology 3 (2005) 61–74. [Google Scholar]
- [48].Sodian R, Lueders C, Kraemer L, Kuebler W, Shakibaei M, Reichart B, Daebritz S, and Hetzer R, Tissue engineering of autologous human heart valves using cryopreserved vascular umbilical cord cells. The Annals of thoracic surgery 81 (2006) 2207–2216. [DOI] [PubMed] [Google Scholar]
- [49].Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, and Hedrick MH, Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 54 (2005) 132–41. [DOI] [PubMed] [Google Scholar]
- [50].Sugimachi K, Roach KL, Rhoads DB, Tompkins RG, and Toner M, Nonmetabolizable glucose compounds impart cryotolerance to primary rat hepatocytes. Tissue Eng 12 (2006) 579–88. [DOI] [PubMed] [Google Scholar]
- [51].Uccelli A, Moretta L, and Pistoia V, Mesenchymal stem cells in health and disease. Nat Rev Immunol 8 (2008) 726–36. [DOI] [PubMed] [Google Scholar]
- [52].Ullah I, Subbarao RB, and Rho GJ, Human mesenchymal stem cells - current trends and future prospective. Biosci Rep 35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Usta OB, Kim Y, Ozer S, Bruinsma BG, Lee J, Demir E, Berendsen TA, Puts CF, Izamis M-L, and Uygun K, Supercooling as a viable non-freezing cell preservation method of rat hepatocytes. PloS one 8 (2013) e69334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Visser T, Pervin B, Uçkan-Çetinkaya D, Wagemaker G, and Aerts-Kaya F, Hypothermic storage of hematopoetic stem cells can be used as an alternative to short-term cryopreservation. Experimental Hematology 53 (2017) S136. [Google Scholar]
- [55].Wagh V, Meganathan K, Jagtap S, Gaspar JA, Winkler J, Spitkovsky D, Hescheler J, and Sachinidis A, Effects of cryopreservation on the transcriptome of human embryonic stem cells after thawing and culturing. Stem Cell Rev 7 (2011) 506–17. [DOI] [PubMed] [Google Scholar]
- [56].Wang W, Penland L, Gokce O, Croote D, and Quake SR, High fidelity hypothermic preservation of primary tissues in organ transplant preservative for single cell transcriptome analysis. BMC Genomics 19 (2018) 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Xu Y, Mawatari K, Konno T, Kitamori T, and Ishihara K, Spontaneous Packaging and Hypothermic Storage of Mammalian Cells with a Cell-Membrane-Mimetic Polymer Hydrogel in a Microchip. ACS Appl Mater Interfaces 7 (2015) 23089–97. [DOI] [PubMed] [Google Scholar]
- [58].Yong KW, Pingguan-Murphy B, Xu F, Abas WA, Choi JR, Omar SZ, Azmi MA, Chua KH, and Wan Safwani WK, Phenotypic and functional characterization of long-term cryopreserved human adipose-derived stem cells. Sci Rep 5 (2015) 9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zanata F, Shaik S, Devireddy RV, Wu X, Ferreira LM, and Gimble JM, Cryopreserved adipose tissue-derived stromal/stem cells: potential for applications in clinic and therapy, Biobanking and Cryopreservation of Stem Cells, Springer, 2016, pp. 137–146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.