In response to the global coronavirus disease 2019 (COVID-19) emergency, clinical trial research assessing the efficacy and safety of clinical candidate interventions to treat COVID-19 are emerging at an unprecedented rate. As of April 21, 2020, well over 500 clinical trials have been registered at the various international and national clinical trial registry sites. Findings from randomised clinical trials that have been published as of April 21, 2020, have investigated the efficacy of lopinavir–ritonavir compared with standard of care,1 hydroxychloroquine compared with best supportive care,2 favipiravir compared with arbidol,3 and lopinavir–ritonavir compared with arbidol.4 Other non-randomised trials have investigated hydroxychloroquine versus hydroxychloroquine combined with azithromycin.5 Over 300 trials are enrolling participants and cover further investigations of the above drugs and promising therapies such as remdesivir, IL-6 inhibitors (tocilizumab and sarilumab), convalescent plasma therapy, stem-cell transfusion, vaccine candidates, several other well known direct acting antiv irals, and traditional Chinese medicines. Most of these trials will offer comparative efficacy data versus standard of care according to local COVID-19 treatment guidelines, but a handful of randomised controlled trials will also provide head-to-head evidence between high profile interventions. The figure shows the network of completed, ongoing, and planned COVID-19 interventional clinical trials of these interventions or intervention groups that are being explored in at least two trials.
Figure.
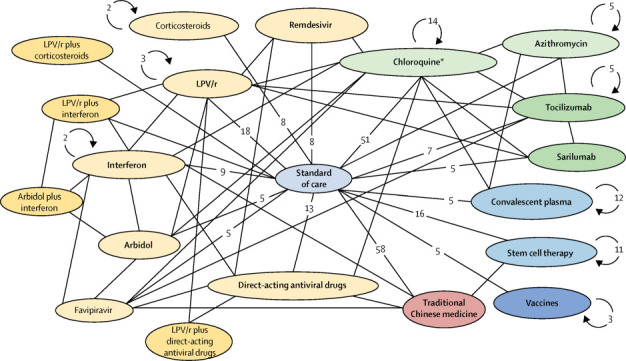
Evidence network of COVID-19 clinical trials of top 15 interventions
Circles (node) represent interventions or intervention groups (categories). Lines between two circles indicate comparisons in clinical trials. The numbers on the lines are the number of clinical trials making the specific comparison. Circular arrows and numbers indicate the number of non-comparative clinical trials in which that intervention is included. A few trials examining combination therapies are excluded from the figure due to space limitations. COVID-19=coronavirus disease 2019. LPV/r (lopinivir–ritonavir). *Includes trials on hydroxychloroquine and chloroquine.
Given the accelerated rate at which trial information and findings are emerging, an urgent need exists to track clinical trials, avoid unnecessary duplication of efforts, and understand what trials are being done and where. In response, we have developed a COVID-19 clinical trials registry to collate all trials. Data are pulled from the International Clinical Trials Registry Platform, including those from the Chinese Clinical Trial Registry, ClinicalTrials.gov, Clinical Research Information Service - Republic of Korea, EU Clinical Trials Register, ISRCTN, Iranian Registry of Clinical Trials, Japan Primary Registries Network, and German Clinical Trials Register. Both automated and manual searches are done to ensure minimisation of duplicated entries and for appropriateness to the research questions. Identified studies are then manually reviewed by two separate reviewers before being entered into the registry. Concurrently, we have developed artificial intelligence (AI)-based methods for data searches to identify potential clinical studies not captured in trial registries. These methods provide estimates of the likelihood of importance of a study being included in our database, such that the study can then be reviewed manually for inclusion. Use of AI-based methods saves 50–80% of the time required to manually review all entries without loss of accuracy. Finally, we will use content aggregator services, such as LitCovid, to ensure our data acquisition strategy is complete. With this three-step process, the probability of missing important publications is greatly diminished and so the resulting data are representative of global COVID-19 research efforts.
Trials for COVID-19 are then mapped according to geographical, trial, patient, and intervention characteristics, when these data are available. These data are stored securely in a backend database and outputs are visualised on a front-end feature.
As trial findings are communicated, these data must be centralised and meta-analysed in real-time. Syntheses of these trials are urgently needed to assist clinicians, researchers, and policy makers to make evidence-informed decisions to minimise the morbidity and mortality due to COVID-19.
Acknowledgments
We declare no competing interests.
References
- 1.Cao B, Wang Y, Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. published online March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Liu L, Liu P. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang Univ (Med Sci) 2020 doi: 10.3785/j.issn.1008-9292.2020.03.03. published onlne March 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C, Huang J, Yin P. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020 doi: 10.1101/2020.03.17.20037432. published onine April 8. (preprint) [DOI] [Google Scholar]
- 4.Yao X, Ye F, Zhang M. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. published online March 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gautret P, Lagier JC, Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. published online March 20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


