Highlights
-
•
COVID-19 in children of Xiangyang city is often family acquired and not serious, with favorable outcomes.
-
•
Asymptomatic children can be diagnosed as pneumonia because of chest CT abnormalities.
-
•
Epidemiologic exposure, SRAS-CoV-2 nucleic acid test (NAT) and lung CT funding provide basis for diagnosis.
Abbreviations: NAT, SARS-CoV-2 nucleic acid test; GGOs, Ground-lass opacities; NAP, nucleic acid positive; LLLL, lower lobe of left lung; LLRL, lower lobe of right lung; ULRL, upper lobe of right lung; ULBL, upper lobe of both lung; Neg, negative; Pos, positive
Keywords: Novel coronavirus disease (COVID-19), SARS-CoV-2, Children
Abstract
Background
Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 has spread around the world, and reports of children with COVID-19 are increasing.
Objectives
To assess clinical profiles of pediatric COVID-19.
Study design
A retrospective analysis was undertaken using clinical data of sixteen children (11 months-14 years) diagnosed with COVID-19 between January 1, 2020 and March 17, 2020 at Xiangyang Central Hospital, Hubei province, China.
Results
All children had positive epidemiologic histories, 12 (12/16, 75 %) involving family units. The illnesses were either mild (5/16, 31.3 %) or ordinary (11/16, 68.8 %), presenting as follows: asymptomatic (8/16, 50 %), fever and/or cough (8/16, 50 %). Four asymptomatic patients (4/16, 25 %) in ordinary cases had chest computed tomography (CT) abnormalities. Leukocyte counts were normal in 14 cases(88 %), but 2 patients (12.5 %) had leukopenia, and 1 (6.3 %) was lymphopenic. There were 11 patients with chest CT abnormalities, some nodular, others small patchy and others ground-glass opacities. In asymptomatic children, the median time to SRAS-CoV-2 nucleic acid test(NAT) positivity once exposed to a family member with confirmed infection was 15.5 days (range, 10–26 days). The median time to first NAT-negative conversion was 5.5 days (range, 1–23 days).
Conclusions
COVID-19 in children of Xiangyang city is often family acquired and not serious, with favorable outcomes. Asymptomatic children can be diagnosed as pneumonia because of chest CT abnormalities. It is essential to actively screen this segment of the population.
1. Background
The 2019 outbreak of coronavirus in Wuhan (Hubei Province of China) has been since attributed to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [[1], [2], [3], [4], [5], [6]]. Termed coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO), it has spawned a Public Health Emergency of International Concern (PHEIC) [7]. At present, there are >100 affected countries and districts [8]. SARS-CoV-2 is the seventh member of the enveloped RNA human coronavirus (HCoV) family, which also includes HCoV-229E, −OC43, -NL63, -HKU1, SARS-CoV, and Middle East respiratory syndrome coronavirus (MERS-CoV) [9]. Earlier data have shown that adult patients with COVID-19 present with fever, dry cough, dyspnea, fatigue, and lymphopenia. Serious pneumonia may also ensue, more often in elderly adult men and those with chronic comorbidities, posing greater risk of severe acute respiratory syndrome and even death [[1], [2], [3], [4], [5], [6]].
Although all humans are susceptible to SARS-CoV-2, it does appear that COVID-19 occurs less and is less severe in children than in adults [10,11]. Reports of pediatric vulnerability are increasing nonetheless [[12], [13], [14], [15], [16]], without comprehensive analysis or conclusive proof. To address this issue, we went through clinical data of our patients. Our findings attest to some distinctive differences, which may help in managing children hereafter.
Objective: To assess clinical profiles of children infected with the 2019 novel coronavirus, SARS-CoV-2 (COVID-19).
2. Study design
2.1. Patient population
Clinical data were collected from 16 children (1 months-14 years) diagnosed with COVID-19 at Central Hospital of Xiangyang city, Hubei province of China between January 31, 2020 and March 17, 2020. Informed consent was obtained for experimentation with human subjects. All subjects met diagnostic guidelines established in China, as stipulated in “Diagnosis and treatment recommendation for pediatric coronavirus disease-19″ (2nd Edition) [17]. Any of the epidemiologic conditions set forth and at least two related clinical provisions were required to define a suspected case. The epidemiologic criteria were as follows: (1) Travel or residence in Wuhan area or other areas showing continuous local spread within 2 weeks of onset; (2) Exposure within prior 2 weeks to fever or respiratory symptoms from Wuhan area or other areas where local spread was ongoing; (3) Close contact with confirmed or suspected cases of COVID-19 within 2 weeks of onset; (4) Disease clusters, comprised of patients (other than the child in question) with fever or respiratory symptoms suggesting or identified as COVID-19; and (5) Newborns of mothers with suspected or confirmed COVID-19. Qualifying clinical manifestations were as follows: (1) Fever, fatigue, dry cough, or some with no fever (2) Abnormal chest imaging (X-ray or computed tomography [CT]); (3) Normal or low total leukocytes counts or lymphopenia in the early phase of disease.
Suspected cases were ultimately confirmed by one of the following diagnostic outcomes, using pharyngeal swabs, sputum, feces, or blood samples: (1) SARS-Cov-2 nucleic acid detected by real-time fluorescence polymerase chain reaction (PCR); (2) Genetic sequencing of virus highly homologous to SARS-Cov-2; or (3) Culture and isolation of SARS-Cov-2 particles.
We gauged the severity of COVID-19 by clinical features, laboratory testing, and chest X-ray imaging. Symptoms of upper respiratory tract infection only, such as stuffy nose, sore throat, and fever, constituted mild disease. Some children were asymptomatic, only identified by SARS-CoV-2 nucleic acid positivity, using pharyngeal swabs and were classified as mild cases. In ordinary disease, fever, cough, fatigue, headache, or myalgia were evident, and there were signs of pneumonia on imaging, but no extreme illness or complications developed. Severe disease is progressive, marked by one of the following: labored breathing (70 times/min in infants or ≥50 times/min in children >1 year old), hypoxic manifestations, disturbed consciousness, mental malaise, lethargy, coma, convulsions, food resistance or feeding difficulties, and even signs of dehydration, coagulation dysfunction, myocardial damage, gastrointestinal dysfunction, sizeable liver enzyme elevations, and rhabdomyolysis syndrome. ARDS, sepsis, and ICU care are referring specifically to the critical disease presentation in COVID-19.
2.2. Data collection
For the SARS-CoV-2 nucleic acid test (NAT), real-time quantitative PCR (RT-qPCR) was used, based on pharyngeal swab and performed 1 day before or on the day of CT studies. Routine laboratory testing of blood count, C-reactive protein (CRP), liver function, and more were also completed 1–2 days prior to or on the day of CT imaging. We reexamined chest CT after body temperatures stable for >3 days, and coughs appreciably controlled. During hospitalization, NAT was reexamined every other day until it showed negative.
3. Results
3.1. Clinical characteristics
Ten of the 16 patients studied (62.5 %) were male and six (37.5 %) were female, ranging in age from 11.5 months to 14 years (median age, 8.5 years). None of them had underlying disease, and all were from the geospatial hotspot of Xiangyang city (second to Wuhan city) in Hubei Province. Twelve patients (75 %) showed disease family clustering, having two or more confirmed infected family members. Among the remaining four cases, one patient had a history travel to Wuhan, and two were in contact with people returning from Wuhan. Mild disease accounted for five cases (31.3 %), and ordinary disease for 11 (68.8 %).
After contact with family members known to be infectious, a median of 15.5 days (range, 10–26 days) elapsed before eight asymptomatic children (50 %) developed NAT positivity. Four of them (4/16, 25 %) were classified as ordinary diseases because of chest CT showing signs of pneumonia and the other four asymptomatic children (4/16, 25 %) were classified as mild diseases with normal chest CT. Clinical manifestations of the other eight patients (50 %) were distributed as follows: fever only (2/16, 12.5 %), fever + cough (3/16, 18.8 %), and cough only (3/16, 18.8 %). The maximum temperature recorded was 38.6 °C, and the febrile course lasted 1–4 days. There were seven patients (44 %) with verifiable pneumonia by chest CT. None of the children experienced dyspnea, vomiting, diarrhea, abdominal pain, or fatigue.
Blood tests were done on admission or the very next day (within 1–2 days of disease onset). Leukocyte counts were normal in 14 children (88 %), but 2 patients (12.5 %) had leukopenia and 1 (6.3 %) was lymphopenic. CRP levels were largely normal, only one patient (6.2 %) with an elevation, but three patients (18.8 %) had increased lactate dehydrogenase (LDH) levels. Otherwise, liver and kidney analytes, myocardial isoenzymes, coagulation indices, electrolytes, blood glucose, and levels of procalcitonin were all within normal ranges for every child. A summary of clinical data is provided in Table 1 .
Table 1.
General charactersitics of sixteen pediatric patients with COVID-19.
| Clinical data (case NO.) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | male | male | male | female | male | female | female | male | male | male | female | male | male | female | male | female |
| Age | 12y | 11y | 3y | 7y | 1y2m | 14y | 11y | 14y | 11y | 9y | 8y | 2y6m | 14y | 7y | 11m15d | 7y |
| Chest CT lesion for first clinical visit | normal | normal | normal | normal | normal | patchy, GGOs at LLLL | nodular shadow at left lower pleura and LLRL | nodular, GGOs at LLLL | patchy, GGOs at ULRL | nodular, GGOs at LLRL | nodular, GGOs at LLLL | patchy, shadow at ULBL | patchy, GGOs at LLRL | patchy, GGOs at middle and LLRL, with consolidation | patchy, GGOs at ULRL | nodular shadow at ULBL |
| Symptom (clinical manifestation) before NAP |
no symptom with NAP 1d | no symptom with NAP 1d | no symptom with NAP 2d | no symptom with NAP 2d | fever 4d | no symptom with NAP 1d | no symptom with NAP 2d | no symptom with NAP 1d | no symptom with NAP 1d | fever 1d | fever cough 1d | cough 4d | cough 3d | cough 3d,fever 1d | cough 5d | cough 4d, fever 1d |
| Family members confirmed with COVID-19 prior to children | parents, sister | grandmother | mother | mother, grandmother | none | mother, aunt | mother | father | mother, grandfather | parents and grandparents | parents and grandparents | mother, grandfather | grandfather | none | none | none |
| Tmax(℃) | 38.3 | 38.6 | 38.6 | 37.7 | 37.4 | |||||||||||
| Duration of fever(days) before NAP | 4 | 1 | 1 | 1 | 1 | |||||||||||
| duration from contact with patients confirmed with COVID-19 to NAP for asymptomatic children(days) | 22 | 14 | 14 | 15 | 16 | 19 | 10 | 26 |
Chest CT images were normal in five patients (31.3 %). Abnormalities identified in the other 11 (68.8 %) involved one lobe (8/16, 50%) or two (3/16, 18.8 %), appearing as nodular (5/16, 31.3 %) or small/patchy (6 cases, 37.5 %) changes. One patient (6.3 %) displayed consolidation, two (12.5 %) had visible air bronchograms, and two (12.5 %) exhibited lesions distributed along bronchovascular bundles, similar to bronchopneumonia. Ground-glass opacities (GGOs) were present in seven patients (43.8 %), without paving-stone pattern; halo signs appeared in four patients (25 %); and lymphadenopathy was encountered in one patient (6.2 %) (Typical cases with Figs. 1 and 2, Figs. 1 and 2, Figs. 3 and 4, Figs. 3 and 4, Fig. 5 ).
Figs. 1 and 2.
Similar chest CT appearance of two siblings from one family..
Figs. 3 and 4.
Asymptomatic patients diagnosed as pneumonia with chest CT abnormalities..
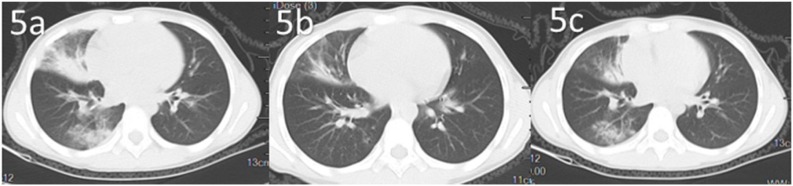
Fig. 5.
Dynamic changes of chest CT of a pediatric patient confirmed COVID-19.
After 9 days of contact with their mother, herself a confirmed case of COVD-19, two siblings (brother and sister) both developed fever and cough in conjunction with small nodular lesions of lung unilaterally, near lateral margins of the lower lobes. Each was regularly shaped, showing a halo sign (Figure1 Figs. 1 and 2a). The inflammatory process resolved after 12 days of treatment (Figure1 Figs. 1 and 2b).
There were two asymptomatic patients diagnosed as pneumonia with chest CT abnormalities. Nodular lesion, GGOs at LLRL and LLRL respectively (Figure3 Figs. 3 and 4).
Another patient admitted with fever and cough had no known family members with COVID-19. Chest CT changes are shown in the course of treatment (Fig. 5a-c). Multiple hazy ground-glass opacities (GGOs) initially observed in middle and lower lobes of right lung were less prominent after 1 week of therapy (middle). After 2 weeks, middle- and lower-lobe GGOs were diminished or gone (right).
3.2. Treatments and outcomes
All children received some type of antiviral treatment as follows: traditional Chinese medicine (TCM) (13/16, 81.3 %), including three recipients of TCM plus lopinavir-ritonavir (orally, twice daily for 1 week); solitary TCM treatment (1/16, 12.5 %); lopinavir-ritonavir only (1/16, 12.5 %); and multidrug regimens of azithromycin (oral/intravenous infusion, once daily for 3–5 days), oseltamivir (orally, twice daily for 5 days), arbidol (orally, twice daily for 5 days), cefamandole (intravenous infusion, twice daily for 7–12 days) (12/16, 75 %) (Table 2 ).
Table 2.
Treatment of patients with COVID-19.
| Clinical data (case NO.) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | ||||||||||||||||
| Lopinavir-ritonavir (oral intake) | YES | YES | YES | YES | ||||||||||||
| Azithromycin (oral intake) | YES | YES | YES | YES | YES | YES | YES | YES | ||||||||
| Azithromycin(intravenous infusion) | YES | |||||||||||||||
| Oseltamivir (oral intake) | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | |||||
| Cefamandole (intravenous infusion) | YES | YES | ||||||||||||||
| Arbidor (oral intake) | YES | YES | YES | YES | YES | YES | ||||||||||
| Traditional Chinese medicine (oral intake) | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES |
Five patients (31.3 %) were admitted with normal chest CTs, including four (25 %) asymptomatic and 1 (6.3 %) with fever and cough. In the other 11 patients (68.8 %), chest CTs confirmed ongoing resolution of pulmonary inflammation within 4–7 days after treatment. Full resolution was achieved 10–14 days after treatment in six patients (37.5 %),
The median time to first NAT-negative conversion was 5.5 days (range, 1–23 days). For two consecutive negative tests, the median time was 6.5 days (range, 2–24 days). Nine patients (56.3 %) immediately turned negative upon re-examination at the hospital, whereas seven (43.7 %) were still positive. After treatment, all children had normal body temperatures, clinical symptoms improved considerably or disappeared, chest CT signs of pneumonia were gone, two consecutive NATs were negative, and all children met established national guidelines for diagnosis and treatment (see Methods). Median hospital stay was 14 days (range, 8–26 days) (Table 3 ). Despite the delayed NAT conversion, chest CT abnormalities and clinical symptoms did not worsen, implying that COVID-19 in children is a mild disease with a good prognosis.
Table 3.
Chest CT and nucleic acid results.
| Clinical data (case NO.) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chest CT lesion for first clinical visit | normal | normal | normal | normal | normal | patchy, GGOs at LLLL | nodular shadow at left lower pleura and LLRL | nodular, GGOs at LLLL | patchy, GGOs at ULRL | nodular, GGOs at LLRL | nodular, GGOs at LLLL | patchy, shadow at ULBL | patchy, GGOs at LLRL | patchy, GGOs at middle and LLRL, with consolidation | patchy, GGOs at ULRL | nodular shadow at ULBL |
| Reexamination of chest CT result | ||||||||||||||||
| After 4-7 days | patchy, GGOs at LLLL reduce | nodular shadow at left lower pleura and LLRL reduce | nodular, GGOs at LLLL reduce | patchy, GGOs at ULRL reduce | nodular, GGOs at LLRL reduce | nodular, GGOs at LLLL reduce | patchy, shadow at ULBL reduce | patchy, GGOs at LLRL reduce | patchy, GGOs at middle and LLRL, with consolidation reduce | patchy, GGOs at ULRL reduce | nodular shadow at ULBL reduce | |||||
| After 8-14days | lesionabsorption | lesionabsorption | lesion absorption | lesion absorption | lesion absorption | lesion absorption | ||||||||||
| NAT | ||||||||||||||||
| First time of re-examine result after hospitalization | Neg | Neg | Pos | Pos | Neg | Pos | Neg | Neg | Pos | Pos | Pos | Neg | Pos | Neg | Neg | Neg |
| Duration of nucleic acid turns negative from disease onset for the first time (days) | 5 | 1 | 17 | 11 | 2 | 22 | 1 | 1 | 19 | 15 | 20 | 3 | 23 | 5 | 2 | 6 |
| Duration of nucleic acid turns negative for 2 consecutive times from disease onset (days) | 6 | 3 | 18 | 14 | 4 | 23 | 2 | 2 | 20 | 16 | 23 | 5 | 24 | 6 | 3 | 7 |
| Duration of hospital stay (days) | 9 | 9 | 18 | 16 | 8 | 25 | 12 | 12 | 21 | 17 | 24 | 12 | 26 | 23 | 11 | 8 |
4. Discussion
Once unleashed in December 2019, COVID-19 due to SARS-CoV-2 spread with ferocity across China and around the world [[1], [2], [3], [4], [5]]. Pathogen detection improved as the outbreak took hold, so reports of pediatric infections began to mount [[12], [13], [14], [15], [16]]. Herein, we have analysed 16 patients of Xiangyang city with laboratory confirmed COVID-19, 12 of whom had immediate family members (sometimes two or more) harbouring the same illness. It is suggested that the extent of epidemics in children reflects the dynamics of family units. Although avoidance of public places and social gatherings under our national prevention and control policies effectively reduced overall morbidity, children clearly rely on parental care. Close contact within the family is thus the likely mode of SARS-CoV-2 infection in children of Xiangyang city, similar to SARS and MERS [18,19]. We are subsequently reminded that prevention and control of pediatric infections is indeed a family effort. Respiratory isolation, hand hygiene, and surface disinfection are all simple yet important preventive measures. Aside from the current lack of medical masks suitable for children, it is quite unrealistic for infants or toddlers to don masks, and many older children are incapable of self-discipline entailed in viral combat. While tending to their own obligations, parents must then do the same for their children, insisting on respiratory protection and hand hygiene.
Half of the children we studied had no clinical manifestations. They were detected by NAT as contacts of confirmed family members. The median incubation time (15.5 days) was also lengthy, enabling a prolonged period of concealment, and four patients had demonstrable CT abnormalities. Such departure from customary adult presentations compounds other diagnostic obstacle. We have not known clearly the pathogenic features of SARS-CoV-2, so by using chest CT, we can effectively identify patients with pneumonia, determine severity of lung lesions and isolate the source of infection [15]. Whereas in our study, eight asymptomatic patients with or without chest CT abnormalities didn’t show any sign of deterioration during the course and their outcomes were good. Hence, we should keep an eye on illness and think twice about CT scans when necessary. Regular use of NAT may be a workable approach to detecting asymptomatic carriers in family units. The rest of the children (50%) examined presented with fever and cough, much like first symptoms of adult infection [[1], [2], [3], [4], [5], [6]]. However, fever in children was of shorter duration and not as high (maximum, <38.6 °C). None of them had early respiratory tract symptoms, and no dyspnea or extrapulmonary issues emerged in the course of disease. We thus concluded that COVID-19 in children is an asymptomatic or mild clinical illness.
In adults with COVID-19, up to 83.2 % are lymphopenic [1,3,4], 33.7 % are leukopenic [1], and 86 % [1] have elevations of CRP, alanine aminotransferase (ALT), aspartate aminotransferase (AST), LDH, procalcitonin, and D-dimer to some extent, especially those with severe disease (primarily LDH and D-dimer) [[1], [2], [3], [4]]. Total leukocyte counts were almost normal in the children we studied, although two were leukopenic and one was lymphopenic. CRP was elevated in one instance (<40 mg/dL), and three patients had LDH elevations. Other laboratory results were all normal. These were minor deviations, compared with those occurring in adults. Lymphopenia reflects consumption of lymphocyte by SARS-CoV-2 and signals severe disease [1,3,4], which is rare in children. Ultimately, laboratory testing in children with COVID-19 yields nonspecific results of little consequence, offering no strong diagnostic support. It may serve to exclude other pathology. Active monitoring is also helpful in determining the severity and progress of ongoing infections.
Early chest CT findings of children with COVID-19 have some similarities to those of adults but differ in ways [[1], [2], [3], [4],20]. Lesions in children commonly occupy lung periphery, sitting close to the pleura. In adults, lesions are likewise shown pleural distributions, positioned at interlobar fissures. However, those in children seem to align with bronchovascular bundles, simulating changes of bronchopneumonia; and although GGOs and consolidation are shared features, single lung involvement by few nodular or small/patchy changes are characteristic of children. Furthermore, the GGO halos are diminutive, consolidation is limited, and air bronchograms are unusual. Adult patients, in contrast, have bilateral broad-ranging lung changes, mainly large patches with paving-stone pattern and halo or reverse-halo signs. These phenomena suggest a more robust inflammatory response in adults, more so than in children. Extrapulmonary manifestations, such as nodal enlargement and pleural effusions that appear in adults are actually rare or absent in children. Finally, the pulmonary lesions in the pediatric patients tend to resolve after treatment, without sequelae, and not reappear; whereas, fibrous cords and secondary diseases may develop in adults. By comparison then, children with COVID-19 have atypical clinical symptoms, showing nonspecific and inconsequential CT changes of lung.
As in patients with SARS and MERS, severe illness is proportionately low in children with COVID-19, and deaths are rare [18.19]. Both SARS-CoV [21] and SARS-CoV-2 gain entry to airway cells via angiotensin-converting enzyme 2 (ACE2) receptors [22,23], triggering massive inflammatory cytokine release and causing tissue necrosis. The resultant immune imbalance may be catastrophic, culminating in systemic inflammatory response syndrome (SIRS) or ARDS. It is still unclear whether the maturity, functionality, or affinity of ACE2 receptors is lower in children than in adults, or whether other mechanisms (eg, accessory receptors) are operant, explaining the many discrepancies in pediatric and adult disease expression. The need for more research is urgent.
All patients in our study received symptomatic antipyretic treatment, but only four were given lopinavir-ritonavir. In retrospect, data on these four children was not distinctive with respect to scope of disease, age, or severely affected family members. Our early inexperience in treating of children during the outbreak prompted some questionable decisions, based on adult guidelines [24]. We now recognize the innocuous course that most children without underlying ailments will follow, so antivirals are not warranted. However, the principles of traditional Chinese medicine are worthy of further study, although recommended by guidelines and experience [17,24,25]. The disease is self-limited, calling for greater focus on supportive treatment, especially a variety of foods that maintain the physical psychologic, and emotional well-being of growing children [[26], [27], [28]].
In summary, COVID-19 in children of Xiangyang city is generally family acquired and often not serious, with a good prognosis. Asymptomatic children can be diagnosed as pneumonia because of chest CT abnormalities. Atypical symptomology and nonspecific laboratory findings tend to muddy the diagnosis, especially if the sole manifestations are chest CT changes. A patient profile incorporating aspects of epidemiologic exposure, NAT, and lung CT funding provides a basis for diagnosis. We must be attentive to the many children who are asymptomatic carriers to prevent and control this pandemic.
Funding
This study was funded by the National Natural Science Foundation of China (No. 81771621), the Natural Science Foundation of Liaoning Province (No. 2019JH8/10300023).
CRediT authorship contribution statement
Wenliang Song: Methodology, Investigation, Data curation, Writing - original draft. Junhua Li: Conceptualization, Resources. Ning Zou: Software, Investigation, Validation. Wenhe Guan: Software, Investigation, Formal analysis. Jiali Pan: Software, Investigation, Visualization. Wei Xu: Conceptualization, Methodology, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
No potential conflict of interest was reported by the authors.
Acknowledgements
Wenliang Song drafted the manuscript. Junhua Li and Wei XU contributed to the idea. Wenliang Song and Wei Xu designed the project. Wei Xu revised the manuscript. All authors contributed to data collection, read and approved to the final draft for publication.
Footnotes
Reference ranges:WBC:5-12×109/L, Lymphocyte count:1.1-3.2×109/L, CRP:0-8mg/L, LDH:120-250U/L.
Contributor Information
Wenliang Song, Email: songwl2002jason@163.com.
Junhua Li, Email: m13797631535@163.com.
Ning Zou, Email: xdzning0215@163.com.
Wenhe Guan, Email: guanwenhe@126.com.
Jiali Pan, Email: cmu107819@163.com.
Wei Xu, Email: tomxu.123@163.com.
References1
- 1.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study [J] Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China[J] JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China[J] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;(February (28)) doi: 10.1056/NEJMoa2002032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Allergy; 2020. Clinical Characteristics of 140 Patients Infected by SARS-CoV-2 in Wuhan, China. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.The International Committee on Taxonomy of Viruses (ICTV) Coronaviridae Study Group. Naming the 2019 Coronavirus. https://talk.ictvonline.org/.
- 7.https://www.who.int/dg/speeches/detail/who-director-general-s-statement-on-ihr-emergency-committee-on-novel-coronavirus-(2019-ncov). Accessed 3/3/2020.
- 8.WHO. Novel Coronavirus (2019-nCoV) Situation Report – 22[EB/OL](2020-02-11)[2020-02-11].https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/.
- 9.Zhu N., Zhang D., Wang W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. Feb 15Epub 2020 Jan 24. Erratum in: Lancet. 2020 Jan 30; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO-China Joint Mission . 2020. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19)https://www.who.int/docs/default-source/coronaviruse/who-china-jointmission-on-covid-19-final-report.pdf Geneva Accessed March 1, 2020. [Google Scholar]
- 11.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 12.Wang D., Ju X.L., Xie F. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua Er Ke Za Zhi. 2020;58(March 2 (4)):E011. doi: 10.3760/cma.j.cn112140-20200225-00138. [Epub ahead of print] Chinese. [DOI] [PubMed] [Google Scholar]
- 13.Sun D., Li H., Lu X.X. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J. Pediatr. 2020;(March (19)) doi: 10.1007/s12519-020-00354-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong Y., Mo X., Hu Y. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;(March (16)):e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 15.Xia W., Shao J., Guo Y. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr. Pulmonol. 2020;(March (5)) doi: 10.1002/ppul.24718. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W., Cui H., Li K. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr. Radiol. 2020;(March (11)) doi: 10.1007/s00247-020-04656-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhm C.H.E.N., FU J.F., SHU Q. second edition. 2020. Diagnosis and Treatment Recommendation for Pediatric Coronavirus disease-19.http://kns.cnki.net/kcms/detail/33.1248.R.20200225.1518.002.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stockman L.J., Massoudi M.S., Helfand R. Severe acute respiratory syndrome in children [J] Pediatr. Infect. Dis. J. 2007;26(1):68–74. doi: 10.1097/01.inf.0000247136.28950.41. [DOI] [PubMed] [Google Scholar]
- 19.Thabet F., Chehab M., Bafaqih H. Middle East respiratory syndrome coronavirus in children[J] Saudi Med. J. 2015;36(April (4)):484–486. doi: 10.15537/smj.2015.4.10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X.F., Gong W., Wang L. Clinical features and high resolution CT imaging findings of preliminary diagnosis novel coronavirus pneumonia. Zhonghuafangshexuezazhi. 2020;54(00):E007. doi: 10.3760/cma.j.issn.1005-1201.2020.0007. [DOI] [Google Scholar]
- 21.Zhang H., Penninger J.M., Li Y. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;(March (3)) doi: 10.1007/s00134-020-05985-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou P., Yang X.L., Wang X.G. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin [EB / OL] bioRxiv. 2020;01:23. doi: 10.1101/2020.01.22.914952. https://www.biorxiv.org/content10.1101/2020.01.22.914952v2.full.pdf [2020⁃02⁃01] [DOI] [Google Scholar]
- 23.Xu X., Chen P., Wang J. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63(March (3)):457–460. doi: 10.1007/s11427-020-1637-5. Epub 2020 Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Health Commission of the People’s Republic of China . 5th trial ed. 2020. Office of National Administration of Traditional Chinese Medicine. Diagnosis and Treatment Program of Novel Coronavirus Infected Pneumonia.http://www.gov.cn/zhengce/zhengceku/2020-02/05/content_5474791.htm [EB/OL]. (2020-02-04)[2020-02-11](in Chinese) [Google Scholar]
- 25.Lin Lili, Yan Hua, Chen Jiabin. Application of metabolomics in viral pneumonia treatment with traditional Chinese medicine. Chin. Med. 2019;12(March (14)):8. doi: 10.1186/s13020-019-0229-x. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez E.E., Mehta N.M. The science and art of pediatric critical care nutrition. Curr. Opin. Crit. Care. 2016;22(August (4)):316–324. doi: 10.1097/MCC.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 27.Béranger A., Pierron C., de Saint Blanquat L. [Communication, information, and roles of parents in the pediatric intensive care unit: a review article] Arch. Pediatr. 2017;24(March (3)):265–272. doi: 10.1016/j.arcped.2016.12.001. Epub 2017 Jan 25. [DOI] [PubMed] [Google Scholar]
- 28.Coats H., Bourget E., Starks H. Nurses’ reflections on benefits and challenges of implementing family-centered care in pediatric intensive care units. Am. J. Crit. Care. 2018;27(January (1)):52–58. doi: 10.4037/ajcc2018353. [DOI] [PMC free article] [PubMed] [Google Scholar]