Abstract
Coronavirus disease 2019 is a pandemic influencing the first half of the year 2020. The virus has rapidly spread to many countries. Studies are rapidly published to share information regarding epidemiology, clinical and diagnostic patterns, and prognosis. The following review condenses the surge of information into an organized format.
Keywords: Coronavirus disease 2019, Severe acute respiratory disease, Epidemiology, Diagnosis, Pandemic
1. Introduction
In December 31, 2019, hospitals in Wuhan, Hubei, China reported a cluster of idiopathic pneumonia cases [1]. The Huanan Seafood Wholesale Market was identified as the origin of the infection, causing the area to shut down. However, a large fluctuation of visitors around the area during the Spring Festival caused the infection to rapidly spread to other regions of China. With the use of real-time reverse transcription polymerase chain reaction (RT-PCR), researchers identified the cause being a novel coronavirus labeled as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), later also called coronavirus disease 2019 (COVID-19) [2,3].
The number of RT-PCR—positive cases rapidly increased [4]. On January 30, 2020, the World Health Organization (WHO) declared COVID-19 a Public Health Emergency of International Concern (PHEIC) and thus a pandemic. During February 2020, mainland China reported 77,780 cases. Thirty-three other countries also reported 2549 cases along with 34 fatalities. World-wide, 80,239 cases occurred with 2700 deaths [5].
2. Viral structure and life cycle
COVID-19 is a single, positive-stranded RNA virus enveloped in a lipid bilayer [6,7]. The lipid bilayer fuses with the host cell membrane, releasing RNA into the cytoplasm and causing translation of various viral proteins. The replicated RNA genome and synthesized viral proteins reassemble into new viruses, which burst out of the cell [8,9].
The virus enters via binding of two proteins. The viral counterpart is the spike-protein (S-protein), a glycoprotein expressed as a homotrimer on the viral envelope [10]. Each S-protein consists of two subunits. S1 subunit includes a receptor-binding domain that targets receptors on host cells, and S2 regulates the membrane fusion. This viral S-protein binds with the human protein receptor ACE2 [11]. ACE2 is abundant in lung, heart, kidney, and adipose tissue [12,13]. Binding of S-protein with ACE2 allows for membrane fusion and introduction of COVID-19 RNA into the cell. The binding of these two proteins serves as a target for potential treatments and vaccinations.
Compared to SARS, COVID-19 uses the same mechanism for entering host cells, but at slower speeds. However, COVID-19 accumulates more in the system compared to SARS. This explains why COVID-19 has a longer incubation period and is more contagious, while SARS presents with more symptoms and disease severity [14].
3. Transmission and infectivity
The spread of COVID-19 is rapid [4]. Transmission is from close contact and droplet. There is scarce evidence to suggest airborne transfer [15]. Very minimal to no RNA concentration is found in airborne samples [16]. No RNA is detected in urine or serum samples of positive patients [17]. Viral RNA can be detected on fomites including plastic [18].
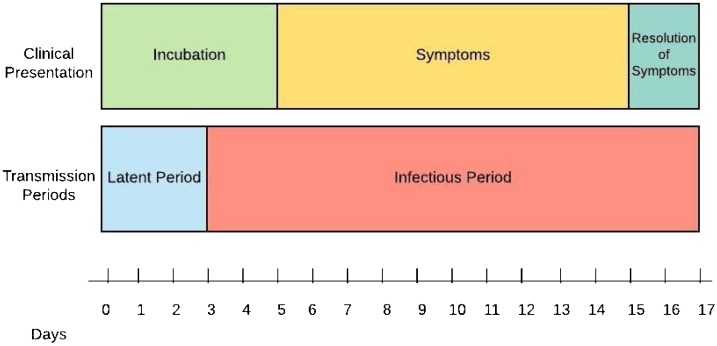
The mean incubation period is about 3–9 days [[19], [20], [21], [22], [23]], with a range between 0–24 days (Fig. 1 ) [24]. The mean serial interval is about 3–8 days, presenting sooner than the end of incubation [21,23,25]. This suggests that one becomes contagious before symptoms present (about 2.5 days earlier from the start of symptoms) [21]. About 44 % of transmission is estimated to occur before symptoms arise [25].
Fig. 1.
Representation of COVID-19 Clinical and Transmission Periods.
Close contact with someone during their infectious period puts one at risk for acquiring the infection. However, the certainty of becoming infected is still unpredictable. Burke et al. tested 445 people that were in close contact (at least 6 feet from the source for a minimum of 10 min) with 10 COVID-19-confirmed patients. After two weeks of testing, only two subjects became positive. Both subjects were household members that practiced isolation from the infected individuals. Five subjects continued to expose themselves constantly with the infected individuals and never became positive. No healthcare workers (222 subjects) became positive [26]. These findings coincide with two other studies [23,27]. Evaluation of all positive cases from mainland China showed 3.8 % being from healthcare workers (1716/44672) [28].
About 18 % of cases remain asymptomatic [[29], [30], [31]]. The potential of asymptomatic patients infecting others is proven by multiple studies concerning clusters [32,33]. They can be asymptomatic and contagious regardless of lab or CT scan findings [20,32,34]. Younger patients tend to remain asymptomatic (even if constantly around an infected individual), while the elderly usually show symptoms [20,31]. It is calculated that about 86 % of infections have remained undocumented, and about 55 % of those cases were contagious [35]. This may be because of the infectious period presenting before symptoms, the frequency of asymptomatic cases, and the poor documented sensitivity of nasopharyngeal RT-PCR [36,37].
Symptoms tend to resolve after 10 days [38]. However, viral shedding continues despite symptoms disappearing [16,17,32,38]. COVID-19 RNA viral shedding persists for about 18 days (by nasopharyngeal swab) or 19 days (via feces) [39]. Mild and asymptomatic cases tend to shed 10 days (between 8–15 days) after symptom resolution [16,32,34], with 90 % resolving after 10 days and nearly all cases resolving after 15 days [16,32]. Severe cases continue shedding up until 25 days after initial symptoms arise. Severe cases also have 60 times more viral load than mild cases [40]. However, the infectious potential based on severity has not been discovered. Due to these findings, the Chinese Municipal Health Commission has recommended against discharging patients until the patient has remained afebrile for three days and RT-PCR becomes negative [41].
4. Clinical features
4.1. Age
Most cases present between ages 30–79 years. Table 1 organizes the prevalence based on age ranges as witnessed by mainland China [28]. These findings reflect a recent meta-analysis [42].
Table 1.
Case Presentation Rate by Age Group.
| Age Group (years) | Case Presentation Rate |
|---|---|
| <10 | 1 % |
| 10–19 | 1 % |
| 20–29 | 8 % |
| 30–79 | 87 % |
| 80+ | 3 % |
4.2. Comorbidities
Table 2 presents the comorbidity rate seen with COVID-19 cases. The most common comorbidity is hypertension (30.7 %). This is followed by diabetes mellitus (14.3 %) and cardiovascular diseases (11.9 %) [24,[43], [44], [45], [46], [47]].
Table 2.
Comorbidity Rates Seen with COVID-19 Cases.
| Comorbidity | Rate |
|---|---|
| Hypertension | 30.7 % |
| Diabetes Mellitus | 14.3 % |
| Cardiovascular Disease | 11.9 % |
| Cerebrovascular Disease | 6.6 % |
| Malignancy | 4.3 % |
| Chronic Liver Disease | 2.8 % |
| Chronic Lung Disease | 2.4 % |
| Chronic Kidney Disease | 2.1 % |
| HIV | 1.4 % |
| Immunodeficiency | 0.2 % |
HIV – Human Immunodeficiency Virus.
There is suspicion regarding whether angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) increase the risk of COVID-19 infection and severity [48]. Similar to SARS, COVID-19 binds to ACE2 to infiltrate cells [48,49]. ACE inhibitors and ARBs increase the level of ACE2 and could therefore increase the infectivity of COVID-19. However, animal models have shown that ACE inhibitors and ARBs modulate the ACE2 levels and therefore decrease the severity of SARS pneumonia [50]. While the question regarding increased infectivity of COVID-19 remains unanswered, the mortality benefits of ACE inhibitors and ARBs for cardiovascular diseases are well-established [49]. Therefore, experts recommend continuing the medications for COVID-19 patients [49,51,52].
4.3. Symptoms
Table 3 shows the rate of symptoms presented with positive cases of COVID-19. The most common symptoms include fever (82.2 %) and cough (61.7 %) [[43], [44], [45], [46],53,54]. These symptoms are similar to other viral respiratory diseases. However, the presentation of myalgia, sore throat, nausea, vomiting, and diarrhea may suggest another infection instead. Viral respiratory co-infection is rare [17,[55], [56], [57], [58]].
Table 3.
Rate of Symptoms Seen with COVID-19 Cases.
| Symptom | Rate |
|---|---|
| Fever | 82.2 % |
| Cough | 61.7 % |
| Fatigue | 44.0 % |
| Dyspnea | 41.0 % |
| Anorexia | 40.0 % |
| Productive Sputum | 27.7 % |
| Myalgia | 22.7 % |
| Sore Throat | 15.1 % |
| Nausea | 9.4 % |
| Dizziness | 9.4 % |
| Diarrhea | 8.4 % |
| Headache | 6.7 % |
| Vomiting | 3.6 % |
| Abdominal Pain | 2.2 % |
5. Laboratory findings
5.1. Common laboratory diagnostic tests
Laboratory values that suggest COVID-19 infection include lymphopenia, prolonged prothrombin time (PT), elevated lactate dehydrogenase (LDH), elevated alanine aminotransferase (ALT), elevated aspartate aminotransferase (AST), elevated D-dimer, elevated neutrophils, eosinopenia, elevated C-reactive protein (CRP), and elevated troponin (including high-sensitivity troponin) [24,[43], [44], [45],47,59,60]. Table 4 displays the frequency of most suggested labs. The most common findings are eosinopenia (<0.02 × 10^9/L) and lymphopenia (<1.5 × 10^9/L) with 78.8 % and 68.7 %, respectively.
Table 4.
Common COVID-19 Laboratory Findings.
| Laboratory finding | Rate |
|---|---|
| Eosinopenia | 78.8 % |
| Lymphopenia | 68.7 % |
| Elevated AST | 63.4 % |
| Elevated C-reactive protein | 60.7 % |
| Elevated PT | 58.0 % |
| Elevated LDH | 47.2 % |
| Elevated D-dimer | 46.4 % |
| Thrombocytopenia | 36.2 % |
| Elevated ALT | 21.3 % |
| Elevated HS-Troponin | 12.5 % |
LDH – Lactate Dehydrogenase; AST – Aspartate Aminotransferase; PT – Prothrombin time; HS-Troponin – High-sensitivity Troponin.
While eosinopenia is linked with COVID-19 infection, its sensitivity and specificity are low at 82 % and 64 %, respectively. This equates to small positive and negative likelihood ratios of 2.29 and 0.28. The combination of lymphopenia and eosinopenia change the sensitivity and specificity to 38.5 % and 75.5 %. Positive and negative likelihood ratios worsen with 1.57 and 0.81, respectively [60].
Troponin elevation is suggestive of infiltration of cardiac tissue [61]. While respiratory-compromising symptoms are present in most cases, cardiac chest pain is also a possibility [59].
5.2. Reverse transcriptase – polymerase chain reaction
RT-PCR remains the gold standard for diagnosing COVID-19. While its specificity is nearly 100 % from having no reported false positive cases or cross-reactivity with other viruses or estranged oligonucleotides [62], the sensitivity is low at 64 % [36,37,63]. This correlates with a high positive likelihood ratio of 64, but a poor negative likelihood ratio of 0.3. Studies have started performing two sequential RT-PCRs to ensure true negative cases [36,37]. RT-PCR tends to present negative-to-positive at a mean of 5.1 days, and positive-to-negative at 6.9 days [36]. Recommendations are to acquire a repeat RT-PCR 3 days after an initial negative result. Factors that may contribute to the low sensitivity of one RT-PCR may be from immature technology, variation of detection by manufacturers, low initial viral load, and improper sampling [36].
While studies recommend two sequential RT-PCRs to ensure true negativity, testing kits are sparse during the pandemic. Some studies suggest employing chest CT scans if the initial RT-PCR is negative. CT scans have a sensitivity of 98 %, despite a lower specificity [37]. The Chinese General Office of National Health Committee initially allowed positive CT scan findings to be diagnostic for COVID-19 without RT-PCR, but this recommendation was removed in a more recent list of recommendations [64,65].
6. Image findings
Imaging modalities may serve as a surrogate to diagnose COVID-19. Chest x-ray abnormalities present in 33 %–60 % of patients, despite most having CT scan findings [66,67]. Chest CT scans hold more potential to diagnose COVID-19 cases.
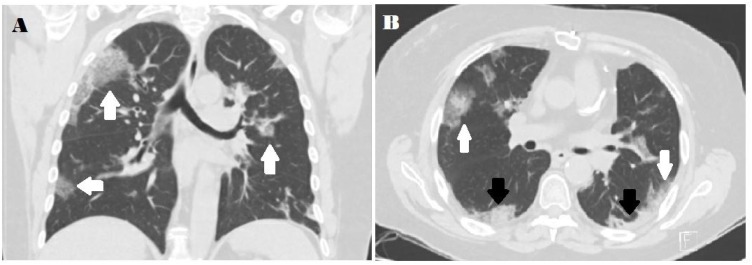
Chest CT scans of COVID-19 cases present with bilateral ground-glass opacification or consolidation (Fig. 2 ). Ground-glass opacification is dominant during early stages and consolidation presents at later stages [68]. More than two lobes are frequently affected with most patients presenting with infiltration in all five lobes. Consolidation rarely present without ground-glass opacification [53,54]. The opacifications typically are rounded and present peripherally in the subpleural area [53,68]. Some studies suggest lower lobe predilection [54,69]. Severe cases present with more consolidation along with architectural distortion, traction bronchiectasis, lymph node enlargement, and pleural effusions [54,[68], [69], [70]].
Fig. 2.
A) Coronal thin-section unenhanced CT image showing ground-glass opacities with a rounded morphology (arrows). B) Axial thin-section unenhanced CT scan showing diffuse bilateral confluent and patchy ground-glass (white arrows) and consolidative (black arrows) pulmonary opacities. Note the peripheral propensity.
CT scan findings, compared to RT-PCR, show a sensitivity of 84 %–98 % and specificity of 80.5 %–25 % [36,37,68]. Combining the data from two studies [36,37], the sensitivity and specificity for CT scans are 88 % and 25 %, respectively. This presents a positive likelihood ratio of 1.17 and a negative likelihood ratio of 0.48. Another study implemented a CT scan algorithm into a machine which produced positive and negative likelihood ratios of 4.3 and 0.2, respectively [68]. This suggests that a negative CT scan confers small-moderate confidence that the case is indeed negative.
CT scan interpretation by radiologists hold a sensitivity of 70–80 % and specificity of 90–100 % [71]. Bai et al. studied whether radiologists could discern COVID-19 cases based on CT scan findings. Using the medians from the study (sensitivity 80 %, specificity 93 %), the positive predictive value and negative predictive value are 92 % and 82 %, respectively. This suggests that during this pandemic, a radiologist stating a CT scan is COVID-19-positive is likely correct; however, if the CT scan is deemed negative, it only can be stated with moderate confidence.
7. Complications
7.1. Acute respiratory distress syndrome
Alveolar cells in the lung contain abundant amounts of ACE2, allowing COVID-19 to harbor within the alveoli [12]. About 41.8 % of patients develop acute respiratory distress syndrome (ARDS) [72]. Diabetes mellitus is a factor associated with the development of ARDS [72]. Other associated comorbidities include hypertension, cardiovascular disease, and chronic kidney disease [72,73]. Laboratory findings associated with the development of ARDS include neutrophilia, lymphopenia, elevated C-reactive protein (high-sensitivity and normal), elevated blood urea nitrogen, elevated d-dimer, prolonged PT, and elevated LDH [72,73]. Patients with ARDS present with higher lactate levels and score high in common risk stratification calculators [73].
About 35.8 %, 45.3 %, and 18.9 % of ARDS cases are mild, moderate, and severe; respectively [73]. Mortality increases with the severity of the disease. Patients greater than 65 years of age present with worse degrees of ARDS and have a higher mortality likelihood [73]. Laboratory markers predicting mortality of COVID-19 ARDS patients include low albumin, elevated blood urea nitrogen, and elevated LDH [72,73].
7.2. Myocardial injury
The most common causes of COVID-19-related death are associated with the lungs and heart [45]. There are two theories explaining the mechanism of myocardial injury occurring with COVID-19. The first theory pertains to the heart having similar ACE2 levels as the lungs [12], allowing viral entry into the myocardial cells [52]. The secondary theory involves a cytokine storm causing myocardial injury [52]. Myocardial injury includes acute coronary syndrome, heart failure, myocarditis, hypotension or shock, and sepsis [74,75]. For definitive characterization of the injury, magnetic resonance imaging and possibly endocardial biopsy is required [74].
Arrhythmias arise with severe COVID-19 cases [61,74,76]. Malignant arrhythmias, including ventricular tachycardia and fibrillation, occur at a rate of 5.9 %, and arise more frequently in patients with elevated troponin levels (17.3 % of patients with elevated troponin) [76].
Heart failure is commonly encountered in severe cases of COVID-19, regardless of previous cardiac history [74,76]. This presents with elevated levels of N-terminal pro-B-type natriuretic peptide (NT pro-BNP) and troponin levels, especially in severe cases [77]. Some suspect pulmonary hypertension causing right heart failure also contributes to these cases [61,74].
Elevated high-sensitivity troponin (HS-troponin) and creatinine kinase – myocardial brand (CK-MB) levels can independently predict severe COVID-19 cases [75,76,78,79]. A recent meta-analysis showed troponin being more elevated in severe cases [80]. CK itself does not predict severity [78]. Patients with elevated HS-troponin (>28 ng/L) and CK-MB are suspected to have myocarditis or heart failure [44,78].
7.3. Acute kidney injury
Acute kidney injury presents with elevated urea and cystatin-C levels in severe COVID-19 infection [72,73,81,82]. There are two hypotheses concerning the cause of acute kidney injury. One is from kidneys harboring more ACE2 levels than the lung or heart, especially in the proximal convoluted tubules. However, COVID-19 RNA is not encountered in the urine [17]. The other theory pertains to injury via a cytokine storm [82].
Patients may acquire continuous renal replacement therapy (CRRT) based on kidney injury severity. Speculation exists regarding CRRT potentially serving as a means of removing large cytokine levels from the system, regardless of kidney injury [81,82].
7.4. Co-infection rate
Table 5 presents the chance of co-infection with another microbe [17,[55], [56], [57], [58]]. Bacteria are more frequently encountered with COVID-19 compared to other viruses.
Table 5.
Virus and Bacterial Co-infection Rate with COVID-19-Positive Cases.
| Source | Lin et al. [57] (Shenzhen, China) 2020 (N = 92) | Xing et al. [58] (Qingdao, China) 2020 (N = 30) | Xing et al. [58] (Wuhan, China) 2020 (N = 38) | Chen et al. [56] (Hubei, China) 2020a | Woelfel et al. [17] (Munich, Germany) 2020 (N = 16) | Ai et al. [55] (Xiangyang, China) 2020 (N = 102) | Rate from Total |
|---|---|---|---|---|---|---|---|
| Viruses | |||||||
| RSV | 3 | 0 | 1 | – | 0 | 0 | 1.44 % |
| Flu A | 0 | 18 | 0 | – | 0 | 0 | 6.47 % |
| Flu B | 0 | 16 | 0 | – | 0 | 0 | 5.76 % |
| Corona NL63 | 0 | – | – | – | 0 | – | 0.00 % |
| Corona 229E | 0 | – | – | – | 0 | – | 0.00 % |
| Corona HKU1 | 1 | – | – | – | 0 | – | 0.93 % |
| Corona OC43 | 0 | – | – | – | 0 | – | 0.00 % |
| Paraflu 1 | 0 | 0 | 0 | – | 0 | – | 0.00 % |
| Paraflu 2 | 1 | – | – | – | 0 | – | 0.93 % |
| Paraflu 3 | 0 | – | – | – | 0 | – | 0.00 % |
| H. Bocavirus | 0 | – | – | – | 0 | – | 0.00 % |
| H. Metapneumovirus | 1 | – | – | – | 0 | – | 0.93 % |
| Adenovirus | 0 | 0 | 0 | – | 0 | 0 | 0.00 % |
| Rhinovirus | 0 | – | – | – | 0 | – | 0.00 % |
| Bacteria | |||||||
| Mycoplasma p. | – | 7 | 1 | 29 | – | 2 | 17.30 % |
| Chlamydia p. | – | 0 | 0 | 22 | – | 3 | 11.90 % |
| Legionella p. | – | 6 | 0 | – | – | – | 8.82 % |
| Coxiella burnetii | – | 0 | 0 | – | – | – | 0.00 % |
N = 44 for Mycoplasma pneumoniae evaluation and N = 40 for Chlamydia pneumoniae evaluation. RSV – Respiratory Syncytial Virus; Flu – Influenza; Corona – Coronavirus; Paraflu – Parainfluenza; H. – Human; p. - pneumoniae.
The three most encountered co-infecting viruses were respiratory syncytial virus (RSV), Influenza A, and Influenza B. RSV presents with a rate of 1.44 %. Influenza A and B presents with a rate of 6.47 % and 5.76 %, respectively. However, the calculated rate of influenza A and B co-infection was heavily influenced by the study conducted in Qingdao, China. Removing the study reduces the rate to 0.00 % for both viruses.
The associated bacteria are those responsible for atypical pneumonia: Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumoniae. No studies exist presenting the rate of other bacteria, including Staphylococcus sp. and Streptococcus sp. IgM against Mycoplasma pneumoniae is most frequently encountered with a rate of 17.30 % [55,56,58].
8. Prognosis
8.1. Risk stratification and survival rate
The case-fatality rate (CFR) continues to change as the pandemic continues. Table 6 presents the CFR in China via age groups [28,83]. Age greater than 60 years is considered a mortality risk factor [28,45,83].
Table 6.
Case-Fatality Rate Organized by Age Group.
| Age group (years) | Case-Fatality Rate |
|---|---|
| Overall | 1.6 % |
| 0–9 | 0.0094 % |
| 10–19 | 0.022 % |
| 20–29 | 0.091 % |
| 30–39 | 0.18 % |
| 40–49 | 0.4 % |
| 50–59 | 1.3 % |
| 60–69 | 4.6 % |
| 70–79 | 8.0 % |
| 80+ | 14.8 % |
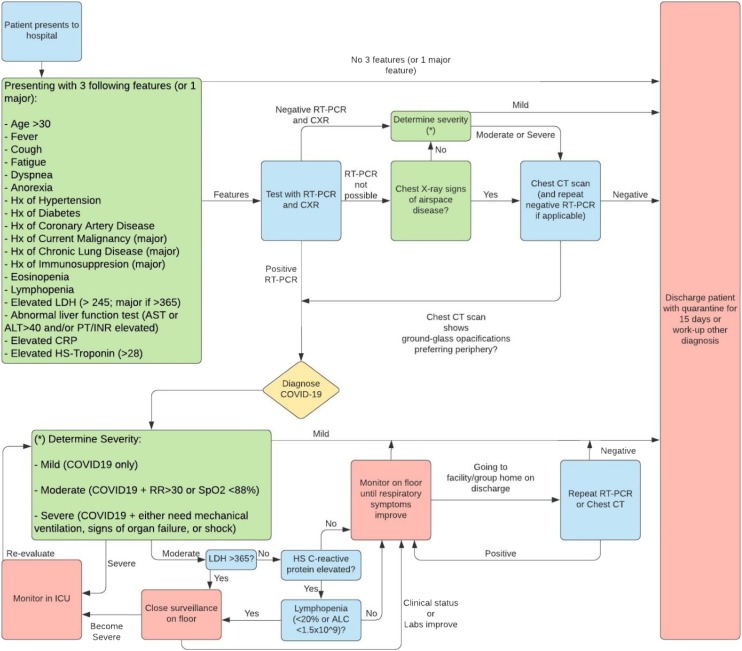
Table 7 presents the risk stratification commonly used in studies [28,46,84,85]. About 81 % are mild cases, 14 % are severe, and 5 % are critical [28]. Mortality for mild, severe, and critical cases are 98 %, 52 %, and 6 % [84,85]. Severe cases have an unpredictable prognosis solely based on clinical presentation. Laboratory markers including LDH, high-sensitivity CRP, and lymphocyte count estimate the prognosis for these cases (Fig. 3 ) [85].
Table 7.
Risk Stratification of COVID-19 Cases.
| Severity | Description |
|---|---|
| Mild | COVID-19 positive |
| Severe | COVID-19 positive + RR > 30 or SaO2<93 % |
| Critical | COVID-19 positive + mechanical ventilation, evidence of multiorgan failure, or shock |
RR-Respiratory Rate; SaO2-Oxygen Saturation; COVID-19-coronavirus infectious disease 2019.
Fig. 3.
COVID-19 Diagnostic and Risk-Stratification Algorithm.
8.2. Prognosis predictors
Comorbidities associated with severe COVID-19 cases include elderly age, hypertension, cardiovascular disease, cerebrovascular disease, and chronic kidney disease [28,43,46,47]. Cardiovascular disease presents with a 10.5 % CFR. Other diseases that present with a high CFR include diabetes (7.3 %), chronic lung diseases (6.3 %), hypertension (6.0 %), and cancer (5.6 %) [28].
Laboratory values contribute to survival prediction. These include elevated LDH, elevated high sensitivity-CRP, and lymphopenia [85,85,86]. A significantly elevated LDH (>365 units/L) presents a positive likelihood ratio of 58 for mortality based on the results by Yan et al. [85]. High sensitivity-CRP also has a positive likelihood ratio of 17, but a negative likelihood ratio of 0. Lymphopenia presents a small positive likelihood ratio of 2.65 and a small-moderate negative likelihood ratio of 0.37 [85,85,86]. Other laboratory values that suggest a high mortality risk if elevated include aspartate aminotransferase (AST), alanine aminotransferase (ALT), D-dimer, neutrophil count, prothrombin time, procalcitonin, and high-sensitivity and regular cardiac troponin [28,[43], [44], [45], [46], [47],59,[84], [85], [86]]. Low monocytes, platelets, and albumin also suggest high mortality risk [28,44,45,47,87].
Some chest CT scan findings, although rare with COVID-19 respiratory disease, suggest a high-risk case. These include architectural distortion, traction bronchiectasis, intrathoracic lymph node enlargement, and pleural effusions [70].
9. Conclusion
The COVID-19 pandemic is rapidly spreading. Case rates and CFRs continue to change. Identifying clinical characteristics, developing and identifying pertinent diagnostic criteria, and providing effective treatment and care are vital for overcoming the pandemic.
Declaration of Competing Interest
No conflict of interest to report.
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020 doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang J., Deng L., Zhang L., Cai Y., Cheung C.W., Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J. Gen. Intern. Med. 2020 doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. JAMA. 2020 doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . 2020. Coronavirus Disease 2019 (COVID-19) Situation Report – 36. [Google Scholar]
- 6.Wu F., Zhao S., Yu B. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu R., Zhao Z., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin P., Du E.Z., Luo W.T. Characteristics of the life cycle of porcine deltacoronavirus (PDCoV) in vitro: replication kinetics, cellular ultrastructure and virion morphology, and evidence of inducing autophagy. Viruses. 2019;11:455. doi: 10.3390/v11050455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qinfen Z. The life cycle of SARS coronavirus in Vero E6 cells. J. Med. Virol. 2004;73(3):332–337. doi: 10.1002/jmv.20095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li F. Structure, function, and evolution of coronavirus spike proteins. Ann. Rev. Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupte M., Boustany-Kari C.M., Bharadwaj K. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:781–788. doi: 10.1152/ajpregu.00183.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su Z., Wu Y. A multiscale and comparative model for receptor binding of 2019 novel coronavirus and the implications of its life cycle in host cells. BioRxiv. 2020 [Google Scholar]
- 15.World Health Organization . World Health Organization; 2020. Rational Use of Personal Protective Equipment for Coronavirus Disease (COVID-19): Interim Guidance. [Google Scholar]
- 16.Liu Y., Ning Z., Chen Y. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan hospitals during COVID-19 outbreak. BioRxiv. 2020 [Google Scholar]
- 17.Woelfel R., Corman V.M., Guggemos W. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel-associated transmission cluster. MedRxiv. 2020 [Google Scholar]
- 18.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan J.F., Yan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tindale L.C., Coombe M., Stockdale J.E. Transmission interval estimates suggest pre-symptomatic spread of COVID-19. MedRxiv. 2020 [Google Scholar]
- 22.Lauer S.A., Grantz K.H., Bi Q. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020 doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pung R., Chiew C.J., Young B.E. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. 2020 doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of 2019 novel coronavirus infection in China. N. Engl. J. Med. 2020 [Google Scholar]
- 25.He X., Lau EHY Wu P. Temporal dynamics in viral shedding and transmissibility of COVID-19. MedRxiv. 2020 doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 26.Burke R.M., Midgley C.M., Dratch A. Active monitoring of persons exposed to patients with confirmed COVID-19 – United States, January—February 2020. MMWR. 2020;69:245–246. doi: 10.15585/mmwr.mm6909e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng V.C.C., Wong S.C., Chen J.H.K. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect. Control Hosp. Epidemiol. 2020 doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 29.Nishiura H., Kobayashi T., Miyama T. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance. 2020 doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng O.T., Marimuthu K., Chia P.Y. SARS-CoV-2 infection among travelers returning from Wuhan, China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai Y., Yao L., Wei T. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou L., Ruan F., Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li R., Pei S., Chen B. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020 doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang Y., Zhang H., Xie J. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiol. 2020 doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L., Gao Y.H., Lou L.L., Zhang G.J. The clinical dynamics of 18 cases of COVID-19 outside of Wuhan, China. Eur. Resp. J. 2020 doi: 10.1183/13993003.00398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lo I.L., Lio C.F., Cheong H.H. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int. J. Biol. Sci. 2020;16:1698–1707. doi: 10.7150/ijbs.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y., Yan L.M., Wan L. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chinese Municipal Health Commission . 2020. The Management of 2019 Novel Coronavirus Infected Pneumonia: Interim Guidance. [Google Scholar]
- 42.Yang J., Zheng Y., Gou X. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on analysis of data of 150 patients from Wuhan, China. Intens. Care Med. Exp. 2020 doi: 10.1007/s00134-020-06028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma C., Gu J., Hou P. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. MedRxiv. 2020 [Google Scholar]
- 48.Diaz J.H. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J. Travel Med. 2020 doi: 10.1093/jtm/taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuster G.M., Pfister O., Burkard T. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun M.L., Yang J.M., Sun Y.P., Su G.H. Inhibitors of RAS might be a good choice for the therapy of COVID-19 pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:e014. doi: 10.3760/cma.j.issn.1001-0939.2020.0014. [DOI] [PubMed] [Google Scholar]
- 51.Thomson G. COVID-19: social distancing, ACE2 receptors, protease inhibitors and beyond? Int. J. Clin. Pract. 2020 doi: 10.1111/ijcp.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020 doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung M., Bernheim A., Mei X. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bernheim A., Mei X., Huang M. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020 doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ai J.W., Chen J.W., Wang Y. The cross-sectional study of hospitalized coronavirus disease 2019 patients in Xiangyang, Hubei province. MedRxiv. 2020 [Google Scholar]
- 56.Chen X., Zheng F., Qing Y. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: a double-center observational study. MedRxiv. 2020 [Google Scholar]
- 57.Lin D., Liu L., Zhang M., Hu Y. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients. Sci. China Life Sci. 2020 doi: 10.1007/s11427-020-1668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xing Q., Li G., Xing Y. Precautions are needed for COVID-19 patients with coinfection of common respiratory pathogens. Lancet. 2020 [Google Scholar]
- 59.Bai T., Tu S., Wei Y. Clinical and laboratory factors predicting the prognosis of patients with COVID-19: an analysis of 127 patients in Wuhan, China. Lancet. 2020 [Google Scholar]
- 60.Li Q., Ding X., Xia G. A simple laboratory parameter facilitates early identification of COVID-19 patients. MedRxiv. 2020 [Google Scholar]
- 61.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care works, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corman V.M., Landt O., Kaiser M. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eur. Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020 doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.General Office of National Health Committee . 2020. Office of State Administration of Traditional Chinese Medicine. Notice on the Issuance of a Program for the Diagnosis and Treatment of Novel Coronavirus (2019-nCoV) Infected Pneumonia (Trial Fifth Edition) [Google Scholar]
- 65.General Office of National Health Committee . 2020. Office of State Administration of Traditional Chinese Medicine. Notice on the Issuance of a Program for the Diagnosis and Treatment of Novel Coronavirus (2019-nCoV) Infected Pneumonia (Trial Sixth Edition) [Google Scholar]
- 66.Yoon S.H., Lee K.H., Kim J.Y. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J. Radiol. 2020;21(4):494–500. doi: 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ng M.Y., Lee E.Y.P., Yang J. Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiol. Cardiothorac. Imag. 2020 doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang S., Kang B., Ma J., Zeng X., Xiao M., Guo J. A deep algorithm using CT images to screen for Corona Virus Disease (COVID-19) MedRxiv. 2020 doi: 10.1007/s00330-021-07715-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gozes O., Frid-Adar M., Greenspan H., Browning P.D., Bernheim A., Siegel E. Rapid AI development cycle for the coronavirus (COVID-19) pandemic: initial results for automated detection and patient monitoring using deep learning CT imaging analysis. ArXiv. 2020 [Google Scholar]
- 70.Zhao W., Zhong Z., Xie X., Yu Q., Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. Am. J. Roentgenol. 2020;214:1–6. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 71.Bai H., Hsieh B., Xiong Z. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020 doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y., Sun W., Li J. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. MedRxiv. 2020 [Google Scholar]
- 74.Tan Z.C., Fu L.H., Wang D.D., Hong K. Cardiac manifestations of patients with COVID-19 pneumonia and related treatment recommendations. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:e005. doi: 10.3760/cma.j.issn.cn112148-20200213-00077. [DOI] [PubMed] [Google Scholar]
- 75.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 76.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen C., Chen C., Yan J.T., Zhou N., Zhao J.P., Wang D.W. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:e008. doi: 10.3760/cma.j.cn112148-20200225-00123. [DOI] [PubMed] [Google Scholar]
- 78.Wu C., Hu X., Song J. Heart injury signs are associated with higher and earlier mortality in coronavirus disease 2019 (COVID-19) MedRxiv. 2020 [Google Scholar]
- 79.Zhou B., She J., Wang Y., Ma X. The clinical characteristics of myocardial injury in severe and very severe patients with 2019 novel coronavirus disease. Lancet. 2020 doi: 10.1016/j.jinf.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog. Cardiovasc. Dis. 2020 doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang X.H., Sun R.H., Chen D.C. Diagnosis and treatment of COVID-19: acute kidney injury cannot be ignored. Zhoughua Yi Xue Za Zhi. 2020;100:e017. doi: 10.3760/cma.j.cn112137-20200229-00520. [DOI] [PubMed] [Google Scholar]
- 82.Xu D., Zhang H., Gong H.Y. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Preprints. 2020 doi: 10.1007/s00134-020-06026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Riou J., Hauser A., Counotte M.J., Althaus C.L. Adjusted age-specific case fatality ratio during the COVID-19 epidemic in Hubei, China, January and February 2020. MedRxiv. 2020 [Google Scholar]
- 84.Yan L., Zhang H.T., Xiao Y. Prediction of criticality in patients with severe Covid-19 infection using three clinical features: a machine learning-based prognostic model with clinical data in Wuhan. MedRxiv. 2020 [Google Scholar]
- 85.Yan L., Zhang H.T., Goncalves J. A machine learning-based model for survival prediction in patients with severe COVID-19 infection. MedRxiv. 2020 [Google Scholar]
- 86.Tan L., Wang Q., Zhang D. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. MedRxiv. 2020 doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen X., Ling J., Mo P. Restoration of leukomonocyte counts is associated with viral clearance in COVID-19 hospitalized patients. MedRxiv. 2020 [Google Scholar]