Abstract
Background
As of March 18, 2020, 13 415 confirmed cases and 120 deaths related to coronavirus disease 2019 (COVID-19) in mainland China, outside Hubei province—the epicentre of the outbreak—had been reported. Since late January, massive public health interventions have been implemented nationwide to contain the outbreak. We provide an impact assessment of the transmissibility and severity of COVID-19 during the first wave in mainland Chinese locations outside Hubei.
Methods
We estimated the instantaneous reproduction number (Rt) of COVID-19 in Beijing, Shanghai, Shenzhen, Wenzhou, and the ten Chinese provinces that had the highest number of confirmed COVID-19 cases; and the confirmed case-fatality risk (cCFR) in Beijing, Shanghai, Shenzhen, and Wenzhou, and all 31 Chinese provinces. We used a susceptible–infectious–recovered model to show the potential effects of relaxing containment measures after the first wave of infection, in anticipation of a possible second wave.
Findings
In all selected cities and provinces, the Rt decreased substantially since Jan 23, when control measures were implemented, and have since remained below 1. The cCFR outside Hubei was 0·98% (95% CI 0·82–1·16), which was almost five times lower than that in Hubei (5·91%, 5·73–6·09). Relaxing the interventions (resulting in Rt >1) when the epidemic size was still small would increase the cumulative case count exponentially as a function of relaxation duration, even if aggressive interventions could subsequently push disease prevalence back to the baseline level.
Interpretation
The first wave of COVID-19 outside of Hubei has abated because of aggressive non-pharmaceutical interventions. However, given the substantial risk of viral reintroduction, particularly from overseas importation, close monitoring of Rt and cCFR is needed to inform strategies against a potential second wave to achieve an optimal balance between health and economic protection.
Funding
Health and Medical Research Fund, Hong Kong, China.
Introduction
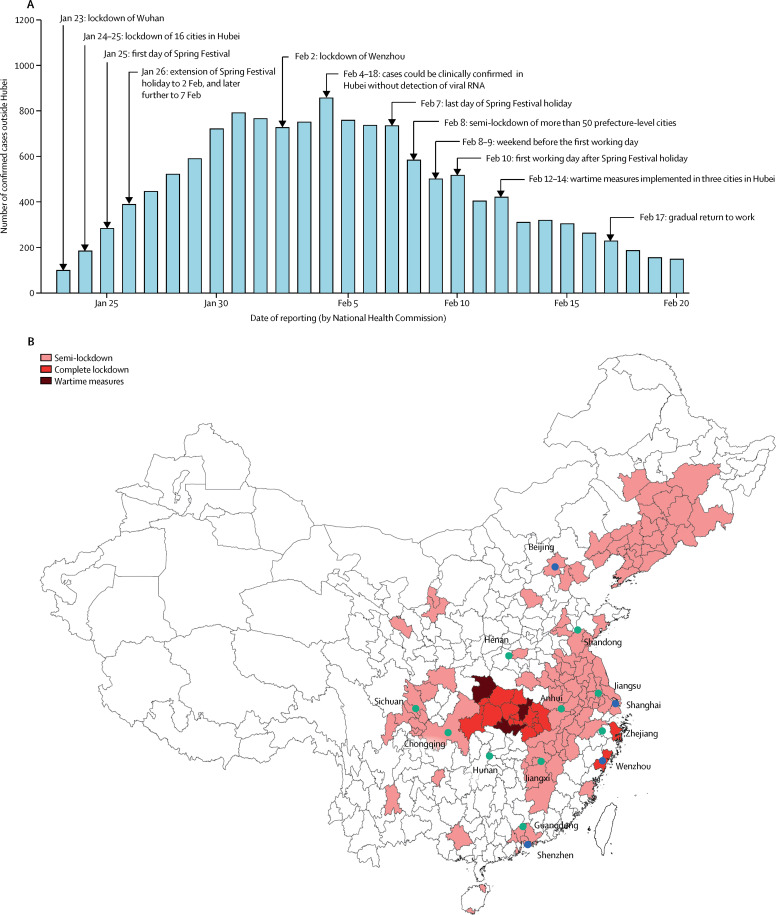
Since the third week of January, 2020, massive public health interventions have been implemented across China to contain the spread of coronavirus disease 2019 (COVID-19; figure 1 ). Wuhan, the epicentre of the outbreak, has been locked down since Jan 23, with 16 of its neighbouring cities in Hubei province included behind the cordon sanitaire shortly thereafter. The national Spring Festival holiday was extended by 8 days to Feb 7, and most schools have remained closed to date. As the Spring Festival holiday ended, stringent social distancing measures and mobility restrictions were coordinated and implemented by the central and local governments in many Chinese megacities (ie, the largest and wealthiest; figure 1), including Beijing (north of Wuhan), Guangzhou and Shenzhen (south), Shanghai and Hangzhou (east), and Chengdu (west). For example, only residents were allowed to enter residential communities, face mask-wearing was made compulsory, and non-essential community services were shut down. Although the aggressive countermeasures appear to have reduced the number of reported cases, the absence of herd immunity against COVID-19 suggests that counts could easily resurge when these interventions are relaxed, as business, factory operations, and schools resume.
Figure 1.
Timeline of events between Jan 23 and Feb 17, 2020
(A) The number of confirmed cases reported outside Hubei province in mainland China. (B) Cities in mainland China that have implemented any lockdowns by Feb 10, and cities (blue) and provinces (green) included in the estimation of the instantaneous reproduction number (Rt). Semi-lockdown was defined as public health control measures that did not quite reach the intensity and comprehensiveness as those implemented in Wuhan. Complete lockdown was defined as measures that were comparable to those implemented in Wuhan (since Jan 23). Wartime measures was defined as residents being unable to get in or out of buildings that come under full closed management.1 Islands in the South China Sea are not shown (appendix p 15).
We have provided an assessment of the transmissibility and severity of COVID-19 in mainland Chinese cities and provinces outside Wuhan and Hubei. We have estimated the instantaneous effective reproduction number (R t) of COVID-19 in Beijing, Shanghai, Shenzhen, and Wenzhou, as well as the ten provinces that have reported the highest number of confirmed cases outside Hubei; namely, Anhui, Chongqing, Guangdong, Henan, Hunan, Jiangsu, Jiangxi, Shandong, Sichuan, and Zhejiang. Additionally, we have estimated the confirmed case-fatality risk (cCFR; the probability of dying among confirmed cases of COVID-19 as officially reported) in these same locations.
The totality of massive social distancing and population behavioural change interventions has huge costs, in terms of both actual expenses and economic opportunity loss, in addition to having traded away individual freedoms and sustained severe societal disruptions.2 Therefore, on the one hand, since Feb 17, the COVID-19 response levels have been progressively relaxed in several provinces, and more than half of the major industrial enterprises in Guangdong, Jiangsu, Zhejiang, and Shanghai have begun to resume operation.3 As of March 16, the epicentres of Wuhan and Hubei began to lift restrictions. On the other hand, COVID-19 has spread to more than 100 countries or regions, and local epidemics have been established in multiple countries, including the USA and most countries in Europe. On March 10, Italy, the worst-hit country outside China, with more than 10 000 cases, imposed a nationwide lockdown, which is expected to be in place until at least early April. Most countries in Europe have imposed stringent control measures to limit social contacts. As COVID-19 continues to spread globally, escalating case importation from overseas or residual infected seeds within China (despite the almost 2-month-long containment policy nationwide), coupled with the resumption of economic activities, a second wave of COVID-19 appears probable. Thus, we simulated the potential consequences of relaxing restrictions in anticipation of a recrudescence of infections.
Research in context.
Evidence before this study
Since Jan 23, 2020, massive public health interventions have been implemented across China to contain the spread of coronavirus disease 2019 (COVID-19). Wuhan has been locked down since Jan 23, 2020, with 16 of its neighbouring cities in Hubei province subsequently included behind the cordon sanitaire shortly thereafter. The national Spring Festival holiday was extended by 8 days to Feb 7, and, to date, most schools have remained closed. Although the aggressive countermeasures appear to have reduced the number of reported cases, the absence of herd immunity against COVID-19 suggests that case counts could easily resurge when these interventions are relaxed, as business, factory operations, and schools resume, given the increasing risk of viral reintroduction, particularly from overseas importation. We searched PubMed and preprint archives for articles published up to March 17, 2020, that contained information about the control measures against COVID-19 outbreak using the terms “coronavirus”, “2019-nCoV”, “COVID-19”, “control measures”, “outside Wuhan and/or Hubei”, and “Chinese New Year”. We found five studies that reported the estimates of the effects of control measures on COVID-19 transmission in Wuhan or Hubei, but no study focused on the regions outside Wuhan or Hubei.
Added value of this study
In regions outside Hubei, the instantaneous reproduction number (R t) of COVID-19 substantially decreased after aggressive control measures were implemented on Jan 23, 2020, and have since remained below 1. Control measures should be relaxed gradually so that the resulting R t would not sustainably exceed 1. Otherwise, the cumulative case count would increase exponentially with the relaxation duration. The confirmed case-fatality risk (cCFR) outside Hubei was 0·98%, but varied substantially among different provinces, probably due to heterogeneity in regional economic development and availability of health-care resources.
Implications of all the available evidence
Although the first wave of COVID-19 in Chinese provinces outside Hubei has abated because of the aggressive non-pharmaceutical interventions, close monitoring of R t and cCFR is needed to inform strategies against a potential second wave, given the increasing risk of viral reintroduction from overseas importation.
Methods
Data
The R t was defined as the average number of secondary cases generated by one primary case with symptom onset on day t. If R t >1 the epidemic is expanding at time t, whereas R t < 1 indicates that the epidemic size is shrinking at time t. Therefore, we identified mainland Chinese cities that had published sufficiently detailed information of the COVID-19 cases confirmed by their local health commission that allowed for estimation of R t—ie, daily number of new imported and local cases by onset date since mid-January, 2020. There were four such cities—Beijing, Shanghai, Shenzhen, and Wenzhou.
As of Feb 29, 2020, there were 411 (Beijing), 337 (Shanghai), 417 (Shenzhen), and 504 (Wenzhou) laboratory-confirmed cases of COVID-19 infections reported. We collated publicly available information of each of these officially confirmed cases to construct the epidemic curve for Beijing4 and Shanghai,5 and a detailed line list for both Shenzhen6 and Wenzhou.7 The National Health Commission definition of a confirmed case is given in the table . We estimated R t and cCFRs in these cities based on the following data sources: (1) for Beijing and Shanghai, the daily number of confirmed cases with and without Wuhan or Hubei travel history were available from the Beijing and Shanghai Municipal Health Commission.4, 5 Dates of symptom onset were also available for 186 (88%) of 212 cases who were reported by Beijing before Feb 3, 2020. (2) For Shenzhen,6 we collected patient-level data, including age, sex, travel history (to Wuhan or Hubei with details), epidemiological links to confirmed cases (with details of their relationships, contact times, and events), residential district, date of exposure (for some cases), presenting symptomatology, dates of symptom onset, admission, confirmation, and discharge. (3) For Wenzhou,7 we were only able to collect patient-level data, including age, sex, travel history (yes or no to Wuhan or Hubei), epidemiological links to confirmed cases (yes or no), residential district, dates of symptom onset, and confirmation. (4) The time between dates of symptom onset (ie, serial interval) of 56 infector–infectee pairs, among which six pairs were from Hubei published by Li and colleagues;8 14 pairs from secondary infections linked to exported cases outside mainland China; two and 23 pairs from the Zhuhai and Shenzhen line lists, respectively; and 11 from a family cluster in Jiangsu, reported by Huang and colleagues9 (appendix pp 7–9). (5) The delay between symptom onset and reporting were calculated for each case in both Shenzhen and Wenzhou. The onset date was not available for 225 (55%) of 411 cases in Beijing and any case in Shanghai. As such, we estimated the onset dates of these cases based on their date of reporting, and assuming that the time between onset and reporting was statistically the same as that of the 186 (45%) of 411 cases in Beijing whose onset dates were known. We used the date when each case was reported on the website of local health commissions as the reporting date. The empirical mean time from symptom onset to reporting was 4·9 days (SD 3·3) for Beijing, 7·6 days (4·2) for Shenzhen, and 6·3 days (4·4) for Wenzhou. (6) The time between onset and death or the time between admission and death for 41 cases in Wuhan (appendix p 10).10
Table.
Case definition of COVID-19 outside Hubei province
| Version 1 (Jan 16, 2020) | Version 2 (Jan 22, 2020) | Version 3 (Jan 23, 2020) | Version 4 (Jan 27, 2020) | Version 5*(Feb 4, 2020) | Version 6 (Feb 19, 2020) and 7 (Mar 4, 2020) | |
|---|---|---|---|---|---|---|
| Clinical criteria | ||||||
| 1 | Fever | Fever | Fever | Fever | Fever or symptoms of respiratory infections, or both | Fever or symptoms of respiratory infections, or both |
| 2 | Radiographic evidence of pneumonia-like patterns, such as areas of consolidation and ground-glass opacity with bilateral peripheral involvement | Radiographic evidence of pneumonia-like patterns, such as areas of consolidation and ground-glass opacity with bilateral peripheral involvement | Radiographic evidence of pneumonia-like patterns, such as areas of consolidation and ground-glass opacity with bilateral peripheral involvement | Radiographic evidence of pneumonia-like patterns, such as areas of consolidation and ground-glass opacity with bilateral peripheral involvement | Radiographic evidence of pneumonia-like patterns, such as areas of consolidation and ground-glass opacity with bilateral peripheral involvement | Radiographic evidence of pneumonia-like patterns, such as areas of consolidation and ground-glass opacity with bilateral peripheral involvement |
| 3 | Low or normal white blood cell count, or low lymphocyte count | Low or normal white blood cell count, or low lymphocyte count | Low or normal white blood cell count, or low lymphocyte count | Low or normal white blood cell count, or low lymphocyte count | Low or normal white blood cell count, or low lymphocyte count | Low or normal white blood cell count, or low lymphocyte count |
| 4 | Antibiotic treatment for 3 days without improvement | .. | .. | .. | .. | .. |
| Epidemiological criteria | ||||||
| 1 | Living in Wuhan or travel history to Wuhan, or direct or indirect exposure to a wet market in Wuhan within 14 days before symptom onset | Living in Wuhan or travel history to Wuhan within 14 days before symptom onset | Living in Wuhan or travel history to Wuhan within 14 days before symptom onset | Living in affected regions (ie, Wuhan or regions with sustained local transmission) or travel history to affected regions within 14 days before symptom onset | Living in affected regions (ie, with any confirmed case) or travel history to affected regions within 14 days before symptom onset | Living in affected regions (ie, with any confirmed case) or travel history to affected regions within 14 days before symptom onset |
| 2 | .. | .. | .. | A link to any confirmed infections | A link to any confirmed infections (ie, positive result from testing of viral RNA) | A link to any confirmed infections (ie, positive result from testing of viral RNA) |
| 3 | .. | Contact with patients with fever and symptoms of respiratory infection from Wuhan within 14 days before symptom onset | Contact with patients with fever and symptoms of respiratory infection from Wuhan within 14 days before symptom onset | Contact with patients with fever and symptoms of respiratory infection from affected regions within 14 days before symptom onset | Contact with patients with fever and symptoms of respiratory infection from affected regions within 14 days before symptom onset | Contact with patients with fever and symptoms of respiratory infection from affected regions within 14 days before symptom onset |
| 4 | .. | .. | Link to any clusters of suspected cases | Link to any clusters of suspected cases | Link to any clusters of suspected cases | Link to any clusters of suspected cases |
| Case confirmation criteria | ||||||
| 1 | Respiratory sample positive by whole-genome sequencing | Respiratory sample positive by sequencing | Respiratory sample positive by sequencing | Respiratory or blood sample positive by sequencing | Respiratory or blood sample positive by sequencing | Respiratory or blood sample positive by sequencing |
| 2 | .. | Respiratory sample positive by RT-PCR | Respiratory sample positive by RT-PCR | Respiratory or blood sample positive by RT-PCR | Respiratory or blood sample positive by RT-PCR | Respiratory or blood sample positive by RT-PCR |
Using the National Health Commission definition, a suspected case was defined as a case that meets three clinical criteria, or two clinical criteria and one epidemiological criterion. A confirmed case was defined as a suspected case that meets one or more case confirmation criteria. COVID-19=coronavirus disease 2019.
In version 5 of the case definition, a confirmed case was defined as a suspected case that met one or more case confirmation criteria; however, between Feb 4 and Feb 18, when version 5 was adopted, a separate category, called clinically confirmed case, applied to cases in Hubei only—ie, defined as a suspected case with radiographic evidence of pneumonia-like pattern, such as areas of consolidation and ground-glass opacity with bilateral peripheral involvement. This separate category led to a sharp increase in the number of confirmed cases in Hubei; however, this change in case definition did not affect our analysis of confirmed cases in cities and provinces outside Hubei.
To estimate R t of the ten provinces with the largest numbers of reported confirmed cases and cCFRs among officially confirmed cases for all provinces, we obtained the daily number of confirmed cases and deaths reported in all 31 provinces since Jan 22, 2020.
Estimating the time between onset and key events
We assumed that the cumulative density function of the time between onset and reporting (F onset-to-reporting), and that between onset and death (F onset-to-death), were both gamma (log-normal distribution was considered in the sensitivity analysis but gamma distribution gave a lower Bayesian information criterion). We estimated these distributions from the data in sources (5) and (6) using a Bayesian approach with non-informative priors in Markov chain Monte Carlo,11 implemented using MATLAB, version 2019b.
Estimating the epidemic curves and Rt
We constructed the epidemic curves of the four cities. First, we plotted the (unadjusted) epidemic curves by date of onset of COVID-19 symptoms, and stratified by whether the cases reported travel history to Hubei (figure 2 ). Because the data of the time between onset and reporting were not available for the ten selected provinces, we assumed that such delay was statistically the same as that in Beijing, which was consistent with the time from onset to confirmation reported in the WHO–China Joint Mission Report.12 To account for the people who had already developed symptoms, but had not yet reported (and thus were not included in the unadjusted epidemic curves), we randomly selected 1000 samples of F onset-to-reporting from its posterior distribution (using the function sample in R with replacement) to infer the true epidemic curves (figure 2) by using the following equation to account for the delay between symptom onset and reporting:
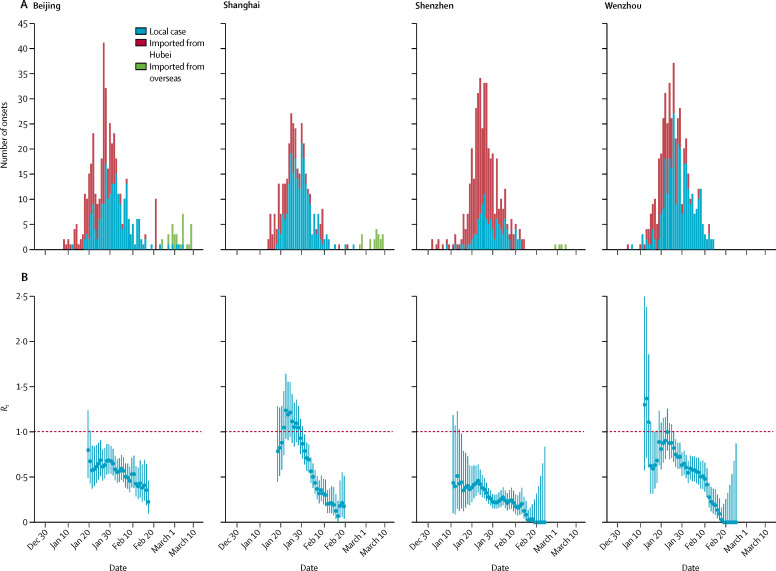
Figure 2.
Estimates of Rt in Beijing, Shanghai, Shenzhen, and Wenzhou
(A) The daily number of symptom onsets in Beijing (411 cases), Shanghai (337 cases), Shenzhen (417 cases), and Wenzhou (504 cases), stratified by local cases (blue), imported cases from Wuhan or Hubei (red). The epidemic curves were estimated from cases reported on or before Feb 29, 2020. The daily number of symptom onsets observed or estimated from reported cases between Feb 29 and March 16, are shown, but not included in the analysis. Imported cases from overseas were reported in Beijing, Shanghai, and Shenzhen since March 1 (green). The date of symptom onset was available for 186 of 212 cases who were reported in Beijing on or before Feb 2, 2020, and for each case in Shenzhen and Wenzhou. The date of symptom onset was not available for the remaining 225 cases in Beijing, and all cases in Shanghai. Therefore, we estimated the date of onset for the 225 cases in Beijing and all cases in Shanghai based on their date of reporting and Beijing's distribution of the time between onset and reporting (which was estimated from the 186 cases reported by Feb 2, in Beijing). (B) The estimates of Rt by date of symptom onset on sliding weekly windows between late January, and Feb 19, 2020, for Beijing and Shanghai, and between mid-January, and Feb 25, 2020, for Shenzhen and Wenzhou (eg, the estimate on Feb 25 was for the week of Feb 22–28). We estimated Rt until Feb 19, because few cases reported in the week of Feb 22–28, and the estimation of the onset dates of these cases was not accurate. Dots show the posterior mean and bars show 95% credible intervals. Rt=instantaneous effective reproduction number.
where n onset(t – a) is the number of onsets at time (t – a) and n report(t – a,t) is the number of onsets at time (t – a) that have been reported by t.
Because travel history information for cases in the ten selected provinces was not publicly available, we assumed that their daily proportion of imported cases from Hubei was similar to each of the four cities (ie, four scenarios for each of the ten provinces; figure 3 and appendix pp 1–2). We applied the method developed by Wallinga and Teunis13 to estimate R t of each inferred epidemic curve between mid-January and Feb 19, using the R package EpiEstim, version 2.2-1.14, 15
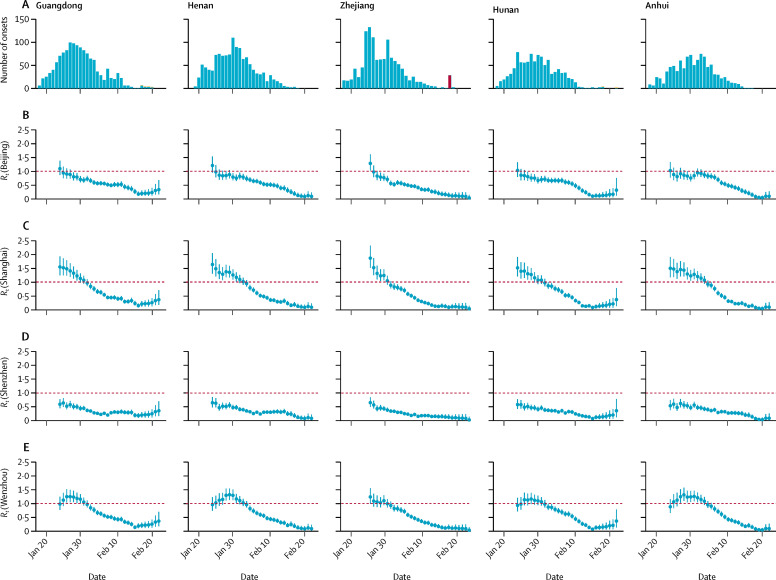
Figure 3.
Estimates of Rt of Guangdong, Henan, Zhejiang, Hunan, and Anhui
(A) The epidemic curves by estimated date of illness onset stratified by reported cases (blue) and estimated cases not reported yet due to the time delay between onset and reporting (yellow). We assumed the distribution of the time between onset and reporting in all provinces was the same as Beijing, with a mean of 4·9 days. The epidemic curves were estimated from cases reported on or before Feb 29, 2020. The Shilifeng prison cluster (red) reported on Feb 21 in Zhejiang was not included in the Rt estimation. (B–E) The estimates of Rt assuming the daily proportion of imported cases from Hubei was the same as Beijing, Shanghai, Shenzhen, and Wenzhou.
Estimating the cCFR
We estimated the cCFR as the ratio of laboratory-confirmed deaths to officially confirmed cases in provinces outside Hubei, adjusting for the time between onset and death.16 We randomly selected 1000 samples of F onset-to-death from its posterior distribution and estimated the number of deaths by onset dates using the following equation:
where d onset(t – a) is the number of onsets at (t – a) who would die of COVID-19 eventually and d death(t – a,t) is the number of onsets at (t – a) that have died by t. The cCFR was estimated by the estimator:
The CI of cCFR was obtained using boot.ci function in the R package boot, version 1.3-24.
Simulating the potential effect of relaxing interventions
We used the following susceptible–infectious–recovered model (appendix p 3) to assess the effect of relaxing non-pharmaceutical and social distancing interventions after the epidemic has been initially brought under control but not eliminated. Stage 1, interventions were implemented, such that R t=1. Stage 2, interventions were relaxed, resulting in R t=R 2>1 when stage 2 began. Stage 3, interventions from stage 1 were again implemented, such that R t=1. Stage 4, interventions more aggressive than those in stages 1 and 3 (when R t=R 4<1 when stage 4 began) were implemented to bring the disease prevalence back to pre-relaxation level (ie, stage 1 level).
Let T i be the duration of stage i and X the cumulative case count by the end of stage 4. We hypothesised that X increased exponentially with T 2 (the duration of intervention relaxation)—ie, health loss; and T 4 (the time required for the prevalence to reach pre-relaxation level during stage 4) was always larger than T 2—ie, economic loss. We tested our hypothesis by examining the simulated values of X and T 4 /T 2 for R 2 at 2·5, 2, and 1·5 (the plausible ranges for COVID-1917, 18) and R 4 at 0·8, 0·5, 0·2, and 0·1, assuming that the mean generation time was 7 days.18, 19 The simulation was done in MATLAB 2019b.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
The inferred epidemic curves and the corresponding estimates of R t on weekly windows between early or mid-January and late February, 2020, for Beijing, Shanghai, Shenzhen, and Wenzhou are shown in figure 2. The same data are shown for the ten selected provinces outside Hubei. In most provinces, the posterior mean of R t gradually decreased from Jan 26 until Feb 7 (figure 3; appendix pp 1–2), which was the weekend before some enterprises and factories began to progressively resume operation on Feb 10 (figure 1), and coastal provinces such as Zhejiang and Guangdong started to have an increased influx of returning migrant workers. R t remained lower than 1 for most provinces up to the week of Feb 19–25, except for Sichuan, which had a slower reduction in the number of reported cases than the other provinces.
More detailed analysis of the four major cities suggested the transmission of COVID-19 was mainly driven by imported cases from Hubei until late January. The posterior mean of R t was greater than 1 in mid-January in Wenzhou, and in late January in Shanghai. R t decreased substantially in Shanghai from the first week of the Spring Festival (figure 1). As one of the cities that is not a provincial capital, but has the highest volume of mobility with Wuhan,17, 20 Wenzhou had the earliest imported cases, as expected. Symptom onset of its first imported case was as early as Dec 24, 2019. Wenzhou was also the first city outside Hubei to be locked down. R t remained lower than 1 for both Beijing and Shenzhen, after discounting the large proportion of imported cases from Hubei, suggesting local transmission of COVID-19 has not been sustained, despite the early importation from Wuhan. The onset date of Shenzhen's first imported case was Jan 1; this case belonged to the first family cluster reported in Guangdong province.6, 21
In all four selected cities, the posterior mean of R t decreased substantially, after massive control measures were implemented on Jan 23, and R t remained lower than 1 after the Spring Festival holidays had ended. Although Beijing, Shanghai, and Wenzhou had few importations from Hubei during the Spring Festival, Shenzhen had a considerable proportion of imported cases from Hubei, even 2 weeks after Wuhan's lockdown. However, from Feb 7 onward, all four cities began to report imported cases from Hubei again, suggesting it might be a challenge to maintain subthreshold R t when economic activities fully resume in the presence of exponentially increasing case importation from locations with ongoing community transmission of COVID-19 (eg, Hubei domestically, and Italy, South Korea, or Iran internationally). The cumulative number of importations from overseas increased from 20 to 353 between March 4 and March 22; most of these importations were reported by megacities such as Beijing, Shanghai, Hangzhou, Guangzhou, and Shenzhen. Containment measures based on isolation and contact tracing will eventually fail if case exportation continues to grow exponentially, which seems probable given that local transmission has already been established in many large countries, including the USA and most European countries.
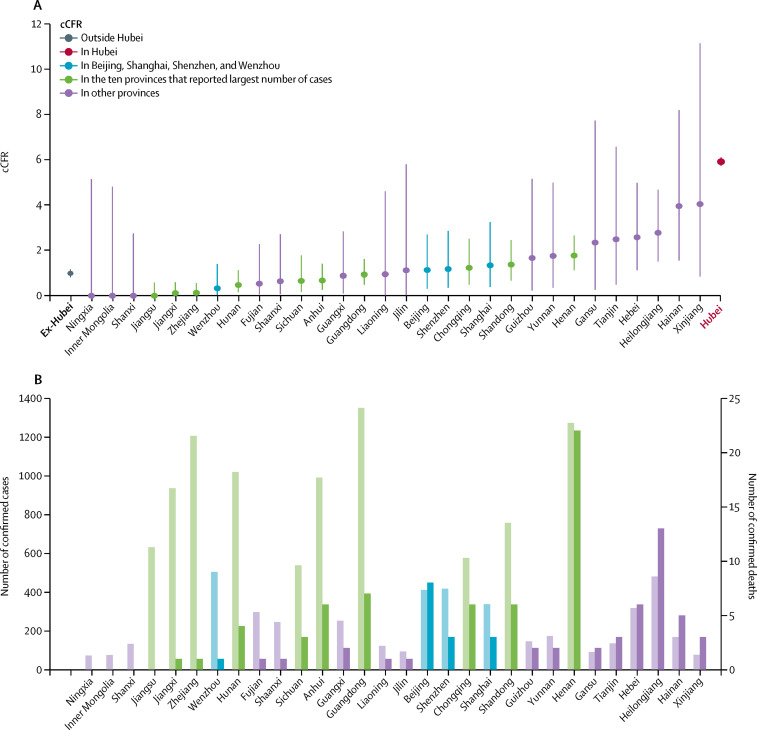
We estimated that cCFR among officially confirmed cases outside Hubei was 0·98% (0·82–1·16), ranging from 0·00% (0·00–0·58) in Jiangsu to 1·76% (1·11–2·65) in Henan among the ten provinces that reported the largest number of confirmed cases (figure 4 ). For reference, Hubei's cCFR was 5·91% (5·73–6·09). Given the intensive, proactive case finding and the sharp drop in reported cases outside Hubei during February, 2020, the ascertainment rates in these cities were probably very high. As such, we posit that cCFR in areas outside Hubei should be roughly comparable to the symptomatic CFR (the probability of dying after developing symptoms) in Wuhan (estimated to be 1·4% [0·9–2·1] in a separate analysis19) after adjusting for the respective age distributions of the reported cases in the different cities and provinces (which was older in Hubei and therefore associated with higher COVID-19 fatality).
Figure 4.
cCFRs in Beijing, Shanghai, Shenzhen, and Wenzhou and in provinces outside Hubei
cCFR in Qinghai and Tibet were not shown because the 95% CIs were wide due to the small number of confirmed cases. cCFR=confirmed case-fatality risk. Ex-Hubei=all provinces outside Hubei.
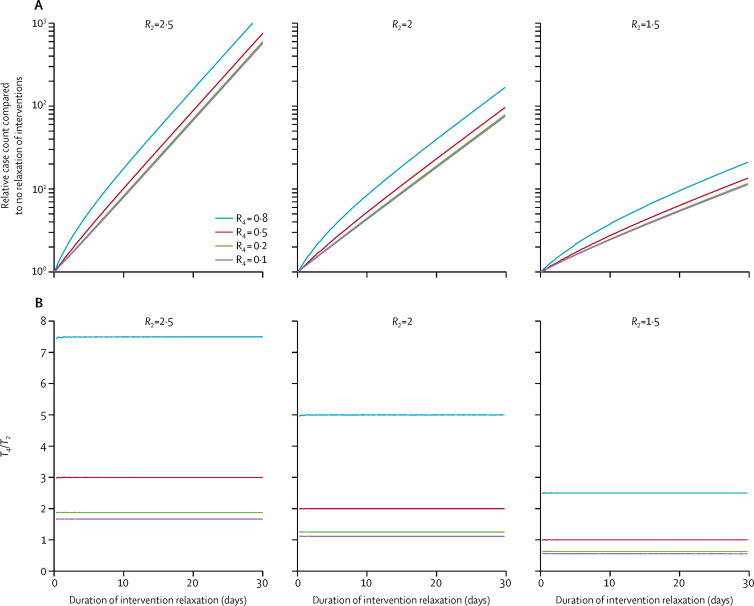
Figure 5 indicates that relaxation of intervention increased the cumulative case count exponentially with the duration of relaxation, even if aggressive interventions could subsequently push prevalence back to pre-relaxation level; and the required duration of such interventions would always be longer than the duration of intervention relaxation unless R t was below 1·5 during intervention relaxation, and could be pushed below 0·5 during stage 4. Taken together, these results suggest that allowing R t to rise above 1 when the epidemic size was still small (ie, no herd immunity) would likely incur both health and economic loss even if aggressive interventions could push the prevalence back to pre-relaxation level afterwards.
Figure 5.
The effect of relaxation of interventions for different scenarios of reproduction numbers
R2 and R4 refer to the reproduction number when stage 2 and 4 began, respectively. (A) Relative case count compared with no relaxation of interventions. (B) The duration of aggressive interventions required to push prevalence back to pre-relaxation level (T4) relative to the duration of interventions relaxation (T2).
Discussion
The findings from our modelling impact assessment suggest that the comprehensive package of non-pharmaceutical interventions China undertook, including social distancing and population behavioural change, has substantially reduced transmissibility of COVID-19 across the country. The daily number of local COVID-19 cases has dropped substantially to nearly zero in areas outside Hubei since late February; however, a second wave of COVID-19 transmission is possible because of viral reintroduction (particularly international importation—eg, from Italy or elsewhere in Europe, Iran, USA, and other rapidly burgeoning secondary epicentres22) that has been exponentially increasing since March, 2020, as well as viral transmissibility that might rebound with the gradual resumption of economic activities, and thus normal levels of social mixing. Close monitoring of the instantaneous effective reproduction number and real-time tuning of policy interventions to ensure a manageable second wave remains the over-riding public health priority.
Early detection of cases is essential. Guangdong province did more than 320 000 RT-PCR tests on those who had attended fever clinics and hospitals over 30 days between January and February, 2020, which was about ten times baseline testing capacity for routine influenza-like illness surveillance during the influenza season of 2018.23 Such a level of testing should be maintained, if not increased, to monitor the real-time point prevalence of COVID-19, so that any possible reintroduction of infected cases could be swiftly identified and isolated, and their contacts traced and quarantined.
Our schematic scenario simulated how control measures could be tuned to minimise the total infected burden, thus corollary adverse health, and social and economic effects, by maintaining the effective reproduction number below the self-sustaining threshold of 1. Our results highlight that as the number of cases would progressively rise when R t > 1, simply tightening control interventions again to maintain R t =1 would not reduce the burden back to its original baseline. Once elevated at the higher level, it could only be pushed back down to the original baseline by extra effort, by driving R t down below unity. Therefore, relaxation of interventions to allow R t > 1 would probably incur both marginally higher health and economic loss, even if the disease prevalence could subsequently be pushed back to pre-relaxation level. Thus, proactively striking a balance between resuming economic activities and keeping R t below 1 is likely to be the optimal strategy until effective vaccines become widely available, despite the fact that control policies, including social distancing, behavioural change, and public awareness, will probably be maintained for some time.24
China could adopt a reinforcement learning approach to achieve such a delicate balance (namely, maximising economic productivity under the R t ≤ 1 constraint) by continuous surveillance of the effect of social mixing and human mobility on the reproduction number of COVID-19 and then tune socioeconomic activities accordingly. In the conventional framework of inferring R t from epidemic curve data (as we have done), the lead time between actual changes in R t and the detection of such changes is roughly equal to the sum of the incubation period and reporting delay (although the latter could be adjusted for, as we have done). We postulate that such lead time might be shortened if reliable analytics could be developed to predict R t from digital proxies for social mixing and human mobility. Specifically, the resumption of economic activities and normal societal functions can be digitally monitored in various ways by the cloud platforms of Alibaba, Baidu, and Tencent.25 As of March 10, the Alipay and WeChat Health Code system had been used more than 1·6 billion times, and its usage patterns reflect the level of economic activities in real time.26 The decline in R t in the four selected cities correlated with the decrease in intracity traffic volumes recorded by Baidu (appendix p 6). Using the intracity traffic volume on Jan 1–15 as the index, to date, the level of economic activities of the four selected cities have been at half the normal pre-outbreak levels. Closely monitoring R t and such digital indices could enable finetuning of control measures to effectively minimise the potential adverse effects of a possible second wave of infections. Given the pivotal role of R t in epidemic control, we suggest that real-time estimates of R t should be presented in the routine COVID-19 dashboards and situation reports for provincial, national, and supranational health agencies.
Keeping close watch of real-time transmissibility will also help to ensure the infection prevalence does not exceed the surge capacity of the health system. For example, even in the most prosperous and well resourced megacities such as Beijing, Shanghai, and Shenzhen, with 5·7, 6·0, and 3·7 hospital beds per 1000 population, respectively, health-care resources are finite.27 If 20% of COVID-19 cases were to require hospital admission28 and infection prevalence was 0·5% at any single point in time, Shenzhen would be required to deploy 27% of its total number of hospital beds. The Chinese health-care system still relies heavily on secondary and tertiary care,29, 30 and hospitals overcrowded with patients with COVID-19 could displace care rendered to other patients who have non-COVID-19 health-care needs. This situation has already been observed in Wuhan and other cities in Hubei.31
We also found that fatality risks, among officially confirmed cases that are adjusted for resolution ascertainment bias, vary by several folds between provinces. The cCFR was relatively low in south and east China, and substantially higher in the north and northwest, which also correlates with provincial per capita gross domestic product (appendix pp 4, 11). Prosperous regions, such as Jiangsu and Zhejiang, had the lowest cCFRs among provinces that had more than 500 confirmed cases, whereas less developed regions, such as Henan, Hebei, and Heilongjiang, had the highest cCFRs. Guangdong, the richest province in the country, has the largest number of migrant workers in China and thus a high proportion of imported cases of COVID-19 from Hubei.12 Guangdong's health-care system, with the second lowest number of hospital beds per 10 000 population, sustained an enormous stress to its surge capacity, which is perhaps reflected by its mid-level cCFR ranking. Similarly, for megacities such as Beijing, Shanghai, Chongqing, and Shenzhen, the cCFRs ranked in the middle, despite their economic prosperity and relatively robust health-care infrastructure (appendix pp 5, 11). Although we did not have data on the proportion of hospital beds occupied during the first wave, given that there were fewer than 1000 confirmed cases in most cities and provinces (compared with approximately 123 600, 139 000, and 45 700 hospital beds overall in Beijing, Shanghai, and Shenzhen, respectively), it was unlikely that the additional burden could have been overwhelming for the health systems outside Hubei.
Several limitations should be noted. First, the estimation of R t was based on the reported number of confirmed cases. Although there might be less under-reporting and ascertainment bias in provinces outside Hubei, our work shows the fundamental importance of a regularly (preferably daily) updated and sufficiently detailed line list. Second, the estimation of R t relied on an accurate estimate of the serial interval distribution, where nearly half of the publicly available paired infector–infectee data points are currently derived from Shenzhen (appendix pp 7–9). Third, travel history or epidemiological links to Hubei were another essential element, because the proportion of imported cases varied over time and across locations. Although most cases in Wenzhou were local cases since Feb 4, the majority of confirmed cases in Shenzhen consisted mostly of imported cases from Hubei even up until Feb 12—more than 2 weeks after the Wuhan lockdown on Jan 23. Fourth, the simulations of intervention relaxation were done for a limited number of illustrative scenarios regarding temporal changes in the reproduction number. We did not attempt to relate which interventions and public responses to the epidemic might correspond to each of these scenarios.
We found that at least 235 (56%) of 417 cases reported in Shenzhen were from clusters, among which more than 80% were household clusters. The proportion was even higher if we considered that some imported cases formed clusters with their close contacts in Hubei (although such information was missing in the Shenzhen line list). Further investigation of the transmission dynamics within households would require detailed data on household composition and demographics of household members,32, 33, 34 importantly including healthy members who were not infected.21
We found that R t estimates were difficult to interpret if we included the two potential superspreading events in the prisons for the R t estimation in Zhejiang and Shandong (figure 3; appendix pp 1–2). However, the number of superspreading events is increasing as COVID-19 spreads outside mainland China; for example, in places of worship in Hong Kong and Daegu of South Korea, in a mental health facility in Daegu, and on the Diamond Princess cruise ship in Japan. A comprehensive database of all confirmed and probable cases in each superspreading event cluster will be essential to understand the transmission of superspreading events. Combining information on COVID-19 transmission in the superspreading events, households, and community would enable better understanding of the overall R t estimates, which would further enhance the effectiveness of control measures, if they are accordingly and precisely designed.
In conclusion, the interventions China implemented in response to the COVID-19 outbreak had a real and dramatic effect on interrupting transmission in all areas outside of Hubei. As economic activity continues to resume in the coming weeks, real-time assessment by monitoring the instantaneous effective reproduction number could allow policy makers to tune relaxation decisions to maintain transmissibility to below the self-sustaining threshold of 1. CFRs vary between provinces, which might be determined by health-care availability, quality, and surge capacity. Therefore, health services planning should be optimised to minimise mortality related to COVID-19.
Acknowledgments
Acknowledgments
We thank Miky Wong and Chi-Kin Lam from the School of Public Health, The University of Hong Kong (Hong Kong, China), for technical support. This research was supported by a commissioned grant from the Health and Medical Research Fund from the Government of Hong Kong.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
JTW, GML, and KL designed the experiments. DL and KL collected data. JTW and KL analysed data. KL, JTW, and GML interpreted the results and wrote the Article.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Cao Y, Zhou L. Hubei district launches wartime control order to better fight virus. Feb 13, 2020. http://www.chinadaily.com.cn/a/202002/13/WS5e44ba07a310128217277470.html
- 2.The Economist . The Economist; Feb 15 2020. How China's coronavirus epidemic could hurt the world economy.https://www.economist.com/leaders/2020/02/13/how-chinas-coronavirus-epidemic-could-hurt-the-world-economy [Google Scholar]
- 3.Caixin . Caixin; Feb 19 2020. China gradually gets back to work in face of worker, material shortages.https://www.caixinglobal.com/2020-02-19/coronavirus-wednesday-update-death-toll-passes-2000-as-russia-announces-ban-on-chinese-citizens-101517506.html [Google Scholar]
- 4.Beijing Municipal Health Commission Daily briefing on the prevention and control of the transmission of the novel coronavirus in Beijing. 2020. http://wjw.beijing.gov.cn/
- 5.Shanghai Municipal Health Commission Press release on the prevention and control of the novel coronavirus in Shanghai. 2020. http://wsjkw.sh.gov.cn/ (in Chinese).
- 6.Shenzhen Municipal Health Commission Confirmed cases of the novel coronavirus pneumonia in Shenzhen. 2020. http://wjw.sz.gov.cn/ (in Chinese).
- 7.Health Commission of Wenzhou Outbreak of novel coronavirus pneumonia in Wenzhou (daily updates) 2020. http://wjw.wenzhou.gov.cn/ (in Chinese).
- 8.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang R, Xia J, Chen Y, Shan C, Wu C. A family cluster of SARS-CoV-2 infection involving 11 patients in Nanjing, China. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30147-X. published online Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Data Platform of Shanghai Observer Line list of 2019-nCoV confirmed fatal cases (from publicly available information) 2020. http://data.shobserver.com/www/index.html#/home
- 11.Gilks WR, Richardson S, Spiegelhalter D. Chapman and Hall/CRC; London: 1995. Markov chain Monte Carlo in practice. [Google Scholar]
- 12.WHO . World Health Organization; Geneva: 2020. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19), 16–24 February, 2020.https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf [Google Scholar]
- 13.Wallinga J, Teunis P. Different epidemic curves for severe acute respiratory syndrome reveal similar impacts of control measures. Am J Epidemiol. 2004;160:509–516. doi: 10.1093/aje/kwh255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cori A, Ferguson NM, Fraser C, Cauchemez S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am J Epidemiol. 2013;178:1505–1512. doi: 10.1093/aje/kwt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson RN, Stockwin JE, van Gaalen RD, et al. Improved inference of time-varying reproduction numbers during infectious disease outbreaks. Epidemics. 2019;29 doi: 10.1016/j.epidem.2019.100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atkins KE, Wenzel NS, Ndeffo-Mbah M, Altice FL, Townsend JP, Galvani AP. Under-reporting and estimates for emerging epidemics. BMJ. 2015;350 doi: 10.1136/bmj.h1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kucharski AJ, Russell TW, Diamond C, et al. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30144-4. published online March 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu JT, Leung K, Bushman M, et al. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med. 2020 doi: 10.1038/s41591-020-0822-7. published online March 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhanwei D, Lin W, Simon C, et al. Risk for transportation of 2019 novel coronavirus disease from Wuhan to other cities in China. Emerging Infect Dis J. 2020;26:5. doi: 10.3201/eid2605.200146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centre for Systems Science Engineering at Johns Hopkins Coronavirus COVID-19 global cases by the Centre for Systems Science Engineering (CSSE) at Johns Hopkins. 2020. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- 23.Kang M, Tan X, Yang Y, Wu J, Zheng H, Song T. Epidemiological characteristics of influenza in Guangdong province, during winter of 2017–2018. Zhonghua Liu Xing Bing Xue Za Zhi. 2018;39:1071–1076. doi: 10.3760/cma.j.issn.0254-6450.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Xu W, Paris C, Reeson A, Li X. Social distance and SARS memory: impact on the public awareness of 2019 novel coronavirus (COVID-19) outbreak. medRxiv. 2020 doi: 10.1101/2020.03.11.20033688. published online March 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.China Academy of Information and Communications Technology Research report of the application of data and intelligence in the prevention and control of the COVID-19 epidemic. 2020. http://www.caict.ac.cn/kxyj/qwfb/ztbg/202003/P020200305495005485729.pdf
- 26.Mozur P, Zhong R, Krolik A. In coronavirus fight, China gives citizens a color code, with red flags. The New York Times. March 1, 2020 https://www.nytimes.com/2020/03/01/business/china-coronavirus-surveillance.html [Google Scholar]
- 27.National Bureau of Statistics of China National data. 2020. http://data.stats.gov.cn/english/
- 28.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China. China CDC Weekly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 29.Meng Q, Mills A, Wang L, Han Q. What can we learn from China's health system reform? BMJ. 2019;365 doi: 10.1136/bmj.l2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Lu J, Hu S, et al. The primary health-care system in China. Lancet. 2017;390:2584–2594. doi: 10.1016/S0140-6736(17)33109-4. [DOI] [PubMed] [Google Scholar]
- 31.Bloomberg Hospitals in China, overwhelmed by coronavirus, turn away patients with other pressing needs. Time. Feb 20, 2020 https://time.com/5788495/china-hospital-shortage/ [Google Scholar]
- 32.Cauchemez S, Donnelly CA, Reed C, et al. Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N Engl J Med. 2009;361:2619–2627. doi: 10.1056/NEJMoa0905498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cauchemez S, Fraser C, Van Kerkhove MD, et al. Middle East respiratory syndrome coronavirus: quantification of the extent of the epidemic, surveillance biases, and transmissibility. Lancet Infect Dis. 2014;14:50–56. doi: 10.1016/S1473-3099(13)70304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cauchemez S, Nouvellet P, Cori A, et al. Unraveling the drivers of MERS-CoV transmission. Proc Natl Acad Sci USA. 2016;113:9081–9086. doi: 10.1073/pnas.1519235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.