Abstract
Background
A rapidly expanding pandemic of the new coronavirus has become the focus of global scientific attention. Data are lacking on the impact of the pandemic caused by the severe acute respiratory syndrome coronavirus 2 on health-related quality of life among patients affected by primary antibody deficiencies (PADs).
Objective
To identify factors impacting the health-related-quality of life (HRQOL) among Italian patients affected by PADs switched to remote assistance at the time of the coronavirus disease 2019 pandemic.
Methods
The quality of life was surveyed in 158 patients with PADs by the Common Variable Immune Deficiency Quality of Life questionnaire, a disease-specific tool, and by the 12-item General Health Questionnaire, a generic tool to assess the risk of anxiety/depression. Since the beginning of the coronavirus disease 2019 epidemic, we shifted all patients with PADs to home therapy, and activated remote visits. Questionnaires were sent by email 4 weeks later. Common Variable Immune Deficiency Quality of Life questionnaire and 12-item General Health Questionnaire data scores were compared with the same set of data from a survey done in 2017.
Results
Of 210 patients, 158 (75%) agreed to participate. The quality of life was worse in the group of patients who were at risk of anxiety/depression at the study time. HRQOL was similar in patients forced to shift from hospital-based to home-based immunoglobulin treatment and in patients who continued their usual home-based replacement. The risk of anxiety/depression is associated with pandemia caused by the severe acute respiratory syndrome coronavirus 2 and with patients' fragility, and not with related clinical conditions associated with common variable immune deficiencies. Anxiety about running out of medications is a major new issue.
Conclusions
The coronavirus disease 2019 epidemic impacted HRQOL and the risk of anxiety/depression of patients with PADs. The remote assistance program was a useful possibility to limit personal contacts without influencing the HRQOL.
Key words: Common variable immunodeficiencies, Health-related quality of life, General Health Questionnaire, Coronavirus, COVID-19 epidemic, COVID-19, Anxiety, Depression, Remote assistance
Abbreviations used: COVID-19, Coronavirus disease 2019; CVID, Common variable immunodeficiency; CVID_QoL, Common Variable Immune Deficiency Quality of Life; GHQ-12, 12-item General Health Questionnaire; HRQOL, Health-related quality of life; IVIG, Intravenous immunoglobulin; PAD, Primary antibody deficiency; QOL, Quality of life; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SCIG, Subcutaneous immunoglobulin
What is already known about this topic? Patients with primary immune deficiency have a poor health-related quality of life. No data are available on the quality of life during the coronavirus disease 2019 pandemic.
What does this article add to our knowledge? Health-related quality-of-life assessments help to identify major issues and patients at risk of anxiety/depression in the coronavirus disease 2019 pandemic.
How does this study impact current management guidelines? The remote assistance program did not have a negative impact on health-related quality of life and on the state of anxiety/depression of patients with primary immune deficiency.
Introduction
An pandemic caused by a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has recently become the focus of scientific attention.1 Humanity is exceptionally susceptible as reported by the World Health Organization.2 The clinical presentation of coronavirus disease 2019 (COVID-19) ranges from an asymptomatic infection to a severe disease with high mortality rate. Immune-competent individuals might clear the infection, whereas this might not happen in patients affected by primary and secondary immune deficiencies. The vast majority of patients affected by primary antibody deficiencies (PADs) might be protected only by avoiding personal contacts because they have reduced number and/or dysfunction in switched memory B cells producing high-affinity antibodies.3, 4, 5, 6 As a consequence, since the beginning of the COVID-19 epidemic in Italy,7 to minimize the risk of infection, we shifted all patients with PADs attending our centers to home therapy and we activated a service of remote visits. To evaluate the impact of (1) the COVID-19 epidemic and (2) the switch from in-person visits to remote visits on the quality of life (QOL) of patients attending our primary immunodeficiency reference centers, we administered 2 questionnaires, the Common Variable Immune Deficiency Quality of Life (CVID_QoL) questionnaire, a specific tool to evaluate the health-related quality of life (HRQOL) of patients with common variable immunodeficiency (CVID),8 and the 12-item General Health Questionnaire (GHQ-12), a generic tool able to assess the risk of anxiety/depression.9 Both questionnaires were administered 4 weeks after the identification of the first Italian patient with COVID-19.
Methods
Objective, study design, and setting
Objective of the study
The objective of our study was to identify factors impacting the HRQOL among Italian patients affected by PADs switched to remote assistance at the time of COVID-19 pandemic.
Study design
On February 24, 2020, the date of the first detection of the COVID-19 infection in Italy, we modified our strategy of providing assistance to patients with PADs by activating the switch of all patients from hospital- to home-settled therapy and by activating a remote assistance service. Because intravenous immunoglobulin (IVIG) cannot be administered at home in Italy, those patients who were previously receiving IVIG were shifted to home-based treatment with subcutaneous immunoglobulin (SCIG) administration. The transfer to home therapy was completed on March 4 when measures to contain the spread of the infection in Italy became mandatory. The remote assistance service started on February 24, and it is still ongoing. The remote service consists of active telephone contacts by the responsible physician to his or her patient every 4 days. The service is also active 24/7 to receive calls from patients. On March 9, we contacted all patients and we sent them questionnaires on the HRQOL assessment by email. On March 18, we closed the study. Verbal informed consent was obtained from all participants. Eligible patients met the following criteria: (1) having a diagnosis of PAD (CVIDs)4; (2) being already enrolled in the Italian Registry for Primary Antibody Deficiencies10; and (3) having participated in the previous QOL survey in 2017. We excluded (1) subjects with incomplete information and (2) patients who were not able to complete the interview.
Setting
The study was conducted in 2 reference centers for PID care located in an area with a high prevalence of COVID-19 infection (Veneto) and one with a low prevalence (Lazio).
Questionnaires
Common Variable Immune Deficiency Quality of Life questionnaire
The CVID_QoL questionnaire is a self-administered questionnaire developed and validated in Italy in 2016.8 The instrument includes 32 items in a Likert-type or forced-choice format and measures health on 3 multi-item dimensions: emotional functioning, relational functioning, and gastrointestinal and skin symptoms. It includes also a summary measure named global CVID_QoL. The emotional functioning dimension includes 19 items on patient feelings. The relational functioning dimension includes 9 items on patients' relationship with relatives and nonaffected people; the gastrointestinal and skin symptoms domain includes 4 items on complications related to gastrointestinal manifestation or skin disease. Response options are formulated using a 5-point scale, with 0 = never and 4 = always, with higher values indicating increasing disability. The CVID_QoL global score and scores for each dimension are defined as the sum of all scores of each item transformed as a percentage of the maximum possible score.
The 12-item General Health Questionnaire
The GHQ-12 is a self-administered 12-item questionnaire designed to measure psychological distress and to detect current nonpsychotic psychiatric disorders, such as depression and anxiety.9 The reliability and validity of the Italian version have been tested in several diseases, including dermatological conditions. Answers are given on a 4-point scale; for instance, the item “in the last weeks, did you feel under strain?” invites the following answers: “no,” “not more than usual,” “more than usual,” and “much more than usual.” When scored with the binary method (0-0-1-1), the GHQ-12 can be used as a screening tool to detect minor nonpsychotic psychiatric disorders, yielding final scores that range from 0 to 12. Operationally, in our study, patients scoring 4 or more were considered as “GHQ-positive” (GHQ+): at risk of anxiety/depression.
Statistical analysis
Categorical data were expressed as frequencies and percentage, and continuous data were reported as means ± SD. For comparisons of mean percentage in CVID_QoL questionnaire scores and in mean GHQ-12 scores between (1) patients who shifted from hospital setting to home setting, (2) CVID-Lazio and CVID-Veneto, and (3) scores obtained in the 2020 and 2017 surveys, we used independent t test. Categorical variables were compared by Fisher exact test. Analyses were performed using the statistical package Stata 11 (Stata Corp, College Station, Texas). P values less than .05 were considered statistically significant.
Results
Patients
On March 9, 2020, a total of 210 patients received by email 2 self-administered questionnaires, along with a cover letter explaining the purposes of the survey. On March 18, 2020, 158 (75%) individuals completed and returned the questionnaires. The characteristics of patients with PAD are summarized in Table I . The mean age at the study time was 47.3 ± 13.8 years (range, 18-77 years), with a 1:1 female:male ratio. All patients were on immunoglobulin replacement prophylaxis. Of 52 patients who were previously treated in a hospital setting by IVIG administration, 32 were shifted to SCIG and 20 to facilitated SCIG replacement in the home setting. One hundred six patients continued to follow their usual subcutaneous therapy at home. The mean age and sex distribution of patients who shifted from a hospital setting to a home setting was not different from that of patients who were already on replacement therapy at home (Table II ).
Table I.
Characteristics of 158 patients with PADs included in the analysis of HRQOL
| Characteristic | n (%) |
|---|---|
| Age (y), range | |
| 18-35 | 35 (22) |
| 36-50 | 57 (36) |
| 51-65 | 48 (30) |
| >66 | 18 (12) |
| Sex (female) | 79 (50) |
| PID referral center | |
| Lazio | 130 (82) |
| Veneto | 28 (18) |
| Setting (home) | |
| SCIG | 61 (38) |
| Facilitated SCIG | 45 (28) |
| Setting (from hospital to home) | |
| From IVIG to SCIG | 32 (21) |
| From IVIG to facilitated SCIG | 20 (13) |
PID, Primary immune deficiency.
Table II.
Characteristics of patients grouped by immunoglobulin replacement setting
| Characteristic | Forced to shift to home therapy | Home therapy (SCIG) | Home therapy (fascilitated SCIG) | P∗ | P† |
|---|---|---|---|---|---|
| Number | 52 | 61 | 45 | ||
| Age (y), mean ± SD | 49.1 ± 15.4 | 46.3 ± 14.4 | 45.5 ± 11.6 | .965 | .340 |
| Sex (female), n (%) | 26 (50) | 29 (48) | 21 (47) | .584 | .846 |
)yJK, Cleary P, Khaw FM, Lim WS5 11 to 22. Check that the citations and references match.t allowed in the text. a pandemic by th
P value for comparison between the group “forced to shift” and “SCIG.”
P value for comparison between the group “forced to shift” and facilitated SCIG.
GHQ-12 to evaluate the risk of anxiety/depression
All 158 patients completed the GHQ-12. The GHQ-12 assessment showed that 42.3% of patients were at risk of anxiety/depression. GHQ-12 positive and GHQ-12 negative patients had a similar age (48.10 ± 2.01.96 vs 45.876 ± 1.876; P = .697) and sex (M:F ratio: 31:39 vs 39:49; P = .075). The percentage of patients at risk of anxiety/depression was not different in those forced to shift to home-based SCIG treatment in comparison to those who continued their usual home treatment (43% vs 38%; P = .610).
CVID_QoL questionnaire to evaluate HRQOL
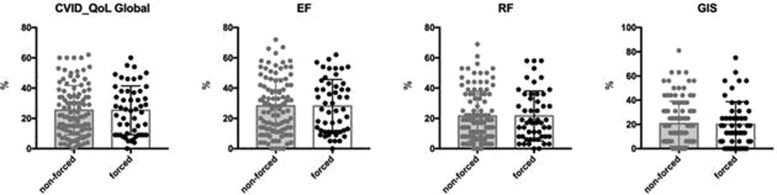
All 158 patients completed the CVID_QoL questionnaire. As previously described,11 woman had a low score in Global CVID_QoL. CVID_QoL Global scores were similar in patients with PADs forced to shift to home-based subcutaneous treatment in comparison to those who continued their usual home-based subcutaneous treatment (Figure 1 ). The 2020 CVID_QoL Global score and its dimensions overlapped those found in the 2017 survey (see Table E1 in this article's Online Repository at www.jaci-inpractice.org).
Figure 1.
CVID_QoL questionnaire scores and change in therapy setting. Patients with PADs forced to shift to home-based subcutaneous treatment and patients with PADs who continued their usual home therapy showed similar CVID_QoL questionnaire scores (Global score, emotional functioning [EF], relational functioning [RF], gastrointestinal and skin symptoms [GIS] dimension scores).
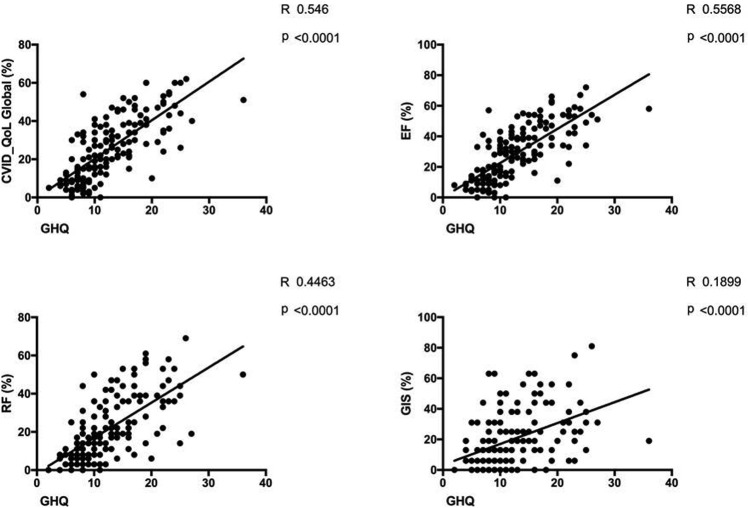
Correlation between GHQ-12 and CVID_QoL questionnaire
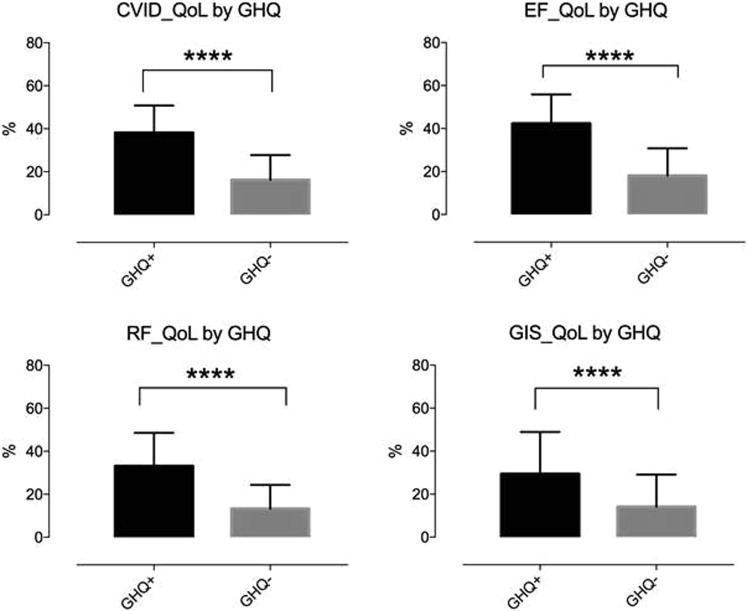
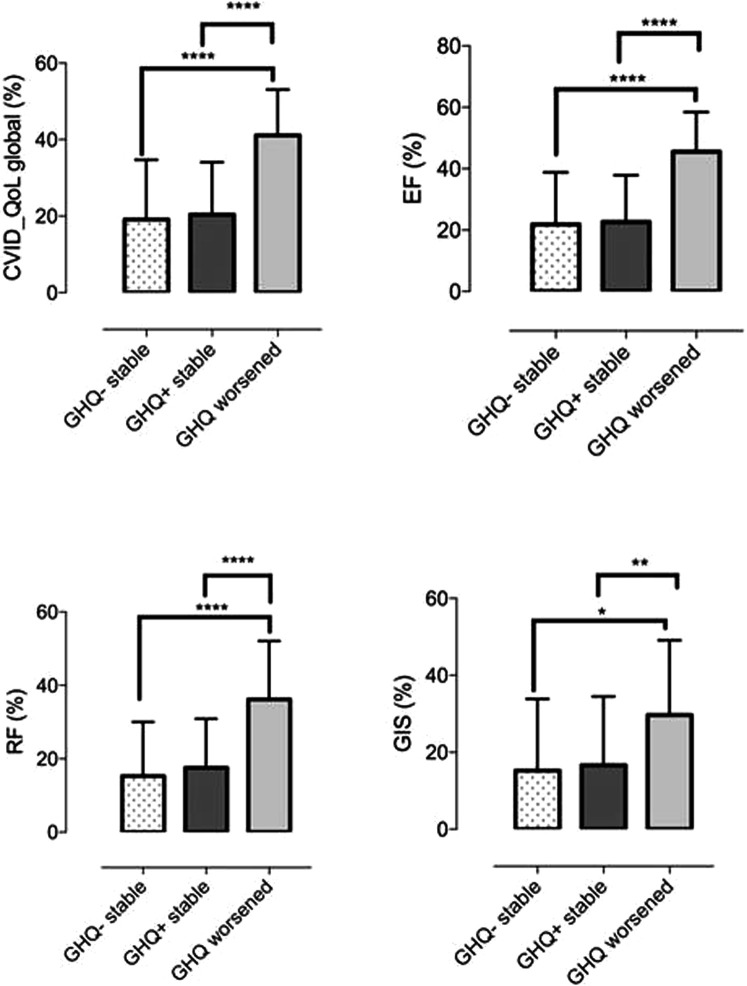
We confirmed8 the previously described direct relation between GHQ-12 and CVID_QoL questionnaire (Global: R = 0.5, emotional functioning: R = 0.6, relational functioning: R = 0.4, GIS: R = 0.2; P < .0001 for all comparisons) (see Figure E1 in this article's Online Repository at www.jaci-inpractice.org). Overall, patients who are not at risk of anxiety/depression had low mean values of HRQOL scores by CVID_QoL questionnaire. In one-third of participants the risk of anxiety/depression worsened (see Figure E2 in this article's Online Repository at www.jaci-inpractice.org). These patients reported higher CVID_QoL questionnaire scores in each dimension in comparison to patients having stable GHQ-positive status and stable GHQ-negative status over time (Figure 2 ). However, being shifted to home therapy was not associated with a worsening of the risk of anxiety/depression (46% vs 40%; P = .752). Similarly, age (48.7 ± 16.4 vs 48.4 ± 12.3 years; P = .928) and female sex (50% vs 50%; P = 1.000) were not related to the worsening in the GHQ-12 score. In addition, we did not record any difference in the risk of anxiety/depression and CVID_QoL questionnaire scores between patients living in north and central Italy, 2 areas with a different prevalence of COVID-19 at the time of our survey (see Table E2 in this article's Online Repository at www.jaci-inpractice.org).
Figure E1.
Correlation between GHQ-12 and CVID_QoL questionnaire scores. Significant linear regression between GHQ-12 score and CVID_QoL dimensions (Global: R = 0.5, EF: R = 0.6, RF: R = 0.4, GIS: R = 0.2, P < .0001, for all comparisons). EF, Emotional functioning; GIS, gastrointestinal and skin symptoms; RF, relational functioning.
Figure E2.
GHQ-12 status and CVID_QoL questionnaire. GHQ-negative patients had lower mean scores on CVID_QoL questionnaire scales than did GHQ-positive patients (P < .0001, for all comparisons).
Figure 2.
CVID_QoL questionnaire scores by GHQ status. Patients who shifted from a GHQ-negative status to a GHQ-positive status showed higher CVID_QoL questionnaire scores (Global score, emotional functioning [EF], relational functioning [RF], gastrointestinal and skin symptoms [GIS] dimensions) in comparison to patients with stable GHQ-positive status and stable GHQ-negative status over time.
CVID_QoL questionnaire questions affecting the GHQ status
To identify the CVID_QoL items influencing the actual patients' risk for anxiety/depression, we selected the items that obtained the “often/always” answer in the 2020 and 2017 surveys, and we calculated the difference between the percentages of patients at risk in the 2 surveys. Questions associated with risk for anxiety/depression were related to the fear of the COVID-19 pandemic and to PAD fragility: to be afraid to get infected, to get sick, to be a sick person, to be tired, and to be weak (Table III ). Questions concerning the clinical conditions associated with CVID such as cough, diarrhea, diet problems, and joints pain appeared to be not relevant for the risk of anxiety/depression (Table IV ). Questions on replacement therapy were not relevant regardless of whether patients switched from hospital therapy to home therapy or being constantly on home therapy: “I was afraid of adverse reactions to immunoglobulin therapy”; “Immunoglobulin therapy bothered me.”
Table III.
CVID_QoL items affecting the actual risk of anxiety/depression
| No. | Question | % |
|---|---|---|
| 30. | I was afraid that I might get infected with other's people illness | 41 |
| 21. | I was afraid of getting sick | 32 |
| 28. | I felt I was a sick person | 32 |
| 13. | I was concerned about my future | 27 |
| 32. | I felt tired | 25 |
| 22. | I felt weak | 24 |
| 18. | I was afraid of dying | 11 |
| 7. | I could not take care of my loved ones as I would like to be able | 10 |
| 15. | I felt less independent than usual | 8 |
| 25. | It was difficult to carry out my usual leisure activities | 7 |
| 16. | I was afraid I might make others sick with my infections | 7 |
| 1. | I felt sad | 5 |
| 8. | I was afraid my health might worsen | 5 |
Data are expressed as percentage of patients at risk of anxiety/depression who answered “often/always” to a given question (difference between 2020 and 2017 surveys).
Table IV.
CVID_QoL items not affecting the actual risk of anxiety/depression
| No. | Question | % |
|---|---|---|
| 6. | I had a cough and/or phlegm | −36 |
| 2. | I had to change my diet | −21 |
| 9. | I had discomfort and/or pain in my joints | −13 |
| 3. | I felt anger | −6 |
| 4. | I had diarrhea | −5 |
| 10. | I need help taking care of myself | −3 |
| 26. | I felt uncomfortable because of my skin problems | −1 |
Data are expressed as percentage of patients at risk of anxiety/depression who answered “often/always” to a given question (difference between 2020 and 2017 surveys).
Surprisingly, about 58% of patients with PADs were not at risk for anxiety/depression. The analysis of CVID_QoL questionnaire identifies similar items (“I was afraid my health might worsen,” “I was afraid of getting sick,” “I felt weak,” “I was afraid that I might get infected with other's people illness,” “I felt tired”), even if the percentage of patients responding “often/always” to each item had a lower frequency (P < .0001, for the items listed). Other items including “I felt I was a sick person,” “I was concerned about my future,” “I was afraid of dying,” “I was afraid I might make others sick with my infections,” “I felt less independent than usual,” and “It was difficult to carry out my usual leisure activities” received a response “often/always” from less than 10% of patients not at risk of anxiety/depression. Also in this group, questions related to clinical conditions such as cough, diarrhea, diet problems, and joints pain had a frequency of “often/always” responses from less than 5% of patients and none reported concerns on items related to the immunoglobulin therapy.
Discussion
Patient-reported outcome measures in clinical practice have been proposed as a means of facilitating doctor-patient communication, uncovering patients' problems, monitoring disease or treatment, and screening for functional problems. We have previously reported a poor HRQOL in patients with PADs by generic instruments, short-form-36 and GHQ-12, and by the CVID_QoL questionnaire, a disease-specific instrument.8 We demonstrated that HRQOL was lower than that reported in generally healthy population and that reported in patients with other chronic disease entities.11 Moreover, we showed that more than one-third of patients were at risk of anxiety/depression at all observation times, a percentage that reached two-thirds of patients, considering only the group of females. Disorders such as anxiety and depression are particularly prevalent in hospital settings and still often go unrecognized. Indeed, we suggested that a comprehensive evaluation of HRQOL assessment should be performed in each patient to document the outcomes of any relevant changes. This is especially true in times of major trouble such as the one being brought by the COVID-19 pandemic. Moreover, patients affected by inborn errors of immunity12 are known to be more vulnerable to infections. Among them, patients with PADs lack antibodies that are important to block microbial infectivity and prevent diseases. The protective role of antibodies in other pandemics was reflected in the severe acute respiratory syndrome, Ebola, and H1N1, when convalescent plasma containing antibodies from patients who recovered from viral infections was used for treatment at the early stage of disease.13, 14, 15 Here, we reported data of a study conducted since the beginning of the epidemic in Italy on the possible and expected changes in the HRQOL between patients with PADs living in an Italian region with a high prevalence of COVID-19 and in an Italian region with a lower prevalence.16 All data were compared with HRQOL scores detected in the same group of patients in 2017, 3 years before the COVID-19 epidemic. The aim of the study was to detect changes in the HRQOL of patients with PADs due to COVID-19 and due to the change in care strategy we adopted. The latter began in late February 2020 by activating the transfer of all patients to home therapy and by activating a remote assistance service as a tool to contain the spread of the infection, to stop travel and exposure, and to permit uninterrupted care of established patients.17 After completing the transfer to home and activating the remote assistance service,18 we sent the HRQOL questionnaires. We had a high rate of responders. One of 3 patients reported a worsening in GHQ-12 assessment, being at risk of anxiety/depression. However, the GHQ status as well as CVID_QoL questionnaire scores were not different in patients forced to shift to home-based SCIG treatment in comparison with those who continued their usual treatment. In addition, we did not find any difference in GHQ status and CVID_QoL questionnaire scores between patients from north and central Italy, 2 areas with a different prevalence of COVID-19 at the time of our survey. Relevant items of the CVID_QoL questionnaire for being GHQ-positive are related to the fear associated with the COVID-19 pandemic, and to the fragility inherent to the condition of being a patient with PAD19: to be afraid to get infected, to get sick, to be a sick person, tired, and weak. Items that did not affect the GHQ-positive status were related to concerns about the CVID itself such as cough, diarrhea, diet problems, and joints pain, all problems previously identified9 , 11 as major drivers of poor QOL in our patients. Anxiety about running out of medications appeared as a major new issue for our patients with PADs, not previously described,20, 21, 22 even if we never faced a problem of shortage. Surprisingly, most patients with PADs were not at risk of anxiety/depression. No patients reported concerns on items related to the immunoglobulin therapy.
Conclusions
As expected, our data stressed the relevance of the COVID-19 pandemic on the QOL of our patients. However, this first survey allowed us to prove that the remote assistance program, as well as the shift from hospital-based IVIG administration route to home-based SCIG administration route, had no impact on the deterioration of QOL and on the risk of anxiety/depression. We are planning to verify these data over time. Indeed, once more22 we underline the importance of conducting a periodical HRQOL assessment in patients with PADs and the need to care for at-risk patients by planning an individualized medical and a psychological support throughout their lifetime and in exceptional cases such as in the 2020 COVID-19 pandemic.
Acknowledgments
We thank all patients for their participation in the study as well as the study team and nurses of the local health units whose enthusiastic collaboration made possible this study. We thank the Jeffery Modell Foundation for supporting our center.
Footnotes
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Online Repository.
Table E1.
CVID_Qol Global score, emotional functioning (EF), relational functioning (RF), and gastrointestinal and skin symptoms (GIS) score dimensions in 2017 and 2020 surveys
| Global score | Survey |
P | |
|---|---|---|---|
| 2017 (%) | 2020 (%) | ||
| 25.9 ±16.6 | 26.5 ± 15.4 | .804 | |
| EF | 28.6 ± 16.7 | 28.6 ± 18.1 | .986 |
| RF | 22.2 ± 15.8 | 22.7 ± 16.5 | .861 |
| GIS | 26.3 ± 18.7 | 24.1 ± 18.5 | .600 |
Table E2.
HRQOL scores in 158 patients with PADs by center
| Questionnaire | Veneto | Lazio | P |
|---|---|---|---|
| CVID_Qol questionnaire Global score, mean ± SD | 26.9 ± 14.8 | 25.5 ± 16.5 | 0.592 |
| GHQ score, mean ± SD | 12.4 (5.1) | 12.6 ± 6.0 | 0.864 |
| GHQ-positive, n (%) | 12 (42.9) | 54 (41.5) | 1.000 |
References
- 1.Fauci A.S., Lane H.C., Redfield R.R. COVID-19—navigating the uncharted. N Engl J Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available from: Accessed April 1, 2020.
- 3.Carsetti R., Rosado M.M., Donnanno S., Guazzi V., Soresina A., Meini A. The loss of IgM memory B cells correlates with clinical disease in common variable immunodeficiency. J Allergy Clin Immunol. 2005;115:412–417. doi: 10.1016/j.jaci.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 4.Bonilla F.A., Barlan I., Chapel H., Costa-Carvalho B.T., Cunningham-Rundles C., de la Morena M.T. International Consensus Document (ICON): common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4:38–59. doi: 10.1016/j.jaip.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carsetti R., Di Sabatino A., Rosado M.M., Cascioli S., Piano Mortari E., Milito C. Lack of gut secretory immunoglobulin A in memory B-cell dysfunction-associated disorders: a possible gut-spleen axis. Front Immunol. 2020;10:2937. doi: 10.3389/fimmu.2019.02937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troilo A., Wehr C., Janowska I., Venhoff N., Thiel J.D., Rawluk J. Non permissive bone marrow environment impairs early B-cell development in common variable immunodeficiency. Blood. 2020;135:1452–1457. doi: 10.1182/blood.2019003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazzerini M., Putoto G. COVID-19 in Italy: momentous decisions and many uncertainties. Lancet Glob Health. 2020;8:e641–e642. doi: 10.1016/S2214-109X(20)30110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinti I., Pulvirenti F., Giannantoni P., Hajjar J., Canter D.L., Milito C. Development and initial validation of a questionnaire to measure health-related quality of life of adults with common variable immune deficiency: the CVID_QoL questionnaire. J Allergy Clin Immunol Pract. 2016;4:1169–1179. doi: 10.1016/j.jaip.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Picardi A., Abeni D., Pasquini P. Assessing psychological distress in patients with skin diseases: reliability, validity and factor structure of the GHQ-12. J Eur Acad Dermatol Venereol. 2001;15:410–417. doi: 10.1046/j.1468-3083.2001.00336.x. [DOI] [PubMed] [Google Scholar]
- 10.Italian Registry for Primary Antibody Deficiencies. www.ipinet.org Available from: Accessed April 1, 2020.
- 11.Tabolli S., Giannantoni P., Pulvirenti F., La Marra F., Granata G., Milito C. Longitudinal study on health-related quality of life in a cohort of 96 patients with common variable immune deficiencies. Front Immunol. 2014;5:605. doi: 10.3389/fimmu.2014.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seidel M.G., Kindle G., Gathmann B., Quinti I., Buckland M., van Montfrans J., ESID Registry Working Party and collaborators The European Society for Immunodeficiencies (ESID) Registry Working Definitions for the Clinical Diagnosis of Inborn Errors of Immunity. J Allergy Clin Immunol Pract. 2019;7:1763–1770. doi: 10.1016/j.jaip.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y., Wong R., Soo Y.O., Wong W.S., Lee C.K., Ng M.H. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.M., Lim W.S., Convalescent Plasma Study Group The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuite A.R., Ng V., Rees E., Fisman D. Estimation of COVID-19 outbreak size in Italy. Lancet Infect Dis. 2020;20:537. doi: 10.1016/S1473-3099(20)30227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 18.Duffy S., Lee T.H. In-person health care as option B. N Engl J Med. 2018;378:104–106. doi: 10.1056/NEJMp1710735. [DOI] [PubMed] [Google Scholar]
- 19.Hajjar J., Kutac C., Rider N.L., Seeborg F.O., Scalchunes C., Orange J. Fatigue and the wear-off effect in adult patients with common variable immunodeficiency. Clin Exp Immunol. 2018;194:327–338. doi: 10.1111/cei.13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rider N.L., Kutac C., Hajjar J., Scalchunes C., Seeborg F.O., Boyle M. Health-related quality of life in adult patients with common variable immunodeficiency disorders and impact of treatment. J Clin Immunol. 2017;37:461–475. doi: 10.1007/s10875-017-0404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pulvirenti F., Cinetto F., Pecoraro A., Carrabba M., Crescenzi L., Neri R. Health-related quality of life in patients with CVID under different schedules of immunoglobulin administration: prospective multicenter study. J Clin Immunol. 2019;39:159–170. doi: 10.1007/s10875-019-0592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinti I., Pulvirenti F. Health-related quality of life and patients’ empowerment in the health care of primary immune deficiencies. J Clin Immunol. 2017;37:615–616. doi: 10.1007/s10875-017-0428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]