Abstract
Clinical and epidemiological evidence has been advanced for human-to-human transmission of the novel coronavirus rampaging the world since late 2019. Outliers in the human-to-human transmission are yet to be explored. In this study, we examined the spatial density and leaned statistical credence to the global debate. We constructed spatial variations of clusters that examined the nexus between COVID-19 attributable deaths and confirmed cases. We rely on publicly available data on confirmed cases and death across Africa to unravel the unobserved factors, that could be responsible for the spread of COVID-19. We relied on the dynamic system generalised method of moment estimation procedure and found a ~0.045 Covid19 deaths as a result of confirmed cases in Africa. We accounted for cross-sectional dependence and found a basis for the strict orthogonal relationship. Policy measures were discussed.
Keywords: COVID-19 attributable deaths, COVID-19 confirmed cases, Spatial variation, System GMM, Africa
Graphical abstract
Highlights
-
•
The paper leaned spatial and statistical credence to COVID-19 confirmed cases and attributable deaths in Africa.
-
•
We rely on density mapping and the system GMM to explain our core observations.
-
•
~0.045 Covid19 deaths are as a result of confirmed cases in Africa.
1. Introduction
Clinical and epidemiological evidence has been advanced for human-to-human transmission of the novel coronavirus rampaging the world since late 2019 (see Kamph, 2020; Shereen et al., 2020; Yeo et al., 2020 for an extensive review). The contemporary disease is spreading exponentially around the world, and the pandemic has been felt in at least 185 countries reporting 1,844,863 confirmed cases with 117,021 deaths as at 14th April 2020 (WHO, 2020a, WHO, 2020b). In this time of the global pandemic, fifty-two (52) African countries have reported one or more confirmed cases of COVID 19 with only Comoros and Lesotho presently with no single confirmed case. As a result of changing medical data, Africa at the time of writing has recorded 10,787 confirmed cases, with 501 deaths. Algeria is the most hardly hit nation with 1983 confirmed cases and 313 deaths, although lesser confirmed cases compared to South Africa (2272) with 27 deaths (WHO, 2020a, WHO, 2020b). In other regions of the world, the United States of America (USA) with 553, 822 confirmed cases and 21,972 deaths remain the hardest-hit nation in America (North and South) and around the world. China, with 83,696 confirmed cases and 3351 deaths, is the hardest hit Asian country. In Europe, Italy, with 159,516 confirmed cases and 20,465 deaths although relatively less compared to confirmed cases in Spain with 169,496 with 17,489 deaths remains the hardest hit because of their superior coronavirus reported death cases. In the middle east region, Iran, with 73,303 confirmed cases and 4585 deaths is the worst-hit nation.
In ascertaining the structures, features, risk factors and severity of the novel coronavirus, clinical and epidemiological evidence has been leaned to human-to-human transmissions (community transmission). However, outliers in the human-to-human transmission could be as a result of clusters that are yet to be explored. By advancing arguments for clusters of the novel coronavirus in Africa, we provided a social dimension to abating the spread of coronavirus diseases. Africa is predominantly famous for overcrowding their megacities like Pretoria, Lagos, Johannesburg, Cairo and Nairobi. Clustering by people either in public gatherings or residential facilities makes them endangered species. Even if social distancing is enforced in the public domain to prevent human-to-human transmission, it may be difficult if not impossible to enforce in an already overcrowded shared resident. By advancing arguments for inevitable human clustering at various residential or sub-groups in Africa, the study provides an econometric analysis of the social dimensions of the spread of coronavirus diseases in Africa. Apparently, this study leads the debate on spatial density and statistical credence of how confirmed cases is statistically related to attributable deaths from coronavirus diseases in Africa. We build upon the work of Sarkodie and Owusu (2020) to examine the phenomenological influence of outliers in the human-human transmission in Africa. The intricacies of these unobserved factors in abating human-to-human transmission of coronavirus disease in Africa underpin this study. We rely on publicly available data for Africa countries confirmed and death cases. We use a novel estimation method capable of accounting for cross-sectional dependence. We found a basis for the strict orthogonal relationship among the variable and accounted for unobserved heterogeneity of the human-to-human transmission.
2. Materials and methods
2.1. Data
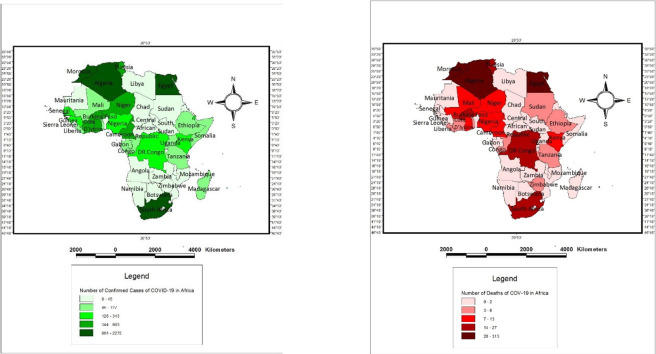
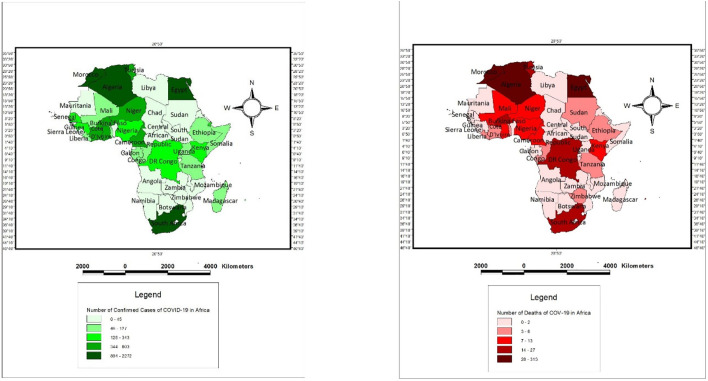
We rely on data obtained from the World Health Organisation (WHO) situation reports 551 through to situation reports 852 (a time plot of 31-time invariant observations). We transformed the daily observations in conformity with conventional panel data estimation procedure (wide to long) as in Sarkodie and Owusu (2020). We relied on health indicators (number of COVID-19 attributable deaths and number of confirmed cases) across 52 Africa states.3 In Fig. I , we presented the spatial density of the number of confirmed cases and the number of deaths from COVID 19 across African states.
Fig. I.
Spatial density of confirmed cases and attributable deaths of COVID-19 Cases across Africa.
2.2. Model
Our empirical strategy in gauging the statistical relationship between the number of attributable COVID-19 deaths and confirmed cases was to estimate a series of baseline fixed effect estimator by assuming that all explanatory variables are strictly exogenous. We proceed to estimate the dynamic panel data system generalised method of moment (GMM) (Arellano and Bover, 1995) and impose (and test) the common factor restrictions to account for the potential endogeneity of the number of COVID-19 attributable deaths and number of confirmed cases across 52 Africa states. The functional relationship is expressed as:
| (1) |
where COVIDdeaths i, t is the total number of attributable COVID-19 deaths in country i over period t; Confrmd Cases i, t represent the number of confirmed cases in country i over period t, t defines the time parameters, and i give the cross-sectional domain of the COVID-19 attributable deaths model.
We begin by imposing the assumption of strict exogeneity on the regressors, leading to violations and inconsistency in our fixed-effect model. We proceed to obtain asymptotically consistent parameter estimates in a single equation dynamic GMM estimators by using a common factor representation (Blundell and Bond, 1998). Our dynamic panel COVID-19 attributable deaths model is specified as:
| (2) |
All other variables remain as earlier defined except ρ, which gives the constant parameter. ω and θ are the output elasticities.
With an attendant, country-specific fixed effect idiosyncratic errors (outliers) predicted upon the model in Eq. (3); we adjusted for violation of strict orthogonal assumptions by introducing the change parameter and by taking the semi-derivatives of the variables to account for variances in units and measurements to specify Eq. (4)
| (3) |
ε i, t contains e i (the country-specific fixed effect that is time-invariant) while μ it is assumed to be independent and normally distributed with zero (0) mean and constant variance σ μ 2 both over time and across cross-sections, i.e., u it ≈ n(0, σ μ 2).
| (4) |
We also accounted for strict orthogonal violations that could be due to first differenced estimation of ordinary least squares since the transformed error term ∆μ it still correlates with COVIDdeaths i, t−1 (a condition exacerbated when both contains μ it−1). The possibility of the E(COVIDdeaths i, t−1 ∆μ it) = 0 ∀ h ≥ 2, t = 3, ……T justify the introduction of lagged variables as instruments in the strict orthogonal assumption relationship (Anderson and Hsiao, 1982; Blundell and Bond, 2000).
We estimated the dynamic system generalised method of moment (GMM) model for some reasons. The real-time reporting informed the observations of coronavirus disease in Africa. We established a linear relationship between the variables of interest which informs our construct along such tangent. The number of cross-sections (countries) is higher than the numbers of time series (time plot of 31-time invariant reports) chosen. We estimated an unbalanced panel data model (predicted on non-occurrences of cases in some situation reports in some African countries).
3. Results and discussions
System GMM, renowned for glowing outcomes with persistent data under trifling assumptions (Arellano and Bover, 1995; Blundell and Bond, 1998), was used to estimate the model of attributable COVID-19 deaths as induced by confirmed cases. The result of the robust two-step estimates of the dynamic system Generalised Method of Moment (GMM) is presented in Table I .
Table I.
System GMM-robust two-step estimates dependent variable: COVIDdeathsi, t.
| Variables | Output elasticities (ωand θ) | Prob. |
|---|---|---|
| ρ | 0.0231 | 0.0017* |
| COVIDdeathsi, t−1 | 0.0123 | 0.0004* |
| Confirmd Casesi, t | 0.0453 | 0.0315** |
| Obs | 1612 | |
| Number of countries | 52 | |
| Cross-sectional dependence | ||
| COVIDdeathsi, t−1 | Confirmd Casesi, t | |
| CD-test value | 8.42** | 9.87** |
| Prob. | 0.03 | 0.04 |
| Slope homogeneity test | ||
| 17.82** | 0.01 | |
| adj | 13.54** | 0.03 |
Note: Parameter estimates and Prob values are reported in the text box. The subsequent tables present the cross-sectional and slope homogeneity parameters. The dependent variable is COVID attributable deaths. ∗P < 0.01, ∗ ∗ P < 0.05, respectively.
Source: Authors Computation
Following Pesaran (2004), we estimated cross-sectional dependence. We rejected the null of no-cross-sectional dependence at a 5% level of significance. We also rejected the null of slope homogeneity at a 5% level of significance using the delta tilde and adjusted delta tilde estimates. We estimated the system GMM to account for cross-sectional dependence among heterogeneous observations.
The one-period lag value of attributable COVID-19 deaths is positive and statistically significant at 1%, implying its percentage increase will result in 0.0123 percentage increase in COVID-19 attributable deaths. The intuition point to the relatively less COVID-19 attributable deaths as induced by previously confirmed cases. Although the lagged factors control for simultaneity bias, we cannot completely rule out the devastating influence of historical information leading to COVID-19 deaths. Types of shared residents, proximity to health care facilities, choice of healthcare utilisation in the time of illness and most importantly during this global pandemic, existing health conditions, as well as the devastating influence of COVID-19 attributable deaths to friends and family in terms of depression, frontline health workers who are saddled with the care and post-death management of patients, are magnanimous in this global pandemic era. In other climes, the number of confirmed cases is positive and statistically significant at 5%, implying 0.0453 percentage increase in COVID-19 attributable deaths in Africa. By way of intuition, a proportionate rise in confirmed cases is attributable to potential deaths by ~0.045. Principally, the cluster of residents and sub-group around regions with high confirmed cases should be dispersed to reduce the wave at which the virus transmits from human to human.
In Table II , we validated the instrumental variable choice in the COVID-19 attributable deaths in Africa. Dynamic panel data estimates are known to suffer from problems of unobserved heterogeneity, dynamic endogeneity and simultaneity bias (Baltagi et al., 2015). System GMM, known explicitly for heteroscedasticity and non-normality assumptions unlike the least squares estimates, assumes linearity and uncorrelated error terms. Our first-order and second-order difference results in favour of the rejection of the null hypothesis in the first-order serial correlation examination and acceptance of the null hypothesis for the second-order serial correlation test. Blundell and Bond (2000) argued that the system GMM estimators requires the presence of first-order serial correlation and not the second-order serial correlation in the residual term. The result of Table II confirms that we obtained appropriate diagnostics. z = − 2.42; p < 0.05 at 5% level of significance in the first order serial coreelation analysis and then no second order serial correlation based on calculated z that is not statistically significant at 5% (z = − 0.96; p > 0.05). The Hansen (1982) J-statistics test result confirmed the model has valid instruments since we fail to reject the null of overidentifying restriction at a 5% level of significance (p > 0.05; i. e p = 0.532). The F-statistics value 122.42 indicates the model is jointly significant at 5% level of significance.
Table II.
Instrumental variable validation.
| Test of joint significance (F-test) | F = 122.42 |
| AR(1) | z = − 2.81 Prob > z = 0.0024* |
| AR(2) | z = − 0.52 pr > z = 0.1152 |
| Hansen J-test for overidentifying restrictions |
Chi2 (2) = 1.88 Prob > chi(2) = 0.627 |
Note: ∗P < 0.01, ∗ ∗ P < 0.05 respectively
Source: Authors Computation
4. Conclusion
This study examined the spatial density of the novel coronavirus disease (COVID-19) across 52 African states and leaned empirical credence to the relationship between confirmed cases and attributable deaths. We presented spatial and statistical evidence based on the situation reports from the World Health Organisation (WHO). We advise the public on the cautious interpretations of our statistical model, which rely on phenomenological models as in most social sciences research and not a clinical procedure that has a confidence interval of 99%. We found Algeria to be the most hardly hit African nation (at the time of writing) by the rampaging virus (estimated in terms of the number of deaths recorded), and also we establish a linear relationship between the number of confirmed cases and the number of attributable deaths. Our findings corroborate the finding of Sarkodie and Owusu (2020) in one of their strings of findings. The study is limited to facts obtainable at the present time of this global pandemic.
CRediT authorship contribution statement
Ibrahim Ayoade Adekunle: Conceptualization, Formal analysis, Data curation, Writing - original draft. Abayomi Toyin Onanuga: Writing - review & editing, Writing - original draft. Olanrewaju Olugbenga Akinola: Resources, Investigation, Formal analysis, Writing - original draft. Olakitan Wahab Ogunbanjo: Investigation, Data curation, Writing - original draft.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Algeria, Angola, Benin, Botswana, Burkina Faso, Burundi, Cabo Verde, Cameroon, Central African Republic, Chad, Congo, Djibouti, Democratic Republic of Congo, Côte d'Ivoire, Equatorial Guinea, Egypt, Eritrea, Eswatini, Ethiopia, Gabon, The Gambia, Ghana, Guinea, Guinea-Bissau, Kenya, Liberia, Madagascar, Malawi, Mali, Mauritania, Mauritius, Morocco, Mozambique, Namibia, Niger, Nigeria, Rwanda, Comoros, Senegal, Seychelles, Sierra Leone, Somalia, South Africa, South Sudan, Sudan, Tanzania, Togo, Tunisia, Uganda, Zambia and Zimbabwe.
References
- Anderson T.W., Hsiao C. Formulation and estimation of dynamic models using panel data. J. Econ. 1982;18(1):47–82. doi: 10.1016/0304-4076(82)90095-1. [DOI] [Google Scholar]
- Arellano M., Bover O. Another look at the instrumental variable estimation of error-components models. J. Econ. 1995;68(1):29–51. doi: 10.1016/0304-4076(94)01642-D. [DOI] [Google Scholar]
- Baltagi B.H., Bun M.J.G., Sarafidis V. The Oxford Handbook of Panel Data. 2015. Dynamic panel data models. (Available at) [Google Scholar]
- Blundell R., Bond S. Initial conditions and moment restrictions in dynamic panel data models. J. Econ. 1998;87(1):115–143. doi: 10.1016/S0304-4076(98)00009-8. [DOI] [Google Scholar]
- Blundell R., Bond S. GMM estimation with persistent panel data: an application to production functions. Econ. Rev. 2000;19(3):321–340. doi: 10.1080/07474930008800475. [DOI] [Google Scholar]
- Hansen L.P. Large sample properties of generalized method of moments estimators. Econometrica. 1982;50(4):1029–1054. doi: 10.2307/1912775. [DOI] [Google Scholar]
- Kamph G. Potential role of inanimate surfaces for the spread of coronaviruses and their inactivation with disinfectant agents. Infection Prevention in Practice. 2020;2(2) doi: 10.1016/j.infpip.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran M.H. Cambridge Working Papers in Economics. 2004. General diagnostic tests for cross-section dependence in panels. (Paper No. 0435) [Google Scholar]
- Sarkodie S.A., Owusu P.A. Investigating the cases of novel coronavirus disease (COVID-19) in China using dynamic statistical techniques. Heliyon. 2020;6(4) doi: 10.1016/j.heliyon.2020.e03747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; 2020. Coronavirus Disease 2019 (COVID-19) Situation Report-55.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200315-sitrep-55-covid-19.pdf?sfvrsn=33daa5cb_8 Available at. [Google Scholar]
- WHO . World Health Organization; 2020. Coronavirus Disease 2019 (COVID-19) Situation Report-85.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200414-sitrep-85-covid-19.pdf?sfvrsn=7b8629bb_4 Available at. [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]