Highlights
-
•
Fever and cough are the most common symptoms in patients with COVID-19.
-
•
The most prevalent comorbidities are hypertension and diabetes which are associated with the severity of COVID-19.
-
•
ARDS and ACI may be the main obstacles to treatment recovery for patients.
-
•
The case severe rate and mortality is lower than that of SARS and MERS.
Abbreviations: COVID-19, corona virus disease; ACE2, angiotensin-converting enzyme 2; SARS, severe acute respiratory syndrome; MERS, middle east respiratory syndrome; ARDS, acute respiratory distress syndrome; ACI, acute cardiac injury; AKI, acute kidney injury; WHO, World Health Organization; CI, confidence interval; RCTs, randomized controlled trials; SD, standard deviation
Keywords: COVID-19, Comorbidities, Symptom, Severity, Mortality
Abstract
Background
Since being first reported in Wuhan, China, in December 8, 2019, the outbreak of the novel coronavirus, now known as COVID-19, has spread globally. Some case studies regarding the characteristics and outcome of patients with COVID-19 have been published recently. We conducted a meta-analysis to evaluate the risk factors of COVID-19.
Methods
Medline, SinoMed, EMBASE, and Cochrane Library were searched for clinical and epidemiological studies on confirmed cases of COVID-19.
Results
The incidence of fever, cough, fatigue, and dyspnea symptoms were 85.6 % (95CI 81.3–89.9 %), 65.7 % (95CI 60.1–71.4 %), 42.4 % (95CI 32.2–52.6 %) and 21.4 % (95CI 15.3–27.5 %). The prevalence of diabetes was 7.7 % (95CI 6.1–9.3 %), hypertension was 15.6 % (95CI 12.6–18.6 %), cardiovascular disease was 4.7 % (95CI 3.1–6.2 %), and malignancy was 1.2 % (95CI 0.5–1.8 %). The complications, including ARDS risk, ranged from 5.6–13.2 %, with the pooled estimate of ARDS risk at 9.4 %, ACI at 5.8 % (95CI 0.7–10.8 %), AKI at 2.1 % (95CI 0.6–3.7 %), and shock at 4.7 % (95CI 0.9–8.6 %). The risks of severity and mortality ranged from 12.6 to 23.5% and from 2.0 to 4.4 %, with pooled estimates at 18.0 and 3.2 %, respectively. The percentage of critical cases in diabetes and hypertension was 44.5 % (95CI 27.0–61.9 %) and 41.7 % (95CI 26.4–56.9 %), respectively.
Conclusion
Fever is the most common symptom in patients with COVID-19. The most prevalent comorbidities are hypertension and diabetes which are associated with the severity of COVID-19. ARDS and ACI may be the main obstacles for patients to treatment recovery. The case severe rate and mortality is lower than that of SARS and MERS.
1. Introduction
The ongoing outbreak of the corona virus disease 2019 (COVID-19) infection has posed significant threats to international health and the economy [[1], [2], [3]]. On 30 January, 2020, the World Health Organization (WHO) declared it to be a Public Health Emergency of International Concern. As of 10 March, 2020, more than 105 countries, 114,253 cases of COVID-19 and 4000 deaths have been reported all over the world, of which the number of confirmed patients in China has gradually decreased, but is increasing rapidly in other countries, especially in Italy, South Korea, and Iran, and there are lots of doctors engaged in combating it [4,5]. The SARS-CoV-2 was first reported in samples of bronchoalveolar lavage fluid from three patients in Wuhan Jinyintan hospital and was confirmed as the cause of COVID-19 on January 24, 2020 [6]. After deep examining the full-length genome, we found that the virus belongs to the beta-coronavirus 2b lineage in the phylogenetic tree [7] and is a new human-infecting beta-coronavirus which had previously not been detected in humans or animals. It is named COVID-19 by the WHO and SARS-CoV-2 by the International Committee on Taxonomy of Viruses as it is similar to the coronavirus responsible for severe acute respiratory syndrome (SARS-CoV) [8]; it shares more than 87.99 % identity sequencing with the Bat SARS-like coronavirus, and it shares more than 80 % identity nucleotide with the original SARS epidemic virus [[9], [10], [11]]. Coronavirus spike (S) glycoproteins promote entry into cells. They are the main target of antibodies and bind with high affinity to the angiotensin-converting enzyme 2 (ACE2) receptor in humans in a manner similar to SARS-CoV [[12], [13], [14]]. However, the SARS-CoV-2 S glycoprotein harbors a furin cleavage site at the boundary between the S1/S2 subunits, which is processed during biogenesis and sets this virus apart from SARS-CoV and SARS-related CoVs [15,16]. At present, research reports that the incubation period in most people range from 1 to 14 days with a median of 5–6 days, but the incubation period may even be as long as 24 days [17]. The reproductive number (R0) for SARS-CoV-2, although still preliminary, is estimated between 2 and 3, suggesting a higher pandemic potential than SARS [18]. Fever or cough may be the major symptom, but asymptomatic individuals have also been identified as potential sources of infection [19]. At present, we think that the new coronavirus is mainly transmitted through respiratory droplets and close contact, but transmission from an asymptomatic carrier appears to be possible [20]. A report of 9 pregnant patients suggests that perinatal transmission is unlikely but larger studies are needed to confirm this finding [21]. Although viral RNA is found in stool, whether it can be transmitted through the fecal-oral route still needs to be confirmed by subsequent investigations [22,23]. Recently, some patients were found to be re-positive after being treated with negative nucleic acid tests twice and symptoms disappeared. It is difficult for us to decrease the severity of COVID-19 owing to the complex structure and unclear physiological mechanism. With the increasing number of confirmed cases, the clinical investigation of patients and antiviral treatment solution has been insufficient, and there is an urgent need to find alternative methods to control the spread of disease. In order to prove more accurate conclusions on the prevalence of comorbidity and relation of clinical characteristics and mortality of patients with COVID-19, we searched the relevant literatures and carried out single arm meta-analysis to describe epidemiological, clinical characteristics, complications, and outcomes of patients confirmed to have 2019-nCoV infection, and to compare the severity between diabetes or hypertension and non-diabetes or non-hypertension patients. Our findings provide vital guidance for current clinical work on the prevention and treatment of 2019-nCoV infection.
2. Methods
Ethical approval or patient consent was not required because the present study was a review of previously published articles.
2.1. Search strategy and study selection criteria
A computerized search spanning January1, 1980 to March 10, 2020 was conducted in Medline, Sino Med, EMBASE, and Cochrane Library databases. The following search terms were used in all possible combinations: ("Corona Virus Disease-2019 "[Mesh] OR “2019 novel coronavirus "[Mesh] OR" SARS-CoV-2 "[Mesh] OR "COVID-19 "[Mesh] OR" 2019-nCoV "[Mesh] The search was limited to human subjects. There was no language limitation. The titles and abstracts of potentially relevant studies identified by the computerized search were reviewed. Full-text articles were obtained for detailed evaluation, and eligible studies were included in the systematic review. The inclusion criteria were the following: randomized controlled trial, clinical trials, and series cases; patients who were of either sex and had been diagnosed with COVID-19; all patients with laboratory-identified SARS-CoV-2 infection who had had both real-time reverse-transcriptase polymerase-chain-reaction detection of SARS-CoV-2 nucleic acid positive in throat swabs or lower respiratory tract, and CT scanning of the lung; inclusion of the epidemiological, clinical characteristics, laboratory and radiological characteristics, and treatment and outcome; clear description of the clinical characteristics such as comorbidities including hypertension, diabetes, cardiovascular disease, and malignancy and the signs and symptoms such as fever, cough, fatigue, and dyspnea; clear description of the outcomes including the major complications such as acute respiratory distress syndrome (ARDS), acute cardiac injury(ACI), acute kidney injury(AKI), shock, and incidence of severity and mortality.
The exclusion criteria were the following: absence of clinical characteristics, treatment outcome, clinical experience, and case reports.
2.2. Data collection and extraction
Two authors independently extracted data by reviewing all titles and abstracts of the searched papers. If any disagreement on the choice of the literature exists, a third evaluator will join in to make the decision. The following information was recorded from the included trials: first author, year of publication, number of participants, and residence of patients. Basic data about gender, age, and diagnosis were extracted and analyzed. To evaluate the proportion of comorbidity such as diabetes, hypertension, cardiovascular disease, and malignancy, the proportion of symptoms such as fever, cough, fatigue, and dyspnea, the proportion of clinical complications including ARDS, ACI, AKI, and shock, and the severity and mortality.
2.3. Quality assessment and risk of bias
Two readers independently extracted and reviewed the data from the enrolled studies to ensure consistency. The quality of the included studies was assessed by the Newcastle–Ottawa Scale. In order to objectively evaluate the publication bias of the included studies, the Egger test with P < 0.05 as the existence of publication bias was performed, and those with larger values were considered as having no publication bias.
2.4. Statistical methods
The single arm meta-analysis of proportions (and 95 %CI) was calculated for the clinical symptoms, complication, outcome, and for each of the selected comorbidities using STATA 15.0. The presence of heterogeneity among the identified studies (Cochran’s Q) and the extent of heterogeneity (I 2 index) were examined, as described previously. All original data included in the literature were first transformed by double arcsine method to make them conform to normal distribution and then analyzed in Stata. The initial conclusion obtained by Meta-analysis was then restored using formula (P=(sin(tp/2)) 2) to reach final conclusion.
2.5. Sensitivity analyses
We performed sensitivity analysis to assess the stability of the results and investigate the influence of each study by omitting a single study sequentially. Using the Egger test, we found no evidence of bias in any of the lag periods.
3. Results
3.1. Included trial characteristics and quality assessment
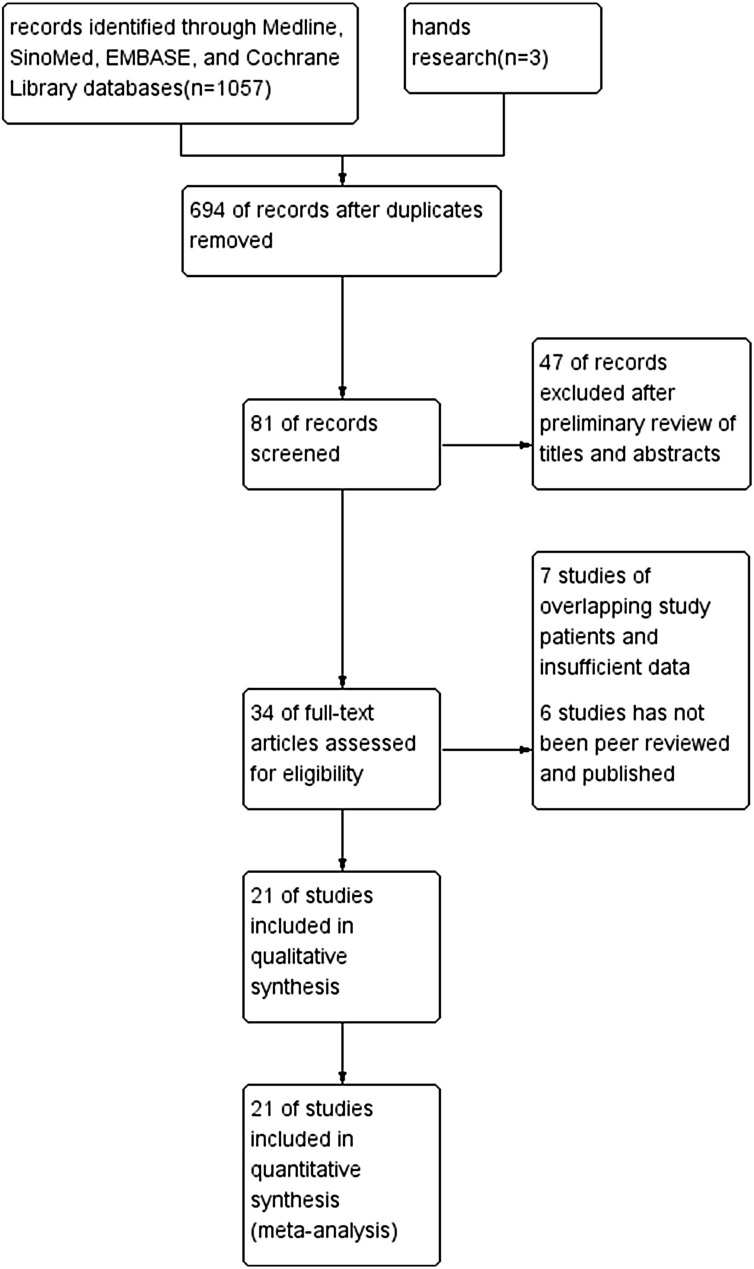
The initial 1057 citations were identified based on a study of the subject and a summary of the literature, of which 694 articles were thereafter excluded because of duplication. After reviewing the title and abstract of the remaining 81 studies, only 34 full-text studies were evaluated for further assessment, and 13 obviously irrelevant records were excluded. Eventually, 21 clinical studies [6,17,[24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42]] were consistent with the inclusion requirements. A detailed study flow-diagram is shown in Fig. 1 . The basic characteristics and quality of the included studies were illustrated in Table 1 .
Fig. 1.
Flow diagram for selection of studies for inclusion in this meta-analysis.
Table 1.
Main characteristics and quality of the included studies.
| Study | Deadline (mm.yy) | City, country | Total patients | Male (%) | Age† (years) | Study design | Quality |
|---|---|---|---|---|---|---|---|
| Chaolin Huang et al. | By Jan 2 2020 | Wuhan,china | 41 | 30(73 %) | 49·0 (41·0–58·0) | Retrospective Study | 7 |
| Jin-jin Zhang et al. | Jan 16 to Feb 3 2020 | Wuhan, China | 140 | 71(51 %) | 57 (25−87) | Retrospective Study | 7 |
| DaweiWang et al. | Jan 1 to Jan 28 2020 | Wuhan, China | 138 | 75(54 %) | 56 (42−68) | Retrospective Study | 7 |
| Nanshan Chen et al. | Jan 1 to Jan 20 2020 | Wuhan, China | 99 | 67(68 %) | 55.5(13.1) | Retrospective Study | 6 |
| Wei-jie Guan et al. | By Jan 29 2020 | 31 provinces,China | 1099 | 640(58 %) | 47 (35–58) | Retrospective Study | 8 |
| Kui Liu et al. | Dec30,2019 -Jan 24 2020 | Wuhan, China | 137 | 61(45 %) | 57 (20–83) | Retrospective Study | 7 |
| Wenjie Yang et al. | Jan17 to Feb 10, 2020 | Wenzhou, China | 149 | 81(54 %) | 45 ± 13 | Retrospective Study | 6 |
| Yingxia Liu et al. | By Jan 21 2020 | Shenzhen, China | 12 | 8(67 %) | 52 ± 17 | Retrospective Study | 5 |
| Xiaobo Yang et al. | by Jan 26 2020 | Wuhan, China | 52 | 35(67 %) | 59 (13) | Retrospective Study | 6 |
| Jian Wu et al. | Jan 22 to Feb 14, 2020 | Jiangsu, China | 80 | 39(49 %) | 46 ± 15 | Retrospective Study | 6 |
| Xi Xu et al. | Jan 23 to Feb 4, 2020 | Guangzhou, China | 90 | 39(43 %) | 50(18–86) | Retrospective Study | 7 |
| Xiao-Wei Xu et al. | Jan 10 to Jan 26 2020 | Hangzhou, China | 62 | 35(56 %) | 41(32−52) | Retrospective Study | 7 |
| Wen Ke et al. | Jan 20 to Feb 8, 2020 | Beijing, China | 46 | 27(58 %) | 41.8 ± 16.3 | Retrospective Study | 6 |
| Sijia Tian et al. | By Feb 10, 2020 | Beijing, China | 262 | 127(48 %) | 47.5(1–94) | Retrospective Study | 7 |
| Chen Lei et al. | Jan, 2020 | Wuhan, China | 29 | 21(72 %) | 56(26–79) | Retrospective Study | 6 |
| Fengxiang Song et al. | Jan 20 to Jan 27, 2020 | Shanghai, China | 51 | 25(49 %) | 49 ± 16 | Retrospective Study | 7 |
| EdwardYoung et al. | Jan 23 to Feb 3, 2020 | Singapore | 18 | 9(49 %) | 47(31−73) | Retrospective Study | 6 |
| Yu-Huan Xu et al. | Jan to Feb, 2020 | Beijing, China | 50 | 29(58 %) | Retrospective Study | 5 | |
| Kunhua Li et al. | Jan to Feb, 2020 | Chongqing, China | 83 | 44(53 %) | 45.5(12.3) | Retrospective Study | 7 |
| Yihui Huang et al. | Dec 2019 to Jan 2020 | Wuhan, China | 34 | 14(41 %) | 56 ± 17 | Retrospective Study | 6 |
| China CDC et al. | by Feb 11 2020 | China | 44,672 | 22,981(51 %) | Retrospective Study | 6 |
Mean (SD) or median (IQR). China CDC, center for disease control and prevention.
3.2. Details of the trial process
Twenty-one studies were selected, with a total of 47,344 patients (24,419 male and 22,925 females), representing approximately 40 % of the WHO confirmed. One clinical trial investigated the characteristics of patients in Singapore and all others came from China, mainly in Wuhan. Systematic analysis of the studies describing the epidemiological, demographic, and clinical features of COVID-19 cases and reporting of the prevalence of a number of chronic diseases in the infectious disease was undertaken. The number of cases in the selected studies varied by approximately 3722-fold and ranged from 12 to 44,672 cases. The sex ratio (male to female) was 1.06 and the overall average age of the subjects was greater than 40 years. Forty-one cases reported in Jinyintan hospital are the earliest confirmed cases, patients treated in Wenzhou hospital almost are mild, but Xiaobo Yang et al. reported 52 cases with COVID-19 that were all critical patients. All studies detailed the pre-treatment biochemical characteristics and subsequent treatment.
3.3. Meta-analysis results
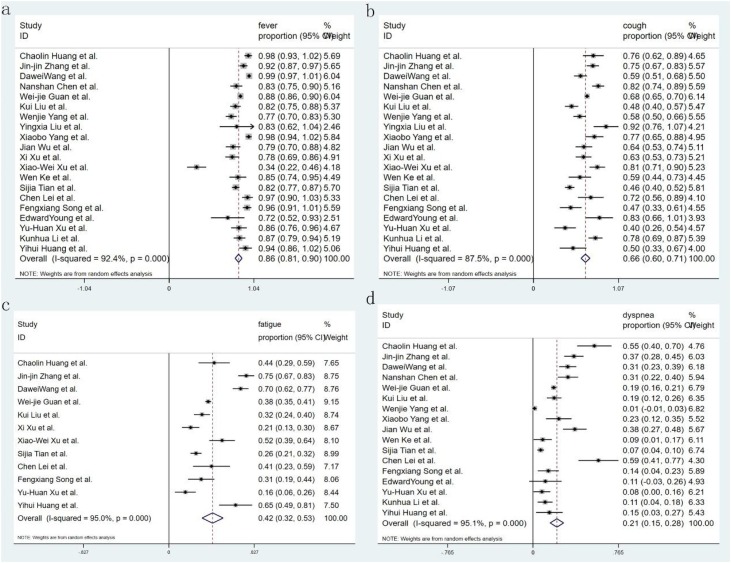
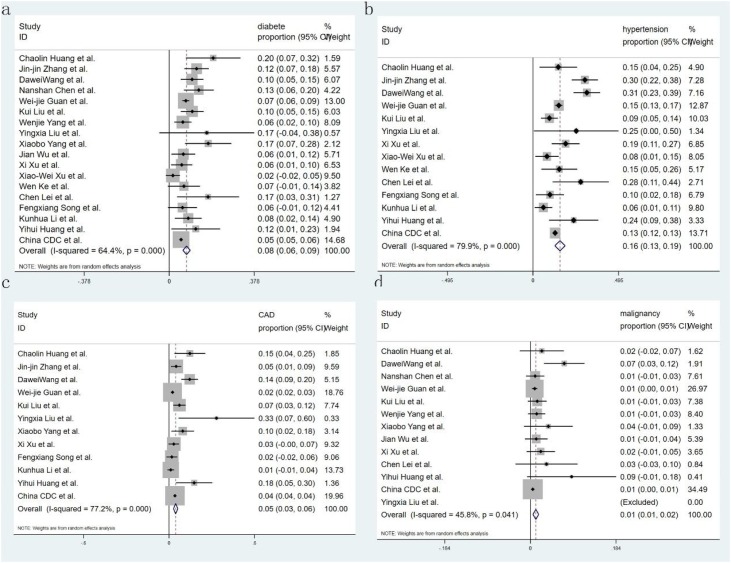
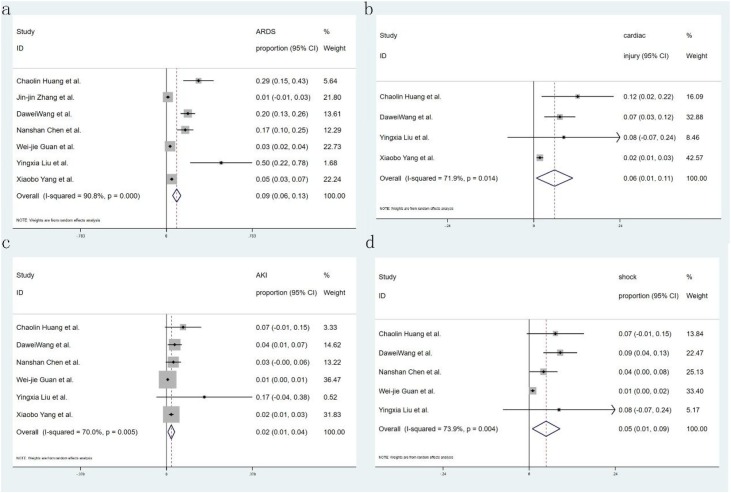
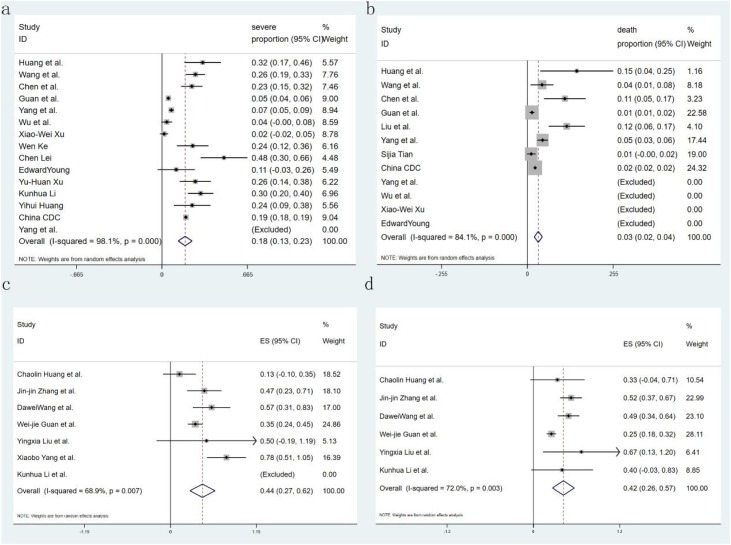
Eighteen studies reported the comorbidity of diabetes, only 7 of them introduced the proportion of severity, 14 studies reported hypertension and 6 of them introduced the proportion of severity, 13 studies reported malignant, and 12 studies reported CAD; with regard to symptoms, 20 studies recorded fever and cough, 12 studies reported fatigue and 15 studies reported dyspnea. Seven studies recorded the complication of ARDS and ACI, and shock was recorded in 4 and 5 studies respectively. Fifteen studies showed critical patients, and 12 research reported mortality. All studies provided incidence data of at least one kind of comorbidity, symptom, or complication. Sixteen forest plots were used to illustrate the prevalence of comorbidities in COVID-19 from the selected studies and to inspect the heterogeneity of the individual findings. Meta-analysis of the identified studies showed that the most prevalent clinical symptoms were fever 85.6 % (95CI 81.3–89.9 %) and cough 65.7 % (95CI 60.1–71.4 %), followed by fatigue 42.4 % (95CI 32.2–52.6 %) and shortness of breath 21.4 % (95CI 15.3–27.5 %) (Fig. 2 ). There was significant heterogeneity (Cochran’s Q) in the estimates of clinical symptoms among the examined studies (p < 0.001) with an I2 index varying from 87.4 % to 95.1 %. The prevalence of diabetes and hypertension comorbidities was 7.7 % (95CI 6.1–9.3 %) and 15.6 % (95CI 12.6–18.6 %), respectively. The incidence of cardiovascular disease and malignancy was 4.7 % (95CI 3.1–6.2 %) and 1.2 % (95CI 0.5–1.8 %), respectively (Fig. 3 ). The case complications including ARDS risk ranged from 5.6 to 13.2 %, with the pooled estimate at 9.4 %. Acute cardiac injury 5.8 % (95CI 0.7–10.8 %), Acute kidney injury 2.1 % (95CI 0.6–3.7 %), and shock 4.7 % (95CI 0.9–8.6 %) were also present (Fig. 4 ). The risks of severity and mortality rate ranged from 12.6 to 23.5 % and from 2.0 to 4.4 %, with pooled estimates at 18.0 and 3.2 %, respectively. The percentage of severe cases in diabetes and hypertension cases was 44.5 % (95CI 27.0–61.9 %) and 41.7 % (95CI 26.4–56.9 %), respectively (Fig. 5 ).
Fig. 2.
Forest plot of the prevalence of symptoms in patients with COVID-19. Weights were calculated from binary random-effects model analysis. (a. fever. b. cough. c. fatigue. d. dyspnea). CI = confidence interval, COVID-19=Corona Virus Disease 2019.
Fig. 3.
Meta-analysis of the proportion of comorbidities in COVID-19 cases. Weights were calculated from binary random-effects model analysis. Values represent proportions of diabetes (a), hypertension (b), CAD (c), and malignancy (d). CAD = coronary artery disease, COVID-19=Corona Virus Disease 2019.
Fig. 4.
Forest plot of the incidence of complications in patients with COVID-19. (a. ARDS. b. ACI. c. AKI. d. shock), CI = confidence interval. ARDS = acute respiratory distress syndrome, ACI = acute cardiac injury, AKI = acute kidney injury, COVID-19=Corona Virus Disease 2019.
Fig. 5.
Forest plot of the incidence of severity and mortality in patients with COVID-19. (a. severe rate. b. mortality), meta-analysis of the severe rate of COVID-19 patients with diabetes (c), or hypertension (d). CI = confidence interval, COVID-19=Corona Virus Disease 2019.
3.4. Sensitivity analysis and publication bias
Sensitivity analysis was performed to assess the stability of pooled results. Among the 21 studies, the significant results were not obviously altered after sequentially omitting each study. In the pooled results, comparing the incidence of mortality after excluding the report by Chaolin Huang et al., the heterogeneity decreased significantly (OR = 0.719, 95 % CI = 0.277–1.865, P = 0.497, I² = 28 %) and showed that there was no significant difference in preventing the ARDS rate between the two groups; hence, it was regarded as a result of heterogeneity. Likewise, the other studies were considered as the source of heterogeneity because the heterogeneity significantly changed and showed that there was no significant difference in preventing ARDS between the two groups when each of these studies were excluded from the pooled results comparing the incidence of ARDS. A sensitivity analysis was conducted to determine whether the exclusion of this study would alter the result, and exclusion of this study from the meta-analysis did not substantially influence the results.
In this part of the study, ARDS was used to assess publication bias. Egger test results showed Pr > jzj = 1.00. Therefore, we believe that the risk of publication bias is low in this meta-analysis.
4. Discussion
4.1. Summary of the main results
The outbreak of COVID-19 has been declared a Public Health Emergency of International Concern by WHO. By March 10, 2020, the epidemic had spread to 25 countries around the world. In accordance with the experience of China, surgical masks are in widespread use which is beneficial to prevent the acquisition of COVID-19. In addition, Italy started taking measures to block traffic and lockdown villages. With the rising incidence of COVID-19 all over the world, feasible and effective messaging about the clinical characteristics of COVID-19 is greatly needed. Therefore, the focus of this analysis was to evaluate the prevalence of symptoms, comorbidity, complications, and different outcomes in China and Singapore. The meta-analysis identified 21 published studies that assessed this proportion. There are few published RCTs because of the rapid development of the epidemic and there are limited medical resources in addition to the presence of uncontrollable risk during treatment. Much of the evidence of effects cannot be adequately studied in randomized trials, such as long-term and rare outcomes. Therefore, we analyzed all retrospective studies in this study. For the main results, we found that the cases reported to date suggest that most are older adults, and there is no difference in susceptibility between male and female. Fever is the most common symptom in patients with 2019-nCoV infection. The most prevalent comorbidities are hypertension and diabetes which are associated with the rapid development of severe illness. ARDS and ACI may be the main obstacles for patients to treatment recovery. The severity and mortality of patients with 2019-nCoV infection is lower than that of Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS), but the prevalence of COVID-19 is much higher than SARS and the MERS.
4.2. Comparison with previous studies
Two coronaviruses have previously caused significant outbreaks associated with more critical disease: the SARS coronavirus in 2002–2003 and the Middle East respiratory syndrome coronavirus that emerged in 2012 [[43], [44], [45]]. A lot of studies have reported the clinical characteristics of patients with COVID-19, but there is a lack of studies on the relation of severity and mortality with comorbidity. Similarly, there is no meta-analysis published on complications for patients COVID-19 infected. Therefore, this is a novel systematic review and meta-analysis. Due to the inadequate evidence, we present this meta-analysis by consolidating multiple studies to enable enhanced clinical decision making in the future. We report here a cohort of patients with laboratory-confirmed 2019-nCoV infection. Patients had serious, sometimes fatal, complications and were admitted to the designated hospital. Clinical presentations greatly resemble SARS-CoV. Patients with severe illness developed ARDS, AKI, ACI, or shock and required ICU admission and oxygen therapy.
4.3. Limitations of the study
However, despite a comprehensive analysis, there are also many limitations that should be taken into consideration in our meta-analysis. First, the studies included in the meta-analysis were not all RCTs. Second, in the literature-included studies, the treatment of every hospital is not completely similar. Third, owing to the treatment by grading of mild, ordinary, and critical, the severity of patients in different hospitals varies greatly. In addition, as the cause and physio pathological mechanism was unknown at the onset of emerging infections, the treatment in Wuhan was only based on clinical experience, leading to a higher complication and mortality. Fourth, partial missing information in a few articles may lead to biased results. We have attempted to contact investigators or study sponsors to verify key study characteristics and obtain missing numerical outcome data. Moreover, clinical and methodological heterogeneities were observed in several parameters in the meta-analysis given the variation in intervention techniques, patient composition, and preferences among different cities. True heterogeneity and poor methodological quality could also lead to an asymmetric plot. In the future, larger, higher quality clinical trials should be conducted, and we will conduct a more detailed subgroup analysis to explore the sources of heterogeneity to obtain a more reliable conclusion and more effort should be made to answer these questions in future studies.
5. Conclusion
COVID-19 is an emerging infectious disease of global public health concern. Our results note the similarity of clinical symptoms and complications between COVID-19 and previous beta-coronavirus infections. The incidence of severity and mortality of COVID-19 is much higher than that of ordinary influenza, and the prevalence of chronic diseases including diabetes and hypertension is rising as populations age and lifestyle and dietary habits change. In addition, we found that diabetes and hypertension are closely related to severity and mortality. Improving the protection against COVID-19 in persons with chronic disorders is essential. Last, but not least, there is a need to limit human-to-human transmission, including reducing secondary infections among close contacts and health-care workers, thus preventing transmission amplification events and preventing further international spread.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102433. Feb 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohrabi C., Alsafi Z., O’Neill N. World Health Organization declares global emergency: a review of the 2019 Novel Coronavirus (COVID-19) Int. J. Surg. (Lond. Engl.) 2020 doi: 10.1016/j.ijsu.2020.02.034. Feb 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanmugaraj B., Malla A., Phoolcharoen W. Emergence of novel coronavirus 2019-nCoV: need for rapid vaccine and biologics development. Pathogens. 2020;9(2) doi: 10.3390/pathogens9020148. Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet (Lond. Engl.) 2020;395(10225):689–697. doi: 10.1016/s0140-6736(20)30260-9. Feb 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y.C., Chen C.S., Chan Y.J. The outbreak of COVID-19: an overview. J. Chin. Med. Assoc. 2020;83(3):217–220. doi: 10.1097/jcma.0000000000000270. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (Lond. Engl.) 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020 doi: 10.1038/s41564-020-0695-z. Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 doi: 10.1038/s41586-020-2012-7. Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benvenuto D., Giovanetti M., Ciccozzi A., Spoto S., Angeletti S. The 2019-new coronavirus epidemic: evidence for virus evolution. J. Med. Virol. 2020;92(4):455–459. doi: 10.1002/jmv.25688. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu F., Zhao S., Yu B. A new coronavirus associated with human respiratory disease in China. Nature. 2020 doi: 10.1038/s41586-020-2008-3. Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020 doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu A., Peng Y., Huang B. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.02.001. Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet (Lond. Engl.) 2020;395(10224):565–574. doi: 10.1016/s0140-6736(20)30251-8. Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science (New York, N.Y.) 2020 doi: 10.1126/science.abb2762. Mar 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wrapp D., Wang N. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020 doi: 10.1126/science.abb2507. Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellewell J., Abbott S., Gimma A. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob. Health. 2020 doi: 10.1016/s2214-109x(20)30074-7. Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai Y., Yao L., Wei T. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. Feb 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020 doi: 10.1016/s1473-3099(20)30113-4. Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Huijun, Guo Juanjuan, Wang Chen. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020 doi: 10.1016/s0140-6736(20)30360-3. February 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W., Du R.H., Li B. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg. Microbes Infect. 2020;9(1):386–389. doi: 10.1080/22221751.2020.1729071. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J.J., Dong X., Cao Y.Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. Feb 19. [DOI] [PubMed] [Google Scholar]
- 25.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (Lond. Engl.) 2020;395(10223):507–513. doi: 10.1016/s0140-6736(20)30211-7. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu K., Fang Y.Y., Deng Y. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020 doi: 10.1097/cm9.0000000000000744. Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang W., Cao Q., Qin L. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020 doi: 10.1016/j.jinf.2020.02.016. Feb 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020 doi: 10.1016/s2213-2600(20)30079-5. Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J., Liu J., Zhao X. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa199. Feb 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X., Yu C., Qu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging. 2020 doi: 10.1007/s00259-020-04735-9. Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu X.W., Wu X.X., Jiang X.G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ke Wen, Li Wengang, Dawei Zhang. Epidemiological and clinical characteristics of 46 newly-admitted coronavirus disease 2019 cases in Beijing. Zhonghua Liuxingbing za zhi. 2020;38 doi: 10.3760/cma.j.cn311365-20200219-00086. 2020-02-26. [DOI] [Google Scholar]
- 35.Tian S., Hu N., Lou J. Characteristics of COVID-19 infection in Beijing. J. Infect. 2020 doi: 10.1016/j.jinf.2020.02.018. Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L., Liu H.G., Liu W. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Chin. J. Tuberculosis Respir. Dis. 2020;43(0):E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005. Feb 6. [DOI] [PubMed] [Google Scholar]
- 37.Liu P., Tan X.Z. 2020. 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Feb 4: 200257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young B.E., Ong S.W.X., Kalimuddin S. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020 doi: 10.1001/jama.2020.3204. Mar 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y.H., Dong J.H., An W.M. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J. Infect. 2020 doi: 10.1016/j.jinf.2020.02.017. Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li K., Wu J., Wu F. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest. Radiol. 2020 doi: 10.1097/rli.0000000000000672. Feb 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Y., Tu M., Wang S. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: a retrospective single center analysis. Travel Med. Infect. Dis. 2020 doi: 10.1016/j.tmaid.2020.101606. Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua liu xing bing xue za zhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. Feb 17. [DOI] [PubMed] [Google Scholar]
- 43.Liu J., Zheng X., Tong Q. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J. Med. Virol. 2020 doi: 10.1002/jmv.25709. Feb 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020 doi: 10.12932/ap-200220-0772. Feb 27. [DOI] [PubMed] [Google Scholar]
- 45.Sun Z., Thilakavathy K. Potential factors influencing repeated SARS outbreaks in China. Int. J. Environ. Res. Public Health. 2020;17(5) doi: 10.3390/ijerph17051633. Mar 3. [DOI] [PMC free article] [PubMed] [Google Scholar]