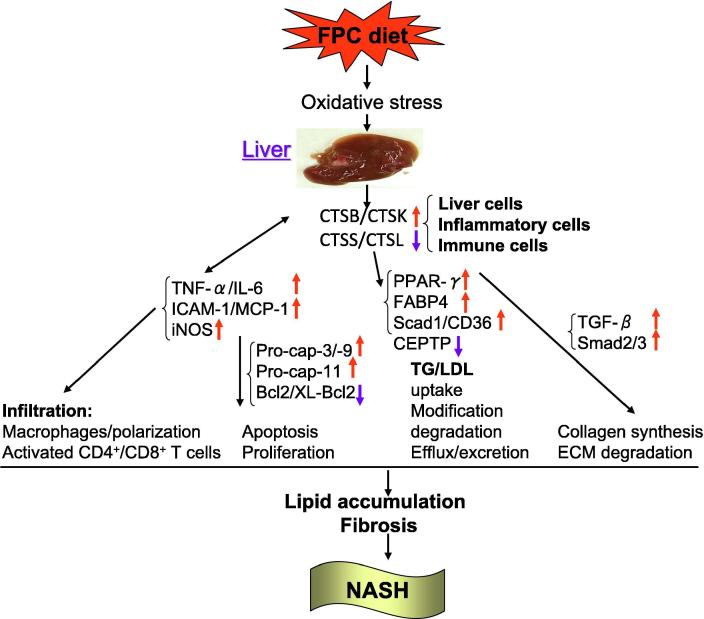
It is well-known that there is close relationship between cardiovascular disease (CVD) and non-alcoholic fatty liver disease (NAFLD). NAFLD, defined as an accumulation of lipid droplets and triglycerides in the liver in the absence of alcohol intake, is becoming one of the most common chronic liver diseases worldwide [1]. Among NAFLD, non-alcoholic steatohepatitis (NASH) has been shown to develop into more severe stages (e.g., hepatocellular carcinoma and cirrhosis), leading eventually to liver failure and transplantation [1]. Thus, the elucidation of the transition from steatosis, known as the non-progressive status of the disease, to NASH is key to a deeper understanding of NAFLD initiation and progression. Lipid accumulation (fatty liver)-based NASH is a metabolic disorder characterized by extensive inflammation and remodeling of the extracellular matrix architecture of the liver organ and associated fibrosis. Although several proteolytic systems such as matrix metalloproteinase (MMP) and serine protease families participate in the pathogenesis of NAFLD and its progression, recent evidence suggests that a ubiquitously expressed cysteine protease is a member of lysosomal cathepsin (CTS) family, which has been implicated in multiple chronic metabolic and inflammatory disorders, including NAFLD, liver fibrosis, atherosclerosis, and cancer (reviewed previously[2]). Human biopsy tissues of liver inflammatory diseases overexpress CTSS, CTSB, and CTSL but show a relatively reduced expression of their endogenous inhibitor, cystatin C, suggesting a shift in the imbalance between CTSs and their inhibitor that favors liver lipid accumulation and fibrosis (Fig. 1). The sera of patients with cirrhosis and hepatocellular carcinoma show greater CTSB and CTSL levels than those of controls. CTSB inactivation suppresses hepatic injury and fibrosis, but its overexpression promotes liver fibrosis [3], [4].
Fig. 1.
Illustration of CTS function in pathogenesis of NAFLD. Cathepsins can originate from hepatocytes, Kupffer cells, macrophages (Mac), and activated T cells under inflammatory conditions. NAFLD, Non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; CTSB, cathepsin B, CTSK, cathpsin K; LDL, low density lipoprotein; TG, triglyceride; Pro-cap-3, pro-caspase-8; FPC, fructose-palmitate-cholesterol; CETP, cholesteryl esters and triglycerides transporter; Ppar-γ, peroxisome proliferator activated receptor-γ; Fabp4, fatty acid binding protein 4; Scad1, short-chain acyl dehydrogenase-1; TGF-β, transforming growth factor-β; TNF-v: tumor necrosis factor-α; MCP-1, monocyte chemoattractant protein-1; ICAM-1, intercellular adhesion molecule-1; iNOS, inducible nitric oxide synthase; IL-6, interleukin-6.
Although accumulating evidence suggests that CTSs play essential roles in the various liver diseases, little is known about the functional relevance of this enzyme family in the pathogenesis of NAFLD. A comprehensive review summarized the roles of the CTS family, especially CTSB and CTSK, in lipid metabolism [5]. If a macrophage takes up oxidized low-density lipoprotein (LDL), it relocates CTSB from the lysosome into the cytosol, probably as a result of the disruption of the lysosomal membrane [6]. CTSs may also be involved in oxidized LDL degradation. Pharmacological inhibition of CTSB suppressed naturally occurring modified LDL degradation in vascular cell lysates by 41% under acidic conditions. Degradation of apoB-100 by CTSK, but not by CTSS, led to the fusion and aggregation of LDL particles and increased LDL’s ability to bind proteoglycans, subsequently leading to the accumulation of extracellular lipid droplets [5]. Now, as discussed in the article by Fang et al. [7] in this issue of Translational Research, upregulated CTSB expression is believed to be an important mediator of lipid accumulation via the regulation of liver lipid transport and lipogenesis in CTSB wild-type (CTSB+/+) NASH mice (Fig. 1). This concept was supported by the findings that fructose-palmitate-cholesterol (FPC) increased levels of fatty acid uptake-related CD36 (known as an extracellular and mitochondrial lipid carrier) and of a peroxisome proliferator-activated receptor-γ gene/protein as well as free-fatty acid transport marker Fabp4 and lipogenesis marker Scad1. These harmful changes were reversed by CTSB deficiency (CTSB−/−) (Fig. 1). A limited experimental study reported that CSTF and CTSS reduced the ability of cholesterol efflux from macrophages by 50% in vitro due to proteolysis of pre-high-density lipoprotein [8]. Thus, these findings indicate that the ability of CTSB to modulate lipid uptake, storage, and efflux is likely to contribute to liver lipid accumulation in NASH mice under experimental conditions.
Fang et al. discussed the different expression patterns of investigated CTSs (CTSB and CSTK CTSS, CTSL) in liver specimens from CTSB+/+ mice fed low-fat and FPC diets, and revealed a possible inflammatory mechanism by which infiltrated inflammatory cells lead to increased CTS-related collagen synthesis and degradation [7]. First the authors showed that, as with inflammatory lesions, liver specimens of CTSB+/+ NASH mice contained not only increased inflammatory cytokines-related genes (tumor necrosis factor-α, monocyte chemoattractant protein-1, intercellular adhesion molecule-1, inducible nitric oxide synthase, and interleukin-1β)- and collagen synthesis-related (transforming growth factor-β, Samd2/3, collagen III)-related genes but also extensive activated macrophages. After Fang et al. characterized the liver metabolic disorder, they reported here for the first time that very high CTSB expression induced by inflammatory cytokines may lead to increased collagen synthesis and accumulation to fibrosis (Fig. 1) [7]. In addition, multiple studies have been performed to explain the underlying mechanisms of liver fibrosis, including excessive proteolysis-related extracellular matrix degradation and fibrosis. Recent lines of evidence highlight the importance of CTSs induced by inflammatory cytokines in the initiation and progression of cardiovascular disease [2] . Augmented CTS expression and activity could provide a proteolytic mechanism for liver remodeling, which is associated with the activation of a cytokine signaling pathway in the case of NAFLD. The importance of this finding, and the increased inflammation-related proteolysis, is supported by the finding that liver specimens from NASH lesions had an abundance of infiltrated adaptive CD4+ and CD8+ T-cells, and these cells also an important source of CTSs.
Fang et al. studied another possible mechanism by which CTS is involved in cell death. In the present study, the authors observed that FPC diet-induced NASH CTSB+/+ mice had increased levels of CTSB and tumor necrosis factor-α in liver tissues; and CTSB−/− reversed these changes [7]. Tumor necrosis factor-α modulates CTSB expression and proapoptotic caspases-3/-9 in macrophages and cancer cells [9]. CTSB has been shown to activate pro-caspase-3, pro-caspase-9, and BH3-interacting domain death agonist as a mechanism to modulate cell apoptosis. Similar to the role of mitochondrial factors in the activation of proapoptotic caspases, CTSB may activate specific proinflammatory caspase-11 based on the data of an affinity purification assay with the biotinylated broad spectrum caspase inhibitor z-VAD-fmk. CTSB also degrades NLRP10, an NACHT, LRR, and PYD domain-containing protein (NLRP) that negatively regulates NLRP3. NLRP3, pro-caspase-3, and pro-caspase-9 form the inflammasome complex, which facilitates the activations of immature caspase-1 and inflammatory cytokines (pro-interleukin-1β and pro-interleukin-18) in oxidative stress-induced apoptosis [10]. In addition, strikingly, a recent comprehensive review highlighted that the antiapoptotic molecules Bcl-2, Bcl-xL, Mcl-1, and X chromosome-linked inhibitor of apoptosis are sensitive to CTSB, CTSL, and CTSH in several human tumor cell lines (reviewed in reference[11]). Recently, we clearly demonstrated that CTSK activity controls vascular smooth muscle apoptosis via the activation of pro-caspase-8 in injury-related neointimal hyperplasia in mice [12], [13]. CTSK-mediated Notch-1 activation induces vascular endothelial apoptosis and proliferation in neovascularization [14]. It has been shown that oxidized low-density lipoprotein induced lysosomal destabilization, leading to the leakage of CTSB and to the activation of caspases and subsequent macrophage apoptosis [11]. Thus, although there is no direct evidence, the CTSB-mediated proapoptotic mechanism may also apply to hepatic cells in the progression of NASH.
The literature highlights the evaluation of serum CTS levels as biomarkers of inflammatory disease, much like the use of MMP. For example, CTSS and CTSK were the first cysteine proteases found to show increased protein levels in human atherosclerotic plaques of aortic aneurysms when cystatin C was decreased [2]. Similarly, CTSB expression also was increased in atherosclerotic complicated lesions containing thrombi of human aortic aneurysms compared to control arteries. Our group obtained evidence of a positive correlation between plasma CTSK and high-sensitivity C-reactive protein levels [15], which reinforces other findings on the association between the CTS family and inflammatory processes, suggesting that the evaluation of circulating CTS levels may be useful for predicting inflammatory diseases such as NAFLD. However, there has been only a limited effort to determine whether increased circulating CTSB levels can serve as specific predictive biomarkers of NAFLD.
Important limitations of the present study include the fact that it used an FEC-induced mouse NASH model, which is still just an animal model that could not fully mimic human NASH or satisfactorily present its molecular mechanism. In the present study, the main findings clearly demonstrated that CTSB deletion prevented liver lipid deposition and fibrosis via the amelioration of liver lipid transport and lipogenesis as well as inflammation. However, the main regulator and cell source for CTSB expression and activation remain unclear, as does the exact role of CTSB in triglyceride and lipid intestinal absorption, transport to liver, and metabolism. One of the important clinical issues requiring consideration is whether human heterozygous and homozygous CTSB exhibit liver-beneficial phenotypes. A larger-scale clinical cohort study including pharmacological intervention with a CTSB inhibitor would be useful to address some of these issues.
It is thus possible that, similar to constant hepatic MMP activation, CTS activation may promote hepatic triglyceride and lipid accumulation as well as the fibrosis process by the negative regulations of lipid metabolism and of interstitial matrix digestion (Fig. 1). These findings have important implications, not only for our understanding of inflammatory NASH but also because they indicated that these proteolytic enzymes may also evolve into useful therapeutic targets in this and other fields such as the treatment of metabolic and inflammatory CVD with NAFLD.
Sources of funding
This work was supported in part by a grant from The Scientific Research Fund of the Chinese Ministry of Education (81770485).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
All authors have read the journal’s policy on disclosure of potential conflicts of interest and have none to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100516.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Segovia-Miranda F., Morales-Navarrete H., Kucken M., Moser V. Three-dimensional spatially resolved geometrical and functional models of human liver tissue reveal new aspects of nafld progression. Nat Med. 2019;25:1885–1893. doi: 10.1038/s41591-019-0660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu H., Du Q., Dai Q. Cysteine protease cathepsins in atherosclerotic cardiovascular diseases. J Atheroscler Thromb. 2018;25:111–123. doi: 10.5551/jat.RV17016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moles A., Tarrats N., Fernandez-Checa J.C. Cathepsin b overexpression due to acid sphingomyelinase ablation promotes liver fibrosis in niemann-pick disease. J Biol Chem. 2012;287:1178–1188. doi: 10.1074/jbc.M111.272393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canbay A., Guicciardi M.E., Higuchi H. Cathepsin b inactivation attenuates hepatic injury and fibrosis during cholestasis. J Clin Invest. 2003;112:152–159. doi: 10.1172/JCI17740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutgens S.P., Cleutjens K.B., Daemen M.J. Cathepsin cysteine proteases in cardiovascular disease. FASEB J. 2007;21:3029–3041. doi: 10.1096/fj.06-7924com. [DOI] [PubMed] [Google Scholar]
- 6.El-Sherbeini M., Geissler W.M., Pittman J. Cloning and expression of staphylococcus aureus and treptococcus pyogenes murd genes encoding uridine diphosphate n-acetylmuramoyl-l-alanine:D-glutamate ligases. Gene. 1998;210:117–125. doi: 10.1016/s0378-1119(98)00059-6. [DOI] [PubMed] [Google Scholar]
- 7.Fang W., Deng Z., Benadjaoud F., Yang C. Cathepsin B deficiency ameliorates liver lipid deposition, inflammatory cell infiltration, and fibrosis after diet-induced nonalcoholic steatohepatitis. Transl Res. 2020 doi: 10.1016/j.trsl.2020.04.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindstedt L., Lee M., Oorni K. Cathepsins f and s block hdl3-induced cholesterol efflux from macrophage foam cells. Biochem Biophys Res Commun. 2003;312:1019–1024. doi: 10.1016/j.bbrc.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Cheng X.W., Shi G.P., Kuzuya M. Role for cysteine protease cathepsins in heart disease: Focus on biology and mechanisms with clinical implication. Circulation. 2012;125:1551–1562. doi: 10.1161/CIRCULATIONAHA.111.066712. [DOI] [PubMed] [Google Scholar]
- 10.Tschopp J., Schroder K. Nlrp3 inflammasome activation: The convergence of multiple signalling pathways on ros production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 11.Cheng X.W., Huang Z., Kuzuya M., Okumura K. Cysteine protease cathepsins in atherosclerosis-based vascular disease and its complications. Hypertension. 2011;58:978–986. doi: 10.1161/HYPERTENSIONAHA.111.180935. [DOI] [PubMed] [Google Scholar]
- 12.Hu L., Huang Z., Ishii H. Plf-1 (proliferin-1) modulates smooth muscle cell proliferation and development of experimental intimal hyperplasia. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.117.005886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H., Meng X., Piao L. Cathepsin s deficiency mitigated chronic stress-related neointimal hyperplasia in mice. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.011994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H., Cheng X.W., Shi G.P. Cathepsin k-mediated notch1 activation contributes to neovascularization in response to hypoxia. Nat Commun. 2014;5:3838. doi: 10.1038/ncomms4838. [DOI] [PubMed] [Google Scholar]
- 15.Li X., Li Y., Jin J. Increased serum cathepsin k in patients with coronary artery disease. Yonsei Med J. 2014;55:912–919. doi: 10.3349/ymj.2014.55.4.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.