Summary
Exit from the cell cycle during the establishment of quiescence and upon cell differentiation requires the sustained inactivation of CDK complexes. Fission yeast cells deprived of nitrogen halt cell cycle progression in pre-Start G1, before becoming quiescent or undergoing sexual differentiation. The CDK inhibitor Rum1 and the APC/C activator Ste9 are fundamental for this arrest, but both are down-regulated by CDK complexes. Here, we show that PP2A-B56Par1 is instrumental for Rum1 stabilization and Ste9 activation. In the absence of PP2A-B56Par1, cells fail to accumulate Rum1, and this results in persistent CDK activity, Ste9 inactivation, retention of the mitotic cyclin Cdc13, and impaired withdrawal from the cell cycle during nitrogen starvation. Importantly, mutation of a putative B56 interacting motif in Rum1 recapitulates these defects. These results underscore the relevance of CDK-counteracting phosphatases in cell differentiation, establishment of the quiescent state, and escape from it in cancer cells.
Subject Areas: Biological Sciences, Biochemistry, Cell Biology
Graphical Abstract

Highlights
-
•
PP2A-B56Par1 is required for cell-cycle arrest and mating upon nitrogen deprivation
-
•
Loss of Par1 impairs degradation of Cdc13 under nitrogen starvation
-
•
Absence of Par1 impedes proper dephosphorylation of Ste9 and accumulation of Rum1
-
•
Mutation of a Rum1 putative PP2A-B56 SLiM depicts similar defects as the loss Par1
Biological Sciences; Biochemistry; Cell Biology
Introduction
In all organisms, from the simplest prokaryote up to humans, cells have the ability to withdraw from the cell cycle when their environment does not support cell proliferation. In metazoans, a majority of the cells lie in a quiescent, non-dividing state prompted by signals emanating from the microenvironment and by the geometrical constraints that occur within tissues (reviewed in Fiore et al., 2018). However, they can re-enter the mitotic cycle upon stimulation when new cells are needed to replace old ones, or in the case of injury (reviewed in O'Farrell, 2011). Thus, maintaining the right balance between quiescence and proliferation is essential for tissue homeostasis and repair. Conversely, loss of this balance can lead to hyperproliferative diseases such as cancer. Cell-cycle exit is also intimately linked to the process of cell differentiation. As cells become more differentiated, their ability to proliferate decreases, to the extent that terminally differentiated cells acquire a post-mitotic state from which they can no longer re-enter the cell cycle. Moreover, the sensing of differentiation signals requires a lengthening of G1 phase, so that cell fate decisions are made before the cell is committed to a new round of division (reviewed in Dalton, 2015). In spite of quiescence and differentiation being different in many aspects, initiation of both processes relies on the sustained inactivation of cyclin-dependent kinase (CDK) complexes during G1, before the passage of the restriction point. Once this point has been traversed, cells engage in an autonomous program and are refractory to environmental signals. CDK inhibitors (CKIs) belonging to the CIP/KIP family (including p21, p27, and p57) are central players in the control of CDK activity during G1 and are essential during quiescence and differentiation (Zhang et al., 1999, Oesterle et al., 2011, Matsumoto et al., 2011). In particular, p21 restrains passage through the restriction point in the absence of mitogenic signaling and is critical to halt cell cycle progression in response to DNA damage (Deng et al., 1995, Barr et al., 2017, Barr et al., 2016, Heldt et al., 2018, Dulić et al., 1994).
In multicellular organisms quiescence serves the purposes of limiting growth, shaping the structure of tissues (O'Farrell, 2011), and protecting the stem cell niche (Cheung and Rando, 2013), whereas in unicellular systems, cell cycle exit is required to survive nutritional deprivation until better conditions arise. In the case of yeast cells, nitrogen starvation not only induces quiescence but also is the trigger for sexual differentiation, mating, and sporulation. Although the signals leading to cell cycle withdrawal in yeast and humans differ, the underlying mechanism for the arrest of cell cycle progression (CDK downregulation) is common to both systems. Hence, work in yeast might unveil new elements of control during quiescence that are also relevant in metazoans.
In this regard, the fission yeast Schizosaccharomyces pombe has proved an excellent model to study cell cycle progression and its modulation by environmental cues. During growth under optimal conditions the S. pombe cell cycle is characterized by a very short G1 phase and a long G2 phase, when most of the growth occurs. However, when the surrounding medium is poor in nitrogen, the distribution of the cell cycle changes dramatically, with a shortening of G2 and the prolongation of G1. In the extreme case of the complete depletion of a source of nitrogen, fission yeast cells arrest their cell cycle progression in G1 phase, before the restriction point (Start in yeast). Upon this initial arrest, they become quiescent or, in the presence of a differentiation stimulus (that is, the presence of a mating partner), they undergo sexual differentiation. The continued repression of CDK activity (which in S. pombe is solely provided by the CDK1 homolog Cdc2) in this situation is critical for the engagement of the transcriptional differentiation program (Kjaerulff et al., 2007) and to prevent commitment to a new round of division.
In the core of this G1 arrest lies the only CKI in fission yeast, Rum1, and the anaphase-promoting complex/cyclosome (APC/C) activator Ste9. They cooperate in the inhibition of G1-S and M-phase CDK complexes and prevent further activation of the M-CDK complex through the targeted degradation of the mitotic cyclin Cdc13 (Correa-Bordes and Nurse, 1995, Stern and Nurse, 1998, Moreno and Nurse, 1994, Kominami et al., 1998b, Kitamura et al., 1998, Yamaguchi et al., 1997, Correa-Bordes, 1997). Of note, Rum1 and Ste9 are themselves counteracted by CDK-mediated phosphorylation (Benito et al., 1998, Blanco et al., 2000), and this regulation results in double-negative feedback loops that are instrumental for the bistable behavior of the system. Under rich conditions, phosphorylation of Rum1 leads to its degradation by the SCFPop1/Pop2 (Skp1-Cullin1-F-box) (Kominami et al., 1998a, Kominami and Toda, 1997), whereas phosphorylation of Ste9 hinders its binding to the APC/C. Altogether this facilitates a rapid increase in CDK activity that drives cells into S-phase. Under restrictive growth conditions, however, the balance is tilted toward Rum1 and Ste9, and this leads to cell-cycle arrest. Here, we investigate whether a protein phosphatase activity contributes to the initial activation of Rum1 and Ste9 that triggers cell cycle exit in fission yeast. By doing so, we reveal a pivotal role of PP2A-B56 enzymes counteracting CDK phosphorylation of Rum1 that has significant consequences for cell differentiation. We characterize their interaction and show that PP2A-B56Par1 is essential for the timely accumulation of Rum1, CDK repression, and activation of Ste9 during the nitrogen starvation response. In addition, we find that this role can be extended to other situations that require stalling of cell cycle progression through G1 and therefore constitutes an important element of CDK control.
Results
PP2A-B56Par1 Activity Is Required for Cell-Cycle Arrest and Mating upon Nitrogen Deprivation
In fission yeast, the sexual differentiation response is closely linked to the sensing of nutritional deprivation that ultimately leads to CDK inhibition and the arrest of cell-cycle progression in G1. Therefore, we reasoned that if a protein phosphatase was required for the sustained downregulation of CDK activity at the end of the cell cycle, its loss would also affect the G1 arrest and mating response. To address this possibility, we investigated the mating efficiency upon nitrogen depletion (calculated as the proportion of zygotes and tetrads present in a homothallic culture) of mutants of the Cdc14-type phosphatase Clp1, of PP1, and of PP2A. PP2A enzymes are multimeric complexes containing a scaffolding A subunit, a catalytic C subunit, and a variable regulatory B subunit, which provides specificity to the complex (Janssens et al., 2008). Hence, we decided to use in our analysis mutants of the two main regulatory subunits of PP2A: pab1 (corresponding to B55) and par1 (the major B56 subunit). A second (minor) B56 subunit, Par2, also contributes to PP2A-B56 activity in the cell. However, its loss does not produce noticeable phenotypic defects in a wild-type (WT) background and only has consequences when combined with the deletion of par1 (Jiang and Hallberg, 2000). Therefore, we did not include the individual par2 mutant in our initial analysis.
In the case of PP1 we analyzed the behavior of the deletion mutant of the major catalytic subunit, Dis2. This mutant and the clp1 mutant did not show any mating defect (clp1Δ cells responded even faster than the WT control strain), whereas PP2A mutants behaved differently. Our group had previously shown that loss of pab1 results in exacerbated conjugation (Martín et al., 2017). Strikingly, in the absence of Par1, fission yeast cells depicted a delayed mating response and their overall mating ability was reduced compared with the rest of the strains analyzed (Figures 1A and 1B). This defect correlated with the impaired induction of the master regulator of meiosis mei2 (Figure S1A) and the reduced phosphorylation of the pheromone-sensing MAPK Spk1 (Figure S1B). This was not the consequence of defective sensing of the nitrogen depletion, because TORC1 inactivation and TORC2 activation occurred normally (Figure 1C). Rather the mating defect correlated with the inability of par1Δ cells to halt cell-cycle progression in G1 (Figure 1D). Hence, these results suggest that PP2A-B56Par1 is required for the cell-cycle arrest that precedes the sexual differentiation response in fission yeast.
Figure 1.
PP2A-B56Par1 Activity Is Required for an Adequate Mating and Cell-Cycle Arrest in G1 upon Nitrogen Depletion
(A) Homothallic WT, pab1Δ, par1Δ, clp1Δ, and dis2Δ cells were incubated at 25°C in the absence of nitrogen, and their mating ability was determined at 0, 8, 24, and 48 h. Mean values of five biological replicates ± SD are shown. Statistical significance of the difference between strains was assessed with a t test assuming two-tailed distribution and unequal variance. ∗∗p < 0.01, and ∗∗∗p < 0.001.
(B) Homothallic WT and par1Δ cells were maintained at 25°C in the absence of nitrogen. Cells were fixed at indicated time points, and pictures were taken after staining the cells with DAPI. Differential interference contrast images were overlaid to determine the cell outline. Arrowheads indicate zygotes and tetrads.
(C) Homothallic WT and par1Δ cells were incubated at 25°C in the absence of nitrogen, and samples were collected at the indicated time points. Phosphorylation at Ser546 of the AKT homolog Gad8 was used as a readout of TORC2 activity. Phosphorylation of Psk1 (S6 kinase in S. pombe) and Rps6 (ribosomal protein S6) were used as a readout of the activity of TORC1, and total Cdc2 (PSTAIR) served as loading control.
(D) Flow cytometric analysis of the DNA content of isolated nuclei from heterothallic WT and par1Δ cells collected at the indicated time points during a time course in the absence of nitrogen.
See also Figure S1.
Loss of PP2A-B56Par1 Impairs the Degradation of Cdc13 (CycB)
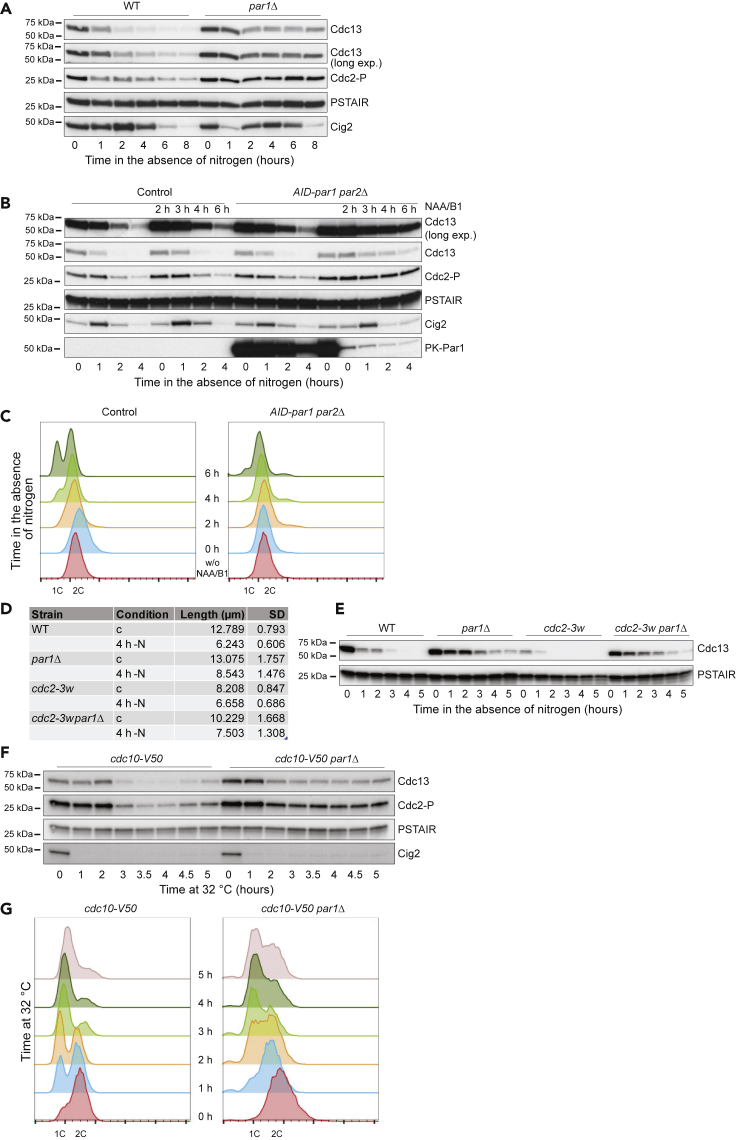
The cell-cycle defect in par1Δ cells could be due to alterations in the kinetics of CDK activity in response to nitrogen starvation. To investigate this possibility we analyzed the levels of the S-phase cyclin Cig2 and the mitotic cyclin Cdc13 (both of them B-type cyclins; Fisher and Nurse, 1995) in a WT and a par1Δ strain. As expected, in the WT background nitrogen deprivation led to a sudden and sustained drop in the levels of Cdc13, which was almost undetectable after 2 h of treatment (Figure 2A). Cig2 behaved differently, increasing slightly during the initial time points and then dropping steadily until its complete disappearance after 6–8 h of starvation (when a majority of the cells were already arrested in G1). In contrast, in the par1Δ mutant, Cdc13 showed an initial decrease, but then it remained stable for the remainder of the experiment (Figure 2A). Cig2 levels were closer to those of the WT strain, and we could only observe a small delay in its disappearance that affected the last time point of our time course. We also determined the presence of Cdc2-Tyr15 phosphorylation in our cells. This conserved inhibitory phosphorylation occurs as the Cdc2-Cdc13 complex is being formed to prevent its premature activation during G2 phase, and it is only removed during mitosis to allow full activation of the complex (Parker et al., 1992, Gould and Nurse, 1989). In addition, the G1-S CDK complex Cdc2-Cig2 is also phosphorylated when DNA replication is halted, and this prevents mitotic entry in the presence of unreplicated DNA (Zarzov et al., 2002). In G1-arrested cells this mark nearly disappeared, because the low level of Cdc13 limits the formation of the Cdc2-Cdc13 complex, which is a prerequisite for its phosphorylation (Figure 2A). Nevertheless, a low level of phosphorylation remained, and this has been previously suggested to be important during the nitrogen starvation response (Wu and Russell, 1997). In agreement with the defective cell-cycle arrest and continuous presence of Cdc13 in par1Δ cells, Tyr15 phosphorylation was detectable throughout the time course, indicating the prevalence of G2 cells in this strain.
Figure 2.
Depletion of Par1 Activity Prevents the Complete Degradation of Cdc13
(A) Homothallic WT and par1Δ cells were incubated at 25°C in the absence of nitrogen, and samples were collected at the indicated time points. Protein levels of Cdc13, Cig2, and phosphorylation of Cdc2 on Tyr15 were followed over the time course by western blot. Total Cdc2 (PSTAIR) served as loading control.
(B) Control cells containing the auxin-inducible degron background (Padh15-skp1-At-Tir1-2NLS Padh15-sk1-Os-Tir1) and nmt41-3PK-miniAID-par1 par2Δ cells in the same genetic background (referred to as AID-par1 par2Δ) were treated or not (as indicated in the figure) with thiamine (0.5 μM) and 1-Naphthaleneacetic acid potassium salt (NAA; 1 mM) 2 h before being incubated in the absence of nitrogen. Samples were collected at the indicated time points. Protein levels of Cdc13 and Cig2, and phosphorylation of Cdc2 on Tyr15, were determined by western blot. B56Par1 depletion was assessed by western blot against its N-terminal 3PK tag. Total Cdc2 (PSTAIR) was used as loading control.
(C) Flow cytometric analysis of the DNA content of control cells containing the auxin-inducible degron background (Padh15-skp1-At-Tir1-2NLS Padh15-sk1-Os-Tir1) and nmt41-3PK-miniAID-par1 par2Δ treated or not with thiamine (0.5 μM) and NAA (1 mM) and collected at the indicated time points during a time course in the absence of nitrogen. At least 7,000 gated single nuclei are represented at each time point.
(D) Heterothallic h-WT, par1Δ, cdc2-3w, and cdc2-3w par1Δ cells were incubated at 30°C in EMM (control) or in EMM-N for 4 h. Fixed cells were measured after staining them with Calcofluor.
(E) Heterothallic h-WT, par1Δ, cdc2-3w, and cdc2-3w par1Δ cells were incubated at 30°C in the absence of nitrogen, and samples were collected at the indicated time points. Protein levels of Cdc13 were assessed by western blot. Total Cdc2 (PSTAIR) was used as loading control.
(F) cdc10-V50 and cdc10-V50 par1Δ cells were incubated at 32°C for the indicated time. Control cells were incubated at 25°C. Western blots show Cdc13 and Cig2 levels, phosphorylation of Cdc2, and total Cdc2 (PSTAIR) as loading control.
(G) Flow cytometric analysis of the DNA content of isolated nuclei from cdc10-V50 and cdc10-V50 par1Δ cells collected at the indicated time points during a time course at 32°C.
See also Figure S2.
The same defects in Cdc13 degradation and Tyr15 phosphorylation dynamics were clear when we used a conditional mutant of par1 in a par2Δ background (nmt41-miniAID-par1 par2Δ, where Par1 is fused to a 68-amino acid fragment of the Auxin-Inducible Degron AID/IAA17) and we depleted Par1 only 2 h before the nitrogen starvation treatment (Figure 2B). Similar to the par1Δ mutant, these cells also failed to arrest their cell-cycle progression in G1 (Figure 2C). Thus, we were confident that this was a bona fide defect due to the loss of par1 and not the consequence of reduced fitness in cells lacking PP2A-B56Par1 activity over many generations.
Accelerated mitotic entry and the subsequent cell size reduction in response to nitrogen deprivation contribute to the cell-cycle arrest in G1, because cells that have not satisfied the size requirement to enter S-phase will delay the G1/S transition (what is known as the G1 cell size control or checkpoint; Novak et al., 1998). We observed that par1Δ cells have a slightly longer generation time than WT cells (5 h 20 min versus 4 h 30 min at 25°C in Edinburgh minimal medium (EMM)). Therefore, we wanted to rule out that the G1 arrest and Cdc13 degradation defect in par1Δ cells was not merely due to a longer residence time in the G2 phase of the cell cycle or to their inability to accelerate mitotic entry and engage the G1 cell size checkpoint. To this end, we combined the par1 deletion with a gain-of-function mutation of the cdk1/cdc2 gene (the cdc2-3w allele) (Fantes, 1981, Enoch and Nurse, 1990). On its own, the presence of this allele results in a decrease in cell length compared with cells containing the WT gene (indicative of premature mitotic entry). The double mutant par1Δ cdc2-3w was shorter than the WT control (Figure 2D). However, this size reduction only marginally ameliorated the Cdc13 degradation defect in response to nitrogen starvation (Figure 2E). We obtained a similar result when we introduced the cdc25-9A allele in the par1Δ background. This allele, mutated in the phosphorylation sites for the checkpoint and stress kinases Cds1, Chk1, and Srk1 (Zeng et al., 1998, Lopez-Aviles et al., 2005), is immune to the cell-cycle arrest in G2 imposed by activation of the DNA and replication checkpoints and in response to stress. Therefore, we excluded the possibility that a slower transit through G2 due to checkpoint activation was responsible for the retention of Cdc13 in the par1Δ mutant (Figure S2A). Given the role of PP2A-B56Par1 in the silencing of the spindle assembly checkpoint (SAC) (reviewed in Saurin, 2018, Hayward et al., 2019), we also analyzed the degradation of Cdc13 during nitrogen starvation in the par1Δ mutant in the absence of the SAC component Mad2. As in the previous cases, this experiment showed that persistent SAC signaling was not the reason for the retention of Cdc13 in nitrogen-starved par1Δ cells (Figure S2B).
Together, these results indicate that the persistence of Cdc13 in par1Δ cells during G1 phase cannot be merely due to defects in the G2 and M phases of the previous cycles but is the result of additional elements of control of the mitotic cyclin being altered in this strain.
Confirming this idea, par1Δ cells also failed to degrade Cdc13 when a G1 arrest was imposed through the inactivation of the MBF transcription factor Cdc10 using a cdc10-V50 allele at restrictive temperature (Figure 2F). In contrast to the nitrogen starvation response, the G1 arrest in this mutant does not require a premature entry into mitosis or the engagement of the G1 cell size checkpoint. Of note, the inability of the par1Δ mutant to fully degrade Cdc13 was accompanied by a failure to sustain the G1 arrest in this mutant (Figure 2G), a phenotype also observed in ste9-deleted cells (Kitamura et al., 1998). Therefore, we conclude that PP2A-B56Par1 activity is required to prevent the passage through Start (the point after which the cell is committed to a new round of division), and this is achieved through the regulation of the degradation of the mitotic cyclin Cdc13.
Deletion of the G1/S Cyclins Rescues the Defects in Cdc13 Degradation and G1 Arrest of par1Δ Cells
Given that par1Δ cells cannot efficiently arrest their cell cycle in G1 or degrade the mitotic cyclin in response to nitrogen starvation we decided to test whether this defect could be rescued by a decrease in the G1/S CDK activity. For this aim we deleted the genes encoding the G1/S cyclins Cig1 and Cig2. This strategy has been previously used to rescue the mating and cell-cycle arrest defects in strains that fail to counteract CDK activity upon nitrogen depletion, such as those containing mutant alleles of wee1, ste9, and rum1 (Wu and Russell, 1997, Kitamura et al., 1998, Martín-Castellanos et al., 1996). These rescues can be understood as the consequence of canceling out the double-negative feedback loops established between CDK complexes and CDK antagonists.
The deletion of cig1 and cig2 does not have a noticeable impact on the cell-cycle distribution of cells growing exponentially in rich medium (Fisher and Nurse, 1996, Mondesert et al., 1996). However, it facilitates the G1 arrest upon nitrogen depletion (Figure 3A, left lower panel). Consequently, the cig1Δ cig2Δ mutant has a higher mating efficiency and degrades Cdc13 more readily than its WT counterpart (Figures 3B–3D). Deletion of cig1 and cig2 in the par1Δ background completely suppressed the G1 arrest and mating defects of the single par1Δ mutant (Figure 3A, right lower panel, 3B and 3D). Remarkably, the triple mutant par1Δ cig1Δ cig2Δ degraded Cdc13 with faster kinetics than the WT strain (Figure 3C). In contrast, individual deletions of either cig1 or cig2 resulted in a partial rescue of the Cdc13 degradation defect observed in par1Δ cells (Figures S3A and S3B). Of the two cyclins, however, Cig2 appeared to have a more prominent role because its deletion in the par1Δ mutant mirrored the behavior of the WT control. Altogether, these findings indicated that PP2A-B56Par1 is important to counteract CDK activity in G1, and therefore we were compelled to investigate its involvement in the regulation of CDK inhibitors.
Figure 3.
Rescue of the Defect in the G1 Arrest, Mating Efficiency, and Degradation of Cdc13 of a par1Δ Mutant by Deletion of the G1/S Cyclins
(A) Homothallic WT, par1Δ, cig1Δ cig2Δ, and cig1Δ cig2Δ par1Δ cells were incubated at 25°C in the absence of nitrogen; cells were collected at the indicated time points, and after processing their DNA content was measured by flow cytometry.
(B) Efficiency of mating of the homothallic strains WT, par1Δ, cig1Δ cig2Δ, and cig1Δ cig2Δ par1Δ after 0, 8, 24, and 48 h upon nitrogen depletion. Mean values of three biological repeats ± SD are shown. Statistical significance of the difference between strains was assessed with a t test assuming two-tailed distribution and unequal variance. ∗p < 0.05, ∗∗∗p < 0.001.
(C) Homothallic WT, par1Δ, cig1Δ cig2Δ, and cig1Δ cig2Δ par1Δ cells were incubated at 25°C in the absence of nitrogen, and samples were collected at the indicated time points. Protein levels of Cdc13 and Cig2 and phosphorylation of Cdc2 on Tyr15 were assessed by western blot. Total Cdc2 (PSTAIR) served as loading control.
(D) Homothallic WT, par1Δ, cig1Δ cig2Δ, and cig1Δ cig2Δ par1Δ cells were maintained in the absence of nitrogen for 8 h. Pictures of fixed cells were taken after staining them with DAPI. Differential interference contrast images were overlaid to determine the cell outline. Arrows indicate zygotes.
See also Figure S3.
The Cdc13 Degradation Defect of par1Δ Cells Correlates with Their Inability to Dephosphorylate Ste9 and Stabilize Rum1
Cdc13 degradation during G1 requires the APC/C activator Ste9 (Kitamura et al., 1998, Blanco et al., 2000, Yamaguchi et al., 1997, Kominami et al., 1998b) and the CKI Rum1 (Correa-Bordes, 1997). Both these CDK-negative regulators are themselves inhibited by CDK-dependent phosphorylation, which leads to dissociation from the APC/C of the former and targeted degradation of the latter (Blanco et al., 2000, Benito et al., 1998).
The cooperation between these two CDK inhibitors is patent during the nitrogen starvation response (and other events leading to G1 arrest), as deletion mutants of either ste9 or rum1 fail to degrade Cdc13 (Figures S4A and S4B).
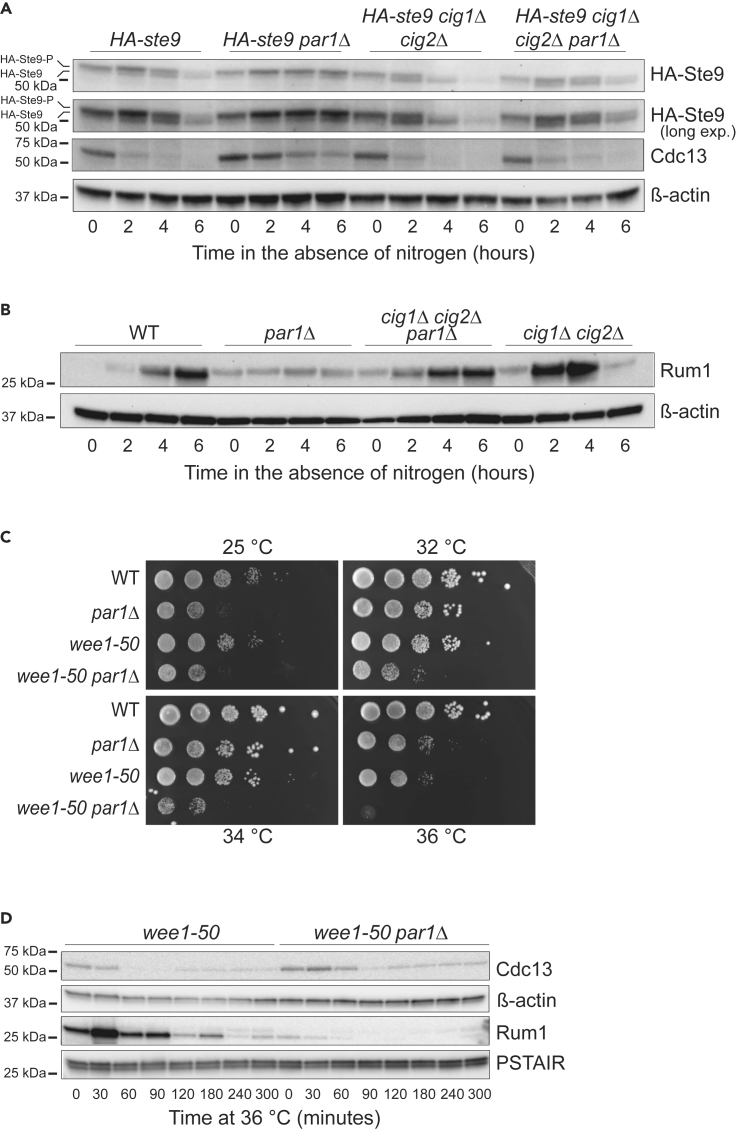
As the par1Δ mutant is comparably defective in downregulating Cdc13, and given the regulation by phosphorylation of both Ste9 and Rum1, we next tested the ability of par1Δ cells to dephosphorylate and stabilize them, respectively, upon nitrogen depletion. Ste9 dephosphorylation can be observed in G1-arrested cells or when CDK activity is exogenously abrogated. Conversely, it becomes phosphorylated during S, G2, and M phases, as CDK activity increases (Blanco et al., 2000, and Figure S4D). These two states can be discerned based on the different electrophoretic mobility of the phosphorylated versus non-phosphorylated protein (Figure S4D). In WT cycling cells, only the phosphorylated form was detected. However, upon nitrogen starvation, an additional, faster-migrating band corresponding to the non-phosphorylated form of Ste9 started to appear 2 h within the treatment and gained intensity as more cells accumulated in G1 (Figures 4A, 7D, S4D, and S4E). In contrast, in the par1Δ strain the slower-migrating band (indicative of phosphorylation) was more prominent throughout the entire time course, and the faster-migrating band was barely present (Figures 4A, S4D, and S4E). Remarkably, we could observe the same behavior in a rum1Δ mutant (Figure S4E). In parallel to Ste9 dephosphorylation, the level of Rum1 started to increase in the WT strain 2 h within the experiment. However, par1Δ cells failed to show a significant accumulation of Rum1 over the 6-h time course (Figure 4B). This was not the consequence of reduced transcription of rum1 in this mutant, because rum1 mRNA was induced to comparable levels in both strains (Figure S4C). Notably, loss of cig1 and cig2 utterly overrode these defects, pointing at PP2A-B56Par1 as the phosphatase in charge of counteracting CDK phosphorylation on Rum1 and Ste9 (Figures 4A and 4B).
Figure 4.
Deletion of par1 Causes a Defect in Ste9 Dephosphorylation and Rum1 Stabilization
(A) Homothallic HA-ste9, HA-ste9 par1Δ, HA-ste9 cig1Δ cig2Δ, and HA-ste9 cig1Δ cig2Δ par1Δ cells were incubated at 25°C in the absence of nitrogen, and samples were collected at the indicated time points. Protein levels of Cdc13 were followed over the time course by western blot. Ste9 was detected by western blot against its N-terminal HA-tag. β-Actin served as loading control. HA, hemagglutinin.
(B) Homothallic WT, par1Δ, cig1Δ cig2Δ, and cig1Δ cig2Δ par1Δ cells were incubated at 25°C in the absence of nitrogen, and samples were collected at the indicated time points. Protein levels of Rum1 were assessed by western blot. β-Actin was used as loading control.
(C) WT, par1Δ, wee1-50, and wee1-50 par1Δ cells were grown in liquid rich medium (yeast extract with supplements (YES)) medium at 30°C and then spotted onto YES plates at serial 10-fold dilutions (from left to right). Plates were incubated at 25°C, 32°C, 34°C, and 36°C for 3 days before pictures were taken.
(D) wee1-50 cells and wee1-50 par1Δ cells were incubated at 36°C, and samples were collected at the indicated time points. Control cells (0 time point) were incubated at 25°C. Western blots show protein levels of Cdc13, Rum1 together with β-actin and Cdc2 (PSTAIR) as loading controls. See also Figure S4.
Figure 7.
Mutation of a Putative SLiM Motif of PP2A-B56 in Rum1 Phenocopies the Deletion of par1
(A) Homothallic WT, par1Δ, and rum1 BM1AA cells were incubated at 25°C in the absence of nitrogen, and their mating efficiency was determined after 0, 8, 24, and 48 h. Mean values of three biological repeats ± SD is shown. Statistical significance of the difference between strains was assessed with a t test assuming two-tailed distribution and unequal variance. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
(B) Flow cytometric analysis of the DNA content of WT and rum1 BM1AA cells collected every 30 min during a time course of 6 h in the absence of nitrogen.
(C) WT and rum1 BM1AA cells were incubated at 25°C in the absence of nitrogen, and samples were collected at the indicated time points. Western blots show protein levels of Cdc13 and Rum1. α-Tubulin was used as loading control.
(D) HA-ste9 and HA-ste9 rum1 BM1AA cells were maintained at 25°C without nitrogen, and samples were collected at the indicated time points. Ste9 was detected by western blot against its N-terminal HA-tag, and β-actin served as loading control. HA, hemagglutinin.
(E) HA-Ste9, HA-Ste9 par1Δ, HA-Ste9 nmt81::rum1 T58A T62A, and HA-Ste9 nmt81::rum1 T58A T62A par1Δ cells were incubated for 24 h in the absence of thiamine for the expression of Rum1 T58A T62A. Cells were washed with four volumes of EMM-N before resuspending them in EMM-N, and samples were collected at the indicated time points. Protein levels of Cdc13 and Cig2, and phosphorylation of Cdc2 Tyr15 were determined by western blot. Total Cdc2 (PSTAIR) was used as loading control.
See also Figure S7.
Finally, indicating that this regulation was not exclusive of the nitrogen starvation response, we observed a genetic interaction between the par1 deletion and the wee1-50 allele (Figure 4C). wee1-50 cells have a shorter G2 phase, which results in premature mitosis (Russell and Nurse, 1987), an effect that is exacerbated with temperature. To avoid the progressive shortening of the cell, this mutant expands the G1 phase, a process that requires Rum1 and Ste9 activities. In their absence, wee1-50 cells become shorter in every cycle, eventually losing their viability (Moreno and Nurse, 1994, Correa-Bordes and Nurse, 1995). Deletion of par1 also affected the survival of the wee1-50 mutant (Figure 4C), and this worsening of the phenotype correlated with the inability of the double wee1-50 par1Δ mutant to accumulate Rum1 (Figure 4D).
All in all, these results suggest that PP2A-B56Par1 is an important regulator of the CDK inhibitors Ste9 and Rum1 and that this regulation is instrumental for the arrest in G1 phase that precedes differentiation and during other special cell cycles that require an expansion of G1.
Overexpression of a PP2A-B56-Specific Small Linear Motif (SLiM) Decoy Phenocopies the Deletion of par1
The aforementioned results strongly suggested that Rum1 and Ste9 are targets of PP2A-B56Par1. PP2A-B56 enzymes recognize a small linear interacting motif (SLiM) found in disordered regions of their substrates (LxxIxE) (Wang et al., 2016, Hertz et al., 2016). To address whether the effects that we had observed upon deletion of par1 were due to the inability of the complex to interact with its substrates we overexpressed a peptide encompassing the optimal sequence of this motif (LEPIPEEPE, based on the RepoMan SLiM and fused to GFP for convenience) (Figure S5). A similar strategy has been used before to prevent the recognition of PP2A-B56 specific targets (Kruse et al., 2018). This construct (Par1-inhibitor, referred to as GFP-B56-SLiM hereafter) expressed from the strong nmt1 promoter was able to recapitulate the phenotype of the par1Δ mutant after 24 h of overexpression (Figure 5A), indicating that it could outcompete PP2A-B56 canonical substrates. Indeed, the cell separation defect typical of par1Δ cells was accentuated in cells expressing the Par1-inhibitor for longer times (Figure 5A), which was reminiscent to the complete loss of B56 activity upon depletion of both Par1 and Par2 (Jiang and Hallberg, 2000). As a control, we overexpressed a mutated version of the Par1-inhibitor where the key residues I4 and E6 had been mutated to Ala (mock-inhibitor, referred to as GFP-B56-SLiM-AA). As expected, this mutant peptide failed to efficiently bind Par1 and to produce a similar phenotype (Figures 5A and 5B). In agreement with our hypothesis, overexpression of the Par1-inhibitor, but not the mock-inhibitor, impaired the degradation of Cdc13 to an extent similar to the par1 deletion (Figure 5C). Therefore, we conclude that overexpression of the Par1-inhibitor can be used as an alternative to the deletion mutant in the analysis of PP2A-B56Par1 phenotypes. More importantly, these results indicate that the defects observed in the par1Δ mutant are due to the inability of PP2A to interact with B56-specific substrates.
Figure 5.
The Defect in the Degradation of Cdc13 in a par1Δ Mutant Is Due to Inability of PP2A to Interact with B56-Specific Substrates
(A) WT and par1Δ cells, and WT cells transformed with either pREP1-GFP-B56-SLiM-AA (mock-inhibitor) or pREP1-GFP-B56-SLiM (Par1-inhibitor), were incubated in EMM in the absence of thiamine for 40 h at 25°C. Subsequently, cells were shifted to fresh EMM (control point) and to EMM-N for 6 h. Cells were fixed and stained with DAPI and Calcofluor before obtaining images.
(B) Overexpressed GFP-B56-SLiM (Par1-inhibitor) and GFP-B56-SLiM-AA (mock-inhibitor) were purified by means of their N-terminal GFP-tag using a GFP trap from cells containing a TAP-tagged allele of par1. A representative experiment shows the co-purifying and the unspecifically pulled down TAP-Par1 detected through western blot against the CBP epitope in the TAP-tag. Western blot against GFP served as control of the GFP pull down.
(C) WT and par1Δ cells, and WT cells transformed with either pREP1-GFP-B56-SLiM-AA (mock-inhibitor) or pREP1-GFP-B56-SLiM (Par1-inhibitor) were incubated in EMM in the absence of thiamine for 40 h at 25°C. Cells were shifted to EMM-N, and samples were collected at the indicated time points. Western blots show protein levels of Cdc13 and Cig2, phosphorylation of Cdc2 on Tyr15, and total Cdc2 (PSTAIR) as loading control. See also Figure S5.
PP2A-B56Par1 Interacts with Rum1 In Vitro
Having delineated the phenotypes upon loss of PP2A-B56Par1 in relation with Rum1 and Ste9, we asked whether they interact. We started by using a linear-motif prediction tool (ELM) (Gouw et al., 2018) to determine putative SLiMs present in Rum1 and Ste9 that could mediate their interaction with PP2A-B56Par1. Analysis of their sequences showed that whereas Ste9 does not contain an obvious PP2A-B56-SLiM that adheres to the strict consensus, Rum1 comprises two potential PP2A-B56-SLiMs. These two motifs (39IDEIPE44 and 80LERCMEE86) surround the two main phospho-sites targeted by CDK (T58 and T62) (Benito et al., 1998) (Figure 6A).
Figure 6.
PP2A-B56 Par1 Interacts with the CDK Inhibitor Rum1
(A) Amino acid sequence of Rum1. The putative SLiMs for PP2A-B56 are in blue and the sites phosphorylated by CDK are in red.
(B) TAP-Par1 was purified using the tandem affinity purification method. 100 ng of Ste9 homogeneous recombinant protein was added to the purified Par1, and after washing and eluting CBP-Par1 with TEV protease (which cleaves the TEV site between the protein A and CBP moieties of the TAP-tag), the bound (elution) and unbound (supernatants) Ste9 was detected by western blot. Untagged WT extract was used as a negative control. Note that, as Ste9 and Par1 have very similar molecular masses, and the protein A present in the TAP-tag is also bound by the rabbit IgGs, signal corresponding to the uncleaved TAP-Par1 appears in the Ste9 blot (marked with an asterisk). In the lower panel, signal coming from the previous incubation with the Ste9 antibody remains in the CBP western blot.
(C) TAP-Par1 and TAP-Par1F314Q were purified using the tandem affinity purification method. 50 ng of Rum1 homogeneous recombinant protein was added to the purified Par1 and TAP-Par1F314Q; after washing and eluting CBP-Par1 with TEV protease, the unbound (supernatants) and the interacting and unspecifically pulled down (elution) Rum1 was detected by western blot. Untagged WT extract was used as a negative control. The amount of pulled down CBP-Par1 and CBP-Par1F314Q were detected by western blot against the CBP-tag.
See also Figure S6.
Following this sequence analysis, we wanted to analyze the interaction between PP2A-B56Par1 Rum1 and Ste9. Neither Rum1 nor Ste9 are abundant proteins, which hampers their purification from yeast native extracts. Hence, to test whether Par1 and Ste9/Rum1 directly interact we expressed and purified Rum1 and Ste9 from E. coli, and the recombinant proteins were subsequently incubated with TAP-tagged Par1 pulled down from fission yeast. As anticipated from the motif analysis, Ste9 binding to Par1 was very weak (Figure 6B), whereas the interaction between Par1 and Rum1 was much stronger (Figure 6C). The same result was obtained when immobilized Rum1 was incubated with TAP-Par1-containing extracts (Figure S6). Moreover, when we incubated Rum1 with TAP-Par1 containing a mutation in one of the residues in the binding pocket previously shown to be critical for the recognition of the LxxIxE motif (Hertz et al., 2016) (TAP-Par1F314Q), this interaction was reduced (Figure 6C). Therefore, we conclude that Rum1 is the most likely a direct substrate of PP2A-B56Par1. Given that Rum1 is needed for counteracting CDK activity during G1, these results support a scenario where PP2A-B56Par1 dephosphorylates and stabilizes Rum1, leading to a drop in CDK activity that ultimately results in the activation of the APC/CSte9.
Mutation of rum1 Putative B56 Binding Motif Phenocopies the par1Δ Mutant
If dephosphorylation of Rum1 by PP2A-B56Par1 is a requirement for its stabilization during G1, then hindering the interaction between the two proteins should lead to a phenotype comparable to that of the par1Δ mutant. To test this idea, we replaced the WT rum1 gene by a mutant allele containing two alanine substitutions at positions 42 and 44, corresponding to the central I and E in the first SLiM (hereon referred to as rum1 BM1AA for Binding Motif 1 I42A E44A). Mutation of these residues led to a decrease in the mating efficiency comparable to the decrease observed in the par1Δ mutant (Figure 7A). In addition, rum1 BM1AA mutant cells were delayed in the G1 arrest following nitrogen starvation and in the degradation of Cdc13 (Figures 7B, 7C, S7A, and S7B). More importantly, this mutant failed to accumulate Rum1 in the cell, even though the mRNA levels were similar to those of the WT strain (Figure S7C) Finally, these observations correlated with a delay in the dephosphorylation of Ste9 (Figure 7D). Of note, concomitant mutation of the second putative binding motif (BM2AA) did not worsen the phenotype of the rum1 BM1AA mutant, but it actually ameliorated its defects (data not shown). Currently, we are missing structural insight into how this second mutation could affect Rum1 functionality and/or stability, and we have not tested the phenotype of the single rum1 BM2AA mutant. However, our results suggest that the first binding motif is sufficient for the interaction with PP2A-B56Par1.
If PP2A-B56Par1-mediated dephosphorylation of Rum1 is an important step for the downregulation of CDK activity during G1, an expected outcome of this model would be that expression of a phospho-null allele (rum1T58AT62A) can rescue the Cdc13 degradation defect of a par1Δ mutant. Indeed, expression of this allele from the nmt81 promoter was sufficient to fully restore the degradation of Cdc13 in par1Δ cells (Figure 7E).
All in all, these observations lead us to the conclusion that PP2A-B56Par1 is the phosphatase in charge of counteracting CDK activity during G1 phase establishment in fission yeast and that this role is mainly executed through the dephosphorylation of the CKI Rum1. These results are summarized in our model (Figure 8).
Figure 8.
Model
(A) (Left) During the nitrogen starvation response rum1 mRNA expression is induced and Rum1 protein is produced. CDK complexes phosphorylate and target newly synthesized Rum1 for degradation, but this phosphorylation is opposed by PP2A-B56Par1, which leads to the stabilization of Rum1. As Rum1 accumulates, this eventually results in the sustained inactivation of CDK complexes. (Right) Owing to the presence of double-negative feedback loops between CDK complexes and their inhibitors, inactivation of CDK complexes leads to the further stockpiling of Rum1 and the termination of the inhibition of Ste9.
(B) (Left) In the absence of PP2A-B56 activity, Rum1 phosphorylation mediated by CDK complexes cannot be counteracted and Rum1 does not accumulate in the cell. (Right) In consequence CDK complexes are not inhibited and can repress APC/C-Ste9 activity, resulting in impaired degradation of Cdc13.
Discussion
In all organisms, cell cycle progression has to be closely coordinated with the sensing of nutritional cues and pro-growth signaling. Even though the cell uses specific mechanisms to sense different perturbations or signals (be it environmental insults or nutritional scarcity) they all converge in the regulation of CDK activity, which ultimately dictates the quiescence/proliferation decision. In later years different protein phosphatases (Cdc14, PP1, PP2A) have been shown to play prominent roles in the regulation of cell cycle transitions by counteracting CDK-dependent phosphorylation events and the amplification of CDK activity (Stegmeier and Amon, 2004, Bouchoux and Uhlmann, 2011, Godfrey et al., 2017, Wu et al., 2009, Mochida et al., 2009, Mochida et al., 2010, Gharbi-Ayachi et al., 2010, Schmitz et al., 2010, Mayer-Jaekel et al., 1994, Manchado et al., 2010, Grallert et al., 2015). In this study we have used the fission yeast response to nitrogen starvation to investigate the involvement of protein phosphatases in the downregulation of CDK activity during the pre-Start G1 arrest that precedes sexual differentiation. By doing so, we revealed an outstanding role of PP2A-B56Par1 in the control of CDK activity during G1 phase.
Fission yeast cells grown in the presence of glucose and a rich nitrogen source have a very short G1 phase and enter S phase shortly after completion of mitosis (so much so that S-phase coincides with cytokinesis). This has been explained as the consequence of cells exiting mitosis having attained the sufficient cell size to satisfy a G1 cell size checkpoint (that under these circumstances is cryptic) (Moreno and Nurse, 1994). From a molecular point of view, this could also be understood as cells exiting mitosis with sufficient CDK activity to progress through Start and initiate DNA replication (Stern and Nurse, 1996, Coudreuse and Nurse, 2010). Indeed, in synchronized mitotic cultures Cig2 (the S-phase cyclin) starts to accumulate as soon as the mitotic cyclin Cdc13 is degraded (Yamano et al., 2000). However, under poor nutritional conditions (a more likely representation of conditions in nature) cell growth is limited and the cell needs to engage molecular brakes that delay or completely halt (in the absence of a nitrogen source) progression through Start. This is mediated by the accumulation of the CKI Rum1 and the activation of the APC/C activator Ste9 that prevent the activation of CDK complexes. Both Rum1 and Ste9 establish double-negative feedback loops with CDK complexes with varying strength depending on the associated cyclin (Benito et al., 1998, Blanco et al., 2000) that render the system bistable (high- versus low-CDK states) and the transitions between these two states irreversible. Here, we have shown that in the absence of PP2A-B56Par1 activity, degradation of Cdc13 is impaired, and this results in the failure to properly arrest cell cycle progression in G1. This defect correlates with the inability of par1Δ cells to dephosphorylate the APC activator Ste9 or to stabilize the CKI Rum1. Moreover, all these defects can be efficiently rescued through the deletion of the G1/S cyclins Cig1 and Cig2, which supports the idea that PP2A-B56Par1 directly controls the dephosphorylation of these CDK inhibitors. This conclusion is also reinforced by the fact that expression of a mutant version of Rum1 lacking a putative B56 SLiM (rum1 BM1AA) depicts similar mating and cell cycle defects to the par1Δ mutant upon nitrogen depletion. Importantly, our conclusions are not only limited to the nitrogen starvation response. The deletion of par1 impairs the extension of G1 phase that is required when the growth during G2 is limited (as in a wee1-50 mutant) or when expression of the genes needed for S-phase is blocked (as in a cdc10-V50 mutant). All this translates to the fact that when PP2A-B56Par1 is not present, the cell is less responsive to the signaling cues indicating that cell cycle progression needs to come to a halt and that CDK activity needs to be kept low.
The budding yeast counterparts of Rum1 and Ste9, Sic1 and Cdh1/Hct1, are dephosphorylated by Cdc14 during mitotic exit (Zachariae, 1998, Visintin et al., 1998, Jaspersen et al., 1999). However, the Cdc14 homolog in fission yeast, Clp1/Flp1, was shown early on not to affect Rum1 and Ste9 phosphorylation status (Cueille et al., 2001). Recent reports indicate that PP2A-B55 is the preferential phosphatase counteracting CDK phosphorylation events, whereas PP2A-B56 is in charge of removing Aurora-dependent (and other mitotic kinases') phosphorylations (Cundell et al., 2016, Godfrey et al., 2017, Saurin, 2018). In the case of Rum1, however, we could not identify a bipartite basic patch mediating the interaction to B55 as described in the study by Cundell et al., whereas two putative B56 binding motifs were present (Hertz et al., 2016). It is worth noting that in fission yeast PP1, PP2A-B55, and PP2A-B56 are sequentially activated upon Cdc13 degradation to bring about mitotic exit (Grallert et al., 2015). As PP2A-B56 is the last in this phosphatase relay, it is not surprising that it is in charge of dephosphorylating a late CDK substrate such as Rum1. Intriguingly, we did not identify any potential B56 binding motif in Ste9, although it was clear that its dephosphorylation was impaired in the par1Δ mutant. This could have different explanations: it could be that in this case binding to B56 is mediated through a motif other than the LxxIxE SLiM described by Hertz et al. or through additional proteins. Alternatively, it could be understood as the result of increased CDK activity in the absence of Par1 due to the failure to stabilize Rum1. Several evidences back this hypothesis: first, the complete suppression of the Ste9 dephosphorylation defect in the triple par1Δ cig1Δ cig2Δ mutant and, second, the fact that either the deletion of rum1 or the mutation of the B56 binding motif impairs the dephosphorylation of Ste9 to a similar extent (Figures S4E and 7D). Nevertheless, in vitro dephosphorylation assays will be needed to determine if Ste9 is a direct PP2A-B56 substrate.
In mammalian cells, a great body of evidence has underscored the importance of the CIP/KIP CKIs p21, p27, and p57 during differentiation and quiescence (Coats et al., 1999, Zhang et al., 1997, Zhang et al., 1998, Zhang et al., 1999, Tury et al., 2011, Gosselet et al., 2007). Coincidentally, B56 subunits were originally shown to be enriched in adult brain and to be induced in neuroblastoma cell lines in response to retinoic acid (McCright et al., 1996). Moreover, PP2A-B56 was one of the signature genes upregulated during the initiation of quiescence induced by contact inhibition in fibroblast cultures (Coller et al., 2006). Of all CIP/KIP members only p57 contains a putative B56 binding motif. Nevertheless, several reports indicate that B56γ can dephosphorylate p27, leaving open the possibility that in this case interaction is mediated by a different motif or through additional proteins. These observations and our results open the possibility that in higher eukaryotes also PP2A-B56 enzymes contribute to the activation of CKIs during cell differentiation and quiescence.
In addition, p21 is an important element controlling the activation of the bistable Rb-E2F switch that dictates passage through the restriction point and that determines the depth of quiescence (Kwon et al., 2017, Moser et al., 2018). p21 sets the threshold of CDK2 activity that governs this transition and the decision to commit to a new round of division or enter a quiescent state (Spencer et al., 2013, Overton et al., 2014). Using a fluorescent sensor of CDK2 activity, Spencer et al. identified two different populations at the end of mitosis: one wherein CDK2 activity started to increase immediately after mitosis from an intermediate level and another population that exited mitosis with low CDK2 activity and that entered a reversible G0 state (Spencer et al., 2013). In the first instance, the level of p21 was low and the decision to commit to a new cell cycle was made at the end of mitosis, much earlier than traditionally believed. Only in cells that exited mitosis with high levels of p21 and low CDK2 activity, mitogenic stimulation was needed to progress through the restriction point. Nevertheless, a later study using fibroblast primary cultures did show that, in this cell type, commitment to a new division was only occurring during G1 phase upon mitogenic stimulation (Schwarz et al., 2018). Although this discrepancy may be due to differences in the experimental setup, it could also indicate that the premature passage through the restriction point is favored in immortalized/cancer-derived cell lines and not the representation of a normal cell cycle regulation. One of the properties of cancer cells is their ability to divide even in the absence of mitogens. Although this can result from the exacerbated signaling of a wealth of pathways, the incomplete downregulation of CDK activity during mitotic exit can clearly also contribute. We have observed that in mitotically synchronized fission yeast cells lacking PP2A-B56 activity mitotic exit occurs in the presence of residual Cdc13 (Nathalia Chica and Sandra Lopez-Aviles, Unpublished Data). Could loss of B56 activity in cancer cells also alter their responsiveness to environmental signals? Given that PP2A regulatory subunits are frequently mutated in cancer (reviewed in Ruvolo, 2016) this is an idea worth investigating in the future.
Limitations of the Study
This study describes a role for PP2A-B56Par1 in mediating the downregulation of CDK activity during G1, which is critical during quiescence and differentiation. In the absence of PP2A-B56Par1 activity, the degradation of the mitotic cyclin Cdc13, the dephosphorylation of the APC activator Ste9, and the stabilization of the CDK inhibitor Rum1 are impaired.
However, the low Ste9 and Rum1 protein level has complicated the execution of in vivo biochemical assays and we have been limited to assays using purified proteins. In the case of Ste9, whose binding to Par1 in vitro was very weak, however clearly affected by the absence of PP2A-B56Par1, we cannot exclude the possibility that it is a direct target, albeit in that case the interaction should be mediated by additional proteins. Still, we lean toward a scenario wherein impaired stabilization of Rum1 in the par1 mutant background results in increased CDK activity and sustained phosphorylation of Ste9. Similarly, although we have seen that mutation of a putative B56 binding motif in Rum1 phenocopies the defects of the par1 deletion, we have not been able to show that the mutation affects the interaction with PP2A-B56Par1 in vivo.
In the future, structural studies and in vitro phosphatase assays will provide further insight into the relationship between PP2A-B56Par1, Rum1, and Ste9.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Sergio Moreno and Jacky Hayles for sharing strains and reagents and for stimulating discussion. We thank Frank Uhlmann, Beata Grallert, Rosa Aligue, Toni Hurtado, and the members of the Lopez-Aviles lab for critical reading of the manuscript. V.S., D.S.-P., N.S., and S.L.-A. are supported by the Centre for Molecular Medicine Norway (NCMM), the Department of Chemistry (N.S.), and the Department of Biosciences (S.L.-A.) at University of Oslo. The research of R.M. is supported by a grant from the Norwegian Research Council awarded to S.L.-A. (FRIMEDBIO 251321). N.S. is supported by a grant from the Norwegian Research Council (FRIMEDBIO 263195).
Author Contributions
Conceptualization: S.L.-A. and R.M.; Methodology: V.S., R.M., D.S.-P., N.S., and S.L.-A.; Investigation: V.S., R.M., and D.S.-P.; Resources: N.S. and S.L.-A.; Writing – Original Draft: S.L.-A. and R.M.; Writing – Review and Editing V.S., R.M., D.S.-P., N.S., and S.L.-A.; Visualization: V.S., R.M., and S.L.-A.; Supervision: S.L.-A.; Funding Acquisition: S.L.-A.
Declaration of Interest
The authors declare no competing interests.
Published: May 22, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101063.
Contributor Information
Ruth Martín, Email: r.m.martin@ncmm.uio.no.
Sandra Lopez-Aviles, Email: sandra.lopez-aviles@ncmm.uio.no.
Supplemental Information
References
- Barr A.R., Cooper S., Heldt F.S., Butera F., Stoy H., Mansfeld J., Novák B., Bakal C. DNA damage during S-phase mediates the proliferation-quiescence decision in the subsequent G1 via p21 expression. Nat. Commun. 2017;8:14728. doi: 10.1038/ncomms14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr A.R., Heldt F.S., Zhang T., Bakal C., Novák B. A dynamical framework for the all-or-none G1/S transition. Cell Syst. 2016;2:27–37. doi: 10.1016/j.cels.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito J., Martín-Castellanos C., Moreno S. Regulation of the G1 phase of the cell cycle by periodic stabilization and degradation of the p25rum1 CDK inhibitor. EMBO J. 1998;17:482–497. doi: 10.1093/emboj/17.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco M.A., Sánchez-Díaz A., de Prada J.M., Moreno S. APC(ste9/srw1) promotes degradation of mitotic cyclins in G(1) and is inhibited by cdc2 phosphorylation. EMBO J. 2000;19:3945–3955. doi: 10.1093/emboj/19.15.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchoux C., Uhlmann F. A quantitative model for ordered cdk substrate dephosphorylation during mitotic exit. Cell. 2011;147:803–814. doi: 10.1016/j.cell.2011.09.047. [DOI] [PubMed] [Google Scholar]
- Cheung T.H., Rando T.A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats S., Whyte P., Fero M.L., Lacy S., Chung G., Randel E., Firpo E., Roberts J.M. A new pathway for mitogen-dependent cdk2 regulation uncovered in p27(Kip1)-deficient cells. Curr. Biol. 1999;9:163–173. doi: 10.1016/s0960-9822(99)80086-4. [DOI] [PubMed] [Google Scholar]
- Coller H.A., Sang L., Roberts J.M. A new description of cellular quiescence. PLoS Biol. 2006;4:e83. doi: 10.1371/journal.pbio.0040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Bordes J. p25rum1 promotes proteolysis of the mitotic B-cyclin p56cdc13 during G1 of the fission yeast cell cycle. EMBO J. 1997;16:4657–4664. doi: 10.1093/emboj/16.15.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Bordes J., Nurse P. p25rum1 orders S phase and mitosis by acting as an inhibitor of the p34cdc2 mitotic kinase. Cell. 1995;83:1001–1009. doi: 10.1016/0092-8674(95)90215-5. [DOI] [PubMed] [Google Scholar]
- Coudreuse D., Nurse P. Driving the cell cycle with a minimal CDK control network. Nature. 2010;468:1074–1079. doi: 10.1038/nature09543. [DOI] [PubMed] [Google Scholar]
- Cueille N., Salimova E., Esteban V., Blanco M., Moreno S., Bueno A., Simanis V. Flp1, a fission yeast orthologue of the s. cerevisiae CDC14 gene, is not required for cyclin degradation or rum1p stabilisation at the end of mitosis. J. Cell Sci. 2001;114:2649–2664. doi: 10.1242/jcs.114.14.2649. [DOI] [PubMed] [Google Scholar]
- Cundell M.J., Hutter L.H., Nunes Bastos R., Poser E., Holder J., Mohammed S., Novák B., Barr F.A. A PP2A-B55 recognition signal controls substrate dephosphorylation kinetics during mitotic exit. J. Cell Biol. 2016;214:539–554. doi: 10.1083/jcb.201606033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton S. Linking the cell cycle to cell fate decisions. TrendsCell Biol. 2015;25:592–600. doi: 10.1016/j.tcb.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C., Zhang P., Wade Harper J., Elledge S.J., Leder P. Mice Lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Dulić V., Kaufmann W.K., Wilson S.J., Tlsty T.D., Lees E., Harper J.W., Elledge S.J., Reed S.I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Enoch T., Nurse P. Mutation of fission yeast cell cycle control genes abolishes dependence of mitosis on DNA replication. Cell. 1990;60:665–673. doi: 10.1016/0092-8674(90)90669-6. [DOI] [PubMed] [Google Scholar]
- Fantes P.A. Isolation of cell size mutants of a fission yeast by a new selective method: characterization of mutants and implications for division control mechanisms. J. Bacteriol. 1981;146:746. doi: 10.1128/jb.146.2.746-754.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore A.P.Z.P., Ribeiro P.de F., Bruni-Cardoso A. Sleeping beauty and the microenvironment enchantment: microenvironmental regulation of the proliferation-quiescence decision in normal tissues and in cancer development. Front. Cell Dev. Biol. 2018;6:59. doi: 10.3389/fcell.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D., Nurse P. Cyclins of the fission yeast Schizosaccharomyces pombe. Semin. Cell Biol. 1995;6:73–78. doi: 10.1016/1043-4682(95)90003-9. [DOI] [PubMed] [Google Scholar]
- Fisher D.L., Nurse P. A single fission yeast mitotic cyclin B p34cdc2 kinase promotes both S-phase and mitosis in the absence of G1 cyclins. EMBO J. 1996;15:850–860. [PMC free article] [PubMed] [Google Scholar]
- Gharbi-Ayachi A., Labbé J.-C., Burgess A., Vigneron S., Strub J.-M., Brioudes E., Van-Dorsselaer A., Castro A., Lorca T. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science. 2010;330:1673–1677. doi: 10.1126/science.1197048. [DOI] [PubMed] [Google Scholar]
- Godfrey M., Touati S.A., Kataria M., Jones A., Snijders A.P., Uhlmann F. PP2A(Cdc55) phosphatase imposes ordered cell-cycle phosphorylation by opposing threonine phosphorylation. Mol. Cell. 2017;65:393–402.e3. doi: 10.1016/j.molcel.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselet F.P., Magnaldo T., Culerrier R.M., Sarasin A., Ehrhart J.-C. BMP2 and BMP6 control p57(Kip2) expression and cell growth arrest/terminal differentiation in normal primary human epidermal keratinocytes. Cell Signal. 2007;19:731–739. doi: 10.1016/j.cellsig.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Gould K.L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989;342:39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Gouw M., Michael S., Sámano-Sánchez H., Kumar M., Zeke A., Lang B., Bely B., Chemes L.B., Davey N.E., Deng Z. The eukaryotic linear motif resource - 2018 update. Nucleic Acids Res. 2018;46:D428–D434. doi: 10.1093/nar/gkx1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grallert A., Boke E., Hagting A., Hodgson B., Connolly Y., Griffiths J.R., Smith D.L., Pines J., Hagan I.M. A PP1-PP2A phosphatase relay controls mitotic progression. Nature. 2015;517:94–U248. doi: 10.1038/nature14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward D., Alfonso Pérez T., Gruneberg U. Orchestration of the spindle assembly checkpoint by CDK1-cyclin B1. FEBS Lett. 2019;246:629–719. doi: 10.1002/1873-3468.13591. [DOI] [PubMed] [Google Scholar]
- Heldt F.S., Barr A.R., Cooper S., Bakal C., Novák B. A comprehensive model for the proliferation–quiescence decision in response to endogenous DNA damage in human cells. Proc. Natl. Acad. Sci. U S A. 2018;115:2532. doi: 10.1073/pnas.1715345115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz E.P.T., Kruse T., Davey N.E., López-Méndez B., Sigurðsson J.O., Montoya G., Olsen J.V., Nilsson J. A conserved motif provides binding specificity to the PP2A-B56 phosphatase. Mol. Cell. 2016;63:686–695. doi: 10.1016/j.molcel.2016.06.024. [DOI] [PubMed] [Google Scholar]
- Janssens V., Longin S., Goris J. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail) Trends Biochem. Sci. 2008;33:113–121. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Jaspersen S.L., Charles J.F., Morgan D.O. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr. Biol. 1999;9:227–236. doi: 10.1016/s0960-9822(99)80111-0. [DOI] [PubMed] [Google Scholar]
- Jiang W., Hallberg R.L. Isolation and characterization of par1(+) and par2(+): two Schizosaccharomyces pombe genes encoding B’ subunits of protein phosphatase 2A. Genetics. 2000;154:1025–1038. doi: 10.1093/genetics/154.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Maekawa H., Shimoda C. Fission yeast Ste9, a homolog of Hct1/Cdh1 and Fizzy-related, is a novel negative regulator of cell cycle progression during G1-phase. Mol. Biol. Cell. 1998;9:1065–1080. doi: 10.1091/mbc.9.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff S., Andersen N.R., Borup M.T., Nielsen O. Cdk phosphorylation of the Ste11 transcription factor constrains differentiation-specific transcription to G1. Genes Dev. 2007;21:347–359. doi: 10.1101/gad.407107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominami K., Ochotorena I., Toda T. Two F-box/WD-repeat proteins Pop1 and Pop2 form hetero- and homo-complexes together with cullin-1 in the fission yeast SCF (Skp1-Cullin-1-F-box) ubiquitin ligase. Genes Cells. 1998;3:721–735. doi: 10.1046/j.1365-2443.1998.00225.x. [DOI] [PubMed] [Google Scholar]
- Kominami K., Seth-Smith H., Toda T. Apc10 and Ste9/Srw1, two regulators of the APC-cyclosome, as well as the CDK inhibitor Rum1 are required for G1 cell-cycle arrest in fission yeast. EMBO J. 1998;17:5388–5399. doi: 10.1093/emboj/17.18.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominami K., Toda T. Fission yeast WD-repeat protein pop1 regulates genome ploidy through ubiquitin-proteasome-mediated degradation of the CDK inhibitor Rum1 and the S-phase initiator Cdc18. Genes Dev. 1997;11:1548–1560. doi: 10.1101/gad.11.12.1548. [DOI] [PubMed] [Google Scholar]
- Kruse T., Biedenkopf N., Hertz E.P.T., Dietzel E., Stalmann G., López-Méndez B., Davey N.E., Nilsson J., Becker S. The ebola virus nucleoprotein recruits the host PP2A-B56 phosphatase to activate transcriptional support activity of VP30. Mol. Cell. 2018;69:136–145.e6. doi: 10.1016/j.molcel.2017.11.034. [DOI] [PubMed] [Google Scholar]
- Kwon J.S., Everetts N.J., Wang X., Wang W., Della Croce K., Xing J., Yao G. Controlling depth of cellular quiescence by an Rb-E2F network switch. Cell Rep. 2017;20:3223–3235. doi: 10.1016/j.celrep.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Aviles S., Grande M., González M., Helgesen A.-L., Alemany V., Sanchez-Piris M., Bachs O., Millar J.B.A., Aligue R. Inactivation of the Cdc25 phosphatase by the stress-activated Srk1 kinase in fission yeast. Mol. Cell. 2005;17:49–59. doi: 10.1016/j.molcel.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Manchado E., Guillamot M., de Cárcer G., Eguren M., Trickey M., García-Higuera I., Moreno S., Yamano H., Cañamero M., Malumbres M. Targeting mitotic exit leads to tumor regression in vivo: modulation by Cdk1, mastl, and the PP2A/B55α,δ phosphatase. Cancer Cell. 2010;18:641–654. doi: 10.1016/j.ccr.2010.10.028. [DOI] [PubMed] [Google Scholar]
- Martín R., Portantier M., Chica N., Nyquist-Andersen M., Mata J., Lopez-Aviles S. A PP2A-B55-mediated crosstalk between TORC1 and TORC2 regulates the differentiation response in fission yeast. Curr. Biol. 2017;27:175–188. doi: 10.1016/j.cub.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Castellanos C., Labib K., Moreno S. B-type cyclins regulate G1 progression in fission yeast in opposition to the p25rum1 cdk inhibitor. EMBO J. 1996;15:839–849. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Takeishi S., Kanie T., Susaki E., Onoyama I., Tateishi Y., Nakayama K., Nakayama K.I. p57 is required for quiescence and maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2011;9:262–271. doi: 10.1016/j.stem.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Mayer-Jaekel R.E., Ohkura H., Ferrigno P., Andjelkovic N., Shiomi K., Uemura T., Glover D.M., Hemmings B.A. Drosophila mutants in the 55 kDa regulatory subunit of protein phosphatase 2A show strongly reduced ability to dephosphorylate substrates of p34cdc2. J. Cell Sci. 1994;107(Pt 9):2609–2616. doi: 10.1242/jcs.107.9.2609. [DOI] [PubMed] [Google Scholar]
- McCright B., Rivers A.M., Audlin S., Virshup D.M. The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J. Biol. Chem. 1996;271:22081–22089. doi: 10.1074/jbc.271.36.22081. [DOI] [PubMed] [Google Scholar]
- Mochida S., Ikeo S., Gannon J., Hunt T. Regulated activity of PP2A–B55δ is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 2009;28:2777–2785. doi: 10.1038/emboj.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S., Maslen S.L., Skehel M., Hunt T. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science. 2010;330:1670–1673. doi: 10.1126/science.1195689. [DOI] [PubMed] [Google Scholar]
- Mondesert O., McGowan C.H., Russell P. Cig2, a B-type cyclin, promotes the onset of S in Schizosaccharomyces pombe. Mol. Cell Biol. 1996;16:1527–1533. doi: 10.1128/mcb.16.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Nurse P. Regulation of progression through the G1 phase of the cell cycle by the rum1+ gene. Nature. 1994;367:236–242. doi: 10.1038/367236a0. [DOI] [PubMed] [Google Scholar]
- Moser J., Miller I., Carter D., Spencer S.L. Control of the restriction point by Rb and p21. Proc. Natl. Acad. Sci. U S A. 2018;115:E8219. doi: 10.1073/pnas.1722446115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak B., Csikasz-Nagy A., Gyorffy B., Chen K., Tyson J.J. Mathematical model of the fission yeast cell cycle with checkpoint controls at the G1/S, G2/M and metaphase/anaphase transitions. Biophys. Chem. 1998;72:185–200. doi: 10.1016/s0301-4622(98)00133-1. [DOI] [PubMed] [Google Scholar]
- Oesterle E.C., Chien W.-M., Campbell S., Nellimarla P., Fero M.L. p27Kip1 is required to maintain proliferative quiescence in the adult cochlea and pituitary. Cell Cycle. 2011;10:1237–1248. doi: 10.4161/cc.10.8.15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Farrell P.H. Quiescence: early evolutionary origins and universality do not imply uniformity. Philos. Trans. R. Soc. B Biol. Sci. 2011;366:3498–3507. doi: 10.1098/rstb.2011.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton K.W., Spencer S.L., Noderer W.L., Meyer T., Wang C.L. Basal p21 controls population heterogeneity in cycling and quiescent cell cycle states. Proc. Natl. Acad. Sci. U S A. 2014;111:E4386. doi: 10.1073/pnas.1409797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L.L., Atherton-Fessler S., Piwnica-Worms H. p107wee1 is a dual-specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc. Natl. Acad. Sci. U S A. 1992;89:2917–2921. doi: 10.1073/pnas.89.7.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P., Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Ruvolo P.P. The broken ‘Off’ switch in cancer signaling: PP2A as a regulator of tumorigenesis, drug resistance, and immune surveillance. BBA Clin. 2016;6:87–99. doi: 10.1016/j.bbacli.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurin A.T. Kinase and phosphatase cross-talk at the kinetochore. Front. Cell Dev. Biol. 2018;6:2964–3023. doi: 10.3389/fcell.2018.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz M.H.A., Held M., Janssens V., Hutchins J.R.A., Hudecz O., Ivanova E., Goris J., Trinkle-Mulcahy L., Lamond A.I., Poser I. Live-cell imaging RNAi screen identifies PP2A–B55α and importin-β1 as key mitotic exit regulators in human cells. Nat. Cell Biol. 2010;12:886–893. doi: 10.1038/ncb2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz C., Johnson A., Kõivomägi M., Zatulovskiy E., Kravitz C.J., Doncic A., Skotheim J.M. A precise cdk activity threshold determines passage through the restriction point. Mol. Cell. 2018;69:253–264.e5. doi: 10.1016/j.molcel.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S.L., Cappell S.D., Tsai F.-C., Overton K.W., Wang C.L., Meyer T. The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell. 2013;155:369–383. doi: 10.1016/j.cell.2013.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmeier F., Amon A. Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu. Rev. Genet. 2004;38:203–232. doi: 10.1146/annurev.genet.38.072902.093051. [DOI] [PubMed] [Google Scholar]
- Stern B., Nurse P. A quantitative model for the cdc2 control of S phase and mitosis in fission yeast. Trends Genet. 1996;12:345–350. [PubMed] [Google Scholar]
- Stern B., Nurse P. Cyclin B proteolysis and the cyclin-dependent kinase inhibitor rum1p are required for pheromone-induced G 1Arrest in fission yeast. Mol. Biol. Cell. 1998;9:1309–1321. doi: 10.1091/mbc.9.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tury A., Mairet-Coello G., DiCicco-Bloom E. The cyclin-dependent kinase inhibitor p57Kip2 regulates cell cycle exit, differentiation, and migration of embryonic cerebral cortical precursors. Cereb. Cortex. 2011;21:1840–1856. doi: 10.1093/cercor/bhq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin R., Craig K., Hwang E.S., Prinz S., Tyers M., Amon A. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell. 1998;2:709–718. doi: 10.1016/s1097-2765(00)80286-5. [DOI] [PubMed] [Google Scholar]
- Wang X., Bajaj R., Bollen M., Peti W., Page R. Expanding the PP2A interactome by defining a B56-specific SLiM. Structure. 2016;24:2174–2181. doi: 10.1016/j.str.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.Q., Guo J.Y., Tang W., Yang C.-S., Freel C.D., Chen C., Nairn A.C., Kornbluth S. PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation. Nat. Cell Biol. 2009;11:644–651. doi: 10.1038/ncb1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Russell P. Roles of Wee1 and Nim1 protein kinases in regulating the switch from mitotic division to sexual development in Schizosaccharomyces pombe. Mol. Cell Biol. 1997;17:10–17. doi: 10.1128/mcb.17.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Murakami H., Okayama H. A WD repeat protein controls the cell cycle and differentiation by negatively regulating Cdc2/B-type cyclin complexes. Mol. Biol. Cell. 1997;8:2475–2486. doi: 10.1091/mbc.8.12.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano H., Kitamura K., Kominami K., Lehmann A., Katayama S., Hunt T., Toda T. The spike of S phase cyclin Cig2 expression at the G1-S border in fission yeast requires both APC and SCF ubiquitin ligases. Mol. Cell. 2000;6:1377–1387. doi: 10.1016/s1097-2765(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Zachariae W. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science. 1998;282:1721–1724. doi: 10.1126/science.282.5394.1721. [DOI] [PubMed] [Google Scholar]
- Zarzov P., Decottignies A., Baldacci G., Nurse P. G(1)/S CDK is inhibited to restrain mitotic onset when DNA replication is blocked in fission yeast. EMBO J. 2002;21:3370–3376. doi: 10.1093/emboj/cdf346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Forbes K.C., Wu Z., Moreno S., Piwnica-Worms H., Enoch T. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature. 1998;395:507–510. doi: 10.1038/26766. [DOI] [PubMed] [Google Scholar]
- Zhang P., Liégeois N.J., Wong C., Finegold M., Hou H., Thompson J.C., Silverman A., Harper J.W., DePinho R.A., Elledge S.J. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature. 1997;387:151–158. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]
- Zhang P., Wong C., DePinho R.A., Harper J.W., Elledge S.J. Cooperation between the Cdk inhibitors p27(KIP1) and p57(KIP2) in the control of tissue growth and development. Genes Dev. 1998;12:3162–3167. doi: 10.1101/gad.12.20.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Wong C., Liu D., Finegold M., Harper J.W., Elledge S.J. p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev. 1999;13:213–224. doi: 10.1101/gad.13.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.