Abstract
Objectives
To describe the clinical characteristics of patients in a Fangcang Hospital.
Methods
Non-critically ill individuals with positive SARS-CoV-2 RT-PCR tests admitted between 7 February and 12 February 2020 to Dongxihu Fangcang Hospital, which was promptly constructed because of the rapid, exponential increase in COVID-19 patients in Wuhan, China, were included; clinical course through to 22 February was recorded.
Results
A total of 1012 non-critically ill individuals with positive SARS-CoV-2 RT-PCR tests were included in the study. Thirty (of 1012, 3.0%) individuals were asymptomatic on admission. During hospitalization, 16 of 30 (53.3%) asymptomatic individuals developed different symptoms. Fourteen of 1012 patients (1.4%) remained asymptomatic from exposure to the end of follow up, with a median duration of 24 days (interquartile range 22–27). Fever (761 of 1012, 75.2%) and cough (531 of 1012, 52.4%) were the most common symptoms. Small patchy opacities (355 of 917, 38.7%) and ground-glass opacities (508 of 917, 55.4%) were common imaging manifestations in chest CT scans. One hundred patients (9.9%) were transferred to designated hospitals due to aggravation of illness. Diarrhoea emerged in 152 of 1012 patients (15.0%). Male, older age, diabetes, cardiovascular diseases, chills, dyspnoea, So2 value of ≤93%, white blood cell counts of >10 × 109/L and large consolidated opacities on CT images were all risk factors for aggravation of illness.
Conclusions
Non-critically ill individuals had different clinical characteristics from critically ill individuals. Asymptomatic infections only accounted for a small proportion of COVID-19. Although with a low incidence, diarrhoea was observed in patients with COVID-19, indicating the possibility of faecal–oral transmission.
Keywords: Clinical characteristics, COVID-19, Fangcang Hospital, Non-critically ill patients, Wuhan
Introduction
The COVID-19 epidemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spread throughout China and to more than 150 other countries [[1], [2], [3], [4], [5]]. As of 21 March 2020, China had 81 054 confirmed cases in total, 50 005 of which were identified in Wuhan. Of all the confirmed cases, about 20% were seriously and critically ill, and 3261 cases died (2508 deaths in Wuhan).
As of 22 February, the final date of our follow up, although 19 927 beds in Wuhan were prepared to treat individuals with COVID-19, with the rapid increase of new cases, there were still many confirmed cases unattended. From the perspective of treating patients and controlling the source of infection, a completely novel model, the Fangcang hospital, emerged, which mainly received non-seriously ill individuals with positive SARS-CoV-2 RT-PCR tests.
In this study, we explore the clinical features of 1012 non-critically ill individuals with a positive SARS-CoV-2 RT-PCR, admitted to Dongxihu Fangcang Hospital, Wuhan. This is the largest study of its kind, to date, and introduces the operation mode of the hospital.
Methods
Study design and participants
In the absence of proven antiviral therapy for COVID-19, effective infection control measures, including patient isolation, are critical. Given the rapid, exponential increase in COVID-19 patients, the prompt construction of temporary Fangcang hospitals was required. Medical personnel at Fangcang hospitals came from all over the country to work shifts lasting between 6 and 8 hours. Fangcang hospitals were usually reconstructed using large-scale spaces such as exhibition halls, gymnasiums and workshops to have the basic functions of medical treatment, logistic support, patient beds and living area. Patients from 7 to 12 February 2020, at Dongxihu Fangcang Hospital in Wuhan, were enrolled in the retrospective study. Dongxihu Fangcang Hospital was managed by the Zhongnan Hospital of Wuhan University. All individuals with COVID-19 recruited in this study were diagnosed according to WHO interim guidance. This study was approved by the institutional ethics board of Zhongnan Hospital of Wuhan University (No. 2020081K). Written informed consent was waived by the institutional ethics board of the hospital for emerging infectious diseases, but oral consent was obtained from all the participants. Clinical courses were followed until 22 February.
Patients were admitted to the Fangcang Hospital according to the following criteria:
-
a)
a positive result confirmed by standard SARS-CoV-2 RT-PCR test
-
b)
age≥16 years with self-care ability
-
c)
respiratory rate <30 with blood oxygen saturation >93%, at rest
-
d)
a negative result for influenza virus RT-PCR test.
Patients were not admitted to the Fangcang Hospital if they showed:
-
a)
a negative result of standard SARS-CoV-2 RT-PCR test
-
b)
age <16 years
-
c)
without self-care ability and ability of daily life activities
-
d)
respiratory rate ≥30 or blood oxygen saturation ≤93%, at rest
-
e)
a positive result for influenza virus RT-PCR test
-
f)
with serious underlying co-morbidities such as acute myocardial infarction, acute intracerebral haemorrhage, acute cerebral infarction, heart failure, renal failure, patients with malignancies in need of comprehensive treatment
-
g)
with a mental disorder such as schizophrenia
-
h)
with a serious psychological disorder such as suicidal tendencies
-
i)
with other infectious diseases such as active tuberculosis
-
j)
excluded by two independent doctors on duty because of conditions that were not mentioned above.
All patients admitted to Dongxihu Fangcang Hospital from 7 to 12 February 2020 were included in this study.
Definitions
Severity of COVID-19Serious illness was defined if satisfying at least one of the following items: (a) breathing rate ≥30/min; (b) pulse oximeter oxygen saturation (Spo 2) ≤93% at rest; (c) ratio of partial pressure of arterial oxygen (Pao 2) to fraction of inspired oxygen (Fio 2) ≤300 mmHg (1 mmHg = 0.133 kPa).
Critical illness was defined if satisfying at least one of the following items: (a) respiratory failure occurred and individual received mechanical ventilation; (b) shock; (c) failure of other organs and received care in the intensive care unit.
Epidemiological historyEpidemiological history in this study was defined as follows:
-
a)
residence in an epidemic area (Wuhan city) in the last month, especially in the last 2 weeks
-
b)
a history of contact with a SARS-CoV-2-positive patient in the last month, especially in the last 2 weeks
-
c)
a history of exposure to a live poultry market or wild animals.
Clinical outcome
Deterioration of COVID-19 Individuals with any one of the following conditions were defined as having deterioration of COVID-19: (a) symptoms persisted or worsened for >7 days; (b) respiratory rate ≥30 or blood oxygen saturation ≤93%, at rest; (c) pulmonary imaging showed that the lesions progressed >50% within 48 hours.
Deterioration of co-morbidities or complications Deteriorated clinical status caused by conditions mentioned from item (f) to item (i) in exclusion criteria during hospitalization were defined as deterioration of co-morbidities or complications.
Discharge
Individuals meeting the following criteria could be discharged: temperature returned to normal for more than 3 days; respiratory symptoms improved significantly; pulmonary imaging showed that acute exudative lesions were significantly improved; with two consecutive negative results for RT-PCR tests for sputum or nasopharynx swab samples (with an interval of at least 24 hours).
Data collection
Basic information (gender, age and co-morbidities), symptoms (fever, chills, runny nose, nasal congestion, sore throat, cough, expectoration, dyspnoea, headache, myalgia, vomiting, diarrhoea and abdominal pain), white blood cell (WBC) counts and results of chest computed tomography (CT) scans and epidemiological data were collected for each patient from electronic medical records. It should be noted that all of the patients' laboratory and radiological examinations were performed before their admission, so not all the patients had related data. All data were checked by two researchers (WX and ZY).
Working procedures of Fangcang Hospital
The flow chart of confirmed patients admitted into Fangcang Hospital is shown in Fig. 1 . The triage flow based on RT-PCR and CT results for outpatients is described in the Supplementary materials (Appendix S1).
Fig. 1.
The flow chart of working procedures of Fangcang hospital.
Statistical analysis
Categorical data were presented as counts and percentages, and continuous data were expressed as mean ± SD, if the data were normally distributed, or expressed as median with interquartile range (IQR) values. Proportions for categorical variables were compared using the χ2 test, and Fisher exact test was used if the data were limited. Comparisons for medians of non-normal distribution data were performed using Mann–Whitney test. All statistical analyses were performed using SPSS (Statistical Package for the Social Sciences, Armonk, NY, USA) Statistics version 24.0 software.
Results
General characteristics of patients with COVID-19
A total of 1012 individuals with non-critically ill COVID-19 were included in the retrospective analysis. The basic information is shown in Table 1 .
Table 1.
Demographics, baseline characteristics and epidemiological history of 1012 non-critically ill individuals with COVID-19 admitted into Dongxihu Fangcang Hospital
| Patients in total (n = 1012) | Patients with aggravation of illness during follow up (n = 100) | Patients without aggravation of illness during follow up (n = 912) | Comparisons between subgroups (p value) | |
|---|---|---|---|---|
| Gender | ||||
| Male | 524 (51.8%) | 62 (62.0%) | 462 (50.7%) | 0.03 |
| Female | 488 (48.2%) | 38 (38.0%) | 450 (49.3%) | |
| Age (years) | ||||
| Range | 16–89 | 21–79 | 16–89 | |
| Median (IQR) | 50 (39–58) | 55.5 (47–62) | 50 (38–58) | <0.01 |
| <18 | 3 (0.3%) | 0 | 3 (0.3%) | <0.01 |
| 18–39 | 257 (25.4%) | 12 (12.0%) | 245 (26.9%) | |
| 40–59 | 516 (51.0%) | 51 (51.0%) | 465 (51.0%) | |
| ≥60 | 236 (23.3%) | 37 (37.0%) | 199 (21.8%) | |
| Chronic medical illness | 114 (11.3%) | 17 (17%) | 97 (10.6%) | 0.06 |
| Hypertension | 46 (4.5%) | 6 (6.0%) | 40 (4.4%) | 0.63 |
| Diabetes | 27 (2.7%) | 7 (7.0%) | 20 (2.2%) | 0.01 |
| Cardiovascular diseases | 15 (1.5%) | 5 (5.0%) | 10 (1.1%) | <0.01 |
| Respiratory system disease | 20 (2.0%) | 2 (2.0%) | 18 (2.0%) | 1 |
| Other diseases | 34 (3.3%) | 3 (3.0%) | 31 (3.4%) | 1 |
| Epidemiological history | ||||
| A history of life in an epidemic area (Wuhan city) in the last month, especially in 2 weeks | 1012 (100%) | 100 (100%) | 912 (100%) | 1 |
| A history of contact with a SARS-CoV-2-positive patient in the last month, especially in 2 weeks | 311 (30.7%) | 25 (25%) | 286 (31.4%) | 0.19 |
| A history of exposure to a live poultry market or wild animals | 0 | 0 | 0 | N/A |
IQR, interquartile range.
The median age of the included patients was 50 years (IQR 39–58 years), ranging from 16 to 89 years. In all, 114 of the 1012 patients (11.3%) had coexisting illness such as hypertension, diabetes, cardiovascular diseases, respiratory system disease and other diseases. All individuals included were at increased risk for COVID-19 with potential or probable exposure and were local residents of Wuhan City or had lived in Wuhan city in the last month.
Of the 1012 patients, 311 (30.7%) were exposed to a SARS-CoV-2-positive patient. No patients had visited a live poultry market or been exposed to wild animals in the last month.
On admission, the most common symptoms was fever (761 of 1012, 75.2%), and 424 of 1012 (41.9%) suffered a moderate fever, 37.3–38.0°C. Cough was also a common symptom, occurring in 531 of 1012 (52.4%). The other symptoms included dyspnoea (231 of 1012, 22.8%), expectoration (220 of 1012, 21.7%), chills (182 of 1012, 18.0%), myalgia (170 of 1012, 16.8%), headache (152 of 1012, 15.0%), diarrhea (152 of 1012, 15.0%), sore throat (144 of 1012, 14.2%), nasal congestion (69 of 1012, 6.9%), runny nose (57 of 1012, 5.6%), abdominal pain (37 of 1012, 3.7%), vomiting (36 of 1012, 3.6%).
Thirty of 1012 (3.0%) patients were asymptomatic from exposure to admission, with a median duration of 10.5 days (IQR 7–13 days). However, during follow up from admission to the end, 16 (53.3%) of the asymptomatic individuals suffered from different symptoms. Fever occurred in 6 (20%), with cough in 8 (26.7%), myalgia in 3 (10%), dyspnoea in 2 (6.7%), runny nose in 1 (3.3%), nasal congestion in 1 (3.3%) and abdominal pain in 1 (3.3%). Fourteen of 1012 patients (1.4%) remained asymptomatic during the whole follow up from exposure to the end, with a median duration of 24 days (IQR 22–27 days).
Duration for all of the 1012 patients from onset of symptoms for symptomatic patients and first positive SARS-CoV-2 RT-PCR test for asymptomatic ones to admission ranged from 1 to 30 days, with a median of 10 days (IQR 10–14 days).
During hospitalization, 46 of 1012 patients (4.5%) had a low So 2 value of 89%–93%.
The WBC counts were performed in 586 of 1012 patients (57.9%) before admission. Most patients (441 of 586, 75.3%) had normal WBC counts, 139 of 586 patients (23.7%) had WBC counts below normal, and 6 of 586 patients (1.0%) had WBC counts above normal.
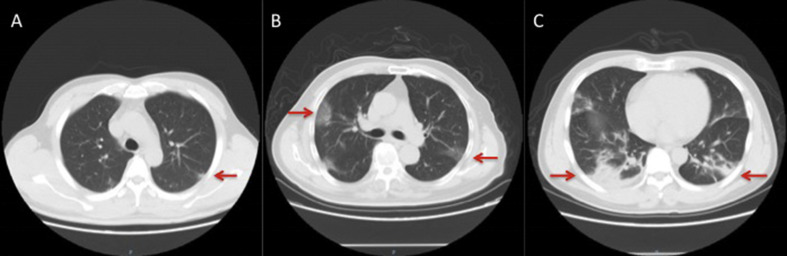
Before admission, the pulmonary imaging of COVID-19 patients was in three categories including small patchy opacities, large ground-glass opacity and large consolidated opacity [[6], [7], [8]] (Fig. 2 ). The proportions of each group were 38.7% (355 of 917), 55.4% (508 of 917) and 5.9% (54 of 917), respectively.
Fig. 2.
Chest CT imaging of individuals with COVID-19. (A) A 42-year-old man with patchy opacities on the 13th day after occupational exposure. (B) A 65-year-old man with a history of contact with COVID-19 patients, showing large ground-glass opacity on the 12th day after exposure. (C) A 48-year-old man exhibiting large consolidated opacity on the 18th day after occupational exposure.
At final follow up, 100 of 1012 patients (9.9%) had been transferred to designated hospitals due to aggravation of their illness, and 93 of 1012 patients (9.2%) had been discharged, with 819 of 1012 patients (80.9%) remaining in hospital. No patients died during the follow up (Table 2 ).
Table 2.
Clinical characteristics and outcome of 1012 non-critically ill individuals with COVID-19 admitted to the Dongxihu Fangcang Hospital
| Patients in total (n = 1012) | Patients with aggravation of illness during follow up (n = 100) | Patients without aggravation of illness during follow up (n = 912) | Comparisions between subgroups (p value) | |
|---|---|---|---|---|
| Signs and symptoms on admission | ||||
| asymptomatic condition | 30 (3.0%) | 2 (2.0%) | 28 (3.1%) | 0.77 |
| fever | 761 (75.2%) | 76 (76.0%) | 685 (75.1%) | 0.85 |
| highest temperature (°C) | ||||
| 37.3–38.0 | 182 (18.0%) | 25 (25.0%) | 157 (17.2%) | 0.05 |
| 38.1–39 | 424 (41.9%) | 42 (42.0%) | 382 (41.9%) | |
| >39 | 155 (of 1012, 15.3%) | 9 (9.0%) | 146 (16.0%) | |
| Chills | 182 (18.0%) | 28 (28.0%) | 154 (16.9%) | <0.01 |
| Runny nose | 57 (5.6%) | 8 (8.0%) | 49 (5.4%) | 0.29 |
| Nasal congestion | 69 (6.9%) | 10 (10.0%) | 59 (6.5%) | 0.20 |
| Sore throat | 144 (14.2%) | 16 (16.0%) | 128 (14.0%) | 0.59 |
| Cough | 531 (52.4%) | 55 (55%) | 476 (52.2%) | 0.59 |
| Expectoration | 220 (21.7%) | 27 (27.0%) | 193 (21.2%) | 0.18 |
| Dyspnoea | 231 (22.8%) | 36 (36.0%) | 195 (21.4%) | <0.01 |
| Headache | 152 (15.0%) | 19 (19.0%) | 133 (14.6%) | 0.24 |
| Myalgia | 170 (16.8%) | 17 (17.0%) | 153 (16.8%) | 0.96 |
| Vomiting | 36 (3.6%) | 6 (6.0%) | 30 (3.3%) | 0.27 |
| Diarrhoea | 152 (15.0%) | 17 (17.0%) | 135 (14.8%) | 0.56 |
| Abdominal pain | 37 (3.7%) | 6 (6.0%) | 31 (3.4%) | 0.30 |
| Duration from onset of symptoms to admission (days) | ||||
| Range | 1–30 | 2–21 | 1–30 | N/A |
| Median (IQR) | 10 (7–14) | 10 (7.5–14) | 10 (7–14) | 0.02 |
| <7 | 152 (15.0%) | 4 (4%) | 148 (16.2%) | <0.01 |
| 7–14 | 641 (63.3%) | 74 (74%) | 567 (62.2%) | |
| >14 | 219 (21.6%) | 22 (22%) | 197 (21.6%) | |
| Blood oxygen saturation during hospitalization, (%) | ||||
| Range | 89–100 | 89–99 | 89–100 | N/A |
| Median (IQR) | 97 (96–98) | 96 (94.5–97) | 97 (96–98) | <0.01 |
| 89–93 | 46 (4.5%) | 15 (15%) | 31 (3.4%) | <0.01 |
| >93 | 966 (95.5%) | 85 (85%) | 881 (96.7%) | |
| WBC counts ( × 109/L) | 586 (of 1012, 57.9%) | 58 (of 100, 58%) | 528 (of 912, 57.9%) | 0.98 |
| Range | 0.67–20.0 | 0.78–20.0 | 0.67–11.1 | N/A |
| Median (IQR) | 4.7 (4.0–6.0) | 4.8 (4.1–6.0) | 4.7 (4.0–6.0) | 0.74 |
| <4 | 139 (of 586, 23.7%) | 14 (of 58, 24.1%) | 125 (of 528, 23.7%) | <0.01 |
| 4–10 | 441 (of 586, 75.3%) | 40 (of 58, 69.0%) | 401 (of 528, 75.9%) | |
| >10 | 6 (of 586, 1.0%) | 4 (of 58, 6.9%) | 2 (of 528, 0.4%) | |
| Chest CT scana | 917 (of 1012, 90.5%) | 86 (of 100, 86%) | 831 (of 912, 91.1%) | 0.09 |
| Small patchy opacities | 355 (of 917, 38.7%) | 37 (of 86, 43.0%) | 318 (of 831, 38.3%) | 0.02 |
| Large ground-glass opacity | 508 (of 917, 55.4%) | 39 (of 86, 45.3%) | 469 (of 831, 56.4%) | |
| Large consolidated opacity | 54 (of 917, 5.9%) | 10 (of 86, 11.6%) | 44 (of 831, 5.3%) | |
| Clinical outcome | ||||
| Transferred to designated hospitals due to aggravation of illness | 100 (9.9%) | 100 (100%) | N/A | N/A |
| Discharge | 93 (9.2%) | N/A | 93 (10.2%) | |
| Remained in hospital | 819 (80.9%) | N/A | 819 (89.8%) | |
| Died | 0 | 0 | 0 | |
IQR, interquartile range.
Only one dominant manifestation on chest CT scan for each patient was recorded by the doctors on duty.
To understand the factors that might be associated with the aggravation of illness and transfer of patients from a Fangcang to a designated hospital, comparisons were made between patients with (Group 1) and without (Group 2) aggravation of illness during follow up (shown in Table 1, Table 2). The details of comparisons are described in the Supplementary material (Appendix S2).
Discussion
Up to 10 March, a total of 16 Fangcang hospitals had been put into use, receiving more than 12 000 non-critically ill patients with COVID-19.
To describe the clinical characteristics and outcomes of patients with COVID-19 in a Fangcang Hospital, this study included 1012 non-critically ill patients infected with SARS-CoV-2 admitted into the Dongxihu Fangcang Hospital. Compared with hospitalized patients in some published studies [[9], [10], [11], [12]], similar to those studies, fever (75.2%) and cough (52.4%) were the most common symptoms, but there were some noteworthy points.
The first was clinical outcome. In a recent study, 32 of 52 (61.5%) individuals admitted into the intensive care unit in Jinyintan Hospital, had died at 28 days, and the median duration from admission to ICU to death was 7 (IQR 3–11) days [12]. In our study, no individuals died during the follow up, and only 10% were transferred to designated hospitals due to aggravation of illness, indicating a significant difference between non-critically ill and critically ill patients. Male gender, older age, diabetes or cardiovascular diseases, chills or dyspnoea, So 2 value of ≤93%, WBC counts of >10 × 109/L and a large consolidated opacity on chest CT images were all risk factors for aggravation of illness and transfer of patients from Fangcang to designated hospitals.
Second, our study revealed that 30 of 1012 (3.0%) patients were asymptomatic on admission, but 14 of 1012 patients (1.4%) remained asymptomatic during the whole follow up, with a median duration of 24 days (IQR 22–27) days. Asymptomatic people might easily be ignored and are potentially infectious. It had been reported that asymptomatic individuals could spread the disease to family members [13], indicating that timely isolation of asymptomatic infected people was important for epidemic prevention and control.
Third, diarrhoea was an atypical symptom of low incidence in COVID-19 patients. In some published studies, incidence of diarrhoea in patients with COVID-19 ranged from 2% to 10% [9,11,14,15]. Our percentage was higher than in those studies, perhaps explained by the use of some drugs such as abidol and moxifloxacin before admission to the Fangcang Hospital. Recent studies found positive SARS-CoV-2-RNA in anal swabs and faecal samples and found that positive results of anal swabs were associated with disease severity [16,17]. Although it was unclear whether there was a definite link between diarrhoea and COVID-19, both studies raise a question concerning whether the gastrointestinal tract might be another site of viral replication, indicating the possibility of faecal–oral transmission.
This study has some limitations. It is a single-centre study. However, patients came from different regions of Wuhan, and the sample size is large. The Fangcang hospital was not a regular hospital, but a temporary one. Routine tests such as biochemical examination and C-reactive protein could not be carried out during the follow up.
There might be a possible association between the features of chest CT manifestation and the course of COVID-19. We could not perform this analysis because of the inability to display CT images in the temporary electronic medical record system and the limited information of our data.
In conclusion, non-critically ill individuals had different clinical characteristics from critically ill patients. Asymptomatic infections only accounted for a small proportion of individuals infected with SARS-CoV-2, but might be a potential source of infection. Although with a low incidence, diarrhoea in patients with COVID-19 should not be ignored, indicating the possibility of faecal–oral transmission.
Transparency declaration
The authors have no conflicts of interest to declare.
Funding
This study is supported by the National Natural Science Fund of China (No. 81600437).
Contributors
ZQ and WX designed the study. WX and ZY collected the epidemiological and clinical data. WX and FJ carried out the patient satisfaction survey. WX, CL, DF, ZR, GL, WF and ZY took part in the data collection. CQ shot the videos about Fangcang hospital. WX drafted the manuscript, and ZQ revised the final manuscript.
Acknowledgements
We thank all the individuals included in this study.
Editor: A. Huttner
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.03.032.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastola A., Sah R., Rodriguez-Morales A.J., Lal B.K., Jha R., Ojha H.C. The first 2019 novel coronavirus case in Nepal. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30067-0. pii: S1473-3099(20)30067-0 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pongpirul W.A., Pongpirul K., Ratnarathon A.C., Prasithsirikul W. Journey of a Thai taxi driver and novel coronavirus. N Engl J Med. 2020 doi: 10.1056/NEJMc2001621. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ki M., Task Force for 2019-nCoV Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Republic of Korea. Epidemiol Health. 2020 doi: 10.4178/epih.e2020007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001191. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin Y.H., Cai L., Cheng Z.S., Cheng H., Deng T., Fan Y.P. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang P., Liu T., Huang L., Liu H., Lei M., Xu W. Use of chest CT in combination with negative RT-PCR assay for the 2019 novel coronavirus but high clinical suspicion. Radiology. 2020:200330. doi: 10.1148/radiol.2020200330. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan Y.N., Qin J. Pre- and posttreatment chest CT findings: 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020:200323. doi: 10.1148/radiol.2020200323. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kui L., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000744. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet. 2020 doi: 10.1016/S2213-2600(20)30079-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu P., Zhu J., Zhang Z., Han Y., Huang L. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa077. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-CoV-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;vol. 368:m606. doi: 10.1136/bmj.m606. Erratum in: BMJ 2020;368:m792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W., Lan Y., Yuan X., Deng X., Li Y., Cai X. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbe. Infect. 2020;9:469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai J., Xu J., Lin D., Yang Z., Xu L., Qu Z. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa198. pii: ciaa198. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.