Abstract
In this study, we used a clinically relevant rat scald burn model to determine the treatment effects of cerium nitrate (CN) for stabilizing burn eschars through reduction of damage-associated molecular patterns (DAMPs), inflammatory cytokines, and bioburden. Forty-two male Sprague–Dawley rats were anesthetized before undergoing a scald burn at 99°C for 6 seconds to create a 10% full-thickness burn. The test groups included sham burn, burn with water bathing, and burn with CN bathing. End point parameters included circulating DAMPs, proinflammatory cytokines, tissue myeloperoxidase activity, and quantification of resident flora in burn skin. The high mobility group protein box 1 was found to be elevated in burn animals at postoperative days (POD) 1 and 7. CN significantly alleviated the increase (P < .05 at POD 1 and P < .01 at POD 7). CN also lessened the heightened levels of hyaluronan in burn animals (P < .05 at POD 7). Additionally, CN significantly reduced the burn-induced increases in interleukin-1β, growth-regulated oncogene/keratinocyte chemoattractant, and macrophage inflammatory protein-1α in burn wounds. The anti-inflammatory effect of CN was also demonstrated in its ability to mitigate the upregulated circulatory xanthine oxidase/dehydrogenase and increased tissue neutrophil infiltration in burn animals. Last, CN suppressed postburn proliferation of resident skin microbes, resulting in a significant 2-log reduction by POD 7. In conclusion, these results suggest that CN attenuates the burn-induced DAMPs, tissue inflammatory responses, and regrowth of resident skin flora, all of which collectively could improve the quality of burn eschar when applied at the point of injury in prolonged field care situations.
INTRODUCTION
The standard care for full-thickness (FT) burns is early eschar excision and graft coverage. However, this approach requires stringent surgical conditions, such as a sizable blood bank, availability of allogeneic or autogenous skin for grafting, a highly skilled burn team, and an appropriately equipped sterile operation room.1,2 These prerequisites for the standard of care are not feasible for prolonged field care during combat operations. Likewise, in some resource-limited regions of the world, the early excisions are often hindered by lack of supplies, equipment, or skilled practitioners. Without treatment, the burn eschar deteriorates, releases damage-associated molecular patterns (DAMPs), and serves as a nidus for infection. Dysregulated inflammatory signaling results locally and systemically. At the extreme, the outcome is multiple organ dysfunction syndrome. Therefore, an alternative field care to stabilize the eschar and decrease harmful eschar-related toxicity and infection of burn wounds is critically needed for austere environment.
Cerium nitrate (CN) has been used since 1976 in various European countries, usually in combination with silver sulfadiazine (CN-SSD), to improve burn wound healing outcomes where early excision is contradicted due to underlying medical conditions.3 The cream temporizes the wound, enabling grafting to be delayed, thereby avoiding extensive surgery at early postburn times that is precluded by medical or other conditions.4 Clinically, CN-SSD converts the eschar into a dry, leathery, pliable, and protective crust with excellent resistance to infection (fewer septic complications) and good long-term adherence to the burn wound.5 In addition, underneath the pliable crust, the wound appeared to be protected from bacterial ingress and drying out, creating an environment favorable for healing.6 After excision, the wound bed was generally clean, healthy, and ready to accept a skin graft, with a graft “take” rate of 90%.7 In a retrospective clinical study that compared SSD with CN-SSD, Vehmeyer-Heeman et al found that the mortality rate decreased from 13.7% (1977–1983, SSD treatment period) to 4.7% (1984–1990, CN-SSD treatment period). Further analysis indicated that CN-SSD was especially superior to SSD for patients with >50% TBSA, within the age group, 0 to 30 years old. Another Dutch research group compared the clinical outcomes of SSD and CN-SSD topical treatment for children with partial-thickness scald burns at two burn centers with one center treating with SSD and the other using CN-SSD. They showed that patients treated with CN-SSD healed faster than patients treated with SSD alone, 13-day vs. 16-day median healing time (P < .01), respectively. CN, without SSD, administered via a bath has been reported to mitigate burn-induced immunosuppression and to improve patient survival. The peripheral blood mononuclear cells from 10 burn patients who received a single bath in CN solution were stimulated ex vivo and produced interleukin-2 (IL-2) in the range of healthy normal persons as opposed to the lower levels in non-CN-bathed burn patients.8–10 Apparent enhanced survival was reported in another study, of 64 burn patients with 30 to 90% TBSA admitted to Kantonsspital, Basel. One 30-minute bath of 40 mM CN in water within 4 hours of admission was reported to improve the survival of high-risk patients, while 80% mortality was expected, only 3.4% mortality was observed with CN treatment.11
In animal studies, CN reduced the lethality of burned skin, mitigated burn-injury-induced immunosuppression, and reduced burn injury progression. First, when healthy mouse skin was removed, burned, and then regrafted, treatment of the burned skin with a 40 mM CN solution prior to regrafting significantly improved the survival of the animals from 10 to 74%.12 Second, in a rat model, a single bathing of FT burns (20% TBSA) in 40 mM CN solution for 30 minutes was as effective as excision with primary closure (on day 2) in mitigating burn-induced elevation of serum IL-6 and tumor necrosis factor-α on days 3 and 7.13 Also, a 30-minute post-burn CN bath reduced the burn-induced rolling, sticking, and transmigrating of leukocytes into tissue distal from the burn, measured immediately after the bath.14 Furthermore, topical CN treatment of steam burns (20 to 25% TBSA) substantially restored the helper-to-suppressor T-cell ratio.15 The precise mechanism of how CN alters the chemical and physical features of eschars has not been completely defined. In burn wounds treated with CN, calcium increased with days of treatment. The data reported in the literature support superficial connective tissue calcification as the potential mechanism(s) underlying the ability of CN to stabilize the eschar.16 Although CN does not cross the cytoplasmic membrane, cerium can displace calcium from molecules to disrupt structures and enzymatic activities extracellularly, as one potential underlying mechanism of reducing inflammation beneath the calcified eschar in the wound bed.3 Recently, aside from increase in expression of cytokines, several reports showed that burn injury induces early release of high mobility group box-1 (HMGB1), one of the more well-characterized DAMPs.17,18 There was a positive correlation between TBSA and HMGB1, with higher levels of circulating HMGB1 being found in nonsurvivors.18 Also, HMGB1 levels were significantly higher in burn patients who had developed sepsis, especially in the nonsurvivors.17 Additionally, a study reported that released DAMPs from burn tissue activated the innate immunity cascade involved in inflammatory signaling.19 In view of early CN treatment potentially limiting signaling from burned tissue that causes immunosuppression and promotes inflammation, we sought to determine whether CN treatment can also limit local release of proinflammatory cytokines and systemic release of DAMPs from eschar tissue, lessening the severity of burn trauma-induced pathophysiological outcomes. We used a clinically relevant rat scald burn model (modified Walker–Mason model) to determine CN treatment effects on the tissue cytokine profile and circulating inflammatory DAMP molecules after an FT burn injury. Additionally, we tested CN treatment effects on resident flora of rat burn skin. Together, the data demonstrated that CN treatment decreased proinflammatory cytokines in the wounds and DAMPs in circulation. CN bathing also suppressed the proliferation of skin flora in the wounds.
METHODS
Animals
Male Sprague–Dawley rats, 3 to 6 months old, weighing between 375 and 400 g were used for the animal burn model. The rats were acclimated in our animal facilities at least 1 week prior to use. The facility’s Institutional Animal Care and Use Committee (IACUC) approved all research conducted in this study. Research was conducted in compliance with the Animal Welfare Act, the implementation of Animal Welfare Regulations, and the principles of the Guide for the Care and Use of Laboratory Animals, National Research Council recommendations. The facility where this research was conducted is fully accredited by AAALAC International.
Burn and Treatment Procedures
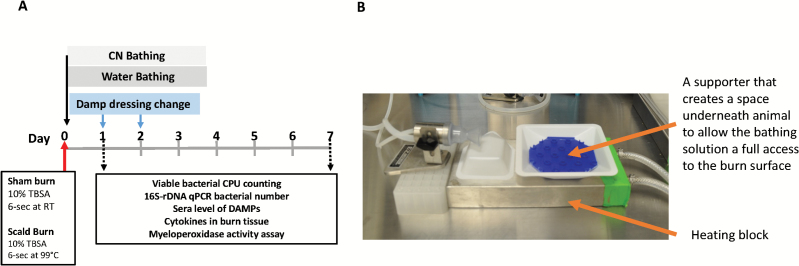
Forty-two rats were randomized into each treatment group. The test groups included sham burn control (n = 6); burn with 30-minute water bathing (37°C) immediately post-burn (n = 18); and burn with 30-minute CN (40 mM; 37°C) bathing immediately post-burn (n = 18). Each test group was further randomized into two subgroups according to end points (postoperative days [POD] 1 and 7) (Figure 1A). One day prior to burn, the rats were anesthetized with 3% isoflurane and the dorsal skin was shaved and depilated with Nair (Church & Dwight, Ewing, NJ). To provide postburn analgesia, a dose of Buprenorphine SR Lab (1.2 mg/kg, Zoopharm Pharmacy) was given subcutaneously. On the burn day, the anesthetization of the rats was induced and maintained with 2.5 to 3% isoflurane for 15 minutes prior to the scald. The animals were then placed into a custom-built, insulated mold with a 5.3 × 9 cm opening equivalent to ~10% TBSA. The mold was immersed into water pre-heated to 99°C in a circulating water bath (Thermo Fisher Scientific, Precision CIR35, Newington, NH) for 6 seconds to create an FT burn on the dorsal skin. The sham burn group animals underwent the same procedure using room temperature water instead of the 99°C water. All treatments started immediately post-burn. For the bathing treatments, rats were positioned supinely with their burned areas completely immersed in a container (12.5 × 12.5 × 2 cm) filled with 37°C 100 ml of filter-sterile 40 mM CN (cerium nitrate hexahydrate; Acros, Fairlawn, NJ) or its vehicle, water, for 30 min. The bathing solutions were kept at 37°C to prevent hypothermia by using a heating block set to 40°C (Figure 1B). For dressing the wounds, a damp dressing (gauze soaked with 40 mM CN for CN-treated animals or gauze soaked with water for vehicle control animals) was used to cover burn wounds and provide additional CN for an extended period of time. For the sham burn, dry gauze was the primary dressing. Tegaderm (3M Healthcare, St. Paul, MN), a semiocclusive bandage, was used to seal the treatments on wounds. The dressings were secured in place for the duration of the experiment using a custom rat jacket.20 Fluid resuscitation to aid in the recovery of the burned animals was injected intraperitoneally (i.p.) with 4 ml of Ringer’s lactate solution immediately after burn and every 12 hours for four times thereafter. The dressings were replaced with fresh ones soaked with CN or water, respectively, at daily intervals for 2 days post-burn with the rats under the inhalant anesthesia regimen described above. The burn wounds were photographed immediately after the burn (POD 0) and again at the end points on POD 1 and 7. Animals were anesthetized and killed at POD 1 and 7. Blood samples were drawn via cardiac puncture and skin samples recovered with dermal biopsy punches (7 mm in diameter).
Figure 1.
(A) Schematic illustration of burn and treatment approaches and end point procedures. Sham burn group consisted of three rats each postoperative days (POD), and burn + water and burn + cerium nitrate (CN) consisted of nine rats each POD. (B) Implementation of rat bathing system. A plastic square weigh boat (12.5 × 12.5 × 2 cm) was used as the basin for delivering the CN treatment (i.e., bathing) to the test animals. The prewarmed bathing solution was kept at 37°C with the heating block set at 40°C.
Multiplex Cytokine/Chemokine Assay
Burned skin (eschar) samples harvested by 7-mm biopsy punches (Acuderm, Fort Lauderdale, FL) were pulverized under liquid nitrogen using a Bessman Tissue Pulverizer (Spectrum, Inc., Rancho Dominguez, CA) and then homogenized in tissue lysis buffer according to the manufacture’s guide using a tissue tearer (IKA work, Inc., Wilmington, NC). After a freeze–thaw cycle and sonication, the samples were centrifuged and supernatants were collected. Total protein concentrations were determined with a Pierce™ BCA protein assay kit (Thermo Scientific, Rockford, IL). A Bio-Rad Rat 23-plex cytokine/chemokines panel (Bio-Rad Laboratories, Hercules, CA) was used to assay 23 different inflammatory mediators according to the manufacture’s guide. The standard curve for each of the cytokines and their quantification was measured using BioPlex 200 (Luminex 100/200, Austin, TX). The cytokine concentrations were calculated/normalized against its protein concentration of each tissue lysate.
Quantification of Resident Skin Flora
The culture-based viable colony forming unit (CFU) counts and the quantitative real-time PCR-based 16S rDNA copy number have been described in detail in our previous publication.20 Briefly, four 7-mm biopsy punches from each burn wound were placed in MagNA Lyser Green Beads tubes (Roche Diagnostics GmbH, Mannheim, Germany) and homogenized with 1-ml phosphate-buffered saline using a FastPrep®-24 Tissue Homogenizer (MP Biomedicals, LLC, Santa Ana, CA). The samples were serially diluted with phosphate-buffered saline and plated on Trypticase soy agar containing 5% sheep’s blood (Becton, Dickinson and Co.) using a WASP 2 Spiral Plater (Microbiology International, Frederick, MD). Viable CFUs were determined using a ProtoCOL 3 Colony Counter (Microbiology International) and plotted as log10 (CFU/g wound tissue) ± SEM.
Fifty microliters from each of the four biopsy homogenates was pooled for analysis by PCR using universal bacterial primers. Bacterial DNA was isolated using the DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA) according to the manufacturer’s instruction. For the quantification of total bacterial load in each wound, the sequences of the universal primers and probe set used were based on the amplification of 16S rDNA primers (forward: 5′-TCCTACGGGAGGCAGCAGT-3′; reverse: 5′-GGACTACCAGGGTATCTAATCCTGTT-3′) and probes [(6FAM)-5′-CGTATTACCGCGGCTGCTGGCAC-3′-MGBNFQ] synthesized by Applied Biosystems (Carlsbad, CA). Genomic P. aeruginosa DNA from the mid-log growth phase culture was isolated to establish a standard curve for bacterial quantification of wound samples. The concentration of isolated genomic DNA was determined using a Quant-iT ds DNA BR Assay Kit (Invitrogen, Carlsbad, CA).
All real-time PCR reactions were performed with a StepOne Plus Real-Time PCR System (Applied Biosystems) using optical grade 96-well plates. StepOne software provided by Applied Biosystems was used to analyze the data. The amount of total genomic DNA in each sample was converted into genome copy number and normalized to the weight of the wound sample.
Enzyme-Linked Immunosorbent Assay Measurement of DAMPs and Xanthine Oxidase/Xanthine Dehydrogenase
Frozen sera from rats (sham, burn treated with water, and burn treated with CN) were thawed on ice and analyzed for HMGB1 (Biotang), cytochrome C, hyaluronan (R&D Systems, MN), histone/DNA (Roche Diagnostics GmbH, Germany), fibronectin (Abcam), and xanthine dehydrogenase/oxidase (G Biosciences, MO) by enzyme-linked immunosorbent assay (ELISA) according to manufacturer’s instructions. Samples, standards, and test controls were manually added to plates, while all other steps were performed using DSX-automated ELISA machine (Dynex Technologies). Samples were assayed in technical replicates at appropriate dilutions, which were previously optimized.
Myeloperoxidase Activity Assay
Tissue myeloperoxidase (MPO) activity has been described previously.21 An MPO detection kit (Cell Technology, Mountain View, CA) was used. Briefly, frozen wound samples harvested by 7-mm biopsy punches were pulverized under liquid nitrogen using a Bessman Tissue Pulverizer and homogenized at 20,000 r.p.m. in 1× assay buffer using a T25 ULTRA-TURRAX. The homogenized tissues were centrifuged at 12,000 r.p.m at 4°C for 15 minutes. A solubilization buffer containing 0.5% hexadecyltrimethylammonium bromide (Sigma) in 1× assay buffer was added to the proteinaceous pellets. Samples were again homogenized as above, sonicated for 30 seconds, and submitted to two freeze–thaw cycles. Fifty microliters of each supernatant was transferred to a fluorescent 96-well plate containing 50-µl reaction cocktail per well (detection reagent and hydrogen peroxide in 1× assay buffer). Serial dilutions of a known quantity of MPO were used to establish the standard curve. After a 30-min incubation, fluorescence with excitation at 530 nm and emission at 590 nm was measured. MPO measurements for each wound were carried out in triplicate.
Statistics
GraphPad Prism 7.03 (GraphPad Software, Inc., San Diego, CA) was used to analyze the data. Results were grouped according to two variables and compared using two-way analysis of variance (two-way ANOVA) with multiple comparisons test. The statistically significant difference among study groups was determined as P ≤ 0.05. Data were plotted as the mean ± standard error (SEM).
RESULTS
General Information
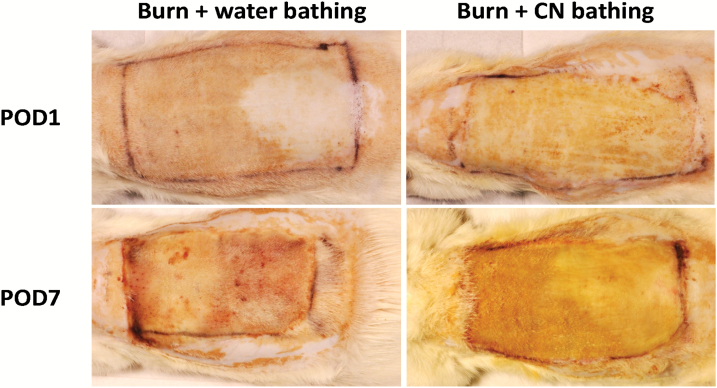
Forty-one rats survived the burn and treatment approaches. One animal failed to recover from anesthesia probably due to hypothermia. In general, the burn wounds created were uniform across all animals. Although the burn skin did not form the leather-like firm eschars by POD 7 as reported in CN-treated human skin, we did observe a yellowish discoloration (another hallmark of CN treatment) in CN-treated burn skin (Figure 2).
Figure 2.
Representative image of full-thickness burn wounds for comparison of burns with or without cerium nitrate (CN) treatment. Note the yellowish discoloration of burn wound skin in CN-treated burns at postoperative days (POD) 1 and 7. Also note that the burn wounds were not desiccated enough to form the typical firm eschar crust reportedly observed in CN-treated burns.
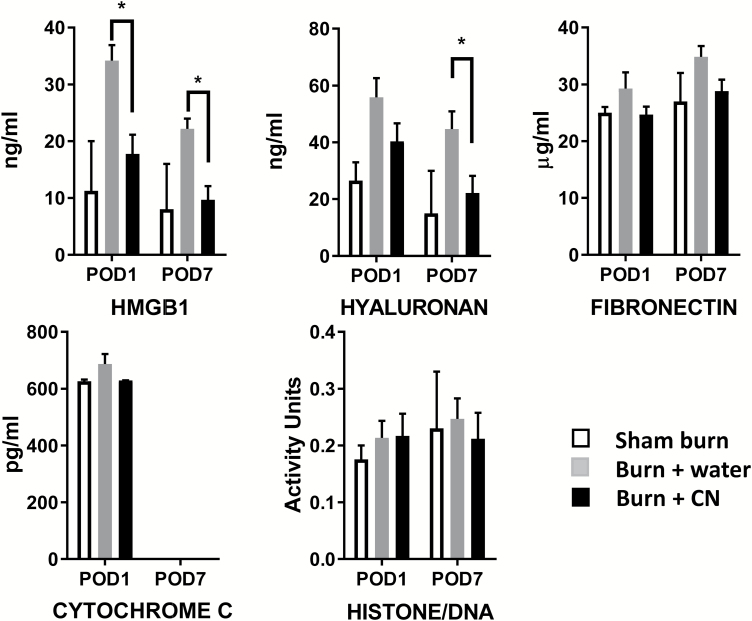
CN Treatment Attenuates Burn-Induced Release of Circulatory DAMPs
Compared with sham burn both at POD 1 and 7, FT burn (10% TBSA; burn + water treatment) resulted in a substantial increase in the circulating HMGB1 (Figure 3). CN treatment (burn + CN) significantly decreased HGMB1 levels (P < .05 at POD 1 and 7, vs. burn + water, respectively). Burn injury also increased hyaluronan levels, an extracellular matrix (ECM) component that peaked on POD 7. CN treatment significantly reduced circulating hyaluronan (P < .05, vs. burn + water) (Figure 3). Additionally, CN treatment trended toward a reduction of circulating fibronectin at both PODs (not statistically significant; Figure 3). In contrast, burn injury did not affect circulating levels of histone/DNA complex and cytochrome C, and therefore, no CN treatment effects were observed (Figure 3).
Figure 3.
Cerium nitrate (CN) reduces circulatory damage-associated molecular pattern (DAMP) levels in burn animals. Serum samples were collected from sham and burn rats with or without CN and assessed for DAMPs listed. Data are expressed as the mean ± SEM for three to six rats per group. *P < .05 burn + CN vs. burn + water; two-way ANOVA with multiple comparisons.
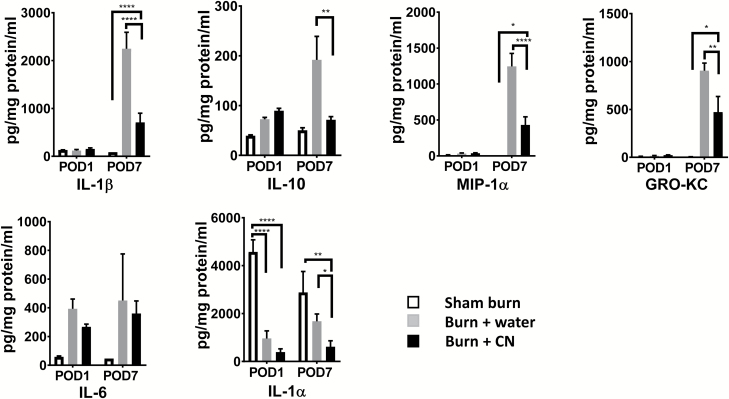
CN Treatment Alleviates the Burn-Induced Increase in Tissue Proinflammatory Cytokines
Among the 23 cytokines surveyed, when compared with sham burn (no burn injury) group, burn injury increased the tissue levels of IL-1β, IL-10, growth-regulated oncogene–keratinocyte chemokine (GRO-KC), and macrophage inflammatory protein-1α (MIP-1α) at POD 7 in the burn group (i.e., burn + water; P < .0001 for IL-1β and MIP-1α, P < .001 for GRO-KC, and P < .01 for IL-10, respectively). CN treatment significantly mitigated the elevated cytokines (P < .0001 for IL-1β and MIP-1α; P < .01 for GRO-KC and IL-10, vs. burn + water, respectively) in burn skin (Figure 4). Conversely, IL-1α levels were much higher in the sham burn animals than in the burn animals regardless the treatment modality (P < .0001, vs. either burn group) at POD 1. At POD 7, high IL-1α levels in sham burn skin remained (Figure 4), implicating its constituent origin within the intact skin.
Figure 4.
Burn induces upregulation of proinflammatory cytokine profiles and cerium nitrate (CN) can mitigate the heightened level of cytokines in burn tissues. The supernatants from homogenized full-thickness (FT) burn skin were collected and assessed by a multiplex cytokine profile panel. Tissue levels of cytokines are expressed as the mean ± SEM for three to six rats per group (****P < .0001, **P < .01, *P < .05 burn + CN vs. respective sham burn or burn + water; two-way ANOVA with multiple comparisons).
CN Suppresses the Burn-Induced Neutrophil Infiltration and Xanthine Oxidase Expression
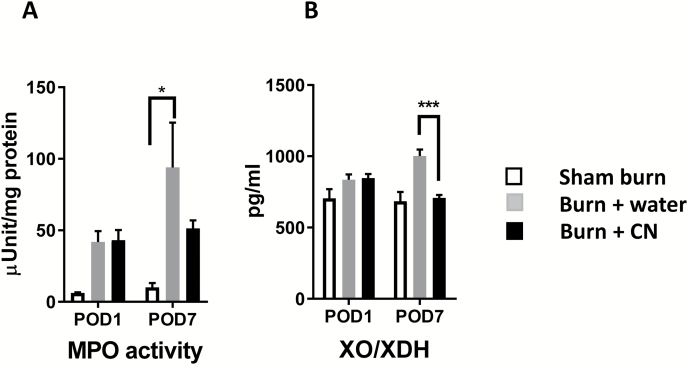
Burn injury caused a several-fold increase in burn tissue MPO activity (93.84 ± 31.35 in burn + water vs. 10.02 ± 3.06 in sham burn, P < .05; mean ± SEM) at POD 7. The MPO activity measurement reflects the amount of tissue neutrophil infiltration. CN treatment reduced the MPO activity from 93.84 ± 31.35 to 51.3 ±5.6, a 47% reduction in burn group (Figure 5A). Xanthine oxidase (XO) is the enzyme that catalyzes the formation of oxygen radicals responsible for skin inflammatory edema following thermal trauma.22 CN also effectively lowered the rise in circulatory level of XO/xanthine dehydrogenase (XDH) (P < .001, vs. burn + water) in burn animals (Figure 5B).
Figure 5.
Cerium nitrate (CN) treatment retards the burn-induced local and systemic inflammation. (A) The supernatants from homogenized burn skin were collected and assessed for myeloperoxidase (MPO) activity. MPO data are expressed as the mean ± SEM for three to nine rats per group (*P < .05 burn + water vs. sham burn; two-way ANOVA). (B) Serum samples were collected from sham and burn rats and assessed for xanthine oxidase/xanthine dehydrogenase (XO/XDH) by ELISA. The data are expressed as the mean ± SEM for three to six rats per group (***P < .001 burn + CN vs. burn + water; two-way ANOVA with multiple comparisons).
CN Treatment Inhibits the Proliferation of Resident Skin Flora in Eschars
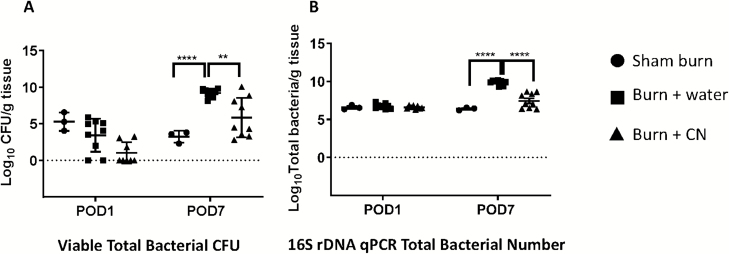
As shown in Figure 6A, there were no significant differences in viable counts (CFU) between sham burn, burn + water, and burn + CN at POD 1. However, by POD 7, bioburden in burned skin increased tremendously (3.23 log CFUs for sham burn to 9.23 log CFUs for burn + water, P < .0001). Treatment with CN limited growth of the bioburden from 9.23 log CFUs to approximately 5.9 log CFUs/gram tissue (P < .01, vs. burn + water). Quantitative PCR using universal primers for determination of total bacterial 16S rDNA copy number (for total bacteria, live and dead) showed a similar reduction in skin bacterial load due to CN treatment (Figure 6B).
Figure 6.
Cerium nitrate (CN) inhibits the proliferation and colonization of resident skin flora in burn skin. (A) Total bacterial CFU recovered from burn tissue at the indicated postoperative days (POD) was determined by serial dilution and plating on regular Trypticase soy blood agar. The data were transformed to log scale and then expressed as the mean ± SEM for three to nine rats per group (****P < .0001 burn + water vs. sham burn group; **P < .01 burn + CN vs. burn + water group; two-way ANOVA with multiple comparisons). (B) Total bacterial numbers were also determined by universal bacterial primers. The data were transformed to log scale and then expressed as the mean ± SEM for three to nine rats per group (****P < .0001 burn + water vs. sham burn group and burn + CN vs. burn + water group, respectively).
DISCUSSION
Major burns are a main cause of civilians and military trauma-related casualties.23 Therapeutic strategies for treatment of burn wounds comprise a complex and time-consuming algorithm including early eschar excision and grafting.24,25 Eschar preservation and staged escharectomy still remain a treatment option in situations where early excision is contraindicated or for those patients who are deemed as medically unfit for the extensive escharectomy surgeries. However, failure to preserve the eschar could result in excessive infection and inflammatory responses that may lead to complications such as sepsis, shock, and even multiorgan dysfunction syndromes.26–28 One of the risks of an untreated eschar (burned tissue) is that it acts as a reservoir for DAMPs29 and their release into circulation activates the innate immunity resulting in inflammatory complications. Another risk is the avascular necrotic tissues could be a hotbed for bacterial colonization, proliferation, and subsequent invasive infection. Additionally, burn wounds induce increased local tissue infiltration of inflammatory cells and release of proinflammatory cytokines that could worsen healing. The significance of our study is that, through the use of a clinically relevant rat burn model, our results demonstrated that CN treatment of acute burn effectively reduced these risk factors for improving burn wound outcomes.
There are multiple studies reporting the release of DAMPs due to trauma30,31 and burn.17–19 These molecules are nuclear or cytosolic proteins constitutively expressed in host cells for carrying out cellular functions. But when released upon tissue damage, these molecules become oxidized and denatured,32 enabling them to activate the innate immune response through their interactions with various receptors of the innate immune system such as toll-like receptors (TLR4 and TLR9) and the receptor for advanced glycation endproducts.33 Some common DAMPs include intracellular proteins such as nuclear proteins HMGB1,34 heat-shock proteins,35 and degradation proteins derived from the ECM such as hyaluronan fragments36 that are generated following tissue injury. There are also nonprotein DAMPs, for example, mitochondrial DNA37 and ATP.38 The results of our rat burn injury showed heightened levels of circulating DAMPs after burn injury, which is corroborated by previously published work. Specifically, we detected heightened levels of circulating HMGB1 and hyaluronan. Fibronectin, another ECM-derived degradation product, showed an increasing trend but not statistically significant. However, we were not able to detect some of the mitochondria-derived DAMPs such as cytochrome C33 nor did we test the presence of mitochondrial DNA in the collected plasma. It is uncertain what contributed to the lack of increased circulating cytochrome C observed in our study. This could be related to the type (or extent) of burn injury, the animal model used, or the timing of plasma collection from the burned rats. It has been reported that some DAMPs can only be detected early post-injury.18
Burn-wounded tissue appears to continuously contribute to systemic inflammation by emanating DAMPs and inflammatory mediators. Unique to this study is that our results demonstrated that topical CN treatment for merely 30 minute post-burn suppressed, at least partially, the levels of circulating HMGB1 and hyaluronan, thereby dampening their contribution to burn-induced systemic inflammation. This opens up a new therapeutic window potentially allowing topical anti-inflammatory treatment for reducing the intensity of the systemic inflammation as the result of burn injury. At present, it is unclear the mechanism(s) by which CN suppressed the release of DAMPs from burn injury site (eschar) into circulation. In our recent unpublished work, using scanning electron microscopy with energy dispersive x-ray spectroscopy, we detected cerium deposition within the frozen sections of CN-treated (40 mM for 30 minutes) ex vivo burn porcine skin. Given what is known in literature about cerium’s ability to displace calcium from pyrophosphate to allow calcium to deposit,3 we speculate that some yet to be determined biochemical interactions might occur between cerium and DAMP molecules, resulting in localizing or trapping these molecules within the eschar, thereby reducing their systemic release. Furthermore, the ability of CN to displace calcium could inactivate calcium-dependent inflammatory signaling molecules such as complement and S100 proteins, underlying the reduced inflammation beneath the calcified eschar.39
We next examined if DAMP blockage due to CN treatment would result in a parallel inhibition of inflammatory cytokines at the tissue level. As expected, we demonstrated that the burn injury caused an apparent elevation in the release of IL-1β, IL-10, GRO-KC, and MIP-1α by POD 7, in which in part mirrors the increase patterns of DAMPs. Moreover, we found that CN treatment significantly mitigated the severity of the cytokine dysregulation. Surprisingly, our IL-1α data displayed a markedly different pattern. At both time points (POD 1 and 7) in this study, there is a high basal level of IL-1α in the unburned animals, whereas lower levels of IL-1α were detected in burned animals. This is likely due to the fact that only healthy keratinocytes within the epidermal layer express IL-1α constitutively.40,41 With the loss of the epidermal layer due to a burn injury, there would be likely less IL-1α to be detected at the tissue level. Notably, our cytokine data were measured directly from burn skin samples, while most other burn-related cytokine data reported in previously published work are on circulating levels of proinflammatory cytokines. The comparison of our data to other ones displayed an obvious temporal variability.19,42–44 Unlike reported early cytokines elevation (from a few hours to POD 3) post-burn that correlated with inflammatory phase, we had not observed the POD 1 spike of burn-related inflammatory cytokines. Usually by POD 3, increase in some cytokines or chemokines became noticeable (data not shown). It was in POD 7 or, thereafter, when the peak concentrations came to burn wound. It is not completely unexpected considering the pathophysiological process taking place in FT burn tissues, which we harvested for these molecular assays. Following burn injury, the necrotized tissue loses its capability to produce cytokines with circulating mediators unable to reach to burn tissues because of capillary occlusion. As a result, postburn inflammatory cytokines may not elevate in necrotic burn tissue at earlier PODs and then gradually increase in their amount as more cytokine-rich exudate is produced by healthy wound bed tissue underneath the eschars and make a way into the circulation-isolating-burn-wound by as late as POD 7. In our study, we repeatedly were not able to detect any significant differences of circulating proinflammatory cytokines in sera between sham burned and scald burned rats (data not shown). This lack of correlation with the previous reports might be related to the extent of burn injury used in these different studies. Heightened levels of circulating cytokines have been reported in severe burn subjects (>30% TBSA) or in cases with confounding diseases such as sepsis.45,46 Our animals received a 10% FT burn, and these smaller burns may not be sufficient to induce systemic proinflammatory cytokines. Nevertheless, the ability of topical CN treatment in reducing tissue production of proinflammatory cytokines implies that cerium, other than its ability to dampen the systemic release of DAMPs, also results in reduction of the proinflammatory potential of burn tissue.
Additionally, CN had been reported to be capable of modulating leukocyte activation following burn injury.14 In alignment with our DAMPs and cytokine data, we found that burn skin had an MPO activity 9.3-fold higher than that of the sham burn skin, and CN treatment decreased this elevated MPO activity by 46% in the CN-treated burns versus the vehicle-treated burns. To further corroborate these findings, a serum ELISA was conducted for XO/XDH. XO and XDH are single gene products, of which XO is identified as the isotype enzyme that responds to burn injury.47 Both increased XO and neutrophil activation are viewed as the oxidant sources in burns.48 XO is a form of xanthine oxidoreductase, which generates reactive oxygen species and therefore plays a key role in the pathogenesis of burn-related tissue damage or organ malfunction. In a relevant clinical observation involving a cohort of 23 burn patients, Filippou et al22 reported a significant elevation of XO at day 4 post-burn, which continued to increase until day 6, the final day of observation. In this study, we showed that 30-minute CN bathing diminished the burn-induced XO increase at POD 7.
Burn is a type of trauma that severely compromises a host’s defensive barrier against the environment. Thermal eschars are ineffective barriers that will quickly deteriorate with time. Skin resident flora are not pathogenic to intact skin but could turn to a pathogenic state once their unchecked proliferation overruns the host’s defense system. During the initial hours and days following a burn, gram-positive staphylococci, members of normal skin flora, often survive the thermal impact and continue to colonize the wound surface to become an active infection.49 Within a week post-burn, bacterial colonization in the burn wound reaches a plateau.50,51 Reduction of the bioburden within the wound may limit the harmful complications of burn infection. With the CN treatment, the growth of the native flora was reduced by more than 3 log compared with the controls over 7 days post-burn (Figure 6). This reduction could result from either direct bactericidal/bacteriostatic activity of CN or its ability to chemically modify the burn eschar. The antimicrobial activity could reduce the initial bacterial numbers or inhibit colonization of the eschar by the native skin flora. Modification, such as calcification, of the burn eschar may also reduce the growth of the normal flora within the eschar.
The antimicrobial activity of CN has been controversial.3 Some research groups reported high levels of antimicrobial activity of CN,52,53 whereas other groups showed that CN only had limited antibacterial activity against common burn pathogens54 or no antibacterial effect at all.55 In our hands, we did not observe any antimicrobial activity of CN in vitro, at the test concentration of 40 mM against Pseudomonas aeruginosa, Staphylococcus aureus, or a mixed culture of normal flora based on the standard zone of inhibition test (data not shown). However, it is possible that during the 30-minute CN bathing the surviving skin flora bacteria after the burn could uptake cerium into their cell cytoplasm, which could cause the inhibition of cellular respiration, oxygen uptake, and glucose metabolism, and eventually cell death similar to the effects of cerium seen on Escherichia coli.56
With this animal study, we provided evidence showing that CN treatment effectively modifies burn skin into a less inflammatory state, decreases circulatory DAMPs that could cause systemic inflammation, and renders the eschars more resistant to bacterial colonization. When surgical removal of eschar is unfeasible, CN treatment could provide an expedient alternative that abates the detrimental impacts of burn eschars and improves the outcome of staged escharectomy and grafting.4 Moreover, CN manifests relatively mild adverse effects shown in a study.57 Methemoglobinemia occurs in only ~10% of patients who received Flammacerium, a silver sulfadiazine cream containing CN. Most cases resolved with cessation of treatment.57 Furthermore, the present study design is not without limitations. One limitation is that the design lacks the capacity to correlate the eschar stabilization by CN with improved burn wound outcome such as hastening wound closure and reducing scarring. An alternative study design using rat deep partial-thickness burn wounds and extending the study timeline beyond day 20 post-burn could provide additional information on eschar stabilization and improved wound healing. In all, CN proves to be a very promising treatment regimen for its ability to stabilize eschars by delaying the need for immediate excision and modulating inflammation, which may prove to be pivotal for military operations during prolonged field care of severe burn injuries.
ACKNOWLEDGMENTS
The authors acknowledge SPC Pineda and CPL Olverson for their excellent technical assistance. The views expressed in this manuscript are those of the author(s) and do not reflect the official policy or position of the U.S. Army Medical Department, Department of the Army, DoD, or the U.S. Government.
Funding
The work is supported in part through Combat Casualty Care Research Directorate, US Army Medical and Development Command and the Naval Medical Research Center’s Advanced Medical Development program (MIPR N3239815MHX040).
Conflicts of interest
The authors declare no competing or financial interests.
REFERENCES
- 1. American Burn Association/American College of Surgeons. Guidelines for the operation of burn centers. J Burn Care Res. 2007;28:134–41. [DOI] [PubMed] [Google Scholar]
- 2. Rowan MP, Cancio LC, Elster EA, et al. Burn wound healing and treatment: review and advancements. Crit Care. 2015;19:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garner JP, Heppell PS. Cerium nitrate in the management of burns. Burns. 2005;31:539–47. [DOI] [PubMed] [Google Scholar]
- 4. Vehmeyer-Heeman M, Tondu T, Van den Kerckhove E, Boeckx W. Application of cerium nitrate-silver sulphadiazine allows for postponement of excision and grafting. Burns. 2006;32:60–3. [DOI] [PubMed] [Google Scholar]
- 5. Monafo WW, Tandon SN, Ayvazian VH, Tuchschmidt J, Skinner AM, Deitz F. Cerium nitrate: a new topical antiseptic for extensive burns. Surgery. 1976;80:465–73. [PubMed] [Google Scholar]
- 6. de Gracia CG. An open study comparing topical silver sulfadiazine and topical silver sulfadiazine-cerium nitrate in the treatment of moderate and severe burns. Burns. 2001;27:67–74. [DOI] [PubMed] [Google Scholar]
- 7. Ross DA, Phipps AJ, Clarke JA. The use of cerium nitrate-silver sulphadiazine as a topical burns dressing. Br J Plast Surg. 1993;46:582–4. [DOI] [PubMed] [Google Scholar]
- 8. Sparkes BG. Mechanisms of immune failure in burn injury. Vaccine. 1993;11:504–10. [DOI] [PubMed] [Google Scholar]
- 9. Sparkes BG. Immunological responses to thermal injury. Burns. 1997;23:106–13. [DOI] [PubMed] [Google Scholar]
- 10. Allgöwer M, Schoenenberger GA, Sparkes BG. Pernicious effectors in burns. Burns. 2008;34 Suppl 1:S1–S55. [DOI] [PubMed] [Google Scholar]
- 11. Scheidegger D, Sparkes BG, Lüscher N, Schoenenberger GA, Allgöwer M. Survival in major burn injuries treated by one bathing in cerium nitrate. Burns. 1992;18:296–300. [DOI] [PubMed] [Google Scholar]
- 12. Kistler D, Hafemann B, Schoenenberger GA, Hettich R. Increased survival rates by topical treatment of burns with cerium nitrate. Eur Surg Res. 1990;22:283–90. [DOI] [PubMed] [Google Scholar]
- 13. Deveci M, Eski M, Sengezer M, Kisa U. Effects of cerium nitrate bathing and prompt burn wound excision on IL-6 and TNF-alpha levels in burned rats. Burns. 2000;26:41–5. [DOI] [PubMed] [Google Scholar]
- 14. Eski M, Deveci M, Celiköz B, Nisanci M, Türegün M. Treatment with cerium nitrate bathing modulate systemic leukocyte activation following burn injury: an experimental study in rat cremaster muscle flap. Burns. 2001;27:739–46. [DOI] [PubMed] [Google Scholar]
- 15. Zapata-Sirvent RL, Hansbrough JF. Postburn immunosuppression in an animal model. III. Maintenance of normal splenic helper and suppressor lymphocyte subpopulations by immunomodulating drugs. Surgery. 1985;97:721–7. [PubMed] [Google Scholar]
- 16. Boeckx W, Blondeel PN, Vandersteen K, De Wolf-Peeters C, Schmitz A. Effect of cerium nitrate-silver sulphadiazine on deep dermal burns: a histological hypothesis. Burns. 1992;18:456–62. [DOI] [PubMed] [Google Scholar]
- 17. Huang LF, Yao YM, Dong N, Yu Y, He LX, Sheng ZY. Association of high mobility group box-1 protein levels with sepsis and outcome of severely burned patients. Cytokine. 2011;53:29–34. [DOI] [PubMed] [Google Scholar]
- 18. Lantos J, Földi V, Roth E, Wéber G, Bogár L, Csontos C. Burn trauma induces early HMGB1 release in patients: its correlation with cytokines. Shock. 2010;33:562–7. [DOI] [PubMed] [Google Scholar]
- 19. Rani M, Nicholson SE, Zhang Q, Schwacha MG. Damage-associated molecular patterns (DAMPs) released after burn are associated with inflammation and monocyte activation. Burns. 2017;43:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brandenburg KS, Weaver AJ Jr, Qian L, et al. Development of Pseudomonas aeruginosa biofilms in partial-thickness burn wounds using a Sprague–Dawley rat model. J Burn Care Res. 2019;40:44–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qian LW, Fourcaudot AB, Yamane K, You T, Chan RK, Leung KP. Exacerbated and prolonged inflammation impairs wound healing and increases scarring. Wound Repair Regen. 2016;24:26–34. [DOI] [PubMed] [Google Scholar]
- 22. Filippou D, Papadopoulos VP, Triga A, et al. Nitric oxide, antioxidant capacity, nitric oxide synthase and xanthine oxidase plasma levels in a cohort of burn patients. Burns. 2007;33:1001–7. [DOI] [PubMed] [Google Scholar]
- 23. Kauvar DS, Wolf SE, Wade CE, Cancio LC, Renz EM, Holcomb JB. Burns sustained in combat explosions in Operations Iraqi and Enduring Freedom (OIF/OEF explosion burns). Burns. 2006;32:853–7. [DOI] [PubMed] [Google Scholar]
- 24. Janzekovic Z. A new concept in the early excision and immediate grafting of burns. J Trauma. 1970;10:1103–8. [PubMed] [Google Scholar]
- 25. Quinby WC Jr, Burke JF, Bondoc CC. Primary excision and immediate wound closure. Intensive Care Med. 1981;7:71–6. [DOI] [PubMed] [Google Scholar]
- 26. Sittig K, Deitch EA. Effect of bacteremia on mortality after thermal injury. Arch Surg. 1988;123:1367–70. [DOI] [PubMed] [Google Scholar]
- 27. Housinger TA, Brinkerhoff C, Warden GD. The relationship between platelet count, sepsis, and survival in pediatric burn patients. Arch Surg. 1993;128:65–6; discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 28. Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg. 1999;178:449–53. [DOI] [PubMed] [Google Scholar]
- 29. D’Arpa P, Leung KP. Toll-like receptor signaling in burn wound healing and scarring. Adv Wound Care (New Rochelle). 2017;6:330–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hauser CJ, Otterbein LE. Danger signals from mitochondrial DAMPS in trauma and post-injury sepsis. Eur J Trauma Emerg Surg. 2018;44:317–24. [DOI] [PubMed] [Google Scholar]
- 31. Vourc’h M, Roquilly A, Asehnoune K. Trauma-induced damage-associated molecular patterns-mediated remote organ injury and immunosuppression in the acutely ill patient. Front Immunol. 2018;9:1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–36. [DOI] [PubMed] [Google Scholar]
- 33. Grazioli S, Pugin J. Mitochondrial damage-associated molecular patterns: from inflammatory signaling to human diseases. Front Immunol. 2018;9:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. [DOI] [PubMed] [Google Scholar]
- 35. Panayi GS, Corrigall VM, Henderson B. Stress cytokines: pivotal proteins in immune regulatory networks; Opinion. Curr Opin Immunol. 2004;16:531–4. [DOI] [PubMed] [Google Scholar]
- 36. Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–81. [DOI] [PubMed] [Google Scholar]
- 37. Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boeynaems JM, Communi D. Modulation of inflammation by extracellular nucleotides. J Invest Dermatol. 2006;126:943–4. [DOI] [PubMed] [Google Scholar]
- 39. Vehmeyer-Heeman M, Van Holder C, Nieman F, Van den Kerckhove E, Boeckx W. Predictors of mortality: a comparison between two burn wound treatment policies. Burns. 2007;33:167–72. [DOI] [PubMed] [Google Scholar]
- 40. Hauser C, Saurat JH, Schmitt A, Jaunin F, Dayer JM. Interleukin 1 is present in normal human epidermis. J Immunol. 1986;136:3317–23. [PubMed] [Google Scholar]
- 41. Gahring LC, Buckley A, Daynes RA. Presence of epidermal-derived thymocyte activating factor/interleukin 1 in normal human stratum corneum. J Clin Invest. 1985;76:1585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Orman MA, Nguyen TT, Ierapetritou MG, Berthiaume F, Androulakis IP. Comparison of the cytokine and chemokine dynamics of the early inflammatory response in models of burn injury and infection. Cytokine. 2011;55:362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Orman MA, Ierapetritou MG, Berthiaume F, Androulakis IP. Long-term dynamic profiling of inflammatory mediators in double-hit burn and sepsis animal models. Cytokine. 2012;58:307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kondo T, Ohshima T. The dynamics of inflammatory cytokines in the healing process of mouse skin wound: a preliminary study for possible wound age determination. Int J Legal Med. 1996;108:231–6. [DOI] [PubMed] [Google Scholar]
- 45. Gauglitz GG, Song J, Herndon DN, et al. Characterization of the inflammatory response during acute and post-acute phases after severe burn. Shock. 2008;30:503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barber RC, Maass DL, White DJ, Horton JW. Increasing percent burn is correlated with increasing inflammation in an adult rodent model. Shock. 2008;30:388–93. [DOI] [PubMed] [Google Scholar]
- 47. Friedl HP, Till GO, Trentz O, Ward PA. Roles of histamine, complement and xanthine oxidase in thermal injury of skin. Am J Pathol. 1989;135:203–17. [PMC free article] [PubMed] [Google Scholar]
- 48. Parihar A, Parihar MS, Milner S, Bhat S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns. 2008;34:6–17. [DOI] [PubMed] [Google Scholar]
- 49. Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19:403–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McManus AT, Mason AD Jr, McManus WF, Pruitt BA Jr. A decade of reduced gram-negative infections and mortality associated with improved isolation of burned patients. Arch Surg. 1994;129:1306–9. [DOI] [PubMed] [Google Scholar]
- 51. McManus AT, Mason AD Jr, McManus WF, Pruitt BA Jr. Twenty-five year review of Pseudomonas aeruginosa bacteremia in a burn center. Eur J Clin Microbiol. 1985;4:219–23. [DOI] [PubMed] [Google Scholar]
- 52. Fox CL Jr, Monafo WW Jr, Ayvazian VH, et al. Topical chemotherapy for burns using cerium salts and silver sulfadiazine. Surg Gynecol Obstet. 1977;144:668–72. [PubMed] [Google Scholar]
- 53. Herruzo-Cabrera R, Garcia-Torres V, Rey-Calero J, Vizcaino-Alcaide MJ. Evaluation of the penetration strength, bactericidal efficacy and spectrum of action of several antimicrobial creams against isolated microorganisms in a burn centre. Burns. 1992;18:39–44. [DOI] [PubMed] [Google Scholar]
- 54. Marone P, Monzillo V, Perversi L, Carretto E. Comparative in vitro activity of silver sulfadiazine, alone and in combination with cerium nitrate, against staphylococci and gram-negative bacteria. J Chemother. 1998;10:17–21. [DOI] [PubMed] [Google Scholar]
- 55. Rosenkranz HS. A synergistic effect between cerium nitrate and silver sulphadiazine. Burns. 1979;5:278–81. [Google Scholar]
- 56. Sobek JM, Talburt DE. Effects of the rare earth cerium on Escherichia coli. J Bacteriol. 1968;95:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kath MA, Shupp JW, Matt SE, et al. Incidence of methemoglobinemia in patients receiving cerium nitrate and silver sulfadiazine for the treatment of burn wounds: a burn center’s experience. Wound Repair Regen. 2011;19:201–4. [DOI] [PubMed] [Google Scholar]