Abstract
We describe a successful bioprosthetic annular stretching in a patient with severe prosthetic aortic valve stenosis from a degenerated 19-mm Mitroflow valve (Sorin Group USA Inc, Arvada, CO, USA). This technique allowed for implantation of a 23-mm Evolut-R Pro valve (Medtronic, Minneapolis, MN, USA) with significant improvement in hemodynamics after prosthetic annular stretching. We have also summarized other case series and case reports which have previously described similar techniques.
<Learning objective: Transcatheter valve-in-valve procedure may not be feasible in certain patients who have a relatively smaller size bioprosthetic valve. Cracking/stretching the annular ring of the smaller prosthetic valve to deploy a larger transcatheter valve is a potential option in these patients. Clinicians must be cognizant of the possible pitfalls, contraindications, and other technical aspects to choose the right patient for this procedure.>
Keywords: Valve-in-valve, Transcatheter aortic valve replacement, Bioprosthetic valve stretching/fracture
Introduction
In certain situations, a valve-in-valve (VIV) procedure may result in suboptimal results (excessive post-procedural mean pressure gradient) due to small internal diameter of the stenosed prosthetic surgical valve. In these cases, bioprosthetic valve annular fracture or stretching (BVF or BVS) has been described in recent years. However, experience with this technique is limited. We describe a case of successful VIV transcatheter aortic valve replacement (TAVR) achieved after BVS of a degenerated Mitroflow valve (Sorin Group USA Inc, Arvada, CO, USA).
Case report
An 85-year-old woman who underwent surgical aortic valve replacement (AVR) 7 years previously presented with symptoms due to progressive degeneration of the 19-mm Mitroflow valve. Cardiac catheterization revealed a mean trans-aortic gradient of 49 mmHg and an effective orifice area (EOA) of 0.8 cm2 with indexed EOA of 0.35 cm2/m2 (3D guided planimetry by echocardiography, Fig. 1, Video 1). Options for re-do AVR were discussed, including a surgical approach, that would entail aortic root replacement for placement of a larger prosthesis. Mortality predicted by Society of Thoracic Surgeons was 3.3%, however it did not take into account the possibility of aortic root replacement to implant a larger valve and other technical challenges encountered during initial AVR (extensive calcifications in the aorta above the valve annulus). TAVR was not initially deemed feasible due to small inner diameter (15.4 mm) of the surgical valve. The patient declined a second surgical AVR. After an extensive evaluation and discussion between the heart team members, the option of provisional TAVR with a 23-mm Evolut-R Pro valve (Medtronic, Minneapolis, MN, USA) with possible BVF/S was considered, to which the patient agreed.
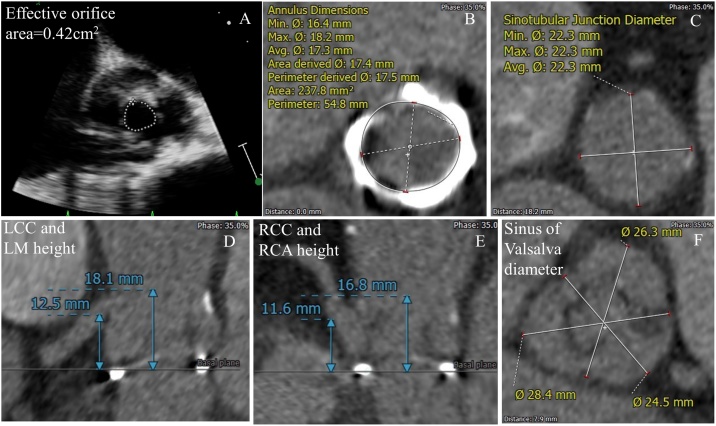
Fig. 1.
(A) Baseline echocardiogram showing degenerated prosthetic valve with severe stenosis. (B) Annulus dimensions. (C) Sino-tubular junction diameter. (D) Left coronary cusp and left main height. (E) Right coronary cusp and right coronary artery diameter. (F) Sinus of Valsalva diameter. LCC, left coronary cusp; LM, left main; RCC, right coronary cusp; RCA, right coronary artery.
On the day of the procedure, the right femoral arterial access was preclosed with 2 Proglides (Abbott, Lake Bluff, IL, USA) and the right femoral arteriotomy site was predilated with a 16-French Cook sheath (Bloomington, IN, USA). We also obtained left femoral arterial access (7-French 30 cm sheath) and right radial arterial access (6-French arterial sheath). Both the coronary arteries were engaged with 6 F guiding catheters. (6-French Judkins right-4 curve guide catheter for the right coronary artery and 6-French Judkins left-4.5 curve guide catheter for the left main). A 4.5-mm x 22-mm Resolute stent (Medtronic) was parked in the left anterior descending artery over a Runthough 0.014” wire (Terumo, Somerset, NJ, USA) via a 6 F Guidezilla II (Boston Scientific, Marlborough, MA, USA). Similarly, a 3.0-mm x 30-mm Integrity stent (Medtronic) was parked in the right coronary artery over a Sport wire (Abbott Vascular) through a 6 F Guidezilla II. This procedure was performed pre-emptively in case of acute closure of the coronary artery during valve deployment or BVF/S. The sheathless 23-mm Evolut R Pro valve was deployed during right ventricular pacing at 100/min over a super-stiff Amplatz wire (Boston Scientific). Due to suboptimal expansion after valve deployment we post-dilated the valve with an 18-mm True balloon (Bard, Tempe, AZ, USA). A residual mean gradient of 33 mmHg was noted after balloon inflation (Video 2). At this time, we elected to proceed with BVF/S using a 20-mm True balloon. Under rapid pacing, this balloon was deployed at 15 atmospheres. A sudden release of the ‘waist’ of the balloon was noted during inflation (Fig. 2, Video 3), which confirmed successful enlargement of the annular ring, possibly due to stretching of the prosthetic annular ring. Even though there was no noticeable break in the continuity of the annular ring on the fluoroscopy, a possible annular fracture could not be completely ruled out. Final mean gradient was 10 mmHg (Fig. 3, Video 4a, b). At this point after confirmation of coronary patency (Video 5a, b) the stents, wires, Guidezillas, and catheters were retrieved and arteriotomy sites were successfully closed using ProGlides (Abbott). No immediate complications were noted. The patient was discharged two days later from the hospital in a stable condition and reported significant symptomatic improvement at follow-up visit in the clinic.
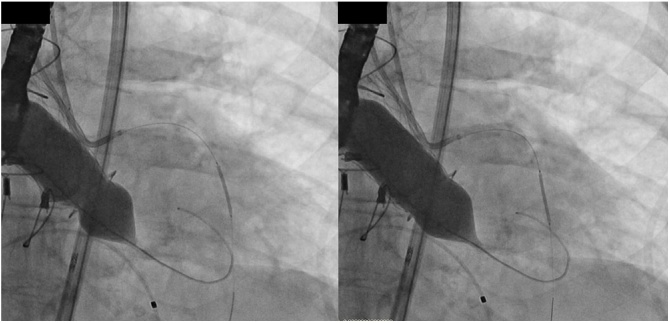
Fig. 2.
Fluoroscopic image showing a ‘waist’ prior to balloon valve fracture. Release of ‘waist’ after high-pressure inflation, suggestive of prosthetic ring fracture/stretching.
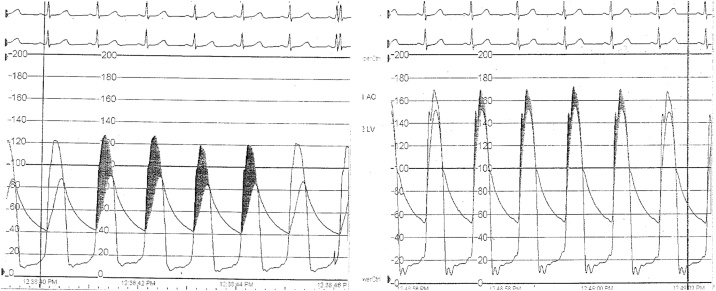
Fig. 3.
Hemodynamic tracings showing a residual gradient after valve-in-valve deployment and subsequent improvement after bioprosthetic valve fracture.
Discussion
This case demonstrates that VIV TAVR can be successfully performed for prosthetic Mitroflow valve stenosis using the BVF/S technique. BVF/S technique has been described for various prosthetic positions and types. This experience is limited either to case reports or case series, of which only 24 cases involved Mitroflow valves to the best of our knowledge. The type of valve prosthesis is an important variable in patient safety and technical success of this procedure. Our case adds to the existing literature.
Not all prosthetic valves are amenable for BVF/BVS. In a bench study by Allen and colleagues BVF could not be achieved for St. Jude Trifecta (St Jude, St Paul, MN, USA) or the Medtronic Hancock II surgical valves using any high pressure balloons [1].
BVF can be performed either before or provisionally after transcatheter valve deployment. BVF after provisional TAVR seems an attractive option as it may not be needed in some cases where it is initially thought to be necessary. However, BVF after TAVR deployment can be associated with the risk of structural damage of the newly deployed valve and in some cases, severe valvular regurgitation. A multicenter case series included 75 patients, including 12 patients with Mitroflow valves [2]. BVF was performed successfully in all patients with significant improvement in gradients after BVF procedure. The most frequent surgical valve was Magna (Edwards Lifesciences, Irvine, California) with size ranging from 19–27 mm. The majority of the patients in this series underwent BVF after VIV implantation (88%). In multivariate analysis BVF after VIV implantation was a predictor of significantly lower gradients compared to BVF prior to valve implantation. Overall mortality in hospital of at 30 days was 2.7% (2/75). Five patients experienced other non-fatal complications. Another case series included 10 patients who underwent BVF for VIV TAVR in patients with degenerated Mitroflow valves [3]. They performed successful BVF prior to valve implantation in all patients. Two patients experienced non-fatal complications. None of the above series or other case reports have reported complications such as annular rupture following BVF procedure in aortic position [3].
Apart from VIV TAVR, technique of BVF has also been described in other positions such as pulmonary/right ventricular outflow tract, tricuspid, and mitral positions [4], [5], [6], [7], [8]. Reports for tricuspid or mitral are limited. This technique has been used for mitral VIV either as an elective or a bail-out option [4], [5]. A series by Shahanavaz and colleagues included 37 patients who underwent VIV procedure for dysfunctional prosthetic pulmonic valves [6]. Successful frame fracture was achieved in 28 patients, while the frame was stretched, but not fractured in 5 patients and unsuccessful in 4 patients.
Another issue relevant in this case relates to the threshold of coronary protection in VIV procedures. BVF/S might increase the risk of coronary obstruction since the degenerated bio-prosthesis could expand more than usual and the pushed leaflet might get closer to the coronary ostium. This patient’s computed tomography was reviewed by outside experts and they suggested protection of both coronaries in view of narrow sinus of Valsalva and also felt the left main and right coronary arteries were relatively low lying. Also, the internal stent frame of Mitroflow valve is particularly likely to promote coronary obstruction [9], especially since BVF/S has unpredictable consequences. Since the stretching was done after full VIV deployment in this case, there would have been no easy options to withdraw the deployed occlusive valve. Hence our best option (especially after review and discussion with outside TAVR experts) was to protect the coronaries by upfront positioning of a coronary stent to facilitate “chimney stenting” if required when withdrawing the stent into the ostium of the occluded vessel. Some of the high-risk markers [9] for coronary obstruction in this patient included: 1) relatively low lying coronary ostia, 2) narrow sinus (left coronary cusp: diameter = 25 mm, height = 18 mm; right-noncoronary cusp: diameter = 26 mm, height = 17 mm, noncoronary-left cusp: diameter = 28 mm, height = 16 mm) and sino-tubular junction measuring 22 X 22 mm] (Fig. 1),3) valve with internal stent frame, 4) bulky leaflets, and 5) non-coaxial surgical valve placement.
While the limited case series discussed above provide an evidence for practicality and safety of this procedure, multiple other questions remain: 1) long-term safety 2) timing of BVF/S (before or after valve placement) 3) possibility of increased risk of procedural complications, especially coronary obstruction and annular rupture 4) comparison of VIV plus BVF/S compared to re-do SAVR 5) rates of leaflet thrombosis after cracking 6) impact on the structural integrity and valve durability.
Conclusion
BVF/S provides a solution for patients who are at a high surgical risk for re-do surgical valve replacement and have a small prosthetic size, which prevents optimal hemodynamic outcome with VIV procedure. However, use of this technique is off-label and is not on the basis of current guideline recommendations. More studies are needed to provide more insights about long-term outcomes in these patients.
Conflict of interest
None related to this manuscript. Other industry relations: Maninder Singh: Received reimbursement from C3 Interventional Academy for attending C3 conference at Orlando, FL, USA (June 2019). Minor reimbursement for attending a workshop: Abiomed and Siemens.
Funding
None
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jccase.2020.01.006.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Allen K.B., Chhatriwalla A.K., Cohen D.J., Saxon J.T., Aggarwal S., Hart A. Bioprosthetic valve fracture to facilitate transcatheter valve-in-valve implantation. Ann Thorac Surg. 2017;104:1501–1508. doi: 10.1016/j.athoracsur.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Allen K.B., Chhatriwalla A.K., Saxon J.T., Cohen D.J., Nguyen T.C., Webb J. Bioprosthetic valve fracture: technical insights from a multicenter study. J Thorac Cardiovasc Surg. 2019;158:1317–1328. doi: 10.1016/j.jtcvs.2019.01.073. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen-Kudsk J.E., Christiansen E.H., Terkelsen C.J., Nørgaard B.L., Jensen K.T., Krusell L.R. Fracturing the ring of small Mitroflow bioprostheses by high-pressure balloon predilatation in transcatheter aortic valve-in-valve implantation. Circ Cardiovasc Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.115.002667. [DOI] [PubMed] [Google Scholar]
- 4.Kamioka N., Corrigan F., Iturbe J.M., Caughron H., Lerakis S., Thourani V. Mitral bioprosthetic valve fracture: bailout procedure for undersized bioprosthesis during mitral valve-in-valve procedure with paravalvular leak closure. JACC Cardiovasc Interv. 2018;11:e21–2. doi: 10.1016/j.jcin.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 5.Kaneko T., Piccirillo B., Golwala H., Trzcinka A., Nyman C., Shook D. Balloon fracture of a surgical mitral bioprosthesis during valve-in-valve transcatheter mitral valve replacement: first-in-human report. Circ Cardiovasc Interv. 2018;11 doi: 10.1161/CIRCINTERVENTIONS.117.006273. [DOI] [PubMed] [Google Scholar]
- 6.Shahanavaz S., Asnes J.D., Grohmann J., Qureshi A.M., Rome J.J., Tanase D. Intentional fracture of bioprosthetic valve frames in patients undergoing valve-in-valve transcatheter pulmonary valve replacement. Circ Cardiovasc Interv. 2018;11 doi: 10.1161/CIRCINTERVENTIONS.118.006453. [DOI] [PubMed] [Google Scholar]
- 7.Brown S.C., Cools B., Gewillig M. Cracking a tricuspid perimount bioprosthesis to optimize a second transcatheter sapien valve-in-valve placement. Catheter Cardiovasc Interv. 2016;88:456–459. doi: 10.1002/ccd.26507. [DOI] [PubMed] [Google Scholar]
- 8.Hensey M., Alenezi A.R., Murdoch D.J., Sathananthan J., Weir-McCall J.R., Wood D. Transcatheter tricuspid valve-in-valve replacement with subsequent bioprosthetic valve fracture to optimize hemodynamic function. JACC Cardiovasc Interv. 2018;11:2226–2227. doi: 10.1016/j.jcin.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Dvir D., Leipsic J., Blanke P., Ribeiro H.B., Kornowski R., Pichard A. Coronary obstruction in transcatheter aortic valve-in-valve implantation: preprocedural evaluation, device selection, protection, and treatment. Circ Cardiovasc Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.114.002079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.