Abstract
Background
As a process of aging, skeletal muscle mass and function gradually decrease. It is reported that ginsenoside Rb1 and Rb2 play a role as AMP-activated protein kinase activator, resulting in regulating glucose homeostasis, and Rb1 reduces oxidative stress in aged skeletal muscles through activating the phosphatidylinositol 3-kinase/Akt/Nrf2 pathway. We examined the effects of Rb1 and Rb2 on differentiation of the muscle stem cells and myotube formation.
Methods
C2C12 myoblasts treated with Rb1 and/or Rb2 were differentiated and induced to myotube formation, followed by immunoblotting for myogenic marker proteins, such as myosin heavy chain, MyoD, and myogenin, or immunostaining for myosin heavy chain or immunoprecipitation analysis for heterodimerization of MyoD/E-proteins.
Results
Rb1 and Rb2 enhanced myoblast differentiation through accelerating MyoD/E-protein heterodimerization and increased myotube hypertrophy, accompanied by activation of Akt/mammalian target of rapamycin signaling. In addition, Rb1 and Rb2 induced the MyoD-mediated transdifferentiation of the rhabdomyosarcoma cells into myoblasts. Furthermore, co-treatment with Rb1 and Rb2 had synergistically enhanced myoblast differentiation through Akt activation.
Conclusion
Rb1 and Rb2 upregulate myotube growth and myogenic differentiation through activating Akt/mammalian target of rapamycin signaling and inducing myogenic conversion of fibroblasts. Thus, our first finding indicates that Rb1 and Rb2 have strong potential as a helpful remedy to prevent and treat muscle atrophy, such as age-related muscular dystrophy.
Keywords: Akt/mTOR signaling, Hypertrophy, Myoblast differentiation, Rb1, Rb2
1. Introduction
Skeletal muscle regeneration declines with aging not only through the intrinsic modifications for self-renewal and differentiation of the muscle stem cells but also through the changes in their environmental niche, which has a direct effect on cell function [1]. Myoblast differentiation is a well-organized multistage process and is regulated by myogenic basic helix-loop-helix transcriptional factors including MRF4, Myf5, MyoD, and myogenin [2], [3]. These myogenic regulatory factors are tightly regulated to ensure efficient cell differentiation. Akt plays an important role as a promyogenic kinase, and Akt signaling is contributed to heterodimerization of MyoD/E-proteins and alteration in chromatin remodeling at muscle-specific loci [4], [5], [6]. Akt/mammalian target of rapamycin (mTOR) signaling is a crucial regulator of skeletal muscle hypertrophy associated with increased protein synthesis [7], [8]. In addition, phosphatidylinositol 3-kinase/Akt pathways prevent muscle atrophy by inhibiting atrophy-related ubiquitin ligases and FoxO transcriptional factors [9], [10]. Interestingly, the loss of skeletal muscle regenerative capacity with aging seems to be reversible, and skeletal muscle functions are important for the betterment of metabolic health and life quality [1]. Understanding molecular regulator mechanisms of precise regulation of muscle stem cell differentiation and mature myotube formation opens up the possibility of devising strategies for slowing the decline of the process and identifying compound(s) to enhance skeletal muscle mass and function.
Ginsenoside Rg1 and Rg2 which are classified as a protopanaxadiol type are the most abundant ginsenosides in Panax ginseng C. A. Meyer [11]. It is reported that Rg1 and Rb2 function as an AMP-activated protein kinase activator and regulate glucose homeostasis [12], [13]. Especially, Rb1 reduces oxidative stress in aged skeletal muscles through activating the phosphatidylinositol 3-kinase/Akt/Nrf2 pathway [14]. However, there are no reports regarding the effects of Rb1 and Rb2 on myoblast differentiation and myotube growth. In the present study, we demonstrated the roles and molecular mechanisms of Rb1 and Rb2 in muscular hypertrophy and myoblast differentiation.
2. Materials and methods
2.1. Cell cultures and immunocytochemistry
Rb1 and Rb2 (purity > 98%) were provided by the Ambo Institute, Korea. Myoblast C2C12 cells, rhabdomyosarcoma (RD) cells, and 293T cells were cultured as described previously [5], [15]. To induce differentiation of the C2C12 myoblasts, cells at near confluence were changed from Dulbecco modified Eagle’s medium (DMEM) containing 15% fetal bovine serum (growth medium) to DMEM containing 2% horse serum (differentiation medium) and myotube formation was observed at 2 or 3 days of differentiation. The differentiated cultures were then immunostained for myosin heavy chain (MHC) antibodies (MF20; Developmental Studies Hybridoma Bank, Iowa, IA, USA) and Alexa Fluor 594–conjugated secondary antibodies (Molecular Probes, Eugene, OR, USA) [16]. To induce hypertrophic myotube growth, the C2C12 cells were differentiated for 2 days and then treated with Rb1 and Rb2 for additional 2 days in the differentiation medium. Additional cell lines, RD and 293T cells were cultured in DMEM containing 10% fetal bovine serum.
2.2. Immunoblotting and immunoprecipitation analysis
For immunoblotting, the cells were lysed in cell lysis buffer (10 mM Tris-HCl [pH7.2]) containing 150 mM NaCl, 1 mM EDTA, 1% NP-40 (Sigma-Aldrich, Louis, MO, USA), and protease inhibitors (Roche Diagnostics, Basel, Switzerland). Each sample was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The primary antibodies are listed in Table 1. For immunoprecipitation, the cells treated with Rg1 and Rg2 were cultured for 36 hours and lysed in cell lysis buffer. Anti-E2A antibody was incubated overnight with the supernatants at 4°C followed by incubation with protein G agarose beads (Roche Diagnostics) and subjected to immunoblotting [15], [16].
Table 1.
Antibodies used in this study.
| Antibody | Dilution factor | Company |
|---|---|---|
| Akt | 1:2000 | Cell Signaling Technology |
| phospho-Akt | 1:1000 | Cell Signaling Technology |
| p70S6K | 1:1000 | Cell Signaling Technology |
| phospho-p70S6K | 1:1000 | Cell Signaling Technology |
| MHC | 1:1000 | Developmental Studies Hybridoma Bank |
| mTOR | 1:1000 | Cell Signaling Technology |
| phospho-mTOR | 1:1000 | Cell Signaling Technology |
| MyoD | 1:500 | Santa Cruz Biotechnology |
| Myogenin | 1:1000 | Santa Cruz Biotechnology |
| E2A | 1:2000 | Santa Cruz Biotechnology |
| 4E-BP1 | 1:1000 | Cell Signaling Technology |
| phospho-4E-BP1 | 1:1000 | Cell Signaling Technology |
| pan-Cadherin | 1:5000 | Sigma-Aldrich |
| α-Tubulin | 1:2000 | Santa Cruz Biotechnology |
MHC, myosin heavy chain; mTOR, mammalian target of rapamycin.
Cell Signaling Technology (Beverly, MA, USA); Developmental Studies Hybridoma Bank (Iowa, IA, USA); Santa Cruz Biotechnology (Santa Cruz, CA, USA); Sigma-Aldrich(St. Louis, MO, USA).
2.3. Statistics
The values were expressed as mean ± standard deviation for at least three independent experiments, as indicated in the figure legends. For comparison between multiple groups, statistical significance was calculated using the Student t test using SPSS software (12.0 version; SPSS, Chicago, IL, USA). The differences were considered statistically significant at or under values of P < 0.05.
3. Results and discussion
3.1. Ginsenoside Rb1 and Rb2 promote the myotube hypertrophy through upregulating Akt/mTOR pathways
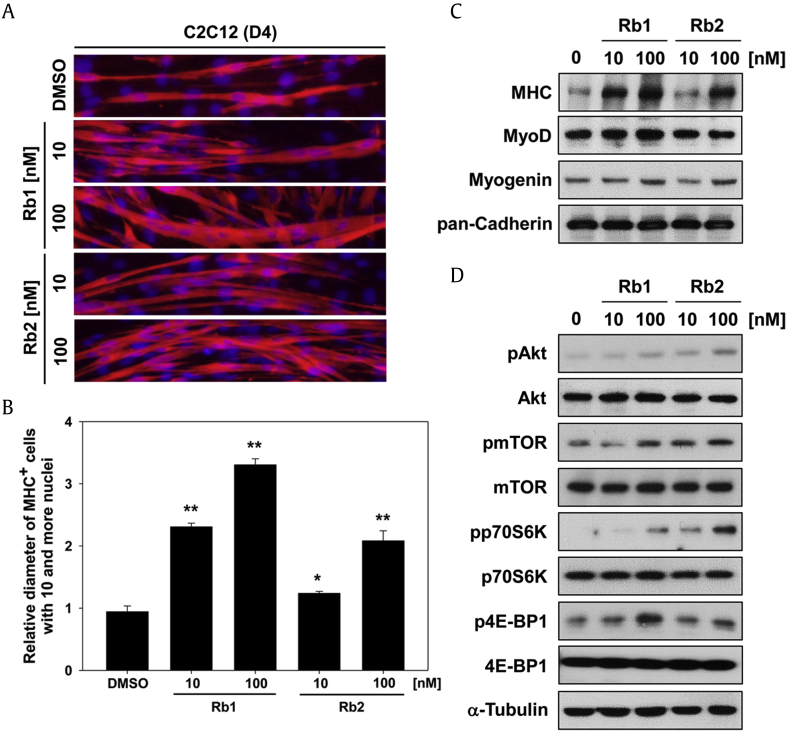
The aging of satellite cells is the loss of skeletal muscle regenerative capacity which is particularly pronounced in the age-related muscular dystrophy, sarcopenia [17]. To examine whether ginsenoside Rb1 and Rb2 could induce myotube formation, the C2C12 cells were induced to differentiate for 2 days (D2) and incubated with Rb1 or Rb2, for additional 2 days (D4). These C2C12 cells were then subjected to MHC immunostaining to identify the formation of myotubes. As a result, the Rb1- or Rb2-treated C2C12 cells formed larger and thicker multinucleated myotubes dose-dependently compared with the control cells (Fig. 1A). Measurement of the myotube diameter showed that Rb1 or Rb2 treatment increased the myotube thickness, especially by 3.31- and 2.08-fold in 100 nM Rb1 and Rb2, respectively (Fig. 1B). To further investigate whether these ginsenosides modulate muscle-specific marker proteins and signaling is involved in muscular hypertrophy, the C2C12 cells were differentiated under the same experimental conditions as previously described. Immunoblotting analysis showed that treatment with Rb1 dramatically increased the expression of MHC and slightly enhanced the expression of MyoD and myogenin at differentiation Day 4 compared with the control cells (Fig. 1C). Similar to Rb1, Rb2 treatment promotes the expression of MHC and myogenin at the same point, whereas MyoD expression was constant in Rb2-treated myotubes. The treatment with Rb1 or Rb2 triggered the phosphorylation of Akt and downstream signal, including mTOR, p70S6K, and 4E-BP1, compared with the control cells (Fig. 1D). Protopanaxatriol ginsenoside Rg1 has been reported to increase myotube growth and prevent dexamethasone-induced myotube atrophy through activation of Akt/mTOR signaling [15]. The insulin growth factor-1 (IGF-1)/Akt signaling pathway inhibits the activation of atrogin and MuRF1, known as the skeletal muscle–specific ubiquitin ligases, and prevents muscular atrophy [8], [10]. The inhibitory mechanism for these ubiquitin ligases is controlled by Akt/mTOR-mediated inhibition of FoxO transcription factors [18]. These results suggest that Rb1 and Rb2 have the potential to elicit myotube hypertrophy depending on Akt/mTOR signaling and could recover the capacity of muscular regeneration.
Fig. 1.
Rb1 and Rb2 enhance Akt/mTOR signaling–mediated myotube hypertrophy. (A) C2C12 cells were induced to differentiate for 2 days and then treated with indicated concentrations of Rb1 or Rb2 for the additional 2 days. The myotube formation was analyzed by MHC immunostaining. 4′,6-diamidino-2-phenylindole (DAPI) was used to visualize nuclei. (B) Average myotube diameter shown in panel (A) was measured using NIS-Elements F software. Data are presented as means determination of 6 fields± 1 SD. *P < 0.05 and **P < 0.01 compared with the control group. (C) Muscle-specific proteins from panel (A) were analyzed by immunoblotting. pan-Cadherin had been used as a loading control. (D) Total and phosphorylated forms of Akt, mTOR, p70S6K, and 4E-BP1 from panel (A) were analyzed by immunoblotting. α-Tubulin was used as a loading control.
MHC, myosin heavy chain; mTOR, mammalian target of rapamycin; SD, standard deviation.
3.2. Ginsenoside Rb1 and Rb2 enhance the early myoblast differentiation through phosphorylation of Akt
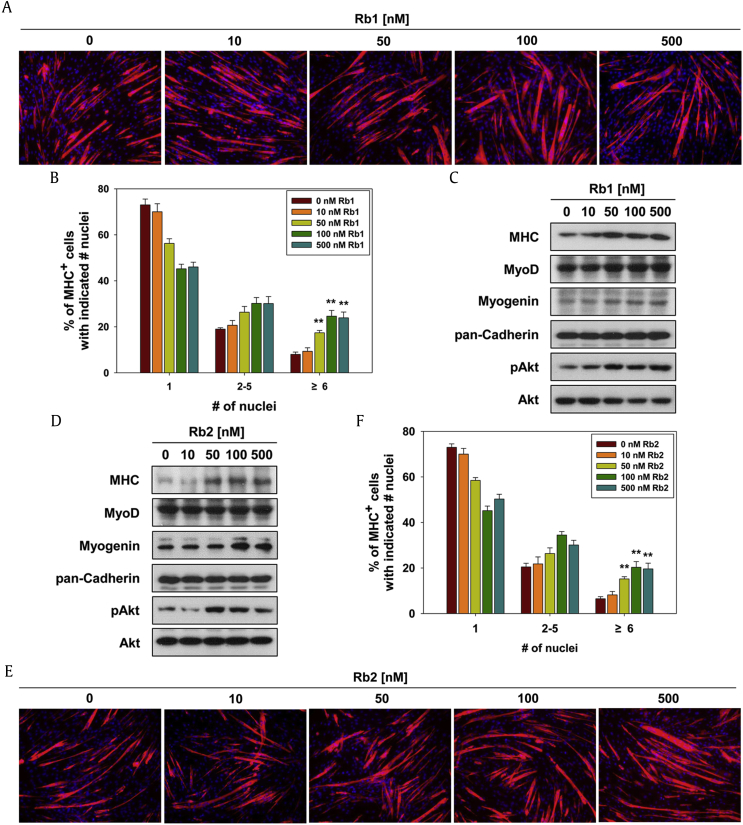
To investigate the effects of Rb1 and Rb2 on early myoblast differentiation, the C2C12 cells were induced to differentiate for D2 at indicated concentrations of Rb1 or Rb2. As assessed by fluorescence immunocytochemistry, Rb1 treatment increased the formation of MHC-positive myotubes with multinuclei in a dose-dependent manner (Fig. 2A). As a result of quantification of the myotube formation, Rb1 treatment significantly increased the proportion of larger myotubes containing six or more nuclei, while it substantially reduced that of mononucleate myocytes dose-dependently, compared with the control (Fig. 2B). Furthermore, treatment with Rb1 enhanced the expression of MHC, MyoD, and myogenin and increased the phosphorylation of Akt, as assessed by immunoblotting analysis (Fig. 2C). Similar to a result of Rb1, the Rb2-treated C2C12 cells showed the incremental expression of MHC and myogenin and increased phosphorylation of Akt in a dose-dependent manner (Fig. 2D). Contrary to Rb1, MyoD expression was constant in the Rb2-treated C2C12 cells. Rb2 treatment elevated the formation of MHC-positive myotubes with six or more nuclei dose-dependently (Fig. 2E and F). The family of myogenic basic helix-loop-helix transcription factors including MyoD is tightly regulated through autoregulatory and cross-regulatory feedback network that ensure efficient cell differentiation and maintenance of the differentiated state of cells [5]. Rb1 and Rb2 seem to regulate mainly MyoD activity rather than expression in muscle stem cell differentiation. Cell adhesion molecule complexes exert the promyogenic effects through activation of Akt signaling which is known as a major promyogenic kinase, along with p38MAPK [5], [19], [20]. Akt interacts with APPL1 and PRK2, a scaffold-like protein, and transduces myogenic signaling into its downstream targets to induce myoblast differentiation [5], [16].These results suggest that the promyogenic effects of Rb1 and Rb2 depend on the activation of Akt at a morphological and biochemical level.
Fig. 2.
Rb1 or Rb2 promote myogenic differentiation through activating a promyogenic kinase, Akt. (A) Rb1-treated C2C12 cells were differentiated for 2 days, followed by MHC immunostaining. (B) The MHC-positive myocytes shown in panel (A) were quantified as the number of nuclei per myotube. The values are represented as means ± SD of three independent experiments. **P < 0.01 compared with the control group. (C) Cell lysates from panel (A) were subjected to immunoblotting with antibodies to MHC, MyoD, and myogenin, phosphorylated Akt, total Akt, and pan-Cadherin used as a loading control. (D) Rb2-treated C2C12 cells were differentiated for 2 days, followed by immunoblotting against antibodies of MHC, MyoD, myogenin, phosphorylated Akt, total Akt, and pan-Cadherin used as a loading control. (E) Cells from panel (D) were immunostained for MHC expression (red) and with DAPI to stain nuclei (blue) to reveal myotube formation. (F) The MHC-positive myocytes shown in panel (E) were quantified as the number of nuclei per myotube. **P < 0.01 compared with the control group.
MHC, myosin heavy chain; SD, standard deviation.
3.3. Rb1 and Rb2 trigger the formation of MyoD/E-protein heterodimerization
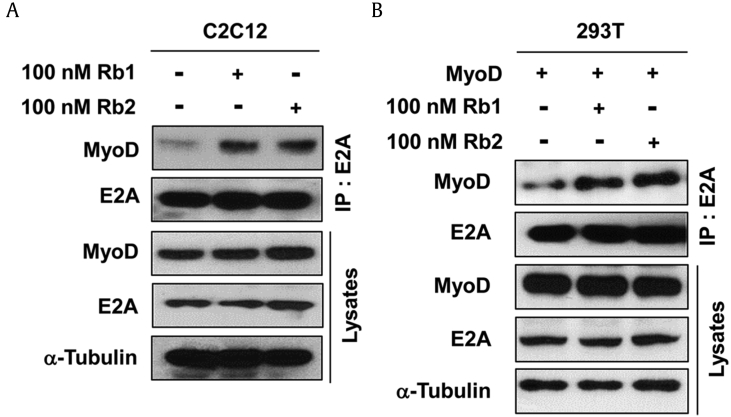
DNA binding of MyoD for expression of muscle-specific genes is induced by heterodimerization with widely expressed E-proteins [21]. To further examine whether Rb1 and Rb2 could induce the heterodimer formation of MyoD and E-proteins, the C2C12 cells were treated with Rb1 or Rb2 for D2, followed by immunoprecipitation analysis. Treatment with Rb1 or Rb2 enhanced the amount of MyoD in the precipitates with E2A antibodies as compared with dimethyl sulfoxide (DMSO) treatment (Fig. 3A). The expression of E2A and MyoD remained constant in the lysates of the C2C12 cells treated with Rb1 or Rb2 relative to the control cells (Fig. 3A). In agreement with the endogenous interaction, ectopically overexpressed MyoD strongly bounds with E2A proteins in the Rb1- or Rb2-treated 293T cells (Fig. 3B). However, the protein levels of MyoD and E2A were constant in the lysates, regardless of the treatment with Rb1 or Rb2. It is reported that MyoD interacts with the Baf60c subunit of the switching/sucrose non-fermenting (SWI/SNF) chromatin–remodeling complex required for the activation of muscle gene transcription [22]. The recruitment of MyoD to the chromatin of muscle-specific genes, an event necessary to enable the chromatin-remodeling activity of the p38MAPK-recruited SWI/SNF complex, was regulated by Akt signaling [23]. These results suggest that myoblast differentiation by treatment with Rb1 and Rb2 is dependent on MyoD activity controlled by Akt signaling.
Fig. 3.
Rb1 and Rb2 enhance the heterodimerization of E-protein with MyoD. (A) C2C12 cells or (B) MyoD-transfected 293T cells were treated with Rb1 or Rb2 and then incubated for 2 days. Cell lysates were subjected to immunoprecipitation with anti-E2A antibodies and then subjected to immunoblotting analysis using anti-MyoD antibodies. α-Tubulin has been used as loading controls.
IP, immunoprecipitation.
3.4. Rb1 and Rb2 induce MyoD-mediated transdifferentiation of the RD cells to myoblasts
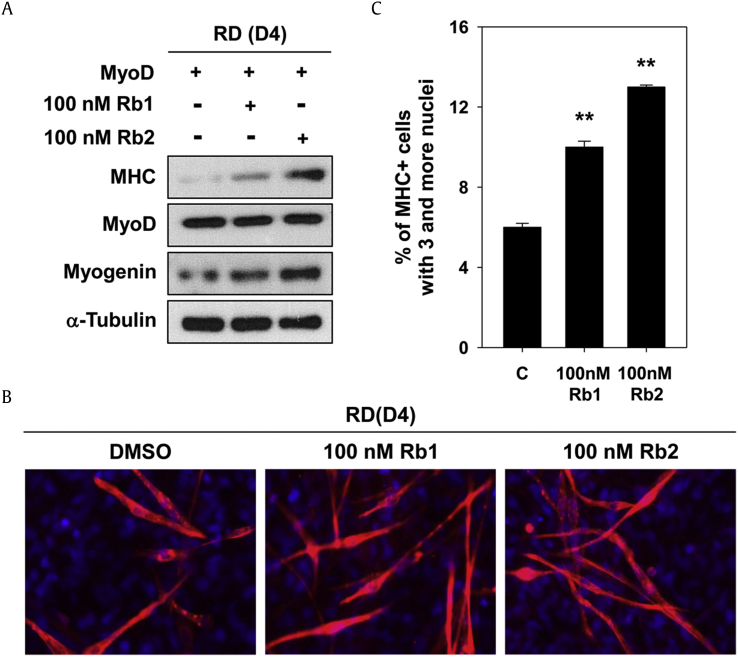
MyoD has been reported to convert many cell types such as adipocytes, chondrocytes, fibroblasts, and osteosarcomas into skeletal muscle cells [24]. To explore whether Rb1 and Rb2 could restore impaired myogenic differentiation of the RD cells (cancer cells of the muscle lineage that have low differentiation capability), the MyoD-transfected RD cells were treated with Rb1 and Rb2 for D4. Immunoblotting analysis reveals that treatment with Rb1 or Rb2 increased the expression of muscle-specific marker proteins, MHC, and myogenin compared with the control cells (Fig. 4A). Furthermore, treatment with Rb1 or Rb2 increased the formation of MHC-positive myotubes containing three or more nuclei in the MyoD-transfected RD cells (Fig. 4B). As a result of quantification of the myotube formation, Rb1 or Rb2 treatment significantly increased the proportion of larger myotubes containing three or more nuclei compared with the control (Fig. 4C). The RD cells express myogenic regulatory factors, while impairing myogenic differentiation on account of the non-functionality of these genes [25]. Reactivation of miR-206, a member of the myomiR family, restores MyoD-dependent myogenic differentiation in the RD cells [26]. Previously, our study revealed that black ginseng induces to implement the myogenic differentiation program of the RD cells into myoblasts through activating Akt/mTOR signaling [27]. In addition, ginsenoside Rg1 induces the MyoD-dependent transdifferentiation of mouse embryonic fibroblasts to myoblasts and myotubes [15]. These data suggest that Rb1 and Rb2 could improve MyoD-mediated myogenic differentiation of the RD cells.
Fig. 4.
Rb1 and Rb2 enhance MyoD-mediated myogenic conversion of RD cells. (A) MyoD-overexpressing RD cells were induced to differentiate with treatment with Rb1 or Rb2 for 4 days. Cell lysates were subjected to immunoblotting with antibodies to MHC, MyoD, myogenin, and α-Tubulin used as a loading control. The experiment was repeated three times with similar results. (B) Cells from panel (A) were immunostained for MHC expression (red) and with DAPI to stain nuclei (blue) to reveal myotube formation. (C) The MHC-positive myocytes shown in panel (B) were quantified as the number of nuclei per myotube. **P < 0.01 compared with the control group.
MHC, myosin heavy chain; RD, rhabdomyosarcoma.
3.5. Co-treatment with Rb1 and Rb2 synergistically enhances myogenic differentiation
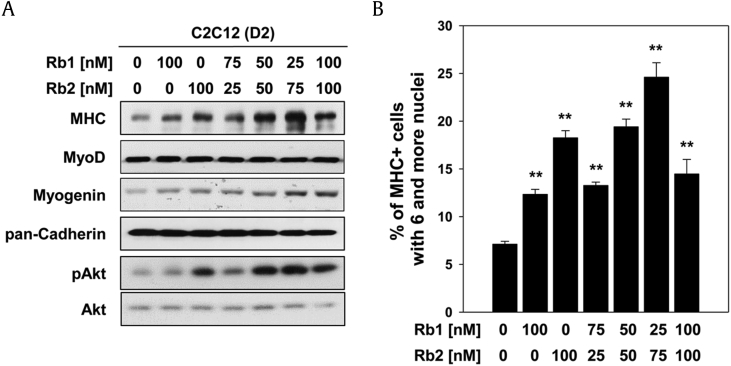
To investigate the combination effect of Rb1 and Rb2 on myoblast differentiation, the C2C12 cells were co-treated with indicated dose combination of Rb1 and Rb2, then induced to differentiation for D2, and subjected to MHC immunostaining. As shown in Fig. 5A, co-treatment with Rb1 and Rb2 showed more effective expression of MHC and myogenin than treatment with each of the ginsenosides, especially, in the combination of 25 nM Rb1 and 75 nM Rb2 and not 100 nM Rb1 and 100 nM Rb2. The effect of a drug has an appropriate concentration, and in some cases, the effect is lowered when the concentration is higher than the appropriate level, which seems to be the case in this study. The expression of MyoD bore a close parallel to the treatment with each of the ginsenosides. Similar to the pattern of MHC expression, the C2C12 cells co-treated with Rb1 and Rb2 showed the incremental phosphorylation of Akt. As a result of quantification of myotube formation under the same experimental conditions as previously described, the C2C12 cells co-treated with Rb1 and Rb2 showed significant increase in the proportion of MHC-positive myotubes containing six or more nuclei compared with the cells treated with each of them (Fig. 5B). Interestingly, co-treatment with the combination of 25 nM Rb1 and 75 nM Rb2 showed approximately 24% of MHC-positive myotubes containing six or more nuclei. Collectively, co-treatment of Rb1 and Rb2 has synergistic effects on Akt-mediated myogenic differentiation, indicating that this combination could be helpful in developing the pharmaceutical or nutraceutical remedy for muscular regeneration.
Fig. 5.
Co-treatment with Rb1 and Rb2 has synergistic effects on myogenic differentiation. (A) C2C12 cells were co-treated with indicated dose combination of Rb1 and Rb2, and then the cells were induced to differentiation for 2 days. The lysates were subjected to immunoblotting analysis against antibodies of MHC, MyoD, myogenin, phosphorylated Akt, total Akt, and pan-Cadherin were used as a loading control. (B) The MHC-positive myocytes were quantified as the number of nuclei per myotube under the same condition of panel (A). **P < 0.01 compared with the control group.
MHC, myosin heavy chain.
4. Conclusions
Our findings provide a molecular action framework of Rb1 and Rb2 that promotes myotube hypertrophy through upregulating Akt/mTOR signaling and enhances Akt-mediated myogenic differentiation, accompanied by the active heterodimerization of MyoD/E-proteins. Furthermore, Rb1 and Rb2 also increment transdifferentiation of RD cells into myoblasts, indicating that Rb1 and Rb2 are strong potential candidates as a helpful remedy to prevent and treat muscle atrophy, such as age-related muscular dystrophy.
Conflicts of interest
All authors have no conflicts of interest.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (NRF-2016R1A2B4014868 to G.U.B. and NRF-2017-R1D1A1B03032839 to S.J.L.).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2019.01.007.
Contributor Information
Gyu-Un Bae, Email: gbae@sookmyung.ac.kr.
Sang-Jin Lee, Email: lee.sangjin74@sookmyung.ac.kr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Domingues-Faria C., Vasson M.P., Goncalves-Mendes N., Boirie Y., Walrand S. Skeletal muscle regeneration and impact of aging and nutrition. Ageing Res Rev. 2016;26:22–36. doi: 10.1016/j.arr.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Molkentin J.D., Olson E.N. Defining the regulatory networks for muscle development. Curr Opin Genet Dev. 1996;6:445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- 3.Sartorelli V., Caretti G. Mechanisms underlying the transcriptional regulation of skeletal myogenesis. Curr Opin Genet Dev. 2005;15:528–535. doi: 10.1016/j.gde.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simone C., Forcales S.V., Hill D.A., Imbalzano A.N., Latella L., Puri P.L. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat Genet. 2004;36:738–743. doi: 10.1038/ng1378. [DOI] [PubMed] [Google Scholar]
- 5.Bae G.U., Lee J.R., Kim B.G., Han J.W., Leem Y.E., Lee H.J., Ho S.M., Hahn M.J., Kang J.S. Cdo interacts with APPL1 and activates Akt in myoblast differentiation. Mol Biol Cell. 2010;21:2399–2411. doi: 10.1091/mbc.E09-12-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lluis F., Ballestar E., Suelves M., Esteller M., Munoz-Canoves P. E47 phosphorylation by p38 MAPK promotes MyoD/E47 association and muscle-specific gene transcription. EMBO J. 2005;24:974–984. doi: 10.1038/sj.emboj.7600528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glass D.J. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol. 2003;5:87–90. doi: 10.1038/ncb0203-87. [DOI] [PubMed] [Google Scholar]
- 8.Rommel C., Bodine S.C., Clarke B.A., Rossman R., Nunez L., Stitt T.N., Yancopoulos G.D., Glass D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 9.Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R., Zlotchenko E., Scrimgeour A., Lawrence J.C., Glass D.J. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 10.Stitt T.N., Drujan D., Clarke B.A., Panaro F., Timofeyva Y., Kline W.O., Gonzalez M., Yancopoulos G.D., Glass D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 11.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 12.Lee K.T., Jung T.W., Lee H.J., Kim S.G., Shin Y.S., Whang W.K. The antidiabetic effect of ginsenoside Rb2 via activation of AMPK. Arch Pharm Res. 2011;34:1201–1208. doi: 10.1007/s12272-011-0719-6. [DOI] [PubMed] [Google Scholar]
- 13.Shen L., Haas M., Wang D.Q., May A., Lo C.C., Obici S., Tso P., Woods S.C., Liu M. Ginsenoside Rb1 increases insulin sensitivity by activating AMP-activated protein kinase in male rats. Physiol Rep. 2015;3 doi: 10.14814/phy2.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhuang C.L., Mao X.Y., Liu S., Chen W.Z., Huang D.D., Zhang C.J., Chen B.C., Shen X., Yu Z. Ginsenoside Rb1 improves postoperative fatigue syndrome by reducing skeletal muscle oxidative stress through activation of the PI3K/Akt/Nrf2 pathway in aged rats. Eur J Pharmacol. 2014;740:480–487. doi: 10.1016/j.ejphar.2014.06.040. [DOI] [PubMed] [Google Scholar]
- 15.Go G.Y., Lee S.J., Jo A., Lee J., Seo D.W., Kang J.S., Kim S.K., Kim S.N., Kim Y.K., Bae G.U. Ginsenoside Rg1 from Panax ginseng enhances myoblast differentiation and myotube growth. J Ginseng Res. 2017;41:608–614. doi: 10.1016/j.jgr.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S.J., Hwang J., Jeong H.J., Yoo M., Go G.Y., Lee J.R., Leem Y.E., Park J.W., Seo D.W., Kim Y.K. PKN2 and Cdo interact to activate AKT and promote myoblast differentiation. Cell Death Dis. 2016;7:e2431. doi: 10.1038/cddis.2016.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sousa-Victor P., Gutarra S., Garcia-Prat L., Rodriguez-Ubreva J., Ortet L., Ruiz-Bonilla V., Jardi M., Ballestar E., Gonzalez S., Serrano A.L. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 18.Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker S.H., Goldberg A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krauss R.S., Cole F., Gaio U., Takaesu G., Zhang W., Kang J.S. Close encounters: regulation of vertebrate skeletal myogenesis by cell-cell contact. J Cell Sci. 2005;118:2355–2362. doi: 10.1242/jcs.02397. [DOI] [PubMed] [Google Scholar]
- 20.Takaesu G., Kang J.S., Bae G.U., Yi M.J., Lee C.M., Reddy E.P., Krauss R.S. Activation of p38alpha/beta MAPK in myogenesis via binding of the scaffold protein JLP to the cell surface protein Cdo. J Cell Biol. 2006;175:383–388. doi: 10.1083/jcb.200608031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkes C.A., Tapscott S.J. MyoD and the transcriptional control of myogenesis. Semin Cell Dev Biol. 2005;16:585–595. doi: 10.1016/j.semcdb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Forcales S.V., Albini S., Giordani L., Malecova B., Cignolo L., Chernov A., Coutinho P., Saccone V., Consalvi S., Williams R. Signal-dependent incorporation of MyoD-BAF60c into Brg1-based SWI/SNF chromatin-remodelling complex. EMBO J. 2012;31:301–316. doi: 10.1038/emboj.2011.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serra C., Palacios D., Mozzetta C., Forcales S.V., Morantte I., Ripani M., Jones D.R., Du K., Jhala U.S., Simone C. Functional interdependence at the chromatin level between the MKK6/p38 and IGF1/PI3K/AKT pathways during muscle differentiation. Mol Cell. 2007;28:200–213. doi: 10.1016/j.molcel.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weintraub H., Tapscott S.J., Davis R.L., Thayer M.J., Adam M.A., Lassar A.B., Miller A.D. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci U S A. 1989;86:5434–5438. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller C., Guttridge D.C. Mechanisms of impaired differentiation in rhabdomyosarcoma. FEBS J. 2013;280:4323–4334. doi: 10.1111/febs.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coda D.M., Lingua M.F., Morena D., Foglizzo V., Bersani F., Ala U., Ponzetto C., Taulli R. SMYD1 and G6PD modulation are critical events for miR-206-mediated differentiation of rhabdomyosarcoma. Cell Cycle. 2015;14:1389–1402. doi: 10.1080/15384101.2015.1005993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S.Y., Go G.Y., Vuong T.A., Kim J.W., Lee S., Jo A., An J.M., Kim S.N., Seo D.W., Kim J.S. Black ginseng activates Akt signaling, thereby enhancing myoblast differentiation and myotube growth. J Ginseng Res. 2018;42:116–121. doi: 10.1016/j.jgr.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.