Abstract
Background
Bisphenol A (BPA), known as an endocrine disruptor, is widely used in the world. BPA is reported to cause inflammation-related diseases. Korean Red Ginseng (KRG) has been used safely in human for a long time for the treatment of diverse diseases. KRG has been reported of its mitigating effect on menopausal symptoms and suppress adipose inflammation. Here, we investigate the protective effect of orally administered KRG on the impacts of BPA in the liver and uterus of menopausal mice model.
Methods
The transcriptome analysis for the effects of BPA on mice liver was evaluated by Gene Expression Omnibus (GEO) database–based data (GSE26728). In vivo assay to evaluate the protective effect of KRG on BPA impact in ovariectomized (OVX) mice were designed and analyzed by RNA sequencing.
Results
We first demonstrated that BPA induced 12 kinds of gene set in the liver of normal mice. The administration of BPA and KRG did not change body, liver, and uterine weight in OVX mice. KRG downregulated BPA-induced inflammatory response and chemotaxis-related gene expression. Several gene set enrichment analysis (GSEA)–derived inflammatory response genes increased by BPA were inhibited by KRG in OVX mice.
Conclusion
Our data suggest that BPA has commonly influenced inflammatory response effects on both normal and OVX mice. KRG protects against BPA impact of inflammatory response and chemotaxis in OVX mouse models. Our comparative analysis will provide new insight into the efficacy of KRG on endocrine disrupting chemicals and OVX mouse.
Keywords: Bisphenol A, Korean Red Ginseng, Transcriptome analysis
1. Introduction
Bisphenol A (BPA; 4,4′-isopropylidenediphenol) is a compound used in the synthesis of phenol resins, polyacrylates, polyesters, epoxy resins, and polycarbonate plastics [1,2]. Many chemicals used in manufacturing, including BPA, have the ability to interrupt the endocrine system, and these materials have been termed endocrine disruptors [3,4]. As an endocrine disrupting chemical, BPA imitates the structure of the endogenous ligand, interfering with the action of the hormone, resulting in a physiological process that can cause hormone-related diseases [5,6]. The side effects of these health problems have been reported in numerous articles. BPA promotes adiposity, inflammation, and gene expression related to lipid synthesis and triglyceride accumulation, and it also induces premature menopause in women by mimicking the endocrine system [[7], [8], [9]]. Although efforts to identify the effects of BPA are ongoing, the impact of BPA on postmenopausal women is not well known. Researchers have attempted to evaluate the effects of endocrine disruptors on bone metabolism after menopause [3], but further investigation into the effects of BPA on postmenopausal women is needed.
Korean Red Ginseng (KRG; Panax ginseng Meyer) has many therapeutic effects; the effects of 6-year-old KRG have been particularly well recognized [10,11]. KRG is a heat-modified product of ginseng radix (root of P. ginseng), with higher contents of ginseng saponins or ginsenosides compared with the original root [11,12]. Ginseng also contains a number of active ingredients, including polysaccharides, phytosterols, peptides, polyacetylenes, fatty acids, and polyacetylenic alcohols, that have various effects on carbohydrate and lipid metabolism, cognition, and angiogenesis, as well as on the neuroendocrine, immune, cardiovascular, and central nervous systems [13,14]. Research to reveal more of the untapped potential of ginseng is ongoing, with more than 6,000 articles regarding traditional uses, chemical constituents, and biological and pharmacological effects of ginseng already published [11,15]. A recent study on its obtunding effect on menopausal symptoms reported that ginseng inhibited ovariectomy-induced obesity, adiposity, and adipocyte hypertrophy by modulating angiogenesis and matrix metalloproteinase activity, as well as also suppressed adipose inflammation in ovariectomized (OVX) mice [16].
We investigated the protective effect of orally administered KRG against the influences of BPA in the liver and uterus of OVX mice. Uterus wet weight is reportedly increased by the estrogenic activity of BPA in normal and OVX rats [17]. We hypothesized that BPA may accelerate lipid synthesis and accumulation in the liver and uterus of OVX mice but that KRG would prevent BPA-induced functional changes. Experiments were designed with a focus on distinguishing gene expression levels and changes in gene ontology (GO) categories. First, we investigated the impact of BPA on CD-1 mouse liver. Then, the impacts of BPA on OVX mouse liver and uterus were identified, and the protective effect of KRG was demonstrated. Our data suggest that BPA commonly influences inflammatory response effects in both normal and OVX mice. We also found that KRG may protect against the impacts of BPA on inflammatory response and chemotaxis in OVX mouse models. Our comparative transcriptome analysis provides new insight into the efficacy of KRG on endocrine disrupting chemicals and menopause.
2. Materials and methods
2.1. Microarray analysis
Gene expression data in every six mice of six-week-old CD-1 mice liver samples treated with low dose of BPA (50 μg/kg/day), treated with high dose of BPA (5,000 μg/kg/day) and treated without BPA for 28 days via food contamination were downloaded from National Center for Biotechnology Information and Gene Expression Omnibus (GEO) database. GEO series accession number GSE26728 was analyzed via the platform of GPL7042, which was published by Marmugi A, Ducheix S, Lasserre F, Polizzi A et al. (2012).
2.2. Gene set enrichment analysis
UP and DOWN gene lists are analyzed together by gene set enrichment analysis (GSEA) software, version 3.0 available from the Broad Institute (http://software.broadinstitute.org/gsea/index.jsp) [18]. Submitted gene list was normalized by the quantile method from the preprocess Core library. GSEA was performed with default algorithm as 1000 permutations, minimum term size of 15, and maximum term size of 500. Annotated gene sets of HALLMARK collection, version 6.2 were used as enrichment input, which gene set were from Molecular Signatures Database (http://software.broadinstitute.org/gsea/msigdb/index.jsp) [19]. Enriched gene sets were assigned based on nominal p-value < 0.05 and FDR q-value < 0.25.
2.3. Materials
BPA (CAS # 80-05-7, ≥ 99%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). KRG was manufactured and kindly provided by the Korea Ginseng Corporation (Daejeon, Korea). The extraction procedure for the KRG followed the international standard production process (ISO 19610). The extract of six-year-old Panax ginseng root (body 75% and root 25%) was prepared by repeated steaming and drying process from the Korea Ginseng Corporation. The extract was freeze-dried, and finally, we obtained a dark-brown powder (KRG). The analysis of KRG was based on the reported method [20]. The phytochemical characteristics of KRG with standard ginsenosides were confirmed by HPLC (High performance liquid chromatography) analysis. Rb1, 5.16 mg/g; Rb2, 1.82 mg/g; Rc, 2.22mg/g; Rd, 0.47 mg/g; Re, 2.16 mg/g; Rf, 0.93 mg/g; Rg1, 2.89 mg/g; Rg2 (s), 0.37 mg/g; Rg2 (r), 0.21 mg/g; Rg3(s), 0.14 mg/g; Rg3(r), 0.08 mg/g and Rh1, 0.13 mg/g. BPA was dissolved in corn oil, whereas KRG was dissolved in triple distilled water.
2.4. In vivo assay
Six-weeks-old female CD-1 mice were OVX and had 1 week of purification period. A total of 49 mice (Control mice; n = 7, Sham; n = 7, OVX; n = 35) were obtained from Doo Yeol Biotech (Seoul, Korea). Except for non-OVX group and sham group, OVX mice were randomly divided into 5 groups (negative control, E2; positive control, BPA, KRG, BPA plus KRG group, n = 7). The animals were kept at a temperature of 23 ± 2°C with 12 hours dark and light cycle and allowed free access to food and water. Body weight was monitored before and after the experiment, and statistical significance between experimental groups and a negative control group were analyzed by ANOVA and the significance level was set at p < 0.05. The dose of BPA and KRG was considered through several references [17,[21], [22], [23], [24], [25]]. Before treatment, the dose range finding assay was conducted independently for one week (data not shown), and finally the BPA dose of 200 mg/kg/day and the KRG dose of 1.2 g/kg/day were determined. After sacrificed, the liver and uterine tissues were carefully amputated, weighed, and stored at −70 °C.
2.5. RNA isolation, library preparation, and sequencing
Total RNA of liver and uterus was isolated using Trizol reagent obtained from Invitrogen (Grand Island, NY, USA). RNA quality was assessed by Agilent 2100 bioanalyzer using the RNA 6000 Nano Chip (Agilent Technologies, Amstelveen, The Netherlands), and RNA quantification was performed using ND-2000 Spectrophotometer (Thermo Fisher Scientific., DE, USA). For control and test RNAs, the construction of library was performed using QuantSeq 3′ mRNA-Seq Library Prep Kit (Lexogen, Vienna., Austria) in accordance with the manufacturer's instructions [26]. QuantSeq 3′ mRNA-Seq reads were aligned using Bowtie2 (Langmead and Salzberg, 2012). The RT (Read Count) data were processed based on quantile normalization method using EdgeR within R (R development Core Team, 2016) using Bioconductor (Gentleman et al, 2004). Gene classification was based on searches carried out by Database for Annotation, Visualization and Integrated Discovery (DAVID) (http://david.abcc.ncifcrf.gov/) and Medline databases (http://www.ncbi.nlm.nih.gov/). The final RNA-Seq data were deposited in the National Center for Biotechnology Information’s GEO database (GSE133430).
2.6. Real-time quantitative reverse transcription–polymerase chain reaction
The expression level of VCAM-1 mRNA and CCR7 mRNA in mice was detected by real-time quantitative reverse transcription–polymerase chain reaction (qRT-PCR) using the RNeasy Mini Kit (Qiagen, Venlo, Netherlands). Relative expression values were obtained using the 2-ΔΔCT method and normalized to the control value for percent fold changes. The forward primer of VCAM-1 was 5′-TGA CAA GTC CCC ATC GTT GA-3′ and the reverse primer was 5′- ACC TCG CGA CGG CAT ATT T -3'. For CCR7, the forward primer was 5′- TCA TTG CCG TGG TGG TAG TCT TCA -3′ and the reverse was 5′-ATG TTG AGC TGC TTG CTG GTT TCG -3'. The forward primer of GAPDH was 5′- CTG CAC CAC CAA CTG CTT AGC -3′ and reverse was 5′- GGG CCA TCC ACA GTC TTC TGG -3'.
2.7. Enzyme-linked immunosorbent assay
Mouse TNF-α enzyme-linked immunosorbent assay kit was purchased from R&D Systems (Minneapolis, MN, USA). Tissues were homogenized and lysated in tissue cell lysis buffer (150mM NaCl, 100mM Tris, 1mM EGTA, 1mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate and protease inhibitor. The pH of the buffer was 7.4).
2.8. GO and pathway analysis
Differentially expressed genes (DEGs) were analyzed base on Excel-based DEG analysis. Genes listed in Venn diagram graphs and gene category graphs were selected considering fold change and normalized RC values (Fold change > 2, Normalized RC (log2) = 3 or 4). Twelve kinds of gene set were selected as shown in Fig. 1, its components were downloaded from Quick GO website (https://www.ebi.ac.uk/QuickGO/). The heat map graph was drawn with Multiexperimental viewer software.
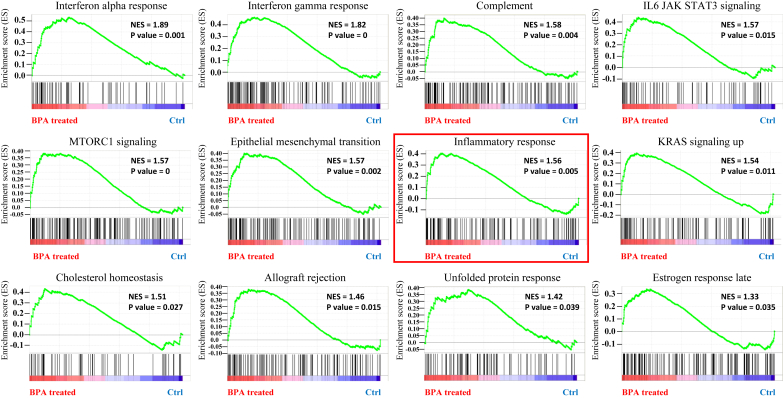
Fig. 1.
Gene set enrichment plot of genes regulated by BPA. GSE26728 includes microarray data of CD-1 mice at 9 weeks of age treated with low and high doses of BPA (50 μg/kg/day and 5,000 μg/kg/day, respectively) for 28 days in their feed. The results of GSEA of DEGs between negative control and BPA treatment groups were visualized using the JavaGSEA package. The most significantly enriched signaling pathways were selected based on the normalized enrichment score (NES), with a nominal P-value < 0.05, and false discovery rate q-value < 0.25. The y axis represents the enrichment score, and the x axis lists genes that showed high levels of expression induced by BPA treatment among the 22,514 total genes. The black bar indicates the locations of the genes in each gene set. BPA, bisphenol A; DEGs, differentially expressed genes.
2.9. Functional annotation analysis
Selected genes in each tissue were submitted to DAVID, version 6.8 software for GO analysis and functional pathway mapping [27]. Submitted gene lists were analyzed by DAVID to distinct GO categories and the significant enrichment was determined by count > 10 and EASE score of p-value < 0.05, which is a modified Fisher exact p-value. Functional pathway database is referred to Kyoto encyclopedia of genes and genomes (https://www.kegg.jp/kegg/). Significantly enriched GO or functional pathways are visualized with -log10 transformation of p-value.
2.10. Statistical analysis
All in vivo data were expressed as mean ± SD using GraphPad Prism 7.0 (Graph Pad Software, La Jolla, CA, USA). Statistical analysis of the data was determined by one-way ANOVA, and p < 0.05 was considered as statistically significance.
3. Results
3.1. BPA induces 12 gene sets in the mouse liver
To analyze the protective effects of KRG against BPA, we first examined the major effects of BPA. We conducted GSEA using a public data set of transcriptome analysis of the mouse liver exposed to BPA from the GEO (accession number GSE26728) to identify signaling pathways that were differentially activated in the liver. A total of 12 gene sets were considered differentially enriched in the BPA treatment group on enrichment of Molecular Signatures Database Collection gene set “h.all.v6.2.symbols.gmt” (Fig. 1). Interestingly, the inflammatory response gene set was regarded as significant with a normalized enrichment score of 1.56 and nominal p-value of 0.005. Other gene sets, including interferon alpha response, interferon gamma response, and IL-6 JAK-STAT signaling factors, which may influence the inflammatory response, also showed significant changes in expression upon BPA treatment. These results indicate that genes involved in the inflammatory response are significantly differentially expressed in GEO-derived BPA-treated CD-1 mouse liver sample.
3.2. KRG with or without BPA did not alter the liver or uterus weight
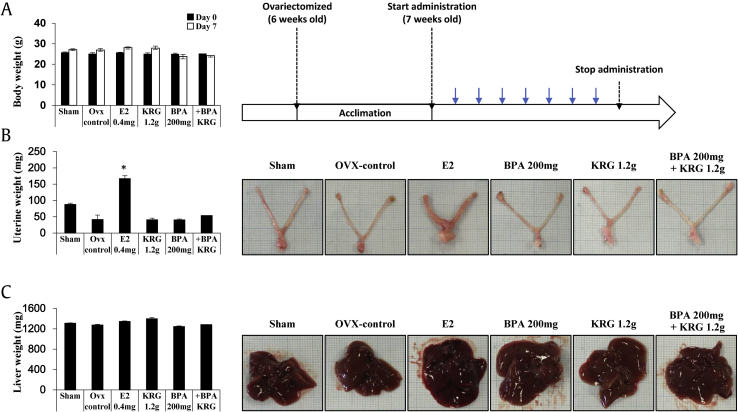
To examine the effects of BPA and the protective effects of KRG, OVX CD-1 mice were treated with BPA alone, KRG alone, and BPA with KRG. As shown in Fig. 2A, there were differences in body weight between the groups, but the degree of change was negligible. The positive control group treated with estradiol (E2) showed significant increases in uterus weight, but KRG and BPA had no significant effects (Fig. 2B). Similarly, KRG and BPA did not cause changes in liver weight (Fig. 2C). KRG and BPA showed no significant effects on body, uterus, or liver weight at the doses and administration period used in the present study.
Fig. 2.
KRG with or without BPA did not alter the liver or uterus weights. Treatment with BPA and KRG was started 1 week after ovariectomy. Ovariectomized CD-1 mice were treated with BPA (200 mg/kg/day) diluted in corn oil and KRG (1.2 g/kg/day) diluted in triple distilled water every day for 7 days via oral administration. Body weight (A), uterine weight (B), and liver weight (C) were recorded. BPA, bisphenol A; KRG, Korean Red Ginseng.
3.3. KRG with or without BPA-induced differential gene expression in the liver and uterus
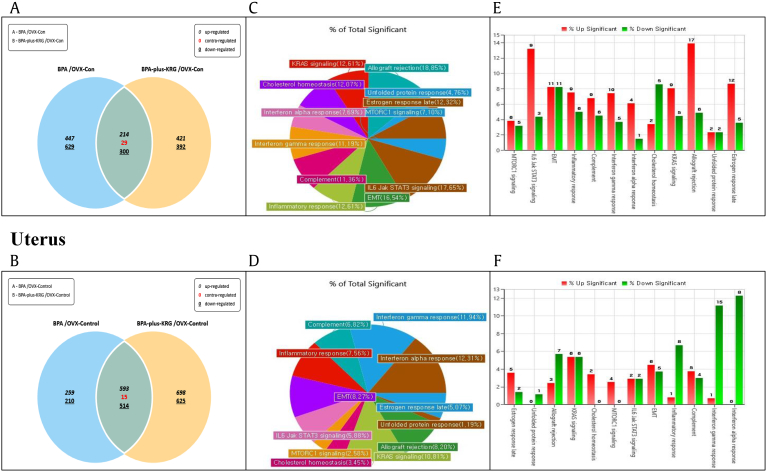
Then, we compared gene expression levels associated with KRG treatment with or without BPA. Liver and uterine tissues were subjected to RNA-sequencing analysis. The genes showing changes in expression in the BPA treatment group and the BPA plus KRG treatment group compared with their levels in the negative control are summarized in a Venn diagram (Fig. 3A and B). We then examined whether the gene set affected by BPA (Fig. 1) differed from that affected by KRG by assessing the numbers of genes in each of the 12 gene sets showing a greater than twofold change in expression induced by simultaneous BPA and KRG treatment vs. treatment with BPA alone (Fig. 3C and D). As a result, fifteen genes (12.61%) related to inflammatory response showed changed expression in the liver; nine genes (Tlr2, Il1b, Emp3, Marco, Pde4b, Cd14, Csf1, Lcp2, F3) were upregulated, whereas six genes (Rgs16, Cdkn1a, Ccl2, Tnfsf15, Pvr,Itga5) were downregulated (Fig. 3E). Nine genes (7.56%) related to inflammatory response showed altered expression in the uterus; one gene (Msr1) was upregulated and eight genes (Cxcl10, Slc1a2, Cxcl9, Tacr1, Il10ra, Inhba, Calcrl, and Myc) were downregulated (Fig. 3F). These results suggest that genes were differentially expressed between the group treated with BPA and the group treated with KRG and BPA.
Fig. 3.
Differential gene expression induced by BPA treatment with or without KRG in mouse liver and uterus. The levels of mRNA transcripts in mouse liver and uterus were determined by the library preparation and sequencing method. Venn diagrams show the classification of DEGs in the BPA group and BPA plus KRG group in the mouse liver (A) and uterus (B). Numbers represent the numbers of genes showing a more than twofold change in expression compared with negative controls. Upper numbers, upregulated genes; lower numbers, downregulated genes; middle numbers, contraregulated genes. Under the same conditions, genes with different expression levels between the BPA plus KRG group and the BPA-only group in the mouse liver (C) and uterus (D) were selected and charted by the percentage of genes in each gene ontology out of the total number of genes. The numbers of genes upregulated or downregulated in the liver (E) and uterus (F) in the BPA plus KRG group are shown in red and green bar graphs, respectively. BPA, bisphenol A; KRG, Korean Red Ginseng; DEGs, differentially expressed genes.
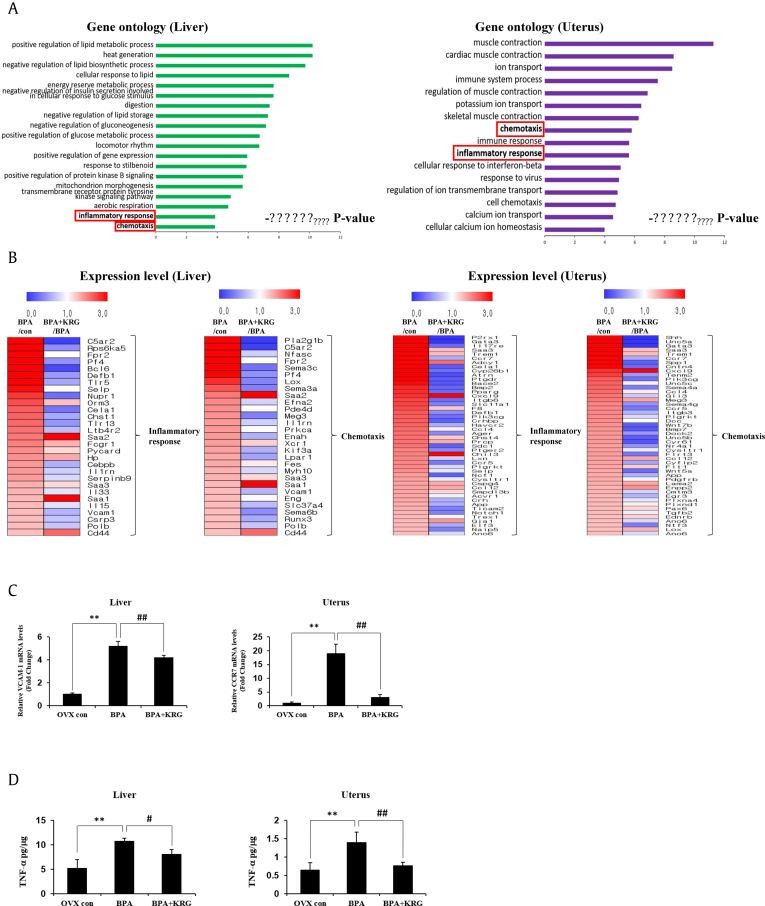
3.4. KRG downregulated BPA-induced expression of genes related to the inflammatory response and chemotaxis in the mouse liver and uterus
To determine the effects of KRG on the effects of BPA, RNA-sequencing data from the liver and uterus were subjected to GO annotation using the DAVID bioinformatics resource. Inflammatory response and chemotaxis-related genes were expressed in both the liver and uterus, although they did not show the highest degrees of significance (Fig. 4A). Moreover, the gene expression levels were examined to determine which components were associated with the inflammatory response and chemotaxis. Genes that showed more than a twofold change in expression in the BPA-treated group were examined in the group treated with both BPA and KRG, and the results are visualized as heat maps (Fig. 4B). The expression levels of most of the genes upregulated by BPA were found to be downregulated by KRG. Among them, the VCAM-1 factor in the liver and the CCR7 factor in the uterus were verified by qRT-PCR (Fig. 4C). Besides, TNF-α, a cytokine factor involved in the inflammatory response, was shown to be elevated by BPA and alleviated by KRG in both liver and uterus (Fig. 4D). Taken together, these database-based annotations indicate that KRG inhibited some portion of the genes related to the inflammatory response and chemotaxis that were upregulated by BPA.
Fig. 4.
Induction of inflammatory response and chemotaxis-related genes by BPA was reduced by KRG. Functional annotation of genes regulated by BPA plus KRG compared with those regulated by BPA alone. Gene Ontology analysis of regulated DEGs in the liver and uterus (A). Genes showing equal expression are marked with red boxes. Heat map plots of DEGs related to the inflammatory response and chemotaxis. The genes upregulated by BPA were identified and evaluated in the BPA plus KRG group and visualized as heat maps (B). Total RNA of the liver and uterus was extracted, and the expression level of the VCAM-1 and CCR7 mRNA was analyzed by real-time PCR (C). TNF-α protein levels in the liver and uterus were analyzed by ELISA (D). ∗P < 0.05, ∗∗P < 0.01. BPA vs OVX-control; #P < 0.05, ##P < 0.01 BPA vs BPA with KRG. BPA, bisphenol A; KRG, Korean Red Ginseng; OVX, ovariectomized; PCR, polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay; DEGs, differentially expressed genes.
3.5. Expression levels of some BPA-induced inflammatory response genes were reduced by KRG
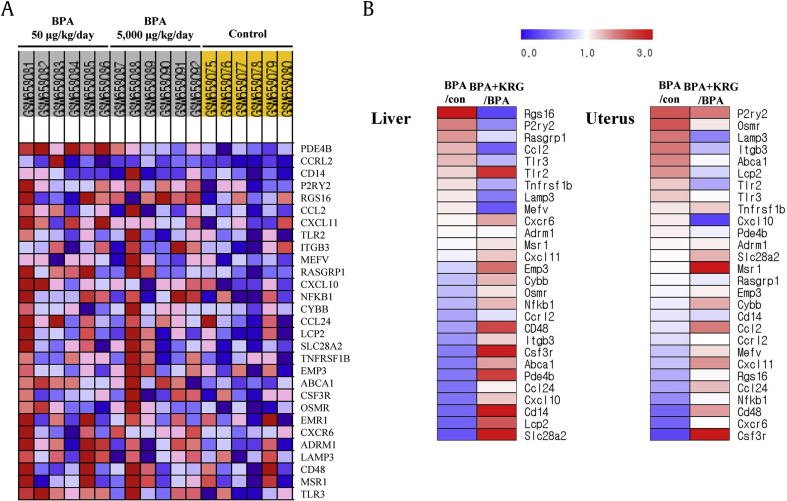
Independent of GO analysis, we identified gene expression changes common to GEO-derived microarray data and RNA-sequencing data. First, we focused on the expression of inflammatory response–related genes that were altered by BPA (Fig. 5A). Each gene upregulated by both low and high doses of BPA was regarded as enriched by BPA. To determine the effects of KRG on the changes induced by BPA, we analyzed the differences in the expression levels of these genes between the BPA group and the KRG plus BPA cotreatment group. The gene expression patterns induced by BPA in the liver and uterus were not all consistent. That is, some genes considered to be upregulated by BPA treatment on CD-1 mice liver derived from GSE26728 showed downregulation on OVX CD-1 mice liver and uterus. Nevertheless, several factors were consistently upregulated by BPA, and the extent of upregulation was reduced by KRG. That is, the increases in Rgs16, P2ry2, Rasgrp1, Ccl2, and Tlr3 expression in the liver and in Itgb3, Abca1, Lcp2, and Tlr2 expression in the uterus were reduced by cotreatment with KRG (Fig. 5B). These results suggest that some BPA-induced inflammatory response–related genes were suppressed by KRG treatment.
Fig. 5.
Induction of inflammatory response–related genes by BPA was reduced by KRG. Heat map of enriched hallmark inflammatory response gene set from GSE26728 (A). The effects of KRG and BPA on the expression of inflammatory response–related genes. The gene set enriched in GSE26728 was collected and applied to the liver and uterus of the BPA group and BPA plus KRG group, and the results are visualized as heat maps (B). BPA, bisphenol A; KRG, Korean Red Ginseng.
4. Discussion
Recently, transcriptome research has earned considerable attention owing to advances in next-generation sequencing [28]. RNA-sequencing (RNA-Seq) is an innovative next-generation sequencing tool for comprehensive transcriptome profiling [29,30]. In contrast to microarray and quantitative PCR analyses, RNA-Seq allows identification of novel transcripts, as well as confirmation of alternative splicing and mutations [31]. GO analysis combined with transcriptome profiling is a great tool for interpreting genome-wide insights.
A previous study reported that KRG lowered urinary BPA levels and BPA-induced malondialdehyde levels [32]. Here, we investigated the beneficial effects of KRG on BPA-induced side effects in an OVX mouse model by examining the transcriptome profile. We first confirmed through gene set enrichment analysis that 12 GO categories, including inflammatory response, had high normalized enrichment scores in BPA-treated normal mouse liver (Fig. 1). The GSE26728 transcriptome data set published by Marmugi et al. (2012) was originally used to examine the lipid accumulation effect of BPA in mouse liver [8]. We hypothesized that the lipid accumulation and inflammatory response effects of BPA in mouse liver might be closely related. In an experiment using the OVX mouse model, we were unable to detect weight changes owing to BPA or KRG treatment in the liver or uterus (Fig. 2). We initially expected that the weight of the uterus would increase owing to the estrogenic effect of BPA. However, in some studies, uterine weight was reduced by BPA [33], and in some experiments, the uterus was enlarged by KRG [23]. These different results are probably owing to differences in mouse strains, age groups, and/or as the doses of chemicals. Interestingly, GO analysis revealed that oral administration of KRG improved the BPA-induced inflammatory response in both the liver and uterus of the OVX mouse model (Fig. 4). Several genes upregulated by BPA in the normal mouse were also upregulated in the OVX mouse model, but KRG suppressed their expression (Fig. 5). This is supported by reports that KRG has anti-inflammatory effects [[34], [35], [36], [37], [38]].
In conclusion, the results of our transcriptome analysis suggest that KRG has a protective effect against BPA in the liver and uterus of the OVX mouse model and provide a comprehensive basis for considering the protective effects of KRG against BPA-induced inflammatory responses.
Conflicts of interest
All authors declare that they have no conflicts of interest.
Acknowledgements
This research was supported by a 2017 grant from the Korean Society of Ginseng to Y.J.L.
References
- 1.Murata M., Kang J.H. Bisphenol A (BPA) and cell signaling pathways. Biotechnology Advances. 2018;36(1):311–327. doi: 10.1016/j.biotechadv.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Vermeirssen E.L., Dietschweiler C., Werner I., Burkhardt M. Corrosion protection products as a source of bisphenol A and toxicity to the aquatic environment. Water Research. 2017;123:586–593. doi: 10.1016/j.watres.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Vitku J., Kolatorova L., Franekova L., Blahos J., Simkova M., Duskova M., Skodova T., Starka L. Endocrine disruptors of the bisphenol and paraben families and bone metabolism. Physiological Research. 2018;67 doi: 10.33549/physiolres.934005. [DOI] [PubMed] [Google Scholar]
- 4.Skledar D.G., Mašič L.P. Bisphenol A and its analogs: do their metabolites have endocrine activity? Environmental Toxicology and Pharmacology. 2016;47:182–199. doi: 10.1016/j.etap.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Park C., Lee J., Kong B., Park J., Song H., Choi K., Guon T., Lee Y. The effects of bisphenol A, benzyl butyl phthalate, and di (2-ethylhexyl) phthalate on estrogen receptor alpha in estrogen receptor-positive cells under hypoxia. Environmental Pollution. 2019;248:774–781. doi: 10.1016/j.envpol.2019.02.069. [DOI] [PubMed] [Google Scholar]
- 6.Acconcia F., Pallottini V., Marino M. Molecular mechanisms of action of BPA. Dose-response. 2015;13(4) doi: 10.1177/1559325815610582. 1559325815610582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang M., Chen M., Wang J., Xu M., Sun J., Ding L., Lv X., Ma Q., Bi Y., Liu R. Bisphenol A promotes adiposity and inflammation in a nonmonotonic dose-response way in 5-week-old male and female C57BL/6J mice fed a low-calorie diet. Endocrinology. 2016;157(6):2333–2345. doi: 10.1210/en.2015-1926. [DOI] [PubMed] [Google Scholar]
- 8.Marmugi A., Ducheix S., Lasserre F., Polizzi A., Paris A., Priymenko N., Bertrand-Michel J., Pineau T., Guillou H., Martin P.G. Low doses of bisphenol A induce gene expression related to lipid synthesis and trigger triglyceride accumulation in adult mouse liver. Hepatology. 2012;55(2):395–407. doi: 10.1002/hep.24685. [DOI] [PubMed] [Google Scholar]
- 9.Vabre P., Gatimel N., Moreau J., Gayrard V., Picard-Hagen N., Parinaud J., Leandri R.D. Environmental pollutants, a possible etiology for premature ovarian insufficiency: a narrative review of animal and human data. Environmental Health. 2017;16(1):37. doi: 10.1186/s12940-017-0242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopalli S.R., Cha K.M., Ryu J.H., Lee S.H., Jeong M.S., Hwang S.Y., Lee Y.J., Song H.W., Kim S.N., Kim J.C. Korean red ginseng improves testicular ineffectiveness in aging rats by modulating spermatogenesis-related molecules. Experimental Gerontology. 2017;90:26–33. doi: 10.1016/j.exger.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. Journal of Ginseng Research. 2015;39(4):287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song H., Lee Y.J. Inhibition of hypoxia-induced cyclooxygenase-2 by Korean red ginseng is dependent on peroxisome proliferator-activated receptor gamma. Journal of Ginseng Research. 2017;41(3):240–246. doi: 10.1016/j.jgr.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K. Effect of ginseng and ginsenosides on melanogenesis and their mechanism of action. Journal of Ginseng Research. 2015;39(1):1–6. doi: 10.1016/j.jgr.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee D.C., Lau A.S. Effects of Panax ginseng on tumor necrosis factor-α-mediated inflammation: a mini-review. Molecules. 2011;16(4):2802–2816. doi: 10.3390/molecules16042802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang S.W., Park J.H., Seok H., Park H.J., Chung J.H., Kim C.J., Kim Y.O., Han Y.R., Hong D., Kim Y.S. The effects of Korea red ginseng on inflammatory cytokines and apoptosis in rat model with chronic nonbacterial prostatitis. BioMed Research International. 2019 doi: 10.1155/2019/2462561. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H., Choi J., Shin S.S., Yoon M. Effects of Korean red ginseng (Panax ginseng) on obesity and adipose inflammation in ovariectomized mice. Journal of Ethnopharmacology. 2016;178:229–237. doi: 10.1016/j.jep.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Takemura H., Ma J., Sayama K., Terao Y., Zhu B.T., Shimoi K. In vitro and in vivo estrogenic activity of chlorinated derivatives of bisphenol A. Toxicology. 2005;207(2):215–221. doi: 10.1016/j.tox.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberzon A., Birger C., Thorvaldsdóttir H., Ghandi M., Mesirov J.P., Tamayo P. The molecular signatures database hallmark gene set collection. Cell Systems. 2015;1(6):417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.In G., Ahn N.G., Bae B.-S., Lee M.W., Park H.W., Jang K.H., Cho B.G., Han C.K., Park C.K., Kwak Y.S. In situ analysis of chemical components induced by steaming between fresh ginseng, steamed ginseng, and red ginseng. Journal of Ginseng Research. 2017;41(3):361–369. doi: 10.1016/j.jgr.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews J.B., Twomey K., Zacharewski T.R. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors α and β. Chemical Research in Toxicology. 2001;14(2):149–157. doi: 10.1021/tx0001833. [DOI] [PubMed] [Google Scholar]
- 22.An B.S., Kang S.K., Shin J.H., Jeung E.B. Stimulation of calbindin-D9k mRNA expression in the rat uterus by octyl-phenol, nonylphenol and bisphenol. Molecular and Cellular Endocrinology. 2002;191(2):177–186. doi: 10.1016/s0303-7207(02)00042-4. [DOI] [PubMed] [Google Scholar]
- 23.Jie D., Ying X., Xiaoping M., Jinna A., Xiudong Y., Zhiqiang L., Na L. Estrogenic effect of the extract of Renshen (Radix Ginseng) on reproductive tissues in immature mice. Journal of Traditional Chinese Medicine. 2015;35(4):460–467. doi: 10.1016/s0254-6272(15)30125-4. [DOI] [PubMed] [Google Scholar]
- 24.Lee J.H., Lee H.J., Yang M., Moon C., Kim J.C., Bae C.S., Jo S.K., Jang J.S., Kim S.H. Effect of Korean Red Ginseng on radiation-induced bone loss in C3H/HeN mice. Journal of Ginseng Research. 2013;37(4):435. doi: 10.5142/jgr.2013.37.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y., Ding J., An J.N., Qu Y.K., Li X., Ma X.P., Zhang Y.M., Dai G.J., Lin N. Effect of the interaction of veratrum nigrum with panax ginseng on estrogenic activity in vivo and in vitro. Scientific Reports. 2016;6:26924. doi: 10.1038/srep26924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim B.-B., Kim M., Park Y.H., Ko Y., Park J.B. Short-term application of dexamethasone on stem cells derived from human gingiva reduces the expression of RUNX2 and β-catenin. Journal of International Medical Research. 2017;45(3):993–1006. doi: 10.1177/0300060517701035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang D.W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols. 2009;4(1):44. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 28.Wang J., Cui C., Fu L., Xiao Z., Xie N., Liu Y., Yu L., Wang H., Luo B. Genomic expression profiling and bioinformatics analysis on diabetic nephrology with ginsenoside Rg3. Molecular Medicine Reports. 2016;14(2):1162–1172. doi: 10.3892/mmr.2016.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z., Gerstein M., Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nature Reviews Genetics. 2009;10(1):57. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang K., Jiang S., Sun C., Lin Y., Yin R., Wang Y., Zhang M. The spatial and temporal transcriptomic landscapes of ginseng, Panax ginseng CA Meyer. Scientific Reports. 2015;5:18283. doi: 10.1038/srep18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satoh Ji, Yamamoto Y., Asahina N., Kitano S., Kino Y. vol. 2014. Disease markers; 2014. (RNA-Seq data mining: downregulation of NeuroD6 serves as a possible biomarker for alzheimer’s disease brains). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang M., Lee H.S., Hwang M.W., Jin M. Effects of Korean red ginseng (Panax Ginseng Meyer) on bisphenol A exposure and gynecologic complaints: single blind, randomized clinical trial of efficacy and safety. BMC Complementary and Alternative Medicine. 2014;14(1):265. doi: 10.1186/1472-6882-14-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan M., Hu M., Lou Y., Wang Q., Mao L., Zhan Q., Jin F. Environmentally relevant levels of bisphenol A affect uterine decidualization and embryo implantation through the estrogen receptor/serum and glucocorticoid-regulated kinase 1/epithelial sodium ion channel α-subunit pathway in a mouse model. Fertility and Sterility. 2018;109(4):735–744. doi: 10.1016/j.fertnstert.2017.12.003. e1. [DOI] [PubMed] [Google Scholar]
- 34.Hong M., Lee Y.H., Kim S., Suk K.T., Bang C.S., Yoon J.H., Baik G.H., Kim D.J., Kim M.J. Anti-inflammatory and antifatigue effect of Korean Red Ginseng in patients with nonalcoholic fatty liver disease. Journal of Ginseng Research. 2016;40(3):203–210. doi: 10.1016/j.jgr.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee H.J., Cho S.H. Therapeutic effects of Korean red ginseng extract in a murine model of atopic dermatitis: anti-pruritic and anti-inflammatory mechanism. Journal of Korean Medical Science. 2017;32(4):679–687. doi: 10.3346/jkms.2017.32.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong C.E., Lyu S.Y. Anti-inflammatory and anti-oxidative effects of Korean red ginseng extract in human keratinocytes. Immune Network. 2011;11(1):42–49. doi: 10.4110/in.2011.11.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saba E., Jeong D., Irfan M., Lee Y.Y., Park S.J., Park C.K., Rhee M.H. Anti-inflammatory activity of Rg3-enriched Korean red ginseng extract in murine model of sepsis. Evidence-Based Complementary and Alternative Medicine. 2018;2018 doi: 10.1155/2018/6874692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song H., Park J., Choi K., Lee J., Chen J., Park H.J., Yu B.I., Iida M., Rhyu M.R., Lee Y. Ginsenoside Rf inhibits cyclooxygenase-2 induction via peroxisome proliferator–activated receptor gamma in A549 cells. Journal of Ginseng Research. 2019;43(2):319–325. doi: 10.1016/j.jgr.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]