Abstract
Korean ginseng (Panax ginseng) is associated with a variety of therapeutic effects, including antioxidative, anti-inflammatory, vasorelaxative, antiallergic, antidiabetic, and anticancer effects. Accordingly, the use of ginseng has reached an all-time high among members of the general public. However, the safety and efficacy of ginseng in transplant recipients receiving immunosuppressant drugs have still not been elucidated. Transplantation is the most challenging and complex of surgical procedures and may require causation for the use of ginseng. In this regard, we have previously examined the safety, immunological benefits, and protective mechanisms of ginseng with respect to calcineurin inhibitor-based immunosuppression, which is the most widely used regimen in organ transplantation. Using an experimental model of calcineurin inhibitor-induced organ injury, we found that ginseng does not affect drug levels in the peripheral blood and tissue, favorably regulates immune response, and protects against calcineurin inhibitor-induced nephrotoxicity and pancreatic islet injury. On the basis of our experimental studies and a review of the related literature, we propose that ginseng may provide benefits in organ transplant recipients administered calcineurin inhibitors. Through the present review, we aimed to briefly discuss our current understanding of the therapeutic benefits of ginseng related to transplant patient survival.
Keywords: cyclosporine A, calcineurin inhibitor, ginseng, transplantation, tacrolimus
1. Introduction
Korean ginseng (Panax ginseng Meyer) has been spent widely as a medicinal and general health supplement for a diversity of diseases worldwide [1,2]. It is one of the most highly regarded herbs in the Orient, where it is used to promote health and general body vigor and prolong life span. The generic name “Panax” originates from the Greek word “panacea,” which denotes “cure all diseases,” and true to its name, ginseng has been certified to have wide medicinal uses, including beneficial effects in diabetes, hypertension, inflammatory diseases, and cancer [[3], [4], [5]], and as an immune modulator [[6], [7], [8], [9]]. Nevertheless, although the therapeutic potential of ginseng has been studied extensively, its effects in transplant recipients taking immunosuppressant drugs remain undetermined.
With respect to transplantation, a calcineurin inhibitor (CNI)-based immunosuppression with cyclosporin A (CsA) and tacrolimus (Tac) is the most widely used regimen in current clinical practice, because this regimen can markedly decrease acute rejection rates and provides excellent early outcomes. However, calcineurin [[10], [11], [12]] lacks T cell specificity, and in addition to its immunosuppressive effects, inhibition by CNIs can induce toxicity [13]. Indeed, the notable side effects associated with the administration of CNIs can hinder the survival of long-term kidney graft and patient, and cause major additional morbidity [14]. This is the case not only in kidney transplantation but also with respect to the transplantation of nonrenal solid organs and in many other diseases requiring therapy with these drugs [15]. Thus, both the nephrotoxic and general toxic effects of CNIs are a major concern in clinicians, pathologists, and scientists [[16], [17], [18]].
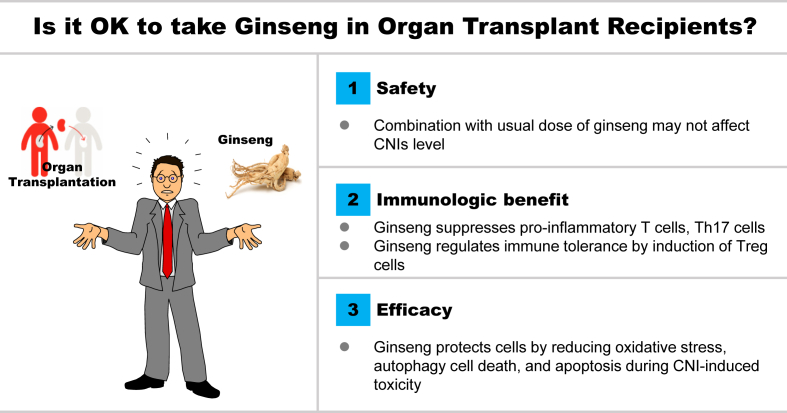
In recent years, ginseng has been shown to have attractive beneficial effects in transplantation-related animal models, such as those for ischemia-reperfusion injury, CsA-induced renal fibrosis, and diabetes mellitus [[19], [20], [21], [22]]. In this paper, we review the literature on ginseng studies that have focused on experimental disease modeling related to the use of CNIs in transplantation. We summarize the safety, immunological benefits, and efficacy of ginseng with respect to the underlying molecular and cellular mechanisms (Fig. 1). This review provides a succinct assessment of our current understanding of the pharmacological benefits of ginseng that conduce to graft survival.
Fig. 1.
Conceptual summary of experimental studies using ginseng and calcineurin inhibitors. CNI, calcineurin inhibitor; Th17, T helper 17 cell; Treg, regulatory T cells.
2. Safety aspects
Transplant patients are generally placed on a regimen of two to three immunosuppressive drugs to prevent graft rejection, along with a wide range of additional drugs for the treatment of other underlying diseases, and to counteract the side effects of CNI treatment. However, as the number of medications patients consume increases, there is a concomitant increase in the likelihood of potentially detrimental drug interactions, and indeed, such interactions have been reported in the literature since the beginning of CsA utilization (1986: Erythromycin; early 1990s; Ketoconazole; 1993 grapefruit interaction). Polypharmacy is an accepted part of a transplant patient’s everyday regimen. Furthermore, in addition to the inevitability of drug interactions in transplant patients, the main concern when using CNIs is their relatively narrow therapeutic windows.
Two main mechanisms that affect the maintenance of therapeutic CNI levels are the activities of the drug-metabolizing enzyme cytochrome P-450 3A4 (CYP3A4) and the drug efflux pump P-glycoprotein (P-gp) [23,24]. Most transplant physicians are well aware of the fact that, by interfering with the activities of CYP3A4 and P-gp, some conventional medications may affect the pharmacokinetic profile of CNIs. To date, however, the question of whether ginseng can elicit similar effects on CNIs remains unanswered.
Ginsenosides, which are the active components of ginseng, are widely agreed to be responsible for both the pharmacological activity and drug interaction potential of ginseng. Although preclinical papers have evaluated the influence of various ginsenosides on CYP, glucuronidation, and drug transport activity, the results obtained in these investigations have been mostly inconclusive, due to differences in the ginseng products used and the adopted methodologies. Moreover, the findings of drug interaction studies in humans have tended to be inconsistent and have largely yielded results that are either negative or suggestive of only weak interactions [25].
We have previously reported on the beneficial effect of red ginseng in chronic CsA-induced renal injury in mice [19]. In humans, 50 ~ 100 mg/kg of ginseng is the manufacturer’s recommended dose, but this dose may differ from that needed for protective action in an experimental mouse model of CsA nephropathy. Therefore, as a preliminary study, we determined the optimal dose of red ginseng in our mouse model. We subsequently examined the effects of three different doses of red ginseng (200, 400, and 800 mg/kg), and 200 and 400 mg/kg of ginseng showed beneficial effects without changing the CsA level, while the highest dose (800 mg/kg) was found to increase the CsA level in the blood and kidney tissues compared with treatment with CsA only. Furthermore, the highest dose of ginseng enhanced the expression of markers for oxidative stress and the level of fibrosis compared with the CsA only and CsA plus 200 or 400 mg/kg red ginseng-treated groups. From this study, we found the protective dose of ginseng in an experimental mouse model of CsA nephropathy. In regards to safety, importantly, we can conclude that the use of large doses of ginseng with CsA increases the side effects of CsA by increasing the CsA levels in the blood and kidneys.
Because little research has been done on drug interactions between CNIs and ginseng, we predicted the drug interactions by searching the literature for known substrates and inhibitors of metabolic enzymes. Other studies using human liver microsomes have shown that Rd is generally able to interact with drugs that are metabolized by CYP2C9 and CYP3A4, whereas CYP2C9 and CYP3A4 are suppressed by Rb1, Rb2, Rc, and Rd [26]. In contrast, clinical studies involving healthy volunteers (who received 25 mg/kg/day of ginseng) found that ginseng does not affect a number of CYP isoforms, including CYP3A4, CYP1A2, CYP2E1, and CYP2D6, although a slight inhibition of CYP2D6 has been demonstrated in elderly individuals [27,28]. There has been no established interaction between ginseng and CNIs, but there is sufficient possibility based on the literature. In spite of the generally favorable findings for ginseng, the possibility that the combination of ginseng and CNIs may cause adverse effects in tissues cannot be ruled out. Therefore, the evidence indicates that ginseng can be considered for use in renal transplant recipients taking CNIs, but patients should be carefully monitored for possible adverse reactions [19].
3. Immunological benefits
Subsequent to kidney transplantation, the alloimmune responses induced by the activation of CD4+ T cells mediate most of the allograft rejections [29]. Therefore, most immunosuppressants have been originated to suppress the activation and differentiation of effector CD4+ T cells to prevent the induction of the alloimmune responses [30,31]. In current clinical practice, the first choice immunosuppressants are CNIs (particularly Tac), and 99.1% of immunosuppressant protocols are CNI-based [32]. This is because CNIs have been shown to be more effective in inhibiting the induction of acute rejection episodes and improving 1-year allograft survival than the azathioprine and steroids [33,34].
In contrast, however, the use of CNIs has not significantly improved long-term allograft survival when compared with that achieved in the azathioprine era [14,35]. Chung et al [32] reported that Tac suppresses T helper type (Th) 1, Th2, and regulatory T (Treg) cells in a Tac level-dependent manner, although it does not inhibit Th17 cells, even at high concentrations. Accordingly, current Tac-based immunosuppression is inadequate for the suppression of Th17 cells in kidney transplant patients, and thus, dysregulation of Th17 and Tregs may be related to the development of chronic allograft dysfunction.
Ginseng is well known as an immune modulator [[6], [7], [8], [9]], and the roots (primarily), stems, and leaves of ginseng, along with their various extracts, have been used for keeping immune homeostasis and promoting resistance to illness or microbial infection via immune system effects. Ginseng has recently received attention as a potential therapy for the prevention of autoimmune immune disease [[36], [37], [38], [39]]. In this respect, ginseng is known to promote the generation of immunosuppressive Tregs through the activation of the transcription factor forkhead box protein P3 and has been demonstrated to have a favorable effect on a mouse model of autoimmune encephalomyelitis and multiple sclerosis. Interestingly, Jhun et al [40] have examined that ginseng could attenuate arthritis in an experimental mice model of collagen-induced arthritis through suppression of Th17 differentiation via preventing the phosphorylation of signal transducer and activator of transcription (STAT) 3 and reciprocally increasing Treg populations.
However, little is currently known with respect to how the immunological functions of ginseng influence the status of transplant patients taking immunosuppressants. In this regard, Heo et al [21] investigated the influence of cotreatment of ginseng with CsA on a CD4+ T cells population and the ability of these cells to provoke cytokines after stimulation with alloantigen or T cell receptor, which served to mimic the conditions characterizing rejection. They found, however, that the cotreatment with red ginseng and CsA had no significant effect on the differentiation of naïve T cells to Th1 or Th2 cells or their ability to produce related cytokines.
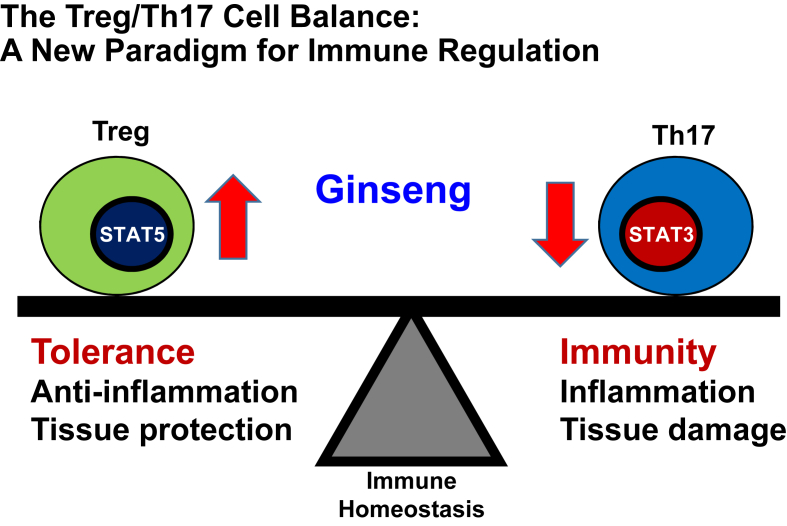
The same authors also examined the reciprocal regulation of Th17 cells and Tregs, whereby the relative activation of STAT3 and STAT5 directly dictates the outcome of IL-17 production in CD4+ T cells [41] during treatment with CsA and red ginseng. Interestingly, they found that the population of Th17 cell and STAT3, which is its transcription factor, were dramatically decreased by red ginseng and that the CsA-induced inhibition of Treg differentiation was significantly recovered by red ginseng.
These results thus provide evidence that supplementation with ginseng can confer a degree of immunological safety in CNI-based immunosuppression, and that ginseng plays as an immune regulator via the reciprocal regulation of Th17 and Treg cells (Fig. 2). These findings accordingly suggest a rationale for the treatment of ginseng in transplant and immune disorder patients taking CNIs.
Fig. 2.
Schematic diagram summarizing the predicted mechanisms whereby ginseng influences immune regulation. Ginseng has immunological benefits via the reciprocal regulation of Th17 and Treg cells during cyclosporin A-induced immunosuppression. Th17, T helper 17 cell; Treg, regulatory T cells.
4. The protective mechanism of ginseng
Chronic allograft injury, the main cause of kidney transplant failure, is a frequent occurrence and often results in the need for dialysis. Renal allograft failure is the most common reason for end-stage kidney disease and is a factor associated with 25% to 30% of patients waiting for kidney transplants [42]. CNI nephrotoxicity is a major contributor to chronic allograft injury, and almost all renal transplant recipients exhibit chronic CsA-induced nephropathy within10 years of the commencement of CNI treatment [43]. The use of CNIs in kidney transplant recipients is also associated with higher incidences of hypertension and dyslipidemia and an increased risk of cardiovascular events [16,44]. Overall, CNI-related complications can lead to poor long-term outcomes in kidney transplant recipients.
Oxidative stress is a common pathway underlying chronic CNI-induced nephropathy [[45], [46], [47]]. High levels of oxidative stress induced by chronic CsA administration have been demonstrated to cause structural and functional kidney impairment via the induction of free radical species in the kidney, which, in turn, may promote apoptotic and autophagic cell death [[48], [49], [50]]. Given that oxidative stress can be an important contributor to the pathogenesis of chronic diseases, the ability of ginseng and its constituent ginsenosides to improve disease symptoms via antioxidant mechanisms is of particular interest. However, the influence of ginseng on chronic CNI-induced oxidative renal injury has yet to be studied in depth. In this review, we describe the few studies that have investigated the effects of ginseng on chronic CNI-induced renal injury in general.
In a study using an experimental mouse model and a cell culture system, Doh et al [19] investigated the effect of red ginseng on chronic CsA-induced nephropathy. This study demonstrated the protective effect of red ginseng against CsA-induced renal injury in mice and treatment was associated with decreased blood urea nitrogen, interstitial inflammation, fibrosis, and apoptosis. Moreover, in vitro and in vivo results revealed that the antioxidant effect of ginseng is associated with a hold in the progression of chronic CsA-induced nephropathy. These findings provided the first evidence of a protective effect of ginseng against CsA-induced renal injury.
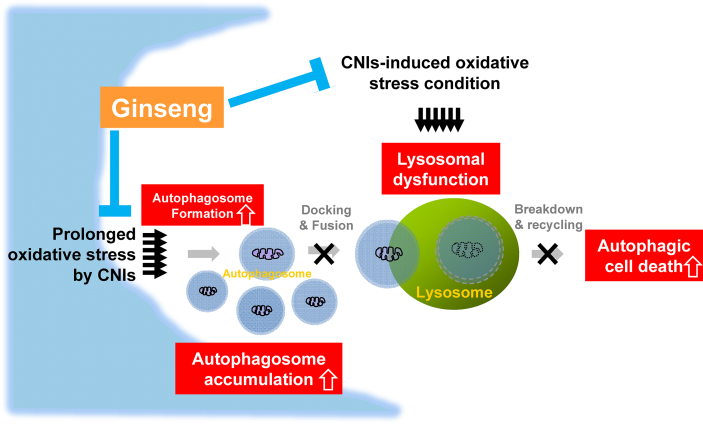
Lim et al, performed further experiments to elucidate the mechanism of protection of ginseng for damage caused by CNI treatment. It is known that prolonged oxidative stress caused by CNI treatment causes excessive autophagosome formation, as an adaptation to stress, as well as lysosomal dysfunction [49,51] (Fig. 3). The excessive autophagosomes resulting from CNI treatment are not effectively degraded due to the impaired lysosomal function (autophagosome fusion with lysosomes and lysosomal degradation). The resulting accumulated autophagosomes in cells lead to autophagic cell death [52]. Under these circumstances, in vivo and in vitro studies showed that ginseng treatment attenuated excess CsA-induced autophagosome formation as measured by autophagosome markers (a phospholipid-conjugated form of microtubule-associated protein light chain 3 and beclin-1) and protected lysosome function [51]. These results showed that the antioxidative effect of ginseng might contribute to reducing CNI-induced autophagic cell death (Fig. 3).
Fig. 3.
Schematic diagram summarizing the predicted mechanism whereby ginseng influences autophagic clearance function. Prolonged oxidative stress induced by tacrolimus augments autophagosome formation as an adaptation to stress and lysosomal dysfunction. However, the autophagosomes are not effectively degraded due to impaired autophagic clearance (lysosomal degradation and autophagosome fusion with lysosomes). The resulting excess production of autophagosomes leads to autophagic cell death. Under these circumstances, ginseng treatment improves the autophagic clearance function by enhancing lysosomal function and autophagosome fusion with lysosomes. CNI, calcineurin inhibitor.
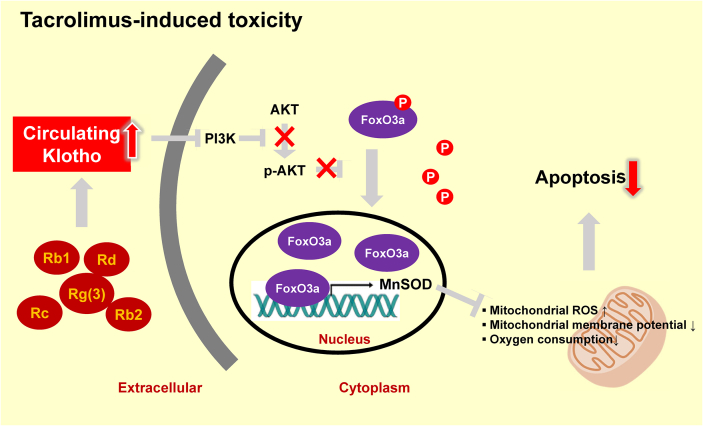
Abundant evidence indicates that oxidative stress also plays a central role in the process of biological aging [53]. In this regard, Klotho has recently been identified as an antiaging gene that is expressed primarily in the kidneys [54,55]. A reduction in the expression of this gene induces an accelerated aging-like syndrome in mice, whereas overexpression extends the life span of these animals [[55], [56], [57]]. To determine whether Klotho is implicated in the mechanism underlying the antiaging effect of ginseng, Lim et al [58] investigated whether ginseng upregulates Klotho and its antiaging signaling in response to Tac-induced oxidative stress. Ginseng was indeed found to reverse the Tac-induced reduction in Klotho levels in the mouse serum and kidney tissues. Tac-induced oxidative stress in an experimental mouse was thus reduced by ginseng treatment, with concomitant functional and histological improvements. In addition, in vivo and in vitro studies revealed that the Tac-induced reduction in Klotho levels promoted the PI3K/AKT-mediated phosphorylation of forkhead box O3a (FoxO3a), which appeared to be present in an inactive form in the cytoplasm. However, restoration of Klotho levels by ginseng induced the nuclear translocation of FoxO3a via the suppression of PI3K/AKT activity and an increase in manganese-dependent superoxide dismutase levels in HK-2 cells. Based on the proposed mechanism, ginseng maintained Klotho expression, thereby protecting against the oxidative damage and apoptotic cell death associated with Tac-induced toxicity. These results, therefore, provided evidence of the protective mechanism of ginseng via the antiaging gene Klotho in response to oxidative stress injury (Fig. 4).
Fig. 4.
Schematic diagram summarizing the predicted mechanism whereby ginseng influences regulation of the antiaging gene Klotho. PI3K/AKT-mediated phosphorylation of FoxO3a is induced by reduced Klotho expression in response to tacrolimus treatment. Under these conditions, FoxO3a appears to be maintained in an inactive form in the cytoplasm. However, the restoration of Klotho levels in response to ginseng treatment induces the nuclear translocation of FoxO3a via the suppression of PI3K/AKT activity and an increase in the levels of MnSOD. By maintaining Klotho expression, ginseng may prevent tacrolimus-induced oxidative damage and apoptotic cell death. These findings provide evidence for the protection mechanism of ginseng via the antiaging gene Klotho against a background of oxidative stress-associated injury. FoxO3a, forkhead box O3; P, phosphorylation; MnSOD, manganese-dependent superoxide dismutase.
As mentioned above, although CNIs are widely used as maintenance immunosuppressants in renal transplant recipients, they can be the cause of notable metabolic abnormalities. In particular, new-onset diabetes after transplantation occurs in 10–25% of the patients taking Tac [59,60], thereby reducing graft survival and increasing the risk of infectious and cardiovascular diseases [61]. Although the pathogenesis of Tac-induced diabetes mellitus is currently unclear, our group and others have demonstrated that the direct toxic effects of Tac on pancreatic beta cells, together with oxidative stress, play key roles in the development of this disease [52,[62], [63], [64]]. Lim et al [20,51] showed that ginseng can alleviate islet dysfunction and decrease oxidative stress and autophagic vacuoles in both CsA- and Tac-induced pancreatic beta-cell injury models. Co-treatment with ginseng decreased autophagosome formation and attenuated lysosomal degradation, accompanied by improved beta-cell viability and insulin secretion in an experimental mouse model and INS-1 cells. Using INS-1 cells, Tac treatment was found to cause mitochondria dysfunction mitochondria (impaired mitochondrial oxygen consumption and ATP production, and increased reactive oxygen species production), whereas ginseng treatment was observed to improve these parameters. These findings thus indicate that ginseng has a favorable modulatory effect on autophagy by reducing the pancreatic beta-cell injury promoted by Tac-induced oxidative stress and that this effect is closely related to the amelioration of mitochondrial function. Accordingly, these results provide evidence for the beneficial effects of ginseng against CNI-induced diabetes mellitus in solid organ transplant recipients.
5. Conclusion
Although the ginseng market is currently undergoing a phase of rapid expansion, the medicinal effects of this plant are still essentially being revealed based on experience. Transplantation is the most challenging and complex of surgical procedures and may require causation for the use of ginseng. Our motivation for reviewing the current literature on the transplantation-related utility of ginseng was to provide practical guidelines for the use of ginseng in transplant patients taking immunosuppressants (Fig. 1). In this regard, using an experimental model of CNI-induced organ injury, we had previously demonstrated that ginseng does not affect drug levels in the peripheral blood and tissue, favorably regulates immune response, and protects against CNI-induced nephrotoxicity and pancreatic islet injury. Similarly, most of the studies discussed in the present review have been based on experimental animal models and/or cell lines. In contrast, few comparable studies have been undertaken using human subjects, despite the fact that ginseng products are widely believed and agreed to contain therapeutic effectiveness when used alone or in a mix with CNIs in the control of graft rejection. Nevertheless, the animal-based experimental studies do provide convincing evidence that ginseng has significant potential for use in patients with immunosuppressant-associated nephropathy and diabetes.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This work was supported by a 2017 grant from the Korean Society of Ginseng funded by the Korea Ginseng Corporation, the Korean Health Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (HI14C3417), and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1A02043014).
References
- 1.Colzani M., Altomare A., Caliendo M., Aldini G., Righetti P.G., Fasoli E. The secrets of Oriental panacea: panax ginseng. J Proteomics. 2016;130:150–159. doi: 10.1016/j.jprot.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Rastogi V., Santiago-Moreno J., Dore S. Ginseng: a promising neuroprotective strategy in stroke. Front Cell Neurosci. 2014;8:457. doi: 10.3389/fncel.2014.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karmazyn M., Moey M., Gan X.T. Therapeutic potential of ginseng in the management of cardiovascular disorders. Drugs. 2011;71:1989–2008. doi: 10.2165/11594300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y., Guo M., Feng Y., Zheng H., Lei P., Ma X., Han X., Guan H., Hou D. Effect of ginseng polysaccharides on NK cell cytotoxicity in immunosuppressed mice. Exp Ther Med. 2016;12:3773–3777. doi: 10.3892/etm.2016.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim Y.R., Yang C.S. Protective roles of ginseng against bacterial infection. Microb Cell. 2018;5:472–481. doi: 10.15698/mic2018.11.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jie Y.H., Cammisuli S., Baggiolini M. Immunomodulatory effects of panax Ginseng C.A. Meyer in the mouse. Agents Actions. 1984;15:386–391. doi: 10.1007/BF01972376. [DOI] [PubMed] [Google Scholar]
- 7.Scaglione F., Ferrara F., Dugnani S., Falchi M., Santoro G., Fraschini F. Immunomodulatory effects of two extracts of Panax ginseng C.A. Meyer. Drugs Exp Clin Res. 1990;16:537–542. [PubMed] [Google Scholar]
- 8.Choi K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 9.Kitts D., Hu C. Efficacy and safety of ginseng. Public Health Nutr. 2000;3:473–485. doi: 10.1017/s1368980000000550. [DOI] [PubMed] [Google Scholar]
- 10.Su Q., Weber L., Le Hir M., Zenke G., Ryffel B. Nephrotoxicity of cyclosporin A and FK506: inhibition of calcineurin phosphatase. Ren Physiol Biochem. 1995;18:128–139. doi: 10.1159/000173910. [DOI] [PubMed] [Google Scholar]
- 11.Halloran P.F., Helms L.M., Kung L., Noujaim J. The temporal profile of calcineurin inhibition by cyclosporine in vivo. Transplantation. 1999;68:1356–1361. doi: 10.1097/00007890-199911150-00023. [DOI] [PubMed] [Google Scholar]
- 12.Kung L., Batiuk T.D., Palomo-Pinon S., Noujaim J., Helms L.M., Halloran P.F. Tissue distribution of calcineurin and its sensitivity to inhibition by cyclosporine. Am J Transplant. 2001;1:325–333. doi: 10.1034/j.1600-6143.2001.10407.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu E.H., Siegel R.M., Harlan D.M., O'Shea J.J. T cell-directed therapies: lessons learned and future prospects. Nat Immunol. 2007;8:25–30. doi: 10.1038/ni1429. [DOI] [PubMed] [Google Scholar]
- 14.Meier-Kriesche H.U., Schold J.D., Srinivas T.R., Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–383. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 15.Ojo A.O., Held P.J., Port F.K., Wolfe R.A., Leichtman A.B., Young E.W., Arndorfer J., Christensen L., Merion R.M. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 16.Olyaei A.J., de Mattos A.M., Bennett W.M. Nephrotoxicity of immunosuppressive drugs: new insight and preventive strategies. Curr Opin Crit Care. 2001;7:384–389. doi: 10.1097/00075198-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Palestine A.G., Austin H.A., 3rd, Nussenblatt R.B. Renal tubular function in cyclosporine-treated patients. Am J Med. 1986;81:419–424. doi: 10.1016/0002-9343(86)90292-5. [DOI] [PubMed] [Google Scholar]
- 18.Myers B.D., Ross J., Newton L., Luetscher J., Perlroth M. Cyclosporine-associated chronic nephropathy. N Engl J Med. 1984;311:699–705. doi: 10.1056/NEJM198409133111103. [DOI] [PubMed] [Google Scholar]
- 19.Doh K.C., Lim S.W., Piao S.G., Jin L., Heo S.B., Zheng Y.F., Bae S.K., Hwang G.H., Min K.I., Chung B.H. Ginseng treatment attenuates chronic cyclosporine nephropathy via reducing oxidative stress in an experimental mouse model. Am J Nephrol. 2013;37:421–433. doi: 10.1159/000349921. [DOI] [PubMed] [Google Scholar]
- 20.Lim S.W., Doh K.C., Jin L., Piao S.G., Heo S.B., Zheng Y.F., Bae S.K., Chung B.H., Yang C.W. Oral administration of ginseng ameliorates cyclosporine-induced pancreatic injury in an experimental mouse model. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heo S.B., Lim S.W., Jhun J.Y., Cho M.L., Chung B.H., Yang C.W. Immunological benefits by ginseng through reciprocal regulation of Th17 and Treg cells during cyclosporine-induced immunosuppression. J Ginseng Res. 2016;40:18–27. doi: 10.1016/j.jgr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim S.W., Doh K.C., Jin L., Jin J., Piao S.G., Heo S.B., Chung B.H., Yang C.W. Ginseng treatment attenuates autophagic cell death in chronic cyclosporine nephropathy. Nephrology (Carlton) 2014;19:490–499. doi: 10.1111/nep.12273. [DOI] [PubMed] [Google Scholar]
- 23.Seifeldin R. Drug interactions in transplantation. Clin Ther. 1995;17:1043–1061. doi: 10.1016/0149-2918(95)80084-0. [DOI] [PubMed] [Google Scholar]
- 24.Saeki T., Ueda K., Tanigawara Y., Hori R., Komano T. Human P-glycoprotein transports cyclosporin A and FK506. J Biol Chem. 1993;268:6077–6080. [PubMed] [Google Scholar]
- 25.Ramanathan M.R., Penzak S.R. Pharmacokinetic drug interactions with panax ginseng. Eur J Drug Metab Pharmacokinet. 2017;42:545–557. doi: 10.1007/s13318-016-0387-5. [DOI] [PubMed] [Google Scholar]
- 26.He N., Edeki T. The inhibitory effects of herbal components on CYP2C9 and CYP3A4 catalytic activities in human liver microsomes. Am J Ther. 2004;11:206–212. doi: 10.1097/00045391-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Gurley B.J., Gardner S.F., Hubbard M.A., Williams D.K., Gentry W.B., Cui Y., Ang C.Y. Clinical assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly: St John's wort, garlic oil, Panax ginseng and Ginkgo biloba. Drugs Aging. 2005;22:525–539. doi: 10.2165/00002512-200522060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurley B.J., Gardner S.F., Hubbard M.A., Williams D.K., Gentry W.B., Cui Y., Ang C.Y. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin Pharmacol Ther. 2002;72:276–287. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- 29.Calvo-Turrubiartes M., Romano-Moreno S., Garcia-Hernandez M., Chevaile-Ramos J.A., Layseca-Espinosa E., Gonzalez-Amaro R., Portales-Perez D. Quantitative analysis of regulatory T cells in kidney graft recipients: a relationship with calcineurin inhibitor level. Transpl Immunol. 2009;21:43–49. doi: 10.1016/j.trim.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Warrens A.N. Pharmacological control of the immune response in renal transplantation. BJU Int. 2002;90:784–791. doi: 10.1046/j.1464-410x.2002.03006.x. [DOI] [PubMed] [Google Scholar]
- 31.Halloran P.F. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 32.Chang J.Y., Yu J., Chung B.H., Yang J., Kim S.J., Kim C.D., Lee S.H., Lee J.S., Kim J.K., Jung C.W. Immunosuppressant prescription pattern and trend in kidney transplantation: a multicenter study in Korea. PLoS One. 2017;12 doi: 10.1371/journal.pone.0183826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hariharan S., Alexander J.W., Schroeder T.J., First M.R. Outcome of cadaveric renal transplantation by induction treatment in the cyclosporine era. Clin Transplant. 1996;10:186–190. [PubMed] [Google Scholar]
- 34.Hariharan S., McBride M.A., Cherikh W.S., Tolleris C.B., Bresnahan B.A., Johnson C.P. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002;62:311–318. doi: 10.1046/j.1523-1755.2002.00424.x. [DOI] [PubMed] [Google Scholar]
- 35.Pascual M., Theruvath T., Kawai T., Tolkoff-Rubin N., Cosimi A.B. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002;346:580–590. doi: 10.1056/NEJMra011295. [DOI] [PubMed] [Google Scholar]
- 36.Hwang I., Ahn G., Park E., Ha D., Song J.Y., Jee Y. An acidic polysaccharide of Panax ginseng ameliorates experimental autoimmune encephalomyelitis and induces regulatory T cells. Immunol Lett. 2011;138:169–178. doi: 10.1016/j.imlet.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Hwang I., Ha D., Ahn G., Park E., Joo H., Jee Y. Experimental autoimmune encephalomyelitis: association with mutual regulation of RelA (p65)/NF-kappaB and phospho-IkappaB in the CNS. Biochem Biophys Res Commun. 2011;411:464–470. doi: 10.1016/j.bbrc.2011.06.195. [DOI] [PubMed] [Google Scholar]
- 38.Oh G.N., Son S.W. Efficacy of Korean red ginseng in the treatment of alopecia areata. J Ginseng Res. 2012;36:391–395. doi: 10.5142/jgr.2012.36.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu D., Liu M., Yang Y., Ma L., Jiang Y., Zhou L., Huang Q., Pi R., Chen X. Ginsenoside Rd ameliorates experimental autoimmune encephalomyelitis in C57BL/6 mice. J Neurosci Res. 2014;92:1217–1226. doi: 10.1002/jnr.23397. [DOI] [PubMed] [Google Scholar]
- 40.Jhun J., Lee J., Byun J.K., Kim E.K., Woo J.W., Lee J.H., Kwok S.K., Ju J.H., Park K.S., Kim H.Y. Red ginseng extract ameliorates autoimmune arthritis via regulation of STAT3 pathway, Th17/Treg balance, and osteoclastogenesis in mice and human. Mediators Inflamm. 2014;2014:351856. doi: 10.1155/2014/351856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X.P., Ghoreschi K., Steward-Tharp S.M., Rodriguez-Canales J., Zhu J., Grainger J.R., Hirahara K., Sun H.W., Wei L., Vahedi G. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Womer K.L., Vella J.P., Sayegh M.H. Chronic allograft dysfunction: mechanisms and new approaches to therapy. Semin Nephrol. 2000;20:126–147. [PubMed] [Google Scholar]
- 43.Nankivell B.J., Borrows R.J., Fung C.L., O'Connell P.J., Allen R.D., Chapman J.R. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 44.de Mattos A.M., Olyaei A.J., Bennett W.M. Nephrotoxicity of immunosuppressive drugs: long-term consequences and challenges for the future. Am J Kidney Dis. 2000;35:333–346. doi: 10.1016/s0272-6386(00)70348-9. [DOI] [PubMed] [Google Scholar]
- 45.Yoon H.E., Yang C.W. Established and newly proposed mechanisms of chronic cyclosporine nephropathy. Korean J Intern Med. 2009;24:81–92. doi: 10.3904/kjim.2009.24.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C., Lim S.W., Sun B.K., Yang C.W. Chronic cyclosporine nephrotoxicity: new insights and preventive strategies. Yonsei Med J. 2004;45:1004–1016. doi: 10.3349/ymj.2004.45.6.1004. [DOI] [PubMed] [Google Scholar]
- 47.Li C., Yang C.W. The pathogenesis and treatment of chronic allograft nephropathy. Nat Rev Nephrol. 2009;5:513–519. doi: 10.1038/nrneph.2009.113. [DOI] [PubMed] [Google Scholar]
- 48.Ghee J.Y., Han D.H., Song H.K., Kim W.Y., Kim S.H., Yoon H.E., Choi B.S., Kim Y.S., Kim J., Yang C.W. The role of macrophage in the pathogenesis of chronic cyclosporine-induced nephropathy. Nephrol Dial Transplant. 2008;23:4061–4069. doi: 10.1093/ndt/gfn388. [DOI] [PubMed] [Google Scholar]
- 49.Lim S.W., Hyoung B.J., Piao S.G., Doh K.C., Chung B.H., Yang C.W. Chronic cyclosporine nephropathy is characterized by excessive autophagosome formation and decreased autophagic clearance. Transplantation. 2012;94:218–225. doi: 10.1097/TP.0b013e31825ace5c. [DOI] [PubMed] [Google Scholar]
- 50.Yang C.W., Faulkner G.R., Wahba I.M., Christianson T.A., Bagby G.C., Jin D.C., Abboud H.E., Andoh T.F., Bennett W.M. Expression of apoptosis-related genes in chronic cyclosporine nephrotoxicity in mice. Am J Transplant. 2002;2:391–399. doi: 10.1034/j.1600-6143.2002.20501.x. [DOI] [PubMed] [Google Scholar]
- 51.Lim S.W., Jin L., Luo K., Jin J., Yang C.W. Ginseng extract reduces tacrolimus-induced oxidative stress by modulating autophagy in pancreatic beta cells. Lab Invest. 2017;97:1271–1281. doi: 10.1038/labinvest.2017.75. [DOI] [PubMed] [Google Scholar]
- 52.Lim S.W., Jin L., Jin J., Yang C.W. Effect of Exendin-4 on autophagy clearance in beta cell of rats with tacrolimus-induced diabetes mellitus. Sci Rep. 2016;6:29921. doi: 10.1038/srep29921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muller F.L., Lustgarten M.S., Jang Y., Richardson A., Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y., Sun Z. Current understanding of klotho. Ageing Res Rev. 2009;8:43–51. doi: 10.1016/j.arr.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T., Ohyama Y., Kurabayashi M., Kaname T., Kume E. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 56.Masuda H., Chikuda H., Suga T., Kawaguchi H., Kuro-o M. Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev. 2005;126:1274–1283. doi: 10.1016/j.mad.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Kurosu H., Yamamoto M., Clark J.D., Pastor J.V., Nandi A., Gurnani P., McGuinness O.P., Chikuda H., Yamaguchi M., Kawaguchi H. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim S.W., Shin Y.J., Luo K., Quan Y., Cui S., Ko E.J., Chung B.H., Yang C.W. Ginseng increases Klotho expression by FoxO3-mediated manganese superoxide dismutase in a mouse model of tacrolimus-induced renal injury. Aging. 2019;11 doi: 10.18632/aging.102137. Albany NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharif A. Preventing and managing hyperglycemia in kidney transplant patients. Curr Opin Nephrol Hypertens. 2012;21:574–579. doi: 10.1097/MNH.0b013e328358d5d0. [DOI] [PubMed] [Google Scholar]
- 60.Valderhaug T.G., Hjelmesaeth J., Hartmann A., Roislien J., Bergrem H.A., Leivestad T., Line P.D., Jenssen T. The association of early post-transplant glucose levels with long-term mortality. Diabetologia. 2011;54:1341–1349. doi: 10.1007/s00125-011-2105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tufton N., Ahmad S., Rolfe C., Rajkariar R., Byrne C., Chowdhury T.A. New-onset diabetes after renal transplantation. Diabet Med. 2014;31:1284–1292. doi: 10.1111/dme.12534. [DOI] [PubMed] [Google Scholar]
- 62.Piao S.G., Lim S.W., Doh K.C., Jin L., Heo S.B., Zheng Y.F., Bae S.K., Chung B.H., Li C., Yang C.W. Combined treatment of tacrolimus and everolimus increases oxidative stress by pharmacological interactions. Transplantation. 2014;98:22–28. doi: 10.1097/TP.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 63.Jin J., Lim S.W., Jin L., Yu J.H., Kim H.S., Chung B.H., Yang C.W. Effects of metformin on hyperglycemia in an experimental model of tacrolimus- and sirolimus-induced diabetic rats. Korean J Intern Med. 2017;32:314–322. doi: 10.3904/kjim.2015.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin J., Jin L., Luo K., Lim S.W., Chung B.H., Yang C.W. Effect of empagliflozin on tacrolimus-induced pancreas islet dysfunction and renal injury. Am J Transplant. 2017;17:2601–2616. doi: 10.1111/ajt.14316. [DOI] [PubMed] [Google Scholar]