Abstract
Tissue fibrosis is an eventual pathologic change of numerous chronic illnesses, which is characterized by resident fibroblasts differentiation into myofibroblasts during inflammation, coupled with excessive extracellular matrix deposition in tissues, ultimately leading to failure of normal organ function. Now, there are many mechanistic insights into the pathogenesis of tissue fibrosis, which facilitate the discovery of effective antifibrotic drugs. Moreover, many chronic diseases remain a significant clinical unmet need. For the past five years, many research works have undoubtedly addressed the functional dependency of ginsenosides in different types of fibrosis and the successful remission in various animal models treated with ginsenosides. Caveolin-1, interleukin, thrombospondin-1 (TSP-1), liver X receptors (LXRs), Nrf2, microRNA-27b, PPARδ-STAT3, liver kinase B1 (LKB1)-AMPK, and TGF-β1/Smads are potential therapy targeting using ginsenosides. Ginsenosides can play a targeting role and suppress chronic inflammatory response, collagen deposition, and epithelial–mesenchymal transition (EMT), as well as myofibroblast activation to attenuate fibrosis. In this report, our aim was to focus on the therapeutic prospects of ginsenosides in fibrosis-related human diseases making use of results acquired from various animal models. These findings should provide important therapeutic clues and strategies for the exploration of new drugs for fibrosis treatment.
Keywords: Chinese herb medicines, Fibrosis, Ginseng, Ginsenosides, Therapeutics
1. Introduction
1.1. The mechanisms of fibrosis in tissues
Fibrosis is a familiar character of numerous organ-targeted diseases and is one of the main causes of mortality and morbidity in countless patients worldwide. It is considered that almost half of human mortality is associated with fibrosis-associated disorder [1]. Fibrosis is regarded as the abundant deposition of extracellular matrix (ECM) including collagen and fibronectin around injured tissue, which can cause distortion of tissue architecture, loss of organ function, and, finally, death, as seen in end-stage liver, kidney, lung, and heart diseases [2]. This pathology ordinarily begins as an abnormal wound repair process response to repeated or chronic tissue damage, regardless of the underlying pathogenesis, and can occur in virtually any solid organ or tissue [3].
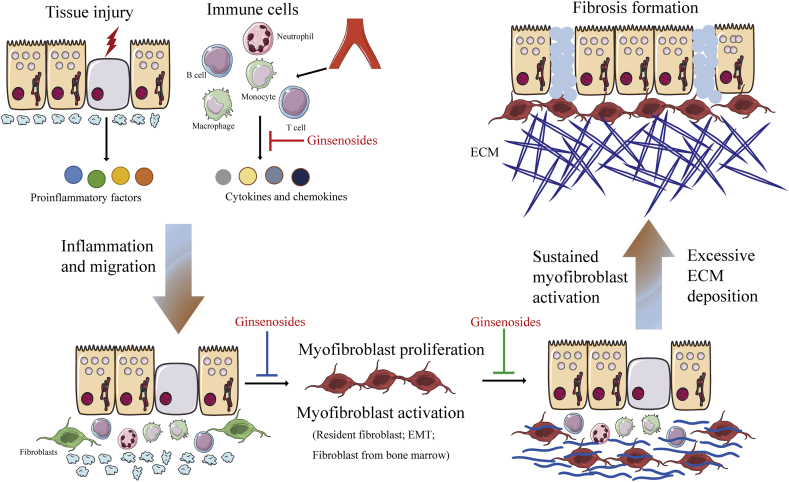
In general, wound healing experiences three broad phases that are temporally overlapping but functionally distinct [4,5]. In a normal situation, following injury, hemostasis is achieved through the formation of a platelet plug and a provisional ECM, accompanied by the threshold of inflammation and recruitment of immune cells. This process initiates the first phase of healing, namely the inflammatory phase. There are much more neutrophils and macrophages infiltration in tissues, which combat possible infections and remove tissue and cell debris. In the inflammatory phase, impaired endothelial cells, epithelial cells, and myofibroblasts produce aberrant matrix metalloproteinases (MMPs) to disrupt basement membrane in local tissues and discharge various cytokines and chemokines, which recruit and activate more different immune cells, including neutrophils, macrophages, T lymphocyte cells, and B lymphocyte cells. These activated leukocytes release proinflammatory, vasoactive, and profibrotic effectors, including transforming growth factor (TGF)-β1, tumor necrosis factor (TNF)-α, and platelet-derived growth factor (PDGF), as well as interleukin (IL)-6 and IL-13, to prompt the proliferative phase of healing [[6], [7], [8], [9]]. It is generally accepted that TGF-β1 has a particularly conspicuous role in inducing the differentiation of precursor cells into myofibroblasts, which rapidly generate a tremendous amount of ECM to maintain the integration of the injured tissue during repair and to improve cell proliferation for the formation of granulation tissues [10]. In the final tissue remodeling and cell maturation phase, activated myofibroblasts provoke the wound contraction; the provisional ECM is degraded and remodeled to rebuild the parenchymal tissue architecture. However, life is not always what we want it to be. An abnormal tissue reconstruction process is more common and can be featured by persistent inflammatory response, necrocytosis, sustained myofibroblasts activation, excessive ECM deposition, and finally a perpetual fibrotic lesion formation (Fig. 1). In most of the cases, chronic inflammation always can lead to tissue fibrosis, which initiates the generation of profibrotic growth factors [5]. In addition to this, aging also is recognized to result in fibrosis in diverse organs [11]. Interestingly, genetic factors are found to cause cystic fibrosis [12] and idiopathic pulmonary fibrosis [13].
Fig. 1.
The cellular and molecular mechanisms of fibrosis and anti-fibrotic property of ginsenosides. Once an injury occurs in an organ, impaired epithelial and/or endothelial cells secrete chemokines and growth factors. Macrophages and monocytes are recruited and activated, which further result in the release of cytokines and chemokines, and further induce fibroblast activation. Activated fibroblasts transform into myofibroblasts and actively synthesize ECM. Once chronic injury, inflammation, and necrosis occur, myofibroblasts are persistently activated and excessive ECM is deposited, finally leading to fibrosis formation. Ginsenosides negatively regulate fibrosis. Red blunted line: an inhibitory effect of ginsenosides on secretion of proinflammatory cytokines from immune cells or injured tissue; blue blunted line: an inhibitory effect of ginsenosides on myofibroblast differentiation, proliferation, and activation; green blunted line: an inhibitory effect of ginsenosides on excessive ECM accumulation and fibrosis formation. Abbreviation: EMT, epithelial–mesenchymal transition; ECM, extracellular matrix.
1.2. Ginseng and ginsenosides
The term “Ginseng” is interpreted from the Chinese “人参” and is extensively used as a restorative drug and generally means Asian ginseng. It has been highly acclaimed in China for more than two thousand years. The Pax family consists of at least nine kinds of ginseng, such as Panax quinquefolius, Panax notoginseng, Panax japonicus, Panax vietnamensis, and Panax trifolius [14].
There are three vital ingredients in ginseng: polysaccharides, saponins, and phenolic compounds [15]. It has been reported that a four year-old Korean ginseng contains ∼5% polysaccharides, ∼3% saponins, and ~0.4% phenolic compounds [16]. Among these elements, saponins have been comprehensively explored and confirmed to trigger various biological effects. Ginsenosides, conventionally identified as saponins, are regarded as the primary bioactive constituents of ginseng [17]. Saponin is a kind of triterpenoidal dammarane glycosides, called ginsenosides Rx in line with their ability to move on TLC plates, with a decline of polarity from "a" to "h" [18]. Based on the location of sugar moieties, ginsenosides can be distinguished into protopanaxadiol type (I-1 type) and protopanaxatriol type (I-2 type).Until now, researchers have identified more than 80 ginsenosides [18]. Among them, ginsenosides Rb1, Rb2, Rg1, Rg2, Rc, Rd, and Re are major ingredients of white and red ginsengs, whereas ginsenosides Rg3, Rg5, and Rg6 are well known to be uniqueness of Korean Red Ginseng (KRG). Table 1 overviews the anti-fibrosis performances of canonical ginsenosides. These contents will be investigated in detail in the latter sections.
Table 1.
Ginsenosides tested in animal or cellular studies for human fibrosis-related diseases
| Ginsenosides | Model | Animal/cell type | Therapeutic target | Output (except fibrosis) | Refs | |
|---|---|---|---|---|---|---|
| Liver fibrosis | Rb1 | CCl4 | Sprague–Dawley rats | The inhibition of hepatic prostaglandin E2 and TIMP-1 | Decreased plasma and hepatic triglyceride, hepatic cholesterol; inhibited IL-1β concentrations | [40] |
| Compound K and/or Rh1 | Non-alcoholic fatty liver | Sprague–Dawley rats | The inhibition of HSCs proliferation and activation; the induction of HSCs apoptosis | Improved hepatic function and abnormal lipid metabolism; alleviated HFD-induced insulin resistance | [50] | |
| Rg1 | Thioacetamide | Sprague–Dawley rats | The inhibition of PDGF-induced proliferation, activation of HSCs, and ROS formation, and NF-κB binding activity | Decreased hepatic hydroxyproline content and lipid peroxidation | [46] | |
| 25-OCH3-PPD | Thioacetamide | C57BL/6 mice; rats HSC-T6 cells | The inhibition of LXRs-P2X7R-mediated NLPR3 inflammasome | Improved hepatic function; inhibited HSCs activation and hepatocyte apoptosis, and proinflammatory cytokines | [43] | |
| Rg1 | Alcohol- and CCl4 | Wistar rats; Primary HSCs of rats | The activation of Nrf2 pathway | Improved liver function; inhibited liver inflammation and HSCs activation; decreased lipid peroxidation and modified antioxidant enzyme activity | [47] | |
| Rb1 | H2O2 | Rats HSC-T6 cells | The inhibition of collagen, TGF-β1, MMP-2, and TIMP-1 | Decreased HSCs proliferation and activation | [48] | |
| 20S-Protopanaxadiol | 10% FBS (primary HSCs) | Human LX-2 cells Primary HSCs of mice | The activation of LKB1-AMPK pathway | Induced HSCs apoptosis and promoted oxidative stress | [52] | |
| 25-OCH3-PPD | Thioacetamide | Kunming mice | The inhibition of JNK and p38-ERK pathway | Decreased the release of inflammatory cytokines | [44] | |
| Rg1 | CCl4 | Kunming mice; rats HSC-T6 cells | The inhibition of the TGF-β/Smad pathway and the activation of Nrf2 | Induced HSCs apoptosis and inhibited intracellular ROS level | [51] | |
| Cardiac fibrosis | Rg1 | TAC | Sprague-Dawley rats | The inhibition of p38 MAPK and the activation of phospho-Akt pathway | Decreased left ventricular hypertrophy; enhanced myocardial angiogenesis | [58] |
| Rb1 | Abdominal aortic coarctation | Sprague-Dawley rats | The inhibition of TGF-β/Smad and ERK signaling pathway and the activation of Akt pathway | Improved cardiac function; decreased cardiac hypertrophy; decreased mitochondrial membrane potential; enhanced the translocation of GLUT4 | [63] | |
| Rg1 | CTEPH | Sprague-Dawley rats | The regulation of MMP-2 and -9 | Decreased right ventricular hypertrophy and immune cell infiltration | [59] | |
| Rh2 | STZ | Sprague-Dawley rats; rat H9C2 cells | The regulation of PPARδ-STAT3 pathway | Improved cardiac function; decreased superoxide ions produced by high glucose | [65] | |
| Rb1 | STZ | Wistar rats; rats primary fibroblast | The inhibition of TGF-β/Smad pathway and the promotion of Smad7 | Improved cardiac function; inhibited cardiac fibroblast to myofibroblast differentiation | [70] | |
| Re | Isoproterenol | Wistar rats | The inhibition of TGF-β/Smad3 pathway | Decreased heart failure; improved cardiac function | [66] | |
| Rg1 | Doxorubicin | C57BL/6J mice | The inhibition of ER stress and autophagy | Improved cardiac function; inhibited cardiac autophagy | [60] | |
| Rb3 | CVB3 | Primary CMVECs of rats | The regulation of Pyk2-PI3K-Akt pathway | Attenuated oxidative stress and preserved endothelial function | [68] | |
| Rd | Pressure overload | C57BL/6 mice; primary cardiac myocytes of rats | The inhibition of ERK and TGF-β1 pathways and the activation of Akt pathway | Improved cardiac function; decreased cardiac hypertrophy; decreased inflammation and oxidative stress | [67] | |
| Renal fibrosis | Rg1 | UUO | Sprague-Dawley rats | The regulation of the Klotho/TGF-β1/Smad pathway | Improved kidney function; prevented EMT | [76] |
| Rb1 and Rc | Cyclosporine A | C57BL/6J mice; human HK-2 cell line | The regulation of oxidative stress pathway | Improved kidney function; inhibited inflammation and the production of proinflammatory cytokines; prevented tubular epithelial cell apoptosis | [81] | |
| Rg1 | DN | Wistar rats | The inhibition of TGF-β1/Smads pathway and oxidative stress | Decreased BUN and SCr; increased anti-oxidative capacity | [78] | |
| Rg1 | UUO | Sprague-Dawley rats | The inhibition of ER stress and unfolded protein response-related apoptotic pathway | Improved renal function; activated ER stress response | [75] | |
| Rg1 | Cyclosporine A | Sprague-Dawley rats | The inhibition of ER stress-triggered tubular cell apoptosis | Decreased tubular epithelial cell apoptosis | [79] | |
| Rg1 | UUO | Sprague-Dawley rats | The regulation of thrombospondin-1 and VEGF expression | Decreased microvessel density; improved tubular atrophy | [74] | |
| Rb1 | UUO | Sprague-Dawley rats | The regulation of oxidative damage and TGF-β1 expression | Increased urinary heme oxygenase-1 level; decreased p47phox expression | [73] | |
| Rg1 | TGF-β1 | Rats NRK-52E cells | The inhibition of ERK pathway | Inhibited the process of EMT | [82] | |
| Rg1 | UUO | Sprague-Dawley rats | The inhibition of TGF-β1/Smads pathway and thrombospondin-1 expression | Inhibited tubular EMT | [72] | |
| Lung fibrosis | Rg1 | Bleomycin | Sprague-Dawley rats | The regulation of TGF-β1 and Caveolin-1 | Decreased lung injury, inflammatory cell infiltration | [87] |
| Rg1 | COPD | Sprague-Dawley rats; human MRC5 fibroblasts | The inhibition of TGF-β1/Smads pathway | Decreased emphysema; inhibited immune cell infiltration; prevented lung fibroblast transdifferentiation | [88] | |
| Total ginsenoside | Bleomycin | BALB/c mice | The inhibition of TGF-β/Smad pathway and the promotion of Smad7 | Reduced the pulmonary coefficient; regulated the MMPs system | [89] | |
| Other fibrotic diseases | Rg3 | Rabbit-ear HS | Primary human HSFs; rabbits | The regulation of collagen fibers accumulation and VEGF expression | Inhibited HS fibroblasts proliferation and induced apoptosis; limited inflammation | [[90], [91], [92], [93]] |
| Rg3 | Keloid scar | Primary human keloid fibroblasts | The inhibition of TGF-β1/Smads and ERK pathway | Inhibited the proliferation, migration, invasion and angiogenesis of keloid fibroblasts | [94] | |
| Rb1 | Rabbit-ear HS | Rabbits | The inhibition of profibrotic proteins and growth factors | Inhibited immune cell infiltration | [95] | |
| Rg3 | Endometriosis | C57BL/6 mice; Primary human HESCs and Ishikawa Cells | The modulation of miR-27b-3p expression | Inhibited cell proliferation and invasion; regulated MMPs expression | [97] |
Abbreviations: CCl4, carbon tetrachloride; TIMP-1, tissue inhibitor of metalloproteinase-1; IL-1β, interleukin-1β; HSCs, hepatic stellate cells; HFD, high fat diet; PDGF, platelet-derived growth factor; ROS, reactive oxygen species; NF-κB, nuclear factor-κB; LXR, liver X receptor; P2X7R, P2X7 receptor; TGF-β1, transforming growth factor-β1; MMP-2, matrix metalloproteinase; FBS, fetal bovine serum; LKB1, liver kinase B1; AMPK, AMP-activated protein kinase; JNK, c-jun N-terminal kinase; ERK, extracellular signal-regulated kinase; TAC, transverse aortic constriction; MAPK, mitogen-activated protein kinase; GLUT4, glucose transporter type 4; CTEPH, chronic thromboembolic pulmonary hypertension; STZ, streptozotocin; PPARδ, peroxisome proliferator-activated receptor δ; STAT3, signal transducer and activator of transcription 3; ER, endoplasmic reticulum; CVB3, coxsackievirus B3; UUO, unilateral ureter obstruction; EMT, epithelial–mesenchymal transition; DN, diabetic nephropathy; BUN, blood urea nitrogen; SCr, serum creatinine; VEGF, vascular endothelial growth factor; COPD, chronic obstructive pulmonary disease; MMPs, matrix metalloproteinase; HS, hypertrophic scarring; HSC: hepatic stellate cell line; LX-2: human hepatic stellate cell line; H9C2: embryonic cardiomyocytes cell line; CMVECs: cardiac microvascular endothelial cells; HK-2: human renal proximal tubular cells; NRK-52E: rat renal tubular epithelial cells; MRC5: human embryonic lung fibroblasts; HSFs: hypertrophic scar fibroblasts; HESCs: human endometrial stromal cells
1.3. Ginsenosides and their therapeutic potential
Many phytochemicals are noted to be anti-cancer candidates in accordance with their low toxicities and their inhibitory effects on inflammation [19]. Previous research works have revealed that consumption of ginseng is closely associated with reduced risk of cancer and good cancer therapeutic effect. The data from case-control studies have indicated that periodical consumption of ginseng could effectively inhibit the development of oral squamous cell cancer, gastric cancer, liver cancer, lung cancer, ovarian cancer, pancreatic cancer, and colorectal cancer [20,21]. A retrospective cohort study including about 4600 healthy individuals found that using ginseng regularly obviously decreased the occurrence of tumor [22]. Results from an epidemiological research of breast cancer showed that periodical usage of ginseng before and after cancer diagnosis could remarkably decrease the risk of tumor recurrence and death from cancer [23]. Likewise, a recent preliminary work demonstrated that taking 800 mg of Panax ginseng each day could effectively improve fatigue caused by cancer [24]. Over the past 40 years, most of the effective anti-tumor constituents in the ginseng have been identified, like ginsenosides Rg3 and Rh2 [25,26]. Ginsenosides have been accepted and emphasized as a potential choice for anticancer treatment. In China, even worldwide, some of them are easy to buy as nonprescription drugs at the pharmacy. In addition, ginseng has been generally considered as safe and is extensively used in the United States and Europe. Apart from showing anticancer attributes, ginsenosides successfully alleviate the progression of atherosclerosis [27], myocardial ischemia/reperfusion injury [28], inflammation [29], and Alzheimer’s [30], Parkinson’s [31], and fibrosis-related diseases.
Fibrosis represents a normal physiological response following injury; but uncontrollable production and accumulation of fibrous ingredients destroys the normal tissue structure and organ function [32]. Thus, it is important to sustain organ physiological function through fibrosis control appropriately. Until now, a few commercial agents have been observed to exert inhibitory effects on fibrosis, such as angiotensin II type 1 receptor blockers (ARB), angiotensin converting enzyme inhibitors (ACEI), metformin, and MMP inhibitors [[33], [34], [35]]. Despite these drugs exhibit certain efficacy against fibrosis, progressive organ dysfunction still occurs in most patients. It is imperative to develop new alternative therapeutics and strategies to relieve fibrosis for clinicians. This review describes recent experimental results of ginsenosides that specially target inflammatory mediators or fibrosis-related signaling pathways and summarizes the possible benefits of ginsenosides as novel regulators of tissue fibrosis.
2. Experimental outcomes of ginsenosides in fibrotic diseases
2.1. Liver fibrosis
Progressive liver fibrosis is one of the hallmark traits of liver impairment, which is a primary reason of mortality in patients with different chronic liver diseases [36]. Liver fibrosis will evolve into liver cirrhosis along with the condition progress, and ultimately patients receive organ transplantation [37]. Chronic hepatic inflammation from chronic hepatitis virus infections, abnormal lipid metabolism, and alcohol abuse contributes to aberrant abundant accumulation of collagen and ECM proteins, finally rising up to severe fibrosis and progression to cirrhosis. In 2009, Peng et al found that the degree of liver fibrosis and collagen deposition area in tissue were strikingly attenuated after Panax notoginseng saponins (PNS) treatment. PNS treatment significantly reduced the generation of growth factors and pro-inflammatory cytokines, whereas elevated the production of anti-inflammatory cytokines [38]. Among more than 30 ginsenosides, ginsenoside Rb1 (C54H92O23) is regarded as the richest ginsenoside in Panax ginseng [39]. A study from Hou et al showed that ginsenoside Rb1 attenuated liver fibrosis by preventing fat deposition, secretion of prostaglandin E2 (PGE2), and production of tissue inhibitor of metalloproteinase-1 (TIMP-1) [40]. Han et al [41] recently put forward a conclusion that ginsenoside 25-OCH3-PPD protected against hepatic fibrosis and inflammation by activating liver X receptors (LXRs) signaling pathway in thioacetamide (TAA)-induced mice. LXRs were reported to be able to promote the resolving of hepatic fibrosis and inflammation through targeting genes involved in cholesterol and lipid metabolism [42]. In addition, 25-OCH3-PPD was found to inhibit the formation and activation of the NLRP3 inflammasome in liver fibrosis [43]. Notably, Su and co-workers supplemented another piece of data that supported the inhibitory effect of 25-OCH3-PPD on inflammation and liver fibrosis [44]. Their findings in combination with previous observations further confirm an advantageous benefit of 25-OCH3-PPD in liver fibrosis treatment.
It is generally known that hepatic stellate cells (HSCs) belong to primary effector cells of fibrosis located perisinusoidally around the space of Disse in liver injury. As one of the pro-fibrogenic cells, HSCs can be activated from quiescent cells to myofibroblast-like cells under inflammatory microenvironment. Activated HSCs further lead to the excessive accumulation of ECM [45]. Thus, how to control the proliferation and activation of HSCs is considered the vital issue for liver fibrosis treatment. Data from rats treated with TAA and cultured HSCs stimulated by PDGF-BB showed that ginsenoside Rg1 significantly prevented HSCs' proliferation and activation, as well as oxidative stress response. Ginsenoside Rg1 reduced PDGF receptor-β expression via down-regulating the nuclear factor-κB activity [46]. Furthermore, in alcohol and CCl4-induced rat liver fibrosis model, activated HSCs and liver inflammation were dramatically decreased in the Rg1-treatment group. The protective effect of Rg1 against fibrosis is related with the upregulation of nuclear translocation of Nrf2 and antioxidant enzymes (SOD, GSH-Px, and CAT) [47]. Moreover, ginsenoside Rb1 was also found to have a prominent inhibitory effect on HSCs by regulating the proliferation and activation, as well as collagen and TGF-β1 expression [48].
Apoptosis of activated HSCs is thought to be the main molecular biological mechanism in the resolution of fibrosis [49]. Chen et al clearly demonstrated that treatment with ginsenoside Rh1 and Compound K either in combination or alone markedly alleviated the liver dysfunction and liver fibrosis caused by high fat diet, which were associated with decreased proliferation and activation of HSCs, and increased HSCs apoptosis [50]. Ginsenoside Rg1 was observed to significantly reduce HSCs proliferation, reverse epithelial–mesenchymal transition (EMT) induced by TGF-β1, and stimulate apoptosis in CCl4-induced liver fibrotic model. Mechanistically, ginsenoside Rg1 shows up a protective action on liver fibrosis via down-regulating the TGF-β/Smad pathway and promoting Nrf2 nuclear translocation [51]. Park et al treated LX-2 cell line and primary HSCs from patients with ginsenoside 20S-protopanaxadiol and observed the change of HSCs number. 20S-protopanaxadiol significantly induced more apoptosis of HSCs through activating LKB1 and the downstream AMPK pathway, indicating it could be a therapeutic candidate for liver fibrosis treatment [52]. Ding et al found that saponins of Panax japonicus distinctly ameliorate liver dysfunction and reduce collagen fibers formation and immune cell infiltration in the fatty liver fibrosis model through the inhibition of the endoplasmic reticulum stress (ERS) and the regulation of inflammation and apoptosis pathways [53].
2.2. Cardiac fibrosis
Cardiac fibrosis is featured by the excessive deposition of ECM-related proteins in the myocardium. It is an essential pathophysiological process existing in nearly all types of cardiac diseases [54]. Cardiac fibrosis damages myocardial structure, disturbs myocardial excitation–contraction coupling, disrupts diastolic and systolic blood pressure, ultimately resulting in the progress of cardiac diseases to heart failure [55,56]. It has been confirmed that the extent of cardiac fibrosis sustains a significant opposite interrelation with outcome, and until now, the only effective treatment for end-stage fibrotic disease is organ transplantation. Considering the limited usability of organ transplantation, the discovery of alternative pharmacologic strategies is still the first priority for researchers.
Ginsenosides have been found to play multiple pharmacological roles on the cardiovascular system [57]. Up to now, most of the previous researches have focused on ginsenoside Rg1. Zhang's group revealed that Rg1 administration significantly decreased transverse aortic constriction (TAC)-induced myocardial fibrosis and left ventricular hypertrophy and maintained myocardial function and the relevant signaling pathways, including Akt and p38 MAPK pathway [58]. In addition, Rg1 also has the effect on the myocardial remodeling in chronic thromboembolic pulmonary hypertension model [59]. Furthermore, Xu et al found that Rg1 treatment could markedly inhibit the deterioration of myocardial function and myocardial fibrotic changes induced by Doxorubicin (DOX) [60]. Wei et al took advantage of polymeric carriers named gelatin microspheres to encapsulate Rg1 and crosslink with genipin, then injected into an infarcted myocardium rat model. Their results suggest that ginsenoside Rg1 as a stabilized angiogenic compound improves myocardial reperfusion and contributes to the maintenance of infarcted left ventricle function [61]. Recently, Li et al investigated the protective effect of Rg1 on myocardial remodeling in a subacute myocardial infarction mouse model, which is associated with down-regulation of α-SMA and MMP-9 [62].
Apart from Rg1, other ginsenosides (Rb1, Rh2, Re, and Rb3) have been described to have a protective action in cardiac function and remodeling. High dose of ginsenoside Rb1 plays an important role in cardiac remodeling via reducing the levels of cardiac fibrosis-related genes (including collagen I, angiotensin II, and periostin), restoring mitochondrial function, and elevating glucose uptake through augmenting GLUT4 translocation [63]. As we know, cardiovascular complications have become a major death reason in diabetic patients. Diabetic cardiomyopathy is primarily featured with cardiac fibrosis and consequent cardiac malfunction [64]. Lo et al [65] observed that increased heart weight/body weight ratio was decreased to a great degree by ginsenoside Rh2 in a streptozotocin (STZ)-stimulated type-1 diabetic rat's model. However, the anti-fibrosis effects of Rh2 could be reversed by peroxisome proliferation-activated receptor δ (PPARδ) GSK0660 administration, implicating that PPARδ signaling pathway may be involved in this process. In addition, GSK0060 or siRNA specific for PPARδ also was able to reverse Rh2-induced down-regulation of fibrosis-related signals, such as signal transducer and activator of transcription 3 (STAT3), fibronectin, and connective tissue growth factor (CCN2) in high glucose-cultured cardiomyocytes. Taking advantage of myocardial fibrosis animal models, Wang et al found that ginsenoside Re treatment could obviously decrease heart weight, myocardial fibrosis, and hydroxyproline content [66]. These effects might be associated with downregulation of TGF-β1 and Smad3 in cardiac tissue. A recently published research study reported that Rd remarkably prevented cardiac hypertrophy induced by pressure overload, fibrotic change, and inflammation [67]. However, unlike the action mechanism of Rg1 [58], Rd improved cardiac remodeling and dysfunction primarily through regulating expression of p-Akt, p-ERK1/2, calcineurin A, and TGF-β1 [67]. Another interesting finding in the study from Yang et al [68] suggests that ginsenoside Rb3 can inhibit cardiac microvascular endothelial–mesenchymal transition following coxsackievirus B3 infection through regulation of the Pyk2/PI3K/Akt pathway.
It is a common phenomenon for drug combinations in the practice of traditional Chinese medicine. “Fu fang” tonics composed by different herbs are often prescribed. This practice is similar to the drug cocktail strategy widely used in Western medicines. Shen et al [69] found that Shen Song Yang Xin Capsule (SSYX) inhibited diabetic myocardial fibrosis through preventing the TGF-β1/Smad3 pathway. Rb1 has been identified as the major ingredient of SSYX. Zhang et al [70] showed that Sheng Mai Yin (SMY) can reduce the risk of Adriamycin-induced myocardial fibrosis through downregulation of inflammatory cytokines and MMPs. Rg1 has been recognized to be the major components of SMY. YiQiFuMai Powder Injection, redeveloped based on SMY, was found to alleviate coronary artery ligation -heart failure through ameliorating cardiac function, collagen deposition, and fibrosis via inhibition of MAPK signaling pathways. The major compounds in YiQiFuMai are ginsenosides and lignans. Broadly speaking, we found that ginsenosides are capable of regulating the development of cardiac fibrosis.
2.3. Renal fibrosis
Chronic kidney disease (CKD) influences billions of population globally and has high mortality. CKD can develop into end-stage kidney disease (ESKD), which is fatal without renal replacement therapy [71]. Kidney fibrosis is defined by aberrant production and accumulation of fibrous ingredients produced by residential fibroblasts. Kidney fibrosis frequently occurs after unilateral ureteral obstruction (UUO) [[72], [73], [74], [75], [76]], diabetic nephropathy [77,78] and cyclosporine A treatment [[79], [80], [81]]. Ginsenosides have shown to exert a renoprotective effect. Xie et al [72] found that ginsenoside Rg1 treatment obviously restrained interstitial fibrosis induced by UUO including tubular injury and collagen accumulation. Intriguingly, Rg1 significantly reduced α-SMA expression and simultaneously improved E-cadherin expression in the obstructed kidney and TGF-β1 induced rat tubular cells (NRK-52E), suggesting that the underlying mechanism of anti-fibrosis might be partly associated with the reversal of tubular EMT [72,82]. Further study from the same UUO model demonstrated that Rg1 also could control renal microvascular integrity to prominently increase peritubular capillary densities through decreasing thrombospondin-1 (TSP-1) expression and increasing vascular endothelial growth factor (VEGF) expression [74]. Additionally, Li et al discovered more possible mechanism of Rg1 in the UUO model. They observed that Rg1 could prevent the renal fibrotic process partly by inhibition of ERS and unfolded protein response (UPR)-associated apoptotic pathway in UUO kidney [75]. Besides this, their results showed that Rg1 could reverse EMT and UUO-induced renal interstitial fibrosis through targeting the Klotho/TGF-β1/Smad pathway in UUO kidney [76]. In addition, our recent new evidence shows that Rg5 could prevent renal tubular cells autophagy by modulating the key proteins in the AMPK-dependent mTOR pathway, as well as the ERK and p38 MAPK pathway in vivo and in vitro (unpublished data).
Cyclosporine A (CsA), as a common clinical immunosuppressive agent, has been widely used for suppressing the rejection response after organ transplantation. Numerous experimental studies have shown that prolonged usage of CsA induces serious side effects, including progressive renal interstitial fibrosis, renal cell apoptosis, immune cell infiltration, and hyalinosis of the afferent arterioles [83]. Doh's group [81] found that Korean Red Ginseng extract treatment could effectively inhibit deterioration of renal function, typical pathologic lesions, and apoptotic cell death through alleviating oxidative stress in a CsA nephropathy model and cell culture model in vitro. The major ingredients of KRG were ginsenoside Rb1 (8.27%) and Rc (3.90%). Following Doh's research, Lim et al further proposed that KRG extract exhibited an inhibitory effect on CsA-induced autophagosome formation and autophagic aggregates, which might be concerned with the activation of the Akt/mTOR pathway [80]. Moreover, ginsenoside Rg1 also clearly manifests the evident anti-apoptosis effect in a chronic CsA nephropathy rat model [79]. The protective actions of ginsenosides on diabetic nephropathy have been observed by several studies. Du et al [77] suggested that PNS administration could protect against kidney injury induced by diabetes possibly through enhancing SIRT1 and suppressing inflammation, as well as activating antioxidant motions. One study even found that ginsenoside Rg1 combination with Astragaloside IV was more effective on inhibiting oxidative stress response and down-regulating the activation of the TGF-β1/Smads signaling cascade in diabetic nephropathy rats [78]. Summarizing the results of the above stated, ginsenosides were suggested as an important option during the treatment of renal fibrosis.
2.4. Pulmonary fibrosis
Pulmonary fibrosis often leads to a chronic irreversible decline in pulmonary function. The leading reasons of pulmonary fibrosis include cigarette smoking, air pollution, and viral infection. Hitherto, no effective treatment has been identified that can restrict the development of pulmonary fibrosis [84]. A few studies have shown the therapeutic potential of ginsenosides in pulmonary fibrosis. Zhang and co-workers identified that PNS treatment apparently ameliorated the cardiopulmonary injury and reduced inflammatory response via regulating the NF-κB signaling pathway [85], which remains consistent with the finding from Tsai et al [86]. Zhan et al [87] showed clearly a remarkable effect of ginsenoside Rg1 on a bleomycin-induced pulmonary fibrosis animal model. Rg1 was found to decrease α-SMA and down-regulate TGF-β1, as well as up-regulate Caveolin-1. Guan et al [88] also confirmed an inhibitory effect of Rg1 in cigarette smoking-induced air fibrosis. Rg1 was found to restrain fibrosis progression through inhibiting the TGF-β1/Smad pathway in in vitro pulmonary fibroblasts and in vivo chronic obstructive pulmonary disease (COPD) rats. Likewise, total ginsenoside exhibits the protective effect on pulmonary fibrosis by bleomycin through interference of the TGF-β1/Smad signaling cascade and MMP system [89].
2.5. Miscellaneous diseases
Hypertrophic scars (HS), or keloids, are one of fibrosis-related disorders. It is hard to handle because surgical treatment or comparable interventions could generate tissue lesion aggravation. In spite of only a few studies mentioned, ginsenosides can be classified as potential therapeutic choices for HS or keloids. Cheng's group confirmed that Rg3 could be recognized as an early intervention and a combining therapeutic agent to suppress inflammatory response and scarring formation [90]. The experimental results from other studies further confirmed the above conclusions [[91], [92], [93]]. Tang et al found that in vitro Rg3 prevented the proliferation of keloid fibroblasts, angiogenesis, and collagen synthesis through regulating TGF-β1/Smads and ERK pathways [94]. Furthermore, Tark and co-workers identified protective action of Rb1 on HS [95]. In a word, these findings suggest ginsenosides treatment be a potential strategy regulating skin fibroblasts proliferation. Moreover, another two independent studies revealed the inhibitory action of PNS on oral submucous fibrosis induced by areca nut extract [96] and the inhibitory action of Rg3 on endometriosis [97].
3. Adverse effects
In most cases, no significant side effects have been found in the supplementation with ginseng or ginsenosides. However, mastalgias and vaginal bleeding have been reported by some female patients [[98], [99], [100]]. Because of its estrogen-like effect, ginseng should be applied with extreme caution in women taking progestogens for the possible worsening of side effects of the latter [98]. Subjects treated with warfarin or other anticoagulants or antiplatelet drugs should avoid taking of ginseng-based supplements because of the high risk of bleedings [101]. Subjects receiving digoxin or corticosteroids should also be cautious when taking ginseng [102]. Moreover, in patients taking high doses of Panax ginseng (more than 2.5 g/day), central nervous system effects have been reported, such as insomnia [101,103], tachyarrhythmias [99], hypertension [104] and nervousness [101,105]. Other reported side effects of Panax ginseng are gastrointestinal disorders and headaches [101].
4. Limitations and future perspectives
Ginsenosides as natural remedies have been broadly approved to exert therapeutic effects regardless of being used alone or in combination with other drugs in various fibrosis-related in vivo animal models and in vitro cells. However, obstacles must be resolved before ginsenosides therapy can reach clinical application. One of the most difficult impediments is lack of well-designed, randomized, placebo-controlled clinical trials for ginsenosides in humans because of their low bioavailability, such as poor water solubility and biomembrane permeability, instability in gastrointestinal tract, and high metabolic rate in the body. The oral bioavailability is a combined effect of numerous body barriers, preparations, and molecular properties. It has been demonstrated that the majority of ginsenosides had low oral bioavailability [106]. When summarizing the factors that have an impact on oral bioavailability, there are the following four remarkable points. The first is regarding the properties of the compounds. Ginsenosides, as a class of natural glycoside compounds, usually have a large molecular weight. It is well known that the smaller the molecular weight, the faster the molecular transport through the biofilm, and therefore ginsenosides tend to be absorbed slowly or even incompletely [107]. The second point is the stability of ginsenosides in the gastrointestinal tract. Most of the ginsenosides are easy to degrade or bio-transform under gastric acid and/or intestinal flora conditions, for which they often exert low oral bioavailability [108]. Third, it is associated with membrane permeability. It is widely believed that the higher the permeability, the better the absorption of compounds. Some ginsenosides have poor oral bioavailability because of their limited permeability of intestinal epithelial cell [109,110], and the membrane permeability relates to molecular weight of compounds ability to form hydrogen bond and lipophilicity [111]. Fourth is the diversity of ginsenosides in intestinal and hepatic first-pass effect. Compounds/drugs that enter the portal vein can be effectively metabolized in the gastrointestinal tract and liver, especially during the first-pass process after absorption, which leads to a decrease of bioavailability [112]. Generally, large molecular weight, low water-solubility, and poor gastrointestinal stability of ginsenosides lead to a significant intestinal and hepatic first-pass effect, then leading to low oral bioavailability. Thus, many efforts have been made on figuring out these issues by building effective drug delivery systems and all sorts of administration routes to enhance the bioavailability of ginsenosides, including fibrous membranes [91], vesicles [113], microsphere delivery systems [61], micelles [114], emulsion delivery systems [115], nanoparticle drug delivery systems [116], and so on. These drug delivery systems will greatly enhance the bioavailability of ginsenosides and are capable to slow down, control, release, or target.
Safety is another issue that needs attention. Because the biological function of most natural products is entirely by identifying their pharmacologically active ingredients, unlike the traditional use as an extract or a combination, it is difficult to ensure safety. Solving these problems requires a lot of work. However, it is worth focusing on the intake of ginseng products by subjects receiving cardiac, antidepressant, and anti-hemorrhagic medications for possible side effects.
Evidence that ginsenosides have significant effects on both cancer treatment and tissue fibrosis is accumulating. These two processes share common biological programs, including the occurrence of inflammation and oxidative stress. Ginsenosides have shown an obvious curative effect on these pathophysiological phenomena, indicating that ginsenosides could be a common treatment of cancer and fibrosis by directing inflammation and oxidative stress. Interestingly, ginsenosides can specifically kill tumor cells and inhibit tumor cell proliferation during cancer treatment; meanwhile ginsenosides are also able to promote tumor metastasis by inhibiting the secretion of MMPs and interfering with matrix remodeling [14]. However, during the treatment of fibrotic diseases, ginsenosides conversely exhibit specific killing of activated myofibroblasts and promote the degradation of ECM through up-regulating the expression of MMPs, as well as effectively protect the parenchymal cells of the organ, which is completely different from the molecular mechanism of treating tumors. It still remains unclear how these different effects of ginsenosides can be induced based on cell types or disease environments. The knowledge pertaining the involved detailed deep mechanisms is sparse, although the functional effects of ginsenosides are well established, particularly regarding ginsenosides involvement in anti-fibrosis. More specific mechanisms of ginsenosides (not limited to signaling pathways) require future further evaluation.
Moreover, ginsenosides present diverse actions, such as anti-oxidative, anti-inflammatory, pro-apoptotic, and immunostimulatory performances, with different molecular mechanisms. With this said, it is often hard to standardize the studies because of the diversity of ginsenosides. The different species, cell type, primary or cultivated, harvest time, and methods of processing could affect the pharmacological effects of ginsenosides, so it will be required to investigate the impact of these variables. Additionally, more in-depth research using a single preparation should explore the detailed cellular and molecular mechanisms of action, the relationship between structure and function, specificity, toxicity, and pharmacokinetics profile in animal models and humans. It is useful for high-throughput expression array to explore the molecular actions of the various ginsenosides and how the different molecular signaling pathways work together. Furthermore, more explorations are needed to investigate whether synthetic or engineered natural ginsenosides can be used as potential candidates for the treatment of fibrotic diseases, especially if they have better bioavailability, efficacy, safety, and affordability. However, these treatments require further investigation through additional animal experiments and large-scale clinical studies. Upcoming studies will greatly improve the therapeutic potential of ginsenosides, consequently further contributing to the promotion of global health.
5. Conclusions
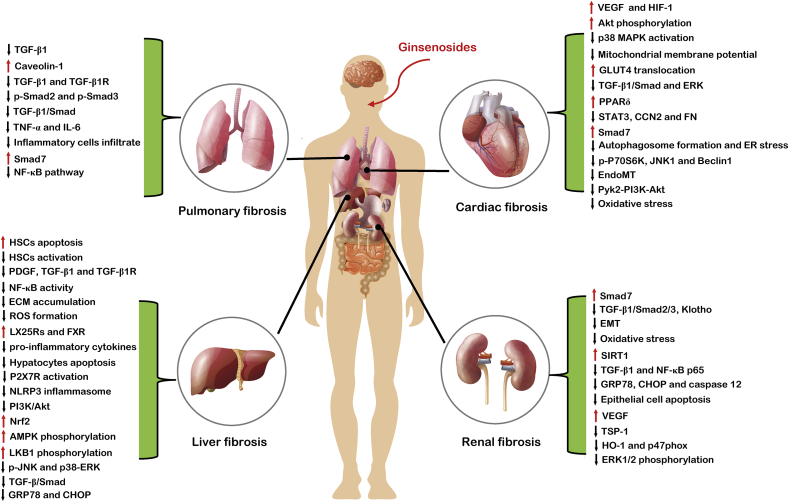
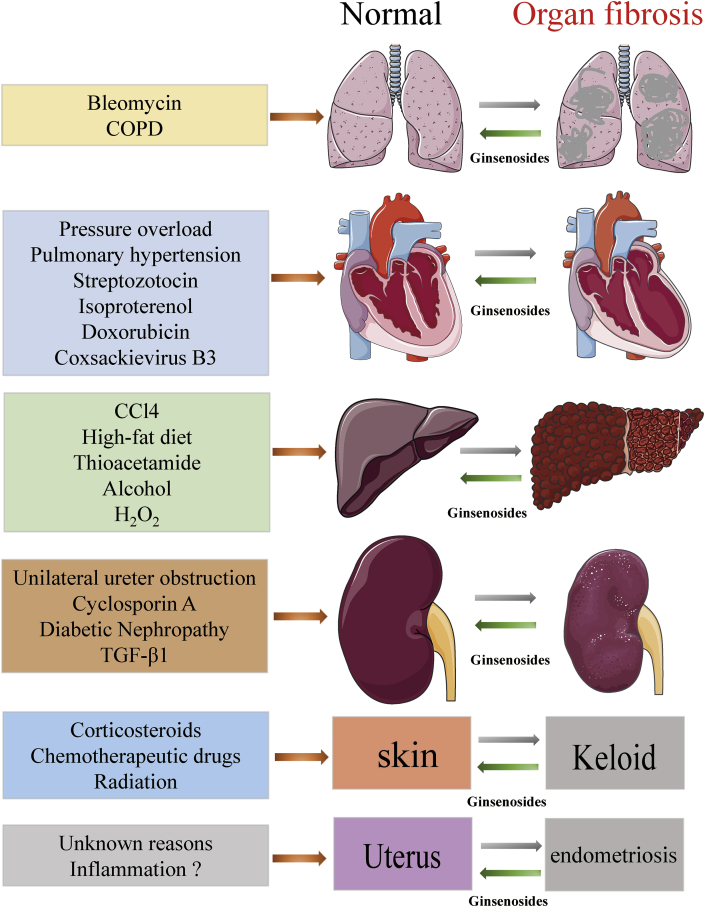
In this review, we make a brief summary on various types of animal studies concerning human diseases that show similar pathological mechanisms, including chronic inflammation and organ fibrosis. Several important phases involved in the pathological process of tissue fibrosis have been identified, all of which may be affected by ginsenosides. As an advantage to anti-fibrosis, ginsenosides are capable to i) alleviate the activation and proliferation of fibrogenic effector cells (generally refer to myofibroblasts); ii) inhibit the excessive accumulation of ECM, including collagen and fibronectin; iii) reduce oxidative stress and inflammation response, as well as fibrotic markers, especially TGF-β1; and iv) decrease the damage to parenchymal cells, including apoptotic and necrotic changes (Fig. 2). Ginsenosides exert their anti-fibrosis effects in numerous organs, including liver, heart, kidney, lung, and others (Fig. 3). Collectively, ginsenosides attenuate excessive accumulation of ECM and fibrosis in multiple organs.
Fig. 2.
Summary and functional network target analysis of ginsenosides, which exert significant anti-fibrotic effects on heart, liver, kidney, and lung via multiple links across regulatory mechanisms and multi-target effects. Abbreviation: VEGF, vascular endothelial growth factor; HIF-1, hypoxia inducible factor-1; MAPK, mitogen-activated protein kinase; GLUT4, glucose transporter type 4; TGF-β1, transforming growth factor beta 1; ERK, extracellular signal-regulated kinase; PPARδ, peroxisome proliferator-activated receptor δ; STAT3, signal transducer and activator of transcription 3; CCN2, connective tissue growth factor; FN, fibronectin; ER, endoplasmic reticulum; JNK, c-Jun N-terminal kinase; EndoMT, endothelial–mesenchymal transition; PI3K, phosphoinositide 3-kinase; EMT, epithelial–mesenchymal transition; SIRT1, sirtuin-1; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; GRP78, glucose-regulated protein 78; CHOP, CCAAT/enhancer-binding protein homologous protein; TSP-1, thrombospondin-1; HO-1, heme oxygenase; TGF-β1R, transforming growth factor beta 1 receptor; TNF-α, tumor necrosis factor α; IL-6, interleukin 6; HSC, hepatic stellate cell; PDGF, platelet-derived growth factor; ECM, extracellular matrix; ROS, reactive oxygen species; LX25Rs, liver X 25 receptors; FXR, farnesoid X receptor; P2X7R, P2X7 receptor; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; Nrf2, nuclear respiratory factor 2; AMPK, AMP-activated protein kinase; LKB1, liver kinase B1. (↓), downregulation or inhibition; (↑), upregulation or activation.
Fig. 3.
Overall protection of ginsenosides against fibrosis in various organs and tissues. Collectively, ginsenosides exert comprehensive protective effects against fibrosis in various organs and tissues, including heart, liver, lung, kidney, etc. These findings demonstrate that ginsenosides may contribute to protection and recovery of multiple organs and tissues after injury. CCl4, carbon tetrachloride; COPD: chronic obstructive pulmonary disease; TGF-β1: transforming growth factor beta 1.
Tissue fibrosis leads to organ dysfunction, and no effective therapeutics is currently available for this situation. For this reason, anti-fibrotic drugs need to be urgently developed for increasing both quality of life and survival rate of patients with fibrosis and related conditions. Abundant studies have repetitively revealed the therapeutic potential of ginsenosides in animal models of fibrosis-associated human diseases. However, to generate more therapeutic options, more continued work are worth taking into account to clarify the unconfirmed chemical composition and regulatory mechanisms, conduct standard clinical trials, and evaluate the possible side effects. Meanwhile, new guidelines about ginseng usage are required to guarantee safety and effectiveness, which is crucial for preserving the heritage and medical knowledge of our ancestries. The research progress and views provided in this review will be helpful for future exploration of ginsenosides in the development of fibrotic-related diseases therapy and to gain more reliable and reproducible data.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This study was supported by “The National Natural Science Foundation of China (Grant No U1304803)”.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2019.12.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wynn T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rockey D.C., Bell P.D., Hill J.A. Fibrosis--a common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 3.Jun J.I., Lau L.F. Resolution of organ fibrosis. J Clin Invest. 2018;128:97–107. doi: 10.1172/JCI93563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 5.Stramer B.M., Mori R., Martin P. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J Invest Dermatol. 2007;127:1009–1017. doi: 10.1038/sj.jid.5700811. [DOI] [PubMed] [Google Scholar]
- 6.Hochreiter-Hufford A., Ravichandran K.S. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5:a008748. doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiskirchen R., Weiskirchen S., Tacke F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol Aspects Med. 2019;65:2–15. doi: 10.1016/j.mam.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thannickal V.J., Lee D.Y., White E.S., Cui Z., Larios J.M., Chacon R., Horowitz J.C., Day R.M., Thomas P.E. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 10.Kim K.K., Sheppard D., Chapman H.A. TGF-beta1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol. 2018;10 doi: 10.1101/cshperspect.a022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapetanaki M.G., Mora A.L., Rojas M. Influence of age on wound healing and fibrosis. J Pathol. 2013;229:310–322. doi: 10.1002/path.4122. [DOI] [PubMed] [Google Scholar]
- 12.Romani L., Oikonomou V., Moretti S., Iannitti R.G., D'adamo M.C., Villella V.R., Pariano M., Sforna L., Borghi M., Bellet M.M. Thymosin alpha1 represents a potential potent single-molecule-based therapy for cystic fibrosis. Nat Med. 2017;23:590–600. doi: 10.1038/nm.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plasschaert L.W., Zilionis R., Choo-Wing R., Savova V., Knehr J., Roma G., Klein A.M., Jaffe A.B. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 2018;560:377–381. doi: 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong A.S., Che C.M., Leung K.W. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat Prod Rep. 2015;32:256–272. doi: 10.1039/c4np00080c. [DOI] [PubMed] [Google Scholar]
- 15.Ru W., Wang D., Xu Y., He X., Sun Y.E., Qian L., Zhou X., Qin Y. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.) Drug Discov Ther. 2015;9:23–32. doi: 10.5582/ddt.2015.01004. [DOI] [PubMed] [Google Scholar]
- 16.Cho C.W., Kim Y.C., Kang J.H., Rhee Y.K., Choi S.Y., Kim K.T., Lee Y.C., Hong H.D. Characteristic study on the chemical components of Korean curved ginseng products. J Ginseng Res. 2013;37:349–354. doi: 10.5142/jgr.2013.37.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Korean Med Sci. 2001;16(Suppl):S28–S37. doi: 10.3346/jkms.2001.16.S.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aung T.N., Qu Z., Kortschak R.D., Adelson D.L. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18030656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yun T.K., Zheng S., Choi S.Y., Cai S.R., Lee Y.S., Liu X.Y., Cho K.J., Park K.Y. Non-organ-specific preventive effect of long-term administration of Korean red ginseng extract on incidence of human cancers. J Med Food. 2010;13:489–494. doi: 10.1089/jmf.2009.1275. [DOI] [PubMed] [Google Scholar]
- 21.Yun T.K. Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat Res. 2003;523–524:63–74. doi: 10.1016/s0027-5107(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 22.Yun T.K., Choi S.Y., Yun H.Y. Epidemiological study on cancer prevention by ginseng: are all kinds of cancers preventable by ginseng? J Korean Med Sci. 2001;16(Suppl):S19–S27. doi: 10.3346/jkms.2001.16.S.S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui Y., Shu X.O., Gao Y.T., Cai H., Tao M.H., Zheng W. Association of ginseng use with survival and quality of life among breast cancer patients. Am J Epidemiol. 2006;163:645–653. doi: 10.1093/aje/kwj087. [DOI] [PubMed] [Google Scholar]
- 24.Yennurajalingam S., Reddy A., Tannir N.M., Chisholm G.B., Lee R.T., Lopez G., Escalante C.P., Manzullo E.F., Frisbee Hume S., Williams J.L. High-Dose Asian Ginseng (Panax Ginseng) for Cancer-Related Fatigue: A Preliminary Report. Integr Cancer Ther. 2015;14:419–427. doi: 10.1177/1534735415580676. [DOI] [PubMed] [Google Scholar]
- 25.Zhou B., Yan Z., Liu R., Shi P., Qian S., Qu X., Zhu L., Zhang W., Wang J. Prospective Study of Transcatheter Arterial Chemoembolization (TACE) with Ginsenoside Rg3 versus TACE Alone for the Treatment of Patients with Advanced Hepatocellular Carcinoma. Radiology. 2016;280:630–639. doi: 10.1148/radiol.2016150719. [DOI] [PubMed] [Google Scholar]
- 26.Li B., Zhao J., Wang C.Z., Searle J., He T.C., Yuan C.S., Du W. Ginsenoside Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of p53. Cancer Lett. 2011;301:185–192. doi: 10.1016/j.canlet.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y., Liu Y., Chen K. Roles and mechanisms of ginsenoside in cardiovascular diseases: progress and perspectives. Sci China Life Sci. 2016;59:292–298. doi: 10.1007/s11427-016-5007-8. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Q., Bao X.Y., Zhu P.C., Tong Q., Zheng G.Q., Wang Y. Ginsenoside Rb1 for myocardial ischemia/reperfusion injury: preclinical evidence and possible mechanisms. Oxid Med Cell Longev. 2017;2017:6313625. doi: 10.1155/2017/6313625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J.H., Yi Y.S., Kim M.Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheng C., Peng W., Xia Z.A., Wang Y., Chen Z., Su N., Wang Z. The impact of ginsenosides on cognitive deficits in experimental animal studies of Alzheimer's disease: a systematic review. BMC Complement Altern Med. 2015;15:386. doi: 10.1186/s12906-015-0894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song L., Xu M.B., Zhou X.L., Zhang D.P., Zhang S.L., Zheng G.Q. A preclinical systematic review of ginsenoside-rg1 in experimental Parkinson's disease. Oxid Med Cell Longev. 2017;2017:2163053. doi: 10.1155/2017/2163053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wynn T.A. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gourdie R.G., Dimmeler S., Kohl P. Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat Rev Drug Discov. 2016;15:620–638. doi: 10.1038/nrd.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin A., Tonelli M., Bonventre J., Coresh J., Donner J.A., Fogo A.B., Fox C.S., Gansevoort R.T., Heerspink H.J.L., Jardine M. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. 2017;390:1888–1917. doi: 10.1016/S0140-6736(17)30788-2. [DOI] [PubMed] [Google Scholar]
- 35.Rangarajan S., Bone N.B., Zmijewska A.A., Jiang S., Park D.W., Bernard K., Locy M.L., Ravi S., Deshane J., Mannon R.B. Metformin reverses established lung fibrosis in a bleomycin model. Nat Med. 2018;24:1121–1127. doi: 10.1038/s41591-018-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Udomsinprasert W., Jittikoon J. Vitamin D and liver fibrosis: molecular mechanisms and clinical studies. Biomed Pharmacother. 2019;109:1351–1360. doi: 10.1016/j.biopha.2018.10.140. [DOI] [PubMed] [Google Scholar]
- 37.Kim W.R., Brown R.S., Jr., Terrault N.A., El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–242. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 38.Peng X.D., Dai L.L., Huang C.Q., He C.M., Yang B., Chen L.J. Relationship between anti-fibrotic effect of Panax notoginseng saponins and serum cytokines in rat hepatic fibrosis. Biochem Biophys Res Commun. 2009;388:31–34. doi: 10.1016/j.bbrc.2009.07.099. [DOI] [PubMed] [Google Scholar]
- 39.Lim W., Mudge K.W., Vermeylen F. Effects of population, age, and cultivation methods on ginsenoside content of wild American ginseng (Panax quinquefolium) J Agric Food Chem. 2005;53:8498–8505. doi: 10.1021/jf051070y. [DOI] [PubMed] [Google Scholar]
- 40.Hou Y.L., Tsai Y.H., Lin Y.H., Chao J.C. Ginseng extract and ginsenoside Rb1 attenuate carbon tetrachloride-induced liver fibrosis in rats. BMC Complement Altern Med. 2014;14:415. doi: 10.1186/1472-6882-14-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han X., Song J., Lian L.H., Yao Y.L., Shao D.Y., Fan Y., Hou L.S., Wang G., Zheng S., Wu Y.L. Ginsenoside 25-OCH3-PPD Promotes Activity of LXRs To Ameliorate P2X7R-Mediated NLRP3 Inflammasome in the Development of Hepatic Fibrosis. J Agric Food Chem. 2018;66:7023–7035. doi: 10.1021/acs.jafc.8b01982. [DOI] [PubMed] [Google Scholar]
- 42.Beaven S.W., Wroblewski K., Wang J., Hong C., Bensinger S., Tsukamoto H., Tontonoz P. Liver X receptor signaling is a determinant of stellate cell activation and susceptibility to fibrotic liver disease. Gastroenterology. 2011;140:1052–1062. doi: 10.1053/j.gastro.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y.L., Wan Y., Jin X.J., Ouyang B.Q., Bai T., Zhao Y.Q., Nan J.X. 25-OCH3-PPD induces the apoptosis of activated t-HSC/Cl-6 cells via c-FLIP-mediated NF-kappaB activation. Chem Biol Interact. 2011;194:106–112. doi: 10.1016/j.cbi.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Su G.Y., Li Z.Y., Wang R., Lu Y.Z., Nan J.X., Wu Y.L., Zhao Y.Q. Signaling pathways involved in p38-ERK and inflammatory factors mediated the anti-fibrosis effect of AD-2 on thioacetamide-induced liver injury in mice. Food Funct. 2019;10:3992–4000. doi: 10.1039/c8fo02405g. [DOI] [PubMed] [Google Scholar]
- 45.Bataller R., Brenner D.A. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geng J., Peng W., Huang Y., Fan H., Li S. Ginsenoside-Rg1 from Panax notoginseng prevents hepatic fibrosis induced by thioacetamide in rats. Eur J Pharmacol. 2010;634:162–169. doi: 10.1016/j.ejphar.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 47.Li J.P., Gao Y., Chu S.F., Zhang Z., Xia C.Y., Mou Z., Song X.Y., He W.B., Guo X.F., Chen N.H. Nrf2 pathway activation contributes to anti-fibrosis effects of ginsenoside Rg1 in a rat model of alcohol- and CCl4-induced hepatic fibrosis. Acta Pharmacol Sin. 2014;35:1031–1044. doi: 10.1038/aps.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lo Y.T., Tsai Y.H., Wu S.J., Chen J.R., Chao J.C. Ginsenoside Rb1 inhibits cell activation and liver fibrosis in rat hepatic stellate cells. J Med Food. 2011;14:1135–1143. doi: 10.1089/jmf.2010.1485. [DOI] [PubMed] [Google Scholar]
- 49.Higashi T., Friedman S.L., Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27–42. doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X.J., Liu W.J., Wen M.L., Liang H., Wu S.M., Zhu Y.Z., Zhao J.Y., Dong X.Q., Li M.G., Bian L. Ameliorative effects of Compound K and ginsenoside Rh1 on non-alcoholic fatty liver disease in rats. Sci Rep. 2017;7:41144. doi: 10.1038/srep41144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei X., Chen Y., Huang W. Ginsenoside Rg1 ameliorates liver fibrosis via suppressing epithelial to mesenchymal transition and reactive oxygen species production in vitro and in vivo. Biofactors. 2018 doi: 10.1002/biof.1432. [DOI] [PubMed] [Google Scholar]
- 52.Park S.M., Jung E.H., Kim J.K., Jegal K.H., Park C.A., Cho I.J., Kim S.C. 20S-Protopanaxadiol, an aglycosylated ginsenoside metabolite, induces hepatic stellate cell apoptosis through liver kinase B1-AMP-activated protein kinase activation. J Ginseng Res. 2017;41:392–402. doi: 10.1016/j.jgr.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan D., Xiang T., Huo Y., Liu C., Wang T., Zhou Z., Dun Y., Zhao H., Zhang C. Preventive effects of total saponins of Panax japonicus on fatty liver fibrosis in mice. Arch Med Sci. 2018;14:396–406. doi: 10.5114/aoms.2016.63260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berk B.C., Fujiwara K., Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gyongyosi M., Winkler J., Ramos I., Do Q.T., Firat H., Mcdonald K., González A., Thum T., Díez J., Jaisser F. Myocardial fibrosis: biomedical research from bench to bedside. Eur J Heart Fail. 2017;19:177–191. doi: 10.1002/ejhf.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen M.N., Kiriazis H., Gao X.M., Du X.J. Cardiac fibrosis and arrhythmogenesis. Compr Physiol. 2017;7:1009–1049. doi: 10.1002/cphy.c160046. [DOI] [PubMed] [Google Scholar]
- 57.Kim J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: a review for use in cardiovascular diseases. J Ginseng Res. 2018;42:264–269. doi: 10.1016/j.jgr.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y.J., Zhang X.L., Li M.H., Iqbal J., Bourantas C.V., Li J.J., Su X.Y., Muramatsu T., Tian N.L., Chen S.L. The ginsenoside Rg1 prevents transverse aortic constriction-induced left ventricular hypertrophy and cardiac dysfunction by inhibiting fibrosis and enhancing angiogenesis. J Cardiovasc Pharmacol. 2013;62:50–57. doi: 10.1097/FJC.0b013e31828f8d45. [DOI] [PubMed] [Google Scholar]
- 59.Li C.Y., Deng W., Liao X.Q., Deng J., Zhang Y.K., Wang D.X. The effects and mechanism of ginsenoside Rg1 on myocardial remodeling in an animal model of chronic thromboembolic pulmonary hypertension. Eur J Med Res. 2013;18:16. doi: 10.1186/2047-783X-18-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu Z.M., Li C.B., Liu Q.L., Li P., Yang H. Ginsenoside Rg1 prevents doxorubicin-induced cardiotoxicity through the inhibition of autophagy and endoplasmic reticulum stress in mice. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19113658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei H.J., Yang H.H., Chen C.H., Lin W.W., Chen S.C., Lai P.H., Chang Y., Sung H.W. Gelatin microspheres encapsulated with a nonpeptide angiogenic agent, ginsenoside Rg1, for intramyocardial injection in a rat model with infarcted myocardium. J Control Release. 2007;120:27–34. doi: 10.1016/j.jconrel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Li Y., Wang L., Dong Z., Wang S., Qi L., Cho K., Zhang Z., Li N., Hu Y., Jiang B. Cardioprotection of salvianolic acid B and ginsenoside Rg1 combination on subacute myocardial infarction and the underlying mechanism. Phytomedicine. 2019;57:255–261. doi: 10.1016/j.phymed.2018.12.040. [DOI] [PubMed] [Google Scholar]
- 63.Zheng X., Wang S., Zou X., Jing Y., Yang R., Li S., Wang F. Ginsenoside Rb1 improves cardiac function and remodeling in heart failure. Exp Anim. 2017;66:217–228. doi: 10.1538/expanim.16-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huynh K., Mcmullen J.R., Julius T.L., Tan J.W., Love J.E., Cemerlang N., Kiriazis H., Du X.J., Ritchie R.H. Cardiac-specific IGF-1 receptor transgenic expression protects against cardiac fibrosis and diastolic dysfunction in a mouse model of diabetic cardiomyopathy. Diabetes. 2010;59:1512–1520. doi: 10.2337/db09-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lo S.H., Hsu C.T., Niu H.S., Niu C.S., Cheng J.T., Chen Z.C. Ginsenoside Rh2 improves cardiac fibrosis via PPARdelta-STAT3 signaling in type 1-like diabetic rats. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Q.W., Yu X.F., Xu H.L., Zhao X.Z., Sui D.Y. Ginsenoside Re improves isoproterenol-induced myocardial fibrosis and heart failure in rats. Evid Based Complement Alternat Med. 2019;2019:3714508. doi: 10.1155/2019/3714508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang N., An X., Lang P., Wang F., Xie Y. Ginsenoside Rd contributes the attenuation of cardiac hypertrophy in vivo and in vitro. Biomed Pharmacother. 2019;109:1016–1023. doi: 10.1016/j.biopha.2018.10.081. [DOI] [PubMed] [Google Scholar]
- 68.Yang L., Liu Q., Yu Y., Xu H., Chen S., Shi S. Ginsenoside-Rb3 inhibits endothelial-mesenchymal transition of cardiac microvascular endothelial cells. Herz. 2019;44:60–68. doi: 10.1007/s00059-017-4628-4. [DOI] [PubMed] [Google Scholar]
- 69.Shen N., Li X., Zhou T., Bilal M.U., Du N., Hu Y., Qin W., Xie Y., Wang H., Wu J. Shensong Yangxin Capsule prevents diabetic myocardial fibrosis by inhibiting TGF-beta1/Smad signaling. J Ethnopharmacol. 2014;157:161–170. doi: 10.1016/j.jep.2014.09.035. [DOI] [PubMed] [Google Scholar]
- 70.Zhang K., Zhang J., Wang X., Wang L., Pugliese M., Passantino A., Li J. Cardioprotection of Sheng Mai Yin a classic formula on adriamycin induced myocardial injury in Wistar rats. Phytomedicine. 2018;38:1–11. doi: 10.1016/j.phymed.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Mutsaers H.A., Olinga P. Editorial: organ fibrosis: triggers, pathways, and cellular plasticity. Front Med (Lausanne) 2016;3:55. doi: 10.3389/fmed.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie X.S., Yang M., Liu H.C., Zuo C., Li Z., Deng Y., Fan J.M. Influence of ginsenoside Rg1, a panaxatriol saponin from Panax notoginseng, on renal fibrosis in rats with unilateral ureteral obstruction. J Zhejiang Univ Sci B. 2008;9:885–894. doi: 10.1631/jzus.B0820024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie X.S., Liu H.C., Yang M., Zuo C., Deng Y., Fan J.M. Ginsenoside Rb1, a panoxadiol saponin against oxidative damage and renal interstitial fibrosis in rats with unilateral ureteral obstruction. Chin J Integr Med. 2009;15:133–140. doi: 10.1007/s11655-009-0133-9. [DOI] [PubMed] [Google Scholar]
- 74.Xie X.S., Liu H.C., Wang F.P., Zhang C.L., Zuo C., Deng Y., Fan J.M. Ginsenoside Rg1 modulation on thrombospondin-1 and vascular endothelial growth factor expression in early renal fibrogenesis in unilateral obstruction. Phytother Res. 2010;24:1581–1587. doi: 10.1002/ptr.3190. [DOI] [PubMed] [Google Scholar]
- 75.Li S.S., Ye J.M., Deng Z.Y., Yu L.X., Gu X.X., Liu Q.F. Ginsenoside-Rg1 inhibits endoplasmic reticulum stress-induced apoptosis after unilateral ureteral obstruction in rats. Ren Fail. 2015;37:890–895. doi: 10.3109/0886022X.2015.1015427. [DOI] [PubMed] [Google Scholar]
- 76.Li S.S., He A.L., Deng Z.Y., Liu Q.F. Ginsenoside-Rg1 protects against renal fibrosis by regulating the Klotho/TGF-beta1/Smad signaling pathway in rats with obstructive nephropathy. Biol Pharm Bull. 2018;41:585–591. doi: 10.1248/bpb.b17-00934. [DOI] [PubMed] [Google Scholar]
- 77.Du Y.G., Wang L.P., Qian J.W., Zhang K.N., Chai K.F. Panax notoginseng saponins protect kidney from diabetes by up-regulating silent information regulator 1 and activating antioxidant proteins in rats. Chin J Integr Med. 2016;22:910–917. doi: 10.1007/s11655-015-2446-1. [DOI] [PubMed] [Google Scholar]
- 78.Du N., Xu Z., Gao M., Liu P., Sun B., Cao X. Combination of Ginsenoside Rg1 and Astragaloside IV reduces oxidative stress and inhibits TGF-beta1/Smads signaling cascade on renal fibrosis in rats with diabetic nephropathy. Drug Des Devel Ther. 2018;12:3517–3524. doi: 10.2147/DDDT.S171286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Q.F., Deng Z.Y., Ye J.M., He A.L., Li S.S. Ginsenoside Rg1 protects chronic cyclosporin a nephropathy from tubular cell apoptosis by inhibiting endoplasmic reticulum stress in rats. Transplant Proc. 2015;47:566–569. doi: 10.1016/j.transproceed.2014.10.047. [DOI] [PubMed] [Google Scholar]
- 80.Lim S.W., Doh K.C., Jin L., Jin J., Piao S.G., Heo S.B., Chung B.H., Yang C.W. Ginseng treatment attenuates autophagic cell death in chronic cyclosporine nephropathy. Nephrology (Carlton) 2014;19:490–499. doi: 10.1111/nep.12273. [DOI] [PubMed] [Google Scholar]
- 81.Doh K.C., Lim S.W., Piao S.G., Jin L., Heo S.B., Zheng Y.F., Bae S.K., Hwang G.H., Min K.I., Chung B.H. Ginseng treatment attenuates chronic cyclosporine nephropathy via reducing oxidative stress in an experimental mouse model. Am J Nephrol. 2013;37:421–433. doi: 10.1159/000349921. [DOI] [PubMed] [Google Scholar]
- 82.Xie X.S., Yang M., Liu H.C., Zuo C., Li H.J., Fan J.M. Ginsenoside Rg1, a major active component isolated from Panax notoginseng, restrains tubular epithelial to myofibroblast transition in vitro. J Ethnopharmacol. 2009;122:35–41. doi: 10.1016/j.jep.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 83.Hong Y.A., Lim J.H., Kim M.Y., Kim E.N., Koh E.S., Shin S.J., Choi B.S., Park C.W., Chang Y.S., Chung S. Delayed treatment with oleanolic acid attenuates tubulointerstitial fibrosis in chronic cyclosporine nephropathy through Nrf2/HO-1 signaling. J Transl Med. 2014;12:50. doi: 10.1186/1479-5876-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Richeldi L., Collard H.R., Jones M.G. Idiopathic pulmonary fibrosis. Lancet. 2017;389:1941–1952. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- 85.Zhang J., Li Q., Shao Q., Song J., Zhou B., Shu P. Effects of panax notoginseng saponin on the pathological ultrastructure and serum IL-6 and IL-8 in pulmonary fibrosis in rabbits. J Cell Biochem. 2018;119:8410–8418. doi: 10.1002/jcb.27045. [DOI] [PubMed] [Google Scholar]
- 86.Tsai K.D., Yang S.M., Lee J.C., Wong H.Y., Shih C.M., Lin T.H., Tseng M.J., Chen W. Panax notoginseng Attenuates Bleomycin-Induced Pulmonary Fibrosis in Mice. Evid Based Complement Alternat Med. 2011;2011:404761. doi: 10.1155/2011/404761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhan H., Huang F., Ma W., Zhao Z., Zhang H., Zhang C. Protective effect of ginsenoside Rg1 on bleomycin-induced pulmonary fibrosis in rats: involvement of caveolin-1 and TGF-beta1 signal pathway. Biol Pharm Bull. 2016;39:1284–1292. doi: 10.1248/bpb.b16-00046. [DOI] [PubMed] [Google Scholar]
- 88.Guan S., Liu Q., Han F., Gu W., Song L., Zhang Y., Guo X., Xu W. Ginsenoside Rg1 ameliorates cigarette smoke-induced airway fibrosis by suppressing the TGF-beta1/Smad pathway in vivo and in vitro. Biomed Res Int. 2017;2017:6510198. doi: 10.1155/2017/6510198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang L., Chen P.P., Luo M., Shi W.L., Hou D.S., Gao Y., Xu S.F., Deng J. Inhibitory effects of total ginsenoside on bleomycin-induced pulmonary fibrosis in mice. Biomed Pharmacother. 2019;114:108851. doi: 10.1016/j.biopha.2019.108851. [DOI] [PubMed] [Google Scholar]
- 90.Cheng L., Sun X., Hu C., Jin R., Sun B., Shi Y., Cui W., Zhang Y. In vivo early intervention and the therapeutic effects of 20(s)-ginsenoside rg3 on hypertrophic scar formation. PLoS One. 2014;9:e113640. doi: 10.1371/journal.pone.0113640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng L., Sun X., Hu C., Jin R., Sun B., Shi Y., Zhang L., Cui W., Zhang Y. In vivo inhibition of hypertrophic scars by implantable ginsenoside-Rg3-loaded electrospun fibrous membranes. Acta Biomater. 2013;9:9461–9473. doi: 10.1016/j.actbio.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 92.Cui W., Cheng L., Hu C., Li H., Zhang Y., Chang J. Electrospun poly(L-lactide) fiber with ginsenoside rg3 for inhibiting scar hyperplasia of skin. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun X., Cheng L., Zhu W., Hu C., Jin R., Sun B., Shi Y., Zhang Y., Cui W. Use of ginsenoside Rg3-loaded electrospun PLGA fibrous membranes as wound cover induces healing and inhibits hypertrophic scar formation of the skin. Colloids Surf B Biointerfaces. 2014;115:61–70. doi: 10.1016/j.colsurfb.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 94.Tang M., Bian W., Cheng L., Zhang L., Jin R., Wang W., Zhang Y. Ginsenoside Rg3 inhibits keloid fibroblast proliferation, angiogenesis and collagen synthesis in vitro via the TGFbeta/Smad and ERK signaling pathways. Int J Mol Med. 2018;41:1487–1499. doi: 10.3892/ijmm.2018.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tark K.C., Lee D.W., Lew D.H., Kang E.H., Roh H., Lee M.C. Effects of ginsenoside Rb1 on hypertrophic scar remodeling in rabbit model. Eur J Pharmacol. 2015;750:151–159. doi: 10.1016/j.ejphar.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 96.Dai J.P., Chen X.X., Zhu D.X., Wan Q.Y., Chen C., Wang G.F., Li W.Z., Li K.S. Panax notoginseng saponins inhibit areca nut extract-induced oral submucous fibrosis in vitro. J Oral Pathol Med. 2014;43:464–470. doi: 10.1111/jop.12158. [DOI] [PubMed] [Google Scholar]
- 97.Kim M.K., Lee S.K., Park J.H., Lee J.H., Yun B.H., Park J.H., Seo S.K., Cho S., Choi Y.S. Ginsenoside Rg3 Decreases Fibrotic and Invasive Nature of Endometriosis by Modulating miRNA-27b: In Vitro and In Vivo Studies. Sci Rep. 2017;7:17670. doi: 10.1038/s41598-017-17956-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Greenspan E.M. Ginseng and vaginal bleeding. JAMA. 1983;249:2018. [PubMed] [Google Scholar]
- 99.Kabalak A.A., Soyal O.B., Urfalioglu A., Saracoglu F., Gogus N. Menometrorrhagia and tachyarrhythmia after using oral and topical ginseng. J Womens Health (Larchmt) 2004;13:830–833. doi: 10.1089/jwh.2004.13.830. [DOI] [PubMed] [Google Scholar]
- 100.Oh K.J., Chae M.J., Lee H.S., Hong H.D., Park K. Effects of Korean red ginseng on sexual arousal in menopausal women: placebo-controlled, double-blind crossover clinical study. J Sex Med. 2010;7:1469–1477. doi: 10.1111/j.1743-6109.2009.01700.x. [DOI] [PubMed] [Google Scholar]
- 101.Coon J.T., Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf. 2002;25:323–344. doi: 10.2165/00002018-200225050-00003. [DOI] [PubMed] [Google Scholar]
- 102.Miller L.G. Herbal medicinals: selected clinical considerations focusing on known or potential drug-herb interactions. Arch Intern Med. 1998;158:2200–2211. doi: 10.1001/archinte.158.20.2200. [DOI] [PubMed] [Google Scholar]
- 103.Scaglione F., Cattaneo G., Alessandria M., Cogo R. Efficacy and safety of the standardised Ginseng extract G115 for potentiating vaccination against the influenza syndrome and protection against the common cold [corrected] Drugs Exp Clin Res. 1996;22:65–72. [PubMed] [Google Scholar]
- 104.Siegel R.K. Ginseng and high blood pressure. JAMA. 1980;243:32. [PubMed] [Google Scholar]
- 105.Siegel R.K. Ginseng abuse syndrome. Problems with the panacea. JAMA. 1979;241:1614–1615. [PubMed] [Google Scholar]
- 106.Biswas T., Mathur A.K., Mathur A. A literature update elucidating production of Panax ginsenosides with a special focus on strategies enriching the anti-neoplastic minor ginsenosides in ginseng preparations. Appl Microbiol Biotechnol. 2017;101:4009–4032. doi: 10.1007/s00253-017-8279-4. [DOI] [PubMed] [Google Scholar]
- 107.Li L., Chen X., Li D., Zhong D. Identification of 20(S)-protopanaxadiol metabolites in human liver microsomes and human hepatocytes. Drug Metab Dispos. 2011;39:472–483. doi: 10.1124/dmd.110.036723. [DOI] [PubMed] [Google Scholar]
- 108.Xu L.Q., Yu H., Yin S.P., Zhang R.X., Zhou Y.D., Li J. Liposome-based delivery systems for ginsenoside Rh2: in vitro and in vivo comparisons. J Nanopart Res. 2015;17 [Google Scholar]
- 109.Han M., Sha X., Wu Y., Fang X. Oral absorption of ginsenoside Rb1 using in vitro and in vivo models. Planta Med. 2006;72:398–404. doi: 10.1055/s-2005-916211. [DOI] [PubMed] [Google Scholar]
- 110.Liu H., Yang J., Du F., Gao X., Ma X., Huang Y., Xu F., Niu W., Wang F., Mao Y. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab Dispos. 2009;37:2290–2298. doi: 10.1124/dmd.109.029819. [DOI] [PubMed] [Google Scholar]
- 111.Artursson P., Ungell A.L., Löfroth J.E. Selective paracellular permeability in two models of intestinal absorption: cultured monolayers of human intestinal epithelial cells and rat intestinal segments. Pharm Res. 1993;10:1123–1129. doi: 10.1023/a:1018903931777. [DOI] [PubMed] [Google Scholar]
- 112.Yanni S., Thakker D.R. Springer; New York: 2007. Prodrugs: absorption, distribution, metabolism, excretion (ADME) issues. [Google Scholar]
- 113.Chen D., Yu H., Mu H., Li G., Shen Y. Novel multicore niosomes based on double pH-sensitive mixed micelles for Ginsenoside Rh2 delivery. Artif Cells Nanomed Biotechnol. 2014;42:205–209. doi: 10.3109/21691401.2013.794358. [DOI] [PubMed] [Google Scholar]
- 114.Xiong J., Guo J., Huang L., Meng B., Ping Q. Self-micelle formation and the incorporation of lipid in the formulation affect the intestinal absorption of Panax notoginseng. Int J Pharm. 2008;360:191–196. doi: 10.1016/j.ijpharm.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 115.Li T., Shu Y.J., Cheng J.Y., Liang R.C., Dian S.N., Lv X.X., Yang M.Q., Huang S.L., Chen G., Yang F. Pharmacokinetics and efficiency of brain targeting of ginsenosides Rg1 and Rb1 given as Nao-Qing microemulsion. Drug Dev Ind Pharm. 2015;41:224–231. doi: 10.3109/03639045.2013.858734. [DOI] [PubMed] [Google Scholar]
- 116.Cai H., Wen X., Wen L., Tirelli N., Zhang X., Zhang Y., Su H., Yang F., Chen G. Enhanced local bioavailability of single or compound drugs delivery to the inner ear through application of PLGA nanoparticles via round window administration. Int J Nanomedicine. 2014;9:5591–5601. doi: 10.2147/IJN.S72555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.