Abstract
Background
Active natural ingredients, especially small molecules, have recently received wide attention as modifiers used to treat neurodegenerative disease by promoting neurogenic regeneration of neural stem cell (NSC) in situ. 20(S)-protopanaxadiol (PPD), one of the bioactive ingredients in ginseng, possesses neuroprotective properties. However, the effect of PPD on NSC proliferation and differentiation and its mechanism of action are incompletely understood.
Methods
In this study, we investigated the impact of PPD on NSC proliferation and neuronal lineage differentiation through activation of the Wnt/glycogen synthase kinase (GSK)-3β/β-catenin pathway. NSC migration and proliferation were investigated by neurosphere assay, Cell Counting Kit-8 assay, and EdU assay. NSC differentiation was analyzed by Western blot and immunofluorescence staining. Involvement of the Wnt/GSK3β/β-catenin pathway was examined by molecular simulation and Western blot and verified using gene transfection.
Results
PPD significantly promoted neural migration and induced a significant increase in NSC proliferation in a time- and dose-dependent manner. Furthermore, a remarkable increase in antimicrotubule-associated protein 2 expression and decrease in nestin protein expression were induced by PPD. During the differentiation process, PPD targeted and stimulated the phosphorylation of GSK-3β at Ser9 and the active forms of β-catenin, resulting in activation of the Wnt/GSK-3β/β-catenin pathway. Transfection of NSCs with a constitutively active GSK-3β mutant at S9A significantly hampered the proliferation and neural differentiation mediated by PPD.
Conclusion
PPD promotes NSC proliferation and neural differentiation in vitro via activation of the Wnt/GSK-3β/β-catenin pathway by targeting GSK-3β, potentially having great significance for the treatment of neurodegenerative diseases.
Keywords: 20(S)-protopanaxadiol, Neural differentiation, Neural stem cell, Proliferation, Wnt/GSK-3β/β-catenin pathway
1. Introduction
The exponential growth of the aging population makes neurodegenerative disease, including Alzheimer's disease (AD) and Parkinson's disease, one of the major threats to human health care [1]. Because the main pathogenesis of neurodegenerative disease is neuron death or deactivation in the brain, a promising therapeutic strategy for the ultimately curing neurodegenerative diseases is neuron regeneration [2] by transplanting neurons differentiated from neural stem cells (NSCs) in vitro or, even better, promoting the neurogenic regeneration of NSCs in situ through proliferation and differentiation of NSCs into functional neurons [3]. However, under normal conditions, NSCs have limited self-renewal and differentiation abilities [4] that normally require growth factor (GF) induction. However, the large amount of GFs required to induce NSCs proliferation and differentiation in vitro may be a carcinogenic risk in vivo after transplantation [5]. Active ingredients extracted from natural medicines, especially small bioactive molecules that freely across the blood–brain barrier (BBB), have recently received wide attention as modifiers used to promote the proliferation and neural differentiation of NSCs.
20(S)-protopanaxadiol (PPD), a derivative of ginsenosides, is one of the active ingredients in ginseng widely used in clinical practice in the treatment of AD. Recently, many studies found that ginsenosides can induce neuronal differentiation in NSCs [6], but passage of large molecules through the BBB to reach the site of the pathological changes associated with AD is difficult. PPD has been shown to penetrate the BBB [7], making it a potential ideal treatment for AD; however, the effect of PPD on NSC proliferation and differentiation and its mechanism of action are incompletely understood.
The Wnt/glycogen synthase kinase (GSK)-3β/β-catenin signaling pathway is found to play a critical role in controlling NSC proliferation and differentiation [8]. β-Catenin is a key regulatory enzyme that is generally phosphorylated and can be degraded by GSK-3β. Hence, GSK-3β is a crucial enzyme in the negative regulation of the Wnt/GSK-3β/β-catenin signaling pathway and can be negatively regulated through phosphorylation at Ser9 [9]. Inactivation of GSK-3β can promote neural differentiation of NSCs [10], indicating that GSK-3β inactivation is an ideal target for NSC induction.
This study investigated the effect of PPD on induction of primary cultured NSCs. The NSC proliferation was monitored using Cell Counting Kit-8 (CCK-8) assay, 5-ethynyl-2′-deoxyuridine (EdU) assay, and neurosphere assay; migration was monitored using the neurosphere method; and differentiation was analyzed by detecting specific neural markers. The Wnt/GSK-3β/β-catenin pathway was studied to investigate the possible mechanism of the effects of PPD using gene-silencing technology. We found that PPD shows the potential to improve the proliferation and neural differentiation of NSCs through stimulating the Wnt/GSK-3β/β-catenin pathway through suppressing the activation of GSK-3β.
2. Materials and methods
2.1. Reagents
PPD was obtained from Chengdu Must Bio-Technology Co., Ltd (Chengdu, China), and the purity is 98.59% assayed by high-performance liquid chromatography (HPLC). Epidermal GF and basic fibroblast GF were obtained from PeproTech (Rocky Hill, NJ, USA). Hank's balanced salt solution, penicillin–streptomycin–neomycin, B27 supplement, and phosphate buffer solution (PBS) were purchased form Thermo Fisher Scientific (Waltham, MA, USA). CCK-8 was purchased from Dojindo Molecular Technologies (Rockville, MD, USA.). A Cell-LightTM EdU apollo643 in vitro kit was purchased from RiboBio (Guangzhou, China). Antinestin antibody, anti–microtubule-associated protein 2 (MAP2) antibody, and anti–glial fibrillary acidic protein (GFAP) antibody were obtained from EMD Millipore Corporation (Temecula, CA, USA). Anti–p-GSK-3β(Ser9) antibody, anti–GSK-3β antibody, anti–non-p-β-catenin (active) antibody, and anti–β-catenin antibody were purchased from Cell Signaling Technology (Beverly, MA, USA). Poly-D-lysine, anti–β-actin antibody, and horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Primary cell culture of NSCs
The experimental method previously described [11] was approved by the Department of Health, the Government of the Hong Kong Special Administrative Region, and researched in consonant with relevant guidelines and requirements of the Animal Ethics Committee at Hong Kong Baptist University. The NSCs were primary cultured from postnatal Day 1 to Day 2 rats (Sprague Dawley) obtained from the Chinese University of Hong Kong. Briefly, the subventricular zone (SVZ) with the thickness of around 200 μm was rapidly removed from the brain, immersed in cold HBSS, manually cut into pieces, digested with trypsin, and maintained at 37°C. After 15 min, the suspension was then filtered through a 40-μm-pore cell strainer, and the filtrate was transferred into a sterile centrifugal tube and centrifuged (1,000 rpm, 5 min) subsequently. The cells at the bottom were resuspended in a neurobasal medium with 20 ng/mL epidermal GF, 20 ng/mL basic fibroblast GF, 2% B27 supplement, and 1% penicillin–streptomycin–neomycin and cultured at 37°C incubator with 5% CO2 and 100% humidity. A half volume of the medium was replaced every 2–3 days. Mechanical passaging was performed when the size of neurospheres was around 150–200 μm in diameter.
2.3. Cell migration analysis
Neurospheres, with the diameter of approximately 200 μm, were seeded onto coverslips coated by poly-D-lysine for 30 min to allow attachment of neurospheres. The neurospheres were then incubated with a variety of concentrations of PPD (10 μM, 20 μM, 40 μM). After 24 h of incubation, the distance of NSCs migrated out of neurospheres was calculated and compared using ImageJ. Immunostaining for nestin (NSC marker) [12] and MAP2 (neuron marker) [13] were also performed as described in the following section.
2.4. Neurosphere assay
Single NSC, dissociated from neurospheres, were seeded into a 96-well plate with 5 × 103 cells/well and various concentrations of PPD (10 μM, 20 μM, 40 μM) without GFs. Images were acquired on day 3 to compare the number and diameter of neurospheres, counting only those with a diameter over 30 μm [14], by ImageJ software.
2.5. CCK-8 assay
Single NSC, dissociated from neurospheres, were seeded into 96-well plates with 5 × 103 cells/well and various concentrations of PPD (10 μM, 20 μM, 40 μM) was added 1 day, 2 days, and 3 days, respectively. CCK-8 assay was used complying with the manufacturer's instructions. After incubation for another 4 h, the absorbance was measured by an automatic microplate reader at 450 nm wavelength (ELx800; BioTek, Winooski, VT, United States).
2.6. EdU assay
EdU assay was performed as per manufacturer's instructions of Cell-LightTM EdU apollo643 in vitro kit and the reference [15]. Single NSC was induced with various concentrations of PPD (10 μM, 20 μM, 40 μM) for 3 days, and EdU (50 μM) was added for 12 h before the end of the culture to label proliferating cells, then fixed by 4% paraformaldehyde (Sigma-Aldrich) for 30 min. Apollo reaction cocktail was added for 30 min, and Hoechst 33342 was used for DNA staining. The analysis of NSC proliferation, represented as the ration of EdU+ to all cells, was performed using randomly selected images obtained by the fluorescence microscope.
2.7. Induction of cell differentiation
Single NSC was seeded at 2 × 105 cells/well on poly-D-lysine–coated 6-well plate. PPD at various concentrations (10 μM, 20 μM, 40 μM) was added to the culture medium and cultured for 7 days. The differentiation of NSC was indicated by detecting the level of protein markers, including nestin, MAP2, and GFAP (astrocyte marker) [13], monitored by immunofluorescent staining and Western blot after 7 days of treatment.
2.8. Immunofluorescent staining
Cells were rinsed twice in PBS and fixed with 4% paraformaldehyde at room temperature for 30 min. The primary antibodies in PBS containing 2% normal goat serum (Vector Laboratories, Burlingame, CA, USA) and 0.1% Triton X-100 (Sigma-Aldrich) was applied overnight at 4°C, followed by rinsing with PBS. Then, specific secondary antibody solutions in PBS was applied for another 3 h at room temperature, followed by rinsing with PBS. 4′,6-diamidino-2-phenylindole (DAPI) (1 μg/ml; Roche, Switzerland) was then used to stain nuclei at 37°C for 15 min, followed by rinsing with PBS. The cells were mounted within a fluorescence mounting medium (Agilent, Santa Clara, CA, USA) and then visualized using a confocal microscope (FluoView FV1000; Olympus, Tokyo, Japan). The primary antibodies used in this study included antinestin antibody (1:1,000), anti-MAP2 antibody (1:1,000), and anti-GFAP antibody (1:1,000).
2.9. Preparation of protein lysates and Western blot assay
Protein lysates were generally extracted from cells using a protein extraction buffer (Novagen, Madison, WI, USA) containing protease inhibitor (Calbiochem, San Diego, CA, USA). After 30 min incubation on ice, protein lysates were clarified by centrifugation at 12,000 g, 10 min. Total protein concentration was routinely determined using protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Total proteins (30 μg) per sample were separated by 10% SDS–polyacrylamide gel electrophoresis and then transferred to a polyvinylidene difluoride (Bio-Rad Laboratories) membrane. After blocked with 5% nonfat milk for 1 h, specific primary antibodies were incubated overnight at 4°C, and then secondary antibodies were further incubated for 1 h. Equal protein sample were verified by β-actin. Chemiluminescent detection was performed using a chemiluminescence detection kit (AbFrontier, South Korea) by the ChemiDoc Touch imaging system (Bio-Rad Laboratories). The primary antibodies used in this study including antinestin antibody (1:1,000), anti-MAP2 antibody (1:1,000), anti-GFAP antibody (1:1,000), anti–p-GSK-3β(Ser9) antibody (1:1,000), anti–GSK-3β antibody (1:1,000), anti–non-p-β-catenin (active) antibody (1:1,000), anti–β-catenin antibody (1:1,000), and anti–β-actin antibody (1:5,000).
2.10. Molecular simulation of interaction between PPD and GSK-3β
Molecular docking was performed to analyzed the mechanism of the binding that occurs between GSK-3β (Protein Data Bank ID (PDB ID): 4ACC) and PPD (CID71620663) by Autodock Vina (Scripps Research Institute, La Jolla, CA, USA) [16]. To perform molecular docking, the GSK-3β protein structure had to be prepared by removing water molecules and bound ligands. Energy minimization of the protein and ligand were performed by yet another scientific artificial reality application (YASARA). The inhibitor-binding domain of GSK-3β also represented its protein–ligand binding site. The star conformation for the molecular dynamics (MD) simulation using assisted model building with energy refinement (AMBER) 03 forcefield was reckoned to be the optimal conformation performed by YASARA [17]. Then, 0.9% NaCl, the solvent of the receptor–ligand complex, was placed in a dodecahedron box with 5 Å between the box and the solute. The initiation of simulated annealing minimizations was set at 298 K, and velocities were scaled down 0.9 every ten steps with a duration of 5 ps. After energy minimization, Berendsen thermostat was used to control the simulation temperature. Every 100 simulation steps, the nonaxial velocities components were reinitialized. In this study, MD simulations of 100-ns were performed with a time step of 2 fs, and coordinates were written every 10 ps of simulation.
2.11. Constitutively active GSK-3β mediated by adenovirus transfection
The adenovirus of pAdM-FH-GFP-GSK-3β(S9A) (Ad-GSK-3β(S9A) for short, 1.2 × 1011 viral particles per milliliter) expressing the constitutively active mutant of GSK-3β (GSK-3β(S9A), with the 9th serine switched to alanine) and control adenovirus vector (Ad-Ctr, 1.0 × 1010 viral particles per milliliter) were obtained from Vigene Bioscience (Rockville, MD, USA). Single NSC was seeded onto a poly-D-lysine–coated 6-well plate at 2 × 105 cells/well and then transfected with Ad-GSK-3β(S9A) with multiplicity of infection of 40 with 6 μg/ml polybrene (Sigma-Aldrich) for 12 h, then the fresh medium was exchanged. Ad-Ctr was applied as a control. Transfected cells were further cultured for at least 48 h and then further experiments were performed. Activation of GSK-3β in infected cells was confirmed by Western blot, and the induction of PPD on NSCs with constitutively active GSK-3β was further determined.
2.12. Statistical analysis
The data were expressed as the means ± standard deviation (SD). The statistical analysis of different groups was performed using one-way analysis of variance. Statistical significance was defined as p < 0.05. The data management was performed using GraphPad Prism software (version 6.0).
3. Results
3.1. Purification and identification of NSCs
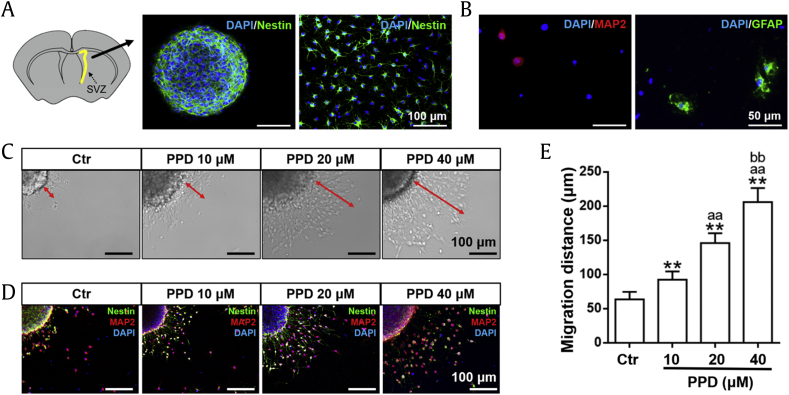
The cells isolated from the SVZ of postnatal day 1 to 2 rats started to form neurospheres after 7 days culturing, and the diameter of neurospheres was increased incrementally over time, defined as passage 1. The neurospheres and dissociated single cells, used in this study, were subcultured and purified from passage 1. The result of immunofluorescent staining showed that the neurospheres and over 90% dissociated single cells were nestin positive (Fig. 1A). After further cultured for 7 days in a culture medium without GFs, MAP2 positive (neurons) and GFAP positive (astrocytes) were detected (Fig. 1B), indicating the multipotency of the primary cultured NSCs.
Fig. 1.
NSC identification and PPD increases NSC migration. (A, B) Primary NSCs were identified by immunofluorescent staining. (A) Schematic diagram indicated the SVZ sampling location (highlighted in yellow). The neurosphere and signal cells subcultured from passage 1 were stained with nestin. Scale bars: 100 μm. (B) After further 7 days of culturing, MAP2-positive and GFAP-positive cells were detected. Scale bars: 50 μm. (C–E) The migration of NSCs was monitored by neurosphere assay after treated with several concentration of PPD for 24h. (C) Image showing the migration distance from the neurosphere, indicated by red arrows. Scale bars: 100 μm. (D) Confocal image visualizing cell migration with the specific markers nestin (green) and MAP2 (red). Scale bars: 100 μm. (E) The migration distance was dose-dependently increased by PPD. Data represent the means ± SD. **p < 0.01, compared with the Ctr group; aap < 0.01, compared with the 10 μM PPD group; bbp < 0.01, compared with the 20 μM PPD group. PPD, 20(S)-protopanaxadiol; NSC, neural stem cell; SVZ, subventricular zone; MAP2, microtubule-associated protein 2; GFAP, glial fibrillary acidic protein; Ctr, control; SD, standard deviation.
3.2. PPD increased NSC migration
After various PPDs at various concentrations (10 μM, 20 μM, 40 μM) was added to the medium, cell migration distance was analyzed. As shown in Fig. 1C, the cells emerged from the edge of neurospheres and migrated along the radial axis. Furthermore, immunofluorescence analysis demonstrated that nestin-positive cells, most of which were also MAP2 positive, were observed in the cell population derived from the neurospheres (Fig. 1D). The migration distance of NSCs was significantly increased with increasing concentration of PPD (Fig. 1E).
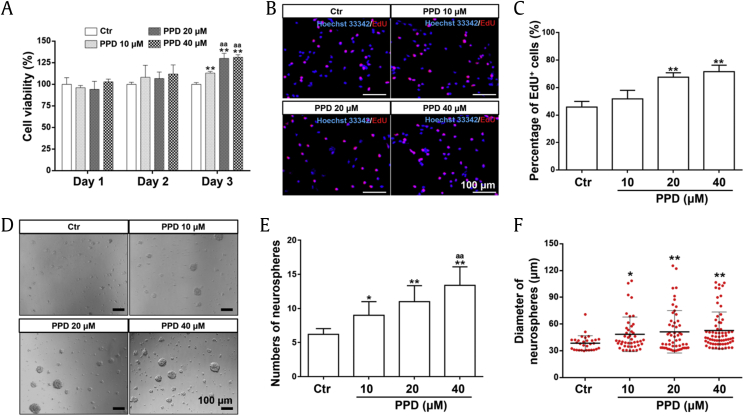
3.3. PPD promoted NSC proliferation
To investigate the proliferation of NSCs induced by PPD, CCK-8, EdU, and neurosphere assays were performed. The CCK-8 assay (Fig. 2A) revealed that the absorbance was slightly but nonsignificantly higher in the PPD-treated group than that in the control group on day 2. After 3 days of treatment, PPD significantly increased cell viability dose-dependently. Specifically, the cell viability in the groups treated with 20 μM and 40 μM PPD was significantly higher than that in the group treated with 10 μM PPD. The proliferation was further measured by the EdU assay. After induction of 3 days, 20 μM and 40 μM PPD significantly increased the percentage of EdU-positive cells compared with the control group (Fig. 2B and C). On the other hand, the neurosphere assay showed that PPD dose-dependently increased the number and the size of neurospheres derived from NSCs (Fig. 2D–F) as observed under a microscope. These results indicated that PPD can significantly promote the proliferation and self-renewal of NSC in vitro.
Fig. 2.
PPD promotes the proliferation of NSCs. The proliferation of NSCs mediated by PPD was determined by CCK-8 assay (A), EdU assay (B, C), and neurosphere assay (D–F). (A) NSCs were treated with different concentrations of PPD (10 μM, 20 μM, 40 μM) for 1, 2, or 3 days, and cell viability was determined by CCK-8 assay. (B–C) The EdU assay was performed to reveal NSC proliferation after induced by PPD for 3 days. (B) The immunofluorescent staining of EdU-positive cells (EdU: staining in red; Hoechst 33342: staining in blue). Scale bars: 100 μm. (C) The percentage of EdU-positive cells was significantly increased. After treatment with PPD for 3 days, cell viability was significantly enhanced. The number and diameter of neurospheres (diameter > 30 μm) was determined after treatment with PPD for 3 days, and the results are shown in D–F. (D) The image showing neurospheres induced by PPD. Scale bars: 100 μm. (E) The number of neurospheres was significantly increased by PPD in a dose-dependent manner. (F) The diameter of neurospheres was significantly and dose-dependently increased by PPD. Data represent the means ± SD. *p < 0.05 and **p < 0.01, compared with the Ctr group; aap < 0.01, compared with the 10 μM PPD group. PPD, 20(S)-protopanaxadiol; NSC, neural stem cell; CCK-8, Cell Counting Kit-8; EdU, 5-ethynyl-2′-deoxyuridine; Ctr, control; SD, standard deviation.
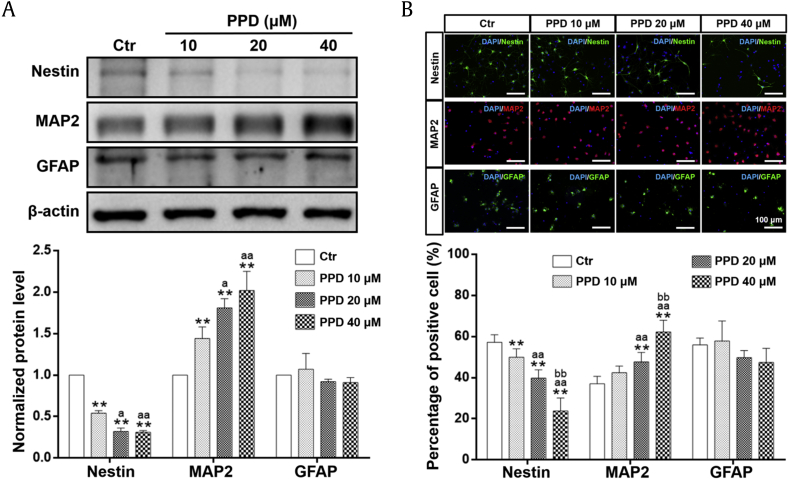
3.4. PPD promoted NSC neural differentiation
NSCs show the potential to differentiate into multiple cell types, primarily neurons and astrocytes. To determine whether PPD could induce NSC differentiation, three types of neural markers were examined: nestin (NSC marker), MAP2 (neuron marker), and GFAP (astrocyte marker). As shown in Fig. 3A, PPD significantly reduced the level of nestin but increased that of MAP2, a dendrite-specific protein in neurons, in a dose-dependent manner. However, PPD at 20 μM and 40 μM showed a tendency to reduce the expression of GFAP. These results showed that PPD can efficiently promote the neural differentiation and suppress astrocyte differentiation of NSCs with an increasing concentration and were confirmed with immunofluorescent staining. PPD reduced the proportion of the number of nestin-positive cells and increased the number of MAP2-positive cells in a dose-dependent manner, whereas decreasing that of GFAP-positive cells (Fig. 3B).
Fig. 3.
PPD selectively induces neural differentiation of NSCs. After NSCs were incubated with different concentrations of PPD for 7 days, PPD significantly promoted NSC differentiation into neurons in a dose-dependent manner monitored by Western blot analysis (A) and immunofluorescent staining (B). (A) PPD (10, 20, 40 μM) significantly downregulated the expression of nestin and upregulated the expression of MAP2, whereas the expression of GFAP showed a downward but nonsignificant trend. (B) The proportion of nestin-positive cells was significantly decreased and the proportion of MAP2-positive cells was increased by PPD treatment (10 μM, 20 μM, 40 μM). Scale bars: 100 μm. Data represent the means ± SD. **p < 0.01, compared with the Ctr group; ap < 0.05 and aap < 0.01, compared with the 10 μM PPD group; bbp < 0.01, compared with the 20 μM PPD group. PPD, 20(S)-protopanaxadiol; NSC, neural stem cell; MAP2, microtubule-associated protein 2; GFAP, glial fibrillary acidic protein; Ctr, control; SD, standard deviation.
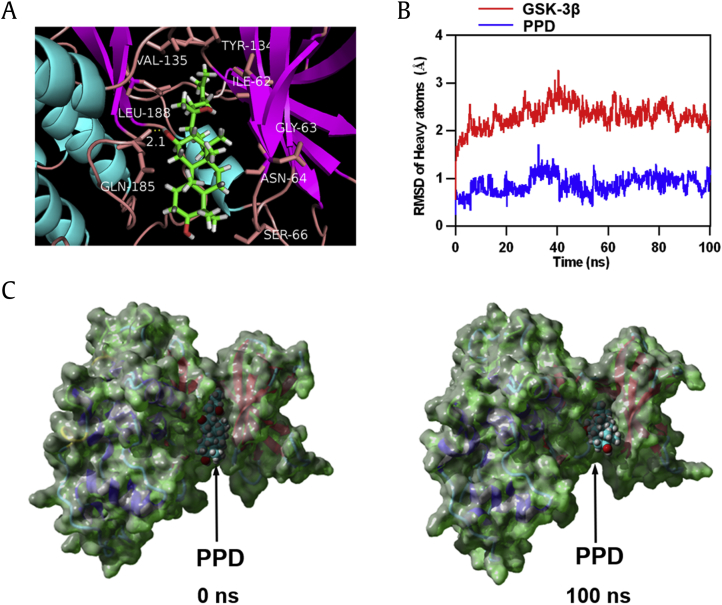
3.5. PPD directly targeted GSK-3β
Molecular docking analysis was performed to explore the interaction between PPD and GSK-3β. The GSK-3β–PPD complex indicated good binding ability with the binding energy −8.874 kcal/mol. As shown in Fig. 4A, based on the binding conformation of the GSK-3β–PPD complex, a hydrogen bond with a distance of 2.1 Å was found to be formed between GLN-185 of GSK-3β and PPD. PPD was also observed to interact with VAL-135, LEU-188, TYR-134, ILE-62, GLY-63, ASN-64, and SER-66 of GSK-3β via van der Waals force. The best conformation of GSK-3β–PPD was analyzed by YASARA to further verify the molecular docking results. Both the heavy atoms root-mean-square deviation track of GSK-3β (Fig. 4B, red line) mildly fluctuated approximately 2 Å during 0–100 ns, and the root-mean-square deviation track of PPD (Fig. 4B, blue line) fluctuated approximately 0.9 Å during MD simulation. In addition, the surface visualization models of the GSK-3β–PPD complex showed that PPD remained stable at the center of the GSK-3β binding site until the end of the 100 ns simulation (Fig. 4C). The molecular docking findings suggested that the binding between GSK-3β and PPD was incredibly stable, strongly indicating that PPD might directly target GSK-3β.
Fig. 4.
Molecular docking and MD simulation of PPD and GSK-3β. (A) Three-dimensional crystal structure of the complex of PPD (CID71620663) within GSK-3β (PDB ID: 4ACC). (B) The RMSD of heavy atoms of the protein (GSK-3β, shown in red) and docking ligand (PPD, shown in blue) versus MD simulation time. (C) The crystal structure of the GSK-3β-PPD complex at 0 and 100 ns. PPD, 20(S)-protopanaxadiol; GSK-3β, glycogen synthase kinase-3β; MD, molecular dynamics; PDB, Protein Data Bank; RMSD, root-mean-square deviation.
3.6. PPD-mediated NSC induction based on the activation of Wnt/GSK-3β/β-catenin pathway
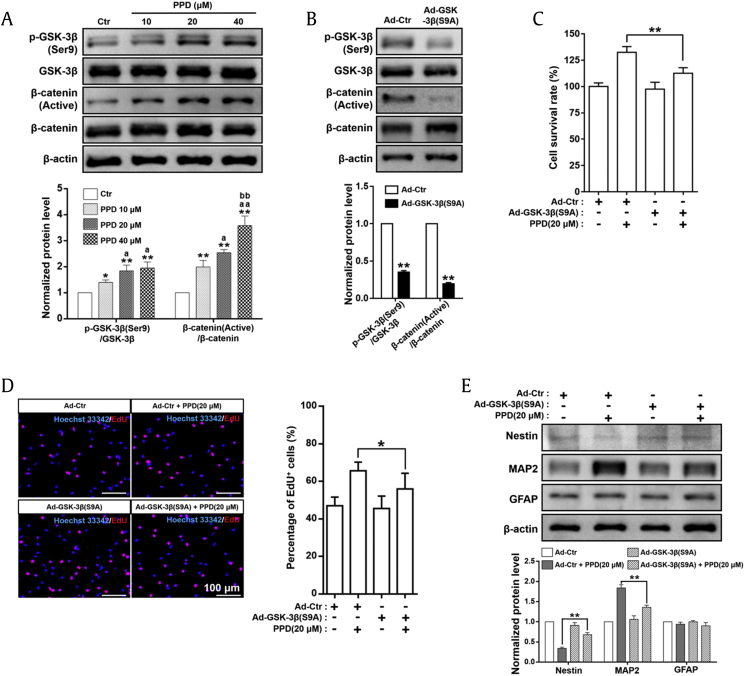
The Wnt/GSK-3β/β-catenin pathway plays a crucial role in regulating the proliferation and differentiation of NSC [8]. GSK-3β is a crucial enzyme in the negative regulation of the pathway and can be inactivated by phosphorylation at Ser9 [p-GSK-3β(Ser9)] [18]. Inactivation of GSK-3β causes the accumulation of active β-catenin in the cytoplasm, leading to activation of the Wnt/GSK-3β/β-catenin pathway to promote NSC proliferation and differentiation [19]. As shown in Fig. 5A, PPD can significantly and dose-dependently increase the p-GSK-3β(Ser9)/GSK-3β and β-catenin (active)/β-catenin expression ratio. These results indicated that NSC proliferation and differentiation induced by PPD may depend on the inhibition of GSK-3β activity and consequently stimulated the Wnt/GSK-3β/β-catenin pathway.
Fig. 5.
PPD-mediated NSC induction depends on the activation of the Wnt/GSK-3β/β-catenin signaling pathway. (A) Western blot analyses and relative optical density of diverse crucial markers in the Wnt/GSK-3β/β-catenin pathway in NSCs treated with PPD for 7 days. PPD significantly increased the p-GSK-3β(Ser9)/GSK-3β and β-catenin (active)/β-catenin ratios. *p < 0.05 and **p < 0.01, compared with the Ctr group; ap < 0.05 and aap < 0.01, compared with the 10 μM PPD group; bbp < 0.01, compared with the 20 μM PPD group. (B) NSCs with constitutively active GSK-3β were successfully established by GSK-3β(S9A) adenovirus transfection confirmed by Western blot analyses in NSCs 2 days after transfection. **p < 0.01, compared with the Ad-Ctr group. Control or GSK-3β(S9A) adenovirus were transfected into NSCs. Forty-eight hours after transfection, cells were treated with 20 μM PPD for further proliferation and differentiation assays. (C) Expression of the constitutively active GSK-3β in NSCs significantly decreased the cell viability induced by PPD as shown by CCK-8 assay. (D) Expression of the constitutively active GSK-3β in NSCs significantly restrained the proliferation induced by PPD as shown by EdU assay. (E) Expression of the constitutively active GSK-3β in NSCs significantly suppressed the PPD-mediated neural differentiation, determined by Western blot analyses of a variety of differentiation markers, including nestin, MAP2, and GFAP. *p < 0.05 and **p < 0.01, compared with the Ad-Ctr + PPD (20 μM) group. Data represent the means ± SD. PPD, 20(S)-protopanaxadiol; NSC, neural stem cell; GSK-3β, glycogen synthase kinase-3β; Ctr, control; Ad, adenovirus; MAP2, microtubule-associated protein 2; GFAP, glial fibrillary acidic protein; CCK-8, Cell Counting Kit-8; EdU, 5-ethynyl-2′-deoxyuridine; SD, standard deviation.
To further confirm the possible mechanism by which PPD mediates NSC proliferation and differentiation, the constitutively active S9A GSK-3β mutant using Ad-GSK-3β(S9A) transfection was performed to inhibit Wnt/GSK-3β/β-catenin pathway. Transduction efficacy was confirmed by detecting the expression of p-GSK-3β(Ser9), as shown in Fig. 5B; the p-GSK-3β(Ser9)/GSK-3β expression ratio in the GSK-3β(S9A) siRNA group was significantly decreased to approximately 30% of that in the control siRNA group, consequently resulting in the β-catenin (active)/β-catenin ratio marked reduced, indicating that Wnt/GSK-3β/β-catenin pathway activation was inhibited by GSK-3β(S9A) adenovirus transfection. As expected, both cell viability (Fig. 5C) and the percentage of EdU-positive cells (Fig. 5D) were significantly lower in PPD-treated NSCsGSK−3β(S9A) than in PPD-treated cells transfected with the control vector. Furthermore, PPD-mediated nestin expression was significantly higher, whereas MAP2 expression was lower (Fig. 5E) in NSCs expressing GSK-3β(S9A). Therefore, overexpression of GSK-3β(S9A), a constitutively active mutant, significantly attenuated PPD-triggered NSC proliferation and differentiation. All of these results suggested that the Wnt/GSK-3β/β-catenin pathway is critical in PPD-mediated NSC proliferation and differentiation.
4. Discussion
Neurodegenerative diseases, of which AD has been confirmed to the most common in the world, are considered to be one of the primary threats to human health and include chronic diseases characterized by the gradual loss of neuronal function and/or structure. According to the World Alzheimer's Disease Report by the World Health Organization, there were approximately 50 million patients with AD in 2017 in the world, and the global number of AD patients will increase to more than 70 million in 2030 [20]. However, the current pharmacotherapy for AD can only alleviate the symptoms of the diseases but cannot provide a permanent cure. In addition, many chemical drugs can cause severe side effects and do not efficiently cross the BBB [21].
The main pathogenesis of neurodegenerative diseases is neuronal cell death in the brain; therefore, neuron replacement might be the most promising method to ultimately cure neurodegenerative diseases. In the present research, we found that PPD was able to promote NSC proliferation and cell migration, which can help NSCs migrate to distant pathological areas [22]. PPD also induced neural differentiation and suppressed the NSC differentiation into astrocytes in a dose-dependent manner. Interestingly, immunofluorescent staining showed many MAP2 and nestin double-stained cells, indicating that some neurons derived from NSC were in the process of maturation [23]. The Wnt/GSK-3β/β-catenin pathway plays a crucial role in stem cell behavior and survival. Inhibition of GSK-3β activity can dramatically affect the proliferation and neural differentiation of NSC [24].
To verify the role of GSK-3β in PPD-mediated NSC induction, molecular docking, and MD simulation was performed to test the ability of PPD binding to GSK-3β directly. The binding energy of the PPD–GSK-3β complex suggested a strong and stable binding between PPD and GSK-3β. A hydrogen bond was formed between GLN-185 of GSK-3β and PPD. Several direct GSK-3β inhibitors have hydrogen bond that interact with GLN-185 [25], [26]. Specifically, PPD interacted with GSK-3β at VAL-135, LEU-188, TYR-134, ILE-62, GLY-63, ASN-64, and SER-66 via van der Waals force, which are the crucial residues in the agonist-binding domain of GSK-3β [27]. In addition, MD stimulation demonstrated that the PPD-GSK-3β–binding conformation was stable. The molecular docking and MD simulation results indicated that PPD directly targets GSK-3β, potentially acting as a GSK-3β inhibitor, which was further confirmed by Western blot analysis. In the present study, we confirmed that PPD significantly suppressed the activity of GSK-3β via up-regulation of phosphorylation at Ser9 and showing that the PPD-mediated proliferation and differentiation were significantly suppressed in NSCs expressing constitutively activate GSK-3β. These results strongly indicated that the induction of NSCs mediated by PPD was due to the inactivation of GSK-3β leading to activation of the Wnt/GSK-3β/β-catenin pathway.
Recently, NSC replacement therapies have been quickly developed as promising treatment strategies for neurodegenerative diseases and other incurable diseases. As the source of NSCs is limited, in vitro induction is required for NSC proliferation and differentiation into functional neurons. However, in natural conditions, NSCs have limited proliferation and differentiation abilities and need to be induced by GFs [28]. Unfortunately, GFs may cause a protentional risk of carcinogenicity in vivo after transplantation in cell replacement therapy [29], [30]. PPD could be used for the efficient and safe induction of NSC proliferation and differentiation as an alternative of GFs. Furthermore, PPD has shown the ability to penetrate the BBB, suggesting that its oral administration for the treatment of AD may be able to repair damaged neurons.
In conclusion, we have revealed that PPD mediates NSC migration, proliferation, and differentiation based on the stimulation of Wnt/GSK-3β/β-catenin pathway. Importantly, the findings may help provide potential novel treatment strategies for AD and other neurodegenerative diseases through neuron repair, and PPD may be used in in vitro GF-free culture to induce NSC proliferation and differentiation to minimize potential biorisk.
Conflicts of interest
The authors declare that they have no conflicts of interests.
Acknowledgments
The authors would like to thank Ms. Samantha Sze Man WONG and Ms. Wing Sze TSE (Biology, HKBU) for their technical supports. This work was financially supported by National Natural Science Foundation of China (NSFC) grants (No. 81703728and 81774100).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2019.03.001.
Contributor Information
Ken K.-L. Yung, Email: kklyung@hkbu.edu.hk.
Shiqing Zhang, Email: shiqingzhang@hkbu.edu.hk.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Stayte S., Vissel B. New hope for devastating neurodegenerative disease. Brain. 2017;140:1177–1179. doi: 10.1093/brain/awx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kikuchi T., Morizane A., Doi D., Magotani H., Onoe H., Hayashi T., Mizuma H., Takara S., Takahashi R., Inoue H. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature. 2017;548:592–596. doi: 10.1038/nature23664. [DOI] [PubMed] [Google Scholar]
- 3.Bray N. Neurodegenerative disease: towards transplant therapy. Nat Rev Neurosci. 2017;18:572. doi: 10.1038/nrn.2017.120. [DOI] [PubMed] [Google Scholar]
- 4.Bizen N., Inoue T., Shimizu T., Tabu K., Kagawa T., Taga T. A growth-promoting signaling component cyclin D1 in neural stem cells has antiastrogliogenic function to execute self-renewal. Stem Cells. 2014;32:1602–1615. doi: 10.1002/stem.1613. [DOI] [PubMed] [Google Scholar]
- 5.Aaronson S.A. Growth factors and cancer. Science. 1991;254:1146–1153. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- 6.Wu J., Pan Z., Cheng M., Shen Y., Yu H., Wang Q., Lou Y. Ginsenoside Rg1 facilitates neural differentiation of mouse embryonic stem cells via GR-dependent signaling pathway. Neurochem Int. 2013;62:92–102. doi: 10.1016/j.neuint.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Musende A.G., Eberding A., Wood C.A., Adomat H., Fazli L., Hurtado-Coll A., Jia W., Bally M.B., Guns E.S.T. A novel oral dosage formulation of the ginsenoside aglycone protopanaxadiol exhibits therapeutic activity against a hormone-insensitive model of prostate cancer. Anticancer Drugs. 2012;23:543–552. doi: 10.1097/CAD.0b013e32835006f5. [DOI] [PubMed] [Google Scholar]
- 8.Selvaraj P., Xiao L., Lee C., Murthy S.R.K., Cawley N.X., Lane M., Merchenthaler I., Ahn S., Loh Y.P. Neurotrophic factor-α1: a key Wnt-β-Catenin dependent anti-proliferation factor and ERK-Sox9 activated inducer of embryonic neural stem cell differentiation to astrocytes in neurodevelopment. Stem Cells. 2017;35:557–571. doi: 10.1002/stem.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patterson A.R., Endale M., Lampe K., Aksoylar H.I., Flagg A., Woodgett J.R., Hildeman D., Jordan M.B., Singh H., Kucuk Z. Gimap5-dependent inactivation of GSK3β is required for CD4+ T cell homeostasis and prevention of immune pathology. Nat Commun. 2018;9:1–15. doi: 10.1038/s41467-018-02897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H.Y., Juo L.I., Lin Y.T., Hsiao M., Lin J.T., Tsai C.H., Tzeng Y.H., Chuang Y.C., Chang N.S., Yang C.N. WW domain-containing oxidoreductase promotes neuronal differentiation via negative regulation of glycogen synthase kinase 3β. Cell Death Differ. 2012;19:1049–1059. doi: 10.1038/cdd.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim D.A., Huang Y.C., Swigut T., Mirick A.L., Garcia-Verdugo J.M., Wysocka J., Ernst P., Alvarez-Buylla A. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 2009;458:529–533. doi: 10.1038/nature07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wojcinski A., Lawton A.K., Bayin N.S., Lao Z., Stephen D.N., Joyner A.L. Cerebellar granule cell replenishment postinjury by adaptive reprogramming of Nestin+ progenitors. Nat Neurosci. 2017;20:1361–1370. doi: 10.1038/nn.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui M., Dai B., Xin J.Y., He J.Q., Feng S.Q. Overexpression of suppressors of cytokine signaling 1 promotes the neuronal differentiation of C17.2 neural stem cells. Cell Physiol Biochem. 2014;33:528–538. doi: 10.1159/000358632. [DOI] [PubMed] [Google Scholar]
- 14.Mori H., Ninomiya K., Kino-oka M., Shofuda T., Islam M.O., Yamasaki M., Okano H., Taya M., Kanemura Y. Effect of neurosphere size on the growth rate of human neural stem/progenitor cells. J Neurosci Res. 2006;84:1682–1691. doi: 10.1002/jnr.21082. [DOI] [PubMed] [Google Scholar]
- 15.Chehrehasa F., Meedeniya A.C., Dwyer P., Abrahamsen G., Mackay-Sim A. EdU, a new thymidine analogue for labelling proliferating cells in the nervous system. J Neurosci Methods. 2009;177:122–130. doi: 10.1016/j.jneumeth.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieger E., Vriend G. YASARA View - molecular graphics for all devices - from smartphones to workstations. Bioinformatics. 2014;30:2981–2982. doi: 10.1093/bioinformatics/btu426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura T., Tanno M. The mPTP and its regulatory proteins: final common targets of signalling pathways for protection against necrosis. Cardiovasc Res. 2012;94:181–189. doi: 10.1093/cvr/cvr302. [DOI] [PubMed] [Google Scholar]
- 19.Lee H.Y., Kléber M., Hari L., Brault V., Suter U., Taketo M.M., Kemler R., Sommer L. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303:1020–1023. doi: 10.1126/science.1091611. [DOI] [PubMed] [Google Scholar]
- 20.Alzheimer’s Association 2017 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2017;13:325–373. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Lleó A., Greenberg S.M., Growdon J.H. Current pharmacotherapy for Alzheimer’s disease. Annu Rev Med. 2006;57:513–533. doi: 10.1146/annurev.med.57.121304.131442. [DOI] [PubMed] [Google Scholar]
- 22.Tong L.M., Fong H., Huang Y. Stem cell therapy for Alzheimer’s disease and related disorders: current status and future perspectives. Exp Mol Med. 2015;47:e151. doi: 10.1038/emm.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 24.Ahn J., Jang J., Choi J., Lee J., Oh S.H., Lee J., Yoon K., Kim S. GSK3β, but not GSK3α, inhibits the neuronal differentiation of neural progenitor cells as a downstream target of mammalian target of rapamycin complex1. Stem Cells Dev. 2014;23:1121–1133. doi: 10.1089/scd.2013.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramer T., Schmidt B., LoMonte F. Small-molecule inhibitors of GSK-3: structural insights and their application to Alzheimer’s disease models. Int J Alzheimers Dis. 2012;2012:381029. doi: 10.1155/2012/381029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann T., Benajiba L., Göring S., Stegmaier K., Schmidt B. Evaluation of improved glycogen synthase kinase-3α inhibitors in models of acute myeloid leukemia. J Med Chem. 2015;58:8907–8919. doi: 10.1021/acs.jmedchem.5b01200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berg S., Bergh M., Hellberg S., Högdin K., Lo-Alfredsson Y., Söderman P., Berg S., Weigelt T., Ormö M., Xue Y. Discovery of novel potent and highly selective glycogen synthase kinase-3β (GSK3β) inhibitors for Alzheimer’s disease: design, synthesis, and characterization of pyrazines. J Med Chem. 2012;55:9107–9119. doi: 10.1021/jm201724m. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y., Tan B., Wang L., Long Z., Li Y., Liao W., Wu Y. Endogenous neural stem cells in central canal of adult rats acquired limited ability to differentiate into neurons following mild spinal cord injury. Int J Clin Exp Pathol. 2015;8:3835–3842. [PMC free article] [PubMed] [Google Scholar]
- 29.Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5:402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saxena N.K., Sharma D. Multifaceted leptin network: the molecular connection between obesity and breast cancer. J Mammary Gland Biol Neoplasia. 2013;18:309–320. doi: 10.1007/s10911-013-9308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.