Abstract
Rationale:
Synthetic psychoactive cathinones (SPCs) are drugs with psychostimulant and entactogenic properties like methamphetamine (MA) and 3,4-methylenedioxymethamphetamine (MDMA). Despite clinical reports of human overdose, it remains to be determined if SPCs have greater propensity for adverse effects than MA or MDMA.
Objectives:
To determine whether the SPCs cathinone (CAT), methcathinone (MCAT), mephedrone (MMC), and methylenedioxypyrovalerone (MDPV) have lower LD50 values than MA or MDMA.
Methods:
Male and female C57Bl/6J mice received single injections of one of 6 doses of a test drug (0–160 mg/kg IP). Temperature and behavioral observations were taken every 20 min for 2 hr followed by euthanasia of surviving mice. Organs were weighed and evaluated for histopathological changes.
Results:
LD50 values for MA and MDMA, 84.5 and 100.9 mg/kg respectively, were similar to previous observations. The LD50 for MMC was 118.8 mg/kg, but limited lethality was observed for other SPCs (CAT, MCAT, MDPV), so LD50 values could not be calculated. For all drugs, death was associated with seizure, when it was observed. Rather than hyperthermia, dose-dependent hypothermia was observed for MMC, MDPV, CAT, and MCAT. Contrary to initial expectations, none of the SPCs studied here had LD50 values lower than MA or MDMA.
Conclusions:
These data indicate that, under the conditions studied here: (1) SPCs exhibit less lethality than MA and MDMA; (2) SPCs impair thermoregulation; (3) effects of SPCs on temperature appear to be independent of effects on lethality.
Keywords: Synthetic psychoactive cathinones, cathinone, methcathinone, mephedrone, methylenedioxypyrovalerone, methamphetamine, methylenedioxymethamphetamine, lethality, temperature regulation
1.0. Introduction
Synthetic psychoactive cathinones (SPCs) are drugs with psychostimulant properties similar to those of methamphetamine (MA) and 3,4-methylenedioxymethamphetamine (MDMA), derived from the naturally occurring molecule cathinone (CAT), found in the khat plant (Catha edulis) (Prosser and Nelson, 2012). Similar to those more well-known stimulants, SPCs show clear potential for abuse ((Javadi-Paydar et al., 2018; Schindler et al., 2016; Vandewater et al., 2015; Watterson et al., 2012a) for review see (Negus and Banks, 2017; Riley et al., In Press; Watterson and Olive, 2017)) and pose a threat to public health (Madras, 2017; Zaami et al., 2018). Their use has been associated with side effects that include neurotoxicity, hyperthermia and lethality (Marusich et al., 2014). However, it is difficult to determine the dangers presented by this new class of drugs from clinical reports alone. Clinical reports are usually single case reports or small studies that may lack confirmatory toxicology. Moreover, clinical circumstances most often involve the ingestion of multiple drugs, making it difficult to evaluate the contribution of particular drugs to these adverse outcomes, and consequently the dangers that individual drugs may pose. Furthermore, illicit drugs have an inconsistent composition that often include drug mixtures, of variable doses, that can create a mismatch between what the patients believe they have consumed and the actual drugs consumed (Araújo et al., 2015). Indeed, hair samples from self-reported MDMA users have been found to contain a wide variety of both SPCs and other amphetamine derivatives (Caudevilla-Gálligo et al., 2013; Palamar et al., 2016). In many cases from those studies samples tested negatively for MDMA but were positive for one or more SPCs, such as methylone or butylone. The possibility of unknowing consumption of SPCs by illicit drug users additionally suggests that the use of these drugs may be more widespread than is currently thought.
SPCs are commonly known as “bath salts”, because they were originally marketed with such labels to circumvent in initial legal restrictions (Spiller et al., 2011), prior to the majority of these drugs being made illegal in the US and Europe. At that time, these drugs were labelled “not-for human consumption” to hide their intended use as recreational drugs and, before 2011, they could be easily purchased in smoke shops and gas stations in the US. Thus, they were also known as “legal highs” and were even presumed by some users to be safe alternatives to illicit drugs such as MA, amphetamine and cocaine (Jerry et al., 2012). Currently, the majority of SPCs are illegal, but analogues are continually emerging as illicit manufacturers attempt to circumvent legal restrictions or to appeal to illicit markets, as is happening for other classes of drugs, including opioids and cannabinoids. These emerging drugs may differ in a number of properties, one of which is potency, which may increase the risk for adverse effects, including death, as it has for the emergence of potent fentanyl analogues as part of the current opioid crisis in the US and elsewhere (Misailidi et al., 2018).
The abuse and addiction potential of SPCs is now well recognized ((Watterson et al., 2014), and see review by Watterson and Olive (2017)), and may be greater than that of MA and MDMA for some SPCs, although many remain to be characterized. Overall, users seek out these drugs because of their similar subjective effects to other drugs of abuse, including MA, MDMA and cocaine (Ashrafioun et al., 2016; Johnson and Johnson, 2014), that include euphoria, alertness, wakefulness, an increased sense of empathy and/or connection with others and orgasm intensification (Rosenbaum et al., 2012). The reported adverse effects of these drugs are similar to other stimulants, and wide in scope, including agitation, tachycardia, palpitations, chest pain, hypertension, violent behavior, hallucinations, paranoia, confusion, mydriasis, vomiting, myoclonus, seizures, organ toxicity, hyperthermia, and lethality (Spiller et al., 2011).
The similarities between the effects of SPCs and other stimulant drugs are not surprising given the strong similarities in their chemical structures and pharmacological activity. SPCs share a phenethylamine pharmacophore with amphetamine (Smith et al., 2017), and really only differ based on the presence of a β-ketone moiety. Like amphetamines, variations in the length of carbon substitutions at the α-carbon and expression of the nitrogen terminus generate analogues that vary in pharmacological effects (Banks et al., 2014), as well as pharmacokinetic properties ((Grecco et al., 2017), for review see Calinski et al. (2019)). The length of the alkyl chain and the presence or absence of a pyrrolidine moiety, along with the β-ketone addition (Namera et al., 2015), have been reported to affect the mechanism of action of CAT analogues and, in particular, for their selectivity to particular monoamine transporters (Simmler et al., 2013). To one degree or another, all of these stimulant drugs increase extracellular norepinephrine, serotonin, or dopamine levels by acting as monoamine transporter inhibitors or monoamine releasers (Baumann et al., 2012). Shorter alkyl chains and modification of the pyrrolidine moiety reduce dopamine transporter activity, for example (Kolanos et al., 2013). The pharmacokinetic properties of cathinones were also altered by extension of the α-alkyl chain, but these effects were different centrally and peripherally (Grecco et al., 2017), increasing Cmax, AUC and t1/2 peripherally, while reducing Cmax and AUC centrally. Although not widely studies yet, it is clear that effects of structural changes on pharmacokinetic properties are likely to contribute to their addictive and toxic potential. However, it remains unclear how particular modifications to the core cathinone structure may alter toxicity or lethality.
At least some SPCs appear to have less neurotoxic potential than MA and MDMA (Angoa-Pérez et al., 2014; Angoa-Pérez et al., 2012), including methylenedioxypyrovalerone (MDPV) and mephedrone (MMC; 4-methyl-methcathinone). Methcathinone (MCAT) produces dopaminergic neurotoxicity, although this is reduced compared to MA. MMC does enhance damage to dopaminergic nerve endings when administered with sub-optimally neurotoxic doses of MA (Angoa-Pérez et al., 2013). In the case of MMC, it has been hypothesized that reduced neurotoxicity is attributable to the 4-methyl group, as the equivalent substitution in MA yields a psychoactive drug devoid of neurotoxic potential. Much still remains to be done to fully characterize the structure-activity relationships underlying the neurotoxic effects of SPCs, and what other actions of these drugs may contribute to their toxicity. As is the case for amphetamines, the effects of these drugs on temperature may be a critical factor influencing their adverse effects. Both MMC and methylone damage serotonergic systems at elevated temperatures (Pantano et al., 2017; Wright Jr et al., 2012), as is the case for amphetamines (Miller and O’Callaghan, 2003; O’Shea et al., 2006). In any case, it remains to be seen if the mechanisms underlying the neurotoxic potential of SPCs is related to the mechanisms underlying the acute neurological and cardiac effects associated with overdose and death.
The psychiatric consequences of SPC overdose may be consistent with the known neural mechanisms of these drugs, but because case reports also describe cardiac, renal, and hepatic injury following SPC overdose, peripheral toxicity has been suggested to be a primary cause of lethality in many cases of acute overdose. Clinical reports that point to cardiac injury as cause of death include reports sinus tachycardia, reduced cardiac output and heart failure (Beck et al., 2018; Marinetti and Antonides, 2013; Spiller et al., 2011). These consequences may be caused by effects of these drugs on the heart itself, myocardial damage due to rhabdomyolysis, or perhaps indirectly through overstimulation of adrenoreceptors in the central nervous system. Reported renal symptoms include high blood urea nitrogen levels, hyperkalemia, dehydration, hyponatremia and rhabdomyolysis resulting in acute renal injury (Benzer et al., 2013; Borek and Holstege, 2012; Eiden et al., 2013). Some overdose cases also present with hepatotoxicity, including post mortem signs of hepatic inflammation (Marinetti and Antonides, 2013). SPCs may act similarly to MA, resulting in elevated hepatic levels of reactive metabolites, mitochondrial impairment, hyperthermia and apoptosis (for review see (Carvalho, M. et al., 2012)). In vitro studies have assessed some potential aspects of SPC toxicity, and shown that a number of SPCs are hepatotoxic (Araújo et al., 2015). MDPV shows a temperature-dependent hepatotoxicity (Valente et al., 2016), and MMC is more cytotoxic in SH-SY5Y cells than MA (Valente et al., 2017). MMC toxicity was partially prevented by dopamine transporter (DAT; Slc6a3) blockade or overexpression of Bcl2. MMC also depletes ATP stores in hepatocytes by disruption of the electron transport chain (Luethi et al., 2017). Although clearly demonstrating several types of toxicity, it remains to be seen which of these effects underlies the acute lethality of SPCs, and whether SPCs have more, or less, potential for lethality compared to MA and MDMA.
To date, only one non-clinical study (Piao et al., 2015) has explicitly examined the lethality of any SPCs. This study found that the LD50 for methylone is slightly lower than that of MA or MDMA. Moreover, methylone lethality was reduced in DAT knockout mice. Moreover, several approaches in that report showed that the mechanisms underlying methylone lethality could be dissociated from methylone effects on temperature, similar to a study of METH-induced lethality (Numachi et al., 2007). This is not to say that temperature does not affect the lethality of these drugs, but rather that the lethal effects of these drugs occur even in the absence of hyperthermia. The experiments described in this report, begin to elucidate the potential mechanisms underlying the lethal effects of SPCs: LD50 studies were conducted with several SPCs, and for comparison, MA and MDMA. Effects on temperature and peripheral organs were also examined.
2.0. MATERIAL AND METHODS
2.1. Subjects
Adult male and female C57Bl/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) at 7 weeks of age. Subjects were housed in groups of 4–5 mice for one week in standard plastic cages (7.5 × 11.75 × 5″) prior to testing for habituation to animal facility conditions. Food (Teklad rodent diet 2916; 4% fat) and water were available ad libitum. Temperature was maintained at 22–23 °C and humidity was maintained at 40–45%. Subjects were tested between 8 and 12 weeks of age (average weight: males, 27.1 ± 0.9; females, 20.5 ± 0.7).
2.2. Drug Treatments:
On test day, mice were taken from the colony and transported to the experimental room where they were allowed to acclimate for 2 hours prior to the first temperature readings. During testing, temperature and humidity remained at the same values as the housing conditions. Water was not available during this time. During this time, they were housed singly in cages identical to their home cages, but with bench paper placed on the bottom of the cages. Rectal temperature was measured twice before injection at twenty-minute intervals, to establish a baseline, and then for two hours after the injections. Subjects (N=5 males, and N=5 females, for each experimental condition) were weighed prior to testing, and injected with one of 6 different drugs (MA, MDMA, MCAT, CAT, MDPV, or MMC) at one of six different doses (20–160 mg/kg IP, calculated as salt, and dissolved in sterile saline). Drugs were kindly provided by the NIDA Drug Supply Program, except for MA, which was purchased from Sigma (St. Louis). These drugs were chosen for study primarily because of their abuse potential, and common use for that purpose, not for structure-activity considerations, something that will need to be addressed in future studies. After each temperature reading, behavioral observations were recorded: general level of activity, presence of stereotypical behavior, aspects of 5-HT behavioral syndrome (e.g. Straub tail, head shakes, ptosis), seizure, tremors, grooming, sniffing, vacuous chewing, and righting. Upon death, either by lethal administration of drug or euthanasia, the following organs were collected: brain, heart, lungs, kidney, intestines, and stomach. Organs were weighed and then immediately immersed in 10% formaldehyde. All experiments were conducted in accordance with all applicable NIH guidelines, and in accordance with The Guide for the Care and Use of Laboratory Animals (National Academies of Sciences Press), under animal protocols approved by the University of Toledo Institutional Animal Care and Use Committee.
2.3. Statistical Analysis:
χ2 tests were initially used to evaluate lethality, comparing sex and dose. As no significant effects of sex were observed, LD50 values were calculated for each drug for the pooled subjects. The same analysis was used to examine the relationship of dose to the incidence of seizure.
Analysis of temperature data was performed using repeated measures ANOVA with TIME as a repeated measure with DOSE and SEX as a between-subjects factor. Temperature data was only analyzed for subjects for which there was complete temperature data, and groups were dropped from the analysis if all measurements were available for less than 4 subjects. Thus, for some drugs (as noted below) only some doses were used in the temperature analysis. Temperature data from male and female subjects were analyzed separately after the initial omnibus ANOVA. Over all effects of drugs on organ weights were performed using two-way ANOVA. Post hoc analyses were performed using Bonferroni comparisons. ANOVA were performed using SPSS 23.0 (IBM). LD50 and ED50 calculations were made using Prism 5.04 (GraphPad Corporation). Temperature and organ weight data are presented as mean ± standard error of the mean (SEM). The α level was 0.05.
3.0. RESULTS
3.1. Lethality
MA and MDMA both induced lethality. Lethal effects were confirmed as significant effects of dose in χ2 tests for MA (χ2[5, 55] = 34.0, p<0.0001) and MDMA (χ2[6, 69] = 49.4, p<0.0001). Sex was not significant in either analysis. The LD50 for MA was estimated to be 105.2 mg/kg calculated as the salt or 84.5 mg/kg as base and MDMA was estimated to be 119.2 mg/kg (salt) or 100.92 mg/kg (base) (Fig. 1). LD50 values could only be calculated for one of the four SPCs tested, MMC, for which there was a significant effect of dose in the X2 test (χ2[5, 60] = 34.0, p<0.0001). Sex was not significant. The LD50 for MMC was estimated to be 143.2 mg/kg (salt) or 118.8 mg/kg (base) (Fig. 1). The only other SPC to induce a lethal effect was MCAT (χ2[5, 60] = 5.1, p=NS), however, this occurred at the highest dose and only in one subject. CAT and MDPV produced no lethal effects at any dose.
Figure 1.
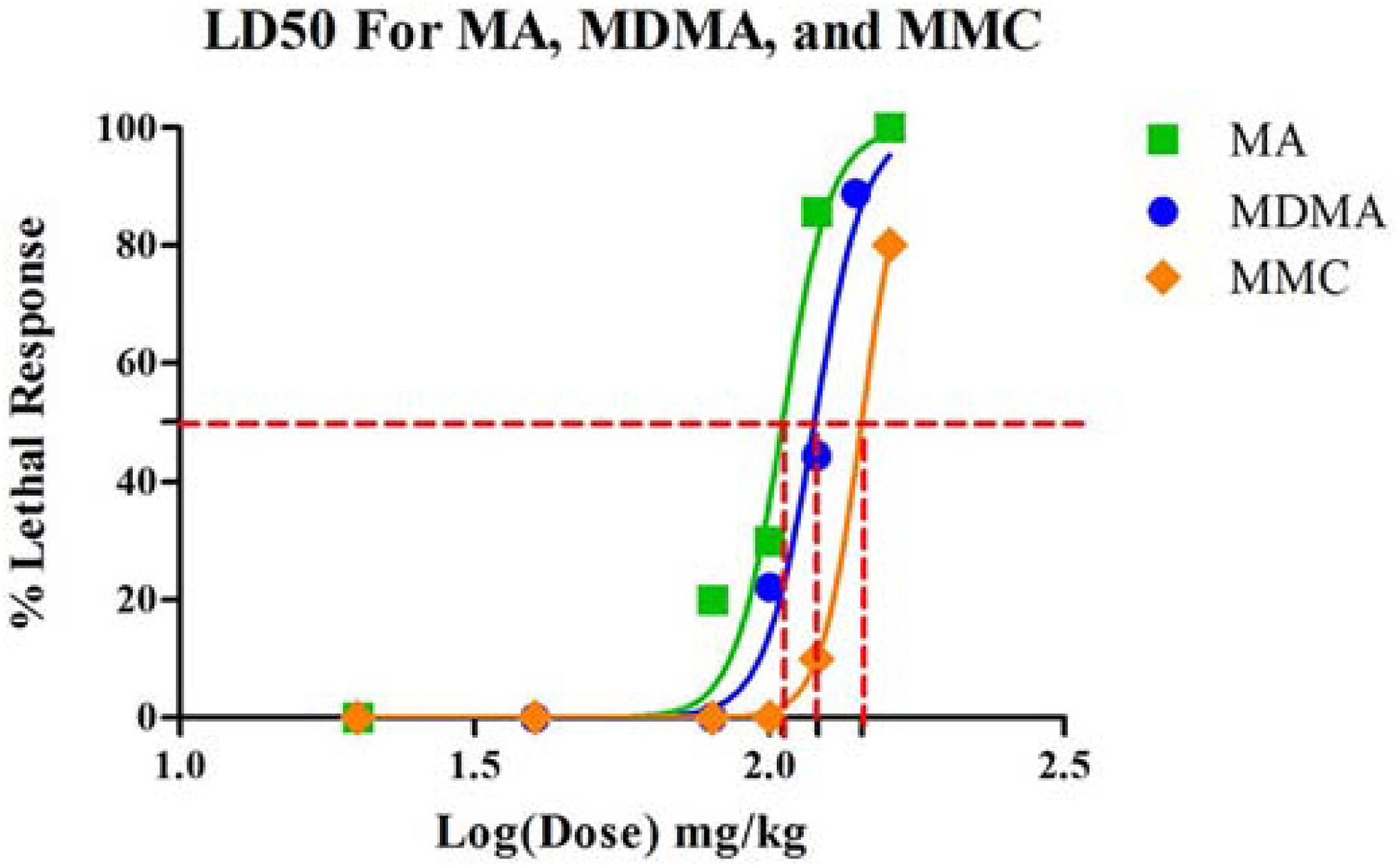
LD50 curves for MA, MDMA and MMC showing relative LD50 values (MMC>MDMA>MA, although all values were similar). Values could not be calculated for CAT, MCAT or MDPV.
Behavioral observations demonstrated a high incidence of hyperlocomotion and stereotypical behavior for all drugs, even from the lowest doses. These behaviors were not always observed for higher doses as these animals lost righting ability and quickly developed seizures. Symptoms of 5-HT behavioral syndrome were sometimes observed, including Straub tail, but were not observed consistently in all subjects for any drug. When lethality did occur, it typically occurred within 20–60 mins of drug administration, generally associated with seizure activity beginning within 5 or 10 minutes after drug administration. This was observed for all cases of lethality for those drugs that did induce lethality at the doses tested.
3.2. Drug-Induced Seizures
MA, MDMA, and MMC induced seizures in a dose dependent manner. Seizure effects were confirmed as significant effects of dose in χ2 tests for MA (χ2[5, 55] = 21.1, p<0.0001), MDMA (χ2[6, 69] = 34.3, p<0.0001), and MMC (χ2[5, 60] = 30.9, p<0.0001). Sex was not significant in either analysis. The ED50 for MA was estimated to be 97.1 mg/kg calculated as the salt or 78 mg/kg as base while MDMA was estimated to be 137.6 mg/kg (salt) or 116.5 mg/kg (base) (Fig. 2). MMC had the second highest incidence of seizure with an estimated ED50 of 124 mg/kg as the salt or 102.9 mg/kg as base, slightly lower than the observed value for MDMA. Sex was not significant.
Figure 2.
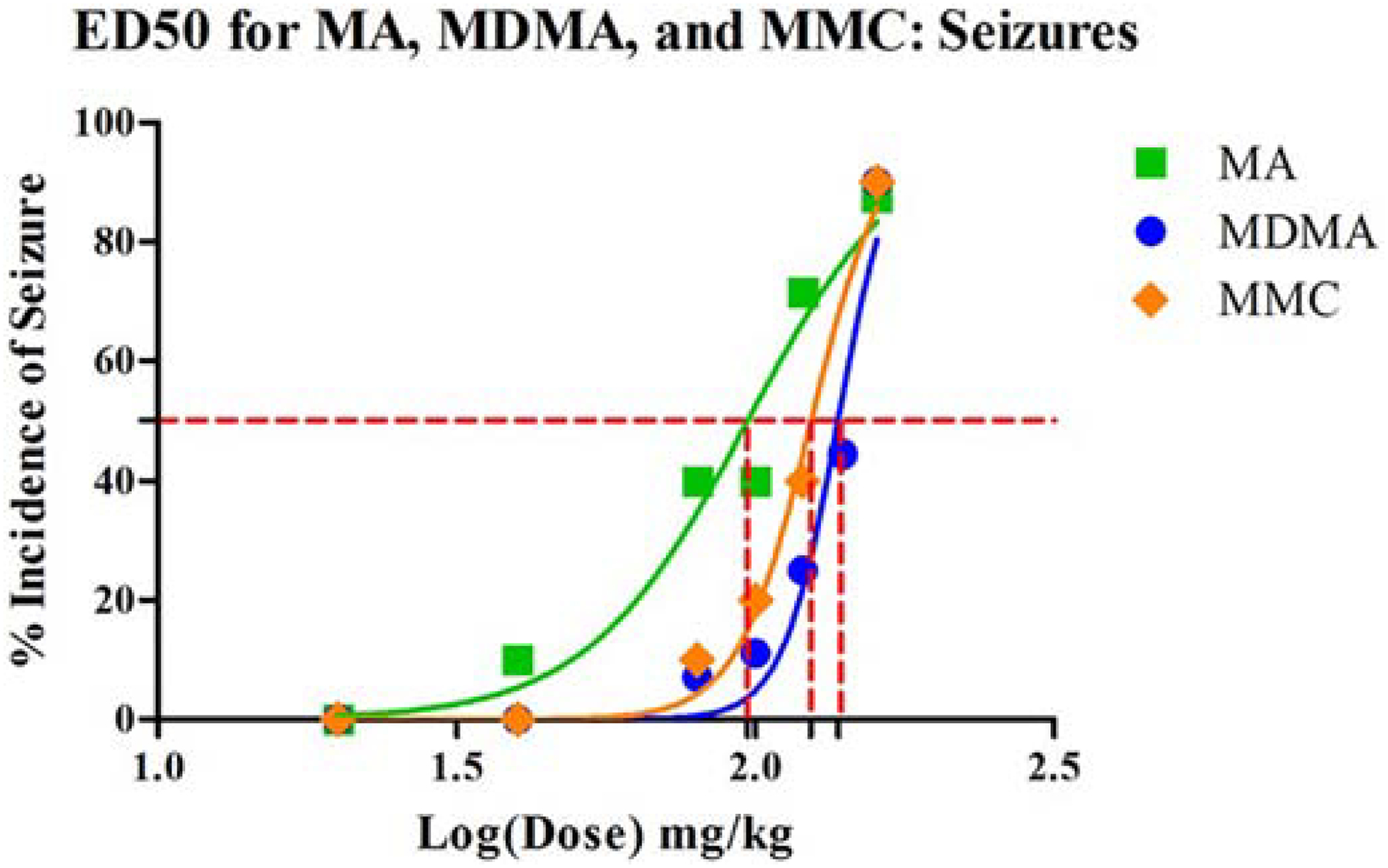
ED50 curves for the incidence of seizure for MA, MDMA an MMC showing relative ED50 values (MDMA>MMC>MA, although all values were similar). Values could not be calculated for CAT, MCAT or MDPV.
3.3. Effect of MA, MDMA and SPCs on Temperature
3.3.1. MA
The effects of MA on temperature were only examined for doses of 20, 40, and 80 mg/kg as higher doses caused lethal effects in most subjects within 2 hrs (Fig. 3A/B). MA administration in male but not female mice increased body temperature. An omnibus repeated measures ANOVA for temperature revealed a significant effect of TIME (F[8,168] = 5.0, p<0.0001) and a significant interaction of TIME × SEX (F[24,200] = 2.8, p<0.0001). In subsequent 1-way ANOVA examining each dose separately, only males at the 40 mg/kg dose showed a significant overall effect of TIME (F[8,80] = 5.38, p<0.0001). Temperature increased after injection by about 1.5 °C, reaching a maximum at the 60 min timepoint (p<0.04 vs. baseline). Temperature subsequently returned to baseline by the 100 min timepoint. Neither the 20 mg/kg dose, nor the 80 mg/kg dose produced significant changes in temperature in males. When compared to baseline, females showed no significant differences in temperature post-injection.
Figure 3.
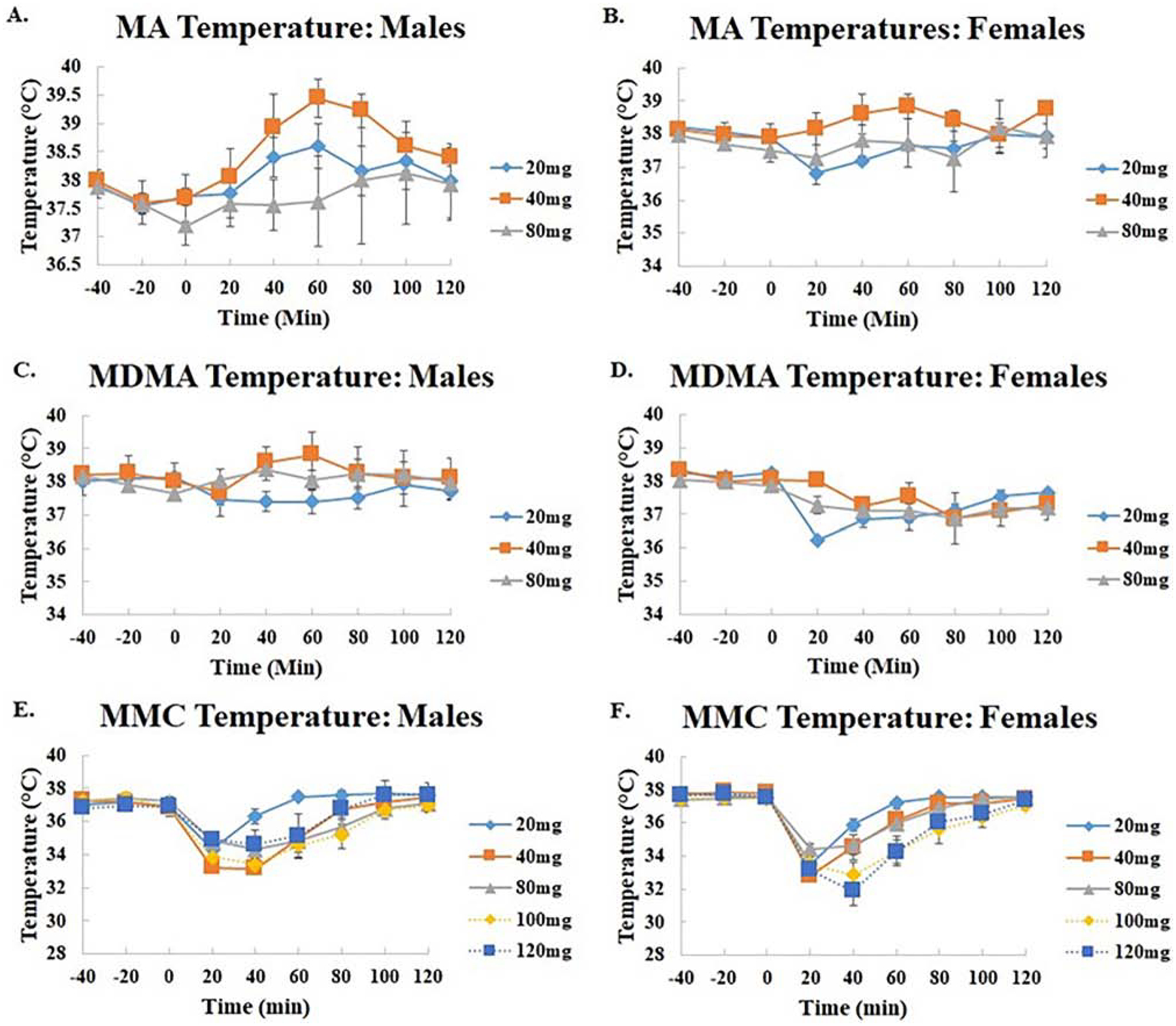
Body temperature in male (A/C/E) and female (B/D/F) mice after administration of MA (A/B; 20, 40, and 80 mg/kg IP), MDMA (C/D; 20, 40, and 80 mg/kg IP), and MMC (E/F; 20–160 mg/kg IP).
3.3.2. MDMA
The effects of MDMA on temperature were only compared for the 20, 40, and 80 mg/kg doses due to lethal effects of MDMA at higher doses. MDMA was found to increase temperature in males while causing hypothermic effects in females (Figs. 3C/D). An omnibus two-way ANOVA found a significant effect of SEX (F[1,26] = 9.0, p<0.006) while a repeated measures ANOVA indicated a significant effect of TIME (F[8,208) = 6.2, p<0.0001), as well as significant interactions of TIME × SEX (F[8,208) = 4.6, p<0.0001) and TIME × DOSE (F[16,208] = 1.7, p<0.05). Post hoc analysis revealed, however, there were no significant differences between doses. Subsequent 1-way ANOVA examining the effect of sex revealed that only females had a significant effect of TIME (F[8,96] = 10.9, p<0.001). When compared to baseline temperatures, a treatment with 20 mg/kg was found to induce a hypothermic response from 20 (p<0.0001) to 80 minutes(p<0.004)) minutes after injection. This effect was not significant at any dose in males.
3.3.3. MMC
Treatment with MMC produced profound hypothermic effects in females as well as producing the greatest temperature decreases in males compared to the five other drugs (Fig. 3 E/F; compare to Figures 3 and 4). An overall ANOVA indicated a significant main effect of DOSE (F[4,39] = 3.6, p<0.01), but there was no main effect of SEX. A repeated measures ANOVA indicated a significant main effect of TIME (F[8,312] = 158.2, p<0.0001) as well as interactions of TIME × SEX (F[8,312] = 3.9, p<0.0001) and TIME × DOSE (F[32, 312] = 4.8, p<0.00001). The data were further examined in separate ANOVA split by sex, which indicated a significant effect of TIME in both males (F[8,160] = 58.1, p<0.0001) and females (F[8,152] = 107.4, p<0.0001). An interaction of TIME × DOSE was also seen for both males (F[32,160] = 2.9, p<0.0001) and females (F[32,152] = 3.3, p<0.0001). Subsequent one-way ANOVA examining each dose separately indicated significant effects of TIME at 20 mg/kg (F[8, 32] = 53.9, p<0.0001), 40 mg/kg (F[8, 32] = 24.3, p<0.0001), 80 mg/kg (F[8, 32] = 17.4, p<0.0001), 100 mg/kg (F[8, 32] = 16.2, p<0.0001), and 120 mg/kg (F[8, 24] = 30.9, p<0.0001) for females and 20 mg/kg (F[8, 32] = 16.0, p<0.0001), 40 mg/kg (F[8, 32] = 106.8, p<0.0001), 80 mg/kg (F[8, 32] = 11.9, p<0.0001), 100 mg/kg (F[8, 32] = 12.5, p<0.0001), and 120 mg/kg (F[8, 32] = 5.2, p<0.0004) for males. Post hoc analysis indicated substantial decreases in temperature in both males and females. At 20 mg/kg, females had hypothermic responses lasting from 20 minutes (p<0.0001) to 40 minutes (p<0.0001) post injection, while males experienced less prolonged effects lasting only 20 minutes (p<0.0001). Administration of 40 mg/kg of MMC produced hypothermic changes extending from 20 minutes (p<0.0001) to 60 minutes (p<0.05) post injection in females and in males (p<0.0001). In females, 80 mg/kg doses produced similar responses with effects peaking at 20 minutes (p<0.0001) post injection and lasting 60 minutes. (p<0.02). A similar, but slightly more prolonged effect was observed in males given this dose, peaking at 20 minutes (p<0.0001) and lasting up until 80 minutes (p<0.03) after administration. When injected with 100 mg/kg MMC, slightly more prolonged hypothermic effects were observed in females which lasted from 20 minutes (p<0.0001) to 60 minutes (p<0.0003). Moreover, males showed similar effects with hypothermic responses lasting from 20 minutes (p<0.0001) to 80 minutes (p<0.03) after injection. Finally, administration of 120mg/kg of MMC produced the most significant and prolonged decreases in temperature in females lasting from 20 minutes (p<0.0001) to 60 minutes (p<0.0001), but this dose only elicited significant hypothermic effects at 20 minutes (p<0.04) post injection in males.
Figure 4.
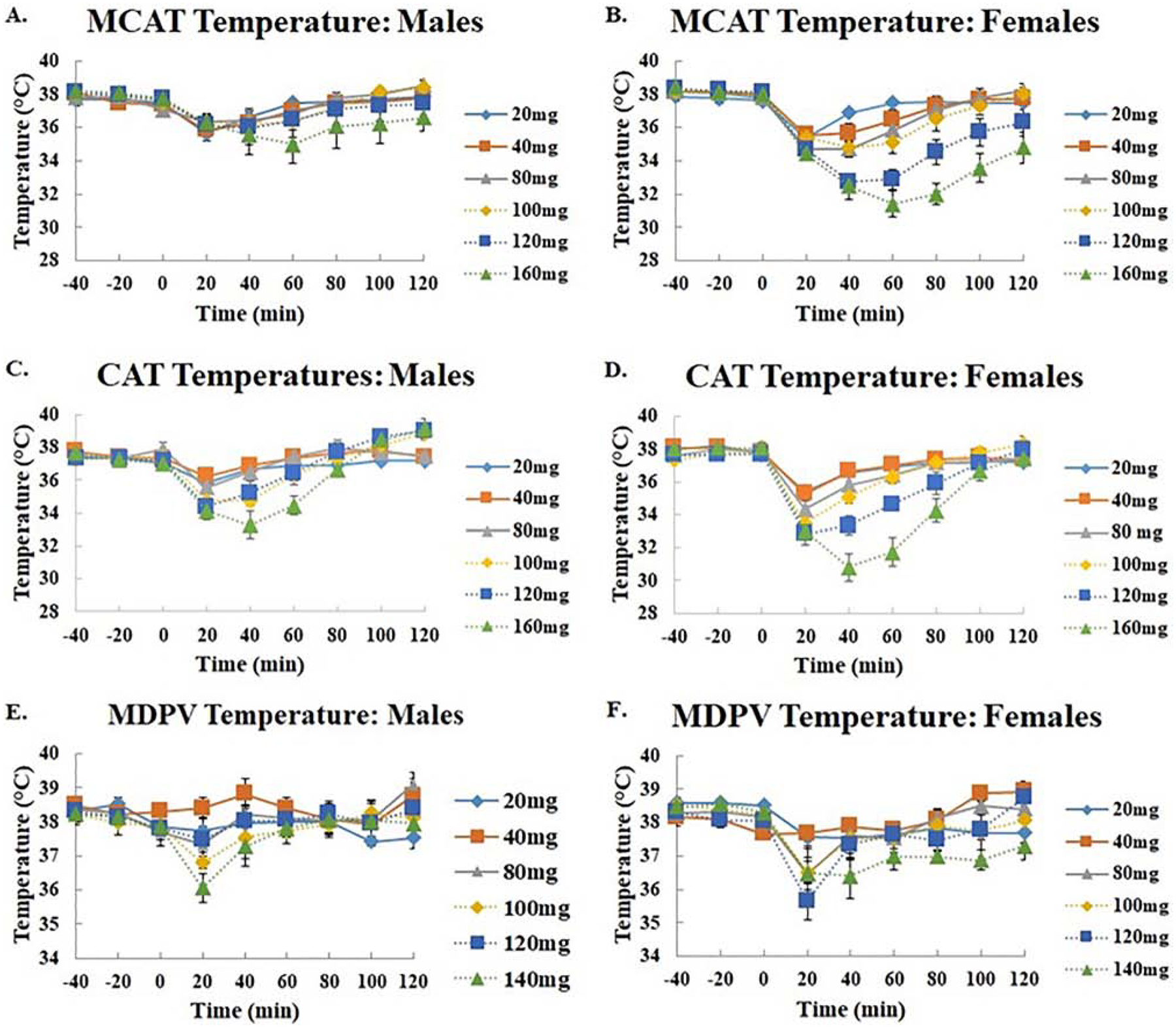
Body temperature in male (A/C/E) and female (B/D/F) mice after administration of MCAT (A/B; 20, 40, 80, 100, 120, and 160 mg/kg IP), CAT (C/D; 20, 40, 80, 100, 120, and 160 mg/kg IP), and MDPV (E/F; 20, 40, 80, 100, 120, and 140 mg/kg IP).
3.3.4. MCAT
Administration of MCAT also induced pronounced hypothermic effects, the severity of which was dependent on both sex and dose (Fig. 4A/B). An overall two-way ANOVA for temperature indicated significant effects of SEX (F[1,47] = 14.2, p<0.0001) and DOSE (F[5,47] = 6.570, p<0.0001), as well as an interaction of SEX × DOSE (F[5,47] = 2.5, p<0.047). A repeated measures ANOVA indicated a significant effect of TIME (F[8,376] = 94.6, p<0.0001) as well as interactions of TIME × SEX (F[8,376] = 13.8, p<0.0001), TIME × DOSE (F[40, 376] = 7.2, p<0.0001), and TIME × SEX × DOSE (F[40, 376] = 1.6, p<0.01).
Subsequent ANOVA examining males and females individually indicated a significant main effect of DOSE (F[5,24] = 9.8, p<0.0001) in females but not males. Additionally, a significant main effect of TIME was found in both males (F[8, 184] = 19.3, p<0.0001) and females (F[8,192] = 98.0, p<0.0001), however, an interaction of TIME × DOSE (F[40,192] = 8.7, P<0.0001) was found in females only. Subsequent ANOVA examining DOSE indicated a significant main effect of TIME for 20 mg/kg (F[8, 32] = 18.0, p<0.0001), 40 mg/kg (F[8, 32] = 8.014, p<0.0001), 80 mg/kg (F[8, 32] = 22.8, p<0.0001), 100 mg/kg (F[8, 32] = 18.3, p<0.0001), 120 mg/kg (F[8, 32] = 28.1, p<0.0001), and 160 mg/kg (F[8, 32] = 32.6, p<0.0001), in females, and 20 mg/kg (F[8, 32] = 7.12, p<0.0001), 40 mg/kg (F[8, 32] = 6.1, p<0.0001), 80 mg/kg (F[8, 32] = 4.6, p<0.001), 100 mg/kg (F[8, 32] = 8.9, p<0.0001), 120 mg/kg (F[8, 32] = 2.3, p<0.05), and 160 mg/kg (F[8, 24] = 2.9, p<0.03) in males. Post hoc analysis of all timepoints compared to baseline indicated that the hypothermic effects MCAT were more prolonged at higher doses in both males and females. Females treated with 20 mg/kg of MCAT experienced hypothermic effects from 20 minutes (p<0.0001) to 40 minutes (p<0.005) post injection (vs baseline) while the duration of this effect was only observed at 20 minutes (p<0.001) in males. Treatment with 40 mg/kg of MCAT again produced pronounced hypothermic effects which lasted longer than the effects of 20 mg/kg MCAT. Females treated with this dose experienced decreases in temperature from 20 minutes (p<0.0001) to 60 minutes (p<0.03) post injection. Similarly, males showed prolonged temperature decreases as well, lasting from 20 minutes (p<0.0001) to 40 minutes (p<0.002) post injection. At 80 mg/kg of MCAT females showed hypothermic effects between 20 minutes (p<0.0001) and 60 minutes (p<0.0001) post injection, while males failed to show significance. Treatment with 100 mg/kg elicited even more prolonged effects in females lasting from 20 minutes post injection (p<0.0001) to 80 minutes post injection. Males at this same dose also experienced prolonged hypothermia lasting from 20 minutes (p<0.002) to 60 minutes (p<0.002) post injection. The hypothermic effects of MCAT were further prolonged at 120 mg/kg in females, starting 20 minutes (p<0.0001) and never fully returning to baseline temperatures even 120 minutes (p<0.02) after injection. Post hoc comparisons did not identify an significant effects in males given 120 mg/kg MCAT. Finally, treatment with 160 mg/kg of MCAT produced the most pronounced prolonged hypothermic effect in females starting at 20 minutes (p<0.0001) post injection and never fully returning to basal temperatures 120 minutes later (p<0.0001). Males experienced some hypothermic response at this dose, peaking at 60 minutes (p<0.02) post injection.
The peak hypothermic effect occurred 60 min after injection in both males and females, however, there was a more pronounced and prolonged effect in females as opposed to males. The most significant difference in temperature among females at this point was observed between the lowest dose of 20mg and the two highest doses of 120mg (p<0.000) and 160mg (p<0.000). When compared to baseline temperatures, all doses induced hypothermic responses 20 minutes post injection (p<0.000) in females. This response was the most prolonged at the 160mg/kg, preventing the return to normothermic temperatures throughout the experiment (p<0.000). In males, no significant differences in dose were observed at any of the time points.
3.3.5. CAT
Treatment with CAT produced a similar hypothermic state to that observed with MCAT, an effect again dependent on both sex and dose (Fig. 4C/D). An overall two way ANOVA indicated significant main effects of both SEX (F[1,45] = 8.5, p<0.006) and DOSE (F[5,45] = 8.2, p<0.0001). A repeated measures ANOVA examining the effect of TIME, showed a significant main effect of TIME (F[8,360] = 168.1, p<0.0001) as well as significant interaction of TIME × SEX (F[8,360] = 12.9, p<0.0001), TIME × DOSE (F[40,360] = 12.9, p<0.0001), and TIME × SEX × DOSE (F[40,360] = 1.5, p<0.03). Post hoc analysis showed that the largest hypothermic effect occurred at a dose of 160 mg/kg when compared to doses of 20 (p<0.0001), 40 (p<0.0001), and 100 mg/kg (p<0.005), but not 120 mg/kg. Additional ANOVA analyzing the data separately for each sex showed significant effects of TIME for females (F[8,192] = 113.4, p<0.0001) and males (F[8,168] = 67.0, p<0.0001) as well as interactions of TIME × DOSE (Females: F[40,192] = 8.4, p<0.0001; Males: F[40, 168] = 5.9, p<0.0001). Subsequent ANOVA examining DOSE separately revealed a significant effect of TIME for females at doses of 20 mg/kg (F[8, 32] = 16.6, p<0.0001), 40 mg/kg (F[8, 32] = 39.9, p<0.0001), 80 mg/kg (F[8, 32] = 16.05, p<0.0001), 100 mg/kg (F[8, 32] = 16.6, p<0.0001), 120 mg/kg (F[8, 32] = 17.5, p<0.0001), and 160 mg/kg (F[8, 32] = 40.5, p<0.0001) and males at doses of 20 mg/kg (F[8, 24] = 4.6, p<0.002), 40mg/kg (F[8, 24] = 3.1, p<0.0001), 80 mg/kg (F[8, 24] = 5.0, p<0.002), 100 mg/kg (F[8, 32] = 22.9, p<0.0001), 120 mg/kg (F[8, 32] = 16.2, p<0.0001), and 160 mg/kg (F[8, 32] = 48.7, p<0.0001). Post hoc comparisons of all timepoints compared to baseline indicated that females showed hypothermic responses at all doses, differing only in the duration of hypothermia, which lasted longer at higher doses. Males showed hypothermic responses, but these responses were not as prolonged as females. Administration of 20 mg/kg CAT in females lead to decreases in temperature 20 minutes (p<0.0001 vs baseline) post injection, which lasted until approximately 60 minutes (p<0.01). Males also had decreased temperatures at this dose, but only at 20 minutes (p<0.005) after administration. In females 40 mg/kg CAT elicited hypothermic responses from 20 minutes (p<0.0001) post injection which lasted the entire duration of the experiment (p<0.01). Conversely, males only showed decreases in temperature at 20 minutes (p<0.007) post injection. Doses of 80 mg/kg produced hyperthermic effects in females, lasting from 20 minutes (p<0.0001) to 60 minutes post injection (p<0.05), but in males hypothermia was only observed at 20 minutes (p<0.004) post injection. Administration of 100 mg/kg of CAT lead to decreased temperatures from 20 minutes (p<0.0001) to 40 minutes (p<0.0002) post injection in males and females. 160 mg/kg injections of CAT induced hypothermic changes in females from 20 minutes (p<0.0001) to 80 minutes (0.0001) after injection. Males showed similar but less prolonged effects at this same dose lasting from 20 minutes (p<0.0001) to 60 minutes (p<0.0001) post injection.
3.3.6. MDPV
Administration of MDPV produced significant hypothermic effects in both males and females, which were most pronounced at the highest doses, primarily at the 20 min time-point, and then returning to basal levels (Fig. 4E/F). The exception here was the highest dose in females, which did not return to baseline levels for nearly the entire assessment period. An omnibus ANOVA for temperature revealed a significant effects of DOSE (F[5,48] = 2.6, p<0.04) and TIME (F[8,384] = 21.2, p<0.0001), and significant interactions with TIME: TIME × SEX (F[8,384] = 3.3, p<0.001) and TIME × DOSE (F[40,384] = 2.1, p<0.0001). The data were then split by sex to further analyze these results in separate ANOVA. Both males (F[8,192] = 5.1, p<0.0001) and females (F[8,192] = 21.7, p<0.0001) showed a significant effect of TIME, however, only females had a significant of TIME × DOSE interaction (F[40,192] = 2.3, p<0.0001). Subsequent ANOVA examining individual doses indicated significant effects of TIME for 40 mg/kg (F[8, 32] = 6.5, p<0.0001), 80 mg/kg (F[8, 32] = 6.2, p<0.0001), 100 mg/kg (F[8, 32] = 3.2, p=0.01), 120 mg/kg (F[8, 32] = 12.35, p<0.0001), and 140 mg/kg (F[8, 32] = 5.0, p<0.001) in females. Males, however, only showed effects of TIME at the 80 mg/kg (F[8, 32] = 2.4, p<0.04) and 140 mg/kg (F[8, 32] = 3.2, p=0.01) doses. Post hoc comparisons of all time-points compared to baseline confirmed that profound hypothermic responses were observed. Females treated with 80 mg/kg (p<0.0003), 100mg/kg (p<0.002), and 120 mg/kg (p<0.0001) of MDPV displayed substantial decreases in temperature 20 minutes after injection compared to baseline. Prolonged hypothermia was observed for doses of 140 mg/kg from 20 (p<0.007) to 40 minutes (p<0.005) post-injection in females. Only a dose of 140 mg/kg, however, was able to elicit hypothermia in males at 20 minutes after injection (p<0.003) compared to baseline. Post hoc means comparisons did not identify any significant differences for the 80 mg/kg dose in males.
3.4. Organ Weights
Organ weight data was normalized according to body weight (Fig. 5). Differences were seen in only the heart and liver and only for MA, MDMA and MMC. MA, MDMA and MMC increased normalized heart weight in a dose dependent manner. MA and MMC, but not MDMA, significantly increased normalized liver weight. A two-way ANOVA for normalized heart weight after treatment with MA indicated a significant effect of DOSE (Fig. 5A; F[5,46] = 2.5, p<0.05) for normalized heart weight. Heart weight increased at intermediate doses but then returned to levels observed at the lower concentrations. The only significant difference in the means comparisons was between the 40 mg/kg MA dose and the 100 and 120 mg/kg MA doses (p<0.05). A two-way ANOVA examining normalized liver weights after MA treatment revealed a significant main effect of DOSE (Fig. 5B; F[5,46] = 16.7, p<0.001). Post hoc means comparisons revealed a significant increase in liver weight at both the 160 mg/kg (p<0.001) and 120 mg/kg (p<0.001) doses when compared to the lowest MA dose. No effect of SEX was observed. MDMA also increased normalized heart rates, as shown by a significant effect of dose (Fig. 5C; F[5,42] = 3.7, p<0.01)). Post hoc means comparisons found that there were significant differences for the 80 and 100 mg/kg IP doses versus the 20 mg/kg dose. MDMA did not significantly affect liver weight (Fig. 5D; F[5,42] = 1.5, NS). MMC administration increased heart weight at the 100 mg/kg dose (Fig. 5E) and liver weight at the 160 mg/kg dose (Fig. 5F). A two-way ANOVA examining normalized heart indicated a significant effect of DOSE (F[5,51] = 5.6, p<0.006). Post hoc means comparisons showed that the largest increase occurred at the 100 mg/kg dose (p<0.02), after which weight again returned to levels observed at lower, non-lethal, doses. A two-way ANOVA examining normalized liver weight showed a significant effect of DOSE (F[5,51] = 4.2, p<0.003). Post hoc means comparisons indicated that the largest differences in liver weight occurred between doses of 160 mg/kg and 20 mg/kg MMC (p<0.05). No differences of SEX were observed.
Figure 5.
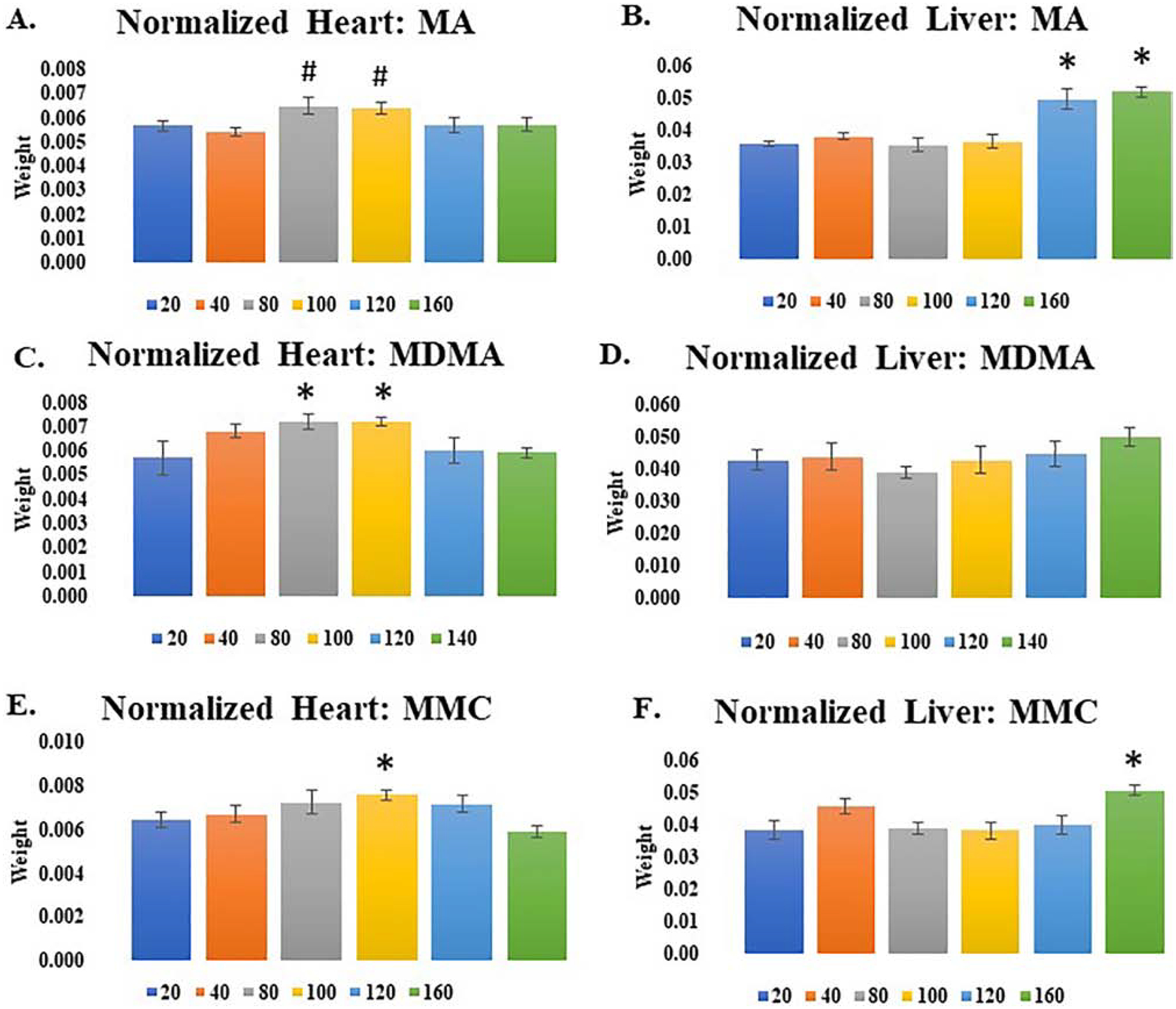
Normalized heart (A/C/E) and liver (B/D/F) weight after administration of MA (A/B; 20–160 mg/kg IP), MDMA (C/D; 20–140 mg/kg IP), and MDPV (E/F; 20, 40, 80, 100, 120, and 140 mg/kg IP). * p<0.05 vs. 20 mg/kg dose. # p<0.05 vs. 40 mg/kg dose.
Histological analysis, by hematoxylin-eosin staining, did not show any changes for most organs examined, but did shown minor changes in cell morphology in the liver (data not presented), similar to those observed by Halpin et al. (2013) for MA at similar timepoints post-injection. Such changes were only observed for MA, MDMA and MMC at lethal doses.
5.0. DISCUSSION
Contrary to initial predictions based upon a previous study that examined the lethal effects of methylone (Piao et al., 2015), none of the SPCs studied here (MMC, CAT, MCAT, and MDPV) were found to induce greater lethality than MA or MDMA (e.g. to have lower LD50 values). Indeed, for several of these drugs (CAT, MCAT, and MDPV) it was not possible to calculate an LD50 value at all over the doses studied. This would seem to suggest, in contrast to expectations based upon clinical reports (for examples see (Fudin et al., 2018)), that these drugs pose less risk of overdose than the more commonly abused amphetamine drugs MA and MDMA. However, as will be discussed here, it is too early to make a broad determination regarding the risks of these drugs and several factors may influence lethality that were not considered in the current experiments.
5.1. Temperature
In a similar manner to the failure of these drugs to induce substantial lethality, these cathinones did not induce hyperthermia, which is one of the primary adverse outcomes of MA and MDMA overdose. MA induced hyperthermia in male, but not female, mice, and MDMA produced only a marginal hyperthermia at one dose in males. In part, this may reflect the conditions used to examine lethality in the present studies – overdose resulting from exposure to a single drug dose. This was an intentional choice for these initial studies, to examine the lethal and toxic effects of these drugs after a single exposure before moving on to studies examining the effects of chronic or repeated exposures in subsequent studies. In particular, hyperthermia is more typically observed using “binge-dosing” regimens (repeated moderate dosing cumulatively equivalent to about an LD50 dose over a period of a few hours). Indeed, most of the studies demonstrating hyperthermia and other adverse effects resulting from MA and MDMA have used these binge-dosing or chronic escalating regimens of drug exposure (for review see Cadet et al. (2007) and Krasnova and Cadet (2009)). It is important to note that even within a single MA binge, the initial dose does not necessarily induce hyperthermia, and indeed can produce hypothermia, while subsequent doses do induce hyperthermia (Granado et al., 2010), suggestive of acute adaptations underlying hyperthermia. A virtually identical pattern was observed recently for both MDMA and methylone (Miner et al., 2017), both drugs exhibiting robust decreases in temperature after the first injection, of 3 °C and 4 °C respectively, but increases in temperature after the fourth injection. The doses of MDMA and methylone tested in that study were in the range of the lowest doses studied in the present experiments. Although those authors did not find effects of MDPV on temperature, they only used a dose of 1 mg/kg IP, well below the range of doses assessed here. MMC has also been found to produce hypothermia acutely, but hyperthermia when administered in a binge-paradigm (Shortall, 2015). Caffeine can also convert MMC-induced hypothermia into hyperthermia (Shortall et al., 2016).
In the present study, MDPV produced pronounced decreases in temperature after single injections of large doses (≈ 2 °C in males and females). These effects were mostly transient, except in females at the highest dose tested. The other cathinones produced even more robust and lasting hypothermia: MCAT (maximal hyperthermia ≈ 3 °C in males and ≈ 7 °C females), CAT (maximal hyperthermia ≈ 5 °C in males and ≈ 7 °C females), and MMC (maximal hyperthermia ≈ 4 °C in males and ≈ 6 °C females). Hypothermic effects were greater in females for all of these cathinones. Susceptibility to changes in body temperature in males and females have been tied to differences in body mass and susceptibility to cutaneous vasodilation in males and females (Ford and Klugman, 1980). Hypothermia is observed after administration of many cathinones under standard temperature conditions in rodents, including MMC (Aarde et al., 2013a; Anneken et al., 2017a; Shortall et al., 2013), MCAT (Anneken et al., 2017a; Anneken et al., 2017b), and MDPV (Aarde et al., 2015). An important factor here may be the ambient temperature. Single injections of MMC (Aarde et al., 2013a; Anneken et al., 2017a; Shortall et al., 2013), MCAT (Anneken et al., 2017a), MDPV (Aarde et al., 2015), and α-pyrrolidinopentiophenone (αPVP; (Aarde et al., 2015; Aarde et al., 2013b)) have been shown to induce hypothermia rather than hyperthermia in rodents at normal room temperature. A direct comparison of MA and MDPV found that MA induced hyperthermia but MDPV did not affect temperature substantially (Aarde et al., 2013b). Lower doses (<10 mg/kg IP) of CAT and MCAT, below those doses examined in the present study, produce a slight (≈ 1 °C) hyperthermia. An important factor here in the observation of hypothermia versus hyperthermia may be the ambient temperature. The current experiments were conducted at “room temperature” (e.g. 22–23 °C) and singly. Ambient temperature and the presence of other animals (also affecting heat dissipation) is known to affect the effects of MDMA and MA on temperature (Fantegrossi et al., 2003; Huether et al., 1997). Similarly, MDPV has been shown to produce hyperthermia at elevated temperatures but not at normal ambient temperature (Gannon et al., 2017), although the robustness of hyperthermia with increased temperature is certainly less that MA (Aarde et al., 2013b). By contrast, MMC potentiated the hyperthermic effects of MA, as well as MA-induced neurotoxicity (Angoa-Pérez et al., 2013). This occurs despite MMC not producing monoaminergic toxicity on its own. Repeated MMC binge injections (at 2 hr intervals), while continuing to produce transient hypothermia, produced an opponent hyperthermia in recovery (Angoa-Pérez et al., 2012). The hypothermia observed here might certainly have affected lethality, indeed it might have exerted a protective effect, although hyperthermia is certainly not necessary for lethality, and vice versa (Numachi et al., 2007; Piao et al., 2015). It remains to be seen whether the effects of the SPCs studied here might produce hyperthermia at higher ambient temperatures, and what effect this might have on their lethality.
5.2. Hepatotoxicity, ammonia, and brain glutamate function
There was evidence for acute hepatotoxicity in response to MA in the present studies based upon increased liver weight and post mortem histology. The histological results are similar to what was observed for MA at a similar time-point by Halpin et al. (2013). Similar results were observed for MMC, but not any of the other SPCs tested. Doses of MA and SPCs that induced evidence of liver toxicity largely corresponded with lethal doses. No evidence of liver toxicity was observed for SPCs that did not induce toxicity. Although liver toxicity was not observed in vivo here for many SPCs, toxicity in primary hepatocytes has been demonstrated for many SPCs, including pentedrone, MDPV, methylone, and 4-methylethcathinone (4-MEC) (Araújo et al., 2015). Numerous factors could influence the observation of liver toxicity, including the route of administration, dosing regimen, ambient temperature, and the presence of hyperthermia/hypothermia. Some of these factors may also differentially affect the observation of hepatotoxicity between in vitro and in vivo studies.
In any case, the observation of hepatotoxicity is consistent with previous observations for MA that also found elevations in plasma concentrations of ammonia (Halpin et al., 2014). Those authors further demonstrated that increased plasma levels of ammonia were associated with increased brain glutamate levels, and that these mechanisms were important mediators of MA neurotoxicity. Neurotoxicity was assessed in terms of the well-known ability of MA to reduce brain tissue concentrations of dopamine and serotonin. This study differed from the present studies in terms of time-course about outcome measures, focusing on sub-lethal binge-dosing and neurotoxicity. However, with regards the connection between hepatotoxicity, plasma ammonia and elevated brain glutamate levels, it is interesting to note, in addition to the present observations of hepatotoxicity at lethal drug doses, that lethality was almost universally associated with seizure in the present experiments, a likely indicator of elevated glutamate function, although this hypothesis will certainly need to be confirmed directly.
Regarding the potential for cathinones to induce neurotoxicity, many studies have failed to show that many cathinones induce neurotoxicity using standard binge-regimen approaches (Angoa-Pérez et al., 2012; Anneken et al., 2015; Lopez-Arnau et al., 2015; Martinez-Clemente et al., 2014; Thomas et al., 2004). These studies examined a wide range of markers for neurotoxic effects, including tissue monoamine levels, markers for monoamine terminals, and markers for reactive gliosis. Given the important role of glutamate in neurotoxicity, this might indicate that these cathinones have less ability to elevate glutamate levels than MA. However, it should be noted that despite a lack of neurotoxicity (Anneken et al., 2015), methylone does induce lethality at doses slightly lower than MA or MDMA (Piao et al., 2015). This might suggest that there may be a divergence between the factors influencing neurotoxicity and acute lethality. Alternatively, some other factor that differed between those studies, such as ambient temperature or species/strain, may influence the observation of lethality or neurotoxicity. These differences might occur in both directions. MCAT, which showed no signs of lethality in the present studies, did produce some neurotoxicity (Anneken et al., 2017b; Sparago et al., 1996), although this was much reduced in comparison to MA.
It has been suggested that cathinone use is associated with a greater incidence of emergency room admissions than other abused drugs (American Association of Poison Control Centers, 2011, Wood et al., 2015, 2013). Among the symptoms noted in reports of cathinone overdoses are hepatic impairments or hepatic failure (Fröhlich et al., 2011; Riyaz et al., 2014; Smith et al., 2013; Somi et al., 2014). Elevated levels of aspartate and alanine aminotransferase are reported in many of these cases (Borek and Holstege, 2012; Kramer et al., 2016; Murray et al., 2012; Thirakul et al., 2017), which are common indicators of liver damage. Post mortem histological analysis of methylone overdose patients has also found evidence of hepatic portal and lobular inflammation (Benzer et al., 2013; Carbone et al., 2013). Even when many of the acute physiological and neurological symptoms associated with cathinone overdose have abated, within about 24 hours of the initial overdose, hepatic impairments can persist, sometimes resulting in hepatic failure in the days following initial hospitalization. One case report of a cathinone overdose involved mildly elevated aspartate and alanine transaminase levels initially, but these levels increased dramatically over the next two days, even as other symptoms abated (Kramer et al., 2016). It is not surprising that cathinones would be associated with hepatic damage since MDMA and MA also produce hepatic impairments (for a review, see Carvalho et al., 2012). Very few studies have examined cathinone toxicity or lethality in vivo. Most of what is known about the hepatotoxic effects of cathinones comes from a few in vitro studies using either primary hepatocytes or hepatic cell lines. These studies have shown that methylone (Nakagawa et al., 2009; Valente et al., 2017), MDPV, pentedrone, and 4-MEC (Valente et al., 2017) are hepatotoxic. Methylone was less toxic than MDMA, while MDPV, pentedrone and 4-MEC were more toxic.
Although much remains to be done to study the cellular toxicity of cathinones, hepatic or otherwise, it certainly appears that cathinones, like MA and MDMA, can damage the liver. It remains to be directly observed whether this is associated with elevated plasma ammonia and brain glutamate function, and whether these effects contribute to lethality. The present study clearly demonstrates that several cathinones have less potential for inducing lethal effects than MA or MDMA. Some evidence here suggests that this may be due, in part, to their propensity to induce liver toxicity, although much remains to be done in this regard, particularly with respect to structural features of cathinones that may contribute to greater liver toxicity and other effects. Reduced lethality was observed for all of the cathinones studied here. Indeed, lethality was so much reduced for CAT, MCAT and MDPV that the LD50 values could not even be calculated. This does not mean that differences in lethality will not be observed for other cathinones, and it will be essential to study a sufficient number of these analogues to identify structure-activity relationships. Moreover, the reduced lethality observed here may not carry over to other situations, relevant to the ways that human consume these drugs, including under conditions of higher ambient temperatures, in the presence of other factors contributing to liver toxicity, and under binge or chronic administration conditions.
Acknowledgements
This work was supported by start-up funds from the University of Toledo (FSH), an Interdisciplinary Research Initiation Award (FSH) from the University of Toledo, and funding from the National Institute on Drug Abuse (NIDA), DA045350 (FSH). We also wish to acknowledge the NIDA Drug Supply Program for supplying most of the drugs used in these studies. The authors wish to acknowledge the assistance of Courtney Hefflinger with these experiments as part of an undergraduate research course at the University of Toledo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- Aarde SM, Angrish D, Barlow DJ, Wright MJ Jr., Vandewater SA, Creehan KM, Houseknecht KL, Dickerson TJ, Taffe MA, 2013a. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addict Biol 18(5), 786–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, & Taffe MA, 2015. In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3, 4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology, 232(16), 3045–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA, 2013b. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology 71, 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Association of Poison Control Centers, 2011. US poison centers raise alarm about toxic substance marketed as bath salts. AAPCC Press Release: http://www.aapcc.org/press/22/. [Google Scholar]
- Angoa-Pérez M, Kane MJ, Herrera-Mundo N, Francescutti DM, Kuhn DM, 2014. Effects of combined treatment with mephedrone and methamphetamine or 3, 4-methylenedioxymethamphetamine on serotonin nerve endings of the hippocampus. Life sciences 97(1), 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Pérez M, Kane MJ, Briggs DI, Francescutti DM, Sykes CE, Shah MM, Thomas DM, Kuhn DM, 2013. Mephedrone does not damage dopamine nerve endings of the striatum, but enhances the neurotoxicity of methamphetamine, amphetamine, and MDMA. Journal of neurochemistry 125(1), 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Pérez M, Kane MJ, Francescutti DM, Sykes KE, Shah MM, Mohammed AM, Thomas DM, Kuhn DM, 2012. Mephedrone, an abused psychoactive component of ‘bath salts’ and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. Journal of neurochemistry 120(6), 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anneken JH, Angoa-Perez M, Kuhn DM, 2015. 3,4-Methylenedioxypyrovalerone prevents while methylone enhances methamphetamine-induced damage to dopamine nerve endings: beta-ketoamphetamine modulation of neurotoxicity by the dopamine transporter. J Neurochem 133(2), 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anneken JH, Angoa-Perez M, Sati GC, Crich D, Kuhn DM, 2017a. Assessing the role of dopamine in the differential neurotoxicity patterns of methamphetamine, mephedrone, methcathinone and 4-methylmethamphetamine. Neuropharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anneken JH, Angoa-Perez M, Sati GC, Crich D, Kuhn DM, 2017b. Dissecting the Influence of Two Structural Substituents on the Differential Neurotoxic Effects of Acute Methamphetamine and Mephedrone Treatment on Dopamine Nerve Endings with the Use of 4-Methylmethamphetamine and Methcathinone. J Pharmacol Exp Ther 360(3), 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo AM, Valente MJ, Carvalho M, Da Silva DD, Gaspar H, Carvalho F, de Lourdes Bastos M, De Pinho PG, 2015. Raising awareness of new psychoactive substances: chemical analysis and in vitro toxicity screening of ‘legal high’packages containing synthetic cathinones. Archives of Toxicology 89(5), 757–771. [DOI] [PubMed] [Google Scholar]
- Ashrafioun L, Bonadio FA, Baik KD, Bradbury SL, Carhart VL, Cross NA, Davis AK, Feuille M, Harper AR, Lackey JH, Lang B, Lauritsen KJ, Leith J, Osborn LA, Rosenberg H, Stock J, Zaturenskaya M, 2016. Patterns of Use, Acute Subjective Experiences, and Motivations for Using Synthetic Cathinones (“Bath Salts”) in Recreational Users. J Psychoactive Drugs 48(5), 336–343. [DOI] [PubMed] [Google Scholar]
- Banks ML, Worst TJ, Rusyniak DE, Sprague JE, 2014. Synthetic cathinones (“bath salts”). Journal of Emergency Medicine 46(5), 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA Jr., Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV, 2012. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 37(5), 1192–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck O, Bäckberg M, Signell P, Helander A, 2018. Intoxications in the STRIDA project involving a panorama of psychostimulant pyrovalerone derivatives, MDPV copycats. Clinical Toxicology 56(4), 256–263. [DOI] [PubMed] [Google Scholar]
- Benzer TI, Nejad SH, Flood JG, 2013. case 40–2013: A 36-year-old Man with Agitation and Paranoia. New England Journal of Medicine 369(26), 2536–2545. [DOI] [PubMed] [Google Scholar]
- Borek HA, Holstege CP, 2012. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3, 4-methylenedioxypyrovalerone. Annals of emergency medicine 60(1), 103–105. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J, 2007. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res 11(3–4), 183–202. [DOI] [PubMed] [Google Scholar]
- Calinski DM, Kisor DF, Sprague JE, 2019. A review of the influence of functional group modifications to the core scaffold of synthetic cathinones on drug pharmacokinetics. Psychopharmacology (Berl) 236(3), 881–890. [DOI] [PubMed] [Google Scholar]
- Carbone PN, Carbone DL, Carstairs SD, Luzi SA, 2013. Sudden cardiac death associated with methylone use. Am. J. Foren. Med. Path 34, 26–28. [DOI] [PubMed] [Google Scholar]
- Carvalho M, Carmo H, Costa VM, Capela JP, Pontes H, Remiao F, Carvalho F, Bastos Mde L, 2012. Toxicity of amphetamines: an update. Arch Toxicol 86(8), 1167–1231. [DOI] [PubMed] [Google Scholar]
- Caudevilla-Gálligo F, Ventura M, Ruiz I, Iciar B, Fornís I, 2013. Presence and composition of cathinone derivatives in drug samples taken from a drug test service in Spain (2010–2012). Human Psychopharmacology: Clinical and Experimental 28(4), 341–344. [DOI] [PubMed] [Google Scholar]
- Eiden C, Mathieu O, Cathala P, Debruyne D, Baccino E, Petit P, Peyriere H, 2013. Toxicity and death following recreational use of 2-pyrrolidino valerophenone. Clinical toxicology 51(9), 899–903. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Godlewski T, Karabenick RL, Stephens JM, Ullrich T, Rice KC, Woods JH, 2003. Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (“ecstasy”) and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology (Berl) 166(3), 202–211. [DOI] [PubMed] [Google Scholar]
- Ford DM, Klugman KP, 1980. Body mass and sex as determining factors in the development of fever in rats. J Physiol 304, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich S, Lambe E, O’Dea J J.I.j.o.m.s., 2011. Acute liver failure following recreational use of psychotropic “head shop” compounds. 180(1), 263–264. [DOI] [PubMed] [Google Scholar]
- Fudin HR, Babin JL, Hong LT, Ku J, May AL, Wisner A, Hall FS, Ray SD, 2018. Drugs of Abuse, in: Ray SD (Ed.) Side Effects of Drugs Annual 40. Elsevier, London, 29–89. [Google Scholar]
- Gannon BM, Williamson A, Rice KC, Fantegrossi WE, 2017. Role of monoaminergic systems and ambient temperature in bath salts constituent 3, 4-methylenedioxypyrovalerone (MDPV) elicited hyperthermia and locomotor stimulation in mice. Neuropharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, O’Shea E, Vicario-Abejon C, Colado MI, Moratalla R, 2010. Selective vulnerability in striosomes and in the nigrostriatal dopaminergic pathway after methamphetamine administration : early loss of TH in striosomes after methamphetamine. Neurotox Res 18(1), 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecco GG, Kisor DF, Magura JS, Sprague JE, 2017. Impact of common clandestine structural modifications on synthetic cathinone “bath salt” pharmacokinetics. Toxicol Appl Pharmacol 328, 18–24. [DOI] [PubMed] [Google Scholar]
- Halpin LE, Gunning WT, Yamamoto BK, 2013. Methamphetamine causes acute hyperthermia-dependent liver damage. Pharmacol Res Perspect 1(1), e00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin LE, Northrop NA, Yamamoto BK, 2014. Ammonia mediates methamphetamine-induced increases in glutamate and excitotoxicity. Neuropsychopharmacology 39(4), 1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huether G, Zhou D, Ruther E, 1997. Causes and consequences of the loss of serotonergic presynapses elicited by the consumption of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) and its congeners. J Neural Transm (Vienna) 104(8–9), 771–794. [DOI] [PubMed] [Google Scholar]
- Javadi-Paydar M, Nguyen JD, Vandewater SA, Dickerson TJ, Taffe MA, 2018. Locomotor and reinforcing effects of pentedrone, pentylone and methylone in rats. Neuropharmacology 134(Pt A), 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerry J, Collins G, Streem D, 2012. Synthetic legal intoxicating drugs: the emerging ‘incense’and ‘bath salt’phenomenon. Cleve Clin J Med 79(4), 258–264. [DOI] [PubMed] [Google Scholar]
- Johnson PS, Johnson MW, 2014. Investigation of “bath salts” use patterns within an online sample of users in the United States. J Psychoactive Drugs 46(5), 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanos R, Solis E Jr, Sakloth F, De Felice LJ, Glennon RA, 2013. “Deconstruction” of the abused synthetic cathinone methylenedioxypyrovalerone (MDPV) and an examination of effects at the human dopamine transporter. ACS chemical neuroscience 4(12), 1524–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer C, Wetzel D, Wijdicks E, 2016. Devastating delayed leukoencephalopathy associated with bath salt inhalation. Neurocrit. Care 24, 454–458. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL, 2009. Methamphetamine toxicity and messengers of death. Brain Res Rev 60(2), 379–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Arnau R, Martinez-Clemente J, Rodrigo T, Pubill D, Camarasa J, Escubedo E, 2015. Neuronal changes and oxidative stress in adolescent rats after repeated exposure to mephedrone. Toxicol Appl Pharmacol 286(1), 27–35. [DOI] [PubMed] [Google Scholar]
- Luethi D, Liechti ME, Krähenbühl S, 2017. Mechanisms of hepatocellular toxicity associated with new psychoactive synthetic cathinones. Toxicology 387, 57–66. [DOI] [PubMed] [Google Scholar]
- Madras BK, 2017. The Growing Problem of New Psychoactive Substances (NPS). Curr Top Behav Neurosci 32, 1–18. [DOI] [PubMed] [Google Scholar]
- Marinetti LJ, Antonides HM, 2013. Analysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: method development, drug distribution and interpretation of results. Journal of analytical toxicology 37(3), 135–146. [DOI] [PubMed] [Google Scholar]
- Martinez-Clemente J, Lopez-Arnau R, Abad S, Pubill D, Escubedo E, Camarasa J, 2014. Dose and time-dependent selective neurotoxicity induced by mephedrone in mice. PLoS One 9(6), e99002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH, 2014. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3, 4-methylenedioxypyrovalerone (MDPV). Neuropharmacology 87, 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP, 2003. Elevated environmental temperature and methamphetamine neurotoxicity. Environ Res 92(1), 48–53. [DOI] [PubMed] [Google Scholar]
- Miner NB, O’Callaghan JP, Phillips TJ, Janowsky A, 2017. The combined effects of 3,4-methylenedioxymethamphetamine (MDMA) and selected substituted methcathinones on measures of neurotoxicity. Neurotoxicol Teratol 61, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misailidi N, Papoutsis I, Nikolaou P, Dona A, Spiliopoulou C, Athanaselis S, 2018. Fentanyls continue to replace heroin in the drug arena: the cases of ocfentanil and carfentanil. Forensic Toxicology 36(1), 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray BL, Murphy CM, Beuhler MC, 2012. Death following recreational use of designer drug “bath salts” containing 3,4-methylenedioxypyrovalerone (MDPV). J. Med. Toxicol 8, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Suzuki T, Tayama S, Ishii H, Ogata A J.A.o.t., 2009. Cytotoxic effects of 3, 4-methylenedioxy-N-alkylamphetamines, MDMA and its analogues, on isolated rat hepatocytes. 83(1), 69. [DOI] [PubMed] [Google Scholar]
- Namera A, Kawamura M, Nakamoto A, Saito T, Nagao M, 2015. Comprehensive review of the detection methods for synthetic cannabinoids and cathinones. Forensic toxicology 33(2), 175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Banks ML, 2017. Decoding the Structure of Abuse Potential for New Psychoactive Substances: Structure-Activity Relationships for Abuse-Related Effects of 4-Substituted Methcathinone Analogs. Curr Top Behav Neurosci 32, 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numachi Y, Ohara A, Yamashita M, Fukushima S, Kobayashi H, Hata H, Watanabe H, Hall FS, Lesch K-P, Murphy DL, 2007. Methamphetamine-induced hyperthermia and lethal toxicity: role of the dopamine and serotonin transporters. European journal of pharmacology 572(2–3), 120–128. [DOI] [PubMed] [Google Scholar]
- O’Shea E, Orio L, Escobedo I, Sanchez V, Camarero J, Green AR, Colado MI, 2006. MDMA-induced neurotoxicity: long-term effects on 5-HT biosynthesis and the influence of ambient temperature. Br J Pharmacol 148(6), 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Salomone A, Vincenti M, Cleland CM, 2016. Detection of “bath salts” and other novel psychoactive substances in hair samples of ecstasy/MDMA/“Molly” users. Drug & Alcohol Dependence 161, 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantano F, Tittarelli R, Mannocchi G, Pacifici R, di Luca A, Paolo Busardò F, Marinelli E, 2017. Neurotoxicity induced by mephedrone: an up-to-date review. Current neuropharmacology 15(5), 738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao Y-S, Hall FS, Moriya Y, Ito M, Ohara A, Kikura-Hanajiri R, Goda Y, Lesch K-P, Murphy DL, Uhl GR, 2015. Methylone-induced hyperthermia and lethal toxicity: role of the dopamine and serotonin transporters. Behavioural pharmacology 26(4), 345–352. [DOI] [PubMed] [Google Scholar]
- Prosser JM, Nelson LS, 2012. The toxicology of bath salts: a review of synthetic cathinones. Journal of Medical Toxicology 8(1), 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley AL, Nelson K, To P, Lopez-Arnau R, Xu P, Wang D, Wang Y, Shen HW, Kuhn DM, Angoa-Perez M, Anneken JH, Muskiewicz DE, Hall FS, In Press. Abuse Potential and Toxicity of the Synthetic Cathinones (i.e. “Bath Salts”) Neuroscience and Biobehavioral Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riyaz S, Imran M, Gleeson D, Karajeh MA, 2014. Khat (Catha edulis) as a possible cause of autoimmune hepatitis. World journal of hepatology 6(3), 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum CD, Carreiro SP, Babu KM, 2012. Here today, gone tomorrow… and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. Journal of Medical Toxicology 8(1), 15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, Baumann MH, 2016. Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology (Berl) 233(10), 1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortall S, 2015. Neuropharmacological properties of the cathinones. University of Nottingham. [Google Scholar]
- Shortall SE, Green AR, Fone KC, King MV, 2016. Caffeine alters the behavioural and body temperature responses to mephedrone without causing long-term neurotoxicity in rats. J Psychopharmacol 30(7), 698–706. [DOI] [PubMed] [Google Scholar]
- Shortall SE, Green AR, Swift KM, Fone KC, King MV, 2013. Differential effects of cathinone compounds and MDMA on body temperature in the rat, and pharmacological characterization of mephedrone-induced hypothermia. Br J Pharmacol 168(4), 966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler L, Buser T, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener M, Liechti M, 2013. Pharmacological characterization of designer cathinones in vitro. British journal of pharmacology 168(2), 458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Williams M, Shaikh M, 2013. Novel psychoactive substances: a novel clinical challenge. BMJ Case Rep 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Blough BE, Banks ML, 2017. Cocaine-like discriminative stimulus effects of amphetamine, cathinone, methamphetamine, and their 3, 4-methylenedioxy analogs in male rhesus monkeys. Psychopharmacology 234(1), 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somi MH, Fatahi E, Panahi J, Havasian MR, 2014. Data from a randomized and controlled trial of LCarnitine prescription for the treatment for Non-Alcoholic Fatty Liver Disease. Bioinformation 10(9), 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparago M, Wlos J, Yuan J, Hatzidimitriou G, Tolliver J, Dal Cason TA, Katz J, Ricaurte G, 1996. Neurotoxic and pharmacologic studies on enantiomers of the N-methylated analog of cathinone (methcathinone): a new drug of abuse. J Pharmacol Exp Ther 279(2), 1043–1052. [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J, 2011. Clinical experience with and analytical confirmation of “bath salts” and “legal highs”(synthetic cathinones) in the United States. Clinical toxicology 49(6), 499–505. [DOI] [PubMed] [Google Scholar]
- Thirakul P, Hair LS, Bergen KL, Pearson JM, 2017. Clinical presentation, autopsy results and toxicology findings in an acute N-ethylpentylone fatality. J. Anal. Toxicol 41, 342–346. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM, 2004. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther 311(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Valente MJ, Araújo AM, Silva R, de Lourdes Bastos M, Carvalho F, De Pinho PG, Carvalho M, 2016. 3, 4-Methylenedioxypyrovalerone (MDPV): in vitro mechanisms of hepatotoxicity under normothermic and hyperthermic conditions. Archives of toxicology 90(8), 1959–1973. [DOI] [PubMed] [Google Scholar]
- Valente M.J.o., Bastos M.d.L., Fernandes E, Carvalho F.l., Guedes de Pinho P, Carvalho M.r., 2017. Neurotoxicity of β-keto amphetamines: deathly mechanisms elicited by methylone and MDPV in human dopaminergic SH-SY5Y cells. ACS chemical neuroscience 8(4), 850–859. [DOI] [PubMed] [Google Scholar]
- Vandewater SA, Creehan KM, Taffe MA, 2015. Intravenous self-administration of entactogen-class stimulants in male rats. Neuropharmacology 99, 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Hood L, Sewalia K, Tomek SE, Yahn S, Johnson CT, Wegner S, Blough BE, Marusich JA, Olive MF, 2012a. The Reinforcing and Rewarding Effects of Methylone, a Synthetic Cathinone Commonly Found in “Bath Salts”. J Addict Res Ther Suppl 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF, 2014. Potent rewarding and reinforcing effects of the synthetic cathinone 3, 4-methylenedioxypyrovalerone (MDPV). Addiction biology 19(2), 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Olive MF, 2017. Reinforcing Effects of Cathinone NPS in the Intravenous Drug Self-Administration Paradigm. Curr Top Behav Neurosci 32, 133–143. [DOI] [PubMed] [Google Scholar]
- Wood DM, Dines AM, Heyerdahl F, Yates C, Giraudon I, Hovda KE, Dargan PI, European Drug Emergencies Network Research Group, 2015. The cathinones are the most commonly reported novel psychoactive substances (NPS) associated with emergency department presentations with acute drug toxicity reported to the European Drug Emergencies Network (Euro-DEN). Clin. Toxicol 53, 355–356. [Google Scholar]
- Wood DM, Greene SL, Dargan PI, 2013. Five-year trends in self-reported recreational drugs associated with presentation to a UK emergency department with suspected drug-related toxicity. Eur. J. Emerg. Med 20, 263–267. [DOI] [PubMed] [Google Scholar]
- Wright MJ Jr, Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, Vandewater SA, Parsons LH, Houseknecht KL, Dickerson TJ, 2012. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PloS one 7(8), e44652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaami S, Giorgetti R, Pichini S, Pantano F, Marinelli E, Busardo FP, 2018. Synthetic cathinones related fatalities: an update. Eur Rev Med Pharmacol Sci 22(1), 268–274. [DOI] [PubMed] [Google Scholar]


