Abstract
Background
Evidence directly evaluating the efficacy of tadalafil vs. tamsulosin for lower urinary tract symptoms (LUTS) secondary to benign prostate hyperplasia (BPH) is limited. We performed a meta-analysis of published studies to assess the comparative effectiveness of tadalafil vs. tamsulosin in treating LUTS suggestive of BPH.
Material/Methods
After performing a comprehensive publication search with PubMed, EMBASE, and Cochrane Controlled Trials Register using the search terms “tadalafil”, “tamsulosin”, “lower urinary tract symptoms”, and “controlled”, 335 articles were screened, out of which 7 randomized controlled trials published up to July 2019 were identified and included in this meta-analysis review.
Results
From 335 screened articles, 7 studies (totalling 1601 patients) were finally included in our analysis. There was no statistically significant difference between tadalafil and tamsulosin in improving the clinical outcomes of total International Prostate Symptom Score (IPSS), voiding subscores, storage subscores, quality of life (QoL) scores, maximum flow rate (Qmax), and postvoid residual urine (PVR), but a statistically significant difference was observed in the International Index of Erectile Function scores (IIEF scores).
Conclusions
Tadalafil and tamsulosin have similar effects in managing LUTS secondary to BPH. Tadalafil is superior to tamsulosin in treating LUTS suggestive of BPH when associated with erectile dysfunction (ED).
MeSH Keywords: Adrenergic alpha-1 Receptor Antagonists, Lower Urinary Tract Symptoms, Phosphodiesterase 5 Inhibitors, Prostatic Hyperplasia
Background
Benign prostate hyperplasia (BPH) is a common urinary disease with pathological feature of non-malignant hyperplasia in prostatic tissue. The progressive proliferation of smooth-muscle cells and epithelial cells in the prostate can cause bladder outlet obstruction, resulting in lower urinary tract symptoms (LUTS) that significantly decreases patients’ quality of life (QoL) by interrupting daily activities and reducing nocturnal sleep quality [1]. Elderly men are more likely to experience LUTS [2], and the prevalence ranges from 10.3% to 25.1% [3–5]. In the United States, nearly 75% of men 60–69 years of age suffer from LUTS [6].
The International Prostate Symptom Score (IPSS) is widely used to diagnose and evaluate the severity of LUTS because of its better accuracy and convenience of use compared with other tools [7]. The IPSS consists of 7 questions assessing urinary symptoms and QoL, and is divided into voiding subscores (obstructive subscores) and storage subscores (irritative subscores). Besides the IPSS, maximum flow rate (Qmax) and postvoid residual urine (PVR) are also valid indexes in evaluating urinary function.
According to the American Urological Association’s clinical practice guidelines, the treatment goals for patients with LUTS should focus on improving the function of both prostate and bladder by alleviating storage and voiding symptoms [7]. With the recent advent of new drugs, pharmacotherapy has played a critical role in reducing urinary symptoms [8]. The medication tamsulosin, which belongs to the class of α1-adrenoceptor antagonists (α1-blockers), has been recommended for the treatment of LUTS, acting by relaxing smooth-muscle tone in the prostate and bladder neck.
Blocking specific receptors (e.g., alpha receptor, phosphodiesterase type-5) can relax smooth muscle in the prostate and bladder neck, which results in improved urinary flow and amelioration of LUTS [9,10]. Tadalafil, a phosphodiesterase type 5 (PDE5) inhibitor, is currently used for the treatment of erectile dysfunction, acting by reducing cyclic guanosine monophosphate (cGMP) in penile tissue via the NO-cGMP pathway [11]. Interestingly, several recent studies have found that tadalafil can relieve both storage and voiding symptoms [11,12], and an increasing number of studies have focused on comparing the efficacy of tadalafil versus tamsulosin in the treatment of LUTS. Therefore, our study compared the clinical efficacy of tadalafil versus tamsulosin in treating LUTS, based on existing evidence.
Material and Methods
Search Strategy
A comprehensive literature search was performed in the databases PubMed, EMBASE, and Cochrane Controlled Trials Register for publications up to July 2019. Search terms were “tadalafil”, “tamsulosin”, “lower urinary tract symptoms”, and “controlled”. We also performed a manual search of urological conference publications to identify potential highly relevant studies. All selected articles were published in English.
Inclusion criteria
The inclusion criteria were: (1) compared tadalafil monotherapy with tamsulosin monotherapy, (2) clinical controlled studies, (3) patients that experienced LUTS suggestive of BPH, (4) valid indices used for evaluating clinical efficacy before and after treatment.
Exclusion criteria
The exclusion criteria were: (1) nonclinical trials, such as reviews, case reports, and letters, (2) duplicate publication, (3) conference abstracts that lack published full text and contain insufficient data.
Data extraction
EndNoteX7 was used to manage the selected articles. Two of the authors independently assessed the title and abstracts of the searched literatures to exclude irrelevant articles and to determine which studies needed further assessment according to the established criteria. Next, the same 2 authors each read the full texts of the screened articles to confirm whether they met the inclusion criteria. Ultimately, the basic characteristics and results of the selected studies were extracted and recorded in the data extraction tables. Discrepancies between the 2 authors were arbitrated by the senior author.
Quality assessment
The Jadad score was used to assess the quality of the selected articles by taking into consideration the following 4 aspects: randomized allocation sequence, allocation concealment, blinding, and quitting. Studies ≥4 points were classified as high quality. The process was accomplished independently by the first 2 authors.
Statistical analysis
We used Review Manager 5.3 (RevMan 5.3) to analyze the extracted data from the 7 included studies in our meta-analysis. Mean difference (MD) and 95% confidence interval (CI) were calculated. Heterogeneity among selected studies was assessed by Q statistic and chi-square test and results are presented as I2 values. A significant heterogeneity among studies was considered as I2>50%, for which a random-effects model was used. Otherwise, a fixed-effects model was used. If necessary, sensitivity analysis was used when heterogeneity was significant (I2>90%).
Results
Search results and characteristics of articles
Initially, 335 articles were screened, among which 10 articles were identified after reading their full text and taking into consideration our inclusion criteria. Out of these 10 articles, 7 randomized controlled trials (RCTs) [13–19] with 1601 subjects met all of the inclusion criteria. The selection process is displayed in Figure 1. The characteristics of the selected articles are listed in Table 1.
Figure 1.
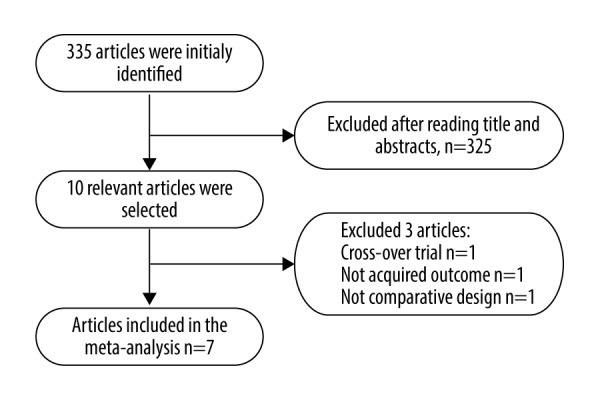
Flow diagram detailing the study selection process.
Table 1.
Characteristics of the seven selected studies in this meta-analysis.
| Author | Year | Country | Intervention | Number (E/C) | Duration (w) | Outcomes | |
|---|---|---|---|---|---|---|---|
| Experimental | Control | ||||||
| Pogula VR [13] | 2019 | India | Tadalafil 5 mg qd | Tamsulosin 0.2 mg qd | 50/50 | 12 | 1), 2), 3), 4), 7) |
| Zhang Z [14] | 2018 | China | Tadalafil 5 mg qd | Tamsulosin 0.4 mg qd | 362/185 | 12 | 1), 2), 3), 4), 7) |
| Karami H [15] | 2016 | Iran | Tadalafil 20 mg qd | Tamsulosin 0.4 mg qd | 60/59 | 12 | 1), 2), 3), 5), 6), 7) |
| Singh DV [16] | 2014 | India | Tadalafil 10 mg qd | Tamsulosin 0.4 mg qd | 44/45 | 12 | 1), 4), 5), 6), 7) |
| Yokoyama O [17] | 2013 | Japan, Korea, China | Tadalafil 5 mg qd | Tamsulosin 0.2 mg qd | 155/152 | 12 | 1), 2), 3), 4), 5), 6) |
| Oelke M [18] | 2012 | Australia, Austria, Belgium, France, Germany, Greece, Italy, Mexico, The Netherlands, and Poland | Tadalafil 5 mg qd | Tamsulosin 0.4 mg qd | 171/168 | 12 | 1), 2), 3), 4), 5), 6) |
| Kim SC [19] | 2011 | Korea | Tadalafil 5 mg qd | Tamsulosin 0.2 mg qd | 51/49 | 12 | 1), 5), 6) |
E – experimental; C – control; 1) IPSS – International Prostate Symptom Score; 2) Voiding Subscores; 3) Storage Subscores; 4) QoL – quality of life; 5) PVR – postvoid residual urine; 6) Qmax – maximum flow rate; 7) IIEF-5 – international index of erectile function-5.
Quality assessment of selected articles
According to the standard of Jadad score, 2 studies [13,16] were low in quality level, while the rest [14,15,17–19] were high. Three studies [13,15,17] used a stochastic approach (random sequence). One study [13] did not use allocation concealment. Only 1 study [14] used a double-blinded approach. Six studies [14–19] described the number of and reasons for subject withdrawals prior to completion of treatment. The quality assessment results of the 7 selected studies are shown in Table 2.
Table 2.
Quality assessment of selected articles.
| Study | Randomized allocation | Allocation concealment | Blinding | Quitting | Total score | Quality level |
|---|---|---|---|---|---|---|
| Pogula VR [13] | AP, random sequence | IP, unused | IP, open-label | Not mentioned | 2 | Low |
| Zhang Z [14] | UC, mentioned | UC, mentioned | AP, double blind | Mentioned | 5 | High |
| Karami H [15] | AP, random chart | UC, mentioned | UC, mentioned | Mentioned | 5 | High |
| Singh DV [16] | UC, mentioned | UC, mentioned | IP, open-label | Mentioned | 3 | Low |
| Yokoyama O [17] | AP, random sequence | UC, mentioned | UC, mentioned | Mentioned | 5 | High |
| Oelke M [18] | UC, mentioned | UC, mentioned | UC, mentioned | Mentioned | 4 | High |
| Kim SC [19] | UC, mentioned | UC, mentioned | UC, mentioned | Mentioned | 4 | High |
AP – appropriate; UC – unclear; IP – inappropriate.
Comparative effectiveness of tadalafil and tamsulosin in treating LUTS total IPSS
Four studies [15,17–19] comprising 863 participants contributed to the meta-analysis of the total IPSS. While the other 3 studies [13,14,16] recorded the result of IPSS, the data were not included in our meta-analysis as the data from these studies were recorded in a different format from the other 4 studies (Pogula recorded IPSS in the form of mean change, while Zhang and Singh recorded IPSS in the form of Least Square Mean±Standard Error). Compared with tamsulosin monotherapy, tadalafil showed no significant difference in reducing IPSS (SMD: 0.16, 95% CI: −1.42 to 1.73, P=0.84, Figure 2).
Figure 2.
Meta-analysis of the total IPSS for tadalafil vs. tamsulosin.
Voiding subscores
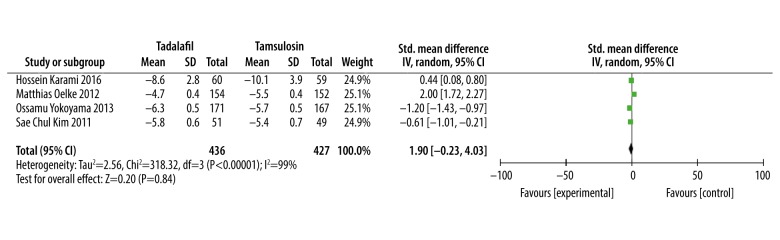
Five studies [14,15,17–19] totalling 1381participants contributed to the meta-analysis of the voiding subscores. Although 2 studies [13,16] showed the results of voiding subscores, the data from those 2 studies were not in valid format for use in our meta-analysis. The meta-analysis results showed there was no statistical difference between tadalafil and tamsulosin in improving voiding subscores (SMD: −0.19, 95% CI: −1.78 to 1.41, P=0.82, Figure 3).
Figure 3.
Meta-analysis of the voiding subscores change using tadalafil vs. tamsulosin.
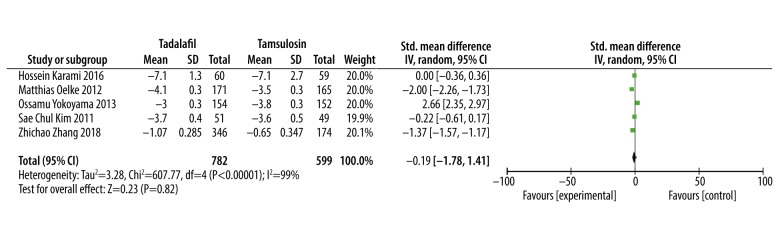
Storage subscores
Five studies [14,15,17–19] involving 1386 patients provided valid data for this meta-analysis, while data from the other 2 studies [13,16] were invalid. The analysis showed that there was no significant difference between tadalafil and tamsulosin in improving storage subscores (SMD: −0.17, 95% CI: −0.59 to 0.26, P=0.45, Figure 4).
Figure 4.
Meta-analysis of the storage subscores changes with tadalafil vs. tamsulosin.
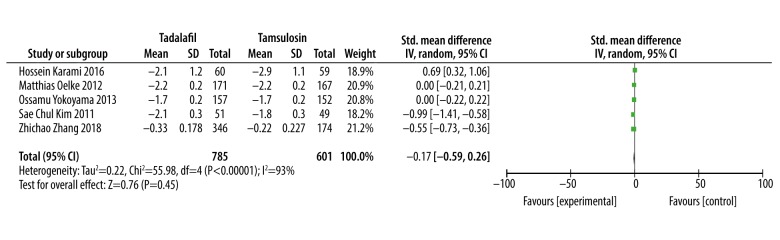
Quality of life (QoL)
Date from 4 studies [14,17–19] were valid for meta-analysis, but the other 3 studies [13,15,16] provided invalid data for meta-analysis. Meta-analysis results of 4 studies involving 1264 patients showed that there was no significant difference in improving quality of life between tadalafil and tamsulosin (SMD: 0.07, 95% CI: −1.85 to 1.99, P=0.95, Figure 5).
Figure 5.
Meta-analysis of QoL changes using tadalafil vs. tamsulosin.
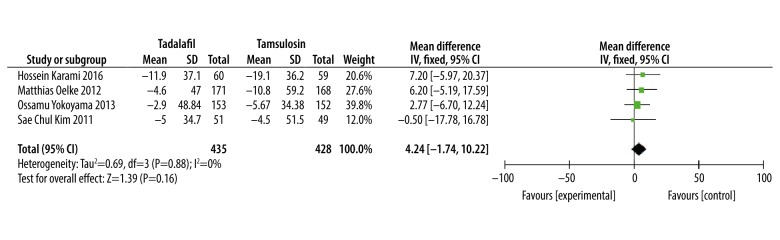
Postvoid residual urine (PVR)
Four studies [15,17–19] involving 863 patients provided valid data for meta-analysis, while data from the other 3 studies [13,14,16] were invalid. Meta-analysis results showed that there was no significant difference in improving PVR with tadalafil versus tamsulosin (WMD: 4.24, 95% CI: −1.74 to 10.22, P=0.88, Figure 6).
Figure 6.
Meta-analysis of PVR changes of tadalafil vs. tamsulosin.
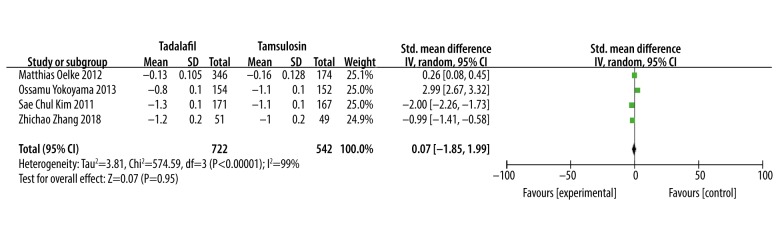
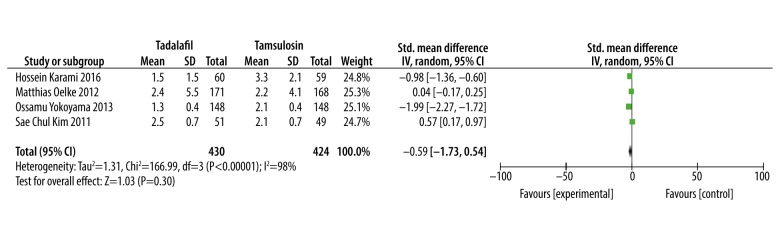
Maximum flow rate (Qmax)
Meta-analysis results of 4 studies [15,17–19] involving 854 patients showed that there was no significant difference in improving Qmax between tadalafil and tamsulosin (SMD: −0.59, 95% CI: −1.73 to 0.54, P=0.30, Figure 7).
Figure 7.
Meta-analysis of Qmax changes of tadalafil vs. tamsulosin.
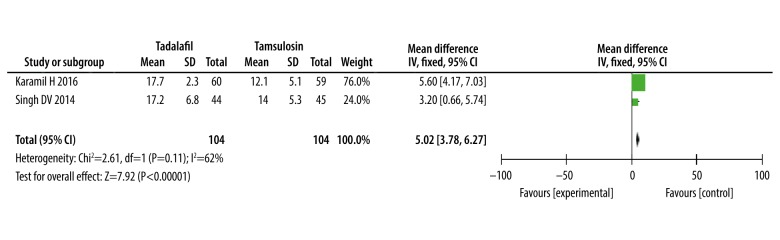
The International Index of Erectile Function scores (IIEF scores)
Only 2 studies [15,16] provided valid data on International Index of Erectile Function scores (IIEF scores). The meta-analysis results show that tadalafil was significantly better than tamsulosin in improving IIEF scores (WMD: 5.02, 95% CI 3.78 to 6.27, P<0.0001, Figure 8).
Figure 8.
Meta-analysis of IIEF scores with tadalafil vs. tamsulosin.
Sensitivity analysis
Except for PVR, the majority of outcomes presented significant heterogeneity (I2>90%). After excluding age heterogeneity (WMD: −0.30,95% CI: −1.61 to 1.01, P=0.65, I2=0), the sensitivity analysis of total IPSS, voiding subscores, storage subscores, QoL, and Qmax, did not alter the treatment effects compared to main analysis.
Discussion
As a first-line treatment for ED, tadalafil has been recently gaining popularity for managing LUTS secondary to BPH. There are few reports in the literature showing that PDE 5 inhibitors (e.g., tadalafil) induce relaxation of smooth-muscle cells in the urethra, prostate, and bladder neck [20]. These mechanisms are believed to help improve vascular endothelial function in patients with male LUTS associated with BPH. Administration of 5 mg tadalafil daily improves endothelial function in patients with benign prostatic hyperplasia. In fact, the first clinical study evaluating whether tadalafil can improve LUTS due to BPH was conducted in 2006 [21], and since then numerous other RCTs were performed to explore the differences between tadalafil and tamsulosin [22]. A prior review pooling 4 RCTs showed that tadalafil is effective in treating LUTS by either monotherapy or combination therapy [23]. However, there still remains insufficient clinical evidence that tadalafil can alleviate LUTS as effectively as tamsulosin, because tamsulosin has always been considered a first-line therapy for managing LUTS. The present systematic review provides a comprehensive evaluation of the comparative effectiveness of tadalafil vs. tamsulosin in treating lower urinary tract symptoms secondary to benign prostate hyperplasia. The primary findings were: (1) Tadalafil and tamsulosin may have similar effects on improving patients’ total IPSS, voiding scores, storage scores, QoL, PVR, and Qmax; (2) Compared to tamsulosin, tadalafil significantly improves erectile function in aging men with both erectile dysfunction and LUTS associated with BPH; (3) In all of the included studies in this meta-analysis review, the treatment duration was 12 weeks, which may be the minimum duration of treatment needed to achieve clinical efficacy using tadalafil for management of LUTS/BPH.
Our meta-analysis supports that tadalafil relieves lower urinary tract symptoms with similar effectiveness as tamsulosin. Firstly, tadalafil improved IPSS (SMD: 0.16, 95% CI: −1.42 to 1.73), revealing that it has a therapeutic effect on LUTS comparable to that of tamsulosin. Second, positive effects with tadalafil on voiding and storage scores were also confirmed by our analysis, as were the improvements in postvoid residual urine (WMD: 4.24, 95% CI: −1.74 to 10.22) and maximum flow rate (SMD: −0.59, 95% CI: −1.73 to 0.54). However, the studies in this review showed no statistically significant improvement in PVR using tadalafil, perhaps due to the small sample size. Hence, a study with a larger sample size is needed to assess the influence of tadalafil on PVR in patients with LUTS. Molecular mechanism studies support the view that tadalafil can alleviate symptoms of LUTS. The nitric oxide NO/cGMP signalling pathway has been confirmed to play an important role in modulating the normal function of bladder and prostate by relaxing smooth muscle, increasing blood perfusion, and regulating afferent nerve activity [24]. As an active messenger in the NO/cGMP pathway, PDE-5 has been shown to play a physiological role in controlling NO/cGMP-regulated bladder smooth muscle relaxation and growth inhibition [25]. Tadalafil, as a long-acting PDE-5 inhibitor, is believed to increase activity of the NO/cGMP pathway and subsequently exert a positive effect on lower urinary tract function [26–28], but existing studies comparing tadalafil and tamsulosin were all RCTs with short treatment duration (the duration of each study in this meta-analysis was 12 weeks). There is still a need for longer-term studies to compare the clinical effects of these drugs. In consideration of the clinical features of LUTS, short trial durations limit reference utility for clinical practice.
Quality of life is important for aging men, and one way to improve it is effectively treating senile male diseases. Both erectile dysfunction and lower urinary tract symptoms negatively affect quality of life in aging men [29,30]. In terms of quality of life, treatment using tadalafil resulted in a significant improvement (SMD: 0.07, 95% CI: −1.85 to 1.99) in the Global Assessment Question, “Has the treatment you have been taking since your last visit improved your urinary symptoms?” and in the International Index of Erectile Function scores (WMD: 5.02, 95% CI 3.78 to 6.27). These results demonstrate that tadalafil can improve quality of life in aging men with BPH by enhancing urinary and erectile function.
Several studies have focused on the efficacy of combination therapy with tadalafil and tamsulosin in managing LUTS and ED simultaneously [14,31], and the results revealed that combination therapy was superior to monotherapy (tamsulosin). Our meta-analysis showed that tadalafil has a comparable effect as tamsulosin in managing LUTS. Moreover, the meta-analysis results demonstrate that tadalafil significantly improved IIEF scores in patients with LUTS and ED vs. tamsulosin. As such, tadalafil monotherapy may be just as effective as tadalafil plus tamsulosin combination therapy in treating patients with both LUTS and ED conditions.
Based on our analysis results and published clinical studies, tadalafil (a phosphodiesterase type-5 inhibitor) and tamsulosin (an alpha-blocker) were both effective in improving lower urinary tract symptoms. Although tamsulosin can inhibit endogenously released noradrenaline on the same smooth-muscle targets as tadalafil, the 2 classes of drugs work by different mechanisms – tadalafil has the dual function of improving erection and urination, while tamsulosin just relieves lower urinary tract symptoms.
Recent data indicates that roughly 40% of men 45 years of age and older showed improvement in both ED and LUTS/BPH following treatment with tadalafil 5 mg once daily for 12 weeks [32]. This is a remarkably shorter treatment duration compared to the recommended treatment duration for tamsulosin, which is stated by guidelines as over 4 years [33]. A 1-year open-label extension study has shown that the efficacy of tadalafil in improving LUTS-BPH was maintained during the full year of therapy [34]. A number of studies have focused on the efficacy of combination therapy with tadalafil and tamsulosin in managing LUTS and erectile dysfunction simultaneously. It is important to note that there is a noticeable difference in drug costs between combination therapy (tamsulosin and tadalafil) and monotherapy (tadalafil). Increased costs along with polypharmacy may put patients at higher risk for medication noncompliance. After all, if ED and LUTS-BPH can be alleviated with monotherapy, the role of combination therapy seems to be unnecessary, since a “kill two birds with one stone” strategy would be advisable.
Several limitations in this meta-analysis should be acknowledged. First of all, it is clear that heterogeneity exists between the aging men with LUTS treated by tadalafil and tamsulosin. This problem can be partially accounted for by the differences in drug dose, baseline characteristics of included studies, and experimental design. Partial data provided by several of the studies were invalid because we could not obtain the full data from the authors, which may have biased our results. Larger sample sizes and longer-duration clinical trials are needed to adequately compare the effectiveness of tadalafil vs. tamsulosin in improving LUTS.
Conclusions
In conclusion, our meta-analysis review shows that tadalafil has a similar therapeutic effect as tamsulosin in managing LUTS suggestive of BPH, and that tadalafil has the added benefit of significantly improving erectile function vs. tamsulosin. Hence, it would be advisable to prescribe tadalafil for patients suffering from both LUTS and ED.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Welch G, Weinger K, Barry MJ. Quality-of-life impact of lower urinary tract symptom severity: Results from the health professionals follow-up study. Urology. 2002;59:245–50. doi: 10.1016/s0090-4295(01)01506-0. [DOI] [PubMed] [Google Scholar]
- 2.Hollingsworth JM, Wilt TJ. Lower urinary tract symptoms in men. BMJ. 2014;349:g4474. doi: 10.1136/bmj.g4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle P, Robertson C, Mazzetta C, et al. The prevalence of lower urinary tract symptoms in men and women in four centres. The UrEpik study. BJU Int. 2003;92:409–14. doi: 10.1046/j.1464-410x.2003.04369.x. [DOI] [PubMed] [Google Scholar]
- 4.Verhamme KMC, Dieleman JP, Bleumink GS, et al. Triumph Pan European Expert Panel. Incidence and prevalence of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in primary care – the Triumph project. Eur Urol. 2002;42:323–28. doi: 10.1016/s0302-2838(02)00354-8. [DOI] [PubMed] [Google Scholar]
- 5.Coyne KS, Sexton CC, Thompson CL, et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: Results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int. 2009;104:352–60. doi: 10.1111/j.1464-410X.2009.08427.x. [DOI] [PubMed] [Google Scholar]
- 6.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: Benign prostatic hyperplasia. J Urol. 2008;179:S75–80. doi: 10.1016/j.juro.2008.03.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–803. doi: 10.1016/j.juro.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 8.Serati M, Andersson KE, Dmochowski R, et al. Systematic review of combination drug therapy for non-neurogenic lower urinary tract symptoms. Eur Urol. 2019;75:129–68. doi: 10.1016/j.eururo.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Kaminetsky J. Comorbid LUTS and erectile dysfunction: Optimizing their management. Curr Med Res Opin. 2006;22:2497–506. doi: 10.1185/030079906x154141. [DOI] [PubMed] [Google Scholar]
- 10.Furuya S, Kumamoto Y, Yokoyama E, et al. Alpha-adrenergic activity and urethral pressure in prostatic zone in benign prostatic hypertrophy. J Urol. 1982;128:836–39. doi: 10.1016/s0022-5347(17)53216-4. [DOI] [PubMed] [Google Scholar]
- 11.Gacci M, Salvi M, Sebastianelli A, et al. The use of a single daily dose of tadalafil to treat signs and symptoms of benign prostatic hyperplasia and erectile dysfunction. Res Rep Urol. 2013;5:99–111. doi: 10.2147/RRU.S31580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapple CR, Roehrborn CG, McVary K, et al. Effect of tadalafil on male lower urinary tract symptoms: an integrated analysis of storage and voiding international prostate symptom subscores from four randomized controlled trial. Eur Urol. 2015;67:114–22. doi: 10.1016/j.eururo.2014.08.072. [DOI] [PubMed] [Google Scholar]
- 13.Pogula VR, Kadiyala LS, Gouru VR, et al. Tadalafil vs. tamsulosin in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: A prospective, randomized study. Cent European J Urol. 2019;72:44–50. doi: 10.5173/ceju.2019.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, Li H, Zhang X, et al. Efficacy and safety of tadalafil 5 mg once-daily in Asian men with both lower urinary tract symptoms associated with benign prostatic hyperplasia and erectile dysfunction: A phase 3, randomized, double-blind, parallel, placebo and tamsulosin-controlled study. Int J Urol. 2019;26:192–200. doi: 10.1111/iju.13828. [DOI] [PubMed] [Google Scholar]
- 15.Karami H, Hassanzadeh-Hadad A, Fallah-Karkan M. Comparing monotherapy with tadalafil or tamsulosin and their combination therapy in men with benign prostatic hyperplasia: A randomized clinical trial. Urol J. 2016;13:2920–26. [PubMed] [Google Scholar]
- 16.Singh DV, Mete UK, Mandal AK, et al. A comparative randomized prospective study to evaluate efficacy and safety of combination of tamsulosin and tadalafil vs. tamsulosin or tadalafil alone in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. J Sex Med. 2014;11:187–96. doi: 10.1111/jsm.12357. [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama O, Yoshida M, Kim SC, et al. Tadalafil once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: A randomized placebo-and tamsulosin-controlled 12-week study in Asian men. Int J Urol. 2013;20:193–201. doi: 10.1111/j.1442-2042.2012.03130.x. [DOI] [PubMed] [Google Scholar]
- 18.Oelke M, Giuliano F, Mirone V, et al. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol. 2012;61:917–25. doi: 10.1016/j.eururo.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Kim SC, Park JK, Kim SW, et al. Tadalafil administered once daily for treatment of lower urinary tract symptoms in Korean men with benign prostatic hyperplasia: Results from a placebo-controlled pilot study using tamsulosin as an active control. Low Urin Tract Symptoms. 2011;3:86–93. doi: 10.1111/j.1757-5672.2011.00088.x. [DOI] [PubMed] [Google Scholar]
- 20.Brock G, Ni X, Oelke M, et al. Efficacy of continuous dosing of tadalafil once daily vs. tadalafil on demand in clinical subgroups of men with erectile dysfunction: A descriptive comparison using the Integrated Tadalafil Databases. J Sex Med. 2016;13:860–75. doi: 10.1016/j.jsxm.2016.02.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez RR, Kaplan SA. Tadalafil for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Expert Opin Drug Metab Toxicol. 2006;2(4):609–17. doi: 10.1517/17425255.2.4.609. [DOI] [PubMed] [Google Scholar]
- 22.Furuya S, Kumamoto Y, Yokoyama E, et al. Alpha-adrenergic activity and urethral pressure in prostatic zone in benign prostatic hypertrophy. J Urol. 1982;128:836–39. doi: 10.1016/s0022-5347(17)53216-4. [DOI] [PubMed] [Google Scholar]
- 23.Chapple CR, Roehrborn CG, McVary K, et al. Effect of tadalafil on male lower urinary tract symptoms: An integrated analysis of storage and voiding international prostate symptom subscores from four randomised controlled trials. Eur Urol. 2015;67:114–22. doi: 10.1016/j.eururo.2014.08.072. [DOI] [PubMed] [Google Scholar]
- 24.Giuliano F, Ückert S, Maggi M, et al. The mechanism of action of phosphodiesterase type 5 inhibitors in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Eur Urol. 2013;63:506–516. doi: 10.1016/j.eururo.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Filippi S, Morelli A, Sandner P, et al. Characterization and functional role of androgen-dependent PDE5 activity in the bladder. Endocrinology. 2007;148:1019–29. doi: 10.1210/en.2006-1079. [DOI] [PubMed] [Google Scholar]
- 26.Andersson KE, de Groat WC, McVary KT, et al. Tadalafil for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: Pathophysiology and mechanism(s) of action. Neurourol Urodyn. 2011;30:292–301. doi: 10.1002/nau.20999. [DOI] [PubMed] [Google Scholar]
- 27.Urios A, Ordoño F, García-García R, et al. Tadalafil treatment improves inflammation, cognitive function, and mismatch negativity of patients with low urinary tract symptoms and erectile dysfunction. Sci Rep. 2019;9(1):1–9. doi: 10.1038/s41598-019-53136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun HY, Lee B, Kim JH. Factors affecting the efficacy and safety of phosphodiesterase 5 inhibitor and placebo in treatment for lower urinary tract symptoms: Meta-analysis and meta-regression. Int Urol Nephrol. 2018;50(1):35–47. doi: 10.1007/s11255-017-1743-3. [DOI] [PubMed] [Google Scholar]
- 29.Choi WS, Heo NJ, Lee YJ, et al. Factors that influence lower urinary tract symptom (LUTS)-related quality of life (QoL) in a healthy population. World J Urol. 2017;35(11):1783–89. doi: 10.1007/s00345-017-2052-2. [DOI] [PubMed] [Google Scholar]
- 30.Teoh JB, Yee A, Danaee M, et al. Erectile dysfunction among patients on methadone maintenance therapy and its association with quality of life. J Addict Med. 2017;11(1):40–46. doi: 10.1097/ADM.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 31.Kim SW, Park NC, Lee SW, et al. Efficacy and safety of a fixed-dose combination therapy of tamsulosin and tadalafil for patients with lower urinary tract symptoms and erectile dysfunction: Results of a randomized, double-blinded, active-controlled trial. J Sex Med. 2017;14(8):1018–27. doi: 10.1016/j.jsxm.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Roehrborn CG, Egan KB, Miner MM, et al. Erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH) combined responders to tadalafil after 12 weeks of treatment. BJU Int. 2016;118(1):153–60. doi: 10.1111/bju.13406. [DOI] [PubMed] [Google Scholar]
- 33.Gravas S, Cornu JN, Gacci M, et al. EAU guidelines of management of non-neurogenic male LUTS. EAU guidelines office; Arnhen, the Netherland: 2019. Published online. [Google Scholar]
- 34.Donatucci CF, Brock GB, Goldfischer ER, et al. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: A 1-year, open-label extension study. BJU Int. 2011;107(7):1110–16. doi: 10.1111/j.1464-410X.2010.09687.x. [DOI] [PubMed] [Google Scholar]