Abstract
Background
Endothelial cell (EC) injury is underlies for the pathogenesis of atherosclerosis (AS). MicroRNAs (miRNAs) have been indicated play important role in modulating AS occurrence and development. However, how miR-328-3p modulates EC injury molecular level for AS remains unclear.
Material/Methods
MiR-328-3p and forkhead box protein O4 (FOXO4) expression were detected using quantitative real-time polymerase chain reaction (qRT-PCR) and western blot. Cell viability was analyzed by Cell Counting Kit-8 (CCK-8) assay. Flow cytometry was utilized to analyze the apoptotic rate. The migration and invasion abilities were measured by Transwell assay. Western blot was applied to examine the expression of C-caspase 3, Beclin, LC3-I, and LC3-II. The levels of interleukin (IL)-1β, IL-10, and tumor necrosis factor-α (TNF-α) were tested by enzyme-linked immunosorbent assay (ELISA) assay and western blot.
Results
MiR-328-3p expression was downregulated in oxidized low-density lipoprotein (ox-LDL) induced human umbilical vein endothelial cells (HUVECs). Overexpressed miR-328-3p obviously alleviated ox-LDL induced inhibition on cell viability, migration and invasion, stimulation on apoptosis, autophagy as well as inflammation in HUVECs. FOXO4 was elevated in ox-LDL HUVECs, and functional assay indicated that FOXO4 aggravated ox-LDL induced HUVECs impairment. In addition, FOXO4 was a target of miR-328-3p in HUVECs; rescue experiments suggested miR-328-3p could protect HUVECs against ox-LDL induced injury via regulating FOXO4.
Conclusions
MiR-328-3p protected vascular endothelial cells against ox-LDL induced injury via targeting FOXO4, suggesting a novel insight for atherosclerosis treatment.
MeSH Keywords: Atherosclerosis, Endothelial Cells, MicroRNAs
Background
Endothelial cells (ECs) are an elementary part of the heart and vasculature and are also the major contributor to the maintenance of vascular homeostasis [1]. Dysfunction of ECs is a key responsibility for the formation of atherosclerotic (AS) which is the foundation for multiple-site vascular disease [2,3]. ECs dysfunction is a complicated and multistage process which includes the injury of ECs represented by abnormal proliferation, apoptosis, autophagy as well as inflammation which has vital implications in the pathogenesis of AS [4–6]. Therefore, it is important for us to understand how EC injury is modulated at the molecular level for AS.
MicroRNAs (miRNAs) are endogenous, non-coding RNA molecules, which inversely regulate the expression of target genes at the post-transcriptional level primarily by binding to the 3′untranslated region (UTR) of mRNAs sequences, thereby controlling transcript stability and/or translation [7]. MiRNAs have been identified to involve in various biological and pathological processes including proliferation, differentiation, metastasis, apoptosis, and tumorigenesis, and are potential targets for cancers treatment [8,9]. MiRNA expression profiles have been shown to differ in many cellular processes related to AS in vivo and in vitro [10]. MiRNAs are related to AS lesion formation and regression, highlighting the potential diagnostic, prognostic and therapeutic roles of miRNAs in AS [11]. MiR-328-3p, a member of miRNAs, has been revealed to mediate anti-tumor effects in several cancers through regulating the target genes or downstream pathway [12–14], Recently, overexpressed miR-328-3p was found to aggravate oxidative stress damage in tricuspid aortic valve ECs, whereas miR-328-3p deletion alleviate oxidative stress damage in bicuspid aortic valve ECs [15]. Xing et al. demonstrated that miR-328-3p was a target of long noncoding RNA (lncRNA)-MEG3, and upregulation of miR-328-3p decreased the expression of insulin-like growth factor 1 receptor (IGF1R) to attenuate proliferation and cell-cycle progression in pulmonary artery smooth muscle cells (PASMCs) [16]. All these studies reveal the potential association of miR-328-3p with vascular diseases. In recent, Wu et al. discovered that miR-328 could ameliorate oxidized low-density lipoprotein (ox-LDL)-induced ECs inflammation, apoptosis as well as oxidative stress response via targeted interaction with HMGB1 in AS [17]. In our study, the functions and potential molecular mechanisms of miR-328-3p in the pathogenesis of AS were investigated.
FOXO4 belongs to the forkhead box O (FOXO) transcription factors family which play a significant role in regulating numerous cellular processes such as cytokine production, immune cell homeostasis, oxidative stress response, metabolism, immunity, cell cycle, and apoptosis involved in the development of AS [18]. Vascular smooth muscle cells (VSMCs) are a major component of arterial walls and its dysfunction is also associate with the AS occurrence [19]. Previous studies have indicated that FOXO4 could activate transcription of the matrix metalloproteinase 9 (MMP9) gene in response to tumor necrosis factor alpha (TNF-α) signaling to promote VSMCs migration [20]. It can also inhibited SMC differentiation by interacting with the transcription factor myocardin [21], which is involved in the pathogenesis of AS. Additionally, Zhang et al. displayed that overexpressed FOXO4 could reverse adiponectin mediated antiatherogenic effect on human aortic ECs via activating NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome [22]. Thus, these research findings suggest an important role for FOXO4 in the occurrence and development of AS.
Ox-LDL is a widely acknowledged factor in the formation of AS [23]. Therefore, our study concentrated on the effects of ox-LDL treatment on miR-328-3p expression and explored the biological function and underlying mechanisms of miR-328-3p in ox-LDL-induced ECs injury in AS.
Material and Methods
Cell culture and treatment of ox-LDL
Human umbilical vein endothelial cells (HUVECs) were purchased from American Tissue Culture Collection (Manassas, VA, USA) and grown in endothelial cell basal medium (EBM-2; Lonza, Walkersville, MD, USA) containing EGM-2MV at 37°C with 5% CO2.
For the establishment of ox-LDL induced AS model in vitro, HUVECs were incubated with 25, 50, 100, or 150 μg/mL of ox-LDL for different time durations (0, 12, 24, or 48 hours) in accordance with the study design. Subsequently, cells were collected for further experiments.
Cell transfection
MiR-328-3p mimic (miR-328-3p), miR-328-3p inhibitor (anti-miR-328-3p), the corresponding negative control (miR-NC, anti-miR-NC), pcDNA3.1-FOXO4 overexpression vector (FOXO4), pcDNA3.1 empty vector (vector), small interfering RNA (siRNA) against FOXO4 (si-FOXO4) or siRNA negative control (scramble) were synthesized by Genepharma (Shanghai, China). The transfection of all oligonucleotides or vectors was conducted by using Lipofectamine 2000 reagent (Invitrogen, ThermoFisher Scientific, Inc., Waltham, MA, USA) in accordance with the standard protocol. Finally, transfected cells were collected for subsequent analysis.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNAs from HUVECs were prepared with TRIzol reagent (Invitrogen). Then complementary DNA was synthesized by using Taqman miRNA Reverse Transcription Kit (Qiagen, Valencia, CA, USA). After that, qPCR analysis was conducted with the SYBR Green PCR Master Mix (Qiagen). The expression of the miRNA and mRNA was normalized using U6 small nuclear B noncoding RNA (U6) or glyceraldehyde 3-phosphate dehydrogenase (GADPH) and calculated by 2−ΔΔCt method. The sequence of primers used in this experiment was exhibited as followed:
-
miR-328-3p forward: 5′-CGGGCCTGGCCCTCTCTGCC-3′ and
reverse: 5′-CAGCCACAAAAGAGCACAAT-3′;
-
U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse: 5′-CGCTTCACGAATTTGCGT-3′;
-
FOXO4 forward: 5′-AGCGACTGACACTTGCCCAGAT-3′ and
reverse: 5′-AGGGTTCAGCATCCACCAAGAG-3′;
-
GADPH forward: 5′-AAGAAGG- TGGTGAAGCAGGC-3′ and
reverse: 5′-GTCAAAGGTGGAGGAGTGGG-3′.
Cells viability assay
HUVECs were seed into 96-well plates (5×103 cell/well) for 24 hours. Subsequently, Cell Counting Kit-8 (CCK-8) reagents (10 μL/well) (Beyotime, Shanghai, China) were added into each well for about 4 hours. Finally, the absorbance was determined by a microplate reader at 450 nm in the indicated time.
Cell apoptosis assay
Transfected HUVECs were resuspended with binding buffer, followed by staining with 10 μL Annexin V-FITC and PI (BD Biosciences, San Jose, CA, USA). Finally, the apoptotic HUVECs were analyzed by FlowJo software.
Western blot analysis
Protein was extracted with the RNA immunoprecipitation (RIP) assay lysate (Beyotime), and then qualified by the Bicinchoninic Acid Method. Subsequently, immunoblot assays were performed using primary antibodies against FOXO4, C-caspase 3, Beclin-1, LC3-I, LC3-II, interleukin (IL)-1β, IL-10, TNF-α, and GAPDH overnight at 4°C and followed incubated with HRP-conjugated secondary antibody at 37°C. Finally, immunoreactive signals were visualized by ECL chromogenic substrate (Beyotime).
Transwell assay
For migration assay, after transfection or treatment, HUVECs were seeded in the Transwell upper chamber with serum-free endothelial cell basal medium, then the lower chamber was added with the 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA)-containing medium. After 24 hours, migrated cells were fixed with 4% formaldehyde and stained with 0.1% crystal violet. Then 6 randomly selected visual fields were counted. For invasion assay, the upper Transwell chambers were pre-coated with the Matrigel (BD Biosciences), and other measurement was similar to the aforementioned steps of cell migration.
Enzyme-linked immunosorbent assay (ELISA)
IL-1β, IL-10, and TNF-α concentrations from the supernatants of HUVECs after appropriate treatment were examined by using commercial IL-1β, IL-10, and TNF-α ELISA kits (R&D Systems, Minneapolis, MN, USA) following the instructions of manufacturer. Finally, the absorbance was detected at 450 nm.
Dual-Luciferase reporter assay
The FOXO4 3′-UTR with wild-type or mutant miR-328-3p binding sites were cloned into the pmirGLO vectors (Promega, Shanghai, China). Then, the constructed vectors combined with miR-328-3p mimics, anti-miR-328-3p, or the corresponding negative controls were co-transfected into HUVECs. After 48 hours, a dual-luciferase reporter assay system (Promega) was employed to detect the relative luciferase activity.
RNA immunoprecipitation (RIP) assay
Magna RNA immunoprecipitation kit (Millipore, Billerica, MA, USA) was applied to investigate the interaction between miR-328-3p and FOXO4 in accordance with the protocols of the manufacturer. HUVECs transfected with miR-NC or miR-328-3p were lysed using RIP assay buffer (Millipore), then cell extracts were coimmunoprecipitated with anti-Ago2, and normal rabbit IgG was used as a negative control. Finally, the enrichment of FOXO4 was extracted and analyzed.
Statistical analysis
All data from at least 3 independently experiments were analyzed with GraphPad (GraphPad Inc., San Diego, CA, USA) and showed as mean±standard deviation (SD). Statistical differenced were analyzed using Student’s t-test and one-way analysis of variance analysis in different groups. P value less than 0.05 exhibited statistically significance.
Results
MiR-328-3p was decreased but FOXO4 was increased in ox-LDL induced HUVECs
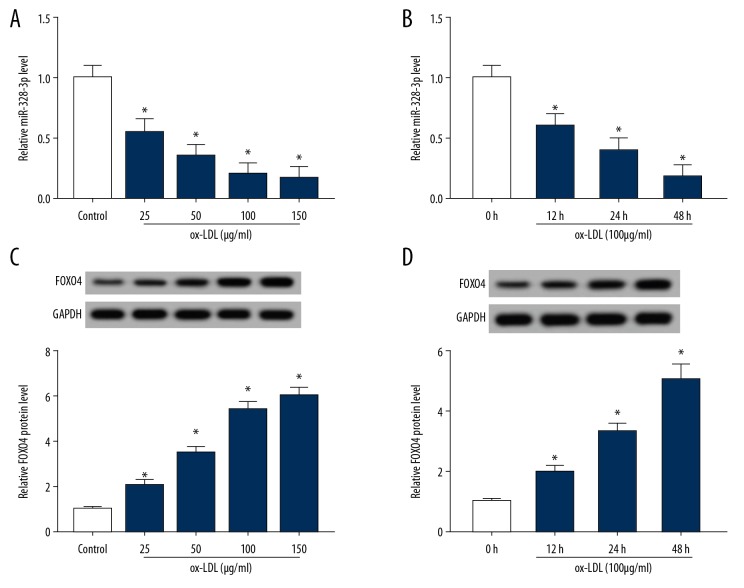
HUVECs were incubated with 25, 50, 100, or 150 μg/mL of ox-LDL for different time durations (0, 12, 24, or 48 hours), then miR-328-3p and FOXO4 expression were detected and the results showed that miR-328-3p expression was downregulated (Figure 1A, 1B) while FOXO4 was upregulated (Figure 1C, 1D) in ox-LDL induced HUVECs in a dose- and time- dependent manner. These results indicated that miR-328-3p decrease or FOXO4 increase might be associated with ox-LDL-induced HUVECs dysfunction.
Figure 1.
MiR-328-3p is decreased but FOXO4 is increased in ox-LDL induced HUVECs. HUVECs were incubated with 25, 50, 100, or 150 μg/mL of ox-LDL for different time durations. (A, B) qRT-PCR analysis of miR-328-3p expression in ox-LDL induced HUVECs. (C, D) Western blot analysis of FOXO4 expression in ox-LDL induced HUVECs. * P<0.05. FOXO4 – forkhead box protein; ox-LDL – oxidized low-density lipoprotein; HUVECs – human umbilical vein endothelial cells; qRT-PCR – quantitative real-time polymerase chain reaction.
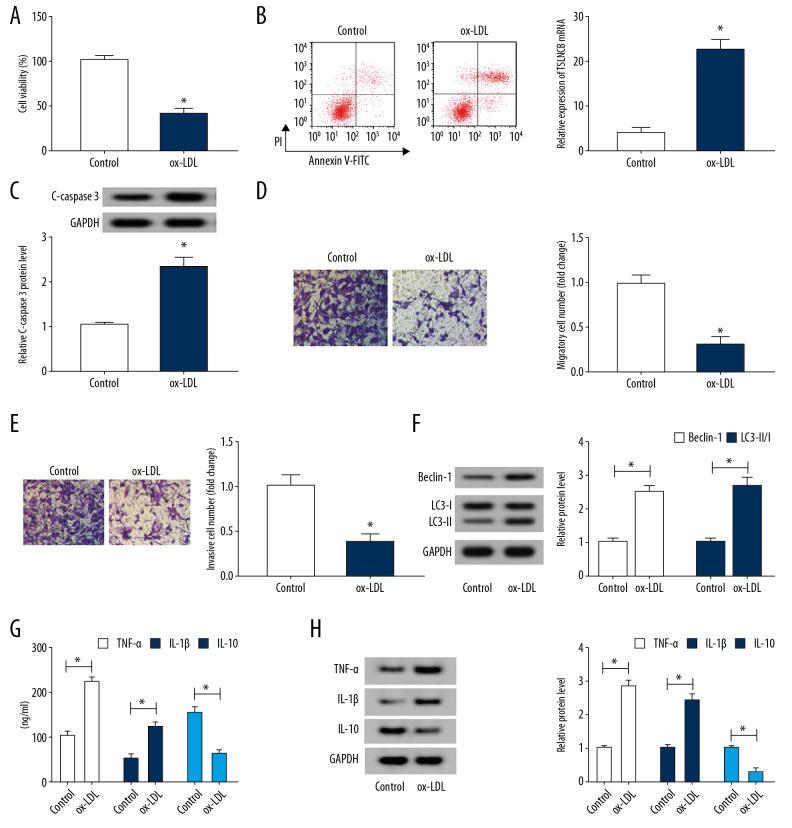
Ox-LDL treatment induced HUVECs injury
When HUVECs were subjected to 100 μg/mL ox-LDL for 24 hours, the level of miR-328-3p or FOXO4 was changed by approximately 50%. Thus, we treated HUVECs with or without 100 μg/mL ox-LDL for 24 hours to analyze the effects of ox-LDL on HUVECs. Subsequently, we found ox-LDL treatment inhibited proliferation (Figure 2A), migration and invasion (Figure 2D, 2E), but induced apoptosis displayed by the increased apoptosis rate (Figure 2B) and C-caspase 3 level (Figure 2C) in HUVECs. Furthermore, the autophagy-related proteins Beclin, LC3-I, and LC3-II were detected and results exhibited ox-LDL treatment promoted the levels of Beclin and the ratio of LC3-II/I (Figure 2F), suggesting ox-LDL induced autophagy in HUVECs. Additionally, inflammatory factors TNF-α, IL-1β, and IL-10 were also measured by ELISA, results exhibited ox-LDL treatment enhanced TNF-α and IL-1β expression but reduced IL-10 expression in HUVECs (Figure 2G), which was consistent with the results of western blot analysis of TNF-α, IL-1β, and IL-10 expression (Figure 2H), indicating ox-LDL induced HUVECs inflammation. These results illustrated that ox-LDL exposure induced HUVECs injury.
Figure 2.
Ox-LDL treatment induces HUVECs injury. HUVECs were subjected to 100 μg/mL ox-LDL for 24 hours. (A) Cell viability was detected by CCK-8 assay. (B) The apoptotic rate was examined by flow cytometry. (C) Western blot analysis of C-caspase 3 expression. (D, E) The migration and invasion abilities were analyzed using Transwell assay. (F) The expression of Beclin, LC3-I and LC3-II was detected using western blot in ox-LDL HUVECs. (G) The levels of TNF-α, IL-1β, and IL-10 were determined by ELISA assay. (H) Western blot analysis of the levels of TNF-α, IL-1β, and IL-10 was performed. * P<0.05. ox-LDL – oxidized low-density lipoprotein; HUVECs – human umbilical vein endothelial cells; CCK-8 – Cell Counting Kit-8; TNF – tumor necrosis factor; IL – interleukin; ELISA – enzyme-linked immunosorbent assay.
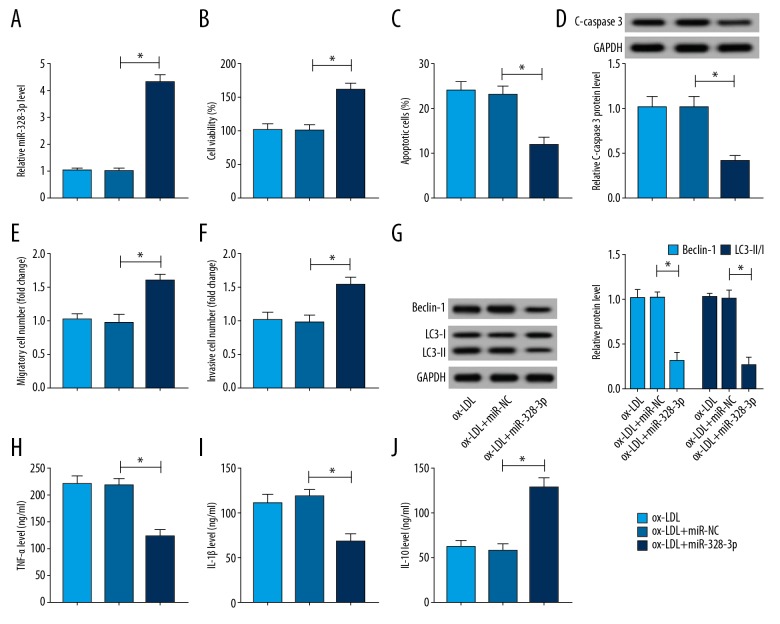
MiR-328-3p alleviated ox-LDL induced HUVECs injury
To investigate the role of miR-328-3p in ox-LDL-induced HUVECs injury, HUVECs were transfected with miR-328-3p or miR-NC prior to ox-LDL treatment. Then the level of miR-328-3p was detected and miR-328-3p was significantly elevated by miR-328-3p mimics in ox-LDL HUVECs (Figure 3A). Immediately, we discovered that overexpressed miR-328-3p obviously abated ox-LDL induced inhibition on cell viability (Figure 3B), migration and invasion (Figure 3E, 3F), stimulation on apoptosis (Figure 3C, 3D), autophagy (Figure 3G), as well as inflammation (Figure 3H–3J) in HUVECs. Thus, we confirmed that miR-328-3p could alleviate ox-LDL induced HUVECs injury.
Figure 3.
MiR-328-3p alleviates ox-LDL induced HUVECs injury. HUVECs were transfected with miR-328-3p or miR-NC prior to ox-LDL treatment. (A) qRT-PCR analysis of miR-328-3p expression. HUVECs were treated with 100 μg/mL ox-LDL for 24 hours. (B) Cell viability was detected using CCK-8 assay. (C) Flow cytometry was used to test cell apoptotic rate. (D) The expression of C-caspase 3 was examined by western blot. (E, F) The migration and invasion abilities were analyzed using Transwell assay. (G) The expression of Beclin, LC3-I, and LC3-II was measured using western blot. (H–J) The levels of TNF-α, IL-1β, and IL-10 were examined by ELISA assay. * P<0.05. HUVECs – human umbilical vein endothelial cells; ox-LDL – oxidized low-density lipoprotein; qRT-PCR – quantitative real-time polymerase chain reaction; CCK-8 – Cell Counting Kit-8; TNF – tumor necrosis factor; IL – interleukin; ELISA – enzyme-linked immunosorbent assay.
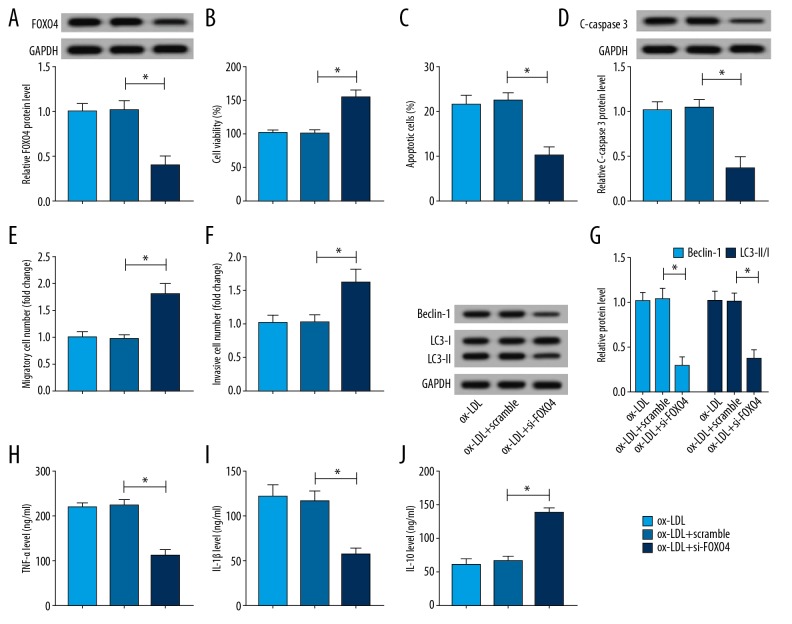
FOXO4 contributed to ox-LDL induced HUVECs injury
To explore whether FOXO4 involved in ox-LDL-induced HUVECs injury, HUVECs were transfected with si-FOXO4 or scramble prior to ox-LDL treatment and a strong decrease of FOXO4 expression in HUVECs transfected with si-FOXO4 was found (Figure 4A). After that, functional experiments were performed and we demonstrated that FOXO4 deletion promoted cells viability (Figure 4B), migration and invasion (Figure 4E, 4F), inhibited apoptosis via repressing C-caspase 3 expression (Figure 4C, 4D), suppressed autophagy reflected by the reduction of Beclin expression and the ratio of LC3-II/I (Figure 4G), as well as repressed inflammation by reducing the level of TNF-α and IL-1β (Figure 4H–4J) in ox-LDL HUVECs. All these results exhibited that FOXO4 could aggravate ox-LDL induced HUVECs injury.
Figure 4.
FOXO4 contributes to ox-LDL induced HUVECs injury. HUVECs were transfected with scramble or si-FOXO4 prior to ox-LDL treatment. (A) The expression of FOXO4 was examined using western blot in ox-LDL HUVECs. HUVECs were subjected to 100 μg/mL ox-LDL for 24 hours. (B) CCK-8 assay was applied to determine cell viability. (C) Flow cytometry was utilized to analyze the cell apoptosis. (D) The expression of C-caspase 3 was measured by western blot. (E, F) Transwell assay was performed to detect cell migration and invasion. (G) The expression of Beclin, LC3-I, and LC3-II was tested using western blot. (H–J) The levels of TNF-α, IL-1β, and IL-10 were examined by ELISA assay. * P<0.05. FOXO4 – forkhead box protein; ox-LDL – ox-LDL, oxidized low-density lipoprotein; HUVECs – human umbilical vein endothelial cells; CCK-8 – Cell Counting Kit-8; TNF – tumor necrosis factor; IL – interleukin; ELISA – enzyme-linked immunosorbent assay.
FOXO4 was a target of miR-328-3p in HUVECs
To explore the mechanism underlying miR-328-3p-mediated protection on ox-LDL induced HUVECs injury. The potential targets of miR-328-3p in HUVECs were searched through Targetscan program. We discovered that FOXO4 might be a target of miR-328-3p with putative binding sites (Figure 5A). Afterwards, dual-luciferase reporter assay showed that miR-328-3p overexpression declined the luciferase activities of the FOXO4-wt reporter vector but not FOXO4-mut reporter vector in HUVECs (Figure 5B). Moreover, the luciferase activities of FOXO4-wt reporter were notably enhanced, but there was no obvious change in FOXO4-mut reporter after miR-328-3p downregulation in HUVECs (Figure 5C). In addition, the RIP assays further identified the direct interaction between miR-328-3p and FOXO4 because of the significantly enrichment of FOXO4 in HUVECs (Figure 5D). We also found FOXO4 expression was repressed by miR-328-3p overexpression but promoted by miR-328-3p inhibition (Figure 5F). Therefore, we confirmed that miR-328-3p directly interacted with FOXO4 and negatively regulated FOXO4 expression in HUVECs.
Figure 5.
FOXO4 is a target of miR-328-3p in HUVECs. (A) The putative binding sites between miR-328-3p and FOXO4 were predicted. (B, C) Dual-luciferase reporter assay in HUVECs after the co-transfection with FOXO4-wt or FOXO4-mut and miR-195-5p mimic or miR-NC or anti-miR-NC or anti-miR-328-3p was conducted. (D) RIP assays were carried out to confirm the interaction of miR-328-3p and FOXO4 to Ago2 in HUVECs. (E) FOXO4 expression was detected using western blot in HUVECs transfected with miR-328-3p or anti-miR-328-3p or the corresponding negative controls. * P<0.05. FOXO4 – forkhead box protein; HUVECs – human umbilical vein endothelial cells; RIP – RNA immunoprecipitation.
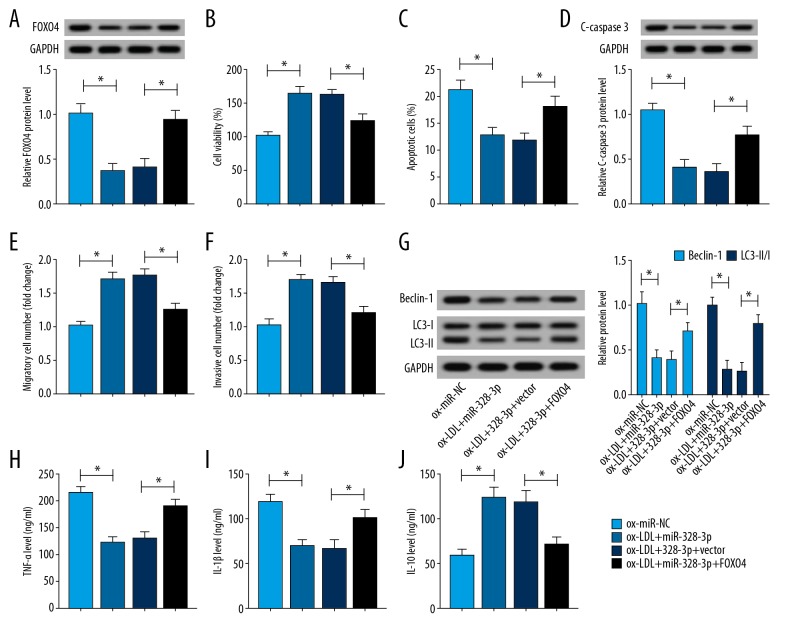
MiR-328-3p protected HUVECs against ox-LDL induced injury via regulating FOXO4
To verify whether FOXO4 implicated in the protection mediated by miR-328-3p on ox-LDL induced HUVECs, HUVECs were transfected with miR-NC, miR-328-3p, miR-328-3p+vector, and miR-328-3p+FOXO4 prior to treatment with ox-LDL. and We found the expression of FOXO4 was inhibited by miR-328-3p mimic transfection, while was restored by FOXO4 pcDNA transfection in HUVECs after transfection (Figure 6A). The rescue assay suggested that overexpressed FOXO4 could reverse miR-328-3p mediated enhancement on cell viability (Figure 6B), migration and invasion (Figure 6E, 6F), inhibition on apoptosis (Figure 6C, 6D), autophagy (Figure 6G) as well as inflammation (Figure 6H–6J) in ox-LDL induced HUVECs. These data suggested that miR-328-3p could alleviate ox-LDL induced HUVECs injury via regulating FOXO4.
Figure 6.
MiR-328-3p protects HUVECs against ox-LDL induced injury via regulating FOXO4. HUVECs were transfected with miR-NC, miR-328-3p, miR-328-3p+vector, miR-328-3p+FOXO4 prior to treatment ox-LDL. (A) The expression of FOXO4 was detected using western blot in ox-LDL HUVECs. HUVECs were treated with 100 μg/mL ox-LDL for 24 hours. (B) CCK-8 assay was used to determine cell viability. (C) Flow cytometry was utilized to determine cell apoptosis. (D) Western blot analysis was conducted to measure C-caspase 3 expression. (E, F) Transwell assay was performed to test cell migration and invasion. (G) The expression of Beclin, LC3-I and LC3-II was tested using western blot. (H–J) ELISA assay was performed to examine the levels of TNF-α, IL-1β, and IL-10. * P<0.05. FOXO4 – forkhead box protein; ox-LDL – oxidized low-density lipoprotein; HUVECs – human umbilical vein endothelial cells; CCK-8 – Cell Counting Kit-8; ELISA – enzyme-linked immunosorbent assay; TNF – tumor necrosis factor; IL – interleukin.
Discussion
Our results demonstrated that miR-328-3p was downregulated while FOXO4 was upregulated in ox-LDL induced HUVECs in a dose- and time-dependent manner, miR-328-3p restoration or FOXO4 depletion reversed ox-LDL-induced inhibition of HUVEC viability, migration, and invasion, as well as stimulation of HUVEC apoptosis, inflammation, and autophagy. Furthermore, our findings have also verified that miR-328-3p targeted the repression of FOXO4, thereby positively impeding the progression of AS.
AS is a chronic inflammatory disease of the arterial wall, initiated by endothelial injury and subendothelial disorder of lipid metabolism, which results in multiple-site vascular disease in the form of cerebrovascular disease, coronary artery, and peripheral arterial [4,10]. The pathogenesis of AS lesion formation is a complex, multistage process, and the initiating phase of AS plaques occurrences as a result of endothelium injury [24]. ECs are the first-line defense against AS. Thus, it is urgently necessary for us to better understand the underlying molecular mechanism in EC injury. At present, investigations are still limited to direct evaluation of EC function, therefore, indirect assessment of mediators arising from EC injury is a main diagnosis of endothelial dysfunction [25]. In recent years, accumulating evidences have revealed the involvement of miRNAs in the balance of AS plaque progression and regression [26]. For example, miR-126 are highly enriched in the ECs, and modulate endothelial phenotypes, particularly the response to migration stimulated by fibroblast growth factor 2 as well as vascular endothelial growth factor [27]. MiR-221/222 cluster is primarily involved in maintaining endothelial integrity and supporting quiescent EC phenotype and appeared to be proatherogenic through inhibiting ECs pro-angiogenic activation, proliferation, and migration [28]. MiR-214-3p regulated ox-LDL-induced autophagy via targeted interactions with autophagy-related protein 5 in ECs of AS [29]. All the studies suggested the important roles of miRNAs on ECs injury in AS. In our research, we demonstrated that miR-328-3p protected ECs against ox-LDL-induced dysfunction.
The FOXO family serves an essential role in the maintenance of vascular stability and the suppression of aberrant vascular outgrowth; the FOXO family, including FOXO4, can regulate EC homeostasis [30]. FOXO4 has been reported to regulate AS by affecting SMCs, bone marrow derived cells, or the expression of NF-κB-activated inflammatory cytokines [31,32]. In this study, we confirmed that FOXO4 regulated the dysfunction of ECs to promote the progression of AS. Moreover, miR-328-3p targeted FOXO4 and exerted protective function in ECs by downregulating FOXO4 expression. In addition, Shang et al. verified that FOXO4 also served as a target of miR-148a-3p to regulate proliferative and migratory function of ECs in AS [33]. Thus, there seems to be a competition or modulating relationship between other miRNAs and miR-328-3p. However, this needs to be verified in future research.
Conclusions
In conclusion, our findings proved that miR-328-3p interacted with FOXO4 to protect ECs against ox-LDL induced impairment in AS, which provided a novel insight and therapeutic strategy for AS. Additionally, the “competing endogenous RNA (ceRNA) hypothesis” has been identified, stating that many lncRNAs functions as ceRNAs by sequestrating target miRNAs; abundant lncRNAs containing similar miRNA target sequences can indirectly modulate miRNAs-target genes through absorbing cytoplasmic miRNAs [16]. LncRNAs have been suggested to be involve in the pathogenesis of AS [34]; the lncRNA-miR-328-3p-FOXO4 needs further study.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Sturtzel C. Endothelial cells. Adv Exp Med Biol. 2017;1003:71–91. doi: 10.1007/978-3-319-57613-8_4. [DOI] [PubMed] [Google Scholar]
- 2.Gimbrone MA, Jr, Topper JN, Nagel T, et al. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann NY Acad Sci. 2000;902:230–39. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239–40. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Horke S, Forstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237(1):208–19. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Wang K, Yang C, Shi J, et al. Ox-LDL-induced lncRNA MALAT1 promotes autophagy in human umbilical vein endothelial cells by sponging miR-216a-5p and regulating Beclin-1 expression. Eur J Pharmacol. 2019;858:172338. doi: 10.1016/j.ejphar.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Kockx M, De Meyer G. Apoptosis in human atherosclerosis and restenosis. Circulation. 1996;93(2):394–95. [PubMed] [Google Scholar]
- 6.Steffens S, Winter C, Schloss MJ, et al. Circadian control of inflammatory processes in atherosclerosis and its complications. Arterioscler Thromb Vasc Biol. 2017;37(6):1022–28. doi: 10.1161/ATVBAHA.117.309374. [DOI] [PubMed] [Google Scholar]
- 7.Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149(3):515–24. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perdas E, Stawski R, Nowak D, et al. The role of miRNA in papillary thyroid cancer in the context of miRNA Let-7 family. Int J Mol Sci. 2016;17(6) doi: 10.3390/ijms17060909. pii: E909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abreu FB, Liu X, Tsongalis GJ. miRNA analysis in pancreatic cancer: The Dartmouth experience. Clin Chem Lab Med. 2017;55(5):755–62. doi: 10.1515/cclm-2017-0046. [DOI] [PubMed] [Google Scholar]
- 10.Raitoharju E, Oksala N, Lehtimaki T. MicroRNAs in the atherosclerotic plaque. Clin Chem. 2013;59(12):1708–21. doi: 10.1373/clinchem.2013.204917. [DOI] [PubMed] [Google Scholar]
- 11.Hosin AA, Prasad A, Viiri LE, et al. MicroRNAs in atherosclerosis. J Vasc Res. 2014;51(5):338–49. doi: 10.1159/000368193. [DOI] [PubMed] [Google Scholar]
- 12.Yan T, Ye XX. MicroRNA-328-3p inhibits the tumorigenesis of bladder cancer through targeting ITGA5 and inactivating PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 2019;23(12):5139–48. doi: 10.26355/eurrev_201906_18178. [DOI] [PubMed] [Google Scholar]
- 13.Shi J, An G, Guan Y, et al. miR-328-3p mediates the anti-tumor effect in osteosarcoma via directly targeting MMP-16. Cancer Cell Int. 2019;19:104. doi: 10.1186/s12935-019-0829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma W, Ma CN, Zhou NN, et al. Up-regulation of miR-328-3p sensitizes non-small cell lung cancer to radiotherapy. Sci Rep. 2016;6:31651. doi: 10.1038/srep31651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poggio P, Songia P, Moschetta D, et al. MiRNA profiling revealed enhanced susceptibility to oxidative stress of endothelial cells from bicuspid aortic valve. J Mol Cell Cardiol. 2019;131:146–54. doi: 10.1016/j.yjmcc.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Xing Y, Zheng X, Fu Y, et al. Long noncoding RNA-maternally expressed gene 3 contributes to hypoxic pulmonary hypertension. Mol Ther. 2019;27(12):2166–81. doi: 10.1016/j.ymthe.2019.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu CY, Zhou ZF, Wang B, et al. MicroRNA-328 ameliorates oxidized low-density lipoprotein-induced endothelial cells injury through targeting HMGB1 in atherosclerosis. J Cell Biochem. 2018 doi: 10.1002/jcb.27469. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Eijkelenboom A, Burgering BM. FOXOs: Signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14(2):83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- 19.Liu T, Xu J, Guo JL, et al. YAP1 up-regulation inhibits apoptosis of aortic dissection vascular smooth muscle cells. Eur Rev Med Pharmacol Sci. 2017;21(20):4632–39. [PubMed] [Google Scholar]
- 20.Li H, Liang J, Castrillon DH, et al. FoxO4 regulates tumor necrosis factor alpha-directed smooth muscle cell migration by activating matrix metalloproteinase 9 gene transcription. Mol Cell Biol. 2007;27(7):2676–86. doi: 10.1128/MCB.01748-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu ZP, Wang Z, Yanagisawa H, et al. Phenotypic modulation of smooth muscle cells through interaction of Foxo4 and myocardin. Dev Cell. 2005;9(2):261–70. doi: 10.1016/j.devcel.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Yuan M, Zhang L, et al. Adiponectin alleviates NLRP3-inflammasome-mediated pyroptosis of aortic endothelial cells by inhibiting FoxO4 in arteriosclerosis. Biochem Biophys Res Commun. 2019;514(1):266–72. doi: 10.1016/j.bbrc.2019.04.143. [DOI] [PubMed] [Google Scholar]
- 23.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation. 2007;116(16):1832–44. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 24.Scott J. The pathogenesis of atherosclerosis and new opportunities for treatment and prevention. J Neural Transm Suppl. 2002;63:1–17. doi: 10.1007/978-3-7091-6137-1_1. [DOI] [PubMed] [Google Scholar]
- 25.Cheng H, Harris RC. Renal endothelial dysfunction in diabetic nephropathy. Cardiovasc Hematol Disord Drug Targets. 2014;14(1):22–33. doi: 10.2174/1871529x14666140401110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feinberg MW, Moore KJ. MicroRNA regulation of atherosclerosis. Circ Res. 2016;118(4):703–20. doi: 10.1161/CIRCRESAHA.115.306300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fish JE, Santoro MM, Morton SU, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272–84. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chistiakov DA, Sobenin IA, Orekhov AN, et al. Human miR-221/222 in physiological and atherosclerotic vascular remodeling. Biomed Res Int. 2015;2015 doi: 10.1155/2015/354517. 354517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paik JH. FOXOs in the maintenance of vascular homoeostasis. Biochem Soc Trans. 2006;34(Pt 5):731–34. doi: 10.1042/BST0340731. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Wang WN, Xu SB, et al. Micro RNA-214-3p: A link between autophagy and endothelial cell dysfunction in atherosclerosis. Acta Physiol (Oxf) 2018;222(3):e12973. doi: 10.1111/apha.12973. [DOI] [PubMed] [Google Scholar]
- 31.Zhou W, Cao Q, Peng Y, et al. FoxO4 inhibits NF-kappaB and protects mice against colonic injury and inflammation. Gastroenterology. 2009;137(4):1403–14. doi: 10.1053/j.gastro.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu M, Zhang Q-J, Wang L, et al. FoxO4 inhibits atherosclerosis through its function in bone marrow derived cells. Atherosclerosis. 2011;219(2):492–98. doi: 10.1016/j.atherosclerosis.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shang L, Quan A, Sun H, et al. MicroRNA-148a-3p promotes survival and migration of endothelial cells isolated from Apoe deficient mice through restricting circular RNA 0003575. Gene. 2019;711:143948. doi: 10.1016/j.gene.2019.143948. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Ruiz I. Atherosclerosis: A new role for lncRNAs in atherosclerosis. Nat Rev Cardiol. 2018;15(4):195. doi: 10.1038/nrcardio.2018.18. [DOI] [PubMed] [Google Scholar]