Abstract
Background
For patients with peritoneal carcinomatosis undergoing cytoreductive surgery with heated intraperitoneal chemotherapy (CRS/HIPEC), incomplete cytoreduction (CCR2/3) confers morbidity without survival benefit. The aim of this study is to identify preoperative factors which predict CCR2/3.
Methods
All patients who underwent curative-intent CRS/HIPEC of low/high-grade appendiceal, colorectal, or peritoneal mesothelioma cancers in the 12-institution US HIPEC Collaborative from 2000 to 2017 were included (n = 2027). The primary aim is to create an incomplete-cytoreduction risk score (ICRS) to predict CCR2/3 CRS utilizing preoperative data. ICRS was created from a randomly selected cohort of 50% of patients (derivation cohort) and verified on the remaining patients (validation cohort).
Results
Within our derivation cohort (n = 998), histology was low-grade appendiceal neoplasms in 30%, high-grade appendiceal tumor in 41%, colorectal tumor in 22%, and peritoneal mesothelioma in 8%. CCR0/1 was achieved in 816 patients and CCR 2/3 in 116 patients. On multivariable analysis, preoperative factors associated with incomplete cytoreduction were male gender [odds ratio (OR) 3.4, p = 0.007], presence of ascites (OR 2.8, p = 0.028), cancer antigen (CA)-125 ≥ 40 U/mL (OR 3.4, p = 0.012), and carcinoembryonic antigen (CEA) ≥ 4.2 ng/mL (OR 3.2, p = 0.029). Each preoperative factor was assigned a score of 0 or 1 to form an ICRS from 0 to 4. Scores were grouped as zero (0), low (1–2), or high (3–4). Incidence of CCR2/3 progressively increased by risk group from 1.6% in zero to 13% in low and 39% in high. When ICRS was applied to the validation cohort (n = 1029), this relationship was maintained.
Conclusion
The incomplete cytoreduction risk score incorporates preoperative factors to accurately stratify the risk of CCR2/3 resection in CRS/HIPEC. This score should not be used in isolation, however, to exclude patients from surgery.
It was not until the mid-20th century with the development of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) that patients with peritoneal metastases had an option to pursue surgical intervention for a potential cure of their disease. Few prospective clinical trials have been able to demonstrate a benefit for the use of CRS/HIPEC in improving survival.1–3 Despite this lack of evidence, the goals of CRS are clear: to achieve complete cytoreduction so that no visible residual tumor remains within the peritoneal cavity (CCR0/CCR1).4,5 In fact, current practice patterns dictate that infusion of intraperitoneal chemotherapy be done only in the setting of CCR0/1 resection, as incomplete cytoreduction (CCR2/3 resection) is not associated with a survival advantage and unnecessary HIPEC may confer significant morbidity.6
Patients undergoing CRS/HIPEC have better outcomes when undergoing CCR0/1 versus CCR2/3 resection across several tumor histologic types. In patients with colorectal cancer, macroscopically complete cytoreduction is associated with improved survival compared with limited cytoreduction.7–10 This pattern is also observed among patients with peritoneal metastases of appendiceal and gastric origin, with significantly improved survival in patients who undergo CCR0/1 compared with CCR2/3 resection.11–15 Although CCR0/1 resection may be associated with improved oncologic outcomes, CRS/HIPEC is associated with perioperative morbidity rates of 25–55% and perioperative mortality rates of up to 7%.6,7,12–14,16–18 Thus, predicting preoperatively which patients are more likely to undergo complete cytoreduction is crucial to spare patients complications from a potentially futile procedure.
Previous groups have attempted to develop models to assess which patients may be more likely to have CCR0/1 resection based on preoperative cross-sectional imaging characteristics, body mass index (BMI), serum concentrations of tumor markers, age, and peritoneal carcinomatosis index (PCI).19–22 Unfortunately, these previous scores are limited in that they are specific to only one histologic type of tumor, use specialized radiological expertise in interpreting preoperative imaging characteristics, or utilize the PCI, which is most faithfully measured intraoperatively. Given the morbidity of CRS/HIPEC and the lack of survival benefit in those patients who undergo CCR2/3 resection, the aim of this study is to use a large, multiinstitutional database to construct a score for preoperatively predicting incomplete cytoreduction to aid in assessment and selection of patients who are being considered for CRS/HIPEC.
METHODS
Patients were included from the US Hyperthermic Intraperitoneal Chemotherapy Collaborative (US-HIPECC) database, a retrospective collaboration of 12 US-based academic tertiary and quaternary referral centers.1 Institutional review board (IRB) approval was obtained at each study site prior to data collection. Patients who underwent exploration for curative-intent CRS ± HIPEC for the top four most common histologic tumor types within the database from 2000 to 2017 were included. Patients who were preoperatively determined to undergo prophylactic or palliative CRS/HIPEC were excluded.
Demographic, perioperative, histopathologic, and outcome data were collected by retrospective review of electronic medical records. Pathology was reviewed by expert gastrointestinal pathologists at each institution. Staging was based on American Committee on Cancer (AJCC) 7th edition guidelines. Histologic types of tumors were categorized into four groups: (1) noninvasive appendiceal tumors [including low-grade appendiceal mucinous neoplasm (LAMN) and high-grade appendiceal mucinous neoplasm (HAMN)], (2) invasive appendiceal tumors (including adenocarcinoma, adenosquamous carcinoma, mixed adeno-neuroendocrine carcinoma, and others), (3) colorectal adenocarcinoma, and (4) peritoneal mesothelioma (all subtypes included). PCI was estimated preoperatively by cross-sectional imaging by radiologists at each respective institution and/or intraoperatively using published guidelines for estimation.23 Presence of ascites was determined using preoperative cross-sectional imaging. Complete cytoreduction was estimated by surgeons at each respective institution at end of CRS and scored as CCR0 (no visible peritoneal disease), CCR1 (remaining tumor nodules < 2.5 mm), CCR2 (remaining tumor nodules 2.5–2.5 cm), or CCR3 (remaining tumor nodules > 2.5 cm). The primary aim is to assess the association between preoperative clinicopathologic variables and incomplete cytoreduction (CCR2/3) and devise a risk score to predict CCR2/3 resection.
Statistical analysis was conducted using SPSS 22.0 software (IBM Inc., Armonk, NY). To create an incomplete-cytoreduction risk score (ICRS), patients in the US-HIPECC database were randomized 1:1 into a derivation cohort or validation cohort using computerized randomization. Chi squared analysis was used to compare categorical variables, and Student’s t test was used for continuous variables. For serum tumor marker concentrations, Youden’s index for each respective receiver operator characteristic (ROC) curve was calculated to determine optimal cut-off values to create a dichotomous variable measured against incomplete cytoreduction. Univariate and multivariable binary logistic regression analyses were used to determine the association of preoperative variables with CCR2/3 in the derivation cohort. The weighted odds ratios (ORs) for variables statistically significantly associated with CCR2/3 (p < 0.05) on multivariable analysis were used to create the ICRS. The validation cohort was subsequently scored using the ICRS. Patients were then categorized into three groups (0 points, 1–2 points, 3–4 points), and the association of each score group with incomplete cytoreduction was calculated. The score was then applied to the validation cohort. Kaplan–Meier survival plots were constructed to determine the association of completeness of cytoreduction or ICRS score group with survival outcomes.
RESULTS
Of the 2372 patients within the US-HIPECC database, 2027 patients met inclusion criteria. Patients were randomized into the derivation cohort (DC, n = 998) and the validation cohort (VC, n = 1029). Demographic, pathologic, and treatment factors are listed in Table 1. The average age was 54.9 years (DC: 54.8 ± 12.4 years, VC: 55.0 ± 12.1 years, p = 0.513). Forty-three percent (n = 871) were male (DC: 44%, VC: 42%, p = 0.492), and mean BMI was 28.1 kg/m2 (DC: 28.4 ± 6.7 kg/m2, VC: 27.8 ± 6.3 kg/m2, p = 0.280). The most common histologic type of tumor was invasive appendiceal cancer in 42% (n = 859, DC: 41%, VC: 44%), followed by noninvasive appendiceal cancer in 27% (n = 555, DC: 30%, VC: 25%), colorectal cancer in 23% (n = 459, DC: 22%, VC: 23%), and peritoneal mesothelioma in 8% (n = 154, DC: 8%, VC: 8%, all p = 0.142). Fourteen percent (n = 278) of patients presented with ascites (DC: 14%, VC: 13%, p = 0.724). By histology, 16% (n = 79) of patients with noninvasive appendiceal cancer, 16% (n = 123) of patients with invasive appendiceal cancer, 7% (n = 32) of patients with colorectal cancer, and 29% (n = 44) of patients with peritoneal mesothelioma had ascites (p < 0.001). The mean CEA level was higher in the DC than VC (entire study population: 108 ± 2507 ng/mL, DC: 190 ± 3507 ng/mL, VC: 22.2 ± 65 ng/mL, p = 0.041), though equal percentages of patients had CEA ≥ 4.2 ng/mL (DC: 48.2% versus VC: 47.1%, p = 0.762). CA-125 and CA19–9 levels were similar between the two cohorts. Complete cytoreduction was carried out in 82% (n = 1656, DC: 82%, VC: 82%, p = 0.946). By histology, 9% (n = 47) of patients with noninvasive appendiceal cancer, 17% (n = 141) of patients with invasive appendiceal cancer, 6% (n = 25) of patients with colorectal cancer, and 18% (n = 25) of patients with peritoneal mesothelioma had incomplete cytoreduction (p < 0.001).
TABLE 1.
Demographic, pathologic, and treatment characteristics of all patients within the entire study cohort, derivation cohort, and validation cohort
| Baseline variable | US-HIPEC (n = 2027) | Derivation cohort (n = 998, %) | Validation cohort (n = 1029, %) | p Value (derivation versus validation) |
|---|---|---|---|---|
| Age (years), mean ± SD | 54.9 ± 12.3 | 54.8 ± 12.4 | 55.0 ± 12.1 | 0.513 |
| Male | 871 (43.0) | 437 (43.8) | 434 (42.2) | 0.492 |
| BMI, mean ± SD | 28.1 ± 6.5 | 28.4 ± 6.7 | 27.8 ± 6.3 | 0.280 |
| Race | 0.629 | |||
| White | 1705 (84.1) | 831 (83.3) | 874 (84.9) | |
| Black | 103 (5.1) | 56 (5.6) | 47 (4.6) | |
| Latino | 71 (3.5) | 33 (3.3) | 38 (3.7) | |
| Functional status | 0.850 | |||
| Independent | 1709 (84.3) | 836 (83.8) | 873 (84.8) | |
| Partially dependent | 38 (1.9) | 18 (1.8) | 20 (1.9) | |
| Histology | 0.142 | |||
| Noninvasive appendiceal | 555 (27.4) | 296 (29.7) | 259 (25.2) | |
| Invasive appendiceal | 859 (42.4) | 405 (40.6) | 454 (44.1) | |
| Colorectal | 459 (22.6) | 221 (22.1) | 238 (23.1) | |
| Peritoneal mesothelioma | 154 (7.6) | 76 (7.6) | 78 (7.6) | |
| Diabetes mellitus | 154 (7.6) | 74 (7.4) | 80 (7.8) | 0.774 |
| Congestive heart failure | 16 (0.8) | 8 (0.8) | 8 (0.8) | 1.000 |
| Dyspnea | 38 (1.9) | 21 (2.1) | 17 (1.7) | 0.576 |
| Liver disease | 21 (1.0) | 9 (0.9) | 12 (1.2) | 0.767 |
| Chronic kidney disease | 24 (1.1) | 9 (0.9) | 15 (1.5) | 0.380 |
| Long-term anticoagulation | 84 (4.1) | 43 (4.3) | 41 (4.0) | 0.799 |
| Tobacco use | 521 (25.7) | 254 (25.4) | 267 (25.9) | 0.714 |
| Alcohol use | 107 (6.3) | 65 (6.5) | 62 (6.1) | 0.507 |
| Symptomatic | 1052 (51.9) | 510 (51.1) | 542 (52.7) | 0.538 |
| Pain | 575 (28.4) | 277 (27.8) | 298 (29.0) | 0.423 |
| Jaundice | 0 (0) | 0 (0) | 0 (0) | – |
| Obstruction | 63 (3.1) | 32 (3.2) | 31 (3.0) | 0.914 |
| Ascites | 278 (13.7) | 141 (14.1) | 137 (13.3) | 0.724 |
| Previous abdominal surgery | 1448 (71.4) | 727 (72.9) | 721 (70.0) | 0.126 |
| Previous cytoreduction | 492 (24.3) | 257 (25.8) | 235 (22.8) | 0.132 |
| Previous HIPEC | 151 (7.4) | 83 (8.3) | 68 (6.6) | 0.165 |
| Preoperative CEA (ng/mL), mean ± SD | 108 ± 2507 | 190 ± 3507 | 22.2 ± 65 | 0.041 |
| CEA ≥ 4.2 ng/mL | 511 (47.6) | 258 (47.1) | 253 (48.2) | 0.762 |
| Preoperative CA 19–9 (U/mL), mean ± SD | 112 ± 502 | 114 ± 404 | 112 ± 591 | 0.915 |
| Preoperative CA-125 (U/mL), mean ± SD | 45.5 ± 100.5 | 46.5 ± 90.8 | 44.5 ± 109.7 | 0.420 |
| CA-125 ≥ 40 U/mL | 165 (26.6) | 80 (25.6) | 85 (27.7) | 0.611 |
| Preoperative albumin (g/dL), mean ± SD | 4.1 ± 0.8 | 4.1 ± 1.0 | 4.1 ± 0.5 | 0.204 |
| Preoperative imaging PCI, mean ± SD | 8 ± 8 | 9 ± 9 | 8 ± 8 | 0.005 |
| Operative PCI, mean ± SD | 14 ± 9 | 14 ± 9 | 14 ± 9 | 0.176 |
| Cytoreduction score | 0.946 | |||
| CCR 0/1 | 1656 (81.7) | 815 (81.7) | 841 (81.7) | |
| CCR 2/3 | 238 (11.7) | 116 (11.6) | 122 (11.9) | |
| Missing | 133 (6.6%) | 67 (6.7%) | 66 (6.4%) | |
| Any complication | 1154 (56.9) | 560 (56.1) | 594 (57.7) | 0.559 |
| Highest Clavien–Dindo grade | 0.933 | |||
| I | 172 (8.5) | 77 (7.7) | 95 (9.2) | |
| II | 593 (29.3) | 300 (30.1) | 293 (28.5) | |
| III | 256 (14.9) | 143 (14.3) | 160 (15.6) | |
| IV | 64 (3.1) | 31 (3.1) | 33 (3.2) | |
| V | 17 (0.8) | 10 (1.0) | 7 (0.7) |
Derivation Cohort
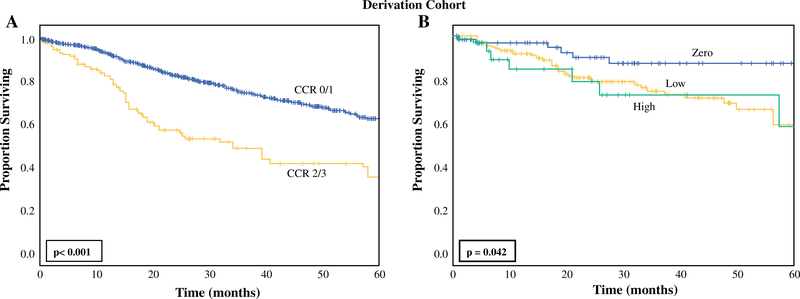
In the DC, incomplete cytoreduction was associated with worse 5-year overall survival (OS) compared with complete cytoreduction (5-year OS: CCR0/1: 63% versus CCR2/3: 36%, p < 0.001, Fig. 1a). Factors statistically significantly associated with increased odds of CC2/3 on univariate analysis included male gender, age, histologic subtype of tumor, prior cardiac event, presence of ascites, any previous abdominal surgery, CA-125 level ≥ 40 U/mL, CA 19–9 ≥ 125 U/mL, and CEA ≥ 4.2 ng/mL (Table 2). Serum albumin concentration ≥ 3.85 g/dL was associated with decreased odds of undergoing CCR2/3 resection on univariate analysis. When these factors were placed into a multivariable model, only male gender [OR 3.4, 95% confidence interval (CI) 1.4–8.6, p = 0.007], presence of ascites (OR 2.8, CI 1.1–7.2, p = 0.028), CA-125 ≥ 40 U/mL (OR 3.3, 95% CI 1.3–8.8, p = 0.012), and CEA ≥ 4.2 ng/mL (OR 3.2, 95% CI 1.1–9.1, p = 0.029) were associated with increased odds of undergoing incomplete cytoreduction.
FIG. 1.
Kaplan–Meier analysis for overall survival in derivation cohort stratified by a completeness of cytoreduction (CCR 0/1: n = 788; CCR 2/3: n = 115) and b score group within the incomplete-cytoreduction risk score (zero: n = 64, low: n = 182, high: n = 33)
TABLE 2.
Univariate and multivariable binary logistic regression for preoperative factors associated with incomplete cytoreduction within the derivation cohort (n = 998)
| Variable | Univariable |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Male | 2.2 | 1.4–3.2 | < 0.001 | 3.4 | 1.4–8.6 | 0.007 |
| Age (continuous, per year) | 1.1 | 1.0–1.1 | 0.015 | 0.6 | 0.2–2.3 | 0.473 |
| Histology | ||||||
| Noninvasive appendiceal | Ref | – | – | Ref | – | – |
| Invasive appendiceal | 1.7 | 1.1–2.7 | 0.035 | 1.1 | 0.4–2.9 | 0.881 |
| Colorectal | 0.7 | 0.4–1.4 | 0.364 | 1.8 | 0.4–8.6 | 0.473 |
| Peritoneal mesothelioma | 2.4 | 1.2–4.9 | 0.014 | 2.4 | 0.6–9.8 | 0.240 |
| Prior cardiac event | 2.2 | 1.1–4.7 | 0.032 | |||
| Ascites | 2.0 | 1.2–3.2 | 0.006 | 2.8 | 1.1–7.2 | 0.028 |
| Previous abdominal surgery | 0.4 | 0.2–0.8 | 0.004 | |||
| Previous cytoreduction | 1.7 | 1.1–2.5 | 0.014 | 2.4 | 0.8–6.8 | 0.109 |
| CA-125 ≥ 40 U/mL | 3.3 | 1.6–6.7 | 0.001 | 3.3 | 1.3–8.8 | 0.012 |
| CA 19–9 ≥ 125 U/mL | 2.3 | 1.1–5.1 | 0.032 | 0.9 | 0.3–2.7 | 0.858 |
| CEA ≥ 4.2 ng/mL | 2.4 | 1.3–4.1 | 0.003 | 3.2 | 1.1–9.1 | 0.029 |
| Albumin ≥ 3.85 g/dL | 0.3 | 0.2–0.5 | < 0.001 | 2.2 | 0.9–5.5 | 0.085 |
Preoperative factors not associated with incomplete cytoreduction were race, BMI, functional status, long-term anticoagulation, congestive heart failure, dyspnea, diabetes mellitus, liver disease, genetic syndrome, alcohol use, smoking use, preoperative imaging PCI, presence of symptoms, and previous HIPEC. Previous abdominal surgery and prior cardiac event were excluded from multivariable analysis due to the small number of patients with this variable available
Incomplete-Cytoreduction Risk Score
When constructing the ICRS, the ORs of the four factors associated with increased odds of undergoing CCR2/3 resection in the DC were considered, viz. male gender, presence of ascites, CA-125 ≥ 40 U/mL, and CEA ≥ 4.2 ng/mL. Because all four factors had an OR of approximately 3 on multivariable analysis, they were assigned equal weight in the ICRS with a score of 1 each. This created a score ranging from 0 to 4. Considering patients who had data available for all four factors in the DC (n = 272), the percentage of patients who underwent incomplete cytoreduction increased with increasing score: 0 points: 2% (n = 1/64), 1 point: 10% (n = 10/102), 2 points: 17% (n = 13/75), 3 points: 38% (n = 11/29), 4 points: 50% (n = 1/2). Based on these percentages, three score groups were formed: zero (0 points), low (1–2 points), and high (3–4 points). When applying these score groups to the DC, there was a statistically significant increase in the percentage of patients undergoing incomplete cytoreduction with increasing score group from 2% for zero (n = 1/64), 13% for low (n = 23/177), to 39% for high (n = 12/31) (p < 0.001, Table 3). On univariate regression, the odds of undergoing CCR2/3 resection increased with increasing score group (zero: reference; low: OR 9.4, 95% CI 1.2–71.2, p = 0.030; high: OR 39.8, 95% CI 4.8–326.1, p = 0.001). On Kaplan–Meier analysis, increasing score group was associated with lower 5-year overall survival (zero: 87.4%; low: 59.1%; high: 58.4%, p = 0.042, Fig. 1b).
TABLE 3.
Incomplete-cytoreduction risk score groups applied to derivation and validation cohorts
| Risk score | Derivation cohort (n = 272) |
Validation cohort (n = 264) |
||||||
|---|---|---|---|---|---|---|---|---|
| Percent CCR2/3 | p Value | OR CCR2/3 (95% CI) | p Value | Percent CCR2/3 | p Value | OR CCR2/3 (95% CI) | p Value | |
| Zero | 2% (n = 1/64) |
< 0.001 | Ref | 2% (n = 1/65) |
0.001 | Ref | ||
| Low (1–2) | 13% (n = 23/177) |
9.4 (1.2–71.2) |
0.030 | 12% (n = 21/169) |
9.1 (1.2–69.0) |
0.033 | ||
| High (3–4) | 39% (n = 12/31) |
39.8 (4.8–326.1) |
0.001 | 27% (n = 8/30) |
23.3 (2.8–196.7) |
0.004 | ||
Validation Cohort
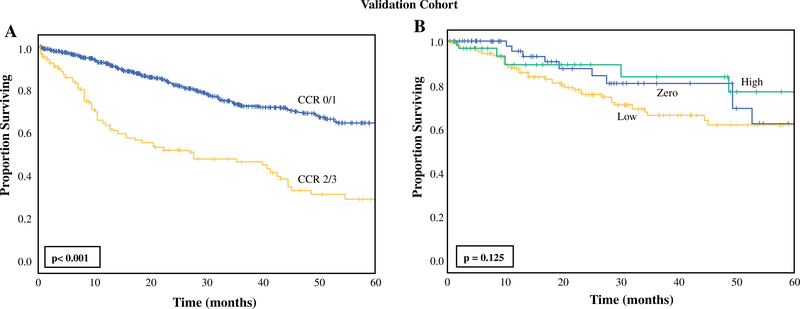
Similar to the DC, patients in the VC with CCR2/3 resection had worse 5-year OS compared with those with CCR0/1 resection (CCR0/1: 63.3% versus CCR2/3: 29.0%, p < 0.001, Fig. 2a). When applying the ICRS to the VC (n = 1029), 264 patients were included in analysis due to availability of variables in the US-HIPECC database. In the VC, increasing score group was associated with an increasing percentage of patients with CCR2/3 resection (zero: 2%, low: 12%, high: 27%, p = 0.001, Table 3). On univariate regression analysis, increasing score group was associated with increased odds of undergoing incomplete cytoreduction (zero: reference; low: OR 9.1, 95% CI 1.2–69.0, p = 0.033; high: OR 23.3, 95% CI 2.8–196.7, p = 0.004). When applying Kaplan–Meier analysis with the score groups to the VC, there was not a statistically significant association of survival with increasing score group (Fig. 2b).
FIG. 2.
Kaplan–Meier analysis for overall survival in the validation cohort stratified by a completeness of cytoreduction (CCR 0/1: n = 813, CCR 2/3: n = 120) and b score group within the incomplete-cytoreduction risk score (zero: n = 62, low: n = 175, high: n = 32)
DISCUSSION
This study presents a novel internally validated incomplete-cytoreduction risk score to predict the risk of incomplete cytoreduction (CCR2/3) in patients undergoing CRS with or without HIPEC. Using patient factors (male gender and presence of preoperative ascites) and preoperative serum tumor markers (CA-125 ≥ 40 U/mL and CEA ≥ 4.2 ng/mL), patients were scored on a scale from zero to four and it was found that our score stratified patients into three score groups to predict the risk of CCR2/3 resection. Use of this score may help improve patient selection for CRS/HIPEC and circumvent the major morbidity associated with the procedure.
For the purposes of this study, four major histologic tumor subtypes were grouped into a single cohort of patients with over 70% having higher-risk tumor histologic types (invasive appendiceal cancer, colorectal cancer, and peritoneal mesothelioma). Indeed, the use of CRS/HIPEC in the setting of peritoneal carcinomatosis for these malignancies has been well described previously,2,7–11,13,14,17,18,24–33 and regardless of histologic subtype, previous studies have reported that completeness of cytoreduction is a major prognostic factor following CRS/HIPEC.11–15 We recognize that grouping these histologic subtypes of tumors together when creating the ICRS may prompt hesitation in its clinical application given their diverse biologic behavior, but it is important to note that factors included in our score have been shown to serve as markers for advanced tumor burden in the setting of peritoneal carcinomatosis of appendiceal cancer, colorectal cancer, and peritoneal mesothelioma.24,34–39 Presence of ascites has different prognostic implications for noninvasive appendiceal neoplasms, where the character of ascites tends to be mucinous, versus the other histologies, where the character of ascites tends to be malignant. The US-HIPECC database does not differentiate between mucinous and malignant ascites preoperatively, as this is difficult to discern using cross-sectional imaging. Further, although noninvasive appendiceal cancer (LAMN/HAMN) is often treated as an entity separate from classic colorectal adenocarcinoma, in our cohort, 9% of patients with this histologic subtype had incomplete cytoreduction.13 Among these noninvasive appendiceal tumors only, all patients who had CCR2/3 resection fell into the high score group (3–4 points), thus suggesting utility of our ICRS even for noninvasive appendiceal tumors. Importantly, the presence of ascites in invasive appendiceal and colorectal cancer mirrored the incidence of incomplete cytoreduction. Lastly, although we did not conduct formal histology-specific analysis of the ICRS, we did account for histologic subtype within our multivariable model, where it was not independently associated with increased risk of incomplete cytoreduction. Future studies will be necessary to validate the ICRS within specific histologic subtypes.
Other groups have published scores to predict complete cytoreduction, though these scores are specific to a single histologic subtype of tumor. Dineen et al. published on the utility of the SPAAT Score, which relies on specific radiologic characteristics seen on cross-sectional imaging for patients with low-grade appendiceal mucinous cancers.21 Chesnias et al. also used radiographic findings to predict incomplete cytoreduction in the setting of ovarian carcinomatosis.19 Although these scores proved to be reliable in their respective studies, adding specific radiographic characteristics which require skilled, expert interpretation may introduce subjectivity to the risk score and prevent its broader applicability. Other groups have reported scores to predict complete cytoreduction for patients with ovarian cancer using data such as presence of ascites, tumor stage, and PCI.20,22 Again, although these scores do provide some utility, factors such as the PCI are most faithfully measured intraoperatively, thus limiting the use of these scores in the preoperative setting. When considering these other scores, we feel that the strength of the ICRS is its use of readily available preoperative data and laboratory measurements which are routinely drawn for patients undergoing CRS/HIPEC.
The potential clinical utility of the ICRS is fourfold. The score can primarily be used as a tool to preoperatively counsel patients on their individual risk of incomplete cytoreduction. This individually tailored approach to risk counseling is especially timely with the added emphasis on “personalized medicine” in the current era. Next, although we were unable to study this in a prospective fashion, the ICRS may be utilized to determine which patients should undergo diagnostic laparoscopy in an effort to visualize the burden of disease prior to laparotomy to determine the feasibility of cytoreduction and potentially spare the patient a nontherapeutic laparotomy. Third, the ICRS can be used to select patients for additional systemic therapy prior to HIPEC in an effort to reduce the burden of disease, which may aid in achieving complete cytoreduction. This necessitates prospective evaluation and is beyond the scope of the present study. Lastly, the ICRS may aid in patient selection for surgery in the case of patients with marginal functional status within the high-risk score group, as patients or surgeons may elect to avoid surgery if the risk of incomplete cytoreduction is substantially high. Although the ICRS is derived from the largest database of its kind published in literature thus far, with over 2000 patients, only 536 were available for final analysis due to the absence of data available for all four preoperative factors included within the score. Considering this limitation and the retrospective format of this study, we feel that the use of the ICRS alone to exclude potential candidates from surgery would not be an appropriate application of this score.
Other limitations include potential serum tumor marker measurement variations between the 12 institutions participating within the US-HIPECC, which may introduce variability in reported values. Surgical conduct and pathologic examination were not standardized across institutions, although all sites are considered high-volume centers for CRS/HIPEC within the USA. Lastly, the ICRS was designed strictly to predict CCR2/3 resection and not survival (Figs. 1b, 2b). The inability of our score groups to discriminate survival differences among the study cohort is likely due to mixed histologic types within each score group.
CONCLUSIONS
This study proposes a novel internally validated risk score to predict preoperatively which patients are more likely to undergo incomplete cytoreduction in CRS ± HIPEC. The score uses male gender, presence of ascites, CA-125 ≥ 40 U/mL, and CEA ≥ 4.2 ng/mL to score and then stratify patients into three score groups: zero (0 points), low (1–2 points), and high (3–4 points). Use of this risk score may help guide preoperative decision-making to help circumvent major morbidity associated with CRS/HIPEC and may also help improve patient selection and preoperative counseling for this procedure. Despite the association of the ICRS with incomplete cytoreduction, it should not be used in isolation to exclude patients from surgery.
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Emory University, The Ohio State University, University of California San Diego, University of Cincinnati, City of Hope National Medical Center, Johns Hopkins University, University of Massachusetts, Mayo Clinic, Medical College of Wisconsin, Moffitt Cancer Center, University of Texas MD Anderson Cancer Center, and University of Wisconsin.
REFERENCES
- 1.Glehen O, Mithieux F, Osinsky D, Beaujard AC, Freyer G,Guertsch PH, et al. Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: a phase II study. J Clin Oncol. 2003;21(5):799–806. [DOI] [PubMed] [Google Scholar]
- 2.Morano WF, Khalili M, Chi DS, Bowne WB, Esquivel J. Clinical studies in CRS and HIPEC: Trials, tribulations, and future directions-A systematic review. J Surg Oncol. 2018;117(2):245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugarbaker PH, Peritoneal metastases, a frontier for progress. Surg Oncol Clin N Am. 2018;27(3):413–424. [DOI] [PubMed] [Google Scholar]
- 4.Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010; 28(1):63–8. [DOI] [PubMed] [Google Scholar]
- 5.Kulu Y, Mueller-Stich B, Buechler MW, Ulrich A. Surgical treatment of peritoneal carcinomatosis: current treatment modalities. Langenbecks Arch Surg. 2014;399(1):41–53. [DOI] [PubMed] [Google Scholar]
- 6.Foster JM, Sleightholm R, Patel A, Shostrom V, Hall B, Neilsen B, et al. Morbidity and mortality rates following cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy compared with other high-risk surgical oncology procedures. JAMA Netw Open. 2019;2(1):e186847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scaringi S, Leo F, Canonico G, Batignani G, Ficari F, Tonelli F.The role of cytoreductive surgery alone for the treatment of peritoneal carcinomatosis of colorectal origin. A retrospective analysis with regard to multimodal treatments. Hepatogastroenterology. 2009;56(91–92):650–5. [PubMed] [Google Scholar]
- 8.Sugarbaker PH, Schellinx ME, Chang D, Koslowe P, von Meyerfeldt M., Peritoneal carcinomatosis from adenocarcinoma of the colon. World J Surg. 1996;20(5): 585–91; discussion 592. [DOI] [PubMed] [Google Scholar]
- 9.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H, 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–32. [DOI] [PubMed] [Google Scholar]
- 10.Verwaal VJ, van Ruth S, de Bree E, van Slooten GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20): 3737–43. [DOI] [PubMed] [Google Scholar]
- 11.Aziz O, Jaradat I, Chakrabarty B, Selvasekar CR, Fulford PE, Saunders MP, et al. Predicting survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for appendix adenocarcinoma. Dis Colon Rectum. 2018;61(7):795–802. [DOI] [PubMed] [Google Scholar]
- 12.Elias D, Laurent S, Antoun S, Duvillard P, Ducreux M, Pocard M, et al. Pseudomyxoma peritonei treated with complete resection and immediate intraperitoneal chemotherapy. Gastroenterol Clin Biol. 2003;27(4):407–12. [PubMed] [Google Scholar]
- 13.Kuncewitch M, Levine EA, Shen P, Votanopoulos KI. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for appendiceal tumors and colorectal adenocarcinomas. Clin Colon Rectal Surg. 2018;31(5):288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugarbaker PH, Chang D, Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6(8):727–31. [DOI] [PubMed] [Google Scholar]
- 15.Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18(6):1575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baratti D, Kusamura S, Cabras AD, Bertulli R, Hutanu I, Deraco M. Diffuse malignant peritoneal mesothelioma: long-term survival with complete cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Cancer. 2013;49(15):3140–8. [DOI] [PubMed] [Google Scholar]
- 17.Deraco M, Casali P, Inglese MG, Baratti D, Pennacchioli E, Bertulli R, et al. Peritoneal mesothelioma treated by induction chemotherapy, cytoreductive surgery, and intraperitoneal hyperthermic perfusion. J Surg Oncol. 2003;83(3):147–53. [DOI] [PubMed] [Google Scholar]
- 18.Gupta N, Asif S, Gandhi J, Rajpurohit S, Singh S. Role of CRS and HIPEC in appendiceal and colorectal malignancies: Indian experience. Indian J Gastroenterol. 2017;36(2): 126–130. [DOI] [PubMed] [Google Scholar]
- 19.Chesnais M, Lecuru F, Mimouni M, Ngo C, Fauconnier A, Huchon C. A pre-operative predictive score to evaluate the feasibility of complete cytoreductive surgery in patients with epithelial ovarian cancer. PLoS One. 2017;12(11):e0187245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowan RA, Eriksson AGZ, Jaber SM, Zhou Q, Iasonos A, Zivanovic O, et al. A comparative analysis of prediction models for complete gross resection in secondary cytoreductive surgery for ovarian cancer. Gynecol Oncol. 2017;145(2): 230–5. [DOI] [PubMed] [Google Scholar]
- 21.Dineen SP, Royal RE, Hughes MS, Sagebiel T, Bhosale P, Overman M, et al. A simplified preoperative assessment predicts complete cytoreduction and outcomes in patients with low-grade mucinous adenocarcinoma of the appendix. Ann Surg Oncol. 2015;22(11):3640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghisoni E, Katsaros D, Maggiorotto F, Aglietta M, Vaira M, De Simone M, et al. A predictive score for optimal cytoreduction at interval debulking surgery in epithelial ovarian cancer: a two-centers experience. J Ovarian Res. 2018;11(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terzi C, Arslan NC, Canda AE, Peritoneal carcinomatosis of gastrointestinal tumors: where are we now? World J Gastroenterol. 2014;20(39):14371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baratti D, Kusamura S, Martinetti A, Seregni E, Laterza B, Oliva DG, et al. Prognostic value of circulating tumor markers in patients with pseudomyxoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2007;14(8): 2300–8. [DOI] [PubMed] [Google Scholar]
- 25.Baumgartner JM, et al. Predictors of progression in high-grade appendiceal or colorectal peritoneal carcinomatosis after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2015;22(5):1716–21. [DOI] [PubMed] [Google Scholar]
- 26.Delhorme JB, Severac F, Averous G, Glehen O, Passot G, Bakrin N, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei of appendicular and extra-appendicular origin. Br J Surg. 2018;105(6):668–676. [DOI] [PubMed] [Google Scholar]
- 27.Govaerts K, Chandrakumaran K, Carr NJ, Cecil TD, Dayal S, Mohamed F, et al. Single centre guidelines for radiological follow-up based on 775 patients treated by cytoreductive surgery and HIPEC for appendiceal pseudomyxoma peritonei. Eur J Surg Oncol. 2018;44(9): 1371–7. [DOI] [PubMed] [Google Scholar]
- 28.Hesdorffer ME, Chabot JA, Keohan ML, Fountain K, Talbot S, Gabay M, et al. Combined resection, intraperitoneal chemotherapy, and whole abdominal radiation for the treatment of malignant peritoneal mesothelioma. Am J Clin Oncol. 2008;31(1): 49–54. [DOI] [PubMed] [Google Scholar]
- 29.Klaver CE, Musters GD, Bemelman WA, Punt CJ, Verwaal VJ, Dijkgraaf MG, et al. Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis; the COLOPEC randomized multicentre trial. BMC Cancer. 2015;15:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam JY, McConnell YJ, Rivard JD, Temple WJ, Mack LA. Hyperthermic intraperitoneal chemotherapy + early postoperative intraperitoneal chemotherapy versus hyperthermic intraperitoneal chemotherapy alone: assessment of survival outcomes for colorectal and high-grade appendiceal peritoneal carcinomatosis. Am J Surg. 2015;210(3):424–30. [DOI] [PubMed] [Google Scholar]
- 31.Loggie BW, Fleming RA, McQuellon RP, Russell GB. Prospective trial for the treatment of malignant peritoneal mesothelioma. Am Surg. 2001;67(10):999–1003. [PubMed] [Google Scholar]
- 32.Solomon D, DeNicola NL, Feferman Y, Bekhor E, Reppucci ML, Feingold D, et al. More synchronous peritoneal disease but longer survival in younger patients with carcinomatosis from colorectal cancer undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2019. [DOI] [PubMed] [Google Scholar]
- 33.Yan TD, Links M, Xu ZY, Kam PC, Glenn D, Morris DL. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei from appendiceal mucinous neoplasms. Br J Surg. 2006;93(10):1270–6. [DOI] [PubMed] [Google Scholar]
- 34.Alexander-Sefre F, Chandrakumaran K, Banerjee S, Sexton R, Thomas JM, Cecil T, et al. Elevated tumour markers prior to complete tumour removal in patients with pseudomyxoma peritonei predict early recurrence. Colorectal Dis. 2005;7(4):382–6. [DOI] [PubMed] [Google Scholar]
- 35.Bruno F, Baratti D, Martinetti A, Morelli D, Sottotetti E, Bonini C, et al. Mesothelin and osteopontin as circulating markers of diffuse malignant peritoneal mesothelioma: a preliminary study. Eur J Surg Oncol. 2018;44(6):792–8. [DOI] [PubMed] [Google Scholar]
- 36.Carmignani CP, Hampton R, Sugarbaker CE, Chang D, Sugarbaker PH. Utility of CEA and CA 19–9 tumor markers in diagnosis and prognostic assessment of mucinous epithelial cancers of the appendix. J Surg Oncol. 2004;87(4):162–6. [DOI] [PubMed] [Google Scholar]
- 37.Di Fabio F, Aston W, Mohamed F, Chandrakumaran K, Cecil T, Moran B. Elevated tumour markers are normalized in most patients with pseudomyxoma peritonei 7 days after complete tumour removal. Colorectal Dis. 2015;17(8):698–703. [DOI] [PubMed] [Google Scholar]
- 38.Taflampas P, Dayal S, Chandrakumaran K, Mohamed F, Cecil TD, Moran BJ. Pre-operative tumour marker status predicts recurrence and survival after complete cytoreduction and hyperthermic intraperitoneal chemotherapy for appendiceal pseudomyxoma peritonei: analysis of 519 patients. Eur J Surg Oncol. 2014;40(5):515–520. [DOI] [PubMed] [Google Scholar]
- 39.van Ruth S, Hart AA, Bonfrer JM, Verwaal VJ, Zoetmulder FA. Prognostic value of baseline and serial carcinoembryonic antigen and carbohydrate antigen 19.9 measurements in patients with pseudomyxoma peritonei treated with cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2002;9(10):961–7. [DOI] [PubMed] [Google Scholar]