Abstract
Circadian rhythms are biological oscillations that occur with an approximately 24 h period and optimize cellular homeostasis and responses to environmental stimuli. A growing collection of data suggests that chronic circadian disruption caused by novel lifestyle risk factors such as shift work, travel across time zones, or irregular sleep-wake cycles has long-term consequences for human health. Among the multiplicity of physiological systems hypothesized to have a role in the onset of pathologies in case of circadian disruption, there are redox-sensitive defensive pathways and inflammatory machinery. Due to its location and barrier physiological role, the skin is a prototypical tissue to study the influence of environmental insults induced OxInflammation disturbance and circadian system alteration. To better investigate the link among outdoor stressors, OxInflammation, and circadian system, we tested the differential responses of keratinocytes clock synchronized or desynchronized, in an in vitro inflammatory model exposed to O3. Being both NRF2 and NF-κB two key redox-sensitive transcription factors involved in cellular redox homeostasis and inflammation, we analyzed their activation and expression in challenged keratinocytes by O3. Our results suggest that a synchronized circadian clock not only facilitates the protective role of NRF2 in terms of a faster and more efficient defensive response against environmental insults but also moderates the cellular damage resulting from a condition of chronic inflammation. Our results bring new insights on the role of circadian clock in regulating the redox-inflammatory crosstalk influenced by O3 and possibly can be extrapolated to other pollutants able to affect the oxinflammatory cellular processes.
1. Introduction
Circadian rhythms are biological oscillations that occur with an approximately 24 h period and are synchronized to cyclic environmental cues, such as light-dark cycles and timing of food intake [1]. In mammals, the circadian system encompasses a central circadian pacemaker in the suprachiasmatic nucleus (SCN) of the hypothalamus which coordinates autonomous peripheral oscillators, localized in almost all tissues and organs, including the liver, heart, skeletal muscle, and skin [2–5]. Functional clock in each of these tissues optimizes cellular homeostasis and responses to environmental stimuli [6]. At the cellular level, the clock is defined as a transcriptional and translational feedback loop oscillator. The core loop consists of the CLOCK:BMAL1 heterodimer, which activates the transcription of period (PER) and cryptochrome (CRY). PER and CRY proteins form heterodimeric complexes that inhibit their own transcription by suppressing the activity of CLOCK:BMAL1 [7]. The molecular clock drives intrinsic daily rhythms of physiology and behavior. The coordinated activity of central and peripheral oscillators can be referred to as the circadian timing system. A growing collection of data suggests that chronic circadian disruption caused by novel lifestyle risk factors such as shift work, travel across time zones, or irregular sleep-wake cycles has long-term consequences for human health [8, 9], causing cellular dysfunction and chronic diseases. Among the multiplicity of physiological systems hypothesized to have a role in the onset of pathologies in case of circadian disruption, there are redox-sensitive defensive pathways and inflammatory machinery [10, 11]. The effects of dysregulated circadian system on cellular defensive enzyme activities were previously reported, and recent investigations have further elucidated the molecular mechanisms that are possibly the link between the circadian clock and the maintenance of cellular redox homeostasis [12]. Moreover, a growing collection of data focus on the deep interconnection between altered redox homeostasis and inflammatory pathways (OxInflammation), since it seems that the imbalance of those two critical pathways can contribute to initiation, development, and progression of several disorders [13–16].
Due to its location and barrier physiological role, the skin is a prototypical tissue to study the influence of environmental insults induced OxInflammation disturbance and circadian system alteration. There are a lot of data suggesting the correlation between chronic and relapsing cutaneous inflammatory diseases, such as psoriasis and atopic dermatitis, and aberrant circadian system [17–19]. Moreover, a growing set of data [2, 20–23] suggests a clear correlation between the cellular circadian system and oxidative stress. Concerning the skin, our group has previously demonstrated that in human keratinocytes, which comprise ~95% of the cells within the epidermis, endogenous circadian clock is involved in the cellular response to oxidative stress. In particular, after the exposure to ozone (O3), one of the most toxic outdoor pollutants, the clock-synchronized keratinocytes exhibit a more efficient antioxidant response compared to arrhythmic ones, attested by a quicker activation of the master defensive cellular transcription factor, nuclear factor erythroid 2-related factor 2 (NRF2) [12].
Starting from these evidences, to further investigate the link between circadian system, redox homeostasis, and inflammation, we envisage a scenario in which we tested the differential response of clock-synchronized or clock-desynchronized keratinocytes, in human keratinocytes exposed to O3 insult in the presence of LPS.
It is well demonstrated that occurrence of oxidative stress contributes to generate an induction of proinflammatory status [24–26], so we hypothesized that circadian deregulation could compromise the crosstalk between these two pathways.
Since NRF2 and NF-κB are the two key transcription factors that regulate cellular responses to oxidative stress and inflammation, we further analyzed the crosstalk between the two transcription factors in the presence of different challenges such as O3 and LPS.
A better understanding of the interactions between the circadian system and the cell physiological pathways is required to better apprehend the role of the clock in pathological development.
2. Materials and Methods
2.1. Cell Culture
HaCaT cell line (obtained from American Type Culture Collection, ATCC) was cultured in DMEM (Lonza, Milan, Italy) supplemented with 10% fetal bovine serum (FBS, EuroClone, Milan, Italy), 1% of L-glutamine (Lonza, Milan, Italy), and 1% of penicillin/streptomycin antibiotics (Lonza, Milan, Italy) at 37°C in 5% CO2. To induce chronic inflammation, cells were seeded into 6 well-plates, cultured to 60-70% confluence, and exposed to lipopolysaccharide (LPS) 0.5 or 1 μM; to synchronize the circadian clock, cells were then treated with 1 μM dexamethasone (dex, Sigma-Aldrich, Hamburg, Germany) for 1 hour.
Ozone treatment was performed in previously LPS-treated-synchronized and control cells 18 hours after dex treatment in accordance with our previous work [12].
2.2. Ozone Exposure
O3 was generated from O2 by electrical corona arc discharge (ECO3 model CUV-01, Torino, Italy), as previously described [27]. The O2–O3 mixture (95% O2, 5% O3) was combined with ambient air and allowed to flow into a Teflon-lined exposure chamber, with the O3 concentration in chamber adjusted to the ppm needed for the experiment and continuously monitored by an O3 detector. Temperature and humidity were monitored during exposures (37°C and 45–55%, respectively).
2.3. Total Protein Extraction
Cells were seeded in 60 mm petri (1.5 × 106 cells). After treatments, cells were detached and washed twice with ice-cold PBS 1x and total cell lysates were extracted in ice-cold solubilization buffer containing 20 mM Tris pH 8, 150 mM NaCl, 1% Triton X-100, 1 mM sodium orthovanadate, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 μg/ml pepstatin, 10 μg/ml phenylmethylsulfonyl fluoride (PMSF), and 5 mM β-glycerophosphate (Sigma, Milan, Italy). After centrifugation (15 000 × g, 15 minutes at 4°C), the supernatants were collected. Protein concentrations were determined using the Bio-Rad protein assay kit (Bio-Rad, Milan, Italy).
2.4. Western Blot Analysis
After protein quantification, 40 μg boiled proteins were loaded into 10% sodium dodecyl sulphate-polycrylamide electrophoresis gels and separated by molecular size. Gels were electroblotted onto nitrocellulose membranes and then blocked for 90 minutes in Tris-buffered saline, pH 7.5, containing 0.5% Tween 20 and 5% (w/v) skim milk powder. Membranes were incubated overnight at 4°C with the appropriate primary antibodies: NRF2 diluted 1 : 1000 (ABE413, Millipore, Billerica, Massachusetts) and NF-κB-p65 diluted 1 : 1000 (sc372 Santa Cruz). The membranes were finally incubated with the peroxidase-conjugated secondary anti-Rabbit antibody (1 : 5000) for 1 hour. The bound antibodies were detected by chemiluminescence (Bio-Rad, Milan, Italy). β-Actin was used as loading control. Images of the bands were digitized using an Epson Stylus SX405 scanner, and the densitometry analysis was performed using ImageJ software.
2.5. Immunocytochemistry
Human keratinocytes were grown on coverslips at a density of 1 × 105 cells/ml and after treatment fixed in 4% paraformaldehyde for 30 min at room temperature as previously described [28]. Cells were permeabilized for 5 min at RT with PBS containing 0.2% Triton X-100, blocked with 1% BSA in PBS at RT for 1 h, and then incubated with primary antibody (NF-κB-p65, 1 : 100, sc372 Santa Cruz; NRF2 1 : 200 ABE413 Millipore) in PBS containing 0.5% BSA at 4°C overnight. After washing, coverslips were incubated with appropriate secondary antibody for 1 h at RT. Nuclei were stained with 1 μg/ml DAPI (4′,6-diamidine-2′-phenylindole dihydrochloride, Sigma-Aldrich, Italy) for 1 min. Coverslips were mounted onto glass slides using antifade mounting medium 1,4 diazabicyclooctane (DABCO) in glycerin. Negative controls for the experiments were performed by omitting primary antibodies. Images were acquired and analyzed with Leica AF CTR6500HS (Microsystems) and analyzed with CellProfiler software.
2.6. NRF2 DNA-Binding Activity
NRF2 to DNA ARE were evaluated using the “TransAM Nrf2” ELISA kit (Active Motif, USA). NRF2 protein presented in cellular extract was incubated with oligonucleotides containing ARE sequencing, immobilized on a 96-well plate. A secondary antibody conjugated with a horseradish peroxidase provides a colorimetric output spectrophotometrically detected at 450 nm.
2.7. RNA Extraction and Gene Expression Analysis
Total RNA was isolated from confluent cells using TRIzol Reagent (Invitrogen), according to the manufacturer's protocol. The amount and quality of isolated RNA were analyzed by BioSpec-nano (Shimadzu, Kyoto, Japan). One microgram of DNase-treated RNA was used to perform cDNA synthesis, using the iScript cDNA Synthesis Kit (Bio-Rad, Milan, Italy). First-strand cDNA was PCR amplified with a CFX Real-Time PCR Detection System (Bio-Rad, Milan, Italy) using SsoFast EvaGreen Supermix (Bio-Rad Laboratories, Hercules, CA, USA). After amplification, a melting curve analysis to confirm the specificity of the amplicons was performed. We used the gene-specific primers for human Ho1, Nqo1, Il1β, I11α, Il6, Il8, and 18 s (Table 1).
Table 1.
| Ho1 | Forward: TTGCTTTGGCGAGCTCTTTT Reverse: TCTGATGCCAAACACCCCA |
|
| |
| Nqo1 | Forward: CTGATCGTACTGGCTCACTC Reverse: AACAGACTCGGCAGGATAC |
|
| |
| Il1β | Forward: ACA GAT GAA GTG CTC CTT CCA Reverse: GTC GGA GAT TCG TAG CTG GAT |
|
| |
| Il1α | Forward: GGAGCTTGTCACCCCAAACT Reverse: TCCGAAGTCAAGGGGCTAGA |
|
| |
| Il6 | Forward: GTAGCCGCCCCACACAGA Reverse: TCTGAGGTGCCCATGCTAC |
|
| |
| Il8 | Forward: GGTGCAGTTTTGCCAAGGAG Reverse: TTCCTTGGGGTCCAGACAGA |
| 18S | Forward: CGAGCCGCCTGGATACC Reverse: CATGGCCTCAGTTCCGAAAA |
The relative levels of each sample were calculated by the 2–ΔΔCT method (where CT is the cycle number at which the signal reaches the threshold of detection) [29]. Each CT value used for these calculations is the mean of three replicates of the same reaction.
2.8. Statistical Analysis
All the results were expressed as means ± SEM. Treatments, sampling time, and their interaction were tested by parametric (one-way ANOVA and unpaired t-test) and nonparametric (Mann-Whitney U-test) tests. Normality was tested by the Kolmogorov–Smirnov test (P < 0.05). Tukey's Multiple Comparison Test was applied as the post hoc test. P values < 0.05 were considered statistically significant. Data were analyzed using the software GraphPad Prism 4.0 (GraphPad Software, Inc., La Jolla, CA).
3. Results and Discussion
On the basis of our previous results [12] showing the association between circadian system and oxidative stress, we explored the crosstalk between NRF2 and NF-κB in clock-synchronized and arrhythmic human keratinocytes exposed to O3.
By Western blot analysis, as shown in Figure 1, we observed a significant increase in NF-κB levels (+33%, P < 0.014, unpaired t-test) right after 30 min of O3 exposure in synchronized cells with respect to the arrhythmic keratinocytes. On note, at 2 hr time point, this effect was completely abolished in unsynchronized cells while was still evident in the synchronized ones. This result indicated that the response to O3 in synchronized cells is more long lasting, as suggested by the higher level of total NF-κB. This data further indicated the possibility that the cells are more prone to reactivate NF-κB (nuclear translocation) in the eventuality of a further challenge. Indeed at this time, it is not possible to discriminate between NF-κB nuclear and cytoplasmic levels.
Figure 1.
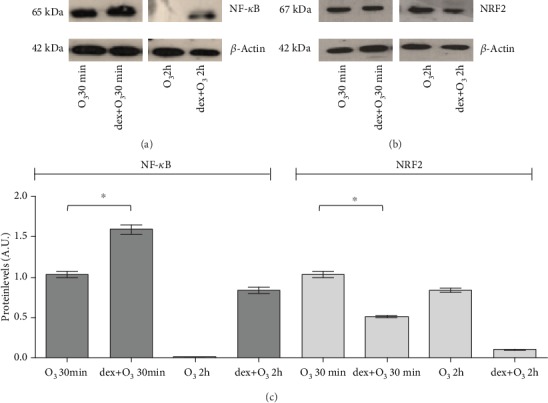
Expression of NRF2 and NF-κB proteins in HaCaT cells exposed to ozone or dex+O3. Samples were harvested at two different time points (30 minutes and 2 hours after the treatments), and the protein expression was measured by Western blot. Representative Western blot of 3 different experiments is depicted in (a) and (b). Quantification of the NRF2 and NF-κB bands is shown in (c). Data are expressed as mean ± SEM from three independent experiments. β-Actin was used as a loading control. ∗P < 0.05.
As the literature strongly indicate the crosstalk between NF-κB and NRF2, we wanted to also determine the effect of O3 and the synchronization on its levels. As depicted in Figure 1(b), NRF2 levels were significantly increased by O3 exposure at earlier time point (30 min) in dex-treated cells with respect to the keratinocytes unsynchronized (+47% P < 0.0085, unpaired t-test). Of note, this trend was also noticed at later time point up to 2 hr. These results suggest that clock synchronization provides a more prompt ability of the cells to increase NRF2 confirming what has been already shown in previous work by Benedusi et al. [12].
Comparing the NF-κB and NRF2 plots, it is easy to observe an opposite trend among the two transcription factors that confirms their interdependency. At this stage, we did not evaluate the nuclear levels of NF-κB and NRF2 because the intent of this study is to understand the role that an inflammatory stimulus (LPS treatment) has on circadian clock modulation of antioxidant pathway in response to O3 insult. Indeed, this issue is shown and discussed below (Figure 2).
Figure 2.
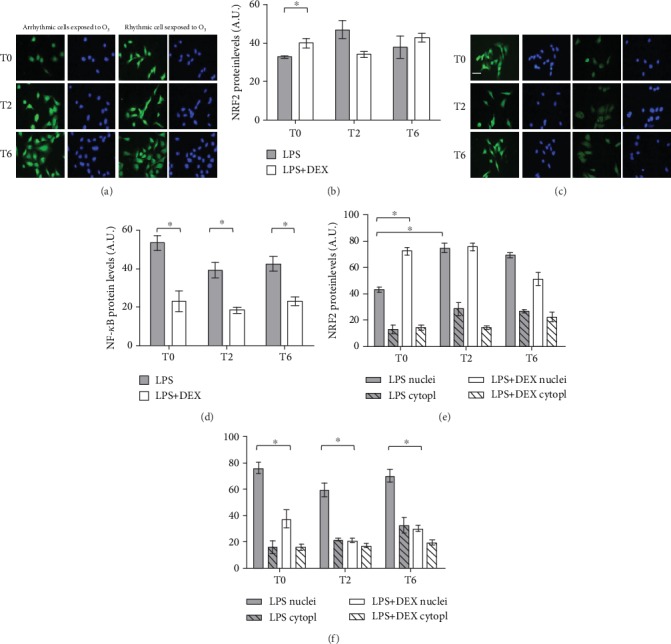
Immunofluorescence for NRF2 and NF-κB in dex-synchronized HaCaT cells and arrhythmic ones chronically inflamed with LPS 1 μg/ml and exposed to O3. Representative pictures of three different experiments are presented. Nuclei (blue) were stained with DAPI. Magnification 40x, scale bar = 50 μm. Samples were harvested at different time points (0, 2, and 6 hours after ozone exposure). Quantification of fluorescence in total HaCaT cells (b, d), cytoplasm, and nucleus fractions (e, f) of NRF2 and NF-κB proteins is shown. Data are expressed as mean ± SEM from three independent experiments. ∗P < 0.05.
To test the appropriate doses of LPS to be used in our experimental protocol, keratinocytes were treated with 0.5 and 1 μM of LPS for 24 hours and cytotoxicity was then evaluated by lactate dehydrogenase (LDH) release assay, in synchronized and arrhythmic cells. No significant cytotoxicity was observed at 0.5 and 1 μM of LPS (data not shown). These data prompted us to use 1 μM as a dose of LPS treatment to induce chronical inflammation without irreversibly damage the cells.
To better elucidate the interplay mechanisms between circadian clock, NRF2, and NF-κB pathways, we induced chronic inflammation in keratinocytes by LPS treatment, and we then analyzed the inflammatory and antioxidant pathways upon acute O3 exposure in clock-synchronized and clock-desynchronized cells.
Since dex used for cell clock synchronization is a steroidal anti-inflammatory drug, we first confirmed that 1-hour dex-treatment on keratinocytes does not significantly alter the inflammatory pathway in terms of NF-κB activation (data not shown). This result is in accordance with previous study [30] where it has been demonstrated that exposure of cutaneous cells to dex induced the transient translocation of NF-κB from the cytoplasm to the nucleus. These changes were transitory, as in cells treated with dex for 1 h, the protein rapidly returned to its cytoplasmic location.
It is well documented that O3 insult in keratinocytes induces cellular defensive mechanisms by the activation of NRF2 [31–33]. Moreover, our previous results demonstrated that in clock entrained cells, the level of NRF2 was significantly lower than in arrhythmic keratinocytes after exposure to 0.2 ppm O3 [12], suggesting a less efficient antioxidant response compared to the entrained cells. Since we observed a specular trend in the NF-κB expression level, we analyzed the crosstalk of the three pathways in entrained and arrhythmic chronically inflamed HaCaT cells after O3 insult.
By immunocytochemistry (Figures 2(a) and 2(b)), we observed an evident increase in the total NRF2 protein level in entrained cells (P = 0.0226; unpaired t-test) immediately after O3 exposure (T0). Conversely, a lower expression of total NF-κB (Figures 2(c) and 2(d)) at all time points was detected in inflamed-synchronized cells, compared to arrhythmic ones (P < 0.003; unpaired t-test).
As both NRF2 and NF-κB mediate the transcriptional response of cells once activated (nuclear levels) [34], we next quantified their subcellular localization after O3 exposure in inflamed-synchronized and desynchronized cells. As expected, nuclear protein levels of both NRF2 and NF-κB raised up immediately after the insult compared to cytoplasm compartment; however, immunofluorescence staining revealed that (Figure 2(e)) NRF2 protein was significantly higher in the nuclear compartment in rhythmic cells with respect to arrhythmic ones (+35% T0; P < 0.007, Mann-Whitney U-test). On the other hand, in arrhythmic cells, NRF2 nuclear translocation reached its maximum level 2 hours later, suggesting a slower and less efficient response (+37% from T0 to T2; P < 0.008, Mann-Whitney U-test). Moreover, in desynchronized cells, we observed a NF-κB higher nuclear level right after O3 insult, when compared to entrained cells (+42%; P < 0.01, Mann-Whitney U-test). This intracellular localization trend of NF-κB was maintained at T2 and T6 (P < 0.01, Mann-Whitney U-test). Taken together, these results underpin the mutual interplay of these two pathways under oxinflammatory conditions.
It is well established that the cellular actions of NRF2 and NF-κB signaling pathways are in opposition [35–37], with reciprocal inhibition mechanistic relationship, but the principal modulator that plays a pivotal role is yet to be defined. Mimicking a scenario of chronic inflammation and circadian deregulation, we speculated that keratinocytes exposed to acute oxidative stress displayed slower defensive response in terms of nuclear translocation of NRF2. Within the nucleus, NRF2 exerts its transcriptional function by binding to the antioxidant response element (ARE 5′-TGACXXXGC-3′) in the promoter region of NRF2 antioxidant target genes (such as NAD(P)H:quinone oxidoreductase1, NQO1, or heme oxygenase 1, HO1) [38].
A TransAM ELISA was performed to endorse the DNA-binding activity of NRF2 upon O3 insult. As shown in Figure 3, immediately after O3 exposure, the DNA-binding activity of NRF2 was higher in synchronized cells with respect to arrhythmic keratinocytes (+37% vs. arrhythmic cells; P = 0.001, unpaired t-test). Differently, in desynchronized cells, DNA-binding activity increased at T2 (+50% vs. T0; P < 0.001, unpaired t-test). At T6, in entrained cells, NRF2 DNA-binding activity declined much faster than in arrhythmic cells, indicating a more efficient antioxidant response in chronically inflamed dex-synchronized human keratinocytes.
Figure 3.
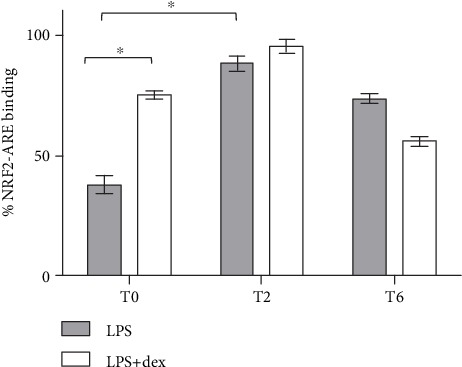
NRF2 binding to DNA antioxidant response element (ARE) in dex-synchronized HaCaT cells and arrhythmic ones chronically inflamed with LPS 1 μg/ml and exposed to O3. Samples were harvested at different time points (0, 2, and 6 hours after ozone exposure). Data are presented as mean ± SEM of three independent experiments in triplicate. ∗P < 0.05.
In this light, as displayed in Figure 4, we explored the expression of NRF2-depending phase II enzymes after O3 exposure in chronically inflamed cells by analyzing the levels of Ho-1 and Nqo1 in entrained and arrhythmic conditions.
Figure 4.

Relative expression (in percentage) of Ho1 (a) and Nqo1 (b) mRNA in dex-synchronized HaCaT cells and arrhythmic ones chronically inflamed with LPS 1 μg/ml and exposed to O3 was determined using qRT-PCR. Samples were harvested at different time points (0, 2, and 6 hours after ozone exposure). For all genes, the constitutively expressed 18S rRNA was used to normalization. For each time point, the mean ± SEM of three independent experiments is shown. ∗P < 0.05.
qRT-PCR analysis of phase II enzyme expression revealed that both genes were strongly induced 2 hours after O3 insult (+60% for Ho1 and +70% for Nqo1; P < 0.005 T2 vs. T0, unpaired t-test) in synchronized keratinocytes. By contrast, neither Ho1 nor Npqo1 gene expression resulted a significant effect after O3 exposure in arrhythmic cells. These results are in agreement with our previous observation of NRF2 nuclear translocation and DNA-binding activity immediately after O3 insult in rhythmic cells.
It is well known that Ho1 have significant anti-inflammatory effects mediated by NRF2 as it has been demonstrated in mouse myoblasts exposed to H2O2 [39] and in mouse peritoneal macrophages treated with LPS [40]. Starting from this knowledge, we analyzed the expression of genes involved in the inflammatory response induced by the activation of NF-κB.
As shown in Figure 5, Il1β, Il8, and Il1α mRNA levels did not change over the different time points neither in rhythmic nor in arrhythmic cells. Intriguingly, Il6 expression profile increases significantly from T0 to T6 in chronically inflamed asynchronous cells exposed to O3 (P = 0.0061; one-way ANOVA), while no differences were observed in entrained cells.
Figure 5.
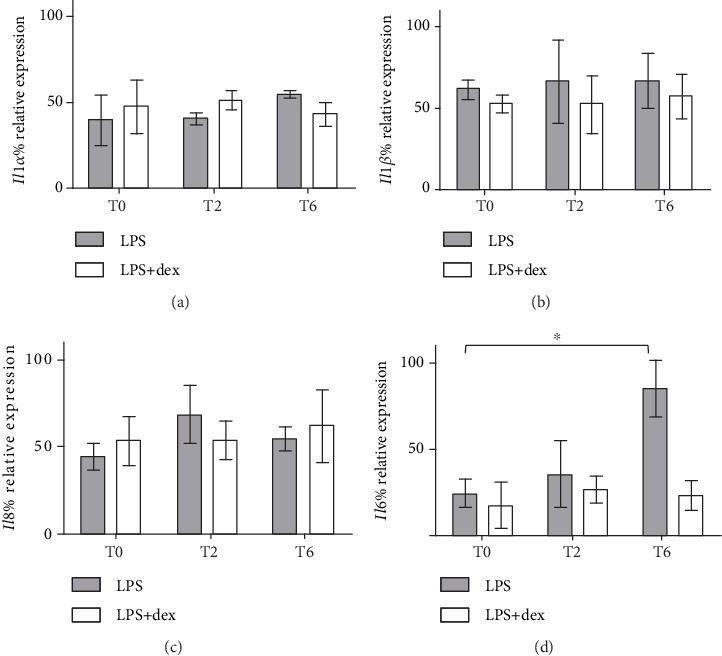
Relative expression (in percentage) of Il1α (a), Il1β (b), Il8 (c), and Il6 (d) mRNA in dex-synchronized HaCaT cells and arrhythmic ones chronically inflamed with LPS 1 μg/ml and exposed to O3 was determined using qRT-PCR. Samples were harvested at different time points (0, 2, and 6 hours after ozone exposure). For all genes, the constitutively expressed 18S rRNA was used to normalization. For each time point, the mean ± SEM of three independent experiments is shown. ∗P < 0.05.
Recent work indicates that circadian and immune functions are highly interconnected [41]. However, the consequences of a disrupted circadian environment for proper immune functions remain unclear. Moreover, IL-6 is a key signal that mediates mutual feedback interactions between inflammation and modulation of peripheral circadian clocks [42]. Our data falls into this picture suggesting that keratinocytes exhibit LPS-induced IL-6 release after oxidative challenge, when the circadian rhythm is disrupted.
In summary (Figure 6), in this experimental scenario, we suggest that a synchronized circadian clock not only facilitates the protective role of NRF2 in terms of a faster and more efficient antioxidant response against environmental insult but also moderates the cellular damage resulting from a condition of chronic inflammation.
Figure 6.
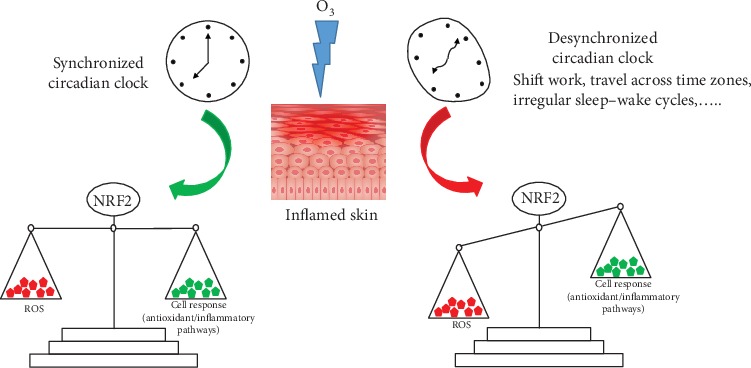
Schematic summary of the discussion and conclusion sections. We suggest the possible role of the circadian clock in O3-induced skin inflammation. In particular, we propose that a synchronized circadian clock facilitates the protective role of NRF2 in terms of a more efficient antioxidant response against environmental insult and of a regulation of the cellular damage resulting from chronic inflammation.
4. Conclusions
The general involvement of aberrant circadian clock has been linked to the development or at least the progression of several pathologies [8, 9]. Preliminary studies have suggested the role of clock synchronization in protecting the skin from exogenous source damage [12]. In particularly, several proinflammatory cutaneous conditions (such as psoriasis, eczema, cutaneous rushes, and atopic dermatitis) have been attributed to outdoor stressor exposure such as O3 [43]. The role of O3 exposure either in developing an inflammatory process or in progressing an inflammation already present is still under investigation although its connection with proinflammatory mediators has been well demonstrated [27]. The present study intends to suggest the possible role of the circadian clock in O3-induced skin inflammation. It is also possible that those results can be extrapolated to other pollutants such as cigarette smoke and particulate matters, as they have been demonstrated to have similar mechanisms of action at the cutaneous levels [44].
Acknowledgments
We thank Andrea Margutti for technical assistance. This work was funded by the University of Ferrara (Italy) to CB (FAR2018-2019) and GV (FAR2018-2019).
Data Availability
the data used to support the findings of this study are available from the corresponding authors upon request.
Disclosure
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
MB and EF conceived and designed the experiments; MB, EF, and AG performed the experiments; MB and EF analyzed the data; CB and GV contributed to reagents, materials, and analysis tools. MB, EF, CB, and GV wrote the manuscript. Elena Frigato and Mascia Benedusi equally contributed to the manuscript. Cristiano Bertolucci and Giuseppe Valacchi equally supervised the work.
References
- 1.Rouyer F. Clock genes: from Drosophila to humans. Bulletin de l'Académie Nationale de Médecine. 2015;7:1115–1131. [PubMed] [Google Scholar]
- 2.Ndiaye M. A., Nihal M., Wood G. S., Ahmad N. Skin, reactive oxygen species, and circadian clocks. Antioxidants & Redox Signaling. 2014;20(18):2982–2996. doi: 10.1089/ars.2013.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harfmann B. D., Schroder E. A., Esser K. A. Circadian rhythms, the molecular clock, and skeletal muscle. Journal of Biological Rhythms. 2015;30(2):84–94. doi: 10.1177/0748730414561638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato S., Solanas G., Peixoto F. O., et al. Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell. 2017;170(4):664–677.e11. doi: 10.1016/j.cell.2017.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyons A. B., Moy L., Moy R., Tung R. Circadian rhythm and the skin: a review of the literature. The Journal of Clinical and Aesthetic Dermatology. 2019;12:42–45. [PMC free article] [PubMed] [Google Scholar]
- 6.Tahara Y., Aoyama S., Shibata S. The mammalian circadian clock and its entrainment by stress and exercise. The Journal of Physiological Sciences. 2017;67(1):1–10. doi: 10.1007/s12576-016-0450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi G., Xie P., Qu Z., et al. Distinct roles of HDAC3 in the core circadian negative feedback loop are critical for clock function. Cell Reports. 2016;14(4):823–834. doi: 10.1016/j.celrep.2015.12.076. [DOI] [PubMed] [Google Scholar]
- 8.Gamble K. L., Resuehr D., Johnson C. H. Shift work and circadian dysregulation of reproduction. Frontiers in Endocrinology. 2013;4:p. 92. doi: 10.3389/fendo.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Touitou Y., Reinberg A., Touitou D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: health impacts and mechanisms of circadian disruption. Life Sciences. 2017;173:94–106. doi: 10.1016/j.lfs.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Polidarová L., Houdek P., Sumová A. Chronic disruptions of circadian sleep regulation induce specific proinflammatory responses in the rat colon. Chronobiology International. 2017;34(9):1273–1287. doi: 10.1080/07420528.2017.1361436. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y., Lv D., Liu W., et al. Disruption of the circadian clock alters antioxidative defense via the SIRT1-BMAL1 pathway in 6-OHDA-induced models of parkinson’s disease. Oxidative Medicine and Cellular Longevity. 2018;2018:11. doi: 10.1155/2018/4854732.4854732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedusi M., Frigato E., Beltramello M., Bertolucci C., Valacchi G. Circadian clock as possible protective mechanism to pollution induced keratinocytes damage. Mechanisms of Ageing and Development. 2018;172:13–20. doi: 10.1016/j.mad.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Valacchi G., Virgili F., Cervellati C., Pecorelli A. OxInflammation: from subclinical condition to pathological biomarker. Frontiers in Physiology. 2018;9:p. 858. doi: 10.3389/fphys.2018.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain T., Tan B., Yin Y., Blachier F., Tossou M. C. B., Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxidative Medicine and Cellular Longevity. 2016;2016:9. doi: 10.1155/2016/7432797.7432797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newsholme P., Cruzat V. F., Keane K. N., Carlessi R., de Bittencourt P. I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. The Biochemical Journal. 2016;473(24):4527–4550. doi: 10.1042/BCJ20160503C. [DOI] [PubMed] [Google Scholar]
- 16.Cordone V., Pecorelli A., Benedusi M., et al. Antiglycative activity and RAGE expression in Rett syndrome. Cells. 2019;8(2):p. 161. doi: 10.3390/cells8020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelfant S., Ozawa A., Chalker D. K., Smith J. G., Jr. Circadian rhythms and differences in epidermal and in dermal cell proliferation in uninvolved and involved psoriatic skin in vivo. The Journal of Investigative Dermatology. 1982;78(1):58–62. doi: 10.1111/1523-1747.ep12497933. [DOI] [PubMed] [Google Scholar]
- 18.Mozzanica N., Tadini G., Radaelli A., et al. Plasma melatonin levels in psoriasis. Acta Dermato-Venereologica. 1988;68(4):312–316. [PubMed] [Google Scholar]
- 19.Bacaksiz A., Akif Vatankulu M., Sonmez O., et al. Non-dipping nocturnal blood pressure in psoriasis vulgaris. Wiener Klinische Wochenschrift. 2012;124(23-24):822–829. doi: 10.1007/s00508-012-0294-y. [DOI] [PubMed] [Google Scholar]
- 20.Wilking M., Ndiaye M., Mukhtar H., Ahmad N. Circadian rhythm connections to oxidative stress: implications for human health. Antioxidants & Redox Signaling. 2013;19(2):192–208. doi: 10.1089/ars.2012.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamaru T., Hattori M., Ninomiya Y., et al. ROS stress resets circadian clocks to coordinate pro-survival signals. PLoS One. 2013;8(12, article e82006) doi: 10.1371/journal.pone.0082006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verma A. K., Singh S., Rizvi S. I. Redox homeostasis in a rodent model of circadian disruption: effect of melatonin supplementation. General and Comparative Endocrinology. 2019;280:97–103. doi: 10.1016/j.ygcen.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Sutton E. F., Beyl R., Early K. S., Cefalu W. T., Ravussin E., Peterson C. M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metabolism. 2018;27(6):1212–1221.e3. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valacchi G., Pecorelli A., Cervellati C., Hayek J. 4-hydroxynonenal protein adducts: key mediator in Rett syndrome oxinflammation. Free Radical Biology & Medicine. 2017;111:270–280. doi: 10.1016/j.freeradbiomed.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 25.Ramos-Tovar E., Muriel P. Free radicals, antioxidants, nuclear factor-E2-related factor-2 and liver damage. Journal of Applied Toxicology. 2019;40(1):151–168. doi: 10.1002/jat.3880. [DOI] [PubMed] [Google Scholar]
- 26.Maamoun H., Benameur T., Pintus G., Munusamy S., Agouni A. Crosstalk between oxidative stress and endoplasmic reticulum (ER) stress in endothelial dysfunction and aberrant angiogenesis associated with diabetes: a focus on the protective roles of heme oxygenase (HO)-1. Frontiers in Physiology. 2019;10:p. 70. doi: 10.3389/fphys.2019.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valacchi G., Pecorelli A., Belmonte G., et al. Protective effects of topical vitamin C compound mixtures against ozone-induced damage in human skin. The Journal of Investigative Dermatology. 2017;137(6):1373–1375. doi: 10.1016/j.jid.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 28.Romani A., Cervellati C., Muresan X. M., et al. Keratinocytes oxidative damage mechanisms related to airbone particle matter exposure. Mechanisms of Ageing and Development. 2018;172:86–95. doi: 10.1016/j.mad.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Livak K. J., Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Castro-Caldas M., Mendes A. F., Carvalho A. P., Duarte C. B., Lopes M. C. Dexamethasone prevents interleukin-1beta-induced nuclear factor-kappaB activation by upregulating IkappaB-alpha synthesis, in lymphoblastic cells. Mediators of Inflammation. 2003;12(1):37–46. doi: 10.1080/0962935031000096953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pecorelli A., Bocci V., Acquaviva A., et al. NRF2 activation is involved in ozonated human serum upregulation of HO-1 in endothelial cells. Toxicology and Applied Pharmacology. 2013;267(1):30–40. doi: 10.1016/j.taap.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Valacchi G., Sticozzi C., Belmonte G., et al. Vitamin C compound mixtures prevent ozone-induced oxidative damage in human keratinocytes as initial assessment of pollution protection. PLoS One. 2015;10(8, article e0131097) doi: 10.1371/journal.pone.0131097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canella R., Benedusi M., Martini M., Cervellati F., Cavicchio C., Valacchi G. Role of Nrf2 in preventing oxidative stress induced chloride current alteration in human lung cells. Journal of Cellular Physiology. 2018;233(8):6018–6027. doi: 10.1002/jcp.26416. [DOI] [PubMed] [Google Scholar]
- 34.Buelna-Chontal M., Zazueta C. Redox activation of Nrf2 & NF-κB: a double end sword? Cellular Signalling. 2013;25(12):2548–2557. doi: 10.1016/j.cellsig.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Kauppinen A., Suuronen T., Ojala J., Kaarniranta K., Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cellular Signalling. 2013;25(10):1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Liu G. H., Qu J., Shen X. NF-κB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2008;1783(5):713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Chen H., Fang Y., Li W., Orlando R. C., Shaheen N., Chen X. L. NFkB and Nrf2 in esophageal epithelial barrier function. Tissue Barriers. 2014;1(5, article e27463) doi: 10.4161/tisb.27463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirotsu Y., Katsuoka F., Funayama R., et al. Nrf2-MafG heterodimers contribute globally to antioxidant and metabolic networks. Nucleic Acids Research. 2012;40(20):10228–10239. doi: 10.1093/nar/gks827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi Y. H. Berberine hydrochloride protects C2C12 myoblast cells against oxidative stress-induced damage via induction of Nrf-2-mediated HO-1 expression. Drug Development Research. 2016;77(6):310–318. doi: 10.1002/ddr.21325. [DOI] [PubMed] [Google Scholar]
- 40.Kuhn A. M., Tzieply N., Schmidt M. V., et al. Antioxidant signaling via Nrf2 counteracts lipopolysaccharide-mediated inflammatory responses in foam cell macrophages. Free Radical Biology & Medicine. 2011;50(10):1382–1391. doi: 10.1016/j.freeradbiomed.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 41.Scheiermann C., Kunisaki Y., Frenette P. S. Circadian control of the immune system. Nature Reviews Immunology. 2013;13(3):190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams K. L., Castanon-Cervantes O., Evans J. A., Davidson A. J. Environmental circadian disruption elevates the IL-6 response to lipopolysaccharide in blood. Journal of Biological Rhythms. 2013;28(4):272–277. doi: 10.1177/0748730413494561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu F., Yan S., Wu M., et al. Ambient ozone pollution as a risk factor for skin disorders. The British Journal of Dermatology. 2011;165(1):224–225. doi: 10.1111/j.1365-2133.2011.10349.x. [DOI] [PubMed] [Google Scholar]
- 44.Pecorelli A., Woodby B., Prieux R., Valacchi G. Involvement of 4-hydroxy-2-nonenal in pollution-induced skin damage. BioFactors. 2019;45(4):536–547. doi: 10.1002/biof.1513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
the data used to support the findings of this study are available from the corresponding authors upon request.


