Key Points
Question
In hospitalized patients with coronavirus disease 2019 (COVID-19), what is the risk of corrected QT (QTc) prolongation when taking hydroxychloroquine with or without azithromycin?
Findings
In a cohort study of 90 hospitalized patients with coronavirus disease 2019, use of hydroxychloroquine with or without azithromycin for treatment of COVID-19 was associated with frequent QTc prolongation, and those taking hydroxychloroquine and azithromycin had greater QT prolongation than those taking hydroxychloroquine alone. One patient developed torsades de pointes.
Meaning
Clinicians should carefully weigh risks and benefits if considering hydroxychloroquine and azithromycin, with close monitoring of QTc and concomitant medication usage.
This cohort study examines the association of hydroxychloroquine or hydroxychloroquine and azithromycin with QT prolongation in adult patients hospitalized with coronavirus disease 2019.
Abstract
Importance
Administration of hydroxychloroquine with or without azithromycin for the treatment of coronavirus disease 2019 (COVID-19)–associated pneumonia carries increased risk of corrected QT (QTc) prolongation and cardiac arrhythmias.
Objective
To characterize the risk and degree of QT prolongation in patients with COVID-19 in association with their use of hydroxychloroquine with or without concomitant azithromycin.
Design, Setting, and Participants
This was a cohort study performed at an academic tertiary care center in Boston, Massachusetts, of patients hospitalized with at least 1 positive COVID-19 nasopharyngeal polymerase chain reaction test result and clinical findings consistent with pneumonia who received at least 1 day of hydroxychloroquine from March 1, 2020, through April 7, 2020.
Main Outcomes and Measures
Change in QT interval after receiving hydroxychloroquine with or without azithromycin; occurrence of other potential adverse drug events.
Results
Among 90 patients given hydroxychloroquine, 53 received concomitant azithromycin; 44 (48.9%) were female, and the mean (SD) body mass index was 31.5 (6.6). Hypertension (in 48 patients [53.3%]) and diabetes mellitus (in 26 patients [28.9%]) were the most common comorbid conditions. The overall median (interquartile range) baseline QTc was 455 (430-474) milliseconds (hydroxychloroquine, 473 [454-487] milliseconds vs hydroxychloroquine and azithromycin, 442 [427-461] milliseconds; P < .001). Those receiving concomitant azithromycin had a greater median (interquartile range) change in QT interval (23 [10-40] milliseconds) compared with those receiving hydroxychloroquine alone (5.5 [−15.5 to 34.25] milliseconds; P = .03). Seven patients (19%) who received hydroxychloroquine monotherapy developed prolonged QTc of 500 milliseconds or more, and 3 patients (3%) had a change in QTc of 60 milliseconds or more. Of those who received concomitant azithromycin, 11 of 53 (21%) had prolonged QTc of 500 milliseconds or more and 7 of 53 (13 %) had a change in QTc of 60 milliseconds or more. The likelihood of prolonged QTc was greater in those who received concomitant loop diuretics (adjusted odds ratio, 3.38 [95% CI, 1.03-11.08]) or had a baseline QTc of 450 milliseconds or more (adjusted odds ratio, 7.11 [95% CI, 1.75-28.87]). Ten patients had hydroxychloroquine discontinued early because of potential adverse drug events, including intractable nausea, hypoglycemia, and 1 case of torsades de pointes.
Conclusions and Relevance
In this cohort study, patients who received hydroxychloroquine for the treatment of pneumonia associated with COVID-19 were at high risk of QTc prolongation, and concurrent treatment with azithromycin was associated with greater changes in QTc. Clinicians should carefully weigh risks and benefits if considering hydroxychloroquine and azithromycin, with close monitoring of QTc and concomitant medication usage.
Introduction
As of April 10, 2020, more than 500 000 cases of coronavirus disease 2019 (COVID-19) have been reported in the United States, with no US Food and Drug Administration–approved treatments to date. Against this backdrop, the use of hydroxychloroquine for COVID-19 treatment has gained traction, appearing in international and domestic therapeutic guidelines. The presumed efficacy and widespread use of hydroxychloroquine stemmed from in vitro evaluations of severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and SARS-CoV-2 and a small prospective study claiming virologic clearance in 6 patients taking hydroxychloroquine with azithromycin. The combination gained further attention after coverage by the lay press; however, subsequent studies have failed to replicate these findings.
Although hydroxychloroquine and azithromycin are generally well-tolerated medications used in clinical practice, both can cause corrected QT (QTc) prolongation. With sweeping usage and perhaps insufficient consideration for comorbidities or concomitant QT-prolonging therapies, the frequency of adverse drug events (ADEs) will likely increase. Furthermore, evidence suggests that patients with underlying cardiac comorbidities are disproportionately affected by COVID-19 and the virus itself provokes myocardial injury. In this study, we aimed to characterize the risk and degree of QT prolongation in patients with COVID-19 in association with their usage of hydroxychloroquine with or without concomitant azithromycin.
Methods
The study was conducted according to Beth Israel Deaconess Medical Center institutional review board standards; informed consent was waived based on the board’s standards. This was a single-center, retrospective, observational study evaluating adults with COVID-19 who were hospitalized at Beth Israel Deaconess Medical Center in Boston, Massachusetts. We included patients admitted between March 1 and April 7, 2020, who received at least 1 day of hydroxychloroquine while inpatients and at least 1 positive COVID-19 nasopharyngeal polymerase chain reaction test result via the Pan Degenerate Amplification and Adaptation (PANDAA) qDx SARS-CoV-2 kit (Aldatu Biosciences). The antimicrobial stewardship team reviewed all hydroxychloroquine orders placed for patients with COVID-19 per internal treatment criteria, which included clinical and radiographic findings, laboratory results, and an electrocardiogram. The standard regimen was 400 mg of hydroxychloroquine twice on day 1, then 400 mg daily on days 2 through 5.
Data were extracted from the electronic medical records and deidentified. Medication administrations, ADEs, and treatment response were reviewed by an infectious disease-specialized pharmacist (N.J.M.) and physician (C.F.Y.). Electrocardiograms were manually evaluated by cardiologists (D.J.S. and T.R.M.) to calculate QTc intervals using the Bazett formula and so-called excess correction method for QRS values greater than 120 milliseconds. The Tisdale score, used to prognosticate QT prolongation in hospitalized patients, was applied retrospectively to evaluate QTc prolongation risk. End points of interest were changes in QTc (ΔQTc) in the cohort and between groups receiving hydroxychloroquine and hydroxychloroquine plus azithromycin, development of prolonged QTc interval to 500 milliseconds or more, and documented ADEs.
Statistical Analysis
Nominal data were described using proportions. Normally distributed discrete data were described with means and SDs, and medians and interquartile ranges (IQRs) were used to represent data that were not normally distributed. Categorical variables were compared with a χ2 or Fisher exact test and described using odds ratios (ORs) and 95% CIs. The Mann-Whitney U test evaluated continuous variables, with a P value of less than .05 to represent the statistical significance threshold. The QTc prolongation risk (≥500 milliseconds) was evaluated in a logistic regression model. Covariates evaluated in the Tisdale score and associated with QTc prolongation in univariate analysis (P < .10) were included in the multivariable analysis. Statistical analyses were performed using SPSS version 25.0 (IBM).
Results
Ninety patients were diagnosed with COVID-19 at a median (IQR) of 8 (5-12) days from the time of symptom onset. The mean (SD) age was 60.1 (16.7) years, 44 (48.9%) were women, and the mean (SD) body mass index (calculated as weight in kilograms divided by height in meters squared) was 31.5 (6.6). The most common comorbidities were hypertension (48 patients [53%]) and diabetes mellitus (26 patients [289%]) (Table 1). Thirty patients (33%) were critically ill at the time of testing, and 23 (26%) were mechanically ventilated. All patients received hydroxychloroquine, and 53 (59%) received hydroxychloroquine plus azithromycin; most patients had at least 1 cardiovascular comorbidity and were taking 2 or more QTc-prolonging medications, and 46 (51%) had a high-risk baseline cumulative Tisdale score of 11 or more points.
Table 1. Baseline Characteristics of 90 Patients Who Initiated Hydroxychloroquine for Coronavirus Disease 2019.
| Characteristic | No. (%) | P value | ||
|---|---|---|---|---|
| Total (n = 90) | Hydroxychloroquine (n = 37) | Hydroxychloroquine and azithromycin (n = 53) | ||
| Age, mean (SD), y | 60.1 (16.7) | 59.5 (15.9) | 60.6 (17.4) | .89 |
| Female | 44 (48.9) | 21 (56.8) | 23 (43.4) | .21 |
| BMI, mean (SD) | 31.5 (6.6) | 30.4 (6.1) | 32.3 (6.9) | .19 |
| Length of stay, median (IQR), da | 5.5 (3.3-8.0) | 5 (4.0-8.0) | 6.5 (3.0-8.8) | .59 |
| Onset illness prior to test, median (IQR), d | 8.0 (5.0-12.0) | 8.5 (5.0-12.0) | 8.0 (5.0-12.0) | .90 |
| Supplemental oxygen required | 64 (71.1) | 24 (64.9) | 40 (75.5) | .28 |
| Radiographic findings of pneumonia | 83 (92.2) | 33 (89.2) | 50 (94.3) | .37 |
| Intensive care at time of testing | 30 (33.3) | 9 (24.3) | 21 (39.6) | .13 |
| Mechanically ventilated at time of testing | 23 (25.6) | 7 (18.9) | 16 (30.2) | .23 |
| ≥2 Systemic Inflammatory Response Syndrome criteria | 57 (63.3) | 20 (54.1) | 37 (69.8) | .13 |
| Vasopressor support | 17 (18.9) | 6 (16.2) | 11 (20.8) | .59 |
| Tisdale score at treatment initiation, median (IQR) | 11.0 (9.0-13.0) | 11.0 (7.5-14.0) | 11.0 (9.0-12.0) | .77 |
| Acute cardiac injuryb | 25 (27.8) | 12 (32.4) | 13 (24.5) | .41 |
| Baseline laboratory values, median (IQR) | ||||
| Serum creatinine, mg/dL (IQR) | 1.0 (0.7-1.3) | 0.9 (0.7-1.3) | 1.0 (0.75-1.3) | .86 |
| White blood cell count, cells/μL | 5.850 (4.475-7.800) | 5.100 (4.100-6.950) | 6.200 (4.950-7.800) | .09 |
| Absolute lymphocyte, count/μL | 0.870 (0.620-1.115) | 0.910 (0.580-1.295) | 0.860 (0.625-1.045) | .65 |
| Maximum temperature on day of treatment initiation, median (IQR), °C | 38.3 (37.1-39.2) | 38.1 (37.3-38.8) | 38.6 (37.8-39.4) | .07 |
| C-reactive protein, mg/dL | 9.30 (5.24-17.5) | 6.41 (1.44-12.8) | 12.5 (7.1-22.7) | .002 |
| Creatine phosphokinase, IU/L | 180 (86-385) | 158 (74- 343) | 210 (102- 430) | .38 |
| D-dimer, μg/mL | 0.769 (0.534-1.191) | 0.775 (0.520-1.490) | 0.769 (0.572-1.164) | .92 |
| Lactate dehydrogenase, IU/L | 364.0 (255.0- 500.5) | 295.0 (243.5-427.5) | 408.0 (312.5- 531.0) | .02 |
| Ferritin, ng/mL | 732.0 (271.5-1627.0) | 585.0 (245.0- 1411.5) | 919.0 (401.0- 1757.0) | .19 |
| Preexisting conditions | ||||
| Hypertension | 48 (53.3) | 18 (48.6) | 30 (56.6) | .48 |
| Congestive heart failure | 9 (10.0) | 3 (8.1) | 6 (11.3) | .73 |
| Diabetes mellitus | 26 (28.9) | 10 (27) | 16 (30.2) | .75 |
| Coronary artery disease | 10 (11.1) | 4 (10.8) | 6 (11.3) | NA |
| Atrial fibrillation | 12 (13.3) | 3 (8.1) | 9 (17.0) | .35 |
| Chronic obstructive pulmonary disease or asthma | 18 (20.0) | 5 (13.5) | 13 (24.5) | .20 |
| Medications | ||||
| Loop diuretic in hospital | 39 (43.4) | 13 (35.1) | 26 (49.1) | .19 |
| Echocardiography, median (IQR), ms | ||||
| Baseline QTc | 455 (430-474) | 474 (454-487) | 442 (427-461) | <.001 |
| Posttreatment QTc peak | 476 (445-500) | 479.5 (443.5-501.5) | 458 (449-492) | .53 |
| ΔQTc | 21 (1-39) | 5.5 (−14 to 31) | 23 (10-40) | .03 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; NA, not available; ΔQTc, change in corrected QT interval.
SI conversion factors: To convert serum creatinine to μmol/L, multiply by 88.4; white blood cells and absolute lymphocytes to 109/L, multiply by 0.001; C-reactive protein to mg/L, multiply by 10; to convert D-dimer values to nmol/L, multiply by 5.476; lactate dehydrogenase to μkat/L, multiply by 0.0167; ferritin to μmol/L, multiply by 1.0; troponin T to μg/L, multiply by 1.0.
45 Patients remained hospitalized at the end of the study.
Peak troponin (corrected troponin T) greater than 0.01 ng/mL.
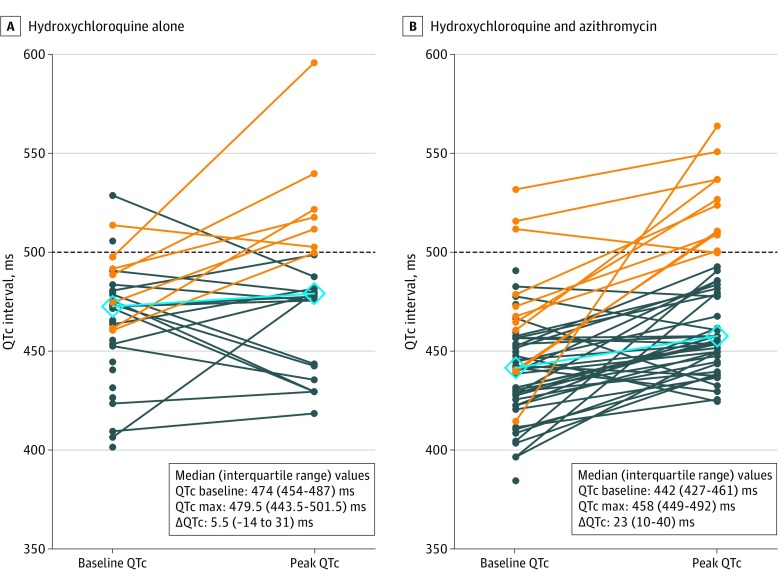
The median (IQR) baseline QTc was 455 (430-474) milliseconds. With treatment, 10 of 90 patients (11%) had ΔQTc of 60 milliseconds or more; 18 (20%) had posttreatment QTc intervals of 500 milliseconds or more. Of 37 patients receiving hydroxychloroquine monotherapy, 7 (19%) developed prolonged QTc of 500 milliseconds or more, and 3 (3%) had ΔQTc of 60 milliseconds or more. With concomitant azithromycin, 11 of 53 patients (21%) had prolonged QTc and 7 (13%) had a ΔQTc of 60 milliseconds or more (Figure; eFigure 1 in the Supplement). Although the baseline QTc was shorter in patients receiving concomitant azithromycin compared with those taking hydroxychloroquine alone (median [IQR], 442 [427-461] milliseconds vs 473 [454-487] milliseconds; P < .001), hydroxychloroquine and azithromycin was associated with a greater change in QTc compared with hydroxychloroquine alone (median [IQR] change, 23 [10-40] milliseconds vs 5.5 [−15.5 to 34.3] milliseconds; P = .03) (Figure). Patients who were critically ill also had a nonsignificantly greater ΔQTc than those who were not (median [IQR] change, 26.5 [11-51] milliseconds vs 16 [−8 to 35] milliseconds; P = .05).
Figure. Individual Changes in Corrected QT (QTc) Interval .
Difference in QTc between individuals at baseline and after use of hydroxychloroquine (A) or hydroxychloroquine and azithromycin (B). Orange lines denote a postadministration QTc of 500 milliseconds or more (while dark blue lines indicate values less than this threshold), and the light blue line with diamonds indicates the median baseline and peak QTc values after drug administration. Max indicates maximum; ΔQTc, change in corrected QT interval.
The likelihood of prolonged QTc (≥500 milliseconds) was greater with concomitant loop diuretic administration (12 of 39 patients [31%] vs 6 of 51 patients [12%]; P = .03), or a baseline QTc of 450 milliseconds or more (15 of 50 patients [30%] vs 3 of 40 patients [8%]; P = .008). Both remained independently associated after controlling for 2 or more Systemic Inflammatory Response Syndrome criteria (Table 2). Age, sex, concomitant QT-prolonging medications administration, and comorbidities did not correlate with a QTc of 500 milliseconds or more. Forty-one patients were discharged, 4 died, and 45 remained hospitalized, with a median follow-up of 9 days. Twenty-one patients had repeated nasopharyngeal polymerase chain reaction testing after a median (IQR) of 3.0 (1.0-6.5) days after starting treatment; 0 of 8 (0%) in the hydroxychloroquine group and 1 of 13 (7.7%) in the hydroxychloroquine and azithromycin group had negative results.
Table 2. Risk of Corrected QT (QTc) Interval Prolongationa.
| Characteristic | Patients, No. (%) | QTc peak ≥500 milliseconds | ||
|---|---|---|---|---|
| Odds ratiob (95% CI) | P value | Adjusted odds ratiob (95% CI) | ||
| Total | 90 (100) | NA | NA | NA |
| Female | 44 (48.9) | 0.80 (0.28-2.26) | .67 | NT |
| Age ≥68 y | 31 (34.4) | 1.70 (0.59-4.89) | .32 | NT |
| 1 QTc-prolonging agent | 24 (26.7) | 1.50 (0.49-4.58) | .55 | NT |
| ≥2 QTc-prolonging agents | 66 (73.3) | 0.67 (0.22-2.04) | .45 | NT |
| Loop diuretic | 39 (43.3) | 3.33 (1.12-9.91) | .03 | 3.38 (1.03-11.08) |
| Baseline QTc ≥450 ms | 50 (55.6) | 5.29 (1.41-19.85) | .008 | 7.11 (1.75-28.87) |
| ≥2 Systemic Inflammatory Response Syndrome criteria | 57 (63.3) | 3.57 (0.95-13.44) | .05 | 3.79 (0.92-15.58) |
| Heart failure | 9 (10.0) | 2.20 (0.49-9.81) | .38 | NT |
| Acute cardiac injury | 25 (27.8) | 1.91 (0.64-5.67) | .24 | NT |
| Serum potassium <4.0 mEq at QTc peak | 27 (30.0) | 1.43 (0.48-4.24) | .14 | NT |
| Serum magnesium <2.0 mg/dL at QTc peak | 19 (21.1) | 0.70 (0.18-2.72) | .75 | NT |
| Intensive care status at time of test | 30 (33.3) | 3.25 (1.12-9.41) | .03 | NT |
| Tisdale score | ||||
| <7 | 7 (7.7) | 0.78 (0.69-0.89) | .34 | NT |
| 7-10 | 37 (41.1) | 0.48 (0.16-1.49) | .20 | NT |
| ≥11 | 46 (51.1) | 3.07 (0.99-9.52) | .05 | NT |
Abbreviations: NA, not applicable; NT, not tested.
SI conversion factors: To convert potassium to mmol/L, multiply by 1.0; magnesium to mmol/L, multiply by 0.4114.
Highest corrected QT interval of 500 milliseconds or more after initiating use of hydroxychloroquine.
The reference value for each odds ratio is the absence of the listed characteristic.
Ten patients (11%) stopped taking hydroxychloroquine prior to day 5 of treatment for QTc prolongation. Possible hydroxychloroquine-associated ADEs included intractable nausea, resolving with medication discontinuation; development of new premature ventricular contractions and right bundle branch block; and a suspected case of hydroxychloroquine-associated hypoglycemia on day 2 of therapy, which was also in the context of poor oral intake. One patient who had hydroxychloroquine and azithromycin discontinued because of QTc prolongation (499 milliseconds) developed torsades de pointes 3 days later (eFigures 2 and 3 in the Supplement) and subsequently developed other ventricular arrhythmias that were treated with lidocaine.
Discussion
Proponents of hydroxychloroquine and chloroquine for COVID-19 treatment cite established safety in patients with autoimmune disorders, in vitro studies, and small nonrandomized clinical trials. However, the patients in these studies are clinically different from patients who were critically ill, infected with COVID-19, and receiving multiple QTc-prolonging medications with extended half-lives, which augment cardiotoxic risks. This was illustrated in a case of torsades de pointes from our cohort. Although hydroxychloroquine and azithromycin administration was discontinued 3 days prior to the event, the patient also had severe acute respiratory distress syndrome, bradycardia, hypothermia, propofol coadministration, and a new cardiomyopathy, raising concerns that the risk of QTc prolongation likely persisted, given the prolonged terminal half-life of each agent (eFigures 2 and 3 in the Supplement).
Hydroxychloroquine is structurally and mechanistically similar to the class IA antiarrhythmic quinidine, which inhibits voltage-gated sodium and potassium channels, prolonging the QT interval and increasing the risk of torsades de pointes and sudden cardiac death. Azithromycin also has been implicated in QTc prolongation and proarrhythmic events; its Food and Drug Administration label highlights the dose-dependent elevation in QTc when combined with chloroquine. Furthermore, enrollment was halted in a treatment arm for high-dose chloroquine plus azithromycin in a randomized clinical trial for patients hospitalized with severe COVID-19 pneumonia because of preliminary safety concerns about excessive cardiotoxicity. Loop diuretics, which were independently associated with prolonged QTc in this study, are also frequently used for severe COVID-19 infection to manage volume and acute respiratory distress syndrome, which should necessitate careful electrolyte management.
Within a 4-week observation period, 21 of 90 patients (23%) treated with hydroxychloroquine or hydroxychloroquine plus azithromycin had either significant QTc prolongation or ΔQTc of 60 milliseconds or greater. This underscores the American College of Cardiology’s recommendation for baseline risk assessment, frequent QTc monitoring, and strict cutoffs for therapy cessation; the Infectious Diseases Society of America voices similar concerns, recommending targeted antiviral therapeutics be limited to clinical trials. Ultimately, curtailing hydroxychloroquine-associated ADEs would require a multidisciplinary effort across medicine, infectious diseases, pharmacy, cardiology, critical care, and health care quality.
Limitations
While hydroxychloroquine and azithromycin administration likely contributed to the observed ADEs, we cannot exclude COVID-19-associated stress cardiomyopathy or myocarditis. Without a control arm, we cannot conclude that hydroxychloroquine and azithromycin conferred increased cardiotoxic risk; however, compared with hydroxychloroquine alone, ΔQTc differences were likely associated with the addition of azithromycin. It remains possible that the true degree of QTc prolongation was underestimated, given clinical practice variation and a limited follow-up period: 45 patients remained hospitalized, and 19 patients had no follow-up electrocardiograms. However, for the observed duration, ΔQTc and prolongation findings aligned with preliminary reports of significant QTc prolongation in 11% to 25% of patients. Higher-risk groups may not have been represented, because institutional guidance recommended against hydroxychloroquine for individuals with prolonged baseline QTc intervals. Numerous factors in this small cohort of adults who had complex, often critical illness could also have confounded clinical and safety end points.
Conclusions
Patients who were hospitalized and receiving hydroxychloroquine for COVID-19 frequently experienced QTc prolongation and ADEs, including a case of torsades de pointes with administration of hydroxychloroquine and azithromycin, which to our knowledge has yet to be reported elsewhere in the literature. There is a critical need for rigorous, large-scale studies and risk-benefit assessment prior to initiating COVID-19 therapeutics, with careful attention to medication interactions, cardiac manifestations, routine electrocardiograms, and electrolyte monitoring.
eFigure 1. Histogram: change in QTc (Δ QTc) and QTc prolongation after hydroxychloroquine administration, with (red, n=47) and without (gray, n=24) azithromycin co-administration.
eFigure 2. Telemetry strip with torsades de pointes following HCQ/AZI for COVID-19 treatment.
eFigure 3. ECG demonstrating prolonged QT prior to developing torsades de pointes.
References
- 1.US Department of Health & Human Services, Centers for Disease Control and Prevention . Coronavirus (COVID-19). Published 2020. Accessed April 10, 2020. https://www.cdc.gov/coronavirus/2019-nCoV/index.html
- 2.Multicenter Collaboration Group of Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for Chloroquine in the Treatment of Novel Coronavirus Pneumonia . [Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(0):E019-E9. doi:10.3760/cma.j.issn.1001-0939.2020.0019 [DOI] [PubMed] [Google Scholar]
- 3.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. doi: 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. Published online March 20, 2020. doi: 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molina JM, Delaugerre C, Le Goff J, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. Published online March 30, 2020. doi: 10.1016/j.medmal.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . The cardiotoxicity of antimalarials: Malaria Policy Advisory Committee Meeting. Published March 24, 2017. Accessed April 21, 2020. https://www.who.int/malaria/mpac/mpac-mar2017-erg-cardiotoxicity-report-session2.pdf
- 7.Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881-1890. doi: 10.1056/NEJMoa1003833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chorin E, Dai M, Shulman E, et al. The QT interval in patients with SARS-CoV-2 infection treated with hydroxychloroquine/azithromycin. Published online April 2, 2020. Accessed April 22, 2020. https://www.medrxiv.org/content/10.1101/2020.04.02.20047050v1
- 9.Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020. Published online March 21, 2020. doi: 10.1161/CIRCULATIONAHA.120.046941 [DOI] [PubMed] [Google Scholar]
- 10.Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479-487. doi: 10.1161/CIRCOUTCOMES.113.000152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grayson ML, Barber BE. Chloroquine and hydroxychloroquine. In: Grayson LM, ed. Kucers’ The Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal and Antiviral Drugs. 7th ed. CRC Press; 2017:3030-3047. [Google Scholar]
- 12.Pfizer Inc . Zithromax. Published 2019. Accessed April 21, 2020. http://labeling.pfizer.com/ShowLabeling.aspx?id=650
- 13.Borba MGS, Val FFA, Sampaio VS, et al. ; CloroCovid-19 Team . Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Netw Open. 2020;3(4.23):e208857. doi: 10.1001/jamanetworkopen.2020.8857 [DOI] [PubMed] [Google Scholar]
- 14.Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19 infection. Published April 11, 2020. Accessed April 21, 2020. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ [DOI] [PMC free article] [PubMed]
- 15.Roden DM, Harrington RA, Poppas A, Russo AM. Considerations for drug interactions on QTc in exploratory COVID-19 (coronavirus Disease 2019) treatment [published online April 10 2020]. J Am Coll Cardiol. 2020;S0735-1097(20)34918-4. doi: 10.1016/j.jacc.2020.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Histogram: change in QTc (Δ QTc) and QTc prolongation after hydroxychloroquine administration, with (red, n=47) and without (gray, n=24) azithromycin co-administration.
eFigure 2. Telemetry strip with torsades de pointes following HCQ/AZI for COVID-19 treatment.
eFigure 3. ECG demonstrating prolonged QT prior to developing torsades de pointes.