Chronic obstructive pulmonary disease (COPD) is a progressive, debilitating respiratory condition and currently the third leading cause of death in the United States (1). Though over 11 million people have been diagnosed with COPD, many more have undiagnosed disease (1). This number is projected to increase further as America’s population ages.
COPD is increasingly being recognized as a major health problem in America’s multicultural black population. Until recent studies such as COPDGene (Genetic Study of the Epidemiology of COPD) (2), which recruited a significant number of black individuals, there have not been many COPD studies inclusive enough of the black population in America to understand how the disease affects or may differ in the black population. Furthermore, evidence indicates that the prevalence and morbidity of COPD vary widely among U.S.-born black individuals versus black immigrants. This brings into question the validity of current knowledge, which largely refers to all “U.S. blacks” as a homogeneous “African American” populace in a majority of studies. This assumption ignores the variations in socioeconomic status, tobacco or biomass smoke exposure, behaviors, access to health care, health insurance coverage, and disease management among black individuals in America.
In this pulmonary perspective article, we define America’s black population and examine the prevalence, risk factors, morbidity, and mortality attributed to COPD among black individuals in America. When information is available, we point to differences among different groups according to their origin, and when possible, we identify future directions for research and interventions in this field.
Defining America’s Black Population
In defining America’s black population, it is important to describe the origin of black individuals in America and the social determinants contributing to their current health status.
The Black Population
The initial origin of America’s black population can be traced back to the 16th century, when a substantial number of Africans were forcibly brought to the United States from West Africa as captives to become slaves (3). The 1860 census counted more than 4 million black individuals, which by the turn of the 20th century (1900) had grown to more than 8 million (3). Black individuals in America are made up of “African Americans” who are identified as direct descendants of captives from West Africa and are citizens of the United States based on the Civil Rights Act of 1866 (4). Another group, black immigrants or foreign-born black individuals, refers to individuals born outside the United States, Puerto Rico, or other U.S. territories to parents who may or may not be U.S. citizens.
According to the 2010 U.S. census, there are 42 million black individuals comprising 14% of the total U.S. population (3). A Pew Research Center analysis of U.S. Census Bureau data showed that 4.2 million black immigrants lived in the United States in 2016, accounting for 9% of the U.S. black population. This number is projected to increase to 16.5% by 2060 (3). In 2016, 8% of black individuals are described as second-generation Americans, meaning they were born in the United States but have at least one foreign-born parent. In total, black immigrants and their children make up approximately one-fifth (18%) of the overall black population in the United States (5) Of note, a subgroup of black individuals that has gone largely uncharacterized is immigrants of Hispanic descent (black Hispanic individuals). They make up 0.4% of the total U.S. population, 2.5% of all African Americans, and 2.5% of all Hispanic Americans (6). Most black Hispanic individuals in the United States are of Puerto Rican descent (32.3%), Central or South American (19.9%), Mexican or Mexican American (19.3%), or from the Dominican Republic (15.0%) (7).
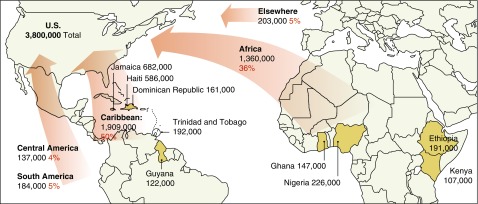
Black immigrants frequently migrate from Caribbean countries such as Jamaica and Haiti, sub-Saharan African countries such as Nigeria and Ethiopia (3), and Central and South American countries (Figure 1). Different U.S. immigration policies have fueled this migration. The Immigration and Nationality Act of 1965 paved a way for skilled immigrant labor and family reunification (8). The Refugee Act of 1980 enabled immigrants from conflict regions such as Ethiopia and Somalia to seek asylum in the United States (9). Finally, the U.S. Immigration Act of 1990 (diversity visa program or U.S. lottery) enabled immigration that mostly benefited immigrants from African countries (10). This diversity of origin must be taken into consideration when describing the epidemiology, including genetic epidemiology, of COPD in black individuals in America.
Figure 1.
Jamaica, Haiti, and Nigeria were the top birthplaces for black immigrants in the United States in 2013. The population and percentage of U.S. foreign-born black individuals by birth region and birth countries that contributed at least 100,000 black immigrants are shown. Note: Foreign-born black individuals include single-race and mixed-race black persons, regardless of Hispanic origin. Africa includes North African and sub-Saharan African countries as defined by Integrated Public Use Microdata Series (IPUMS). Source: Pew Research Center tabulations of the 2013 American Community Survey (1% IPUMS). Adapted from Reference 3.
Health Status of Black Individuals
Over the past few years, there have been significant efforts to improve the health status of black individuals in America and eliminate the long history of health disparities (11). Unfortunately, black individuals still experience poor health disproportionately compared with other racial/ethnic groups in the United States (11). The Institute of Medicine’s 2003 report titled “Unequal Treatment” (12) found that black individuals (and Hispanic individuals) tend to receive a lower quality of health care than their non-Hispanic white (NHW) counterparts across a range of disease areas. In addition, it is reported that black individuals are more likely than NHW individuals to receive less–than-adequate medical services (9).
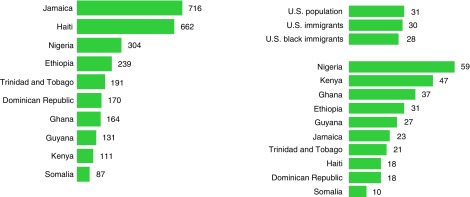
Black America is disproportionately impacted by low socioeconomic status (poverty) and lower rates of health insurance coverage. The September 2017 U.S. Census Bureau report on income and poverty (13) showed the poverty rate for the U.S. population as a whole at 12.7%; for black individuals, it was 22.0%, Hispanic individuals 19.4%, and NHW individuals 8.8% (13). The report showed that NHW individuals had the lowest uninsured rate at 6.3%, with uninsured rates for black individuals and Hispanic individuals at 10.5% and 16.0%, respectively (13). Other social determinants of health, such as educational attainment (high school diploma or higher), show lower rates of 22.5% in Black individuals compared with 36.2% in NHW individuals (14). Overall, 28% of black immigrants, compared with 31% of the total U.S. population, have a college degree or more, with black immigrants from African nations being more likely than NHW individuals or the total U.S. population to have a college degree or higher (14). Despite these figures, there are still significant differences even among this group; for example, 59% of foreign-born black individuals from Nigeria have a bachelor’s or advanced degree, compared with just 10% of black immigrants from Somalia (5) (Figure 2).
Figure 2.
(Left) Jamaica, Haiti, and Nigeria were the top birthplaces for black immigrants in the United States in 2016. The top 10 largest black immigrant groups are shown (data shown as total foreign-born black population in the United States in 2016 from the given countries, in thousands). Source: Pew Research Center tabulations of the 2016 American Community Survey (Integrated Public Use Microdata Series [IPUMS]). (Right) Overall, 28% of black immigrants have a college degree, but this varies widely by country of origin. The percentage of those living in the United States ages 25 and older with a bachelor’s or advanced degree in 2016 is shown. At bottom right, data for the top 10 largest black immigrant groups are shown. Note: Foreign-born black individuals include single-race and mixed-race black persons, regardless of Hispanic origin. Source: Pew Research Center tabulations of the 2016 American Community Survey (IPUMS). Adapted from Reference 5.
COPD in U.S. Black Individuals
Black individuals in the United States may be preferentially affected by COPD, with their prevalence and morbidity varying widely among subgroups because of biological, socioeconomic, and cultural factors. The subsections below examine the prevalence, lung function, risk factors, diagnosis, and management of COPD in the U.S. black population.
Prevalence
Overall, 6.3% of U.S. adults (projected at 15 million) have been diagnosed with COPD, with an age-adjusted prevalence of 6.0% (15). Self-reported COPD is estimated at 6.1% in black individuals, compared with 6.3% and 4%, respectively, for NHW and Hispanic individuals (15). Data from 1980 to 2000 show that rates of death due to COPD rose faster by 87% in black individuals than 67% in NHW individuals (16), with higher rates of hospitalization and emergency department (ED) visits for COPD but lower rates of physician office visits (16). In the following years from 2000 through 2014, the age-adjusted rates of death due to COPD declined by 21.1% in NHW males and by 24.4% in black males but increased for black females by 4.2% with no change in rates for NHW females (17). Though the death rate was higher for black men than for black women throughout the period, the sex gap decreased (17). With U.S. black individuals having a lower prevalence of COPD than NHW individuals in the United States, this prevalence is expected to rise if the prevalence of smoking continues to remain higher in U.S. black individuals than in NHW individuals.
Several reports have demonstrated that “African Americans” develop COPD with less cumulative smoking and at younger ages, suggesting greater susceptibility to the damaging effects of tobacco smoke (18). Chatila and colleagues (19), as well as data from the National Emphysema Treatment Trial (20), showed black patients to be younger at presentation with lower cumulative tobacco smoke exposure, despite comparable lung function. An analysis of data from the COPDGene study reported interactions between social, environmental, and genetic risk factors commonly associated with early COPD among black participants (21), which may explain why black individuals have less cumulative smoking history for similar disease severity. In a review of these studies, there was no stratification of U.S. black individuals into U.S. born or foreign born to allow for a comparison of possible differences according to the country of origin or age at immigration. However, data from the BOLD (Burden of Obstructive Lung Disease) study suggest that COPD prevalence may vary substantially by county of origin, with a COPD prevalence of 6.9% in Nigeria (22) as compared with 21.8% in the Caribbean (Trinidad and West Indies) (23). In a study by Barr and colleagues (24) using data from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL), there was no evidence that prevalence of COPD differed by Hispanic descent or varied by age at immigration after accounting for differences in history of asthma and smoking.
Lung Function
It is also important to realize that any estimate for the prevalence of lung disease among black individuals will necessarily also be influenced by lung function assessments. Studies have shown that black adults and children have lower lung function than NHW individuals (25). Hankinson and colleagues (26) derived spirometric reference equations for NHW, black, and Hispanic individuals based on asymptomatic, nonsmoking male and female participants of NHANES III (Third National Health and Nutrition Examination Survey). When adjusted for standing height, FVC and FEV1 were similar in NHW and Hispanic individuals with lower values in black individuals. This racial difference has been attributed in part to anthropometric factors of smaller trunk/leg ratio in black individuals (26).
This association between black individuals and lower lung function has been explored in several studies. The Health ABC (Health, Aging and Body Composition) study (27) examined “African Americans” who had a mixed ancestry of approximately 80% African ancestry and 20% European ancestry. The study demonstrated that smoking has a greater impact on reduced lung function among “African Americans” with high African ancestry (defined as greater than the median value of 80.8%) than in those with low African ancestry (≤80.8%), suggesting that individuals with high African ancestry may be particularly susceptible to the impact of cigarette smoking on the decline of FEV1 (27). The CARDIA (Coronary Artery Risk Development in Young Adults) study also noted that African ancestry was inversely related to FEV1 and FVC (28). Also, in the HCHS/SOL study and the Multi-Ethnic Study of Atherosclerosis Lung Study, results showed that Hispanic individuals of non-Mexican background, most of whom were of Puerto Rican and Dominican background, had lower FEV1 and FVC than Mexican Americans because of their substantially higher proportion of African ancestry (29).
This relationship between lung function and people of African ancestry further highlights the need to integrate country of birth or origin in the determination of lung function. Similarly, a lower baseline lung function has been identified as a risk factor for accelerated lung function loss, which could be related to a greater risk of COPD among America’s black population (27).
Risk Factors
Tobacco Use and Exposure
Cigarette smoking is the primary risk factor for COPD in the developed world, explaining 85–90% of cases (30). Results of the 2013 National Survey on Drug Use and Health showed 29.8% of U.S. black adults reported current use of tobacco in 2013 (31). Tobacco use also varies among U.S. black individuals, with marked variability in smoking habits among U.S.-born black individuals and foreign-born black individuals. Using data from the 1990 to 1994 National Health Interview Survey, with participation of 16,738 U.S.-born and foreign-born black individuals between 18 and 64 years of age, King and colleagues (32) showed major differences in the smoking behavior of U.S.-born and foreign-born black individuals (Table 1). Recent data from the National Health Interview Survey 2012 to 2015 (Table 2) also show similar trends. U.S.-born black individuals were far more likely than foreign-born black individuals to be smokers, even after controlling for differences in sociodemographic indicators (32). Therefore, combining data from U.S.-born and foreign-born black individuals may underestimate cigarette smoking in certain black populations because foreign-born black individuals have lower smoking prevalence (32). It is therefore imperative that these groups be separated as U.S.-born versus foreign-born black individuals, and even among the foreign born, the countries of origin may be as important.
Table 1.
Unadjusted Percentage Distribution of Smoking Status, by Race and Nativity: United States, Average Annual Figures, 1992–1995
| Smoking Status* | Total Population† | U.S.-born Black | Foreign-born Black | U.S.-born White | Foreign-born White |
|---|---|---|---|---|---|
| Numbers in thousands | |||||
| All persons | 166,980 | 19,275 | 1,407 | 137,972 | 8,325 |
| Unadjusted percentage distribution (SE) | |||||
| Total | 100 | 100 | 100 | 100 | 100 |
| Current | 26.1 (0.24) | 27.8 (0.63) | 11.4 (1.72) | 26.2 (0.27) | 22.6 (0.87) |
| Former | 24.9 (0.21) | 15.2 (0.47) | 10.7 (1.94) | 26.5 (0.24) | 23.0 (0.85) |
| Never smoked | 49.0 (0.26) | 57.0 (0.70) | 77.9 (2.49) | 47.3 (0.29) | 54.4 (1.03) |
Because of rounding, figures may not add to 100%. Source: National Health Interview Surveys, 1992–1995. Adapted from Reference 66.
Includes persons 18 years of age and older.
All non-Hispanic black and white persons and all nativity groups.
Table 2.
Unadjusted Percentage Distribution of Smoking Status, by Race and Nativity: United States, Average Annual Figures, 2012–2015
| Smoking Status | Total Population | U.S.-born Black | Foreign-born Black | U.S.-born White | Foreign-born White |
|---|---|---|---|---|---|
| Total | 100 | 100 | 100 | 100 | 100 |
| Current | 16.9 (16.6–17.3) | 19.4 (18.6–20.2) | 6.6 (5.4–8.1) | 18.8 (18.3–19.3) | 14.5 (13.0–19.3) |
| Former | 23.0 (21.6–22.3) | 14.9 (14.2–15.6) | 8.2 (6.7–10.0) | 25.9 (25.4–26.4) | 25.4 (23.6–27.2) |
| Never smoked | 61.1 (60.7–61.6) | 65.7 (64.8–66.7) | 85.3 (83.0–87.3) | 55.3 (54.7–55.9) | 60.2 (58.1–62.1) |
Data are shown as unadjusted percentage distribution (95% confidence interval). Source: National Health Interview Survey, 2012–2015. Adapted from Reference 67.
On average, black individuals smoke fewer cigarettes and start smoking cigarettes at an older age, but they still are more likely to die of smoking-related diseases than NHW individuals (33). The reason for the increased likelihood of smoking-related diseases despite a lower prevalence of cigarette smoking is unclear, but a potential factor is that U.S. black individuals appear to have greater nicotine intake from tobacco smoke and lower renal clearance of cotinine (an indicator of recent exposure to tobacco smoke), with cotinine half-life shown to be elevated in black as compared with NHW individuals (34, 35).
Despite lower prevalence of active smoking, U.S. black children and adults are more likely to be exposed to secondhand smoke than any other racial or ethnic group (36). This has been partially explained by higher degrees of poverty among black than NHW individuals (13) and a higher likelihood of living in multiunit dwellings (37), with higher concentrations of cotinine detected among multiunit inhabitants than in those living in single-family dwellings, even when no household member smokes (38). Thus, black nonsmokers generally have higher cotinine concentrations than nonsmokers of other races/ethnicities (33).
Genetics
Several studies have shown a significant genetic contribution to COPD (39). The most well-recognized genetic risk factor is alpha-1 antitrypsin deficiency, which affects 1–2% of those with COPD and is more common among NHW than black individuals (40). Though genome-wide association studies have implicated genetic polymorphisms in alpha-1 antitrypsin deficiency pathogenesis and other genetic factors such as transforming growth factor-β1, tumor necrosis factor-α, glutathione S-transferase, and SERPINA1 (serpin family A member 1) have been linked to COPD development (41, 42), few studies have examined genetic risk factors in populations of African ancestry. Polymorphism at the 16th amino acid residue of the β2-adrenergic receptor is associated with adverse effects of β-agonist use in patients with asthma, as observed in a retrospective, genotype-stratified analysis of published studies (43, 44). Also, results of the Salmeterol Multicenter Asthma Research Trial questioned the safety of long-acting β-agonists, particularly in African American patients with asthma (45). Despite the growing interest in identification of genetic risks for obstructive lung disease, there is very little information available exploring their role in explaining the risk for COPD or early-onset COPD in U.S. black individuals.
Air Pollution, Biomass, and Occupational Exposures
The effect of air pollution on morbidity and mortality has varied among the different racial groups in the United States (46). Occupational exposure to noxious substances such as dusts, chemicals, and smoke from biomass fuels has been associated with the development of COPD in many studies (47), with occupational exposures estimated to cause 15–31% of COPD cases among never smokers (46). The contribution of occupational exposures to COPD risk may be higher among minorities (black individuals and Hispanics), owing to their higher exposure while working in hazardous industries than exposure among nonminority workers (48). The impact of biomass fuels on airflow obstruction may also predominantly impact black populations, given the higher prevalence of biomass exposure in resource-poor settings. This increased biomass exposure was observed in the BOLD study by Amaral and colleagues (22), who found that the use of biomass fuels for cooking and heating ranged between 47% and 96% in the black population studied in three sub-Saharan African countries and 70% in the U.S. site (Lexington, KY). Even with this high biomass exposure, the study concluded that airflow obstruction assessed by post-bronchodilator spirometry was not associated with use of solid fuels for cooking or heating in low-/middle- and high-income sites. Of note, no analysis was done to differentiate the U.S. site of the study by race; thus, there is a need for more specific studies on the impact of biomass fuels as it relates to household air pollution specifically among U.S. black individuals.
Diagnosis
According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) consensus statement, a diagnosis of COPD should be considered and confirmed by spirometry in any adult (>40 yr) at risk (e.g., cigarette smoker) and with symptoms of cough, sputum production, or dyspnea (30). Studies collectively suggest that approximately 70% of COPD worldwide may be undiagnosed or underdiagnosed (49). Underdiagnosis in COPD may occur when patients do not communicate their symptoms to a physician or when physicians fail to attribute symptoms to COPD and thus do not appropriately order spirometry (49). In both the NHANES III and NHANES 2007–2012 surveys, being of an increased age and a member of a racial/ethnic minority group (U.S. black or Hispanic) increased the odds of being undiagnosed (50). Also, undiagnosed COPD can be attributed to patients and primary care physicians who are not aware of and therefore have a low index of suspicion for COPD, sometimes leading to misdiagnosis (50). In most cases, they fail to recognize the silent nature of early disease stages or to make adequate use of spirometry, as observed in a study by Kesten and colleagues (51). Notably, that study did not investigate whether use of spirometry to confirm diagnosis differed in NHW or U.S. black individuals. Owing to inability to provide preventive measures, underdiagnosis of COPD has been shown to impact survival, causing increased mortality and functional limitation, as well as low FEV1, which has been used to monitor decline in lung function in smokers especially. Common reasons for misdiagnosis or underdiagnosis of COPD in black individuals may include lack of reference values for spirometric measures; language barriers in cases of non–English-speaking black immigrants; and inadequate access to health care based on socioeconomics, geography, and differences in health beliefs and attitudes.
Disease Management
The principles of COPD management, as recommended by the GOLD initiative (52), include assessing and monitoring disease, reducing risk factors, and managing stable COPD and its exacerbations. Implementing these management strategies requires proper diagnosis of COPD, as well as availability and affordability of healthcare services, which currently are of poor quality and less accessible (53) among U.S. black individuals. In their prospective multicenter study, Tsai and colleagues (54) found that compared with NHW patients, African American patients appeared to have greater barriers to accessing COPD care, as suggested by being uninsured, a decreased likelihood of having a primary care provider, and more frequent ED visits. Currently, the ED is frequently the main source of care for black compared with NHW individuals (55). These findings of racial inequality in treatment also has been reported in Veterans Affairs hospitals (56), a system that, like the ED, provides equal access to care for minority patients.
In the treatment of COPD exacerbations, multiple factors, including cultural, socioeconomic, and biological influences, could account for the differences in acute COPD exacerbation severity between U.S.-born black individuals and NHW individuals. Sarrazin and colleagues (57) reported that “African Americans” admitted to Veterans Affairs hospitals with COPD exacerbations were more likely to be admitted to the ICU and receive mechanical ventilation, supporting the conclusion that “African Americans” may be more prone to severe exacerbations. A study of treatment of COPD exacerbations in the ED also reported that “African American” patients were more likely to be uninsured and less likely to have a primary care provider than NHW patients (54), thus explaining their frequent use of the ED for COPD management. Hence, socioeconomic disparities could affect treatment and subsequent severity of acute exacerbations in that lower socioeconomic status is a known predictor of lower lung function and is more common among African Americans (48). Some data have also suggested that racial disparities in home oxygen prescriptions, influenza vaccine administration, and referral for smoking cessation education could contribute to greater severity of COPD exacerbations seen in black individuals (57).
Smoking cessation is of prime importance in COPD management, with the prevalence of smoking cessation interventions higher among NHW individuals than African Americans. Even though African Americans tend to be highly motivated to quit (58), their being more prone to nicotine dependence has been suggested as a reason (59). Nonadherence to therapy is common in patients with COPD, but black individuals are more likely than NHW individuals to be nonadherent to treatment, as observed in a study by Krauskopf and colleagues (60) in participants with COPD. In that study, 58% of the participants were nonadherent to their COPD medications. They were younger, more likely to be black or Hispanic, more likely to have an income at or below the federal poverty level, had fewer years of formal education, and had greater COPD severity scores. They also reported greater concerns about medication effectiveness and being more emotionally affected by COPD. In participants with high adherence, the more commonly held belief was that their regimen would control their COPD or that they understood their disease, whereas those with low adherence were more likely to report being confused about the management of their illness.
Conclusions
Major diversity in clinical presentation and disease progression exists within COPD, and these differences are seen in outcomes such as symptoms, exacerbations, therapeutic response, and rate of disease progression or even death. Recent studies, such as SPIROMICS (Subpopulations and Intermediate Outcomes in COPD Study) (61) and COPDGene (2), have gathered clinical, physiological, radiological, biological, and genetic data on subjects with COPD to aid understanding of these phenotypic differences seen when comparing “African Americans” and NHW individuals with COPD (62). In “African Americans,” COPD is a known predictor of decline in health-related quality of life (63), worsening dyspnea, shorter 6-minute walk distance, and more hospitalized exacerbations than in NHW individuals (64). Also, radiologic differences on a quantitative computed tomographic scan show less severe emphysema despite similar impairments in lung function (20), leading to poorer quality of life and exercise capacity (64). In addition, comorbidities such as cardiovascular diseases, metabolic syndrome, gastroesophageal reflux disease, osteoarthritis, and osteoporosis, when present, were associated with higher risk for worse outcomes among “African Americans” with COPD (65). Although data are beginning to accumulate on how COPD susceptibility and management may differ among black individuals, it is difficult to fully extrapolate this information to America’s black population, given differences that exist between U.S.-born black individuals and foreign-born black individuals, including black Hispanic individuals. The use of “African American” to describe all U.S. black individuals in a majority of studies no longer captures the heterogeneity of the U.S. black population and so should be curtailed. The use of the term “U.S. black individuals” could be used to be more inclusive moving forward, given the potential genetic, social, cultural, and socioeconomic differences between these patient populations. Other subgroups, such as Africans who migrated to America in the 19th century and who still describe themselves as, for example, “Nigerian American,” “Ethiopian American,” or Hispanic black are groups rarely mentioned in studies. This need for further stratification was added by the U.S. Census Bureau in their 2020 questionnaires with a question on origin that includes whether the respondent is black and identifies as African American, Jamaican, Haitian, Nigerian, or Ethiopian. Though this might help in the interim, it might not be realistic moving forward, owing to the increasing admixing of races. This will likely lead to the medical community totally abandoning racial partition and trying to embrace the bold new realm of personalized medicine, individual genetics, and epigenetics in COPD medical management.
There is also an urgent need to collect solid data to allow the design of effective preventive and therapeutic interventions. First steps should be to ensure the inclusion of black individuals in both observational and interventional studies, with appropriate collection of data on place of birth, country of origin, tobacco use habits, biomass smoke exposure, and socioeconomic status to better capture the diversity within this racial group. Reference values for spirometric measures of lung function for black individuals must be used. Differences in behaviors of tobacco use and adherence to therapy comparing foreign-born black individuals with U.S.-born black individuals must be considered. Eliciting and addressing illness beliefs specifically about medication concerns while linking counseling about COPD medication adherence with discussions about side effects and long-term benefits of medication use may prove to be an efficient mechanism for targeted counseling about adherence to COPD treatment. Finally, because black individuals in America are disproportionately impacted by low socioeconomic status (poverty) and lower health insurance rates, increasing health insurance coverage and developing culturally sensitive policies to address healthcare barriers (i.e., low health literacy, nonadherence to therapy, patient beliefs, and cultural competency of healthcare providers) will help improve the care of America’s black patients with COPD.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201810-1909PP on February 21, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.American Lung Association. How serious is COPD. [updated 2011; accessed 2018 Feb 27]. Available from: https://www.lung.org/lung-health-and-diseases/lung-disease-lookup/copd/learn-about-copd/how-serious-is-copd.html.
- 2. COPD Genetic Epidemiology (COPDGene) study [accessed 2018 Feb 27]. Available from: http://www.copdgene.org/study-design.
- 3.Anderson M. A rising share of the U.S. black population is foreign born; 9 percent are immigrants; and while most are from the Caribbean, Africans drive recent growth. Washington, DC: Pew Research Center; 2015. [Google Scholar]
- 4.The Civil Rights Act of 1866. [accessed 8 Apr 2018]. Available from: http://teachingamericanhistory.org/library/document/the-civil-rights-act-of-1866/
- 5.Anderson M, López G. Key facts about black immigrants in the U.S. Washington, DC: Pew Research Center; 2018 Jan 24 [accessed 2018 Feb 4]. Available from: http://www.pewresearch.org/fact-tank/2018/01/24/key-facts-about-black-immigrants-in-the-u-s/ [Google Scholar]
- 6.Humes KR, Jones NA, Ramirez RR.Overview of race and Hispanic origin: 2010Report No. C2010BR-02. Suitland, MD: U.S. Census Bureau; 2011Mar [accessed 2018 Apr 3]. Available from: https://www.census.gov/library/publications/2011/dec/c2010br-02.html [Google Scholar]
- 7.LaVeist-Ramos TA, Galarraga J, Thorpe RJ, Jr, Bell CN, Austin CJ. Are black Hispanics black or Hispanic? Exploring disparities at the intersection of race and ethnicity. J Epidemiol Community Health. 2012;66:e21. doi: 10.1136/jech.2009.103879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Immigration and Nationality Act of 1965. [accessed 2018 Feb 4]. Available from: http://immigrationtounitedstates.org/594-immigration-and-nationality-act-of-1965.html.
- 9.The Refugee Act. [accessed 2018 Feb 4]. Available from: https://www.acf.hhs.gov/orr/resource/the-refugee-act.
- 10.Kent MM. Immigration and America’s black population. Popul Bull. 2007;62(4) [Google Scholar]
- 11.Mullins CD, Blatt L, Gbarayor CM, Yang HW, Baquet C. Health disparities: a barrier to high-quality care. Am J Health Syst Pharm. 2005;62:1873–1882. doi: 10.2146/ajhp050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smedley BD, Stith AY, Nelson AR.editors. Unequal treatment: confronting racial and ethnic disparities in health care Washington, DC: National Academies Press; 2003 [PubMed] [Google Scholar]
- 13.Semega JL, Fontenot KR, Kollar MA. Income and poverty in the United States: 2016. Current Population Reports. Report No. P60-259. U.S. Census Bureau; Washington, DC: U.S. Government Printing Office; 2017. [Google Scholar]
- 14.Ryan CL, Bauman K. Educational attainment in the United States: 2015. Current Population Reports. Report No. P20-578. Suitland, MD: U.S. Census Bureau; Mar 2016. [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC) Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:938–943. [PubMed] [Google Scholar]
- 16.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Surveill Summ. 2002;51:1–16. [PubMed] [Google Scholar]
- 17.Ni H, Xu J. COPD-related mortality by sex and race among adults aged 25 and over: United States, 2000–2014. NCHS Data Brief. 2016;(256):1–8. [PubMed] [Google Scholar]
- 18.Dransfield MT, Davis JJ, Gerald LB, Bailey WC. Racial and gender differences in susceptibility to tobacco smoke among patients with chronic obstructive pulmonary disease. Respir Med. 2006;100:1110–1116. doi: 10.1016/j.rmed.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Chatila WM, Wynkoop WA, Vance G, Criner GJ. Smoking patterns in African Americans and whites with advanced COPD. Chest. 2004;125:15–21. doi: 10.1378/chest.125.1.15. [DOI] [PubMed] [Google Scholar]
- 20.Chatila WM, Hoffman EA, Gaughan J, Robinswood GB, Criner GJ National Emphysema Treatment Trial Research Group. Advanced emphysema in African-American and white patients: do differences exist? Chest. 2006;130:108–118. doi: 10.1378/chest.130.1.108. [DOI] [PubMed] [Google Scholar]
- 21.Foreman MG, Zhang L, Murphy J, Hansel NN, Make B, Hokanson JE, et al. COPDGene Investigators. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPDGene Study. Am J Respir Crit Care Med. 2011;184:414–420. doi: 10.1164/rccm.201011-1928OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amaral AFS, Patel J, Kato BS, Obaseki DO, Lawin H, Tan WC, et al. Burden of Obstructive Lung Disease (BOLD) Collaborative Research Group. Airflow obstruction and use of solid fuels for cooking or heating: BOLD results. Am J Respir Crit Care Med. 2018;197:595–610. doi: 10.1164/rccm.201701-0205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thorington P, Rios M, Avila G, Henry J, Haynes C, Pinto Pereira LM, et al. Prevalence of chronic obstructive pulmonary disease among stable chronic disease subjects in primary care in Trinidad, West Indies. J Thorac Dis. 2011;3:177–182. doi: 10.3978/j.issn.2072-1439.2011.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barr RG, Avilés-Santa L, Davis SM, Aldrich TK, Gonzalez F, II, Henderson AG, et al. Pulmonary disease and age at immigration among Hispanics: results from the Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2016;193:386–395. doi: 10.1164/rccm.201506-1211OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harik-Khan RI, Fleg JL, Muller DC, Wise RA. The effect of anthropometric and socioeconomic factors on the racial difference in lung function. Am J Respir Crit Care Med. 2001;164:1647–1654. doi: 10.1164/ajrccm.164.9.2106075. [DOI] [PubMed] [Google Scholar]
- 26.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 27.Aldrich MC, Kumar R, Colangelo LA, Williams LK, Sen S, Kritchevsky SB, et al. Health ABC and CARDIA Studies Groups. Genetic ancestry-smoking interactions and lung function in African Americans: a cohort study. PLoS One. 2012;7:e39541. doi: 10.1371/journal.pone.0039541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar R, Seibold MA, Aldrich MC, Williams LK, Reiner AP, Colangelo L, et al. Genetic ancestry in lung-function predictions. N Engl J Med. 2010;363:321–330. doi: 10.1056/NEJMoa0907897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaVange L, Davis SM, Hankinson J, Enright P, Wilson R, Barr RG, et al. Spirometry reference equations from the HCHS/SOL (Hispanic Community Health Study/Study of Latinos) Am J Respir Crit Care Med. 2017;196:993–1003. doi: 10.1164/rccm.201610-1987OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 31.Substance Abuse and Mental Health Services Administration (SAMHSA), Center for Behavioral Health Statistics and Quality. 2013 National Survey on Drug Use and Health: detailed tables, Table 2.21B. Rockville, MD: SAMHSA; 2014. [Google Scholar]
- 32.King G, Polednak AP, Bendel R, Hovey D. Cigarette smoking among native and foreign-born African Americans. Ann Epidemiol. 1999;9:236–244. doi: 10.1016/s1047-2797(98)00052-0. [DOI] [PubMed] [Google Scholar]
- 33.American Lung Association. Too many cases, too many deaths: lung cancer in African Americans. Washington, DC: American Lung Association; 2010. [Google Scholar]
- 34.Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, et al. Centers for Disease Control and Prevention (CDC) Tobacco use among middle and high school students—United States, 2011–2014. MMWR Morb Mortal Wkly Rep. 2015;64:381–385. [PMC free article] [PubMed] [Google Scholar]
- 35.Pérez-Stable EJ, Herrera B, Jacob P, III, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280:152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 36.Homa DM, Neff LJ, King BA, Caraballo RS, Bunnell RE, Babb SD, et al. Centers for Disease Control and Prevention (CDC) Vital signs: disparities in nonsmokers’ exposure to secondhand smoke—United States, 1999–2012. MMWR Morb Mortal Wkly Rep. 2015;64:103–108. [PMC free article] [PubMed] [Google Scholar]
- 37.King BA, Babb SD, Tynan MA, Gerzoff RB. National and state estimates of secondhand smoke infiltration among U.S. multiunit housing residents. Nicotine Tob Res. 2013;15:1316–1321. doi: 10.1093/ntr/nts254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC) Secondhand smoke (SHS) facts. 2015 [accessed 2018 Mar 1]. Available from: http://www.cdc.gov/tobacco/data_statistics/fact_sheets/secondhand_smoke/general_facts/index.htm.
- 39.Silverman EK, Chapman HA, Drazen JM, Weiss ST, Rosner B, Campbell EJ, et al. Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease: risk to relatives for airflow obstruction and chronic bronchitis. Am J Respir Crit Care Med. 1998;157:1770–1778. doi: 10.1164/ajrccm.157.6.9706014. [DOI] [PubMed] [Google Scholar]
- 40.de Serres FJ. Worldwide racial and ethnic distribution of alpha1-antitrypsin deficiency: summary of an analysis of published genetic epidemiologic surveys. Chest. 2002;122:1818–1829. doi: 10.1378/chest.122.5.1818. [DOI] [PubMed] [Google Scholar]
- 41.Celedón JC, Lange C, Raby BA, Litonjua AA, Palmer LJ, DeMeo DL, et al. The transforming growth factor-β1 (TGFB1) gene is associated with chronic obstructive pulmonary disease (COPD) Hum Mol Genet. 2004;13:1649–1656. doi: 10.1093/hmg/ddh171. [DOI] [PubMed] [Google Scholar]
- 42.DeMeo DL, Mariani TJ, Lange C, Srisuma S, Litonjua AA, Celedón JC, et al. The SERPINE2 gene is associated with chronic obstructive pulmonary disease. Am J Hum Genet. 2006;78:253–264. doi: 10.1086/499828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, et al. The effect of polymorphisms of the β2-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000;162:75–80. doi: 10.1164/ajrccm.162.1.9907092. [DOI] [PubMed] [Google Scholar]
- 44.Taylor DR, Drazen JM, Herbison GP, Yandava CN, Hancox RJ, Town GI. Asthma exacerbations during long term beta agonist use: influence of β2 adrenoceptor polymorphism. Thorax. 2000;55:762–767. doi: 10.1136/thorax.55.9.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- 46.Hnizdo E, Sullivan PA, Bang KM, Wagner G. Airflow obstruction attributable to work in industry and occupation among U.S. race/ethnic groups: a study of NHANES III data. Am J Ind Med. 2004;46:126–135. doi: 10.1002/ajim.20042. [DOI] [PubMed] [Google Scholar]
- 47.Zeger SL, Dominici F, McDermott A, Samet JM. Mortality in the Medicare population and chronic exposure to fine particulate air pollution in urban centers (2000-2005) Environ Health Perspect. 2008;116:1614–1619. doi: 10.1289/ehp.11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dransfield MT, Bailey WC. COPD: racial disparities in susceptibility, treatment, and outcomes. Clin Chest Med. 2006;27:463–471. doi: 10.1016/j.ccm.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Diab N, Gershon AS, Sin DD, Tan WC, Bourbeau J, Boulet LP, et al. Underdiagnosis and overdiagnosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198:1130–1139. doi: 10.1164/rccm.201804-0621CI. [DOI] [PubMed] [Google Scholar]
- 50.Martinez CH, Mannino DM, Jaimes FA, Curtis JL, Han MK, Hansel NN, et al. Undiagnosed obstructive lung disease in the United States: associated factors and long-term mortality. Ann Am Thorac Soc. 2015;12:1788–1795. doi: 10.1513/AnnalsATS.201506-388OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kesten S, Chapman KR. Physician perceptions and management of COPD. Chest. 1993;104:254–258. doi: 10.1378/chest.104.1.254. [DOI] [PubMed] [Google Scholar]
- 52.Global Initiative for Chronic Obstructive Lung Disease (GOLD) GOLD strategy for the diagnosis, management and prevention of COPD. 2018 [accessed Aug 2018]. Available from: www.goldcopd.org.
- 53.Smedley BD, Stith AY, Nelson AR Institute of Medicine (US); Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 54.Tsai CL, Camargo CA., Jr Racial and ethnic differences in emergency care for acute exacerbation of chronic obstructive pulmonary disease. Acad Emerg Med. 2009;16:108–115. doi: 10.1111/j.1553-2712.2008.00319.x. [DOI] [PubMed] [Google Scholar]
- 55.Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data. 2007;386:1–32. [PubMed] [Google Scholar]
- 56.Jha AK, Shlipak MG, Hosmer W, Frances CD, Browner WS. Racial differences in mortality among men hospitalized in the Veterans Affairs health care system. JAMA. 2001;285:297–303. doi: 10.1001/jama.285.3.297. [DOI] [PubMed] [Google Scholar]
- 57.Sarrazin MV, Cannon KT, Rosenthal GE, Kaldjian LC. Racial differences in mortality among veterans hospitalized for exacerbation of chronic obstructive pulmonary disease. J Natl Med Assoc. 2009;101:656–662. doi: 10.1016/s0027-9684(15)30974-3. [DOI] [PubMed] [Google Scholar]
- 58.Royce JM, Hymowitz N, Corbett K, Hartwell TD, Orlandi MA COMMIT Research Group. Smoking cessation factors among African Americans and whites. Am J Public Health. 1993;83:220–226. doi: 10.2105/ajph.83.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kirkpatrick P, Dransfield MT. Racial and sex differences in chronic obstructive pulmonary disease susceptibility, diagnosis, and treatment. Curr Opin Pulm Med. 2009;15:100–104. doi: 10.1097/MCP.0b013e3283232825. [DOI] [PubMed] [Google Scholar]
- 60.Krauskopf K, Federman AD, Kale MS, Sigel KM, Martynenko M, O’Conor R, et al. Chronic obstructive pulmonary disease illness and medication beliefs are associated with medication adherence. COPD. 2015;12:151–164. doi: 10.3109/15412555.2014.922067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, et al. SPIROMICS Research Group. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182:598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolinsky FD, Malmstrom TK, Miller JP, Andresen EM, Schootman M, Miller DK. Antecedents of global decline in health-related quality of life among middle-aged African Americans. J Gerontol B Psychol Sci Soc Sci. 2009;64:290–295. doi: 10.1093/geronb/gbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han MK, Curran-Everett D, Dransfield MT, Criner GJ, Zhang L, Murphy JR, et al. COPDGene Investigators. Racial differences in quality of life in patients with COPD. Chest. 2011;140:1169–1176. doi: 10.1378/chest.10-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Putcha N, Han MK, Martinez CH, Foreman MG, Anzueto AR, Casaburi R, et al. the COPDGene® Investigators. Comorbidities of COPD have a major impact on clinical outcomes, particularly in African Americans. Chronic Obstr Pulm Dis (Miami) 2014;1:105–114. doi: 10.15326/jcopdf.1.1.2014.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lucas JW, Barr-Anderson DJ, Kington RS. Health status of non-Hispanic U.S.-born and foreign-born black and white persons: United States, 1992–95. National Center for Health Statistics. Vital Health Stat. 2005;10(226) [PubMed] [Google Scholar]
- 67.Division of Health Interview Statistics, National Center for Health Statistics, Centers for Disease Control and Prevention. 2015 National Health Interview Survey (NHIS) public use data release: survey description. Hyattsville, MD: US Department of Health and Human Services; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.