Dear Editor,
Interleukin 17C (IL-17C) is a cytokine produced by epithelial cells, including keratinocytes, in response to multiple stimuli1 such as IL-17A, IL-17F, TNF-α, bacterial stimuli and toll-like receptor agonists1. When produced in keratinocytes, IL-17C mediates the production of inflammatory molecules, including IL-1β, IL-8, CXCL1, and IL-36γ. In addition, IL-17C induces further elevation in IL-17A and IL-17F in Th17 cells in a pro-inflammatory positive amplification loop2,3. These mechanisms contribute to the feed-forward inflammatory cascade described in psoriasis and atopic dermatitis (AD)2. Elevated levels of IL-17A and IL-17F have been identified in lesional tissue of Hidradenitis Suppurativa (HS) patients4, associated with epidermal psoriasiform patterning. Here, we questioned whether epithelial derived cytokines (such as IL-17C) are also produced in HS lesional tissue, given the existing commonalities in IL-17A and IL-17F signalling and psoriasiform epidermal patterning between the three disorders.
Examination of lesional, perilesional and non-lesional skin biopsies from 8 untreated individuals with clinically diagnosed moderate-to-severe HS (Hurley Stage 2 and 3) were examined using quantification of IL-17A, IL-17C and IL-17F mRNAs done in parallel with control skin (healthy volunteers), and psoriasis vulgaris lesional skin. Immunohistochemistry (IHC) and mRNA analysis was performed according to previously published protocols3. As presented in Figure 1, we observed a significant elevation of IL-17C mRNA in all HS samples including in lesional, perilesional and unaffected tissue compared to site-matched healthy controls (Fig 1A and Fig 1B). These changes were comparable to the elevations seen in psoriasis, wherein the role of IL-17C has been well defined (Fig 1A). IHC localised IL-17C to the supra-basal epidermis (Fig 1A) with particular accentuation of the stratum corneum and stratum granulosum, a visible gradient toward the stratum corneum, as well as diffuse dermal staining. Semi-quantitative visual scoring identified a significant difference between basal epidermal staining in unaffected tissue and lesional/perilesional tissue (Fig 1C), with supra-basal staining increasing from unaffected to perilesional and lesional tissue (Figs 1B and 1C). However, in some patients with pronounced epidermal acanthosis, pan-epidermal staining for IL-17C was present (not shown). in a similar pattern to psoriasis vulgaris (Fig 1A).
Figure 1:
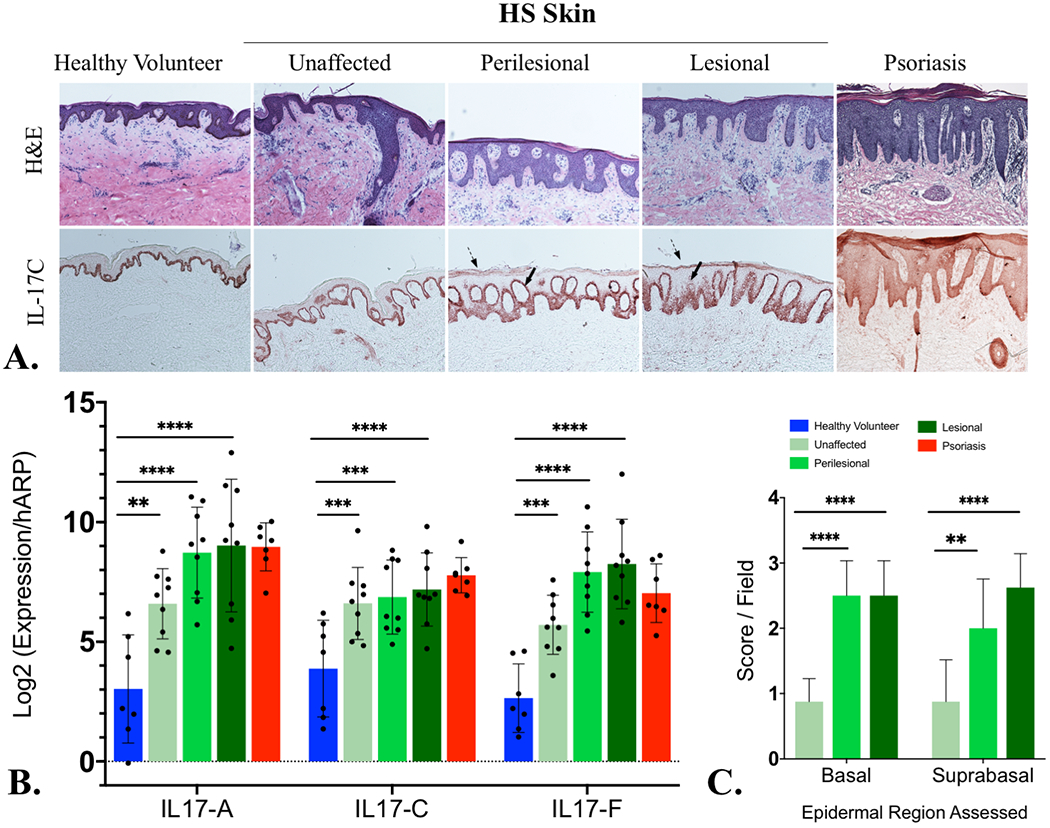
A) Interleukin 17C (IL-17C) localizes to the keratinocytes of psoriasiform epidermal hyperplasia in Hidradenitis Suppurativa by immunohistochemistry (Fig 1A) with increased IL-17C in suprabasal and granular layer of HS lesional skin. Diffuse dermal and epidermal staining is indicative of IL-17C protein diffusing into the surrounding dermis and epidermis at levels comparable with psoriasis. B) mRNA levels of IL-17C are significantly elevated (using one-way ANOVA) in lesional, perilesional and unaffected skin compared with healthy controls (Fig 1B) and comparable to the levels seen in psoriasis skin (Fig 1B). mRNA elevations of IL-17A and IL-17F are provided (Fig 1B) for comparison. Semiquantitative scoring of IL-17C IHC staining identifies statistically significant elevation (by one-way ANOVA) in perilesional and lesional tissue compared to unaffected tissue (Fig 1C).
Key: *= p>0.05; **= p<0.01; ***=p<0.001; ****= p<0.0001
NB: All statistical tests using one-way ANOVA were adjusted for multiple comparisons using the Benjamini-Hochberg method.
mRNA levels of IL-17C in lesional, perilesional and unaffected HS tissues were not significantly different from one another. Significant differences were only seen against (p<0.05) against healthy controls (Fig 1B). It is understood that apocrine gland rich skin (such as axillary, inguinal and sub-mammary tissues which have predilections to the development of HS) has an increased Th17 signal compared to other body sites6. Our use of site matched control skin accounts for this immuno-topographical variation in Th17 activity and highlights the significant elevation of IL-17C even in clinically “unaffected” tissue. IHC staining illustrates the development of a trans-epithelial IL-17C gradient in perilesional and lesional HS tissues compared with unaffected tissues. The presence of such a transepithelial gradient is documented as a potent neutrophil chemoattractant in other conditions including pustular psoriasis4,5. Our results suggest that the supra-basal localisation of IL-17C and establishment of a trans-epithelial gradient may be functionally important in the development of clinical disease, as opposed to absolute IL-17C levels in HS tissues; particularly as C/EBP β and C/EBP σ transcription factors that mediate signalling of IL-17A,C and F isoforms are more concentrated in differentiated keratinocytes of the spinous and granular layers7.
We present the first identification of IL-17C in HS. Extrapolating from known mechanistic pathways in psoriasis and AD, IL-17C may contribute to the upregulation of other keratinocyte derived cytokines and inflammatory mediators including IL-36y, IL-32, CXCL1, CXCL8 and LCN2. IL-36y is elevated in HS4 and is highly upregulated in other pustular disorders including generalized pustular psoriasis (GPP)5. Interactions between IL-17C and IL-36y are central to the pathogenesis of disease given effect of keratinocyte-derived factors in promoting trans-epithelial neutrophil migration. Given the purulent nature of HS this interaction may also be shared with HS, although further investigation is required to validate this hypothesis. In conclusion, the identification of IL-17C in epidermal keratinocytes of HS is a novel finding, identifying a potential new therapeutic target for the inflammatory drive in HS tissue.
Acknowledgments
Conflicts of Interest: J. G. Krueger has received research support (grants paid to institution) from AbbVie, Amgen, BMS, Boehringer, EMD Serono, Innovaderm, Kineta, LEO Pharma, Novan, Novartis, Paraxel, Pfizer, Regeneron, and Vitae and personal fees from AbbVie, Acros, Allergan, Aurigne, BiogenIdec, Boehringer, Escalier, Janssen, Lilly, Novartis, Pfizer, Roche, and Valeant. The other authors declare they have no relevant conflicts of interest.
Funding and Disclosures: J.W.F. was supported in part by grant # UL1 TR001866 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program. K.N. was supported by a MSTP grant from the National Institute of General Medical Sciences of the NIH under award number T32GM007739 to the Weill Cornell/Rockefeller/Sloan Kettering Tri-Institutional MD-PhD Program.
References:
- 1).Ramirez-Carozzi V, Sambandam A, Luis E, Lin Z et al. IL-17C regulates the innate function of epithelial cells in an autocrine manner Nat Immunol 2011;12(12);1159–1166 [DOI] [PubMed] [Google Scholar]
- 2).Guttman-Yassky E, Krueger JG IL-17C: A unique Epithelial Cytokine with Potential for Targeting across the Spectrum of Atopic Dermatitis and Psoriasis JID 2018;138(7):1467–1469 [DOI] [PubMed] [Google Scholar]
- 3).Hawkes JE, Chan TC Krueger JG Psoriasis Pathogenesis and the Development of Novel, targeted Immune Therapies J Allergy Clin Immunol 2017;140(3):645–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Witte Handel E, Wolk K, Tsaousi A, Irmer ML et al. The IL-1 Pathway is Hyperactive in Hiradenitis Suppurativa and Contributes to Skin Infiltration and Destruction J Invest Dermatol 2019;139(6):1294–1305 [DOI] [PubMed] [Google Scholar]
- 5).Johnston A, Xing X, Wolternik L, Barnes D et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis J Allergy Clin Immunol 2017;140(1):109–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Jenei A, Dajnoki Z, Medgyesi B, Gaspar K Beke G, Kinyo A et al. Apocrine Gland-Rich Skin Has a Non-Inflammatory IL-17-Related Immune Milieu, that Turns to Inflammatory IL-17-Mediated Disease in Hidradenitis Suppurativa J Invest Dermatol 2019. 139(4):964–968 [DOI] [PubMed] [Google Scholar]
- 7).Chiricozzi A, Nograles KE, Johnson-Huang LM, Fuentes-Duculuan J, Cardinale I Bonifacio et al. IL-17 Induces an Expanded Range of Downsdtream Genes in Reconstituted Human Epidermis Model PLoS One 9(2):e90284. [DOI] [PMC free article] [PubMed] [Google Scholar]


