Abstract
The role of several risk factors, such as pollution, consumption of alcohol, age, sex and obesity in cancer progression is undeniable. Human malignancies are mainly characterized by deregulation of cyclin-dependent kinases (CDK) and cyclin inhibitor kinases (CIK) activities. Viruses express some onco-proteins which could interfere with CDK and CIKs function, and induce some signals to replicate their genome into host’s cells. By reviewing some studies about the function of CDK and CIKs in cells infected with oncoviruses, such as HPV, HTLV, HERV, EBV, KSHV, HBV and HCV, we reviewed the mechanisms of different onco-proteins which could deregulate the cell cycle proteins.
Keywords: CDK, CIKs, Cancer, Virus
Introduction
Cell division is controlled by various elements [1–10], especially serine/ threonine protein kinase complexes, called cyclin-dependent kinases (CDKs), and cyclins, whose expression is prominently regulated by the binding to CDK inhibitors [11, 12]. In all eukaryotic species, these genes are classified into different families. It is well-established that the complexes of cyclin and CDK could regulate the distribution of cells in different phases of the cell cycle through modulating the transition of cells toward each phase. By constructing a complex with cyclinE and cyclinA, CDK2 facilitates the progression of S phase. Much of our recent knowledge about the significant role of CDKs and CDK inhibitors is emanated from studying RbyE2F pathway, which resulted in the discovery of the principal substrates of these proteins, such as Rb, p107, p130, E2F-1, and DP-1 [13–15]. It has been investigated that when these mentioned substrates are phosphorylated, CDKS bind tightly to their especial motif (the RXL motif) [16–18]. This finding highlights the key role of the phosphorylation in the entrance of the cells to the S phase of the cell cycle [19].
CDK genes are classified in mammalian cells into different classes of CDKs, especially some important regulatory ones (The regulatory CDKs play important roles in mediating cell cycle). Each of these CDKs could interact with a specific cyclin and thereby regulating the expression of different genes [20, 21]. Classical cell cycle CDKs, Cdk4, Cdk6, Cdk2 and Cdk1 regulate the transitions through the different phases of the cell-division cycle, and activating of these genes are at least partially mediated by the control of multiple transcription factors (TFs) or regulatory elements such as the retinoblastoma protein (Rb). The other group of regulatory CDKs includes CDKs 10, 11, 12, 14, 40 16, 19, 5, 7, 8, and 9. Cdk10 and Cdk11 control transcription by phosphorylating TFs, hormone receptors and associated regulators (HRs), or splicing factors (SPFs) while Cdk7, Cdk9 and Cdk12 directly phosphorylate the C42 terminal domain (CTD) of RNA polymerase II (RNAPII), thus modulating the different phases of generation of transcripts. The Mediator complex is specifically regulated by Cdk8 or the highly related Cdk19. Cdk7 functions as a CDK-activating kinase (CAK) by directly phosphorylating several of the CDKs mentioned above. Cdk5 displays many functions in the cell, but it is better known for its function in the control of neuron-specific proteins such as Tau. The members of the Cdk14 subfamily, such as Cdk14 itself or Cdk16, are activated at the membrane by cyclin Y and also participate in many different pathways, such as Wnt-dependent signaling or signal transduction in the primary cilium. CKI family, comprised from two main genes, particularly targets CDKs through its phosphorylation and halts the transition of cells from different phase of the cell cycle, leading to cell cycle arrest. The first gene family associated with CKIs is INK4 gene family (p16INK4a, p15INK4b, p18INK4c, and p19INK4d), which can interact with CDK4 and CDK6, and prevent their activity. The second family of genes is composed of p21Cip1/Waf1/Sdi1 [22, 23], p27Kip1 [24, 25], and p57Kip2 [26] that can interfere with cyclin D-, E-, A-, and B53 CDK complexes [27]. Sharing a similar N-terminal domain, all these molecules could bind to the cyclins and CDKs; however, given to their different structure, each CKI participates in a distinct cell function [21]. Mounting body of evidence has shown that in cancer cells the alteration of the expression level of CDKs and CDK inhibitors may provide a platform for malignant cells giving them the opportunity to proliferate vigorously. The association between CDKs/CDK inhibitors with viral onco-protiens has been investigated in numerous studies. For instance, a recent report suggested that avian reovirus p17 protein is a virus-encoded CDK inhibitor (V-CDKI) and downregulates CDK7/cyclin H complex [28]. In the present study, we did an intensive literature review to shed light on the underlying mechanisms through which viral onco-protiens regulate the expression of CDKs and CDk inhibitors, leading to tumor progression.
Human papillomaviruses
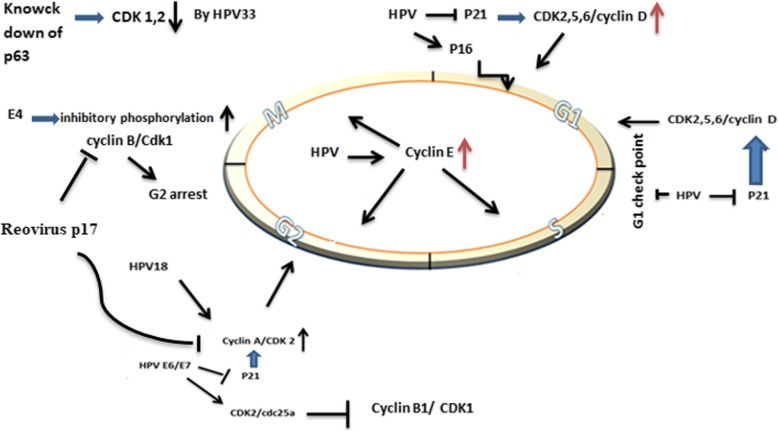
Human papillomaviruses, comprising over 100 genotypes, are non-enveloped and double-stranded DNA viruses [29]. More than 30 years ago, it has been assumed that HPV may participate in different types of cancers, including cervical, anus, oropharyngeal cancer. The results of studies indicated that there is a direct relationship between high-risk strains of HPV (HPV16, 18, 31, 33, 45, 52, and 58) and the incidence of human cancers [30]. Once HPV infected skin and mucosal surfaces, this virus not only initiates replication in the undifferentiated, proliferating cells of the basal layer but also through stimulating the host cell to divide, HPV increases its genome widely. Although the underlying mechanisms responsible for HPV replication are not entirely clear, it is suggested that some oncoproteins, such as E6 and E7 are involved in this process [31]. E6 and E7 can deregulate proteins, which control mitosis. After binding to p53, E6 destroys this tumor suppressor protein by exploiting the ubiquitin pathway. It has also been indicated that E7 could separate Rb from E2F and subsequently lead to the cell cycle progression [32]. Moreover, the results of some other studies suggested that HPV onco-proteins could interact with cell cycle regulatory proteins, such as CDKs, and CDK inhibitors. There are some evidence showing that E7 can prevent the activity of tumor suppressor, called p21Cip1 in cervical cancer [33]. E7 either expressed in high-risk strains of HPV (HPV16 and HPV31) or in the low-risk strain of the virus (HPV6b) could increase the activity of CDK2. The analysis of E7 protein sequences in these strains revealed that amino acids 9 to 38 participate in the induction of CDK2 activation. It has been reported that the 640 ng Glutathione-S-transferase (GST)–E7 can directly activate CDK2/cyclin A histone H1 kinase. Although GST-16E7 could stimulate CDK5/p25 complex, this protein has no inductive potential on CDC2/cyclin B activity [34]. By binding to the carboxy-terminal end of p21, HPV-16E7 blocks the activity of p21 on proliferating cell nuclear antigen (PCNA)-dependent DNA replication [35]. In addition, HPV-18 E1^E4 can promote both cyclin E and cyclin A/CDK 2 through binding to RXL motif [36]. The activation of CDKs may be up-regulated by HPV-16 E7. HPV-16E7 increases the expression level of cyclin E both transcriptionally and post transcriptionally, stimulating the entrance of cells into S and G2/M phase of the cell cycle [37]. The expression of CKI depends on the context of the cells. Although the expression of CKI is up-regulated in hyperplasia and koilocytes, its expression is down-regulated in cervical carcinoma. The expression of p16 also is increased in neoplastic cells [38]. HPV E6 could regulate the expression of p21 through down-regulation of p53 [39]. By changing the expression of cyclin/CDK, HPVE6 impairs G2 check point, which in turn lead to chromosomal instability [40]. The expression level of both p21WAF1/CIP1 and p27KIP1 can be negatively regulated in cervical lesions [41]. In a study conducted by Cho et al., it has been indicated that while the cyclin E was over-expressed in cervical lesions, the expression of cyclin D was decreased as result of p2lWAFI/CIPI and p27KIP1 down-regulation [42]. The association between HPV E6 and E7 proteins and cell cycle regulatory proteins has been well-established in another study conducted by Kim et al. They reported that treatment of TC-1 cells, expressing both HPV E6 and E7 proteins, with bortezomib and celecoxib could trigger apoptotic cell death through MAPK-mediated suppression of cyclin D1 and CDK2 [43]. The results of precise analysis also showed that knocking down p63 expression in differentiating HPV31 positive keratinocytes decreased the expression of cyclins A, B, and E, as well as Cdc25c, Cdk1 and Cdk2 [44]. Additionally, the high levels of cytoplasmic cyclin B1 and Cdk1 in HPV18 positive keratinocytes may induce G2/M cell cycle arrest [45]. It has also been demonstrated that E7 protein can increase the expression level of CDK2 by interacting with cyclin A, cyclin E, and cdc25a phosphatase [46–48] (Fig. 1) (Table 1).
Fig. 1.
The schematic diagram representing the effects of HPV on CDK and CIKs
Table 1.
The different effects of oncovirus on CDK and CIKs
| Viral pathogen | Viral Factor | HDAC/Mechanism/Effect | References |
|---|---|---|---|
| HPV | E7 | p21Cip1 suppression | Shin MK et al.,2009 [33] |
| HPV | E7 | CDK2/cyclin A activation | He W et al.,2003 [34] |
| HPV16 | E7 | block the effect of p21on (PCNA)- | Funk J et al.,1997 [35] |
| HPV-18 E1^E4 | E1^E4 | both cyclin E and cyclin A/CDK 2 activation | Ding Q et al.,2013 [36] |
| HPV-16 | E7 | Cyclin E activation- decrease in CIKs except, p21 and p16 | Martin L et al.,1998 [37] |
| HPV | E6 | Decrease in p21 and up-regulation of cyclin E/CDK2 | Syrjänen SM et al.,1999 [39] |
| HPV31 | cyclins A, B, and E, Cdc25c, Cdk1 and Cdk2 activation | Mighty, K.K et al.,2011 [44] | |
| HPV18 | Increase the levels of cytoplasmic cyclin B1 and inactivation of cyclin-dependent kinase1(Cdk1) | Wang, H.K et al.,2009 [45] | |
| High | E7 | Promotion of Cyclin A/E/ CDK2 | Nguyen, C.L et al.,2008 [46], Katich, S.C et al.,2001 [48] |
| HTLV | Decrease in p21WAF1/CIP1 | Moles R et al.,2015 [49] | |
| HTLV | Down-regulation of p27KIP1 | Cereseto A et al.,1999 [50] | |
| HTLV-I | Tax | Cyclin A limitation | Kibler KV et al.,2001 [51] |
| HTLV | P30 | Binding with cyclin A and cdk2, and preventing enter in S phase | Baydoun HH et al.,2010 [52] |
| HTLV | Increase in cyclin D/ CDK2 and inhibition of INK4 | Grassmann R et al.,2005 [53] | |
| HTLV | cyclin D2/ CDK4,6 | Santiago F et al.,1999 [54] | |
| HTLV | p18ink4c inactivation | Suzuki T et al.,1999 [55] | |
| HTLV | CDK4 | Haller K et al.,2002 [56] | |
| EBV | EBNA3C | cyclinA/CDK2 is activation | Knight JS et al.,2004 [57] |
| EBV | EBNA3C | Suppression of p16/INK4a | Parker GA et al.,1996 [58, 59] |
| EBV | EBNA3C | Targeting of SCF/skp2 E3 | Kumar P et al.,2009 [60], Iwahori S et al.,2009 [61] |
| EBV | EBNA3C | Repressing the repress p21WAF1/CIP1, p14ARF and p16INK4a | Tursiella ML et al.,2014 [62] |
| EBV | BZLF1 | Increase in p21WAF1/CIP1 | Sato Y et al.,2010 [63] |
| EBV | Up-regulation of cdc-2, cyclin E, D23, and cyclin D2 | Hollyoake M et al., 1995 [64] | |
| EBV | LMPA 2A and MYC | Suppress the p27 | Kamonwan Fish et al.,2014 [65] |
| EBV | Increase in CDKN2A | Vo QN et al.,2002 [66] | |
| KSHV | K-cyclin | CDK6 | Li, M et al.,1997 [67] |
| KSHV | K-cyclin | CDK9 | Chang PC et al.,2007 [68] |
| KSHV | v-cyclin | P27 | Moore PS et al.,2001 [69] |
| KSHV | p21 | Van Dross R et al.,2005 [70] | |
| HBV | HBx | Suppression of P21 | Han,J et al.,2000 [71] |
| HBV | HBX | cyclinA/CDK2 | Bouchard M et al.,2001 [72] |
| HBV | HBX | Methylation of P16 | Jung JK et al.,2007 [73] |
| HCV | HCV core | Inhibition of CDK7 | Ohkawa K et al.,2004 [74] |
| HCV | HCV core | Activation of CDK2 | Ohkawa K et al.,2004 [74] |
| HCV | HCV core | cyclinA, E, D1, CDK2 and CDK4 | Bahnassy AA et al.,2011 [75] |
| HCV | NS5A | P16,P21,P57 | Arima N et al.,2001 [76], Wagayama H et al.,2001 [77], Shackel NA et al.,2002 [78] |
| Avian Reovirus | p17 | Activation of v-CDKI | Huang W et al., 2017 [79] |
| Avian Reovirus | P17 | CDK inhibitor (V-CDKI) | Kozak R et al., 2017 [80] |
| Avian Reovirus | P17 | v-CDKI | Chiu HC et al., 2018 [28] |
| Avian Reovirus | P17 | suppression of CDK1/cyclin B1, CDK2/cyclin A2, CDK2/cyclin E1, and CDK6/cyclin D1 complexes by direct binding to CDKs, cyclins or complexes | Huang W et al. 2017 [79] |
| Avian Reovirus | P17 | Suppression of the CDK7/cyclin H complex by promoting p53 and cyclin H interaction. | Huang W et al. 2017 [79] |
Human T-lymphotropic viruses and human endogenous retroviruses
Human T-lymphotropic viruses (HTLV-1 and HTLV-2) are among those oncoviruses that are supposed to be involved in different types of cancers. These viruses belong to retroviridae family and are spread worldwide [81, 82] with some endemic areas such as North-Eastern Iran [83, 84]. These viruses possess positive-sense RNA genome that is further converted into DNA by a reverse transcriptase enzyme and then integrated into human genome [85]. It is estimated that 20 millions of people in the world are infected with HTLV; however, they do not display any clinical symptoms in their life time [86]. Basically, HTLV is a congenital infection. It could also be transmitted through blood transfusion, sharing syringe, breastfeeding and unprotected sexual intercourse [87]. TAX and human T-cell leukemia virus type 1 bZIP factor (HBZ) are the most important oncoprotins encoded by HTLV [88]. TAX functions as a trans-activator of viral replication, but it is often inactivated in infected cells [89]. In contrast, HBZ which is responsible for viral latency is expressed in the infected cells [90]. The association between Cyclin-dependent kinase/or Cyclin-dependent kinase inhibitor with HTLV is established in several studies. It has been indicated that the expression level of p21WAF1/CIP1 is down-regulated in HTLV infected cells [49]. Moreover, in most ALT and HTLV infected cells, the expression of cyclin E/CDK2 is increased as a result of the suppression of p27KIP1 [50]. By regulating cAMP responses, it has been demonstrated that Tax protein could restrict the activity of Cyclin A promoter [51]. Moreover, HTLV P30 halts the entrance of the cells into the S phase of the cell cycle by binding to cyclin A and cdk2 [52]. As a results of the down-regulation of p21 and INK4 in HTLV infected cells, the expression of cyclin E/ CDK2 and cyclin D/CDK2 is elevated [53]. Of note, the increase in the activity of cyclin D2/ CDK4,6 in infected T cell is coupled with the activation of DNA replication and cell proliferation [54]. Another mechanism by which HTLV1 could increase the expression of CDK4 is mediated through transcriptional suppression of p16 INK4a-mediated p15 INK4b expression. Furthermore, HLTV could bind to the E-Box promoter and thereby repress the expression of p18ink4c in infected cell. HTLV also could increase the expression of CDK4, one of the main regulators of cell growth [55], by directly binding to its N-terminal [91]. Once CDK4 is over-expressed, this kinase can phosphorylate Rb and increase its cellular protein expression level [56]. The association between HTLV and the expression level of CDKs has been reported in cell line studies. It was delineated that HTLV-I infected T-cell lines displayed an over-expression in p21Waf1/Cip1/Sdi1, cyclin D2, p18Ink4, highlighting that HTLV could potently increase the expression of CDK inhibitors in malignant cells [92].
Another member of retroviridea family, which has integrated its genome into human germ line about 30–40 million years ago is Human endogenous retroviruses (HERVs). It is estimated that the genome of this virus constructed about 8% of human genome. The over-expression of its association genes has been observed in some types of human cancer [93]. Mounting body of evidence has claimed that HERVs could alter the expression of CDK and CIK. In breast cancer cells, it is demonstrated that HERV-K gene could change the expression level of CDK4 and CDK6 [94]. Moreover, it is reported that HERV-K decreased the expression of p16/CDK4 through BRAF-MEK-ERK signaling pathway in melanoma [95]. (Fig. 2) (Table 1).
Fig. 2.
Different mechanisms which show the reciprocal interactions between HTLV and CDK/CIKs in different cancers
Epstein-Barr virus (EBV)
Epstein-Barr virus (EBV) is one of the etiologic infection agents which is responsible for a wide range of human malignancies, such as Burkitt’s lymphoma, nasopharyngeal carcinoma, post-transplant, AIDS-associated lymphomas, and Hodgkin’s disease [96]. This virus transforms B lymphocytes with EBV nuclear antigen 3C (EBNA3C) into an immortalized cell [97]. It is assumed that EBNA3C could have an impact on the expression of CDK and CKIs, foremost, p16INK4A [58]. Moreover, it has been suggested that by separating p27 from the complex of cyclin A/CDK2, EBNA3C binds to Carboxy-terminus of cyclin A, and increase in the activity of cyclin A/CDK2 complex can be observed [57]. The other mechanism which has been attributed to this virus is mediated through Ras signaling pathway, as EBNA3C could inactivate p16/INK4a and transform embryonic fibroblast. This function is similar to the mechanism exploited by E7 papilloma and E1A adenovirus [59]. During the viral lytic replication, EBNA3C recruits SCF/skp2 E3 ubiquitin ligase and thereby, decreases the amount of p27 in cells which subsequently leads to the activation of cyclin A/CDK2 [60, 61]. As another mechanism, it has been demonstrated that EBNA3C can repress p21WAF1/CIP1, p14ARF and p16INK4a, stimulating cell proliferation [62].
At the early stages of EBV lytic stage, BZLF1, an immediate-early viral gene of the Epstein–Barr virus increases the expression of p21WAF1/CIP1 through not only elevating the expression of p53 but also through binding to the promoter of p21WAF1/CIP1 [63]. In a study conducted by Hollyoake et al., it has been reported that there is an over-expression in cdc-2, cyclin E, D23, and cyclin D2 in EBV-mediated immortalized cells [64]. EBV can denature and phosphorylate p21WAF1 with stabilizing Pim-1 protein in S phase. This virus also can degrade p27KIP1 by modulating the expression of Skp2, one of the subunits of SCF ubiquitin–protein ligase complexes [98]. Moreover, the unique cross talk between EBV latent membrane protein (LMP 2A) and Myc, a family of wide range of onco-proteins, can suppress p27kip1, and facilitate the G1-S transition [65]. Several studies also suggested that there is a tight correlation between the expression of EBV-associated NPC, EBV- LMP-1 and EBER-1 and p16, CDK4, cyclin D1, and Rb. The significant role of aforementioned proteins in different malignancies, including colorectal and nasopharyngeal carcinoma is well-established [99]. Moreover, EBV infection is associated with the increment in the expression of CDKN2A in gastric cancer [66] (Fig. 3) (Table 1).
Fig. 3.
EBV can cause different effects on cyclin and CDK/CLKs
Herpes virus type 8 (KSHV)
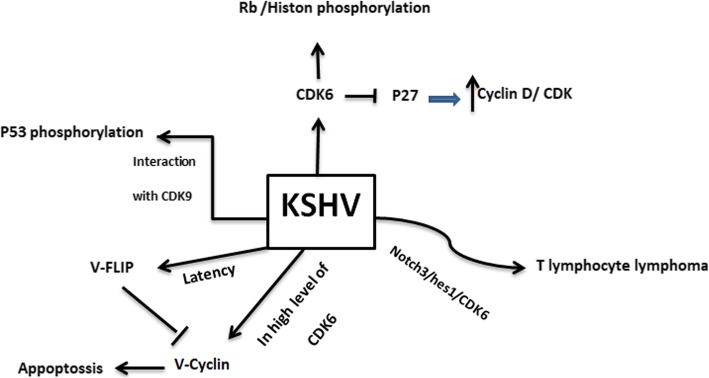
Herpes virus (KSHV), or human herpes virus 8 (HHV-8), is one of the members of herpes viridea family that accounts for the development of malignancies in patients suffering from AIDS [100]. This virus could also cause non-Hodgkin B-cell lymphoma (PEL), [101] and lymphoproliferative disorder (MCD) [102]. Sharing 30% similarity between K-cyclin, expressed by KSHV, and D-type cyclins leads to the interaction between these two proteins [103]. K-cyclin is able to make a complex with Cdk6, phosphorylate Rb and histone H1 in both G1 and S phases of the cell cycle. This function is similar to the activity of cyclinA/CDK2. Also p27 is one of the main targets of K-cyclin [67]. Moreover, it is suggested that K-cyclin and CDK9 can suppress the activity of p53, through its phosphorylation, leading to tumor progression [68]. Although K-cyclin is considered as an onco-protein, this protein can also lead to the induction of apoptosis in the high level of CDK6 [104]. Integrating with Notch3 and Hes1 signaling pathways, KSHV can interfere with T cell maturity, and initiate lymphoma [105, 106]. HHV-8 can cause primary lymphomas through suppression of p27KIP1 via Ser10 and Thr187 phosphorylation [107]. Dross et al. also confirmed that KSHV could exert an inhibitory effect on the expression of p21 and p27KIP1 [70]. During latency phases, ORF 72 expresses v-cyclin, a homologue of D-type cyclin, which is associated with CDK2, CDK4, CDK9. Indeed, nucleophosmin can be phosphorylated by V-cyclin CDK6, and control the latency phase [108]. Furthermore, it has been reported that v-cyclin-CDK6 is not sensitive to the Cip/Kip and INK4, and can phosphorylate Rb protein and Cdc6 [109]. It is assumed that v-cyclin may inhibit p27 and directly phosphorylate H1 histone [69]. In contrast, V-Flip can prevent the activity of V-cyclin, and induce the autophagy [110] (Fig. 4) (Table 1).
Fig. 4.
herpes virus type 8 (KSHV) regulates the expression of CDK and CIKs
Human hepatitis B virus (HBV)
Human hepatitis B virus (HBV) is one of the major causes of hepatic disease all around the world. Severe liver disease can also be occurred by co-infection of other types of hepatitis viruses [111–114]. By having a DNA genome, HBV encodes a wide variety of onco-proteins which can deregulate the expression of CDK and CIKs. Hepatitis B virus X protein (HBx) is one of these proteins that affect the pregenome transcription and HBV replication [115]. HBx possesses two important sites, Ser-101 and Met-130, which can activate and repress this protein, respectively. Although the signaling pathway through which HBx regulate the expression of cell cycle regulatory proteins has not been elucidated, some studies have suggested that HBx has a tight association with p21 [71, 116]. It is assumed that Met-130 represses the expression of p21 through suppression of the cellular transcription factor, Sp1 [117]. While several studies claimed that Hbx could repress the expression of p21, other reports suggested that HBx increases the expression of this CDK inhibitor [118]. Src tyrosine kinases-mediated activation of cyclinA and consequently CDK2 may be another mechanism through which HBx may regulate cell proliferation and transition of cells from G1 to S phase of the cell cycle [72].
HBV also can express another onco-protein, called large surface antigen (LHBS). This protein is able to increase the phosphorylation of Rb, employs c-Jun activation Domain-Binding Protein 1 signaling factor to deregulate p27Kip1, and exhibits CDK2 activity [119]. The ability of this protein to activate CDK1 and CDK2 is similar to HBx protein [120]. It is worth to mention that HBx can induce p16 hypermethylation, CDK4/6 up-regulation, Rb phosphorylation, and E2F and DNMT1 activity [73] (Fig. 5) (Table 1).
Fig. 5.
The regulation of cells CDK and CIKs expression by HBV and HCV
Human hepatitis C virus (HCV)
Hepatitis C virus (HCV), one of the major causes of hepatitis and liver cancer, possesses a positive-strand RNA that can regulate the expression level of CDK-activating kinase (CAK) [121, 122]. Although HCV could express wide range of proteins, its core protein possesses the most ability to interfere with the progression of the cell cycle. More than 10 HCV core proteins has been identified with the ability to modulate apoptosis pathways, as well as diverse biological activities [123]. Some studies suggested that this protein can inhibit the expression of CDK7. In addition, HCV could impair the cell cycle machinery through suppressing of CDK-activating kinase (CAK), CDK2 and CAK complex [74]. By cooperating with HBV, HCV core protein decreases the expression of p21 through influencing on the transforming growth Factor-L responsive element and Sp1 site of the p21 promoter [124]. The increase in the expression level of cyclinA, E, D1, CDK2 and CDK4, in the patients who are infected with HCV, could be exploit as a biomarker for the detection of hepatitis cancerous cells [75]. Another protein encoded by HCV which may have an impact on the regulation of CDK and CIK is NS5A. This protein increases the expression pf p21, as well [76]. In HCV infected patients, there is also an increase in the expression of CDK inhibitors, including p16 and p57 [77, 78] (Fig. 5) (Table 1).
Reovirus
Respiratory Enteric Orphan virus, commonly known as the reovirus, is an oncolytic virus. Both mammalian [125–127] and avian reoviruses [28, 79, 80] are under evaluation as cancer potential therapeutics. Current efforts focus on increasing the intrinsic capacity of mammalian reovirus to kill cancer cells, thwart cell division, optimizing the efficacy of reovirus combination therapies, and evaluating the effect of reovirus on immunotherapy [125–127]. However, a recent report suggested that avian reovirus p17 protein is a virus-encoded CDK inhibitor (v-CDKI). The ARV p17 protein suppresses CDK1/cyclin B1, CDK2/cyclin A2, CDK2/cyclin E1, and CDK6/cyclin D1 complexes by direct binding to CDKs, cyclins or complexes. In addition, the ARV p17 protein suppresses the CDK7/cyclin H complex by enhancement of p53 and cyclin H interaction [28, 80]. Furthermore, avian reovirus p17 protein has been demonstrated to function like v-CDKI, [28] which shuttles between the cytoplasm and the nucleus [128] to perform specific duties in mediating cancer cell cycle and growth [28]. Avian reovirus protein p17 is a nucleoporin Tpr Suppressor and can activate p53, p21 and PTEN and inactivates the PI3K/AKT/mTOR and ERK signaling pathways [79, 129]. In fact, the ARV p17 protein suppresses Tpr, leading to activation of p53, thereby activating p21 and PTEN as well as suppression of the PI3K/AKT/mTOR and ERK signaling pathways [129]. Also p17 may change some cell cycle pathway [28, 130, 131], such as, triggering autophagy by activating phosphatase [79, 132], altering cellular translation and so forth [133]. (Fig. 6).
Fig. 6.
The effect of P17 reovirus on cancerous signaling pathway
Conclusion
Dysregulation of the cell cycle through alteration in the expression CDK and CIKs has long been considered as a classic hallmark of cancer growth. Given to the importance of CDK and CIKs, we allocated this study to evaluate the effect of viral onco-proteins on the expression level of these proteins. Overviewing these mechanisms can highlight not only the management strategies but also the therapeutic approaches against these viral infections.
Acknowledgements
Not applicable.
Declaration
This study has been conducted in Department of the School of Medicine Shahid Beheshti University of Medical.
Abbreviations
- CDKs
Cyclin-dependent kinases
- CKIs
Cyclin-dependent inhibitors
- HPV
Human papillomaviruses
- HERVs
Human endogenous retroviruses
- EBV
Epstein-Barr virus
- HCV
Hepatitis C virus
- HBV
Human hepatitis B virus
- KSHV
Herpes virus type 8
- HBx
HBV X protein
- LHBS
Large surface antigen
- LMP1
Latent membrane protein 1
- HTLV-1
Human T cell leukemia virus type 1
- HTLV
Human T-lymphotropic viruses
Authors’ contributions
E.F, H.G and SH.T designed the study. All authors read and approved the final version of the manuscript.
Funding
No funding.
Availability of data and materials
Please contact author for data requests.
Ethics approval and consent to participate
This study was approved by the Shahid Beheshti University of Medical Sciences”.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shaian Tavakolian, Email: shaian.tvk@gmail.com.
Ebrahim Faghihloo, Email: faghihloo@gmail.com.
References
- 1.Yasbolaghi Sharahi J, Aliakbar Ahovan Z, Taghizadeh Maleki D, Riahi Rad Z, Riahi Rad Z, et al. In vitro antibacterial activity of curcumin-meropenem combination against extensively drug-resistant (XDR) bacteria isolated from burn wound infections. Avicenna J Phytomed. 2020;10(1):3–10. [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmoudi M, Taghavi-Farahabadi M, Namaki S, Baghaei K, Rayzan E, et al. Exosomes derived from mesenchymal stem cells improved function and survival of neutrophils from severe congenital neutropenia patients in vitro. Hum Immunol. 2019;80(12):990–998. doi: 10.1016/j.humimm.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Faghihloo E, Saremi MR, Mahabadi M, Akbari H, Saberfar E. Prevalence and characteristics of epstein–barr virus- associated gastric cancer in Iran. Arch Iran Med. 2014;17(11):767–770. [PubMed] [Google Scholar]
- 4.Mirzaei H, Goudarzi H, Eslami G, Faghihloo E. Role of viruses in gastrointestinal cancer. J Cell Physiol. 2018;233(5):4000–4014. doi: 10.1002/jcp.26194. [DOI] [PubMed] [Google Scholar]
- 5.Faghihloo E, Yavarian J, Jandaghi NZ, Shadab A, Azad TM. Genotype circulation pattern of human respiratory syncytial virus in Iran. Infect Genet Evol. 2014;22:130–133. doi: 10.1016/j.meegid.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Pormohammad A, Azimi T, Falah F, Faghihloo E. Relationship of human herpes virus 6 and multiple sclerosis: a systematic review and meta-analysis. J Cell Physiol. 2018;233(4):2850–2862. doi: 10.1002/jcp.26000. [DOI] [PubMed] [Google Scholar]
- 7.Faghihloo E, Akbari A, Adjaminezhad-Fard F, Mokhtari-Azad T. Transcriptional regulation of E-cadherin and oncoprotein E7 by valproic acid in HPV positive cell lines. Iran J Basic Med Sci. 2016;19(6):601–607. [PMC free article] [PubMed] [Google Scholar]
- 8.Taghavi-Farahabadi M, Mahmoudi M, Hashemi SM, Rezaei N. Evaluation of the effects of mesenchymal stem cells on neutrophils isolated from severe congenital neutropenia patients. Int Immunopharmacol. 2020;83:106463. doi: 10.1016/j.intimp.2020.106463. [DOI] [PubMed] [Google Scholar]
- 9.Bahramian A, Khoshnood S, Shariati A, Doustdar F, Chirani AS, Heidary M. Molecular characterization of the pilS2 gene and its association with the frequency of Pseudomonas aeruginosa plasmid pKLC102 and PAPI-1 pathogenicity island. Infect Drug Resist. 2019;12:221–227. doi: 10.2147/IDR.S188527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahramian A, Shariati A, Azimi T, Sharahi JY, et al. First report of New Delhi metallo-β-lactamase-6 (NDM-6) among Klebsiella pneumoniae ST147 strains isolated from dialysis patients in Iran. Infect Genet Evol. 2019;69:142–145. doi: 10.1016/j.meegid.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 11.Draetta G. Cell cycle control in eukaryotes: molecular mechanisms of cdc2 activation. Trends Biochem Sci. 1990;15(10):378–383. doi: 10.1016/0968-0004(90)90235-4. [DOI] [PubMed] [Google Scholar]
- 12.Norbury C, Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- 13.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 14.Khajehzadeh M, et al. Insight to the molecular mechanisms of the osmolyte effects on mycobacterium tuberculosis pyrazinamidase stability using experimental studies, molecular dynamics simulations, and free energy calculation. Int J Mycobacteriol. 2018;7(3):268–274. doi: 10.4103/ijmy.ijmy_64_18. [DOI] [PubMed] [Google Scholar]
- 15.Russo AA, Jeffrey PD, Patten AK, Massague J, Pavletich N. Nature (London) 1996;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- 16.Vlach J, Hennecke S, Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Saha P, Kornbluth S, Dynlacht BD, Dutta A. Mol Cell Biol. 1996;16:4673–4682. doi: 10.1128/mcb.16.9.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams PD, Sellers WR, Sharma SK, Wu AD, Nalin CM, Kaelin WG., Jr Mol Cell Biol. 1996;16:6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chevalier S, Blow JJ. Cell cycle control of replication initiation in eukaryotes. Curr Opin Cell Biol. 1996;8:815–821. doi: 10.1016/s0955-0674(96)80082-2. [DOI] [PubMed] [Google Scholar]
- 20.Pines J. Cyclins and cyclin-dependent kinases: take your partners. Trends Biochem Sci. 1993;18:195–197. doi: 10.1016/0968-0004(93)90185-p. [DOI] [PubMed] [Google Scholar]
- 21.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 22.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 23.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 24.Polyak K, Kato J, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-CDK inhibitor, links transforming growth factor β and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 25.Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 26.Lee MH, Reynisdottir I, Massague J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- 27.Besson A, Dowdy SF, Roberts JM. CDK Inhibitors: Cell Cycle Regulators and Beyond. Genes Dev. 1999;13:1501–1512. [Google Scholar]
- 28.Chiu HC, Huang WR, Liao TL, Chi PI, Nielsen BL, et al. Mechanistic insights into avian reovirus p17-modulated suppression of cell cycle CDK-cyclin complexes and enhancement of p53 and cyclin H interaction. J Biol Chem. 2018;293(32):12542–12562. doi: 10.1074/jbc.RA118.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taheri F, Goudarzi H, Faghihloo E. Aneuploidy and oncoviruses. Rev Med Virol. 2019;29(6):e2076. doi: 10.1002/rmv.2076. [DOI] [PubMed] [Google Scholar]
- 30.Mousavi SR, Hemmat N, Bannazadeh Baghi H, Derakhshani A, Tommasi S, Brunetti O, et al. Signaling pathways in cervical Cancer Chemoresistance: are microRNAs and long-noncoding RNAs the Main culprits? Preprints. Yamagata Med J. 2015;33(2):61–69.
- 31.Faghihloo E, Sadeghizadeh M, Shahmahmoodi S, Mokhtari-Azad T. Cdc6 expression is induced by HPV16 E6 and E7 oncogenes and represses E-cadherin expression. Cancer Gene Ther. 2016b. 10.1038/cgt.2016.51. [DOI] [PubMed]
- 32.Hemmat N, Baghi HB. The interaction of human papillomaviruses and adeno-associated viruses in suppressive co-infections. Infect Genet Evol. 2019;73:66–70. doi: 10.1016/j.meegid.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Shin MK, Balsitis S, Brake T, Lambert PF. Human papillomavirus E7 oncoprotein overrides the tumor suppressor activity of p21Cip1 in cervical carcinogenesis. Cancer Res. 2009;69(14):5656–5663. doi: 10.1158/0008-5472.CAN-08-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He W, Staples D, Smith C, Fisher C. Direct activation of cyclin-dependent kinase 2 by human papillomavirus E7. J Virol. 2003;77(19):10566–10574. doi: 10.1128/JVI.77.19.10566-10574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Funk J, Waga S, Harry J, Espling E, Stillman B, Galloway D. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 1997;11(16):2090–2100. doi: 10.1101/gad.11.16.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding Q, Li L, Whyte P. Human papillomavirus 18 E1^E4 protein interacts with cyclin a/CDK 2 through an RXL motif. Mol Cell Biochem. 2013;373:29–40. doi: 10.1007/s11010-012-1472-y. [DOI] [PubMed] [Google Scholar]
- 37.Martin L, Demers G, Galloway D. Disruption of the G1/S transition in human papillomavirus type 16 E7-expressing human cells is associated with altered regulation of cyclin E. J Virol. 1998;72(2):975–985. doi: 10.1128/jvi.72.2.975-985.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim YT, Cho NH, Park SW, Kim JW. Underexpression of cyclin-dependent kinase (CDK) inhibitors in cervical carcinoma. Gynecol Oncol. 1998;71(1):38–45. doi: 10.1006/gyno.1998.5134. [DOI] [PubMed] [Google Scholar]
- 39.Syrjänen SM, Syrjänen KJ. New concepts on the role of human papillomavirus in cell cycle regulation. Ann Med. 1999;31(3):175–187. doi: 10.3109/07853899909115976. [DOI] [PubMed] [Google Scholar]
- 40.Yim EK, Park JS. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res Treat. 2005;37(6):319–324. doi: 10.4143/crt.2005.37.6.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zehbe I, Rätsch A, Alunni-Fabbroni M, Burzlaff A, Bakos E, Dürst M, Wilander E, Tommasino M. Overriding of cyclin-dependent kinase inhibitors by high and low risk human papillomavirus types: evidence for an in vivo role in cervical lesions. Oncogene. 1999;18(13):2201–2211. doi: 10.1038/sj.onc.1202549. [DOI] [PubMed] [Google Scholar]
- 42.Cho NH, Ahn HJ, Kim YT, Kim JW. Correlation of GI Cyclins and Cyclin-dependent kinase inhibitors relative to human papillomavirus infection in the uterine cervical lesions. Int J Surg Pathol. 1999;7(2):61–71.
- 43.Kim JE, Lee JI, Jin DH, Lee WJ, Park GB, Kim S, et al. Sequential treatment of HPV E6 and E7-expressing TC-1 cells with bortezomib and celecoxib promotes apoptosis through p-p38 MAPK-mediated downregulation of cyclin D1 and CDK2. Oncol Rep. 2014;31(5):2429–2437. doi: 10.3892/or.2014.3082. [DOI] [PubMed] [Google Scholar]
- 44.Mighty KK, Laimins LA. P63 is necessary for the activation of human papillomavirus late viral functions upon epithelial differentiation. J Virol. 2011;85:8863–8869. doi: 10.1128/JVI.00750-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang HK, Duffy AA, Broker TR, Chow LT. Robust production and passaging of infectious HPV in squamous epithelium of primary human keratinocytes. Genes Dev. 2009;23:181–194. doi: 10.1101/gad.1735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen CL, Munger K. Direct association of the HPV16 E7 oncoprotein with cyclin a/CDK2 and cyclin E/CDK2 complexes. Virology. 2008;380:21–25. doi: 10.1016/j.virol.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen DX, Westbrook TF, McCance DJ. Human papillomavirus type 16 E7 maintains elevated levels of the CDC25A tyrosine phosphatase during deregulation of cell cycle arrest. J Virol. 2002;76:619–632. doi: 10.1128/JVI.76.2.619-632.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katich SC, Zerfass-Thome K, Hoffmann I. Regulation of the CDC25A gene by the human papillomavirus type 16 E7 oncogene. Oncogene. 2001;20:543–550. doi: 10.1038/sj.onc.1204130. [DOI] [PubMed] [Google Scholar]
- 49.Moles R, Bellon M, Nicot C. STAT1: a novel target of miR-150 and miR-223 is involved in the proliferation of HTLV-I-transformed and ATL cells. Neoplasia. 2015;17:449–462. doi: 10.1016/j.neo.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cereseto A, Washington Parks R, Rivadeneira E, Franchini G. Limiting amounts of p27Kip1 correlates with constitutive activation of cyclin E-CDK2 complex in HTLV-I-transformed T-cells. Oncogene. 1999;18:2441–2450. doi: 10.1038/sj.onc.1202567. [DOI] [PubMed] [Google Scholar]
- 51.Kibler KV, Jeang KT. CREB/ATF-dependent repression of cyclin a by human T-cell leukemia virus type 1 tax protein. J Virol. 2001;75:2161–2173. doi: 10.1128/JVI.75.5.2161-2173.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baydoun HH, Pancewicz J, Bai X, Nicot C. HTLV-I p30 inhibits multiple S phase entry checkpoints, decreases cyclin E-CDK2 interactions and delays cell cycle progression. Mol Cancer. 2010;9:302. doi: 10.1186/1476-4598-9-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grassmann R, Aboud M, Jeang KT. Molecular mechanisms of cellular transformation by HTLV-1 tax. Oncogene. 2005;24(39):5976–5985. doi: 10.1038/sj.onc.1208978. [DOI] [PubMed] [Google Scholar]
- 54.Santiago F, Clark E, Chong S, Molina C, Mozafari F, Mahieux R, et al. Transcriptional up-regulation of the Cyclin D2 gene and Acquisition of new Cyclin-Dependent Kinase Partners in human T-cell leukemia virus type 1-infected cells. J Virol. 1999;73(12):9917–9927. doi: 10.1128/jvi.73.12.9917-9927.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki T, Narita T, Uchida-Toita M, Yoshida M. Down-regulation of the INK4 family of Cyclin-dependent kinase inhibitors by tax protein of HTLV-1 through two distinct mechanisms. Virology. 1999;259(2):384–391. doi: 10.1006/viro.1999.9760. [DOI] [PubMed] [Google Scholar]
- 56.Haller K, Wu Y, Derow E, Schmitt I, Jeang KT, Grassmann R. Physical interaction of human T-cell leukemia virus type 1 tax with Cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol Cell Biol. 2002;22(10):3327–3338. doi: 10.1128/MCB.22.10.3327-3338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knight JS, Robertson ES. Epstein-Barr virus nuclear antigen 3C regulates cyclin a/p27 complexes and enhances cyclin A-dependent kinase activity. J Virol. 2004;78(4):1981–1991. doi: 10.1128/JVI.78.4.1981-1991.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parker GA, Crook T, Bain M, Sara EA, Farrell PJ, Allday MJ. Epstein-Barr virus nuclear antigen (EBNA3c) is an immortalizing ancoprotein with simila properties to adenovirus E1A and papillomavirusE7. Oncogene. 1996;13(12):2541–2549. [PubMed] [Google Scholar]
- 59.Parker GA, Crook T, Bain M, Sara EA, Farrell PJ, Allday MJ. Epstein-Barr virus nuclear antigen (EBNA)3C is an immortalizing oncoprotein with similar properties to adenovirus E1A and papillomavirus E7. Oncogene. 1996;13:2541–2549. [PubMed] [Google Scholar]
- 60.Kumar P, Murakami M, Kaul R, Saha A, Cai Q, Robertson ES. Deregulation of the cell cycle machinery by Epstein-Barr virus nuclear antigen 3C. Future Virol. 2009;4(1):79–91. doi: 10.2217/17460794.4.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwahori S, Murata T, Kudoh A, Sato Y, Nakayama S, Isomura H, et al. Phosphorylation of p27Kip1 by Epstein-Barr virus protein kinase induces its degradation through SCFSkp2 ubiquitin ligase actions during viral lytic replication. J Biol Chem. 2009;284(28):18923–18931. doi: 10.1074/jbc.M109.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tursiella ML, Bowman ER, Wanzeck KC, Throm RE, Liao J, Zhu J, et al. Epstein-Barr virus nuclear antigen 3A promotes cellular proliferation by repression of the cyclin-dependent kinase inhibitor p21WAF1/CIP1. PLoS Pathog. 2014;10(10):e1004415. doi: 10.1371/journal.ppat.1004415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato Y, Shirata N, Murata T, Nakasu S, Kudoh A, Iwahori S, et al. Transient increases in p53-responsible gene expression at early stages of Epstein-Barr virus productive replication. Cell Cycle. 2010;9(4):807–814. doi: 10.4161/cc.9.4.10675. [DOI] [PubMed] [Google Scholar]
- 64.Hollyoake M, Stühler A, Farrell P, Gordon J, Sinclair A. The Normal cell cycle activation program is exploited during the infection of quiescent B lymphocytes by Epstein-Barr Virus1. Cancer Res. 1995;55(21):4784–4787. [PubMed] [Google Scholar]
- 65.Fish K, Chen J, Longnecker R. Epstein-Barr virus latent membrane protein 2A enhances MYC-driven cell cycle progression in a mouse model of B lymphoma. Blood. 2014;123(4):530–540. doi: 10.1182/blood-2013-07-517649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vo QN, Geradts J, Gulley ML, Boudreau DA, Bravo JC, Schneider BG. Epstein-Barr virus in gastric adenocarcinomas: association with ethnicity and CDKN2A promoter methylation. J Clin Pathol. 2002;55(9):669–675. doi: 10.1136/jcp.55.9.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li M, Lee H, Yoon DW, Albrecht JC, Fleckenstein B, Neipel F, Jung JU. Kaposi’s sarcoma-associated herpesvirus encodes a functional cyclin. J Virol. 1997;71:1984–1991. doi: 10.1128/jvi.71.3.1984-1991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang PC, Li M. Kaposi’s sarcoma-associated Herpesvirus K-Cyclin interacts with Cdk9 and stimulates Cdk9-mediated phosphorylation of p53 tumor suppressor. J Virol. 2008;82(1):278–290. doi: 10.1128/JVI.01552-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moore PS, Chang Y. Molecular virology of Kaposi's sarcoma-associated herpesvirus. Philos Trans R Soc Lond Ser B Biol Sci. 2001;356(1408):499–516. doi: 10.1098/rstb.2000.0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Dross R, Yao S, Asad S, Westlake G, Mays DJ, Barquero L, et al. Constitutively active K-cyclin/cdk6 kinase in Kaposi sarcoma – associated Herpesvirus – infected cells. J Natl Cancer Inst. 2005;97(9):656–666. doi: 10.1093/jnci/dji113. [DOI] [PubMed] [Google Scholar]
- 71.Han J, Yoo HY, Choi BH, Rho HM. Selective transcriptional regulations in the human liver cell by hepatitis B viral X protein. Biochem Biophys Res Commun. 2000;272:525–530. doi: 10.1006/bbrc.2000.2801. [DOI] [PubMed] [Google Scholar]
- 72.Bouchard M, Giannakopoulos S, Wang EH, Tanese N, Schneider RJ. Hepatitis B virus HBx protein activation of cyclin A-cyclin-dependent kinase 2 complexes and G1 transit via a Src kinase pathway. J Virol. 2001;75(9):4247–4257. doi: 10.1128/JVI.75.9.4247-4257.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jung JK, Arora P, Pagano JS, Jang KL. Expression of DNA methyltransferase 1 is activated by hepatitis B virus X protein via a regulatory circuit involving the p16INK4a-cyclin D1-CDK 4/6-pRb-E2F1 pathway. Cancer Res. 2007;67(12):5771–5778. doi: 10.1158/0008-5472.CAN-07-0529. [DOI] [PubMed] [Google Scholar]
- 74.Ohkawa K, Ishida H, Nakanishi F, Hosui A, Ueda K, Takehara T, et al. Hepatitis C virus core functions as a suppressor of cyclin-dependent kinase-activating kinase and impairs cell cycle progression. J Biol Chem. 2004;279(12):11719–11726. doi: 10.1074/jbc.M308560200. [DOI] [PubMed] [Google Scholar]
- 75.Bahnassy AA, Zekri AR, Loutfy SA, Mohamed WS, Moneim AA, Salem SE, et al. The role of cyclins and cyclin dependent kinases in development and progression of hepatitis C virus-genotype 4-associated hepatitis and hepatocellular carcinoma. Exp Mol Pathol. 2011;91(2):643–652. doi: 10.1016/j.yexmp.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 76.Arima N, Kao CY, Licht T, Padmanabhan R, Sasaguri Y, Padmanabhan R. Modulation of cell growth by the hepatitis C virus nonstructural protein NS5A. J Biol Chem. 2001;276:12675–12684. doi: 10.1074/jbc.M008329200. [DOI] [PubMed] [Google Scholar]
- 77.Wagayama H, Shiraki K, Yamanaka T, Sugimoto K, Ito T, Fujikawa K, Takase K, Nakano T. p21WAF1/CTP1 expression and hepatitis virus type. Dig Dis Sci. 2001;46:2074–2079. doi: 10.1023/a:1011977923941. [DOI] [PubMed] [Google Scholar]
- 78.Shackel NA, McGuinness PH, Abbott CA, Gorrell MD, McCaughan GW. Insights into the pathobiology of hepatitis C virus-associated cirrhosis: analysis of intrahepatic differential gene expression. Am J Pathol. 2002;160:641–654. doi: 10.1016/S0002-9440(10)64884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang W, Chi P, Chiu H, Hsu J, Nielsen B, Liao T, Liu H. Avian reovirus p17 and σA act cooperatively to downregulate Akt by suppressing mTORC2 and CDK2/cyclinA2 and upregulating proteasome subunit PSMB6. Sci Rep. 2017;7:5226–5244. doi: 10.1038/s41598-017-05510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kozak R, Hattin L, Biondi M, Corredor J, Walsh S, et al. Replication and Oncolytic activity of an avian Orthoreovirus in human hepatocellular carcinoma cells. Viruses. 2017;9(4):90. doi: 10.3390/v9040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chenari M, Norouzi M, Ghalichi L, Rezaee A, Yari A, Alavian SM, Jazayeri SM. Characterization of overt and occult hepatitis B virus infection among HTLV-1 positive healthy carriers in the northeast of Iran;an HTLV-I endemic area. J Med Virol. 2014;86(11):1861–1867. doi: 10.1002/jmv.24046. [DOI] [PubMed] [Google Scholar]
- 82.Hemmat N, Bannazadeh BH. Effects of neglect and stigmatisation on post-therapy behaviour of patients who are HIV-positive. Sex Transm Infect. 2019;95(7):548. doi: 10.1136/sextrans-2019-054031. [DOI] [PubMed] [Google Scholar]
- 83.Cook LB, Taylor GP. HTLV-1 and HTLV-2 prevalence in the United States. J Infect Dis. 2014;209(4):486–487. doi: 10.1093/infdis/jit558. [DOI] [PubMed] [Google Scholar]
- 84.Farid R, Farid F, Rezaee SA. Prevalence of HTLV-1 infection in northeast of Iran. Retrovirology. 2015;12(Suppl 1):O7.
- 85.Cook LB, Melamed A, Niederer H, Valganon M, Laydon D, et al. The role of HTLV-1 clonality, proviral structure, and genomic integration site in adult T-cell leukemia/lymphoma. Blood. 2014;123:3925–3931. doi: 10.1182/blood-2014-02-553602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Thé G, Kazanji M. An HTLV-I/II vaccine: from animal models to clinical trials? J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13(Suppl 1):S191–S198. doi: 10.1097/00042560-199600001-00029. [DOI] [PubMed] [Google Scholar]
- 87.Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24(39):6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 88.Matsuoka M, Yasunaga J. Human T-cell leukemia virus type 1: replication, proliferation and propagation by tax and HTLV-1 bZIP factor. Curr Opin Virol. 2013;3(6):684–691. doi: 10.1016/j.coviro.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 89.Hanon E, Hall S, Taylor GP, Saito M, Davis R, Tanaka Y, et al. Abundant tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-I) is prevented by cytotoxic T lymphocytes. Blood. 2000;95(4):1386–1392. [PubMed] [Google Scholar]
- 90.Philip S, Zahoor MA, Zhi H, Ho YK, Giam CZ. Regulation of human T-lymphotropic virus type I latency and reactivation by HBZ and Rex. PLoS Pathog. 2014;10(4):e1004040. doi: 10.1371/journal.ppat.1004040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li J, Li H, Tsai MD. Direct bind of the N terminal of HTLV-1 tax oncoprotein to cyclin4 is a dominant path to stimulate the kinase mortality. Biochemistry. 2003;42(22):6921–6928. doi: 10.1021/bi034369n. [DOI] [PubMed] [Google Scholar]
- 92.Akagi T, Ono H, Shimotohno K. Expression of cell-cycle regulatory genes in HTLV-I infected T-cell lines: possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4 and p21Waf1/Cip1/Sdi1. Oncogene. 1996;12(8):1645–1652. [PubMed] [Google Scholar]
- 93.Ramanian RP, Wildschutte JH, Russo C, Coffin JM. Identification, characterization, and comparative genomic distribution of the HERV-K (HML-2) group of human endogenous retroviruses. Retrovirology. 2011;8:90. doi: 10.1186/1742-4690-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johanning GL, Malouf GG, Zheng X, Esteva FJ, Weinstein JN, Wang-Johanning F, et al. Expression of human endogenous retrovirus-K is strongly associated with the basal-like breast cancer phenotype. Sci Rep. 2017;7:41960. doi: 10.1038/srep41960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dong J, Huang G, Imtiaz R, Xu F. The Potential Importance of K Type Human Endogenous Retroviral Elements in Melanoma Biology. 2012. [Google Scholar]
- 96.Ambinder RF. Hum. Lymphotropic viruses associated with lymphoid malignancy: Epstein-Barr and HTLV-1. Hematol Oncol Clin North Am. 1990;4:821–833. [PubMed] [Google Scholar]
- 97.Taghizadeh Maleki D, Goudarzi AM, Golrokh Mofrad M, Faghihloo E. Viral infections in intensive care unit patients. Novel Biomed. 2019;7(2):84–95. [Google Scholar]
- 98.Hui KF, Yiu SPT, Tam KP, AKs C. Viral-Targeted Strategies Against EBV-Associated Lymphoproliferative Diseases. Front Oncol. 2019;9:81. doi: 10.3389/fonc.2019.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang W, Zeng Z, Zhou Y, Xiong W, Fan S, Xiao L, et al. Identification of aberrant cell cycle regulation in Epstein–Barr virus-associated nasopharyngeal carcinoma by cDNA microarray and gene set enrichment analysis. Acta Biochim Biophys Sin Shanghai. 2009;41(5):414–428. doi: 10.1093/abbs/gmp025. [DOI] [PubMed] [Google Scholar]
- 100.Boshoff C. Kaposi virus scores cancer coup. Nat Med. 2003;9:261–262. doi: 10.1038/nm0303-261. [DOI] [PubMed] [Google Scholar]
- 101.Boulanger E, Gerard L, Gabarre J, Molina JM, Rapp C, Abino JF, Cadranel J, Chevret S, Oksenhendler E. Prognostic factors and outcome of human herpesvirus 8-associated primary effusion lymphoma in patients with AIDS. J Clin Oncol. 2005;23:4372–4380. doi: 10.1200/JCO.2005.07.084. [DOI] [PubMed] [Google Scholar]
- 102.Oksenhendler E, Boulanger E, Galicier L, Du MQ, Dupin N, Diss TC, Hamoudi R, Daniel MT, Agbalika F, Boshoff C, Clauvel JP, Isaacson PG, Meignin V. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood. 2002;99:2331–2336. doi: 10.1182/blood.v99.7.2331. [DOI] [PubMed] [Google Scholar]
- 103.Cannell E, Mittnacht S. Viral encoded cyclins. Semin Cancer Biol. 1999;9:221–229. doi: 10.1006/scbi.1999.0090. [DOI] [PubMed] [Google Scholar]
- 104.Ojala PM, Tiainen M, Salven P, Veikkola T, Castanos-Velez E, Sarid R, Biberfeld P, Makela TP. Kaposi’s sarcoma-associated herpesvirus-encoded v-cyclin triggers apoptosis in cells with high levels of cyclin-dependent kinase 6. Cancer Res. 1999;59:4984–4989. [PubMed] [Google Scholar]
- 105.Ojala PM, Yamamoto K, Castanos-Velez E, Biberfeld P, Korsmeyer SJ, Makela TP. The apoptotic v-cyclin-CDK6 complex phosphorylates and inactivates Bcl-2. Nat Cell Biol. 2000;2:819–825. doi: 10.1038/35041064. [DOI] [PubMed] [Google Scholar]
- 106.Pekkonen P, Järviluoma A, Zinovkina N, Cvrljevic A, Prakash S, Westermarck J, et al. KSHV viral cyclin interferes with T-cell development and induces lymphoma through Cdk6 and notch activation in vivo. Cell Cycle. 2014;13(23):3670–3684. doi: 10.4161/15384101.2014.964118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sarek G, Järviluoma A, Ojala PM. KSHV viral cyclin inactivates p27KIP1 through Ser10 and Thr187 phosphorylation in proliferating primary effusion lymphomas. Blood. 2006;107(2):725–732. doi: 10.1182/blood-2005-06-2534. [DOI] [PubMed] [Google Scholar]
- 108.Verschuren EW, Jones N, Evan GI. The cell cycle and how it is steered by Kaposi’s sarcoma-associated herpesvirus cyclin. J Gen Virol. 2004;85:1347–1361. doi: 10.1099/vir.0.79812-0. [DOI] [PubMed] [Google Scholar]
- 109.Swanton C, Mann DJ, Fleckenstein B, Neipel F, Peters G, Jones N. Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature. 1997;390:184–187. doi: 10.1038/36606. [DOI] [PubMed] [Google Scholar]
- 110.Leidal AM, Cyr DP, Hill RJ, Lee PW, McCormick C. Subversion of autophagy by Kaposi sarcoma associated herpesvirus impair oncogene induced senescenses. Cell Host Microbe. 2016;19(6):901. doi: 10.1016/j.chom.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 111.Benvegnu L, Alberti A. Patterns of hepatocellular carcinoma development in hepatitis B virus and hepatitis C virus related cirrhosis. Antiviral Res. 2001;52:199–207. doi: 10.1016/s0166-3542(01)00185-1. [DOI] [PubMed] [Google Scholar]
- 112.Koike K, Kobayashi M, Gondo M, Hayashi I, Osuga T, Takada S. Hepatitis B virus DNA is frequently found in liver biopsy samples from hepatitis C virus-infected chronic hepatitis patients. J Med Virol. 1998;54:249–255. [PubMed] [Google Scholar]
- 113.Shibata Y, Nakata K, Tsuruta S, Hamasaki K, Hayashida Y, Kato Y, Nakao K, Eguchi K. Int J Oncol. 1999;14:1153–1156. doi: 10.3892/ijo.14.6.1153. [DOI] [PubMed] [Google Scholar]
- 114.Villa E, Grottola A, Buttafoco P, Colantoni A, Bagni A, Ferretti I, Cremonini C, Bertani H, Manenti F. Am J Gastroenterol. 2001;96:2973–2977. doi: 10.1111/j.1572-0241.2001.04670.x. [DOI] [PubMed] [Google Scholar]
- 115.Murakami S. Hepatitis B virus X protein: a multifunctional viral regulator. J. Gastroenterol. 2001;36:651–660. doi: 10.1007/s005350170027. [DOI] [PubMed] [Google Scholar]
- 116.Park US, Park SK, Lee YI, Park JG, Lee YI. Hepatitis B virus-X protein upregulates the expression of p21waf1/cip1 and prolongs G1 to S transition via a p53-independent pathway in human hepatoma cells. Oncogene. 2000;19:3384–3394. doi: 10.1038/sj.onc.1203674. [DOI] [PubMed] [Google Scholar]
- 117.Kwun HJ, Jang KL. Natural variants of hepatitis B virus X protein have differential effects on the expression of cyclin-dependent kinase inhibitor p21 gene. Nucleic Acids Res. 2004;32(7):2202–2213. doi: 10.1093/nar/gkh553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yen A, Sturgill R, Varvayanis S, Chern R. FMS (CSF-1 receptor) prolongs cell cycle and promotes retinoic acid-induced hypophosphorylation of retinoblastoma protein, G1 arrest and cell differentiation. Exp Cell Res. 1996;229:111–125. doi: 10.1006/excr.1996.0349. [DOI] [PubMed] [Google Scholar]
- 119.Hsieh YH, Su IJ, Wang HC, Tsai JH, Huang YJ, Chang WW, et al. Hepatitis B virus pre-S2 mutant surface antigen induces degradation of cyclin-dependent kinase inhibitor p27Kip1 through c-Jun activation domain-binding protein 1. Mol Cancer Res. 2007;5(10):1063–1072. doi: 10.1158/1541-7786.MCR-07-0098. [DOI] [PubMed] [Google Scholar]
- 120.Benn J, Schneider RJ. Hepatitis B virus HBx protein deregulates cell cycle checkpoint controls. Proc Natl Acad Sci U S A. 1995;92(24):11215–11219. doi: 10.1073/pnas.92.24.11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Choo QL, Richman KH, Han JH, Berger K, Lee C, Dong C, Gallegos C, Coit D, Medina-Selby A, Barr PJ, Weiner AJ, Bradley DW, Kuo G, Houghton M. Proc Natl Acad Sci U S A. 1991;88:2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yoshida T, Hanada T, Tokuhisa T, Kosai K, Sata M, Kohara M, Yoshimura A. J Exp Med. 2002;196:641–653. doi: 10.1084/jem.20012127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tsutsumi T, Suzuki T, Shimoike T, Moriya K, Shintani Y, Fujie H, Matsuura Y, Koike K, Miyamura T. Hepatology. 2002;35:937–946. doi: 10.1053/jhep.2002.32470. [DOI] [PubMed] [Google Scholar]
- 124.Han HJ, Jung EY, Lee WJ, Jang KL. Cooperative repression of cyclin-dependent kinase inhibitor p21 gene expression by hepatitis B virus X protein and hepatitis C virus core protein. FEBS Lett. 2002;518(1–3):169–172. doi: 10.1016/s0014-5793(02)02694-7. [DOI] [PubMed] [Google Scholar]
- 125.Kemp V, Hoeben RC, van den Wollenberg DJ. Exploring Reovirus plasticity for improving its use as Oncolytic virus. Viruses. 2016;8(1):4. doi: 10.3390/v8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao X, Chester C, Rajasekaran N, He Z, Kohrt HE. Strategic combinations: the future of Oncolytic Virotherapy with Reovirus. Mol Cancer Ther. 2016;15(5):767–773. doi: 10.1158/1535-7163.MCT-15-0695. [DOI] [PubMed] [Google Scholar]
- 127.Phillips MB, Stuart JD, Rodríguez Stewart RM, Berry JT, Mainou BA, et al. Current understanding of reovirus oncolysis mechanisms. Oncolytic Virother. 2018;7:53–63. doi: 10.2147/OV.S143808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chiu HC, Huang WR, Wang YY, Li JY, Liao TL, et al. Heterogeneous Nuclear Ribonucleoprotein A1 and Lamin A/C Modulate Nucleocytoplasmic Shuttling of Avian Reovirus p17. J Virol. 2019;93:e00851-19. [DOI] [PMC free article] [PubMed]
- 129.Huang W, Chiu H, Liao T, Chuang K, Shih W, et al. Correction: Avian Reovirus Protein p17 Functions as a Nucleoporin Tpr Suppressor Leading to Activation of p53, p21 and PTEN and Inactivation of PI3K/AKT/mTOR and ERK Signaling Pathways. PLoS ONE. 2015;10(9):e0138627. [DOI] [PMC free article] [PubMed]
- 130.Liu HJ, Lin PY, Lee JW, Hsu HY, Shih WL. Retardation of cell growth by avian reovirus p17 through the activation of p53 pathway. Biochem Biophys Res Commun. 2005;336(2):709–715. doi: 10.1016/j.bbrc.2005.08.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chiu H-C, Huang W-R, Liao T-L, Wu H-Y, Munir M Shih W-L, et al. Suppression of Vimentin Phosphorylation by the Avian Reovirus p17 through Inhibition of CDK1 and Plk1 Impacting the G2/M Phase of the Cell Cycle. PLoS ONE. 2016;11(9):e0162356. [DOI] [PMC free article] [PubMed]
- 132.Chi PI, Huang WR, Lai IH, Cheng CY, Liu HJ. The p17 nonstructural protein of avian reovirus triggers autophagy enhancing virus replication via activation of phosphatase and tensin deleted on chromosome 10 (PTEN) and AMP-activated protein kinase (AMPK), as well as dsRNA-dependent protein kinase (PKR)/eIF2α signaling pathways. J Biol Chem. 2013;288(5):3571–3584. doi: 10.1074/jbc.M112.390245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ji WT, Wang L, Lin RC, Huang WR, Liu HJ. Avian reovirus influences phosphorylation of several factors involved in host protein translation including eukaryotic translation elongation factor 2 (eEF2) in Vero cells. Biochem Biophys Res Commun. 2009;384(3):301–305. doi: 10.1016/j.bbrc.2009.04.116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact author for data requests.