Abstract
Parental responses to their children are crucially influenced by stress. However, brain-based mechanistic understanding of the adverse effects of parenting stress and benefits of therapeutic interventions is lacking. We studied maternal brain responses to salient child signals as a function of Mom Power (MP), an attachment-based parenting intervention established to decrease maternal distress. Twenty-nine mothers underwent two functional magnetic resonance imaging brain scans during a baby-cry task designed to solicit maternal responses to child’s or self’s distress signals. Between scans, mothers were pseudorandomly assigned to either MP (n = 14) or control (n = 15) with groups balanced for depression. Compared to control, MP decreased parenting stress and increased child-focused responses in social brain areas highlighted by the precuneus and its functional connectivity with subgenual anterior cingulate cortex, which are key components of reflective self-awareness and decision-making neurocircuitry. Furthermore, over 13 weeks, reduction in parenting stress was related to increasing child- versus self-focused baby-cry responses in amygdala–temporal pole functional connectivity, which may mediate maternal ability to take her child’s perspective. Although replication in larger samples is needed, the results of this first parental-brain intervention study demonstrate robust stress-related brain circuits for maternal care that can be modulated by psychotherapy.
Maternal caregiving requires sensitive and contingent response for each mother to her own child’s distress signals, such as cries. Maternal sensitivity thus involves a complex array of brain activity to support the required thoughts and behaviors, including recognition and acknowledgment of signal salience, reflective self-awareness, and emotion regulation toward social attachment (Ainsworth & Bell, 1970; Bowlby, 1958; Swain, Mayes, & Leckman, 2004). Studies of brain activity as a function of listening to baby-cry, especially in the early postpartum, have established responses in adaptable social brain circuits, highlighting those concerned with emotion response and regulation, in concert with empathy and theory of mind or consideration of others’ minds (Barrett & Fleming, 2011; Swain, Kim, et al., 2014; Swain, Lorberbaum, Kose, & Strathearn, 2007). The experience of psychosocial stress in the life of the mother may interfere with the brain responses that support adaptive early parenting.
Elucidating the brain mechanisms that underlie maternal thoughts and behaviors promises to inform our understanding of the atypical neural responses that have been identified in mothers who suffer from psychopathology (Feldman et al., 2009), as several brain imaging studies of mothers with depression have demonstrated (Moses-Kolko, Horner, Phillips, Hipwell, & Swain, 2014). Specifically, it has been shown that increased response in the lenticular nucleus and left medial prefrontal cortex to own baby-cry was associated with more depressive symptoms and more anxious intrusive thoughts in a group of 12 healthy mothers who were scanned at approximately 3 weeks postpartum (Swain et al., 2008). In addition, own versus generic baby-cry among depressed versus nondepressed mothers was associated with reduced reward circuit activity (including the caudate and nucleus accumbens; Laurent & Ablow, 2012a). Furthermore, higher depressive symptom severity was also associated with decreased activity in left orbitofrontal, dorsal anterior cingulate, and medial superior frontal regions. Findings from these studies are consistent with allied research showing that depression dampens neural responses when mothers attended to noninfant negative emotional stimuli in emotion regulation circuits (Moses-Kolko et al., 2010; Silverman et al., 2007) and that women’s response to attachment figures in these areas is also related to the Adult Attachment Interview measures (Yaseen, Zhang, Muran, Winston, & Galynker, 2016). This paper presents maternal brain responses to child distress as a function of their own level of stress and in response to attachment-based therapeutic parenting intervention.
Among mothers, in addition to differences in functional reactivity in specific brain regions, it has also been suggested that functional connectivity for social and emotional brain circuits may differ and adapt according to depression, stress, experience, and attachment (Kim, Strathearn, & Swain, 2016). For example, women with postpartum depression, as compared to healthy controls, demonstrated decreased functional connectivity between left amygdala and subgenual anterior cingulate cortex (sgACC)/ventromedial prefrontal cortex while viewing fearful and threatening faces (Moses-Kolko et al., 2010). This was interpreted as a lack of top- down emotion regulation of negative emotions among depressed mothers. In a study that used positive baby picture stimuli, depression and trait anxiety was inversely related to amygdala–insula connectivity, which was interpreted as reduced processing of socially and emotionally relevant stimuli, interoception, and evaluation of subjective emotional experience (Wonch et al., 2016). In addition, work using attachment measures connected to parental brain responses to infant stimuli underlines the likely key role of the amygdala. For example, maternal amygdala response to their own infants’ sadness as compared to happiness using picture stimuli was blunted with unresolved early life trauma (Kim, Fonagy, Allen, & Strathearn, 2014) and hyperresponsive to own infant crying with insecure attachment (Riem, Baker-mans-Kranenburg, van IJzendoorn, Out, & Rombouts, 2012). The capacity to utilize top-down regulation of negative emotions, and to attend to and accurately process the social and emotional reactions of one’s own infant are critical elements contributing to sensitive early parenting (Dayton, Huth-Bocks, & Busuito, 2016). Thus, prior work in this area suggests that maternal distress may inhibit the adaptive neurological functioning that supports early parenting. To our knowledge, this study is the first to examine either task-dependent brain responses or connectivity before and after a therapeutic intervention designed to decrease maternal stress and depression.
Mom Power (MP) is an attachment-based parenting intervention that is equipped with cognitive behavioral therapy techniques. MP aims to strengthen maternal capacity for emotion regulation through mindfulness and distress tolerance practices. It provides mothers with pedagogical training and in-session practice on positive parenting, child developmental needs, self-reflection, and perspective taking. It has been shown that mothers undergoing MP exhibit reduced parenting stress, improved mental health (less depression and anxiety), and increased bonding and emotional responsiveness toward their children (Muzik et al., 2015, 2016). We therefore hypothesize that MP reduction of stress would be associated with increased maternal brain response to own versus generic baby-cry in social brain circuits known to be critical for empathy, motivation for sensitive caring, reflective mentalization (the ability to sensitively consider and understand the mental state of her child), and self-awareness. Furthermore, we expect that the connectivity between brain regions associated with empathy and emotion regulation would increase as a function of the MP intervention. Finally, using a novel task that elicits maternal responses to own-child distress signals versus self-focused distress, we explore a possible mechanism to explain brain function, stress response, mental focus, and associated treatment effects among mothers of young children.
Methods
Procedures
Participants were recruited from community health clinics, primary care clinics, and University of Michigan hospitals. All participants were mothers living with at least one biological child. All of them received an initial depression screening, and completed the Parenting Stress Index ((PSI; Abidin, 1995) and other questionnaires around the time of two functional magnetic resonance imaging (fMRI) scanning sessions, with a 12- to 15-week interval between the two scans. The MP group, but not the control group, received the MP intervention between the first (Time 1 [T1]) and second (Time 2 [T2]) scans. All procedures were approved by the University of Michigan’s Institutional Review Board.
Participants
Mothers (n = 29, mean age = 31.64 years, SD = 7.93) of young children (mean age = 2.53 years, SD = 2.37) were recruited and pseudorandomly assigned to MP intervention group (MP, n = 14) or a control group (control, n = 15).
Measures
Maternal depression.
Depression ratings were obtained for each mother based on the Postpartum Depression Screening Scale (Beck & Gable, 2001). The Postpartum Depression Screening Scale is a 35-item validated self-report questionnaire asking about depressive symptoms in the previous 2 weeks that yields a total symptom count (possible range = 35–175) with a clinical cutoff score of >80 indicating depression diagnosis risk. In the current sample (n = 29), there were 14 mothers scoring above the clinical cutoff (referred to as depressed mothers) and 15 mothers below the cutoff (referred to as nondepressed mothers). The relatively high rate of depression in this sample may reflect the fact that these participants were recruited from clinics where relatively more mothers at risk can be found.
Parenting stress.
Around the times of each of the two scans, each participant reported on her own level of parenting stress on the PSI (Abidin, 1995), a 36-item self-report questionnaire with high reliability across diverse populations, including coefficient a of 0.95 with concurrently reported psychological symptoms (Reitman, Currier, & Stickle, 2002). In the current sample, the Cronbach a was 0.93.
fMRI task
Baby-cry task.
The fMRI task for this paper was adapted from our previous work on parents in the postpartum period (Swain & Lorberbaum, 2008; Swain et al., 2007) for use with mothers of older children rather than the first few months postpartum. It has also previously been used to study non-mothers (Kim, Ho, Evans, Liberzon, & Swain, 2015). The baby-cry stimuli were recordings of infant cries that were gathered during the discomfort of a diaper change. Mothers were instructed to listen to the baby-cry stimuli using different frames of reference: (a) listening passively; (b) listening and imagining that their own child were crying; and (c) listening and imagining that that they, the mothers, were the ones crying. In this way, the protocol pulls for different types of empathic concern. To facilitate these distinctions, during the scanning session, the baby-cry stimuli were presented randomly with descriptive primers before each 30-s block of baby-cry sound to simulate (a) passive listening of generic baby-cry (generic baby-cry), (b) listening to baby-cry as if it were your own baby’s (own baby-cry), (c) listening to baby-cry as if it were yourself crying (self baby-cry), and (d) the control sound (white noise). There were two runs of 4.5 min/run, wherein there were three 30-s blocks for each of the four conditions. The fixation period was 10 s long between each of the blocks. The white noise and generic baby-cry conditions have been used in prior work as the standard conditions in the infant cry paradigm (De Pisapia et al., 2013; Laurent & Ablow, 2012a, 2012b; Swain et al., 2007; Venuti et al., 2012). A particularly novel component of this work is that it includes conditions that pull specifically for responses to own infant (own baby-cry) and to distress within the self (self baby-cry). These stimuli are therefore more personally salient to each mother and are designed to elicit a more robust emotional reaction to the personal identity of the baby-cry.
MP intervention.
MP intervention consists of 3 individual sessions and 10 group sessions (Muzik, Rosenblum, & Schuster, 2010). Groups were led by two trained facilitators, and fidelity was monitored via weekly reflective supervision as well as video review of 20% of all sessions using a fidelity monitoring scale (Schuster, 2013). Fidelity was formally assessed using a 5-point Likert scale (5 = highest fidelity) for both content (i.e., adherence to manual=content) and framework (i.e., maintaining the therapeutic framework dedicated to creating a therapeutic milieu based in attachment theory and trauma informed care). Fidelity was found to be excellent across clinicians for both content (M = 4.02, SD = 0.72) and framework (M = 3.85, SD = 0.69).
The MP curriculum parallels the Protective Factors Framework (CSSP, 2015), which is based on core theoretical pillars of attachment-based parenting education, self-care, mother–child interaction practice, and social support (Muzik et al., 2010). Parents learn how to empathically understand and respond to a child’s emotional needs and experiences; how to repair disruptions in the relationship; how to support the child’s emotional development via parent–child emotional coregulation; how to empathically understand a child’s emotional needs and experiences; how to coregulate a child’s emotions; and how to create an atmosphere of warmth, joy, and delight in which their child can learn and grow, which is sometimes summarized as providing a secure base and safe haven for their child (Bowlby, 1978). In addition, parents learn how to practice mindfulness and explore what experiences from the past might impact their parenting and what current experiences may be affecting their children. For a detailed description of the parenting intervention, please see Muzik et al. (2015, 2016). Group participants received transportation incentives ($5 per session), a family-style meal during the group time, and a final $15 incentive if they attended at least 7 of the 10 group sessions.
Control group.
The control condition also involved two individual sessions but instead of 10 weekly in-person group sessions, the control group participants received 10 weekly mailings of the MP curriculum content. Mailings included a prestamped postcard for the mother to send back indicating that the week’s material had been read. Participants were compensated $5 for each postcard returned, and an additional $15 if they returned 7 out of 10 postcards. Thus, both groups received the same remuneration, and both had access to the MP curriculum content. This was not, however, an active control condition because it lacked the social content of the intervention.
Prescan ratings on auditory stimuli used in the fMRI task.
Immediately before the scanning, the participants listened to all 30-s auditory stimuli that would be used in the fMRI task and answered questions on a 5-point Likert scale immediately after listening to each stimulus: for the noise stimuli, there were two questions, “What is the intensity of this noise? (1 = very low, 3 = middle, 5 = very strong) and “How annoying is this noise?” (1 = not at all, 3 = moderate, 5 very annoying); for the infant cries, “What is the intensity of this cry?” (1 = very low, 3 = middle, 5 = very strong) and “How annoying is this cry?” (1 = not at all, 3 = moderate, 5 = very annoying) and “If this crying baby was in the same room with you, how much would you like to move closer to or away from this baby” (1= very much away, 3 = neutral, 5 = very much closer).
fMRI and structural MRI.
During the fMRI scanning session, each participant was positioned in a supine orientation with her head positioned in a head coil. Visual stimuli were presented with E-Prime (PST, Inc., Pittsburgh, PA) via a goggle system and Nordic NeuroLab audio system. Behavioral responses were recorded by a button glove attached to the participant’s right hand and linked to the E-Prime system. All fMRI scanning sessions were performed with a 3.0 Tesla Philips magnetic resonance imaging scanner using a standard eight-channel radiofrequency SENSE head coil with the following acquisitions: (a) a high-resolution T1 scan was acquired to provide precise anatomical localization (resonance time = 9.8 ms, echo time = 459 ms, fractional anisotropy = 8°, field of view = 256 mm, slice thickness of 1.0 mm, 180 slices with 288 × 288 matrix per slice); (b) two runs of T2*-weighted echo planar imaging sequence with blood oxygenation level dependent contrast (190 frames per run, resonance time = 2000 ms, echo time = 30 ms, functional anisotropy = 90°, field of view = 220 mm, 42 contiguous axial slices, slice thickness = 2.8 mm with 64 × 64 matrix per slice, voxel size = 3.44 × 3.44 2.8 mm3) were acquired for whole-brain fMRI blood oxygenation level dependent signal measures during the experimental task.
Data processing and analysis
The fMRI data were preprocessed and analyzed using statistical parametric mapping software (SPM8; Welcome Department of Imaging Neuroscience, London). Five images at the beginning of each fMRI run were discarded to account for magnetic equilibrium. Slice timing correction was performed using a middle slice as a reference (Slice 21). After slice time correction, images within each run were realigned to the mean image of the first run to correct for movement. Realigned functional images and structural image were spatially normalized using DARTEL method in SPM8. The normalized functional images were resliced to 2 × 2 × 2 mm voxels. Images were then spatially smoothed using a Gaussian filter with a full-width half-maximum value of 8 mm. Following preprocessing, a first-level fixed effect model was constructed with a matrix of regressors modeling each trial type (noise, generic baby-cry, own baby-cry, and self baby-cry). Each individual participant’s contrasts of interest at the first level were submitted to second-level random effects analyses of general linear models (GLMs). A model of flexible factorial design was used to examine the two-way analysis of variance, with treatment (MP vs. control), time (pre-and posttreatment), and the Treatment × Time interaction effects. To examine the neurocorrelates of parenting stress, the T2–T1 change in SPM contrast images were computed for each participant and regressed against predictors of the changes in PSI, controlling for the baseline PSI at T1. All statistical parametric maps in the second level models were controlled for whole brain multiple comparison at the cluster level for voxel-wise intensity threshold at p = .005 and cluster-wise size threshold as determined by Analysis of Functional NeuroImages software (Cox, 1996) version 16.1.3.
Results
Group characteristics
The MP and control groups differed in their ages (MP mean age = 27.19, SD = 6.69; control mean age = 35.80, SD = 2.53); F (1, 27) 11.84, MSerror = 45.39, p < 0.01, but not in the number of children (MP mean = 1.36, SD = 0.50; control mean = 1.53, SD= 0.64); F (1,27) = 0.68, MSerror = 0.33, p = 0.42, or their youngest child’s age (MP mean = 1.78, SD = 2.01; control mean = 2.26, SD =2.53); F (1, 27) = 0.32, MSerror = 5.26, p = 0.58. Both MP and control groups included mothers with current depressive mood and mothers without. Specifically, the MP group had seven depressed mothers and seven nondepressed mothers, and the control group had seven depressed mothers and eight nondepressed mothers. The high rate of depression may reflect recruitment from clinics rather than community. The distribution of depressed and nondepressed mothers were balanced between the MP and control groups (X2 0.032, p = .86).
Self-reported ratings on the auditory stimuli
The participants’ ratings of the auditory stimuli’s (intensity and annoyance for noises and cries, and avoidance/approach for cries only) before each of the two scans were submitted to a GLM repeated measurement model, using depression (depressed or nondepressed) and treatment (MP or control) as between-subject independent variables, time as a within-subject independent variable, and the age of the youngest child as a covariate. There were no significant main effects or interaction effects of any independent variables on any of the ratings. These results suggest that task-dependent effects on brain responses during the fMRI task were unlikely to be due to individual differences in how mothers perceived the stimuli.
MP decreases parenting stress
The total scores of PSI were submitted to a GLM repeated measurement model, using depression (depressed or nondepressed) and treatment (MP or control) as between-subject independent variables, time as a within-subject independent variable, and the age of the youngest child as a covariate. There was a main effect of depression on PSI, with the depressed mothers showing higher PSI than the nondepressed mothers (depressed mean = 91.54, SE = 5.19; nondepressed mean = 63.14, SE = 5.25); F (1, 19) = 14.27, MSerror = 610.830, p = .001. There were no significant main effects of other independent variables. There was a marginally significant Treatment × Time interaction effect, where the MP group, as compared to the control, showed greater reduction in PSI over time, F (1, 19) = 4.28, MSerror = 104.627, p = 0.052 (see Figure 1 and Figure 2, left panel), and this pattern was prominent in the depressed mothers. There were no other significant interaction effects see Figure 2, right panel).
Figure 1.
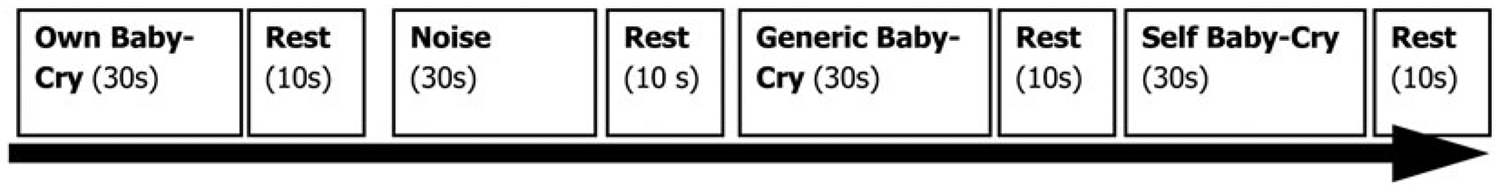
The baby-cry task design. See the Method section for details.
Figure 2.
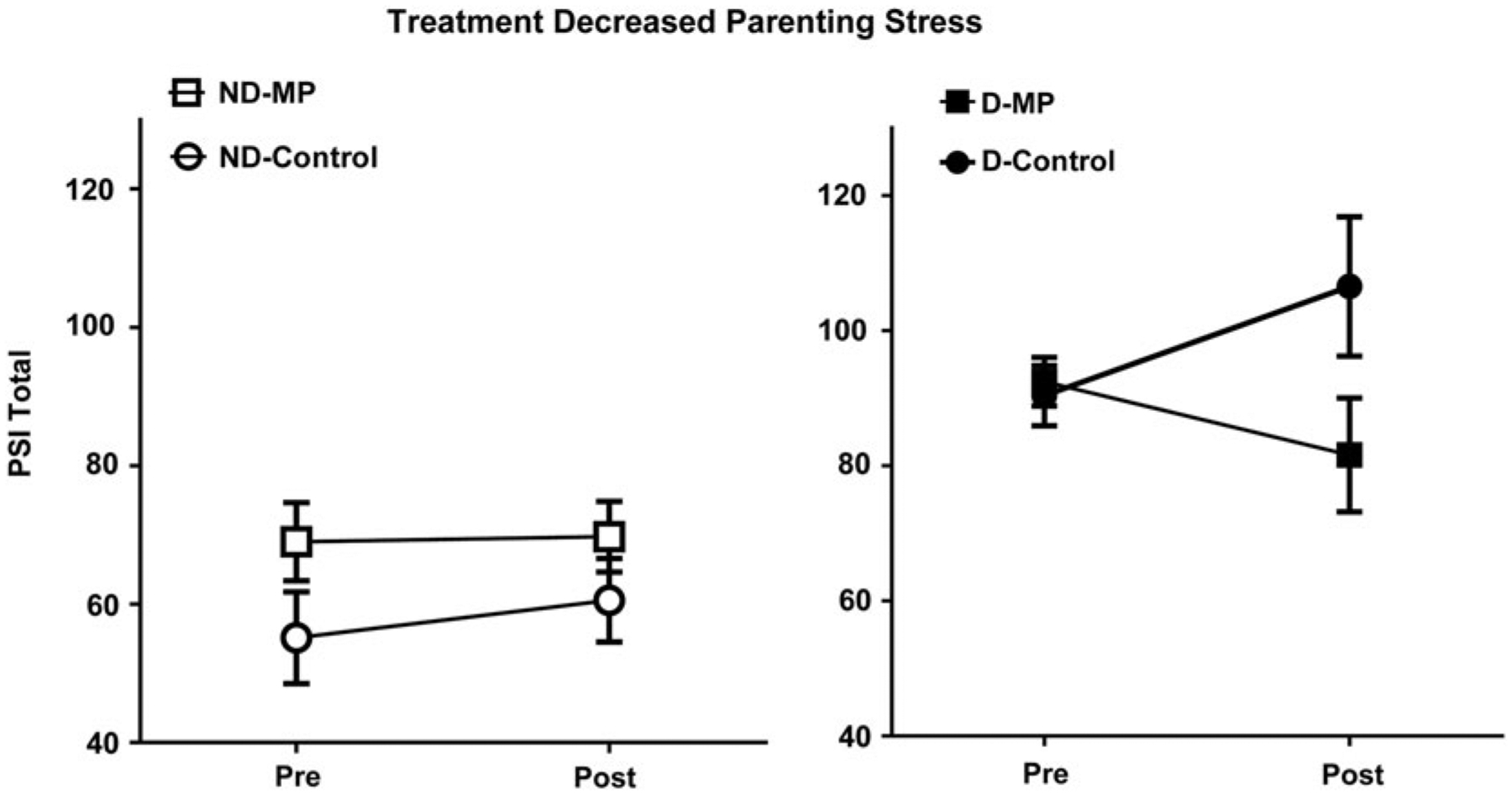
Mom Power (MP) parenting treatment (vs. control group) decreased parenting stress, especially in mothers with depression. Mothers were grouped according to whether they were depressed or not depressed (filled for depressed, open for nondepressed) and whether they received 12-week MP treatment or control (squares for MP, circles for control). MP intervention goals involve group psychoeducation sessions aimed at increasing stress coping skills and reflective capacity toward sensitive parenting.
Baby-cry task main effects
There are four contrasts of interest that were examined among the conditions of noise, generic baby-cry, own baby-cry, self baby-cry, and noise in the fMRI task, including (a) generic baby-cry versus noise, to examine the neural responses specific to infant cries as a distress signal; (b) own baby-cry versus generic baby-cry, to examine the neural responses specific to own child’s distress signal; (c) self baby-cry versus generic baby-cry, to examine the neural responses specific to self’s distress signal; and (d, e) own baby-cry versus self baby-cry, to examine the differential neural responses to child’s and self’s distress signals. To validate the task design, the main effects of these contrasts, pooling all scans across all participants and sessions, were examined and results summarized in Table 1 and Figure 3. The infant cry as a distress signal activated bilateral posterior insula, dorsomedial prefrontal cortex, and sgACC, in addition to sensory cortices that are typically activated by baby-cry (Kim et al., 2016). Compared to generic baby-cry, own baby-cry preferentially activated cortical areas related to attention and cognitive control, while self baby-cry preferentially activated subcortical caudate along with other cortical areas. When directly contrasting between own baby-cry and self baby-cry, the former preferentially activated cortical areas related to ventral attention network, while the latter preferentially activated cortical areas related to default mode network (see Figure 3).
Table 1.
Main effects of task conditions
| Brain Region | Side | MNI Coordinates | No. of Voxels | Z Score | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Generic Baby’s Cry Versus Noise | ||||||
| Superior temporal gyrus | R | 58 | −8 | −2 | 1956 | 7.30 |
| L | −62 | −20 | 4 | 1834 | 6.32 | |
| Insula, posterior | L | −44 | −20 | 2 | 469 | 6.09 |
| R | 48 | −14 | 4 | 414 | 5.97 | |
| Midbrain | R/L | 12 | −18 | −8 | 407 | 4.02 |
| Anterior cingulate cortex, subgenual | R | 14 | 34 | 0 | 166 | 3.33 |
| Prefrontal cortex, dorsomedial | R/L | −6 | 38 | 44 | 193 | 3.29 |
| Your Baby’s Cry Versus Generic Baby’s Cry | ||||||
| Inferior parietal lobule | L | −64 | −48 | 20 | 478 | 4.41 |
| Middle temporal gyrus | L | −48 | −60 | 12 | 245 | 3.36 |
| Inferior frontal gyrus/precentral | L | −36 | 4 | 34 | 206 | 3.23 |
| Your Self’s Cry Versus Generic Baby’s Cry | ||||||
| Middle temporal gyrus, posterior | L | −42 | −50 | 16 | 1759 | 4.76 |
| Superior frontal gyrus | R/L | −8 | 10 | 62 | 626 | 4.73 |
| Occipital lobe | L | −28 | −74 | −8 | 336 | 4.01 |
| Middle temporal gyrus, anterior | L | −50 | −4 | −20 | 381 | 3.79 |
| Middle frontal gyrus/precentral | L | −34 | 4 | 42 | 705 | 3.78 |
| Middle/inferior frontal gyrus | R | 50 | 26 | 14 | 258 | 3.67 |
| Caudate | R | 26 | −4 | 18 | 179 | 3.63 |
| Inferior frontal gyrus | L | −40 | 34 | −8 | 593 | 3.30 |
| Your Baby’s Cry Versus Your Self’s Cry | ||||||
| Inferior parietal lobule | L | −66 | −32 | 32 | 268 | 4.46 |
| Inferior frontal gyrus | R | 56 | 36 | 4 | 152 | 3.68 |
| Insula | L | −38 | 0 | −12 | 163 | 3.36 |
| Your Self’s Cry Versus Your Baby’s Cry | ||||||
| Middle temporal gyrus, posterior | L | −38 | −64 | 24 | 297 | 3.87 |
| Temporal pole | L | −46 | 4 | −30 | 146 | 3.53 |
| Superior frontal gyrus | L | −12 | 28 | 58 | 169 | 3.43 |
Figure 3.
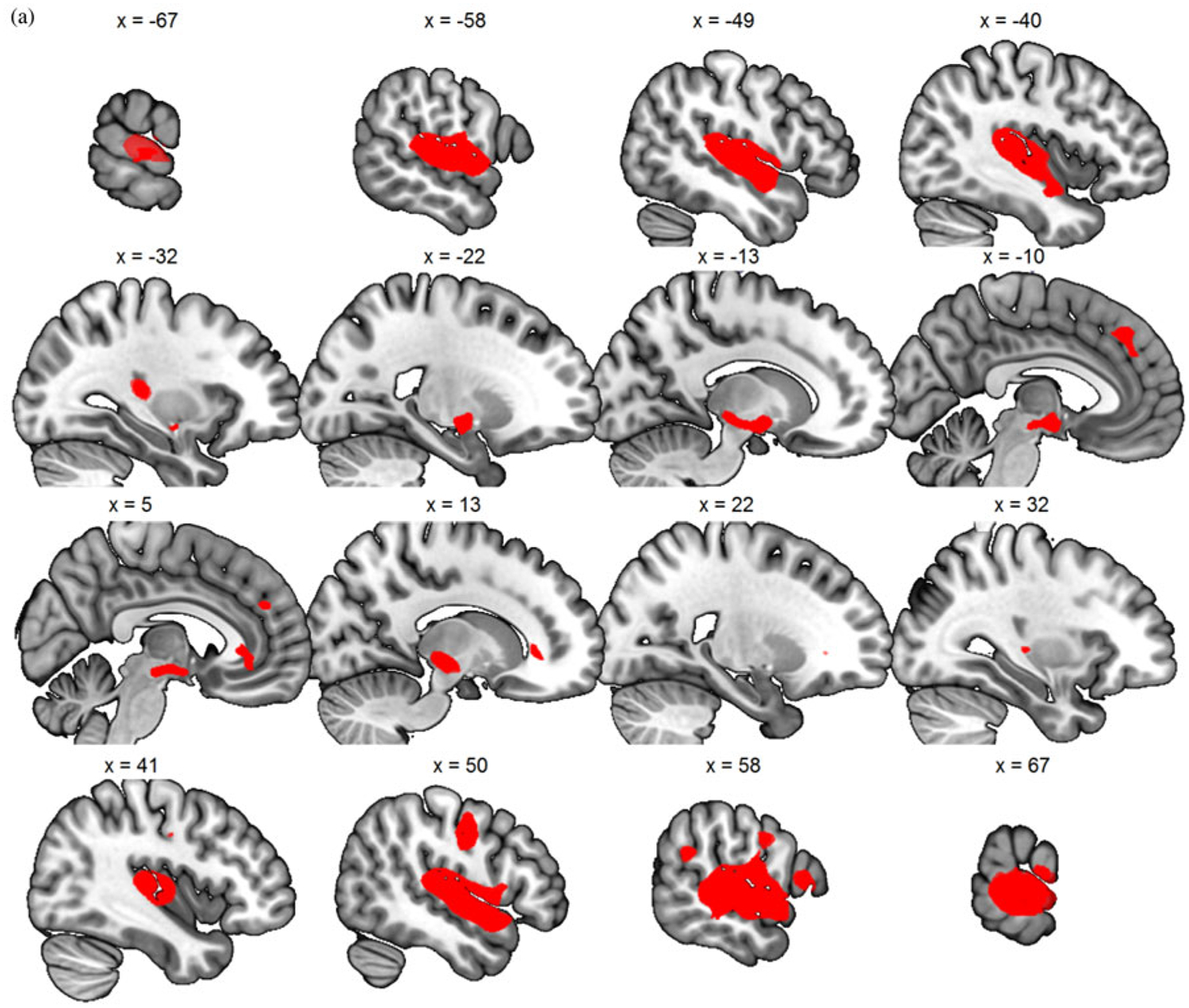
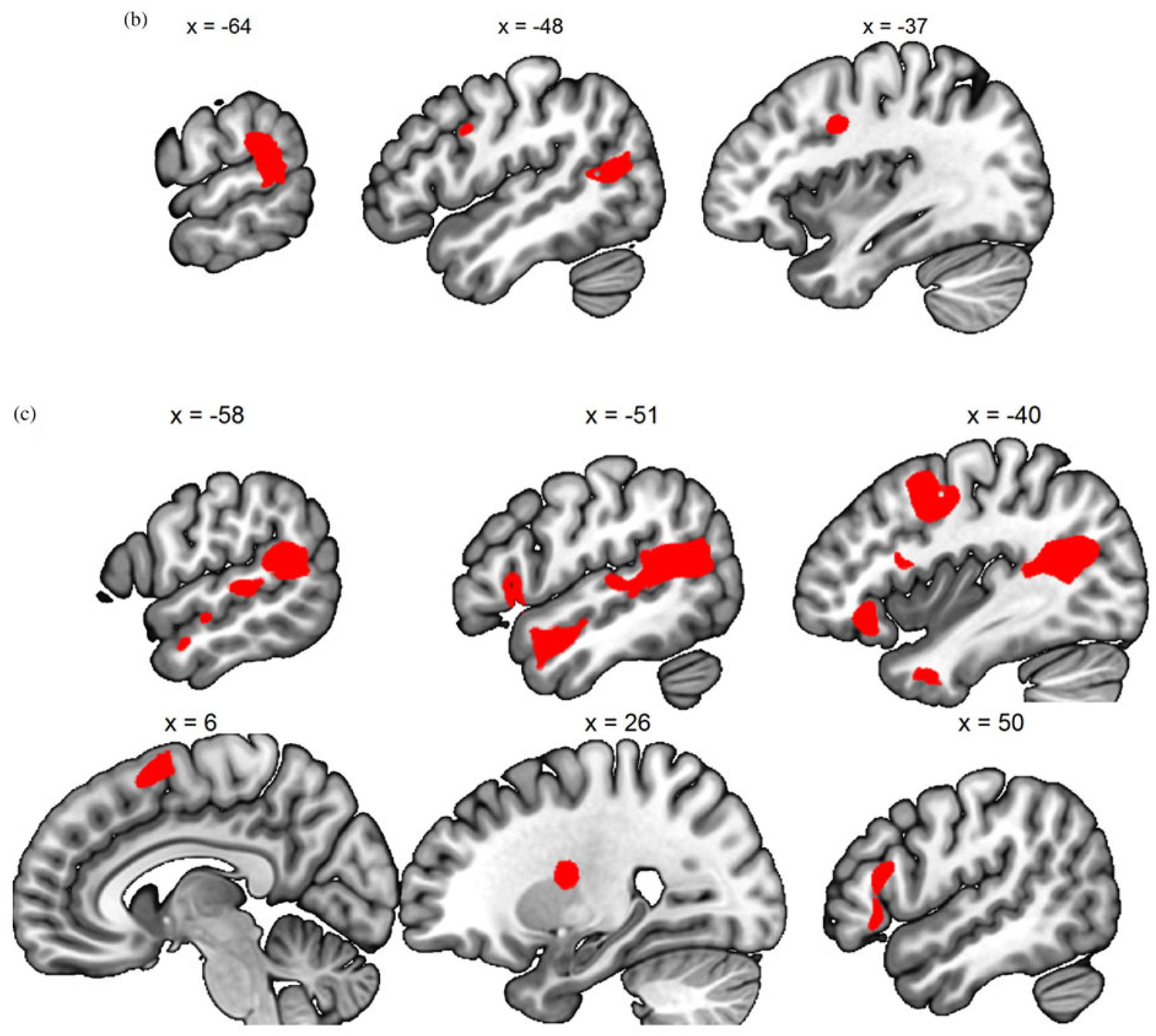
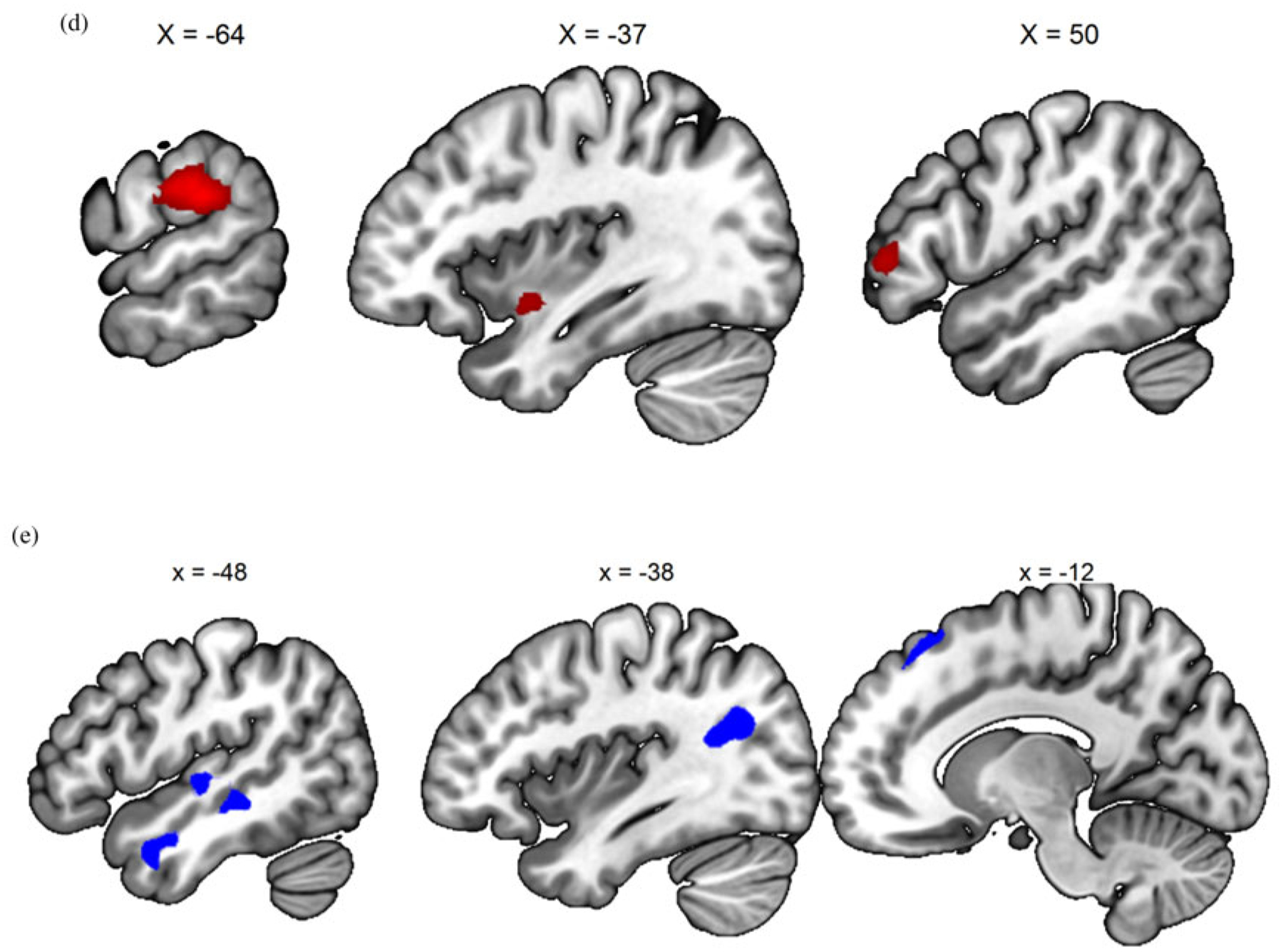
(Color online) (a) Generic baby-cry versus noise, (b) own baby-cry versus generic baby-cry, (c) self baby-cry versus generic baby-cry,(d) own baby-cry > self baby-cry, (e) self baby-cry > own baby-cry.
MP changed neural response to baby-cry
Treatment and time were used as two independent variables in the SPM8’s flexible factorial model to examine how MP versus control groups’ maternal brain responses differentially changed over time (a Group × Time interaction effect). For each contrasts of interest, we found no significant clusters in the generic baby-cry versus noise and own baby-cry versus self baby-cry. The visual cortex showed increasing activity for both own baby-cry versus generic baby-cry and self baby-cry versus generic baby-cry contrasts. Moreover, mothers in MP, but not control, showed increasing own baby-cry versus generic baby-cry activity over time (see Table 2 and Figure 4a, b).
Table 2.
Treatment Group ×Time interaction effects, controlling for Depression and Treatment × Depression interaction
| Brain Region | Side | MNI Coordinates | No. of Voxels | Z Score | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Generic Baby’s Cry Versus Noise | ||||||
| None | ||||||
| Your Baby’s Cry Versus Generic Baby’s Cry | ||||||
| Fusiform | L | −28 | −44 | −18 | 461 | 4.61 |
| Precuneus | R/L | −10 | −42 | 48 | 336 | 3.93 |
| Visual cortex/fusiform | R/L | 14 | −80 | 16 | 1898 | 3.74 |
| Your Self’s Cry Versus Generic Baby’s Cry | ||||||
| Visual cortex | R | 12 | −62 | 6 | 816 | 3.36 |
| Your Baby’s Cry Versus Your Self’s Cry | ||||||
| None | ||||||
Figure 4.
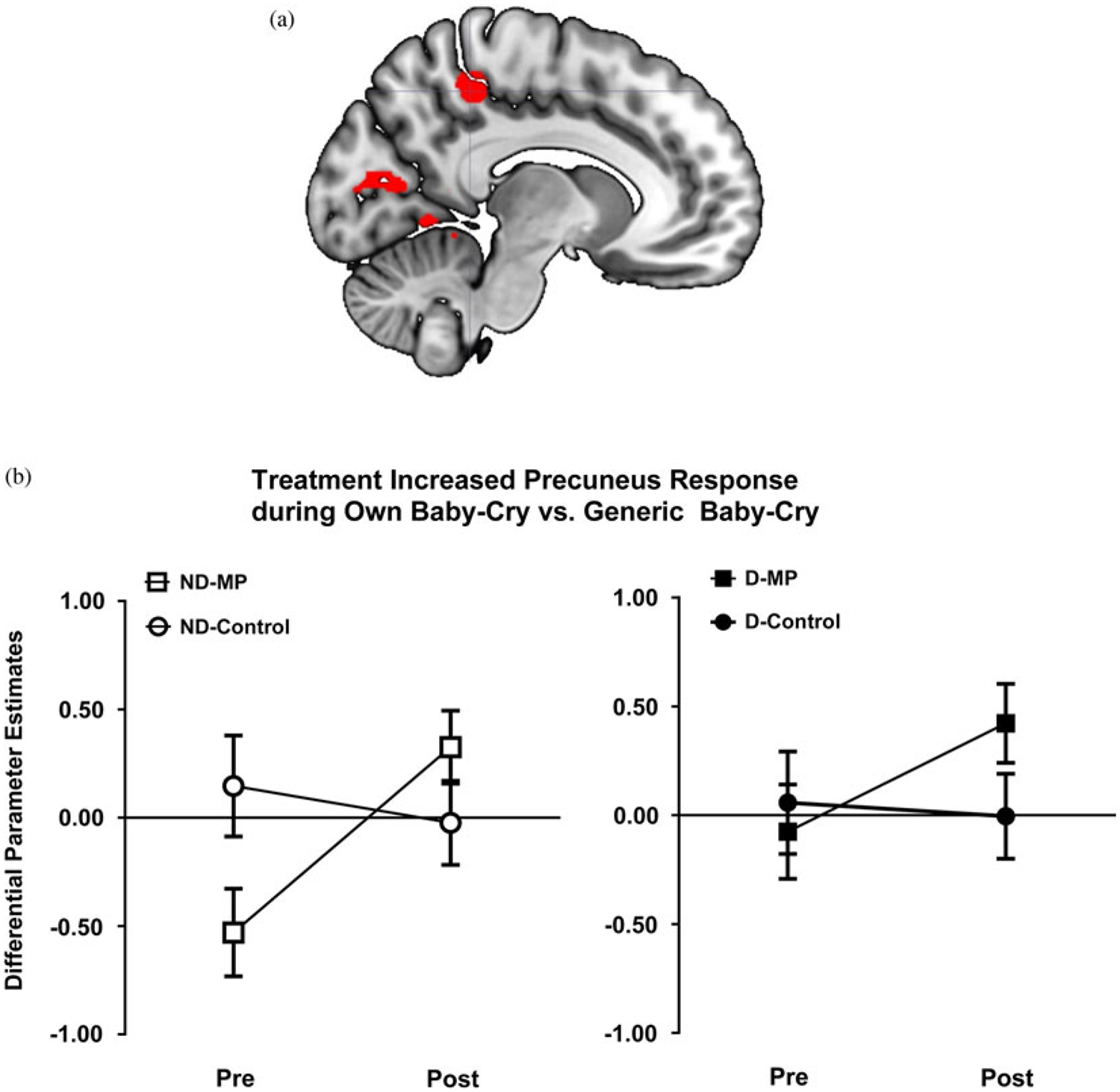
(Color online) (a) Brain image highlighting precuneus region, which showed increased own baby-cry versus generic baby-cry activity with Mom Power (MP) parenting intervention and was also used for further functional connectivity analysis. (b) MP treatment (vs. control group) increased own baby-cry versus generic baby-cry response in the precuneus. Mothers were grouped according to whether they were depressed (filled for depressed, open for nondepressed) and whether they received MP (squares for MP, circles for control).
Neurocorrelates of changes in parenting stress
To examine the neurocorrelates related to the change in parenting stress over 13 weeks, we computed the differential brain map by subtracting the SPM contrast image at T1 from that at T2, and regressed the T2–T1 difference images against the T2–T1 change in PSI total score (dPSI), controlling for the PSI at T1. The results are summarized in Table 3. The T2–T1 increase in own versus generic baby-cry differential response in the precuneus was associated with T2–T1 reduction in PSI (see Figure 5).
Table 3.
Neurocorrelates showing associations between Time 2 and Time 1 change in brain response and change in parenting stress index (dPSI), controlling for baseline PSI
| Brain Region | Side | MNI Coordinates | No. of Voxels | Z Score | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Generic Baby’s Cry versus Noise, Positive Association | ||||||
| Middle frontal gyrus | R | 46 | 26 | 32 | 181 | 3.80 |
| Supplemental motor area | R | 22 | 14 | 62 | 213 | 3.62 |
| Generic Baby’s Cry Versus Noise, Negative Association | ||||||
| None | ||||||
| Your Baby’s Cry Versus Generic Baby’s Cry, Positive Association | ||||||
| None | ||||||
| Your Baby’s Cry Versus Generic Baby’s Cry, Negative Association | ||||||
| Middle frontal gyrusa | R | 34 | 52 | 6 | 1167 | 4.31 |
| Superior temporal gyrus | L | −68 | −28 | 2 | 243 | 3.84 |
| Posterior cingulate cortex | R | 18 | −50 | 10 | 334 | 3.65 |
| Precuneus | R/L | −8 | −68 | 44 | 788 | 3.52 |
| Superior temporal gyrus | R | 58 | −46 | 18 | 429 | 3.36 |
| Precentral | R | 40 | −8 | 54 | 248 | 3.23 |
| Your Self’s Cry Versus Generic Baby’s Cry, Positive Association | ||||||
| None | ||||||
| Your Self’s Cry Versus Generic Baby’s Cry, Negative Association | ||||||
| Supplemental motor areaa | R | 24 | 16 | 62 | 619 | 4.72 |
| Middle/inferior frontal gyrusa | R | 32 | 34 | 38 | 499 | 4.44 |
| Insula, anterior | R | 30 | 22 | −2 | 148 | 4.41 |
| Inferior frontal gyrus/precentral | L | −58 | 4 | 22 | 461 | 4.03 |
| Occipital lobe | R | 14 | −72 | 10 | 949 | 3.91 |
| Superior temporal gyrus | L | −64 | −46 | 12 | 278 | 3.61 |
| Your Baby’s Cry Versus Your Self’s Cry, Positive or Negative Associations | ||||||
| None | ||||||
The cluster largely overlapped with those showing positive association with generic baby’s versus noise.
Figure 5.
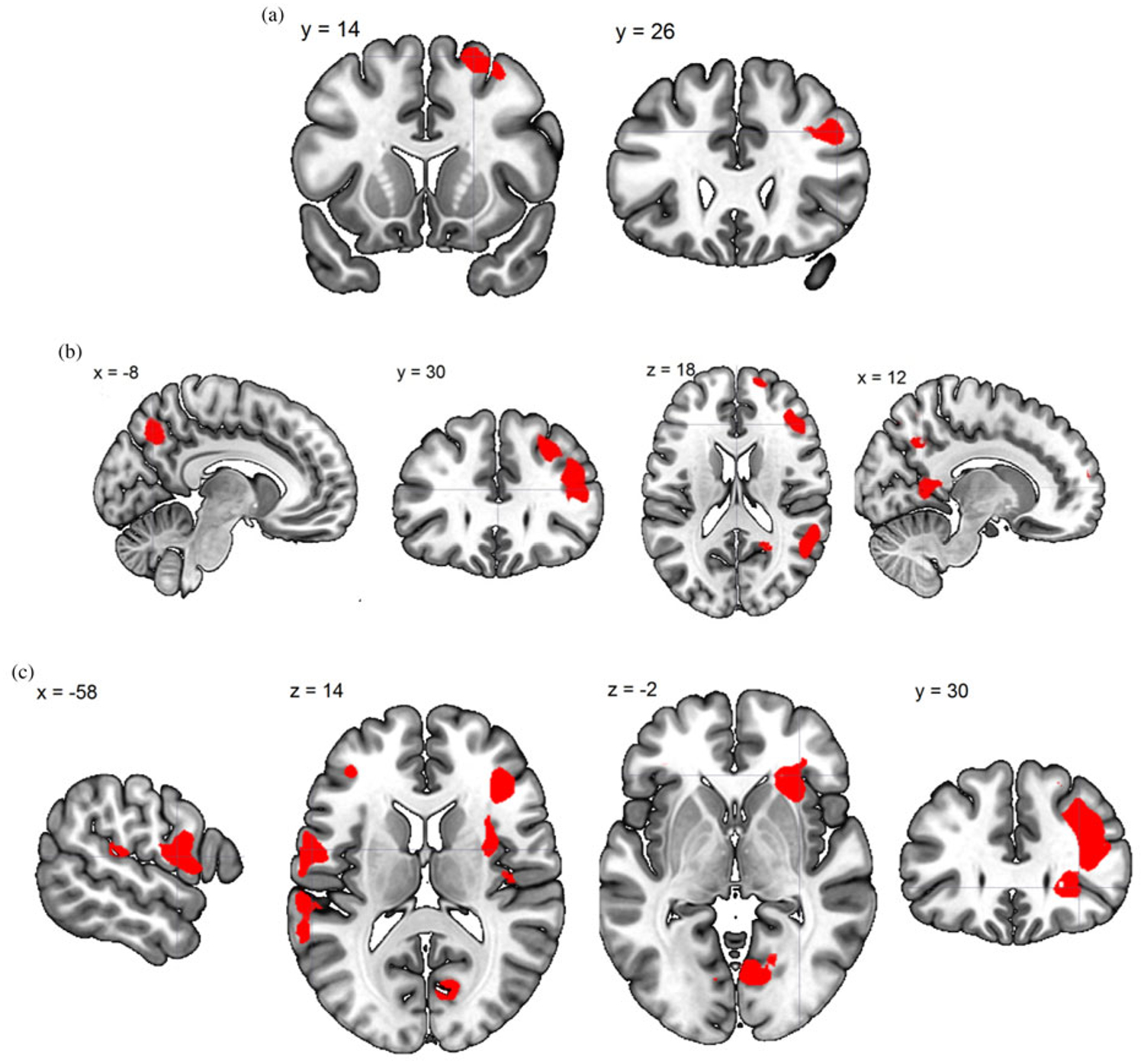
(Color online) Time 2–Time 1 change in brain response. (a) Generic baby-cry versus noise directly associated with change in Parenting Stress Index total score (dPSI). (b) Own baby-cry versus generic baby-cry inversely associated with dPSI. (c) Self baby-cry versus generic baby-cry inversely associated with dPSI.
MP treatment increased precuneus–sgACC functional connectivity in response to child’s versus self’s distress signals
Given that results demonstrated that the precuneus was implicated in the effects of MP treatment, we decided to perform a generalized psychological–physiological interaction analysis (gPPI; McLaren, Ries, Xu, & Johnson, 2012) to examine how task-dependent functional connectivity with this precuneus locus was related to the T2–T1 change related to the MP treatment and parenting stress. With regard to the treatment by time effect, we found that sgACC showed significant own baby-cry versus self baby-cry task-dependent functional connectivity with the precuneus (seed). As both sgACC and precuneus are nodes in the default-mode network, this result suggests that, over time, MP treatment enhanced the participants’ node-to-node connectivity within the default-mode network while they were relating to their child’s distress signals, as compared to while they were relating to their own distress signals (Figure 6).
Figure 6.
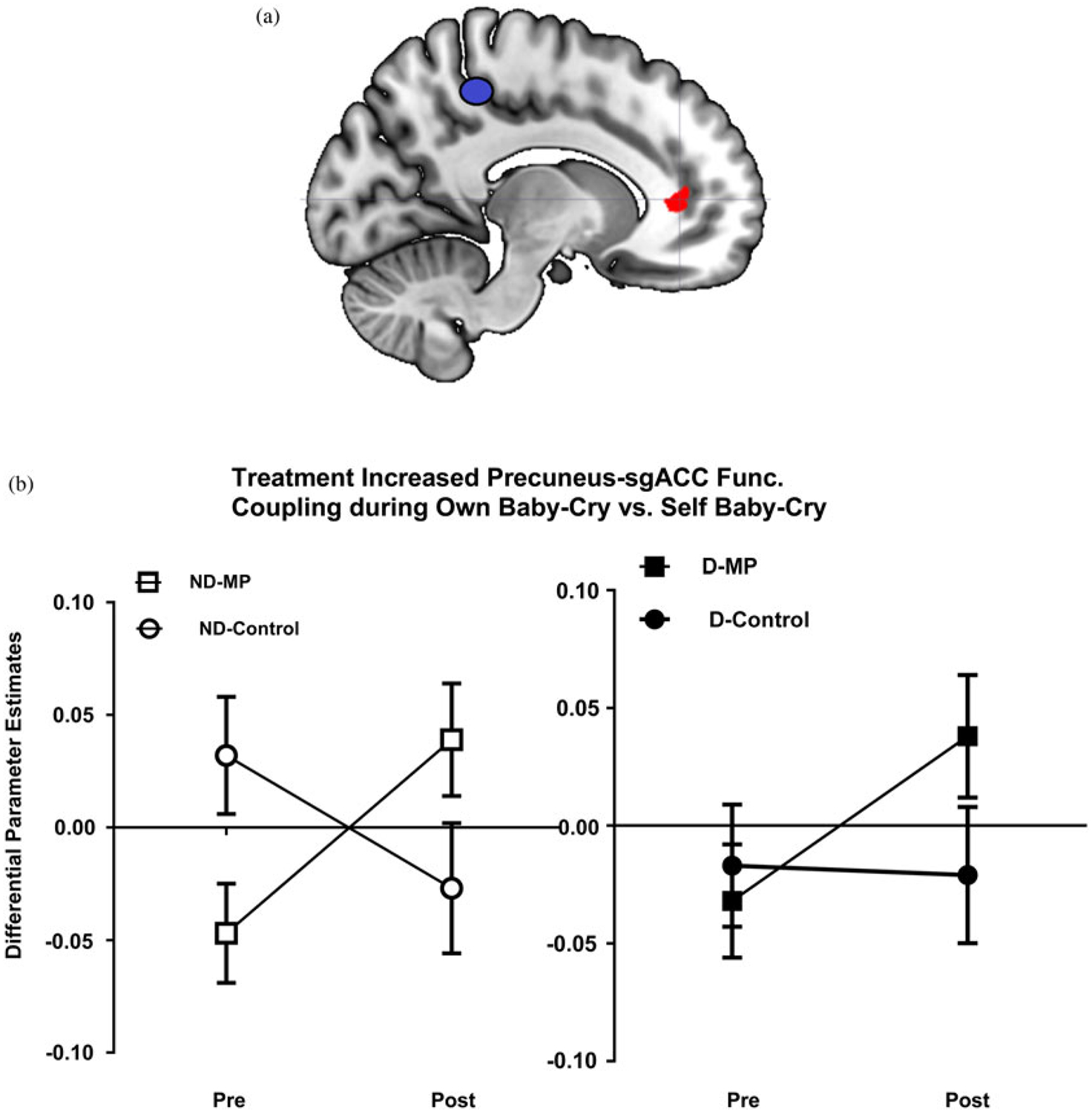
(Color online) (a) Mom Power (MP) parenting intervention versus control increased differential functional connectivity between the precuneus (as the seed) and subgenual anterior cingulate cortex in the own baby-cry versus self baby-cry contrast (care for own baby vs. preoccupation with self. (b) MP treatment (vs. control group) increased the functional connectivity between the precuneus (seed) and the subgenual anterior cingulate cortex. Mothers were grouped according to whether they were depressed (filled for depressed, open for nondepressed) and whether they received MP (squares for MP, circles for control).
Changes in parenting stress and precuneus-based functional connectivity
To investigate how T2–T1 dPSI related to task-dependent functional connectivity with the precuneus, we performed gPPI with the precuneus seed derived from Table 4. The results are summarized in Table 4 and Figure 7, and include regions of inverse relation to stress responding to own baby-cry of the medial frontal gyrus and middle frontal gyrus.
Table 4.
Neurocorrelates showing associations between Time 2 and Time 1 change in task-dependent functional connectivity with precuneus (seed) and change in parenting stress index (dPSI), controlling for baseline PSI
| Brain Region | Side | MNI Coordinates | No. of Voxels | Z Score | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Generic Baby’s Cry Versus Noise, Positive Association | ||||||
| Rolandic operculum/insula | R | 46 | −2 | 14 | 137 | 3.88 |
| Generic Baby’s Cry Versus Noise, Negative Association | ||||||
| None | ||||||
| Your Baby’s Cry versus Generic Baby’s Cry, Positive Association | ||||||
| None | ||||||
| Your Baby’s Cry Versus Generic Baby’s Cry, Negative Association | ||||||
| Medial frontal gyrus/supplemental motor area | L | −8 | −28 | 58 | 144 | 3.35 |
| Middle frontal gyrus/precentral | L | −36 | −2 | 52 | 205 | 3.28 |
| Your Self’s Cry Versus Generic Baby’s Cry, Positive or Negative Association | ||||||
| None | ||||||
| Your Baby’s Cry Versus Your Self’s Cry, Positive Association | ||||||
| None | ||||||
| Your Baby’s Cry Versus Your Self’s Cry, Negative Association | ||||||
| Middle cingulate cortex, anterior | R | 30 | −16 | 42 | 646 | 3.80 |
| L | −6 | −12 | 42 | 456 | 3.44 | |
Figure 7.
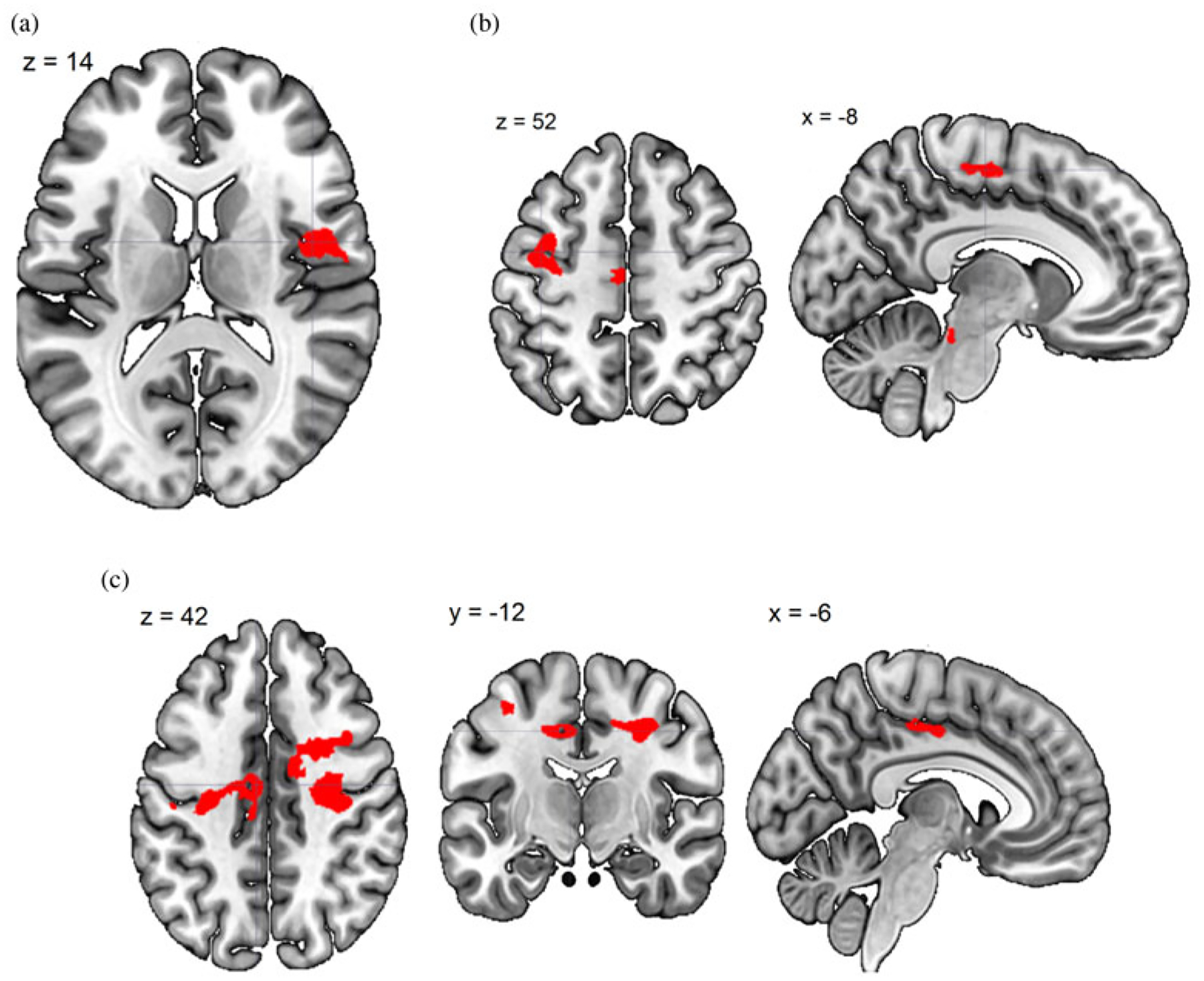
(Color online) Time 2–Time 1 change in task-dependent functional connectivity (generalized psychological-physiological interaction analysis) with precuneus. (a) Generic baby-cry versus noise directly associated with change in PSI total score (dPSI). (b) Own baby-cry versus generic baby-cry inversely associated with dPSI. (c) Own baby-cry versus self baby-cry inversely associated with dPSI.
Changes in parenting stress and amygdala-based functional connectivity
Given sensitivity of the amygdala to attachment-related emotional stimuli including infant stimuli (Kim et al., 2016), we explored connectivity using an anatomically defined amygdala seed. Specifically, we examined how changes in amygdala-based functional connectivity were associated with changes in parenting stress in the entire group of mothers (n = 29). The results are summarized in Table 5 and depicted in Figure 8a and b. We found that the T2–T1 change in own baby-cry versus self baby-cry task-dependent functional connectivity between the left amygdala and left temporal pole was highly correlated with the T2–T1 reduction in parenting stress. That is, the greater T2–T1 increase in the own baby-cry versus self baby-cry differential coupling between the left amygdala and temporal pole, the greater the reductions in parenting stress over time. All of those participants who showed a reduction in parenting stress over time demonstrated an increase in the own baby-cry versus self baby-cry functional connectivity (n = 9) in the green shaded quadrant in Figure 8c, and the majority of these participants were treated with MP (n = 7). In contrast, the participants in the red shaded quadrant of Figure 8c demonstrated an increase in their parenting stress and a decrease in the task-dependent connectivity, with the most extreme case being a depressed mother in the control group. There was a trend that MP is particularly beneficial for mothers with depression, suggested by the finding that, when only depressed mothers were considered, there were more participants in the MP group (n = 5) than in the control group (n = 1) who demonstrated a reduction in parenting stress (PSI), and more participants in the control group (n = 4) than in the MP group (n = 2) who failed to demonstrate a reduction in parenting stress(X2 = 3.086, p = .078).
Table 5.
Neurocorrelates showing associations between Time 2 and Time 1 change in task-dependent functional connectivity with left amygdala (seed) and change in parenting stress index (dPSI), controlling for baseline PSI
| Brain Region | Side | MNI Coordinates | No. of Voxels | Z Score | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Generic Baby’s Cry Versus Noise, Positive Association | ||||||
| Inferior parietal lobule | L | −50 | −52 | 44 | 219 | 4.70 |
| Lentiform nucleus | R | 22 | −8 | 2 | 376 | 4.26 |
| Ventral Striatum | L/R | −6 | 2 | −2 | 189 | 3.86 |
| Midbrain | R | 6 | −34 | −8 | 247 | 3.86 |
| Middle temporal gyrus | R | 56 | −48 | −4 | 210 | 3.38 |
| Generic Baby’s Cry Versus Noise, Negative Association | ||||||
| None | ||||||
| Your Baby’s Cry Versus Generic Baby’s Cry, Positive or Negative Association | ||||||
| None | ||||||
| Your Self’s Cry Versus Generic Baby’s Cry, Positive or Negative Association | ||||||
| None | ||||||
| Your Baby’s Cry Versus Your Self’s Cry, Positive Association | ||||||
| None | ||||||
| Your Baby’s Cry Versus Your Self’s Cry, Negative Association | ||||||
| Temporal pole | L | −48 | 8 | −22 | 291 | 3.88 |
Figure 8.
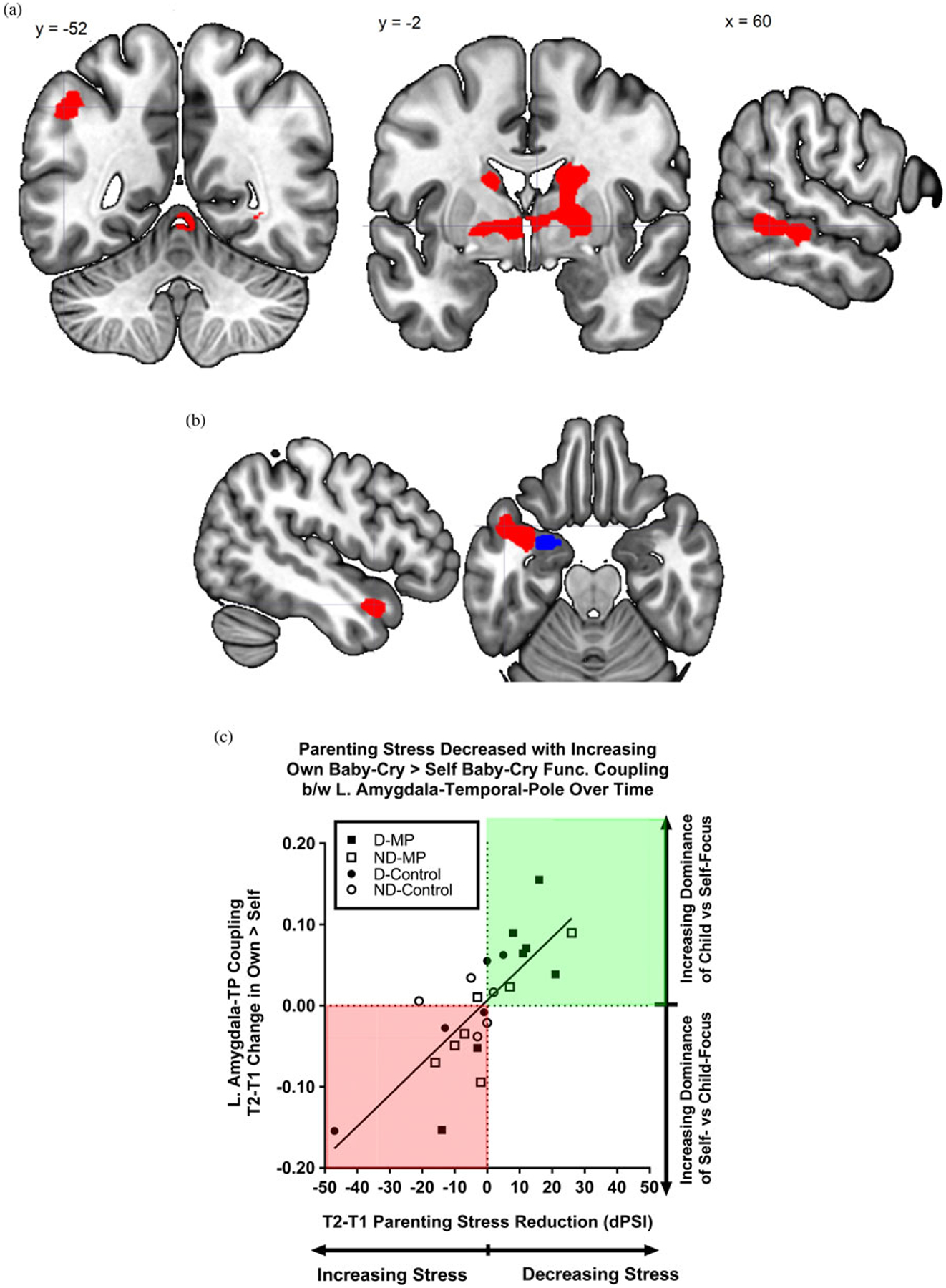
(Color online) Time 2–Time 1 change in task-dependent functional connectivity (generalized psychological-physiological interaction analysis) with left amygdala. (a) Generic baby-cry versus noise directly associated with change in PSI total score (dPSI). (b) Brain image highlighting the Time 2–Time 1 change in own baby-cry versus self baby-cry differential functional connectivity between the left amygdala seed (blue online only) and the temporal pole (red online only) that was associated with the Time 2–Time 1 reduction in parenting stress (dPSI).(c) Parenting stress is decreased with functional coupling between amygdala and temporal pole for own versus self baby-cry that increases over time. Scatterplot of the reduction in PSI (dPSI) on the x-axis and increase in amygdala–temporal pole’s own baby-cry versus self baby-cry differential functional coupling strength (y axis) from Time 1 to Time 2. Mothers were grouped according to whether they were depressed or not depressed (filled for depressed, open for nondepressed) and whether they received MP (squares for MP, circles for control). Of note, all mothers who showed increased own versus self baby-cry functional connectivity had a reduction in parenting stress over time (green shading online only), and none of those who decreased this differential functional connectivity reported reduced parenting stress over time (red shading online only).
Discussion
The results of this study provide preliminary evidence that care-provoking baby-cry distress signals evoke a pattern of neural responses among mothers that vary based on the identity of who is crying. A parenting intervention, MP, decreased stress for the participants in the current study. Thus, we were positioned to identify neurocorrelates that may serve as brain-based mechanisms of therapeutic changes. Exploration of functional brain changes associated with MP revealed increased functional connectivity in brain circuits involved in reflective self-awareness and mood regulation. Finally, functional connectivity between salience and mental-state-attribution circuits shifts according to the focus of the task. Specifically, we found that functional connectivity in the precuneus–sgACC and amygdala–temporal pole during the child’s versus mother’s own distress signal condition was associated with decreased stress over time. These results support the notion that preferential responding to child’s distress signals over those of one’s own distress signals may serve as a protective factor to downregulate the mothers’ parenting stress. Thus, parenting treatment benefits appear to be reflected in aspects of brain function and connectivity during parent response to child distress. Understanding which maternal brain-circuits change due to intervention may help to identify biomarkers for future studies of brain plasticity to improve treatment efficacy.
Distinct from most previous work on baby-cry brain responses, this study was conducted with mothers who were, on average, a few years into the postpartum period. Even though the participants did not listen to the actual baby-cry from their own child but were rather asked to conceptualize the identity as generic, own child, or themselves, our results suggested that the maternal brain responded to own baby-cry and generic baby-cry in a way that is consistent with the previous findings and robust given whole-brain correction (Table 1). In summary, generic baby-cry versus control noise activated motivational brain area (midbrain), visceral and interoception areas (bilateral posterior insula), affective valuation areas (sgACC), and social brain regions (dorsomedial prefrontal cortex). This is in accord with previous work using this task on nonparents (Kim, Ho, et al., 2015), in which insula responses to generic baby-cry versus noise were significantly reduced by the experience of chronic childhood poverty.
Over the baseline of the response to generic baby-cry, when the mothers listened to own baby-cry, the results that we found were consistent with the literature on unique care responses to one’s own baby versus a generic baby (Swain, 2011). They include regions of the inferior parietal lobule and middle temporal gyrus, which may be interpreted as important for social and language comprehension (Decety & Lamm, 2007). If baby-cry may be considered the infant’s primary mode of social communication, then brain function to decipher meaning may be critical for sensitive responses. The inferior frontal gyrus activity may serve to volitionally plan for caregiving response to child’s distress (Haggard, 2008). The self baby-cry, as compared to own baby-cry, elicited activity in the default mode network (DMN; Yeo et al., 2011). Activity in this functional connectivity network may underlie many aspects of self-related social cognition, attention, and empathy for another’s suffering (Otti et al., 2010). For example, the middle temporal gyrus cluster we report has been linked to self-awareness (Goldberg, Harel, & Malach, 2006), a key faculty that is augmented with parenting interventions such as MP. In addition, own baby-cry versus self baby-cry included activity in the ventral attention network, according to Yeo et al. (2011), which underlies tonic alertness (Sadaghiani & D’Esposito, 2015) and salience processing (Seeley et al., 2007).
Parenting interventions such as MP focus on improving reflective mentalization, increasing parental self-awareness, and reducing parenting stress. Accordingly (Table 2), we found that MP intervention upregulated the differential responses in precuneus and visual processing areas to own versus generic baby-cry over the course of the intervention, as compared to control. The precuneus is a major node in DMN related to social cognition and self-related processing (Yeo et al., 2011). Allied work found that maternal warmth was associated with greater neural responses to videos of their own infants in the precuneus, visual areas, the insula, and the medial frontal gyrus (Wan et al., 2014). Our connectivity analysis of precuneus seed connectivity that varies inversely with stress (Table 4) also revealed the medial frontal gyrus. These findings suggest that maternal warmth and reduced stress are associated with greater responses to mothers’ own infants compared to unfamiliar infants in brain areas involved in social and sensory information processing connected to the precuneus.
Moreover, the precuneus’ differential own versus self baby-cry functional connectivity with another major node in the DMN, sgACC, was also increased by MP intervention, as compared to control. As sgACC and precuneus are robustly connected within the DMN during resting state and self-related process in psychopathology (Whitfield-Gabrieli & Ford, 2012), MP may have supported the mother to invest added personal relevance to the child in the child-oriented (own baby-cry) task over and above her baseline personal relevance investment to herself in the self-oriented condition (self baby-cry).
We also found that several brain areas typically included in the DMN (Yeo et al., 2011) that were present in the correlation analysis between the T2–T1 change in PSI and the T2–T1 change the own versus generic baby-cry, controlling for PSI at T1 (Table 3). Within DMN regions such as the bilateral superior temporal gyrus, posterior cingulate cortex, and precuneus, time-dependent increased activity was associated with decreased parenting stress over time. This led to our use of a precuneus seed (Table 4) that revealed connectivity change over time in another DMN region (medial frontal cortex), for which activity (own vs. generic baby-cry) was inversely related to stress. Thus, the DMN may serve a protective role to reduce parenting stress that this role may be augmented with parenting interventions.
It is interesting to consider how maternal behaviors in response to baby-cry and associated mother–infant bonding may be particularly susceptible to stress and attachment. On the one hand, stress is critical to survival of offspring for heightened vigilance and threat detection to ensure own-baby survival across mammalian species (Barrett & Fleming, 2011; Strathearn & Kim, 2013). On the other hand, if stress becomes too high, it may contribute to anxiety and depression and involve alterations of amygdala function in particular (Pizzagalli, 2014). In addition then to the DMN, we consider the partly overlapping salience network that included the amygdala. The amygdala is key to brain functions across emotional and stressful tasks (LeDoux, 2000, 2012). In the current parental brain literature, cross-sectional studies have shown its importance in responding to different baby stimuli. For example, own baby-cry activations of the amygdala at 2–4 weeks predict maternal sensitivity at 3–4 months (Kim et al., 2011). In addition, mothers who engaged in more synchronous interactions showed more coherent activations of the amygdala/nucleus accum-bens in response to their infant’s video stimuli, and these activations correlated with maternal plasma oxytocin (Atzil, Hendler, & Feldman, 2011; Strathearn, Fonagy, Amico, & Montague, 2009). Similarly, maternal amygdala responses to own-baby emotional face stimuli correlated with maternal reflective functioning (empathy; Lenzi et al., 2009). In addition, amygdala activity given negative versus positive feedback after a parenting decision was a function of the personal distress aspect of empathy (Ho, Konrath, Brown, & Swain, 2014). Furthermore, amygdala responses to own versus unfamiliar positive infant image paradigm was reduced among mothers suffering more concurrent depression, anxiety, and distressed attachment-related feelings about their infant (Barrett et al., 2012). Finally, unresolved trauma is associated with reduced amygdala response to emotionally charged own infant images (Kim et al., 2014) and increased response to own infant crying according to insecure attachment (Riem et al., 2012). It seems likely that amygdala activity is critical to parenting thoughts and behaviors al-though responses vary according to the stimuli used as well as variables of stress, mood, and attachment.
Although the amygdala does appear to be a critical structure for parenting, stress, and intervention, it is likely not the key to understanding all maternal brain activity and plasticity. For example, amygdala response to own versus other baby-cry in the first weeks postpartum was not related to mothers’ perception of her own early life maternal experience (Kim, Leckman, Mayes, Newman, et al., 2010) or child socioemotional outcomes at 18–24 months of age (Kim, Rigo, et al., 2015). Instead, hippocampus and frontal brain regions were among brain regions important for these individual differences. Furthermore, amygdala response to own baby-cry at 18 months postpartum did not vary according to early postpartum depression (Laurent & Ablow, 2012a). However, in the only longitudinal study published to date on maternal brains in early postpartum, amygdala gray matter volume increased from 2–4 weeks to 3–4 months postpartum in proportional to positive perceptions of her own baby (Kim, Leckman, Mayes, Feldman, et al., 2010). Thus, the amygdala may be part of a motivational–emotional limbic network that “labels” certain key stimuli with an emotional valance according to certain environmental or historical factors (Cardinal, Parkinson, Hall, & Everitt, 2002) that would be important for parents to read their infant’s signals and provide sensitive and synchronous parenting (Atzil, Hendler, Zagoory-Sharon, Winetraub, & Feldman, 2012). We thus proceeded with a standard amygdala seed for connectivity analyses during the baby-cry task.
We report that changes in baby-cry task-dependent functional connectivity with the amygdala over time were directly associated with parenting stress. Specifically, as summarized in Table 5, T2–T1 increase in generic baby-cry versus noise differential functional connectivity between the left amygdala (as the seed) and the areas of midbrain, striatum, inferior parietal lobule, and middle temporal gyrus were found to be associated with increasing parenting stress. These regions are both part of the dorsal attention network (Yeo et al., 2011) with primarily functions to sustain attention to selected objects (Corbetta & Shulman, 2002). The midbrain and striatum in addition to the amygdala mediate anxiety emotions and stress (Pohlack, Nees, Ruttorf, Schad, & Flor, 2012). Thus, mothers who experienced increasing parenting stress may be showing a generalization of their stress response to generic baby-cry.
Conversely, we found that the change in own versus self baby-cry differential functional connectivity between the left amygdala and left temporal pole was inversely related to the change in parenting stress. Specifically, the T2–T1 decrease in parenting stress was associated with a T2–T1 increase in own versus self baby-cry differential connectivity (Figures 8b and 8c). It has been found that individuals with damage to both the amygdala and the temporal pole fail to form proper associations between affective traits and faces (Todorov & Olson, 2008), so our results may suggest that both the amygdala and the temporal pole are crucial in social affective parenting functioning. Furthermore, the concerted activity between these two brain regions may be a protective factor against parenting stress. All mothers who showed increased own versus self baby-cry functional connectivity between these two brain regions had a reduction in their parenting stress over time (green shading in Figure 8c), and none of those who decreased this differential functional connectivity reported reduced parenting stress over time (red shading in Figure 8c). Moreover, the MP intervention group, as compared to the control group, were more effective in reducing the parenting stress in mothers with depressed mood and increasing the amygdala-temporal pole connectivity in own versus self baby-cry.
In addition, for confirmation of these temporal pole findings, future work may find it fruitful to examine connectivity between amygdala and other brain regions that may mediate different aspects of parenting and parenting change. For example, own baby-cry response was directly associated with maternal mental state talk but not with more global aspects of observed caregiving (Hipwell, Guo, Phillips, Swain, & Moses-Kolko, 2015). It will also be interesting for future research to dissect unique contributions of amygdala subregions that mediate different environmental stressors and treatments, along the lines of work on anxiety during resting state (Etkin, Prater, Schatzberg, Menon, & Greicius, 2009) or in auditory processing according to sound characteristics (Ball et al., 2007). Finally, it will be interesting for future research to explore the impact of MP on resting-state connectivity for mothers and children. For example, default mode network activity has been shown to be diminished by the chronic early life stress of poverty (Sripada, Swain, Evans, Welsh, & Liberzon, 2014). Perhaps such stress is moderated by parenting and amenable to parenting interventions with potentially long-term benefits.
Strengths, advantages, and limitations
This study is the first to present results of functional brain imaging pre/post a therapeutic parenting psychotherapy intervention that reduced parenting stress. We validated a baby-cry task variation that is flexible for research throughout childhood. Although we did assess ratings of baby-cry, the inclusion of more postscan parenting interviews might offer more helpful information on the mothers’ experience of baby-cry in the scanner and how realistic the experience was even though the cry recordings were not actually from their own baby or self. Although the distributions of depressed and nondepressed mothers were balanced between the MP and control groups, a larger sample size in depressed mothers will be needed in future research on depression and parenting. This study did not include other adult caregivers, including fathers that are the subject of a growing literature (Kim, Rigo, et al., 2014; Swain, Dayton, Kim, Tolman, & Volling, 2014). In addition, diagnostic psychiatric tools, more detailed assessments of parental thoughts and behaviors, and coded measurements of actual parent behavior and child outcomes (Kim, Mayes, Feldman, Leckman, & Swain, 2013) are lacking in this study. Correlations with such measures could separate brain responses from more generally important attention and sensory processing from those related to specific dimensions of mental health, parenting competence, and infant outcome with even greater translational significance to ascertain high-risk mothers, provide optimal therapy for mothers, and inform the science of child health risk and resilience development (Feldman & Eidelman, 2009).
Significance and conclusions
This study partially replicates previous studies with findings suggesting that mothers show patterns of neural response specific to the conceptualized identity of the baby-cry distress signals. Responses appear similar to mothers in the early postpartum period listening to recently recordings of their own baby crying (Kim et al., 2016) highlighted by the precuneus in this paper. Therapeutic MP parenting intervention reduced parenting stress and altered brain activity in response to baby-cry for own versus generic baby-cry contrast in the precuneus. Controlling for baseline stress, decreases in parenting stress were related to own baby-cry responsive social brain areas, including the precuneus. Whole-brain connectivity analysis of precuneus revealed connectivity changes with decreasing stress and MP parenting treatment. Further analysis of amygdala response revealed connectivity with the temporal pole that increased with own baby stimulus and decreasing stress. This paper suggests brain-based mechanisms through which a parenting intervention that reduces stress involves brain circuits known to be important for reflective mentalization on self versus other emotion regulation and decision making.
Acknowledgments
This article is supported by the Brain and Behavior Research Foundation (to J.E.S.), the State of Michigan, Department of Community Health (2009–2010, to M.M.); the University of Michigan’s Injury Center (Center for Disease Control and Prevention U49/CE002099); the Center for Human Growth and Development (to J.E.S.); the Robert Wood Johnson Foundation Health and Society Scholar Awards (to J.E.S. and M.M.); and the National Institutes for Health National Center for Advanced Translational Sciences via the Michigan Institute for Clinical Health Research UL1TR000433 (to J.E.S., S.S.H., C.J.D., K.L.R., and M.M.).
References
- Abidin R (1995). Parenting Stress Index. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Ainsworth MD, & Bell SM (1970). Attachment, exploration, and separation: Illustrated by the behavior of one-year-olds in a strange situation. Child Development, 41, 49–67. [PubMed] [Google Scholar]
- Atzil S, Hendler T, & Feldman R (2011). Specifying the neurobiological basis of human attachment: Brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology, 36, 2603–2615. doi: 10.1038/npp.2011.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Zagoory-Sharon O, Winetraub Y, & Feldman R (2012). Synchrony and specificity in the maternal and the paternal brain: Relations to oxytocin and vasopressin. Journal of the American Academy of Child & Adolescent Psychiatry, 51, 798–811. doi: 10.1016/j.jaac.2012.06.008 [DOI] [PubMed] [Google Scholar]
- Ball T, Rahm B, Eickhoff SB, Schulze-Bonhage A, Speck O, & Mutschler I (2007). Response properties of human amygdala subregions: Evidence based on functional MRI combined with probabilistic anatomical maps. PLOS ONE, 2, e307. doi: 10.1371/journal.pone.0000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, & Fleming AS (2011). Annual Research Review: All mothers are not created equal: Neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry, 52, 368–397. [DOI] [PubMed] [Google Scholar]
- Barrett J, Wonch KE, Gonzalez A, Ali N, Steiner M, Hall GB, & Fleming AS (2012). Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Social Neuroscience, 7, 252–268. doi: 10.1080/17470919.2011.609907 [DOI] [PubMed] [Google Scholar]
- Beck CT, & Gable RK (2001). Further validation of the Postpartum Depression Screening Scale. Nursing Research, 50, 155–164. [DOI] [PubMed] [Google Scholar]
- Bowlby J (1958). The nature of the child’s tie to his mother. InternationalJournal of Psychoanalysis, 39, 350–373. [PubMed] [Google Scholar]
- Bowlby J (1978). Attachment theory and its therapeutic implications. Adolescent Psychiatry, 6, 5–33. [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, & Everitt BJ (2002). Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience & Biobehavioral Reviews, 26, 321–352. [DOI] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3, 201–215. doi: 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- CSSP. (2015). Strengthening Families™: A Protective Factors Framework. Retrieved from http://www.cssp.org/reform/strengtheningfamilies
- Dayton CJ, Huth-Bocks AC, & Busuito A (2016). The influence of interpersonal aggression on maternal perceptions of infant emotions: Associations with early parenting quality. Emotion, 16, 436–448. doi: 10.1037/emo0000114 [DOI] [PubMed] [Google Scholar]
- Decety J, & Lamm C (2007). The role of the right temporoparietal junction in social interaction: How low-level computational processes contribute to meta-cognition. Neuroscientist, 13, 580–593. [DOI] [PubMed] [Google Scholar]
- De Pisapia N, Bornstein MH, Rigo P, Esposito G, De Falco S, & Venuti P (2013). Sex differences in directional brain responses to infant hunger cries. NeuroReport, 24, 142–146. doi: 10.1097/WNR.0b013e32835df4fa [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, & Greicius MD (2009). Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry, 66, 1361–1372. doi:66/12/1361[pii] 10.1001/archgenpsychiatry.2009.104 [DOI] [PubMed] [Google Scholar]
- Feldman R, & Eidelman AI (2009). Biological and environmental initial conditions shape the trajectories of cognitive and social-emotional development across the first years of life. Developmental Science, 12, 194–200. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, & Gilboa-Schechtman E (2009). Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of the American Academy of Child & Adolescent Psychiatry, 48, 919–927. [DOI] [PubMed] [Google Scholar]
- Goldberg II, Harel M, & Malach R (2006). When the brain loses its self: Prefrontal inactivation during sensorimotor processing. Neuron, 50, 329–339. doi: 10.1016/j.neuron.2006.03.015 [DOI] [PubMed] [Google Scholar]
- Haggard P (2008). Human volition: Towards a neuroscience of will. NatureReviews Neuroscience, 9, 934–946. [DOI] [PubMed] [Google Scholar]
- Hipwell AE, Guo C, Phillips ML, Swain JE, & Moses-Kolko EL (2015). Right frontoinsular cortex and subcortical activity to infant cry is associated with maternal mental state talk. Journal of Neuroscience, 35, 12725–12732. doi: 10.1523/jneurosci.1286-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SS, Konrath S, Brown S, & Swain JE (2014). Empathy and stress related neural responses in maternal decision making. Frontiers in Neuroscience, 8, 152. doi: 10.3389/fnins.2014.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, & Swain JE (2011). Breastfeeding, brain activation to own infant cry, and maternal sensitivity. Journal of Child Psychology and Psychiatry, 52, 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Ho SS, Evans GW, Liberzon I, & Swain JE (2015). Childhood social inequalities influences neural processes in young adult caregiving. Development and Psychobiology, 57, 948–960. doi: 10.1002/dev.21325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, & Swain JE (2010). The plasticity of human maternal brain: Longitudinal changes in brain anatomy during the early postpartum period. Behavioral Neuroscience, 124, 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Newman MA, Feldman R, & Swain JE (2010). Perceived quality of maternal care in childhood and structure and function of mothers’ brain. Developmental Science, 13, 662–673. doi: 10.1111/j.1467-7687.2009.00923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Mayes L, Feldman R, Leckman JF, & Swain JE (2013). Early postpartum parental preoccupation and positive parenting thoughts: Relationship with parent–infant interaction. Infant Mental Health Journal, 34, 104–116. doi: 10.1002/Imhj.21359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Rigo P, Leckman JF, Mayes LC, Cole PM, Feldman R, & Swain JE (2015). A prospective longitudinal study of perceived infant outcomes at 18–24 months: Neural and psychological correlates of parental thoughts and actions assessed during the first month postpartum. Frontiers in Psychology, 6, 1772. doi: 10.3389/fpsyg.2015.01772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Rigo P, Mayes LC, Feldman R, Leckman JF, & Swain JE (2014). Neural plasticity in fathers of human infants. Social Neuroscience, 9, 522–535. doi: 10.1080/17470919.2014.933713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Strathearn L, & Swain JE (2016). The maternal brain and its plasticity in humans. Hormones and Behavior, 77, 113–123. doi: 10.1016/j.yhbeh.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Fonagy P, Allen J, & Strathearn L (2014). Mothers’ unresolved trauma blunts amygdala response to infant distress. Social Neuroscience, 9, 352–363. doi: 10.1080/17470919.2014.896287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, & Ablow JC (2012a). A cry in the dark: Depressed mothers show reduced neural activation to their own infant’s cry. Social Cognitive & Affective Neuroscience, 7, 125–134. doi: 10.1093/scan/nsq091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, & Ablow JC (2012b). The missing link: Mothers’ neural response to infant cry related to infant attachment behaviors. Infant Behavior and Development, 35, 761–772. doi: 10.1016/j.infbeh.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155–184. doi: 10.1146/annurev.neuro.23.1.155 [DOI] [PubMed] [Google Scholar]
- LeDoux J (2012). Rethinking the emotional brain. Neuron, 73, 653–676. 10.1016/j.neuron.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, Macaluso E, Iacoboni M, Lenzi GL, & Ammaniti M (2009). Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cerebral Cortex, 19, 1124–1133. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, & Johnson SC (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61, 1277–1286. doi: 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Horner MS, Phillips ML, Hipwell AE, & Swain JE (2014). In search of neural endophenotypes of postpartum psychopathology and disrupted maternal caregiving. Journal of Neuroendocrinology, 26, 665–684. doi: 10.1111/jne.12183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, & Phillips ML (2010). Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. American Journal of Psychiatry, 167, 1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzik M, Rosenblum KL, Alfafara EA, Schuster MM, Miller NM, Waddell RM, & Kohler ES (2015). Mom Power: Preliminary outcomes of a group intervention to improve mental health and parenting among high-risk mothers. Archives of Women’s Mental Health. Advance online publication. doi: 10.1007/s00737-014-0490-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzik M, Rosenblum KL, & Schuster MM (2010). Mom Power 10 Week Curriculum. Unpublished manuscript, University of Michigan, Department of Psychiatry. [Google Scholar]
- Muzik M, Rosenblum KL, Schuster MM, Kohler ES, Alfafara EA, & Miller NM (2016). A mental health and parenting intervention for adolescent and young adult mothers and their infants. Journal of Depression and Anxiety, 5, 233–239. doi: 10.4172/2167-1044.1000233 [DOI] [Google Scholar]
- Otti A, Guendel H, Laer L, Wohlschlaeger AM, Lane RD, Decety J, … Noll-Hussong M (2010). I know the pain you feel—How the human brain’s default mode predicts our resonance to another’s suffering. Neuroscience, 169, 143–148. doi: 10.1016/j.neuroscience.2010.04.072 [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA (2014). Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annual Review of Clinical Psychology, 10, 393–423. doi: 10.1146/annurev-clinpsy-050212-185606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlack ST, Nees F, Ruttorf M, Schad LR, & Flor H (2012). Activation of the ventral striatum during aversive contextual conditioning in humans. Biological Psychology, 91, 74–80. doi: 10.1016/j.biopsycho.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Reitman D, Currier RO, & Stickle TR (2002). A critical evaluation of the Parenting Stress Index—Short Form (PSI-SF) in a Head Start Population. Journal of Clinical Child and Adolescent Psychology, 31, 384–392. doi: 10.1207/S15374424JCCP3103_10 [DOI] [PubMed] [Google Scholar]
- Riem MME, Bakermans-Kranenburg MJ, van IJzendoorn MH, Out D, & Rombouts S (2012). Attachment in the brain: Adult attachment representations predict amygdala and behavioral responses to infant crying. Attachment & Human Development, 14, 533–551. doi: 10.1080/14616734.2012.727252 [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, & D’Esposito M (2015). Functional characterization of the cingulo-opercular network in the maintenance of tonic Aaertness. Cerebral Cortex, 25, 2763–2773. doi: 10.1093/cercor/bhu072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster M (2013). Mom Power Fidelity Scale. Unpublished manuscript.
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27, 2349–2356. doi: 10.1523/jneurosci.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman ME, Loudon H, Safier M, Protopopescu X, Leiter G, Liu X, & Goldstein M (2007). Neural dysfunction in postpartum depression: An fMRI pilot study. CNS Spectrums, 12, 853–862. [DOI] [PubMed] [Google Scholar]
- Sripada RK, Swain JE, Evans GW, Welsh RC, & Liberzon I (2014). Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology, 39, 2244–2251. doi: 10.1038/npp.2014.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, & Montague PR (2009). Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology, 34, 2655–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, & Kim S (2013). Mothers’ amygdala response to positive or negative infant affect is modulated by personal relevance. Frontiers in Neuroscience, 7, 1–10. doi: 10.3389/fnins.2013.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE (2011). The human parental brain: In vivo neuroimaging. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35, 1242–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Dayton CJ, Kim P, Tolman RM, & Volling BL (2014). Progress on the paternal brain: Theory, animal models, human brain research, and mental health implications. Infant Mental Health Journal, 35, 394–408. doi: 10.1002/imhj.21471 [DOI] [PubMed] [Google Scholar]
- Swain JE, Kim P, Spicer J, Ho SS, Dayton CJ, Elmadih A, & Abel KM (2014). Approaching the biology of human parental attachment: Brain imaging, oxytocin and coordinated assessments of mothers and fathers. Brain Research, 1580, 78–101. doi: 10.1016/j.brainres.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, & Lorberbaum JP (2008). Imaging the human parental brain. Neurobiology of the Parental Brain, 6, 83–100. doi: 10.1016/B978-0-12-374285-8.00006-8 [DOI] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, & Strathearn L (2007). Brain basis of early parent-infant interactions: Psychology, physiology, and in vivo functional neuroimaging studies. Journal of Child Psychology and Psychiatry, 48, 262–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Mayes LC, & Leckman JF (2004). The development of parent-infant attachment through dynamic and interactive signaling loops of care and cry. Behavioral and Brain Sciences, 27, 472–473. [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, & Leckman JF (2008). Maternal brain response to own baby-cry is affected by cesarean section delivery. Journal of Child Psychology and Psychiatry, 49, 1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, & Olson IR (2008). Robust learning of affective trait associations with faces when the hippocampus is damaged, but not when the amygdala and temporal pole are damaged. Social Cognitive & Affective Neuroscience, 3, 195–203. doi: 10.1093/scan/nsn013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuti P, Caria A, Esposito G, De Pisapia N, Bornstein MH, & de Falco S (2012). Differential brain responses to cries of infants with autistic disorder and typical development: An fMRI study. Research in Developmental Disabilities, 33, 2255–2264. doi: 10.1016/j.ridd.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan MW, Downey D, Strachan H, Elliott R, Williams SR, & Abel KM (2014). The neural basis of maternal bonding. PLOS ONE, 9, e88436. doi: 10.1371/journal.pone.0088436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Ford JM (2012). Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology, 8, 49–76. doi: 10.1146/annurev-clinpsy-032511-143049 [DOI] [PubMed] [Google Scholar]
- Wonch KE, de Medeiros CB, Barrett JA, Dudin A, Cunningham WA, Hall GB, … Fleming AS (2016). Postpartum depression and brain response to infants: Differential amygdala response and connectivity. Social Neuroscience. Advance online publication. doi: 10.1080/17470919.2015.1131193 [DOI] [PubMed] [Google Scholar]
- Yaseen ZS, Zhang X, Muran JC, Winston A, & Galynker II (2016). Comparison of brain activity correlating with self-report versus narrative attachment measures during conscious appraisal of an attachment figure. Frontiers in Human Neuroscience, 10, 90. doi: 10.3389/fnhum.2016.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hol-linshead M, … Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 1125–1165. doi: 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]


