Abstract
Objectives
This study sought to determine the frequency and magnitude of impaired systolic deformation in heart failure with preserved ejection fraction (HFpEF).
Background
Although diastolic dysfunction is widely considered a key pathophysiologic mediator of HFpEF, the prevalence of concomitant systolic dysfunction has not been clearly defined.
Methods
We assessed myocardial systolic and diastolic function in 219 HFpEF patients from a contemporary HFpEF clinical trial. Myocardial deformation was assessed using a vendor-independent 2-dimensional speckle-tracking software. The frequency and severity of impaired deformation was assessed in HFpEF, and compared to 50 normal controls free of cardiovascular disease and to 44 age- and sex-matched hypertensive patients with diastolic dysfunction (hypertensive heart disease) but no HF. Among HFpEF patients, clinical, echocardiographic, and biomarker correlates of left ventricular strain were determined.
Results
The HFpEF patients had preserved left ventricular ejection fraction and evidence of diastolic dysfunction. Compared to both normal controls and hypertensive heart disease patients, the HFpEF patients demonstrated significantly lower longitudinal strain (LS) (−20.0 ± 2.1 and −17.07 ± 2.04 vs. −14.6 ± 3.3, respectively, p < 0.0001 for both) and circumferential strain (CS) (−27.1 ± 3.1 and −30.1 ± 3.5 vs. −22.9 ± 5.9, respectively; p < 0.0001 for both). In HFpEF, both LS and CS were related to LVEF (LS, R = −0.46; p < 0.0001; CS, R = −0.51; p < 0.0001) but not to standard echocardiographic measures of diastolic function (E’ or E/E’). Lower LS was modestly associated with higher NT-proBNP, even after adjustment for 10 baseline covariates including LVEF, measures of diastolic function, and LV filling pressure (multivariable adjusted p = 0.001).
Conclusions
Strain imaging detects impaired systolic function despite preserved global LVEF in HFpEF that may contribute to the pathophysiology of the HFpEF syndrome. (LCZ696 Compared to Valsartan in Patients With Chronic Heart Failure and Preserved Left-ventricular Ejection Fraction; NCT00887588) (J Am Coll Cardiol 2014;63:447–56) © 2014 by the American College of Cardiology Foundation
Keywords: cardiac biomarkers, diastolic heart failure, echocardiography, mechanics, systolic strain
Heart failure with preserved ejection fraction (HFpEF) is a prevalent and growing public health problem associated with significant morbidity and an increased risk of in-hospital, short-term, and long-term mortality (1,2). Impairment in LV diastolic function has been proposed as a key pathophysiologic mediator (3–5). However, the role of concomitant systolic dysfunction despite preserved left ventricular ejection fraction (LVEF) has not been well characterized, but may help inform future treatment strategies by defining subphenotypes in this heterogeneous population. Indeed, prior studies suggest that LV longitudinal function assessed by tissue Doppler imaging may be impaired in HFpEF (6–11). However, tissue Doppler-based assessment of LV longitudinal function is angle dependent and typically assesses only mitral annular motion.
More recently, B-mode speckle tracking has allowed for quantitative assessment of LV deformation, and abnormalities of strain and strain rate have been described in HFpEF in several small single-center studies (12–15). We employed myocardial deformation imaging to determine the frequency, severity, and correlates of impaired systolic function among patients with HFpEF enrolled in a contemporary multicenter clinical trial. Specifically, we hypothesized that despite preserved LVEF, abnormal strain would be prevalent in HFpEF, differentiate HFpEF from asymptomatic hypertensive heart disease (HHD), and would relate to levels of N-terminal pro-brain natriuretic peptide (NT-proBNP), a soluble biomarker of myocardial wall stress with prognostic relevance in HFpEF, independent of measures of diastolic function.
Methods
Patient population
The PARAMOUNT (Prospective Comparison of ARNI With ARB on Management of Heart Failure With Preserved Ejection Fraction Trial) study enrolled patients with signs and symptoms of heart failure (HF), New York Heart Association class II to IV symptoms, LVEF ≥45%, and NT-proBNP level >400 pg/ml. Patients were randomly allocated to receive either the angiotensin-receptor neprilysin inhibitor (ARNI) LCZ696 or valsartan over a period of 12 weeks. The study protocol was approved by all individual site institutional review boards and ethics committees, and all recruited patients gave written informed consent. Details of the inclusion and exclusion criteria, study design and primary findings have been previously reported (16). Screening NT-proBNP was established by a tabletop device at point of care, local laboratory, or central laboratory. No NT-proBNP data were available for the HHD group or control population.
Control group
We screened the Brigham and Women’s Hospital’s echocardiography database to retrospectively identify normal control subjects. Echocardiographic examinations were clinically indicated for 1 of the following reasons: murmur, evaluation of LV function, syncope, or atypical chest pain. Normal echocardiograms were defined as normal LV size and geometry, normal LVEF (>55%), normal left atrial volume index (LAVi) (<29 ml/m2) (17), no stenotic valvular lesion, and no abnormal valvular regurgitation. Electronic medical records were reviewed for prevalent cardiovascular disease (stroke, coronary artery disease, myocardial infarction, revascularization, heart failure, arrhythmia, peripheral artery disease), cardiovascular risk factors (hypertension, diabetes mellitus, hyperlipidemia, smoking, renal dysfunction), systemic disease (such as cancer, infections, autoimmune disorders), or any pharmacotherapy. Subjects were excluded if any of these were identified. In all, 2,100 echocardiographic examinations and medical records performed between 2010 and 2012 were screened to identify 50 controls of similar age and sex distribution as our HFpEF cohort.
Hypertensive group with diastolic dysfunction but no HF
We identified 44 patients with hypertension and diastolic dysfunction matched to the HFpEF population for age and sex. They were selected from patients enrolled in the EXCEED (Exforge Intensive Control of Hypertension to Evaluate Efficacy in Diastolic dysfunction) trial. Details of the inclusion and exclusion criteria, study design, and primary findings have been previously published (18,19). Briefly, the EXCEED trial was a multicenter, open-label study of patients ≥45 years of age with a history of uncontrolled systolic hypertension, preserved LVEF (≥50%), and echocardiographic evidence of diastolic dysfunction. Patients with HF symptoms, secondary hypertension, diabetes, atrial fibrillation, a vascular event within the prior 6 months, serum creatinine >2.0 mg/dl, or nephrotic syndrome were excluded. All participants underwent echocardiography at enrollment, which was analyzed centrally by the same core laboratory as the PARAMOUNT study (Brigham and Women’s Hospital, Boston, Massachusetts).
Echocardiographic analyses
All sonographers at participating sites underwent central training in the details of the echocardiographic views and techniques at study investigator meetings. Echocardiograms were performed at study enrollment and were sent on digital storage media to the echocardiography core laboratory at Brigham and Women’s Hospital. Conventional echocardiographic analysis including 2-dimensional, Doppler, and tissue Doppler were performed by technicians blinded to clinical information and treatment assignment using an offline analysis work station, as previously described in detail (20). Ventricular volumes were calculated by the modified Simpson’s method using the apical 4- and 2-chamber views, and LVEF was derived from volumes in the standard manner (17). The LV mass was calculated from LV linear dimensions and indexed to body surface area as recommended by American Society of Echocardiography guidelines. Left ventricular hypertrophy was defined as LV mass indexed to body surface area (LVMi) >115 g/m2 in men or >95 g/m2 in women. The relative wall thickness (RWT) was calculated from LV end-diastolic dimension and posterior wall thickness. The left atrial (LA) volume was measured by the biplane area-length method using apical 4- and 2-chamber views at the end-systolic frame preceding mitral valve opening, and was indexed to body surface area to derive LAVi. Early transmitral velocity (E wave) was measured by pulsed wave Doppler from the apical 4-chamber view with the sample volume positioned at the tip of the mitral leaflets. Tissue Doppler derived peak longitudinal systolic shortening velocity (S′) was obtained in the apical 4-chamber view at the lateral and septal mitral annulus and averaged. Peak left ventricular relaxation velocity (E’) was obtained from the lateral and septal mitral annulus and averaged. The E/E’ ratio was calculated as E wave divided by E’ velocities. Diastolic dysfunction grade was derived from mitral inflow E/A ratio, tissue Doppler septal E’, and deceleration time (21). All measurements were performed in triplicate.
Digitally acquired baseline echocardiography images in Digital Imaging and Communications in Medicine (DICOM) format with acceptable image quality were uploaded to the TomTec system (Munich, Germany) for further deformational analyses (Cardiac Performance Analysis software, TomTec). These methods have been validated against magnetic resonance imaging and sonomicrometry (22,23), and we have previously reported excellent reproducibility (24–26). A total of 219 patients of the total PARAMOUNT patient population of 301 participants (73% of total enrolled) had adequate echocardiographic image quality for deformational analysis by B-mode speckle tracking. Unacceptable image quality was defined as lack of a full cardiac cycle, >1 segment dropout, digital format other than DICOM, missing view, or significant foreshortening of the left ventricle. As compared to the 219 patients with image quality adequate for strain analysis, the 82 excluded patients were less frequently female (45% vs. 61%), had a lower prevalence of chronic obstructive pulmonary disease (6% vs. 16%), a higher prevalence of diabetes (49% vs. 34%), and a lower LVEF (56% vs. 59%, p = 0.006). No significant differences were noted in other clinical or echocardiographic measures, including age, NT-proBNP level, LV mass index, LAVi, E’, and E/E’. (Detailed information on included and excluded patients are given in Online Table S1.)
For deformation analysis, endocardial borders were traced at the end-diastolic frame in apical views and at an end-systolic frame in short-axis views. End diastole was defined by the QRS complex or as the frame after mitral valve closure. The software tracks speckles along the endocardial border throughout the cardiac cycle. Peak longitudinal strain (LS) and peak circumferential strain (CS) were computed automatically generating regional data from 6 segments and an average value for each view. For patients in sinus rhythm, analyses were performed on a single cardiac cycle; and for patients in atrial fibrillation, strain values were calculated as the average of 3 cardiac cycles. Peak average LS was measured in the apical 4-chamber and apical 2-chamber views (in 6 segments from each view) and averaged, and peak average CS was obtained from 6 segments measured in the short-axis view at the midpapillary level.
All strain analysis on HFpEF, HHD, and normal control subjects were performed by a single investigator. Intra-observer variability for LS and CS was assessed in a sample of 30 randomly selected patients. Coefficient of variation was 6.8% and 8.1% for LS and CS, respectively. Intraclass correlation coefficients were 0.95 for LS (95% confidence interval: 0.91 to 0.98) and 0.94 for CS (95% confidence interval: 0.91 to 0.98).
Statistical analyses
Descriptive statistics for continuous variables are expressed as mean and standard deviation for normally distributed variables and median and interquartile range for non-normally distributed data. Categorical variables are presented as percentages. Comparison of echocardiographic measures between HFpEF versus HHD and normal controls was performed using Student t tests, Wilcoxon rank-sum tests, or chi-square tests, as appropriate. The relationship between average LS and CS and clinical characteristics, echocardiographic measures, electrocardiographic parameters, and NT-proBNP was assessed using linear regression or nonparametric trend tests. Abnormal LS and CS was defined as >1 SD or >2 SD below the mean value of normal controls.
The NT-proBNP was log-transformed due to its skewed distribution. Pearson correlation coefficient was used to assess the relationships between log-transformed NT-proBNP and strain measures. Multivariable linear regression was used to determine the relationship between strain measures and NT-proBNP after adjustment for potential confounders. All p values were 2-sided, with p < 0.05 used to define statistical significance. Statistical analyses were performed using STATA version 11.2 (Stata Corp., College Station, Texas).
Results
Of 301 patients randomized in the PARAMOUNT study, 219 (73%) had echocardiographic images in appropriate format and of adequate quality for speckle-tracking analysis (Online Table S1). Baseline patient characteristics of the 219 included patients are summarized in Table 1. The average age was 71 ± 9 years, and the majority of patients were female, white, and had a history of hypertension. Half had a history of prior HF hospitalization. In addition to diuretic use (100%), which was a required inclusion criterion, rates of therapy with an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker (92%) and beta-blockers (80%) were high. The median NT-proBNP level was markedly elevated (894 pg/ml, interquartile range: 526 to 1,457 pg/ml).
Table 1.
Baseline Characteristics According to Quartiles of Longitudinal Strain
| Overall (n = 219) | LS Quartile 1 (n = 55) | LS Quartile 2 (n = 55) | LS Quartile 3 (n = 55) | LS Quartile 4 (n = 54) | p Value for Trend | |
|---|---|---|---|---|---|---|
| Longitudinal strain, % | −14.6 ± 3.3 | −26.5 to −16.5 | −16.4 to −14.8 | −14.8 to −12.3 | −12.2 to −7.4 | |
| Clinical characteristics | ||||||
| Age | 72 (66–78) | 70 (66–76) | 74 (70–80) | 73 (66–79) | 70 (60–78) | 0.226 |
| Female | 61 | 67 | 53 | 64 | 59 | 0.86 |
| White race | 83 | 93 | 85 | 89 | 65 | <0.001 |
| Medical history | ||||||
| Hypertension | 90 | 91 | 93 | 95 | 90 | 0.97 |
| Diabetes mellitus | 34 | 33 | 33 | 24 | 48 | 0.22 |
| Renal disease, eGFR<60 ml/kg/1.73 m2 | 37 | 38 | 36 | 45 | 28 | 0.51 |
| Coronary heart disease | 42 | 27 | 51 | 47 | 44 | 0.11 |
| Prior MI | 19 | 13 | 27 | 16 | 21 | 0.55 |
| Prior HF hospitalization | 50 | 36 | 49 | 53 | 63 | 0.006 |
| Systolic BP, mm Hg | 136 (128–145) | 139 (127–146) | 139 (130–147) | 136 (124–150) | 132 (128–140) | 0.46 |
| Diastolic BP, mm Hg | 80 (71–84) | 75 (68–85) | 78 (73–85) | 80 (72–82) | 80 (75–82) | 0.12 |
| Body mass index, kg/m2 | 29.7 (26.1–33.6) | 30.9 (27.2–34.0) | 29.1 (25.8–32.9) | 29.8 (26.1–36.8) | 27.5 (24.6–31.7) | 0.03 |
| Body surface area, m2 | 1.85 (1.68–2.00) | 1.90 (1.72–2.03) | 1.85 (1.73–1.96) | 1.83 (1.70–2.07) | 1.75 (1.61–1.95) | 0.022 |
| Electrocardiogram | ||||||
| Heart rate, beats/min | 66 (60–75) | 62 (58–73) | 64 (55–74) | 68 (60–75) | 71 (64–81) | 0.001 |
| LBBB | 5 | 2 | 4 | 7 | 9 | 0.06 |
| Atrial fibrillation | 29 | 35 | 35 | 24 | 22 | 0.08 |
| Biomarkers | ||||||
| NT-proBNP, pg/ml | 894 (526–1,457) | 771 (419–1,036) | 946 (540–1,454) | 999 (582–1,615) | 941 (663–2,119) | 0.005 |
| Echocardiographic characteristics | ||||||
| LV structure | ||||||
| LVEDVi, ml/m2 | 58.4 (50.5–67.9) | 55.4 (49.4–64.8) | 58.6 (50.5–73.0) | 57.4 (50.8–66.3) | 63.3 (54.6–72.7) | 0.034 |
| LVESVi, ml/m2 | 23.2 (19.1–29.6) | 20.5 (16.8–24.6) | 22.8 (19.9–30.6) | 24.5 (18.1–28.8) | 28.7 (22.4–34.3) | <0.001 |
| RWT | 0.37 (0.33–0.40) | 0.37 (0.34–0.41) | 0.37 (0.33–0.42) | 0.36 (0.32–0.40) | 0.36 (0.32–0.40) | 0.12 |
| LVMi, g/m2 | 74.1 (63.2–90.7) | 71.0 (60.0–85.8) | 75.4 (62.3–95.1) | 73.0 (63.2–84.5) | 81.1 (66.8–96.0) | 0.036 |
| Concentric remodeling, % | 13 | 15 | 11 | 13 | 14 | 0.92 |
| Concentric hypertrophy, % | 8 | 9 | 13 | 4 | 6 | 0.24 |
| Eccentric hypertrophy, % | 7 | 4 | 11 | 4 | 10 | 0.51 |
| Systolic function | ||||||
| LVEF, % | 59.2 (53.7–63.6) | 63.3 (58.9–67.0) | 59.8 (54.1–62.2) | 58.1 (53.5–62.7) | 54.0 (49.6–59.3) | <0.001 |
| LVEF <50% | 11 | 0 | 5 | 11 | 26 | <0.001 |
| LVEF 50% –55% | 22 | 11 | 20 | 22 | 34 | 0.005 |
| LVEF >55% | 68 | 89 | 75 | 67 | 40 | <0.001 |
| LV stroke volume, ml | 61.4 (52.6–76.5) | 66.4 (53.7–82.9) | 67.7 (54.6–79.7) | 60.5 (51.9–71.7) | 59.2 (50.3–71.2) | 0.003 |
| S’ mean, cm/s | 6.29 (5.24–7.21) | 6.51 (5.86–7.66) | 6.34 (5.33–7.04) | 5.61 (4.96–6.69) | 6.25 (4.88–7.24) | 0.009 |
| Diastolic function | ||||||
| E’ lateral, cm/s | 7.4 (5.4–9.0) | 7.3 (5.2–9.4) | 7.4 (5.8–8.9) | 7.1 (5.3–8.8) | 7.5 (5.4–9.2) | 0.94 |
| E’ septal, cm/s | 5.3 (4.2–6.8) | 5.4 (4.4–6.6) | 5.2 (4.5–6.8) | 5.2 (4.0–7.4) | 5.4 (4.2–6.8) | 0.64 |
| E/E’ ratio (septal) | 14.7 (11.5–18.8) | 15.7 (12.1–19.1) | 14.2 (11.1–18.1) | 14.2 (11.4–20.1) | 15.3 (11.5–22.6) | 0.84 |
| LAVi, ml/m2 | 33.9 (26.8–43.0) | 34.2 (27.9–45.0) | 35.1 (28.1–44.7) | 31.5 (24.0–41.2) | 34.1 (27.4–40.5) | 0.49 |
Values are mean ± SD, median (interquartile range), or n. The p value for trend across quartiles of longitudinal strain (LS) is averaged from apical 4- and 2-chamber views.
BP = blood pressure; E/E’ ratio = mitral inflow to mitral relaxation velocity ratio; eGFR = estimated glomerular filtration rate; E’ lateral = lateral mitral relaxation velocity; E’ septal = septal mitral relaxation velocity; HF = heartfailure; LAVi = left atrial volume index; LBBB = left bundle branch block; LVEDVi = leftventricularend-diastolicvolume index; LVEF = leftventricularejection fraction; LVESVi = left ventricular end-systolic volume index; LVMi = left ventricular mass index; MI = myocardial infarction; NT-proBNP = N-terminal pro-brain natriuretic peptide; RWT = relative wall thickness.
Among the normal control group (n = 50), the mean age was 69 ± 7 years, 68% were female, the majority was white, and their mean body mass index was 25.9 ± 3.9 kg/m2. All patients in the control group were free of hypertension, diabetes, hyperlipidemia, smoking, coronary artery disease, and structural or valvular heart disease, and were not taking any cardiovascular medication. Echocardiographic analysis showed normal-sized ventricles, wall thickness, and left atrial size (LAVi 21.3 ± 5.5 ml/m2). Left ventricular ejection fraction was normal (61 ± 3%), and there was no evidence of diastolic dysfunction (E’ lateral: 9.0 ± 2.2).
Among the 44 age- and sex-matched HHD patients, the average age was 71 ± 8, 61% were female, the majority was2 white, and their mean body mass index was 28.5 ± 4.8 kg/m. Mean blood pressure was 165/85 mm Hg. Echocardiographic analysis showed normal-sized ventricles (mean left ventricular end-diastolic volume 100 ± 17 ml) with preserved LVEF (mean 56 ± 3%). The LV mass index was 73.5 ± 16.1 g/m2. By definition, all patients had evidence of diastolic dysfunction with a mean E/E’ of 9.4 ± 2.2 and LAVi of 26.6 ± 3.7 ml/m2. (A comprehensive summary of clinical and echocardiographic characteristics of the normal control, the HHD, and the HFpEF group is provided in Online Table S2.)
Conventional 2-dimensional and Doppler echocardiographic findings in the overall HFpEF cohort are shown in Table 1. Diastolic dysfunction was present in 95% of patients, with 66% having grade II or III diastolic dysfunction. Median septal E/E’ was 14.7 (11.5−18.8) and two-thirds presented with enlarged left atria using a cutoff of 29 ml/m2 (median LAVi 33.9 (26.8−43.0) ml/m2) (19). Despite the high prevalence of diastolic abnormalities and signs of increased LV filling pressure, LV volumes, mass, and geometry were normal in most subjects, with only 15% demonstrating LV hypertrophy and 21% demonstrating concentric remodeling or hypertrophy.
HFpEF versus controls
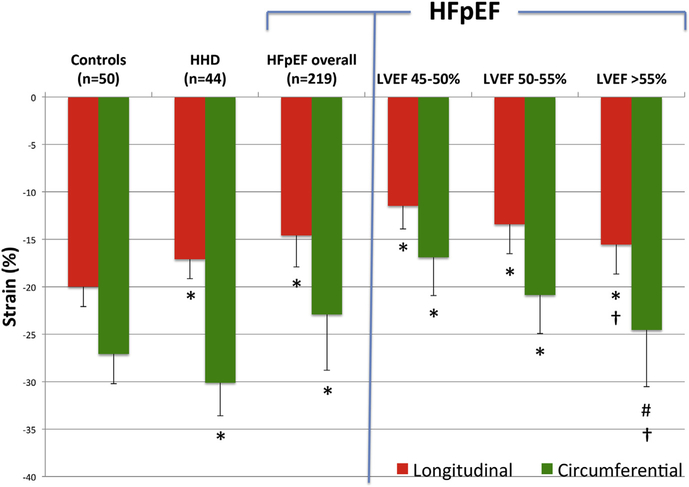
Although global systolic pump function (LVEF) did not differ significantly between the PARAMOUNT study patients and normal controls (59 ± 8% versus 61 ± 3%, respectively; p = 0.09), HFpEF patients demonstrated significantly lower LS and CS (LS, p < 0.0001; CS, p < 0.0001) (Fig. 1, Table 2). We observed a relationship between LVEF and both LS (Pearson correlation = −0.46, p < 0.001) and CS (Pearson correlation = −0.51, p < 0.001). However, both LS and CS remained significantly lower among HFpEF patients compared to controls after adjusting for LVEF (p < 0.001 for both LS and CS) (Fig. 1, Table 2) and after excluding subjects with LVEF <55% (p < 0.0001 for LS, p = 0.0002 for CS). Patients with evidence of ischemic heart disease had worse LS and CS as compared to those HFpEF patients without ischemic heart disease.
Figure 1. Average Longitudinal and Circumferential Systolic Strain.
Average longitudinal strain (red bars) and circumferential systolic strain (green bars) among normal controls (n = 50), hypertensive heart disease (HHD) patients (n = 44), heart failure with preserved ejection fraction (HFpEF) patients overall (n = 219), and in 3 categories HFpEF based on left ventricular ejection fraction (LVEF). *p < 0.0001 compared to controls and between HHD and HFpEF overall for longitudinal strain and circumferential strain. #p = 0.0002 compared to controls. †LVEF-adjusted p < 0.001 compared to controls.
Table 2.
Percentage of Patients With Abnormal Strain*
| Controls | HHD | HFpEF Overall | HFpEF by LVEF Category |
p Value (HHD vs. Control) | p Value (HFpEF vs. Control) | p Value (HFpEF vs. HHD) | |||
|---|---|---|---|---|---|---|---|---|---|
| EF <50% | EF 50%–55% | EF>55% | |||||||
| Longitudinal strain | n = 50 | n = 44 | n = 219 | n = 23 | n = 47 | n = 149 | |||
| Mean ± SD, % | −20.0 ± 2.1 | −17.07 ± 2.04 | −14.6 ± 3.3 | 11.5 ± 2.5 | −13.5 ± 3.1 | −16.6 ± 3.5 | <0.0001 | <0.0001† | <0.0001 |
| Percent abnormal | |||||||||
| >1 SD below normal (−17.9) | 61.4% | 71% | 100% | 92% | 64% | ||||
| >2 SD below normal (−15.8) | 29.6% | 54.3% | 100% | 81% | 43% | ||||
| Circumferential strain | n = 50 | n = 41 | n = 146 | n = 14 | n = 35 | 142 | |||
| Mean ± SD, % | −27.1 ± 3.1 | −30.1 ± 3.5 | −22.9 ± 5.9 | 16.9 ± 4.0 | −21.0 ± 4.1 | −25.3.5 ± 4 | <0.0001 | <0.0001† | <0.0001 |
| Percent abnormal | |||||||||
| >1 SD below normal (−24) | 2.4% | 51% | 100% | 71% | 42% | ||||
| >2 SD below normal (−20.9) | 0% | 31% | 79% | 54% | 20% | ||||
> 1 or 2 standard deviations from the mean value for normal controls.
p < 0.001 when adjusted for left ventricular ejection fraction (LVEF).
EF = ejection fraction; HFpEF = heart failure with preserved ejection fraction; HHD = hypertensive heart disease.
To further investigate the role of ischemic heart disease in the observed differences in LV deformation, we performed a sensitivity analysis excluding all patients with a history of myocardial infarction, coronary artery disease, revascularization procedures, and anginal symptoms, and all patients with an LVEF <55%. In the remaining 91 patients without any evidence of myocardial ischemia and an LVEF ≥55%, both LS and CS remained significantly lower as compared to controls (HFpEF vs. controls: LS, −15.7 [−18.0 to −13.8] vs. −19.9 [−21.3 to −18.3], p < 0.0001; CS, −24.2 [−29.0 to −20.4] vs. −26.9 [−28.5 to −25.0], p = 0.0007).
HFpEF versus HHD
Compared to HHD, the HFpEF group demonstrated significantly lower LS (p < 0.0001) and CS (p < 0.0001) (Fig. 1, Table 2). Interestingly, when compared to controls, the HHD group demonstrated significantly lower LS (p < 0.0001) but higher CS (p < 0.0001).
Prevalence of abnormal strain in HFpEF
Abnormal LS and CS was present in 66.7% and 40.4% of HFpEF patients, respectively, when abnormal was defined as >2 SD below the mean value of controls (Table 2). In analyses stratified by LVEF (<50%, 50% to 55%, and >55%), the proportion of patients with abnormal LS and CS was greatest in the lowest LVEF category. The LS was more frequently abnormal than the CS, a pattern that held across all LVEF categories (Table 2). The magnitude of impairment in LS was also more prominent than the magnitude of impairment in CS (average relative reduction compared to controls of 27% and 15%, respectively).
Longitudinal strain in HFpEF
Worse LS was significantly associated with nonwhite race, a history of HF hospitalization, higher heart rate, ischemic etiology, and lower LVEF (Table 1). No significant association was noted between LS and sex, cardiovascular comorbidities, or pharmacotherapy. Importantly, LS was not associated with systolic or diastolic blood pressure, and 70% of patients with normal blood pressure at the time of echocardiography had abnormal LS. Worse LS was significantly associated with lower LVEF (p < 0.001), stroke volume (p = 0.003), and S’ (p = 0.009). The association with LVEF remained significant when LVEF was stratified into categories (LVEF <50%, p < 0.001; LVEF 50% to 55%, p = 0.005; LVEF >55%, p < 0.001) (Table 1). Worse LS was also associated with higher LV end-systolic volume index (p < 0.001), LV end-diastolic volume index (p = 0.03), and LV mass index (p = 0.04). There was no association between LS and echocardiographic measures of diastolic function (Table 1).
Circumferential strain in HFpEF
Patients with worse CS were more likely to have a history of HF hospitalization, coronary heart disease, and prior myocardial infarction. Worse CS was also associated with lower systolic blood pressure but not with age, race, or heart rate. Like LS, lower CS was associated with lower LVEF, lower stroke volume, and higher LV end-systolic volume index. The association with LVEF remained significant after stratification by LVEF category (LVEF <50%, p < 0.001; LVEF 50% to 55%, p = 0.012; LVEF >55%, p < 0.001) (Table 3). There was no association between CS and S’ (p = 0.40). Similar to LS, there was no association between CS and measures of diastolic function. The CS was related to LV geometry, with worse CS being significantly related to lower RWT (Table 3). Similarly, in a multivariable model accounting for clinical covariates and echocardiographic measures of cardiac structure and function, LV mass index was significantly associated with CS (p = 0.02).
Table 3.
Baseline Characteristics According to Quartiles of Circumferential Strain
| Overall (n = 146) | CS Quartile 1 (n = 37) | CS Quartile 2 (n = 36) | CS Quartile 3 (n = 38) | CS Quartile 4 (n = 35) | p Value for trend | |
|---|---|---|---|---|---|---|
| Circumferential strain, % | −22.9 ± 5.9 | −40.9 to −25.9 | −25.9 to −22.1 | −22.1 to −18.9 | −18.9 to −11.1 | |
| Clinical characteristics | ||||||
| Age | 73 (66–79) | 74 (67–78) | 71 (64–79) | 74 (66–78) | 72 (66–79) | 0.71 |
| Female | 62 | 59 | 75 | 63 | 49 | 0.24 |
| White race | 84 | 92 | 75 | 84 | 83 | 0.51 |
| Medical history | ||||||
| Hypertension | 90 | 95 | 89 | 89 | 88 | 0.40 |
| Diabetes mellitus | 37 | 35 | 36 | 37 | 41 | 0.61 |
| Renal disease, eGFR<60 ml/kg/1.73 m2 | 35 | 39 | 19 | 38 | 43 | 0.40 |
| Coronary heart disease | 38 | 30 | 25 | 40 | 59 | 0.006 |
| Prior MI | 17 | 5 | 17 | 16 | 32 | 0.005 |
| Prior HF hospitalization | 51 | 27 | 53 | 58 | 66 | 0.001 |
| Systolic BP, mm Hg | 134 (127–144) | 140 (132–147) | 135 (130–150) | 127 (120–140) | 133 (128–140) | 0.03 |
| Diastolic BP, mm Hg | 80 (70–84) | 78 (74–85) | 80 (70–88) | 78 (70–80) | 79 (70–83) | 0.88 |
| Body mass index, kg/m2 | 29.9 (25.8–33.6) | 30.0 (26.5–34.0) | 28.4 (24.3–32.0) | 30.9 (26.3–34.5) | 30.1 (25.6–32.7) | 0.81 |
| Body surface area, m2 | 1.86 (1.68–2.02) | 1.94 (1.72–2.09) | 1.75 (1.67–1.95) | 1.85 (1.75–1.99) | 1.88 (1.66–2.03) | 0.74 |
| Electrocardiogram | ||||||
| Heart rate, beats/min | 66 (60–75) | 63 (59–72) | 69 (61–77) | 67 (60–75) | 68 (60–75) | 0.14 |
| Atrial fibrillation | 32 | 30 | 39 | 29 | 31 | 0.89 |
| Biomarkers | ||||||
| NT- proBNP, pg/ml | 945 (513–1,561) | 1,036 (540–1,834) | 833 (457–1,495) | 836 (482–1,459) | 951 (726–1,796) | 0.86 |
| Echocardiographic characteristics | ||||||
| LV structure | ||||||
| LVEDVi, ml/m2 | 58.4 (50.7–67.6) | 56.5 (49.6–65.1) | 58.3 (53.5–68.2) | 58.4 (47.9–66.0) | 61.5 (50.9–73.8) | 0.48 |
| LVESVi, ml/m2 | 22.9 (19.0–29.4) | 20.3 (16.6–24.9) | 24.1 (20.0–30.8) | 22.6 (19.0–28.9) | 28.2 (22.1–35.1) | 0.001 |
| RWT | 0.36 (0.33–0.41) | 0.37 (0.35–0.43) | 0.39 (0.34–0.42) | 0.35 (0.33–0.40) | 0.34 (0.32–0.39) | 0.016 |
| LVMi, g/m2 | 73.8 (62.1–90.6) | 73.8 (58.3–91.8) | 73.6 (63.3–86.5) | 71.8 (62.2–90.6) | 75.0 (65.1–90.7) | 0.79 |
| Concentric remodeling, % | 15 | 14 | 23 | 11 | 12 | 0.53 |
| Concentric hypertrophy, % | 8 | 14 | 6 | 3 | 9 | 0.37 |
| Eccentric hypertrophy, % | 7 | 8 | 6 | 8 | 6 | 0.83 |
| Systolic function | ||||||
| LVEF, % | 59.9 (53.7–64.1) | 63.1 (60.5–67.1) | 59.1 (54.3–64.3) | 59.3 (53.3–62.6) | 54.2 (49.6–60.3) | <0.001 |
| LVEF <50% | 8 | 0 | 6 | 3 | 27 | <0.001 |
| LVEF 50%–55% | 24 | 11 | 20 | 32 | 33 | 0.014 |
| LVEF >55% | 68 | 89 | 74 | 65 | 39 | <0.001 |
| LV stroke volume, ml | 61.1 (52.1–78.4) | 68.3 (57.7–85.2) | 59.7 (55.1–73.1) | 61.5 (51.1, 73.2) | 56.6 (47.6, 75.7) | 0.02 |
| S’ mean, cm/s | 6.17 (5.21–7.07) | 6.22 (5.49–7.30) | 6.11 (5.24–6.91) | 6.49 (5.24, 7.21) | 5.83 (4.89, 7.35) | 0.40 |
| Diastolic function | ||||||
| E’ lateral, cm/s | 7.1 (5.1–8.8) | 7.0 (5.4–8.7) | 7.0 (4.6–10.2) | 7.2 (5.2–8.3) | 7.4 (5.4–9.4) | 0.70 |
| E’ septal, cm/s | 5.3 (4.2–7.1) | 5.3 (4.3–6.3) | 5.2 (4.2–7.4) | 5.1 (4.2–7.1) | 6.0 (4.0–7.6) | 0.88 |
| E/E’ ratio (septal) | 14.8 (11.7–19.1) | 14.2 (11.2–18.3) | 13.8 (11.4–19.0) | 15.8 (13.1–22.4) | 14.2 (11.7–19.0) | 0.55 |
| LAVi, ml/m2 | 34.4 (27.7–44.1) | 33.7 (28.8–45.0) | 38.2 (28.1–46.8) | 36.5 (28.4–40.9) | 33.4 (24.4–44.1) | 0.60 |
Values are mean ± SD, median (interquartile range), or n. The p value for trend across quartiles of CS (circumferential strain) is from the parasternal short-axis view.
Abbreviations as in Table 1.
Association of strain and NT-proBNP
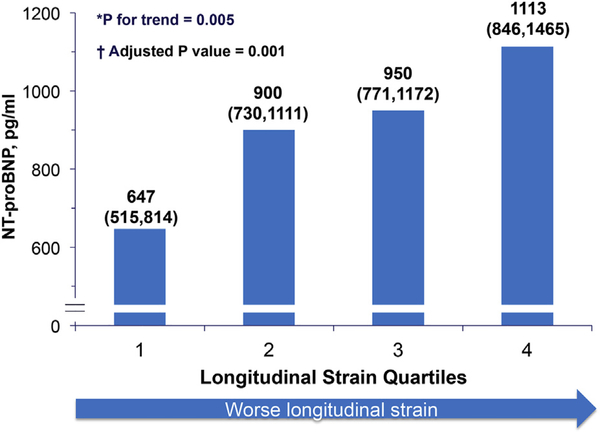
Worse LS (modeled both as categorical variable in quartiles and continuously) was associated with higher NT-proBNP levels, both when modeled continuously (Pearson correlation 0.20, p = 0.005) (Fig. 2) and categorically (as quartiles; p for trend = 0.005). The inverse relationship between LS and NT-proBNP remained significant after adjusting for age, sex, systolic and diastolic blood pressure, body mass index, LVEF, LAVi, E/E’, atrial fibrillation, and estimated glomerular filtration rate (adjusted p = 0.001). This robust relationship also remained significant when adjusting for E’ instead of E/E’ (p = 0.001) or when adding E′ (p = 0.001) or S′ (p = 0.002) to the model. In contrast to LS, contemporary measures of diastolic function (E′ and LAVi) were not independently associated with NT-proBNP, nor was a history of ischemic heart disease or presence of EF <55%. The inverse association of LS with NT-proBNP, however, remained significant in the subgroup of patients without ischemic heart disease and with EF ≥55%. The CS was not associated with NT-proBNP.
Figure 2. Association of Longitudinal Systolic Strain and NT-proBNP.
Association of longitudinal systolic strain (quartiles) and N-terminal pro-brain natriuretic peptide (NT-proBNP), geometric means and 95% confidence intervals. *Trend test performed using log-transformed NT-proBNP data. †Analysis adjusted for age, sex, systolic and diastolic blood pressure, body mass index, E/E’, left ventricular ejection fraction, left atrial volume index, atrial fibrillation, and estimated glomerular filtration rate.
Discussion
Principal findings
This study of 219 patients with HFpEF enrolled in a contemporary international multicenter clinical trial has 3 major findings. First, LV LS and CS are significantly reduced in HFpEF compared to normal controls and to age- and sex-matched hypertensive patients with diastolic dysfunction. Second, the prevalence of reduced LS and CS in HFpEF is high. Although LS and CS are significantly related to LVEF, the impairment in LS and CS in HFpEF persists even when restricted to patients with EF >55% or to patients without coronary heart disease. More than half of HFpEF patients with an LVEF ≥55% had reduced LS. Neither LS nor CS were related to standard echocardiographic measures of diastolic function (E’ or E/E’). Third, LS is significantly and independently associated with NT-proBNP level, a prognostically relevant biomarker in HFpEF.
Systolic dysfunction in HFpEF
Although LVEF is the most commonly used and accepted measure of systolic function, it is highly load dependent and relatively insensitive to subtle abnormalities of LV function (8,27). Indeed, some studies involving select HFpEF patients have failed to demonstrate abnormalities in systolic performance, reflected in stroke work, preload recruitable stroke work, and peak (+)dP/dt (28). In contrast, several other studies evaluating multiple noninvasive measures of LV systolic function by standard echocardiographic techniques, such as LV midwall fractional shortening or mitral annular plane systolic displacement, indicate that systolic function may not be uniformly normal in HFpEF (11,29). The reason for these discrepancies are unclear but may be related to the systolic measures evaluated and differences in the HFpEF patients studied. Early data employing tissue Doppler suggest that longitudinal systolic function may be abnormal despite preserved LVEF in conditions predisposing to HF and in HFpEF (6,11). However, tissue Doppler imaging faces technical limitations including preload and afterload dependence and is limited in its ability to assesses different planes of LV deformation other than longitudinal (30). In addition, prior studies in HFpEF have been largely limited to single-center experiences with small series of select patients (12–15).
Speckle-tracking echocardiography is a relatively new technique, largely independent of angle of incidence, tethering, and cardiac translation, which allows for quantification of myocardial deformation in multiple planes. During systole, the components of LV deformation include longitudinal shortening, radial thickening, and circumferential shortening (31). These planes of deformation are thought to be related to LV myocardial fiber orientation, which is primarily in the longitudinal direction sub-endocardially and primarily in an oblique orientation sub-epicardially (32). Our findings demonstrate a high prevalence of impaired LV longitudinal function in HFpEF, even among patients with LVEF >55%, with worse LS significantly related to higher NT-proBNP levels even after adjusting for LVEF and diastolic measures. NT-proBNP is a powerful prognostic discriminator in HFpEF (33). Longitudinal strain predicts outcome in low LVEF patients independent of LVEF (24,25). Whether impaired longitudinal deformation has prognostic significance in HFpEF remains to be determined.
Our data further suggest impairment in LV circumferential deformation in HFpEF. Conditions predisposing to HFpEF, such as hypertension or diabetes, are characterized by reduced longitudinal strain but an increase in circumferential function (34–36), which has been proposed as a compensatory mechanism to preserve LVEF (37). Our findings suggest that reduced LV CS partially distinguishes patients with HFpEF from asymptomatic persons with similar comorbidities. This hypothesis is also supported by prior studies demonstrating a progressive decrease of global CS from normal to HFpEF to HFrEF groups even after adjustment for LV end-systolic wall stress (12).
The underlying pathophysiology in patients with HFpEF has been commonly believed to involve impairment of diastolic function, with increased passive chamber stiffness (38,39). However, the marked phenotypic and pathophysiologic heterogeneity characterizing this syndrome is now well recognized. Traditional noninvasive markers of diastolic dysfunction are absent in approximately one-third of patients enrolled in large HFpEF trials (40,41). Indeed, in the PARAMOUNT trial, although the majority of patients demonstrated some echocardiographic findings of diastolic abnormalities at rest, frankly elevated filling pressure—based on an E/E’ ratio ≥15—was present in only 49% of the patients. Similarly, the prevalence of concentric ventricular remodeling was very low. These observations suggest that abnormalities other than concentric hypertrophy and elevated filling pressure (assessed as E/É ≥15 at rest) may contribute to the pathogenesis of HFpEF. Our findings of lower LV strain, a measure of LV systolic function that was not correlated with diastolic indices, and its independent association with NT-proBNP suggest a contribution of systolic dysfunction despite preserved LVEF in at least a subset of patients with HFpEF.
Study limitations
Strain analysis was not possible in all patients enrolled in the PARAMOUNT trial, although no significant systematic differences were noted between patients included or excluded from this analysis. Studies were performed at 65 sites and on echocardiography machines from a variety of vendors. However, all studies were recorded digitally, and quantitative analysis was performed centrally at a blinded core laboratory. All echocardiograms were performed in a resting condition, which limits the ability to assess the relationship between LS and impaired functional capacity, an important hallmark of the HFpEF syndrome. Patients enrolled in this contemporary HFpEF clinical trial may not be representative of HFpEF patients in the community, because of specific clinical trial inclusion and exclusion criteria. Future studies with clinical outcomes will be essential to understand the clinical relevance of our findings.
Conclusions
Systolic impairment in LV longitudinal and circumferential deformation is prevalent in HFpEF. Worse LS, in particular, is associated with higher NT-proBNP. Our findings suggest that abnormalities of LV systolic function measured by strain imaging may contribute to the HFpEF syndrome. These findings may help inform future studies to identify pathophysiologically relevant subgroups of patients within this heterogeneous syndrome.
Supplementary Material
Acknowledgments
Drs. Solomon, Shah, Zile, Pieske, Voors, Packer, and McMurray have received research support from and/or have consulted for Novartis. Dr. Pieske is on the Steering Committee of the PARAMOUNT study and receives moderate compensation. Dr. Lefkowitz is an employee of Novartis. All other authors have reported they have no relationships relevant to the contents of this paper to disclose. Drs. Kraigher-Krainer and Shah contributed equally to this manuscript.
Abbreviations and Acronyms
- CS
circumferential strain
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HHD
hypertensive heart disease
- LA
left atrial
- LAVi
left atrial volume index
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- LS
longitudinal strain
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- RWT
relative wall thickness
Footnotes
APPENDIX
For supplemental tables, please see the online version of this article.
REFERENCES
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis 2005;47:320–32. [DOI] [PubMed] [Google Scholar]
- 3.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail 2011;13:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulus WJ, Tschoepe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007;28:2539–50. [DOI] [PubMed] [Google Scholar]
- 5.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure—abnormalities inactiverelaxationandpassivestiffnessoftheleftventricle.NEnglJMed 2004;350:1953–9. [DOI] [PubMed] [Google Scholar]
- 6.Yip G, Wang M, Zhang Y, Fung JW, Ho PY, Sanderson JE. Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefinition? Heart 2002;87: 121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu CM, Lin H, Yang H, Kong SL, Zhang Q, Lee SW. Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation 2002;105:1195–201. [DOI] [PubMed] [Google Scholar]
- 8.Aurigemma GP, Zile MR, Gaasch WH. Contractile behavior of the left ventricle in diastolic heart failure: with emphasis on regional systolic function. Circulation 2006;113:296–304. [DOI] [PubMed] [Google Scholar]
- 9.Vinereanu D, Lim PO, Frenneaux MP, Fraser AG. Reduced myocardial velocities of left ventricular long-axis contraction identify both systolic and diastolic heart failure—a comparison with brain natriuretic peptide. Eur J Heart Fail 2005;7:512–9. [DOI] [PubMed] [Google Scholar]
- 10.Bruch C, Gradaus R, Gunia S, Breithardt G, Wichter T. Doppler tissue analysis of mitral annular velocities: evidence for systolic abnormalities in patients with diastolic heart failure. J Am Soc Echocardiogr 2003;16:1031–6. [DOI] [PubMed] [Google Scholar]
- 11.Petrie MC, Caruana L, Berry C, McMurray JJ. “Diastolic heart failure” or heart failure caused by subtle left ventricular systolic dysfunction? Heart 2002;87:29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yip GW, Zhang Q, Xie JM, et al. Resting global and regional left ventricular contractility in patients with heart failure and normal ejection fraction: insights from speckle-tracking echocardiography. Heart 2011;97:287–94. [DOI] [PubMed] [Google Scholar]
- 13.Carluccio E, Biagioli P, Alunni G, et al. Advantages of deformation indices over systolic velocities in assessment of longitudinal systolic function in patients with heart failure and normal ejection fraction. Eur J Heart Fail 2011;13:292–302. [DOI] [PubMed] [Google Scholar]
- 14.Tan YT, Wenzelburger F, Lee E, et al. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol 2009;54:36–46. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J 2008;29:1283–9. [DOI] [PubMed] [Google Scholar]
- 16.Solomon SD, Zile M, Pieske B, et al. , for the Prospective Comparison of ARNI With ARB on Management of Heart Failure With Preserved Ejection Fraction (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012;380:1387–95. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group. J Am Soc Echocardiogr 2005;18:1440–63. [DOI] [PubMed] [Google Scholar]
- 18.Solomon SD, Verma A, Desai A, et al. Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension 2010;55:241–8. [DOI] [PubMed] [Google Scholar]
- 19.Hassanein A, Desai A, Verma A, et al. EXCEED: Exforge-Intensive Control of Hypertension to Evaluate Efficacy in Diastolic Dysfunction: study rationale, design, and participant characteristics. Ther Adv Cardiovasc Dis 2009;3:429–39. [DOI] [PubMed] [Google Scholar]
- 20.Solomon SD, Foster E, Bourgoun M, et al. Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: MADIT-CRT. Circulation 2010;122:985–92. [DOI] [PubMed] [Google Scholar]
- 21.Redfield MM, Jacobsen SJ, Burnett JC Jr., Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 22.Amundsen BH, Helle-Velle T, Edvardsen T, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 2006;47:789–93. [DOI] [PubMed] [Google Scholar]
- 23.Pirat B, Khoury DS, Hartley CJ, et al. A novel feature-tracking echocardiographic method for the quantification of regional myocardial function: validation in an animal model of ischemia-reperfusion. J Am Coll Cardiol 2008;51:651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin SH, Hung CL, Uno H, et al. Mechanical dyssynchrony after myocardial infarction in patients with left ventricular dysfunction, heart failure, or both. Circulation 2010;121:1096–103. [DOI] [PubMed] [Google Scholar]
- 25.Hung CL, Verma A, Uno H, et al. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J Am Coll Cardiol 2010;56:1812–22. [DOI] [PubMed] [Google Scholar]
- 26.Knappe D, Pouleur AC, Shah AM, et al. Dyssynchrony, contractile function, and response to cardiac resynchronization therapy. Circ Heart Fail 2011;4:433–40. [DOI] [PubMed] [Google Scholar]
- 27.Carabello BA. Evolution of the study of left ventricular function: everything old is new again. Circulation 2002;105:2701–3. [DOI] [PubMed] [Google Scholar]
- 28.Baicu CF, Zile MR, Aurigemma GP, Gaasch WH. Left ventricular systolic performance, function, and contractility in patients with diastolic heart failure. Circulation 2005;111:2306–12. [DOI] [PubMed] [Google Scholar]
- 29.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol 2009;54:410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging: a new prognosticator for cardiovascular diseases. J Am Coll Cardiol 2007;49:1903–14. [DOI] [PubMed] [Google Scholar]
- 31.Shah AM, Solomon SD. Myocardial deformation imaging: current status and future directions. Circulation 2012;125:244–8. [DOI] [PubMed] [Google Scholar]
- 32.Buckberg G, Mahajan A, Saleh S, Hoffman JI, Coghlan C. Structure and function relationships of the helical ventricular myocardial band. J Thorac Cardiovasc Surg 2008;136:578–89. [DOI] [PubMed] [Google Scholar]
- 33.Anand IS, Rector TS, Cleland JG, et al. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circ Heart Fail 2011;4:569–77. [DOI] [PubMed] [Google Scholar]
- 34.Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricular contraction and relaxation in patients with cardiovascular risk factors: a study with two-dimensional strain imaging. J Am Soc Echocardiogr 2008;21:1138–44. [DOI] [PubMed] [Google Scholar]
- 35.Fang ZY, Leano R, Marwick TH. Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clin Sci (Lond) 2004;106:53–60. [DOI] [PubMed] [Google Scholar]
- 36.Imbalzano E, Zito C, Carerj S, et al. Left ventricular function in hypertension: new insight by speckle tracking echocardiography. Echocardiography 2011;28:649–57. [DOI] [PubMed] [Google Scholar]
- 37.Shah AM, Solomon SD. Phenotypic and pathophysiological heterogeneity in heart failure with preserved ejection fraction. Eur Heart J 2012;33:1716–7. [DOI] [PubMed] [Google Scholar]
- 38.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 2011;32:670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westermann D, Kasner M, Steendijk P, et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation 2008; 117:2051–60. [DOI] [PubMed] [Google Scholar]
- 40.Persson H, Lonn E, Edner M, et al. Diastolic dysfunction in heart failure with preserved systolic function: need for objective evidence: results from the CHARM Echocardiographic Substudy—CHARMES. J Am Coll Cardiol 2007;49:687–94. [DOI] [PubMed] [Google Scholar]
- 41.Zile MR, Gottdiener JS, Hetzel SJ, et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation 2011;124: 2491–501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.