Abstract
PURPOSE
Adult T-cell lymphoma/leukemia (ATL) is a rare and aggressive peripheral T-cell malignancy caused by human T-cell lymphotropic virus-1 infection, which occurs in areas of high prevalence, predominantly in Japan and the Caribbean basin. Most ATL literature is derived from Japan and little is published about Caribbean patients. We describe the clinicopathologic characteristics and treatment outcomes of our Caribbean patients who have ATL at the State University of New York Downstate Medical Center and Kings County Hospital.
PATIENTS AND METHODS
We conducted a retrospective analysis of our patients with ATL who were diagnosed between 2005 and 2017. Medical records were reviewed for clinicopathologic data and treatment outcomes. The final analysis included acute and lymphomatous subtypes only. For the univariable analysis, outcomes were calculated by using a log-rank test, and survival curves were estimated by the Kaplan-Meier method.
RESULTS
We identified 63 patients with acute (55%) and lymphomatous (45%) subtypes, 95% of whom had Ann Arbor stage III to IV disease. The median age was 54 years, and the study population was predominantly female (65%). Most patients (82%) received first-line etoposide, cyclophosphamide, vincristine, doxorubicin, and prednisone (EPOCH) or cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) chemotherapy (10%) with an overall response rate of 46%. The median overall survival was 5.5 months, and the median progression-free survival was 4 months. Incidence of atypical immunophenotype (32%) was higher than previously reported in the Japanese literature and was associated with worse survival (P = .04). Abnormal cytogenetics correlated with shorter progression-free survival (P < .05).
CONCLUSION
We describe here the clinicopathologic characteristics and treatment outcomes of our Caribbean patients with aggressive ATL, which is largely chemotherapy resistant, and the challenges of treating a population with unmet medical needs.
INTRODUCTION
Adult T-cell lymphoma/leukemia (ATL) is a rare and aggressive mature peripheral T-cell lymphoma caused by the human T-cell lymphotropic virus type 1 (HTLV-1), which in America reflects migration patterns from endemic areas.1 ATL incidence in Japan, the Caribbean, and central Brooklyn in the United States, which has a sizeable number of immigrants from the Caribbean, is about 86, 20, and 3.2 cases per 100,000 people, respectively.2,3
ATL is classified into four distinct subtypes on the basis of the Shimoyama criteria: smoldering, chronic, acute, and lymphomatous.4 Smoldering and chronic ATL follow an indolent course with median survival of 4 to 5 years, whereas acute and lymphomatous subtypes have a dismal prognosis with less than 1-year survival despite aggressive therapy.5,6 Given the absence of a standard treatment for ATL, a clinical trial is the preferred option, although the most common strategy remains doxorubicin-based chemotherapy.7-9
Most ATL literature is derived from the Japanese population and less is known about the Caribbean population.8,10-12 Our institutions at State University of New York (SUNY) Downstate Medical Center and Kings County Hospital serve a predominantly Caribbean population in central Brooklyn, New York. We describe here the clinicopathologic characteristics and treatment outcomes of our Caribbean patients with ATL, an under-represented population with high unmet medical needs.
PATIENTS AND METHODS
We performed a retrospective descriptive study of all patients diagnosed with ATL at SUNY Downstate Medical Center and Kings County Hospital between January 2005 and January 2017 after approval by the Institutional Review Board. Diagnosis of ATL was confirmed by clinical history, pathology, and serum HTLV-1 antibody positivity. Patients with pathology not confirmed at our institutions were excluded. Medical records were reviewed for clinicopathologic data and treatment outcomes. We included only patients who had ATL of the acute or lymphomatous subtypes in our final analysis because these groups had most available data. Survival and treatment response were assessed according to 2009 ATL Consensus Criteria.4 Overall survival (OS) was defined from the time of initial diagnosis (diagnosis of acute or lymphomatous subtypes if progression was from chronic or smoldering subtypes) to death or discharge to hospice or last hospital or clinic censor date (with documentation of refractory or progressive disease). Progression-free survival (PFS) was defined from time of initial therapy to death or progression of disease or relapse, whichever occurred first.
CONTEXT
Key Objective
What are the clinical-pathologic characteristics and treatment outcomes of Caribbean patients with adult T-cell lymphoma/leukemia (ATL) in New York City hospitals?
Knowledge Generated
Despite moderate response to doxorubicin-based chemotherapy (overall response rate of 46%), remissions were short and survival was poor (median overall survival 5.5 months). Atypical immunophenotype in our Caribbean patients with ATL was higher than previously reported (30%) and associated with worse outcomes.
Relevance
ATL, especially in Caribbean patients, remains a fatal disease with many challenges and high unmet need.
Demographic data, clinicopathologic features, and treatment responses were summarized by using descriptive measures. Treatment outcomes were compared by using independent t tests or nonparametric tests, depending on the normality of the distribution. Analysis outcomes were calculated by the log-rank test, and survival curves were assessed by the Kaplan-Meier method. Statistical analysis was performed by using IBM SPSS Statistics Version 23 (Chicago, IL).
RESULTS
Baseline Characteristics
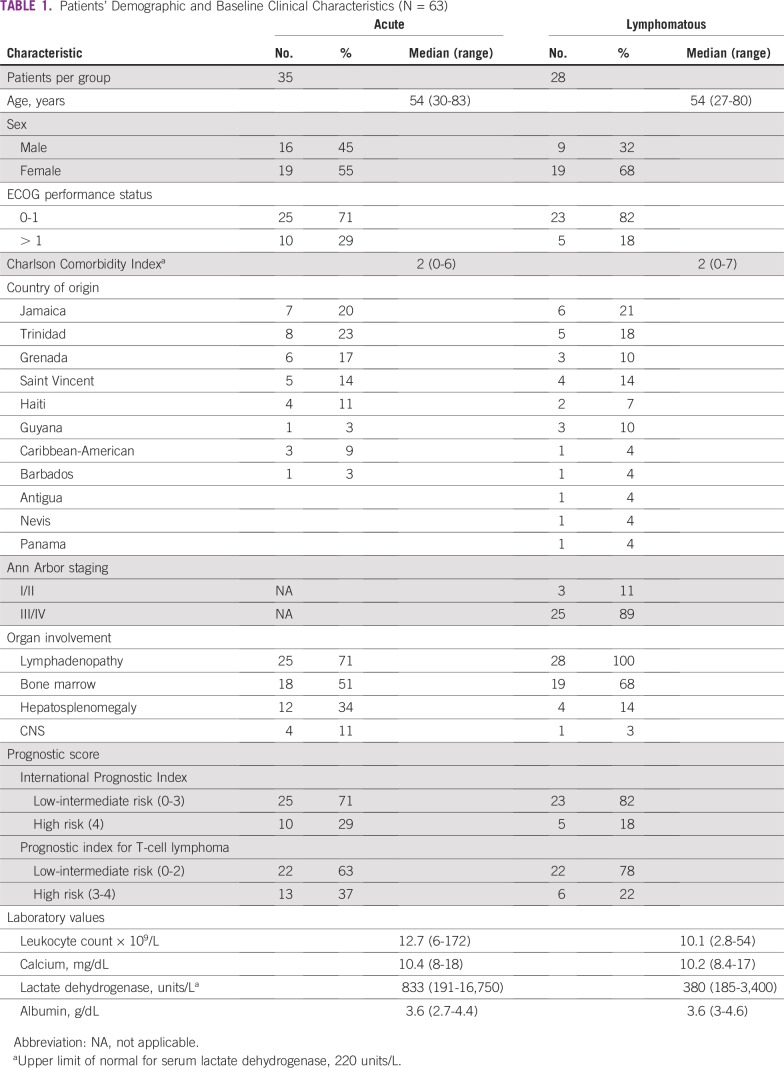
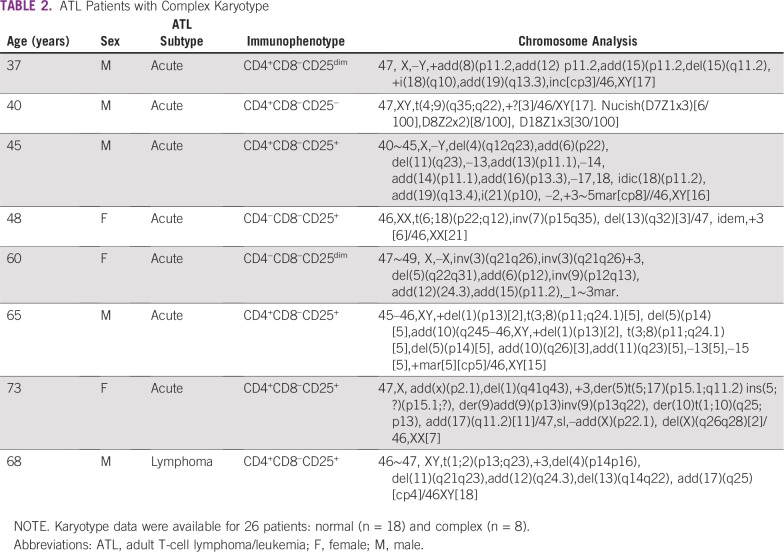
We identified 63 patients with ATL who had acute (55%) or lymphomatous (45%) subtypes. Two patients with smoldering subtype and one with chronic subtype were not included in the analysis. Three other patients progressed to acute subtype (two patients from chronic disease and one from smoldering disease). Baseline clinical characteristic are summarized in Table 1. The median age at presentation was 54 years, and the study population was predominantly female (65%). Of the patients with lymphomatous subtype, 95% had Ann Arbor stage III to IV disease. Most patients present with lymphadenopathy and bone marrow involvement and had International Prognostic Index (IPI) low-intermediate scores. Of 26 patients with reported karyotype, eight (31%) were abnormal. Aneuploidy was observed in several chromosomes including +3, –13, –14, –15, –17, –18, –22, –X, and –Y, with recurrent +3 noted in four of the eight patients (Table 2).
TABLE 1.
Patients’ Demographic and Baseline Clinical Characteristics (N = 63)
TABLE 2.
ATL Patients with Complex Karyotype
Treatment Response
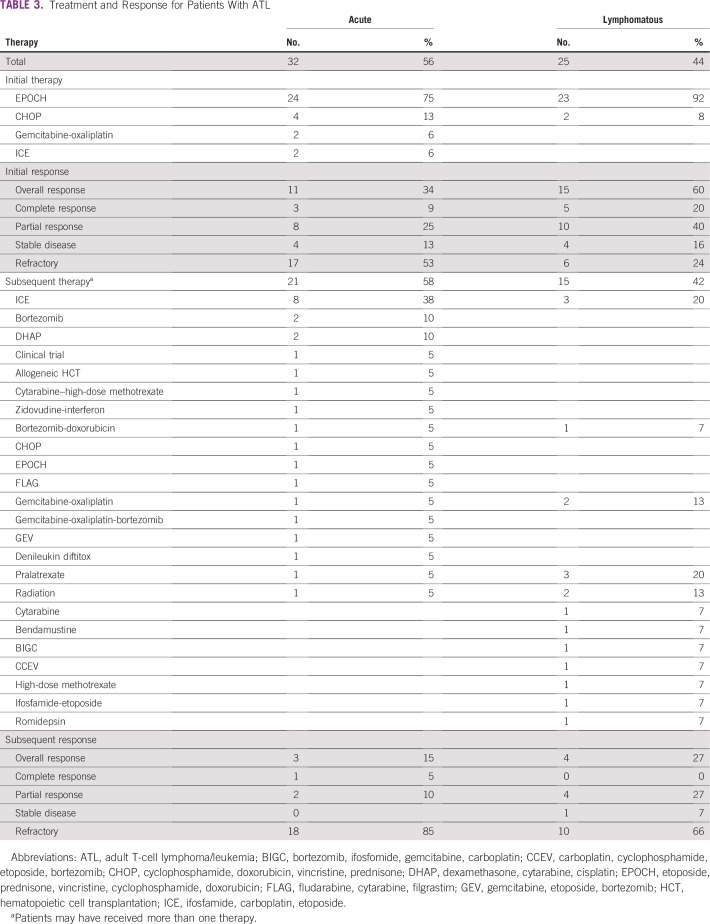
Six patients (9%) received best supportive care because of either poor performance status or patient preference. Of 57 patients who received first-line chemotherapy, 82% received etoposide, cyclophosphamide, vincristine, doxorubicin, and prednisone (EPOCH) and 10% received cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP; Table 3). The overall response rate (ORR) to first-line chemotherapy was 46% (EPOCH, n = 47 [43%] and CHOP, n = 6 [66%]), including 14% of patients with a complete response (CR) and 32% with a partial response (PR), and the median duration of response was 2 months. Of 50 patients (87%) with relapsed/refractory (R/R) disease, 36 patients (63%) received salvage chemotherapy with an ORR of 20% (CR, [n = 1]; PR [n = 6]). Notably, 40% of patients had refractory disease. None had first-line antiviral therapy, and one patient received allogeneic hematopoietic cell transplantation (HCT) as salvage therapy.
TABLE 3.
Treatment and Response for Patients With ATL
Survival
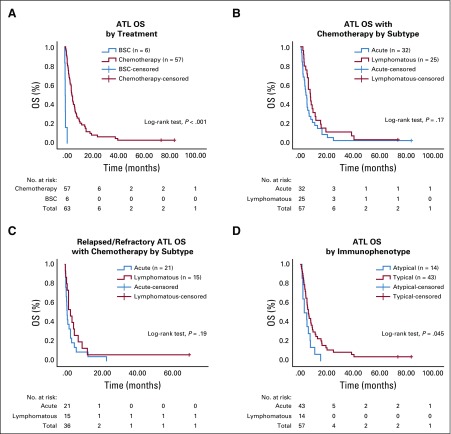
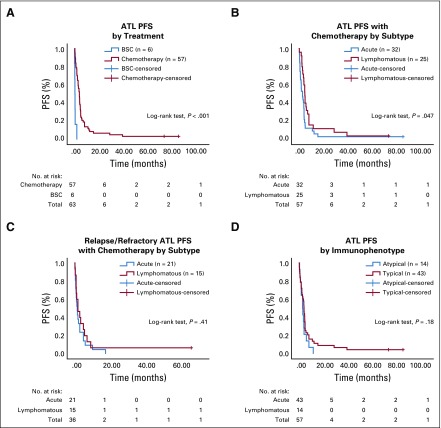
Among patients who received chemotherapy, the median OS was 5.5 months (range, 0.5 to 81.5 months), and the median PFS was 4.0 months (range, 0.8 to 81.5 months; Figs 1 and 2). Despite better response to chemotherapy in patients who had the lymphomatous subtype compared with patients who had the acute subtype, the median OS did not differ significantly between the two subtypes (P = .17). No survival difference was noted between the CHOP and EPOCH therapy groups (P = .07). In the R/R setting, the median OS was 2.2 months (range, 0.2 to 64 months), and the median PFS was 2 months (range, 0.2 to 64 months). Two patients remain alive with prolonged CR after EPOCH therapy (acute subtype: OS, 81.5 months) or EPOCH followed by radiation therapy (stage I lymphomatous subtype: OS, 71.7 months).
FIG 1.
Kalpan-Meier graphs of overall survival (OS) of patients with adult T-cell lymphoma/leukemia (ATL) stratified by (A) chemotherapy v best supportive care (BSC), (B) acute v lymphomatous subtype, (C) acute v lymphomatous subtype when relapsed or refractory, and (D) typical v atypical immunophenotype.
FIG 2.
Kalpan-Meier graphs of progression free survival (PFS) of patients with adult T-cell lymphoma/leukemia (ATL) stratified by (A) chemotherapy v best supportive care (BSC), (B) acute v lymphomatous subtype, (C) acute v lymphomatous subtype when relapsed or refractory, and (D) typical v atypical immunophenotype.
In univariable analysis, the following factors were associated with shorter OS and PFS (P < .05): bone marrow involvement, lactate dehydrogenase (LDH) more than twice the upper limit of normal, and hypercalcemia > 13 mg/dL. Splenomegaly and abnormal cytogenetics correlated with shorter PFS (P < .05), but not OS (P = .42). Normal LDH correlated with OS beyond 1 year (P < .05).
Fourteen patients (32%) had atypical immunophenotype (IP): the typical IP being CD4+CD8−CD25+. The distribution of atypical IP was as follows: CD4−CD8−CD25+ (n = 3), CD4+CD8+CD25+ (n = 4), CD4+CD8−CD25dim (n = 6), and CD4+CD8−CD25− (n = 1). Atypical IP correlated with worse survival (P = .045; Fig 1D).
DISCUSSION
Literature on patients in North America with ATL remains scarce, and our study of 63 Caribbean patients represents only the fourth published cohort.8,11,12 There is no widely accepted standard treatment for acute or lymphomatous subtypes of ATL, although doxorubicin-based chemotherapy remains the most common treatment.6 In a Japanese cohort of patients with ATL, LSG15, an intensive multiagent chemotherapy was compared with once-every-two-weeks CHOP. Despite higher CR rates with LSG15 (40% v 24% with CHOP), there was no statistically significant improvement in survival rates (median OS, 12.7 v 11 months [P = .085] for LSG15; median PFS, 7 v 5.4 months [P = .1] for CHOP).9
In our experience, patients who received first-line chemotherapy, mostly with EPOCH (82%), had an ORR of 46%, but nearly all patients eventually relapsed or progressed quickly, precluding maintenance therapy. Outcome was dismal, with a median OS of 5.5 months (2 months for R/R disease) and 1-year OS rate of 20%, consistent with other American ATL series.8,11,12 Older patients with ATL and R/R disease or those who required intensive care unit support have an especially poor prognosis.13
At our institution, antiviral therapy was used primarily in the indolent forms of ATL. The role of antiviral therapy with zidovudine and interferon-alfa has been previously debated in acute and lymphomatous subtypes and was not adopted as our institutional treatment practice. However, more recent studies suggest primary benefit in non-lymphomatous subtypes; unfortunately, most patients inevitably relapse and require cytotoxic therapy.14,15 Allogeneic HCT remains the only curative option for chemotherapy-sensitive disease with 1-year OS of 20% to 30%.16-18 Other North American physicians perform allogeneic HCT in 2% to 11% of their patients with ATL.8,11,12 In our series, HCT was underutilized (one patient) because of the patient’s rapid clinical deterioration, brief duration of treatment response, socioeconomic status, and unavailable stem cell donors.
Few studies evaluate the effect of comorbidity index on survival in T-cell lymphoma but not specifically in ATL.19,20 In our series, Charlson comorbidity index, IPI score, and performance status did not statistically correlate with survival. However, bone marrow involvement, elevated LDH, and hypercalcemia predicted worse outcomes concordant with many prognostic scoring systems.21
The typical IP of ATL is CD4+CD7−CD8−, with strong co-expression of CD25.22 In Japanese patients with ATL, atypical IP has been reported in 10% to 20% of patients and has been linked to a more aggressive disease in a single case series.23 In North American patients with ATL, the incidence of atypical IP ranged between 17% and 22% in two reports, but the prognostic significance has never been reported.12,24 In our cohort, we noted a higher incidence of atypical IP (n = 14 [32%]) with adverse survival outcome despite chemotherapy; however, this warrants prospective validation.25
The Japanese ATL series showed that chromosomal aneuploidy, multiple breaks, and karyotype complexity adversely correlated with shorter survival.26 Our study is the second to report the impact of karyotype on survival in Caribbean patients with ATL. One series suggested worse survival with complex karyotype.27 In our cohort, abnormal cytogenetics (eight of 26 patients) was more common in acute subtype (88%) and correlated with shorter PFS (P < .05), but not OS (P = .42). Aneuploidy was observed in several chromosomes including +3, –13, –14, –15, –17, –18, –22, –X, and –Y, with recurrent +3 noted in four of the eight patients.
Several Japanese ATL cohorts have longer survival (median OS, 9 to 12 months) compared with our cohort despite aggressive chemotherapy.9,28 Genomic profiling of Japanese patients with ATL uncovered a spectrum of HTLV-driven mutations in T-cell receptor NF-κB signaling and downstream pathways.29 In contrast, recent genomic profiling of a North American Caribbean ATL cohort has demonstrated a higher burden of epigenetic and histone-modifying genes (in particular, EP300 mutations) with compromised TP53 function and fewer mutations in T-cell receptor NF-κB and JAK/STAT pathways.30 Differences in biology and treatment regimen and access to HCT partly explain disparate outcomes between Japanese and North American ATL cohorts and remain an area of active research.6,31
Several novel therapeutic approaches in ATL include checkpoint inhibition and anti-CCR4 antibody (mogamulizumab). In the R/R ATL setting, checkpoint inhibition with nivolumab in ATL was halted because of accelerated disease progression, and it prompted caution regarding the use of immune therapy.32 Mogamulizumab in a North American ATL cohort led to an ORR of 11% whereas a Japanese ATL cohort had an ORR of 50%; this remains an area of active investigation.31 Future trials incorporating chemotherapy with antiviral therapy or novel small molecular inhibitors may show potential promise.15
In conclusion, our large cohort of Caribbean patients with ATL shows a chemotherapy-resistant disease with dismal prognosis. It is essential to further understand the disease biology to guide novel therapeutic strategies and improve patient outcomes.
ACKNOWLEDGMENT
We thank Nikhil Mukhi, MD, Mohamed Alshal, MD, and Charles Kim, MD, for their help in collecting data.
Presented at the 60th American Society of Hematology Annual Meeting and Exposition, San Diego, CA, December 1-4, 2018.
Supported by a Professional Development Award from the New York State/United University Professions Joint Labor Management Professional Development Committee (E.C.).
AUTHOR CONTRIBUTIONS
Conception and design: Edwin Chiu, Bachar Samra, Eric Tam, Babak Baseri, Carol Luhrs, Ahmed Sawas, Evelyn Taiwo, Gurinder Sidhu
Administrative support: Bachar Samra, Carol Luhrs, Ahmed Sawas
Provision of study materials or patients: Bachar Samra, Jason Gonsky
Collection and assembly of data: Edwin Chiu, Bachar Samra, Eric Tam, Bo Lin, Gurinder Sidhu
Data analysis and interpretation: Edwin Chiu, Bachar Samra, Eric Tam, Babak Baseri, Bo Lin, Jason Gonsky, Ahmed Sawas, Evelyn Taiwo, Gurinder Sidhu
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Eric Tam
Stock and Other Ownership Interests: Celgene
Jason Gonsky
Stock and Other Ownership Interests: Pfizer, Johnson & Johnson
Ahmed Sawas
Consulting or Advisory Role: Kirin Pharmaceuticals, Pharmacyclics
Speakers' Bureau: Seattle Genetics, Gilead Sciences, Daiichi Sankyo
Research Funding: Affimed Therapeutics (Inst), Trillium Therapeutics (Inst), Viracta Therapeutics (Inst), Astellas Pharma (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ohsima K, Jaffe ES, Yoshino T, et al., editors. Adult T-cell leukaemia/lymphoma, In Swerdlow SH, Campo E, Harris NL, et al (eds): WHO Classification of Tumours of Haematopoetic and Lymphoid Tissues, World Health Organization Classification of Tumours (ed 4). Lyon, France, IARC, 2017. [Google Scholar]
- 2.Levine PH, Dosik H, Joseph EM, et al. A study of adult T-cell leukemia/lymphoma incidence in central Brooklyn. Int J Cancer. 1999;80:662–666. doi: 10.1002/(sici)1097-0215(19990301)80:5<662::aid-ijc5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Mehta-Shah N, Ratner L, Horwitz SM. Adult T-cell leukemia/lymphoma. J Oncol Pract. 2017;13:487–492. doi: 10.1200/JOP.2017.021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma: A report from the Lymphoma Study Group (1984-87) Br J Haematol. 1991;79:428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 5.Suzumiya J, Ohshima K, Tamura K, et al. The International Prognostic Index predicts outcome in aggressive adult T-cell leukemia/lymphoma: Analysis of 126 patients from the International Peripheral T-Cell Lymphoma Project. Ann Oncol. 2009;20:715–721. doi: 10.1093/annonc/mdn696. [DOI] [PubMed] [Google Scholar]
- 6.Cook LB, Fuji S, Hermine O, et al. Revised Adult T-Cell Leukemia-Lymphoma International Consensus Meeting Report. J Clin Oncol. 2019;37:677–687. doi: 10.1200/JCO.18.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips AA, Harewood JCK. Adult T cell leukemia-lymphoma (ATL): State of the art. Curr Hematol Malig Rep. 2018;13:300–307. doi: 10.1007/s11899-018-0458-6. [DOI] [PubMed] [Google Scholar]
- 8.Phillips AA, Shapira I, Willim RD, et al. A critical analysis of prognostic factors in North American patients with human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: A multicenter clinicopathologic experience and new prognostic score. Cancer. 2010;116:3438–3446. doi: 10.1002/cncr.25147. [DOI] [PubMed] [Google Scholar]
- 9.Tsukasaki K, Utsunomiya A, Fukuda H, et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol. 2007;25:5458–5464. doi: 10.1200/JCO.2007.11.9958. [DOI] [PubMed] [Google Scholar]
- 10.Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: A proposal from an international consensus meeting. J Clin Oncol. 2009;27:453–459. doi: 10.1200/JCO.2008.18.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malpica L, Pimentel A, Reis IM, et al. Epidemiology, clinical features, and outcome of HTLV-1-related ATLL in an area of prevalence in the United States. Blood Adv. 2018;2:607–620. doi: 10.1182/bloodadvances.2017011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zell M, Assal A, Derman O, et al. Adult T-cell leukemia/lymphoma in the Caribbean cohort is a distinct clinical entity with dismal response to conventional chemotherapy. Oncotarget. 2016;7:51981–51990. doi: 10.18632/oncotarget.10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Killoran K, Aspergis GA, Gorga J, et al. Outcome of patients with adult T cell lymphoma/leukemia admitted to the medical intensive care unit. Chest. 2010;138 (suppl; abstr 298A) [Google Scholar]
- 14.Bazarbachi A, Plumelle Y, Carlos Ramos J, et al. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol. 2010;28:4177–4183. doi: 10.1200/JCO.2010.28.0669. [DOI] [PubMed] [Google Scholar]
- 15.Marino-Merlo F, Mastino A, Grelli S, et al. Future perspectives on drug targeting in adult T cell leukemia-lymphoma. Front Microbiol. 2018;9:925. doi: 10.3389/fmicb.2018.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Utsunomiya A, Miyazaki Y, Takatsuka Y, et al. Improved outcome of adult T cell leukemia/lymphoma with allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:15–20. doi: 10.1038/sj.bmt.1702731. [DOI] [PubMed] [Google Scholar]
- 17.Ishida T, Hishizawa M, Kato K, et al. Allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia-lymphoma with special emphasis on preconditioning regimen: A nationwide retrospective study. Blood. 2012;120:1734–1741. doi: 10.1182/blood-2012-03-414490. [DOI] [PubMed] [Google Scholar]
- 18.Phillips AA. Hematopoietic stem cell transplantation for adult T cell leukemia-lymphoma: Who is the best candidate? Leuk Lymphoma. 2017;58:1–3. doi: 10.1080/10428194.2016.1246730. [DOI] [PubMed] [Google Scholar]
- 19.Ellin F, Jerkeman M, Törnqvist J, et al. Impact of comorbidity on survival in peripheral T-cell lymphomas: A Swedish Lymphoma Registry study. Hematol Oncol. 2018;36:159–165. doi: 10.1002/hon.2428. [DOI] [PubMed] [Google Scholar]
- 20.Zhao H, Wang T, Wang Y, et al. Comorbidity as an independent prognostic factor in elderly patients with peripheral T-cell lymphoma. OncoTargets Ther. 2016;9:1795–1799. doi: 10.2147/OTT.S93687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutiérrez-García G, García-Herrera A, Cardesa T, et al. Comparison of four prognostic scores in peripheral T-cell lymphoma. Ann Oncol. 2011;22:397–404. doi: 10.1093/annonc/mdq359. [DOI] [PubMed] [Google Scholar]
- 22.Uchimaru K.Adult T-cell Leukemia/LymphomainWatanabe T, Fukushima T.(eds)Immunophenotype Tokyo, Japan: Springer Japan; 201767–81. [Google Scholar]
- 23.Kamihira S, Sohda H, Atogami S, et al. Phenotypic diversity and prognosis of adult T-cell leukemia. Leuk Res. 1992;16:435–441. doi: 10.1016/0145-2126(92)90168-7. [DOI] [PubMed] [Google Scholar]
- 24.Salib M, Pintilie M, Wang R, et al. Treatment outcome of North American patients with adult T-cell leukemia/lymphoma: Princess Margaret Cancer Centre experience. Blood. 2016;128 (abstr 3010) [Google Scholar]
- 25.Samra B, Chiu E, Lin B, et al. Atypical immunophenotype predicts worse prognosis in adult T-cell lymphoma/leukemia: A retrospective study of 63 Caribbean patients at a New York City tertiary center. Blood. 2018;132(Suppl 1):2906. [Google Scholar]
- 26.Itoyama T, Chaganti RS, Yamada Y, et al. Cytogenetic analysis and clinical significance in adult T-cell leukemia/lymphoma: A study of 50 cases from the human T-cell leukemia virus type-1 endemic area, Nagasaki. Blood. 2001;97:3612–3620. doi: 10.1182/blood.v97.11.3612. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y, Murty VV, Leeman-Neill R, et al. Cytogenetic analysis of adult T-cell leukemia/lymphoma: Evaluation of a Caribbean cohort. Leuk Lymphoma. 2019;60:1598–1600. doi: 10.1080/10428194.2018.1538506. [DOI] [PubMed] [Google Scholar]
- 28.Katsuya H, Ishitsuka K, Utsunomiya A, et al. Treatment and survival among 1594 patients with ATL. Blood. 2015;126:2570–2577. doi: 10.1182/blood-2015-03-632489. [DOI] [PubMed] [Google Scholar]
- 29.Kataoka K, Nagata Y, Kitanaka A, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304–1315. doi: 10.1038/ng.3415. [DOI] [PubMed] [Google Scholar]
- 30.Shah UA, Chung EY, Giricz O, et al. North American ATLL has a distinct mutational and transcriptional profile and responds to epigenetic therapies. Blood. 2018;132:1507–1518. doi: 10.1182/blood-2018-01-824607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips AA, Fields PA, Hermine O, et al. Mogamulizumab versus investigator’s choice of chemotherapy regimen in relapsed/refractory adult T-cell leukemia/lymphoma. Haematologica. 2019;104:993–1003. doi: 10.3324/haematol.2018.205096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratner L, Waldmann TA, Janakiram M, et al. Rapid progression of adult T-cell leukemia-lymphoma after PD-1 inhibitor therapy. N Engl J Med. 2018;378:1947–1948. doi: 10.1056/NEJMc1803181. [DOI] [PubMed] [Google Scholar]