Abstract
Neoadjuvant treatment (NAT) followed by total mesorectal excision is currently considered the standard of treatment for rectal adenocarcinoma. The degree of pathologic treatment response (pTR) correlates significantly with the recurrence free survival and overall survival (OS). However, it remains unclear which clinical and pathologic factors are associated with a more robust response to NAT, including showing pathologic complete response (pCR). Chemokine receptor 4 (CXCR4) overexpression has been associated with unfavorable OS in some studies. In this study, we sought to evaluate the clinicopathologic determinants of pTR in neoadjuvant treated rectal adenocarcinoma (NAT-RA). We retrospectively identified 91 patients who underwent pre-treatment diagnostic biopsy, NAT, and surgical resection at our institution. The archival slides were reviewed for pathologic features in the pre-treatment biopsies and for assessment of pTR in the resection specimens according to the current College of American Pathologist (CAP)’s guidelines. pCR was obtained in 16.5% of the cases, whereas 20.9% had near pCR, 30.8% had partial response, and 31.9% had a poor/no response. CXCR4 immunohistochemical analysis was also performed on the pre-treatment biopsies. Lower pre-treatment cT-stage (p=0.019) and pre-treatment AJCC cTNM stage groups (p=0.004), longer time interval between completion of NAT and resection (p=0.022), and presence of tumor-infiltrating lymphocytes in the pre-treatment biopsies (p=0.019) were significantly associated with a better pTR. CXCR4 nuclear expression was associated with a lower percentage of residual tumor (p=0.036). Pre-treatment CEA levels, tumor differentiation, CAP treatment response groups and lower percentage of residual tumor were associated with a better OS.
Keywords: Rectal adenocarcinoma, neoadjuvant treatment, NAT, treatment response, complete response, CXCR4, tumor infiltrating lymphocytes, TILs
1. INTRODUCTION
Colorectal cancer (CRC) remains the third most common cancer in the United States, with approximately 135,430 new cases and 50,260 deaths in 20171. Rectal adenocarcinoma (RA) differs from other CRCs, given its unique anatomy and treatment modalities available. The anatomic location of the rectum in the pelvis and lack of a serosal lining increases the risk of local invasion and distant metastasis compared to the rest of the colon2,3. RA most commonly presents with locally advanced (stage II-III) disease, for which neoadjuvant treatment (NAT) followed by total mesorectal excision (TME) and adjuvant chemotherapy is currently considered the standard of care2–5.
Pathologic complete response (pCR) following NAT is achieved in approximately 20% of RA cases2,3,5, and another 60% of patients show some degree of tumor regression6. The degree of pathologic treatment response (pTR) strongly correlates with recurrence-free survival (RFS), with a 5-year RFS rate of 95% for pCR compared to 61% for minor response5. It remains unclear, however, which patients respond best to NAT and, moreover, achieve a pCR. With the increasing interest in non-operative management in RA, prediction of response to NAT has become a critically important clinical question7,8.
We sought to evaluate which clinicopathologic factors in the pre-treatment diagnostic biopsies for RA could predict treatment response. Pre-treatment clinical stage, pre-treatment carcinoembryonic antigen (CEA), histologic differentiation, tumor budding (TB), tumor infiltrating lymphocytes (TILs), and desmoplastic reaction are among other factors that are currently being considered in multiple other neoplasms to show a prognostic association9–20. Similarly, the novel marker C-X-C chemokine receptor 4 (CXCR4) is a membrane bound heptahelical receptor which is low or absent in healthy tissues but highly expressed in multiple tumor types21,22. CXCR4 binds to its corresponding ligand chemokine stromal-derived factor 1α (SDF-1α) (CXCL12)23, and its overexpression is associated with chemotaxis, invasion, angiogenesis and proliferation, independent of the specific tumor histologic findings22,24. The goal of the study was to identify factors that are predictive of a pCR or a near-complete response.
2. MATERIALS AND METHODS
2.1. Data Collection
A retrospective review was performed for patients with a confirmed pathologic diagnosis of RA followed by NAT and total mesorectal excision at our institution. A total of 91 patients were identified from 2008 to 2016, for which the archival hematoxylin and eosin (H&E) stained slides from both the diagnostic biopsy and the surgical resection were available. Clinical data were obtained from a prospectively maintained quality improvement rectal cancer database. Approval from the Institutional Review Board of Washington University School of Medicine was obtained prior initiating the study.
2.2. Clinicopathologic Parameters
Demographic and clinical parameters included gender, age at diagnosis, pre-treatment clinical T-, N-, and M-stages, pretreatment CEA levels, NAT modality, duration of NAT, time interval from end of treatment to surgical resection, RFS and overall survival (OS). NAT modalities for this cohort of patients included four different regimens: chemoradiation, short-course radiation alone, short-course total neoadjuvant therapy, and other modalities. Chemoradiation consisted of 28 fractions of 180cGy of pelvic radiation delivered 5 days per week for 6 weeks with concurrent single agent chemotherapy. Short-course radiation consisted of 5 consecutive treatments of 500cGy of pelvic radiation followed by surgery within 2 weeks. Short-course total neoadjuvant therapy included a combination of a short-course radiation followed by 2–6 months of multi-agent FOLFOX-equivalent chemotherapy. The other modalities included chemotherapy alone and long-course radiotherapy alone.
All the archival H&E slides of the diagnostic RA biopsies were examined, and the following pathologic findings were noted: identifiable precursor lesion if any (tubular adenoma, tubulovillous adenoma, villous adenoma, traditional serrated adenoma, or other), tumor histologic differentiation (well-, moderately-, or poorly-differentiated), intratumoral budding (TB), tumor infiltrating lymphocytes (TILs), type of desmoplastic reaction if any, lymphovascular invasion (LVI), perineural invasion (PNI), and mitotic count per 10/high-power field (HPF). TB was defined according to the International Tumor Budding Consensus Conference 2016 (ITBCC) as a single tumor cell or cell clusters of up to 4 tumor cells17. TILs was defined as the presence of >4 intratumoral lymphocytes per HPF12,25. The type of desmoplastic reaction was classified as myxoid/immature desmoplastic stroma, collagenous/mature desmoplastic stroma or absent for significant stromal response12.
All the slides of the surgical resection specimens were examined for each case. The presence or absence of pCR was assessed and a percentage of residual tumor was assigned to each case by eyeballing after reviewing all tumor bed slides submitted. The pTR was also recorded as indicated in the current CAP guideline for primary carcinoma of the colon and rectum (version 4.0.1.0) as: group 0 (no viable cancer cells), group 1 (single cells or rare small groups of cancer cells), group 2 (residual cancer with evident tumor regression, but more than single cells or rare small groups of cancer cells), and group 3 (extensive residual tumor with no evident tumor regression)26. The pathologic stage was established according to the American Joint Committee on Cancer (AJCC) staging system (8th edition)27. The resection specimens were grossed according to our institutional protocol: when an evident tumor was identified at least 1 block per cm was included. If the tumor measured less than 3 cm, the entire lesion was submitted, and if no tumor was grossly identified the entire recognizable tumor bed/area of fibrosis was submitted.
All the biopsy and resection slides were reviewed by two pathologist independently involved in recording the findings. The method followed was objective and reproducible, since only minor differences arose in a small subset of cases which were easily resolved by re-review.
2.3. Immunohistochemistry
The archival formalin-fixed paraffin embedded (FFPE) blocks of the diagnostic endoscopic biopsy were available in 85 patients and were used for immunohistochemical (IHC) analysis. In cases where more than one FFPE block or biopsy parts were available, one representative block per case was selected. IHC was performed using mouse monoclonal IgG2 antibodies against human CXCR4 (R&D systems, clone #44716) on 5 μm unstained whole slides. Invasive breast caricnoma, renal cell carcinoma, and non-small cell lung carcinoma were used as positive controls23,28. Inflammatory cells present in the biopsy served as internal positive control23. Appropriate negative controls were used. The location of reactivity was recorded as cytoplasmic only, nuclear only, or cytoplasmic and nuclear, as previously reported29. The intensity of the reactivity was interpreted as weak, intermediate, or strong, and an estimated percentage of reactivity in the tumor cells was also noted.
2.4. Statistical Analysis
The clinical characteristics were summarized using descriptive statistics. Continuous and categorical variables were compared by Kruskal-Wallis test and Chi-square test, respectively. OS was defined as the years from the date of surgery to death. Alive patients were censored at the last follow-up. RFS was defined as the years from the date of surgery to recurrence. Patients without recurrence were censored at the last follow-up. Recurrence free probabilities were calculated using Kaplan-Meier plot. Differences between groups were determined by logrank test. Cox proportional-hazards models were used to evaluate the relationship of the interested variables for OS and RFS analysis. The proportionality assumption was tested by adding a time-dependent covariate for each variable. The variables with p<0.25 from the univariate models were considered in the multivariable model. The stages included pre-treatment clinical N-stage, T-stage, and cTNM prognostic stage groups, and post-treatment pathologic N-stage, T-stage, and ypTNM prognostic stage groups. Given the possible correlation among these, ypTNM prognostic stage groups had the highest priority. The final multivariable model was built using the backward stepwise selection approach to identify all significant risk factors. Factors significant at a 10% level were kept in the final model. All statistical tests were two-sided using an α = 0.05 level of significance. SAS Version 9.4 (Cary, NC) was used to perform all statistical analyses.
3. RESULTS
3.1. Patient Characteristics
The demographic and clinical characteristics are summarized in Table-1. The male-to-female ratio was 1.4:1 with a mean age of diagnosis of 58.6 ±12.3 years. The pretreatment CEA level was available in 77 patients with a mean value of 11.0 ±32.3 ng/mL (reference range: 0.0 – 5.0 ng/mL). The majority of the patients were pre-treatment clinical stage T3 (73.6%). Clinical node-positive disease was more prevalent than node-negative disease (62.7%). The majority of patients received chemoradiation (51.7%) followed by short course total neoadjuvant therapy (29.7%), short course radiation alone (13.2%), and other treatment modalities (5.5%) including chemotherapy alone (2 cases, 2.2%) and radiotherapy alone (3 cases, 3.3%). The time interval from end of treatment to surgical resection was available in 84 patients with a mean duration of 94.3 ±91.8 days. The mean clinical follow-up time was 4.1 ±2.4 years.
Table-1.
Patient and Tumor Characteristics (N = 91)
| n, % | |
|---|---|
| Gender | |
| Male | 53, 58.2% |
| Female | 38, 41.8% |
| Age at diagnosis (mean, SD) | 58.6 years, 12.3 |
| Pre-treatment cT-stage | |
| T1 | 2, 2.2% |
| T2 | 11, 12.1% |
| T3 | 67, 73.6% |
| T4 | 11, 12.1% |
| Pre-treatment cN-stage | |
| N0 | 34, 37.4% |
| N1 | 44, 48.4% |
| N2 | 13, 14.3% |
| Pre-treatment cM-stage | |
| M0 | 81, 89.0% |
| M1 | 10, 11.0% |
| Pre-treatment cTNM prognostic stage groups | |
| I | 9, 9.9% |
| II | 23, 25.3% |
| III | 49, 53.9% |
| IV | 10, 11.0% |
| Pre-treatment CEA level, ng/mL (n, mean, SD) | 77, 11.0, 32.3 |
| NAT modality | |
| Chemoradiation | 47, 51.7% |
| Total neoadjuvant therapy | 27, 29.7% |
| Short course radiotherapy alone | 12, 13.2% |
| Other | 5, 5.5% |
| Time interval from end of NAT to resection, days (n, mean, SD) | 84, 94.3, 91.8 |
| Precursor lesion | |
| Tubular adenoma | 39, 42.9% |
| Tubulovillous adenoma | 19, 20.9% |
| Villous adenoma | 6, 6.6% |
| Not identified | 27, 29.7% |
| Histologic differentiation | |
| Well | 14, 15.4% |
| Moderate | 70, 76.9% |
| Poor | 7, 7.7% |
| Intratumoral budding | |
| No | 62, 68.1% |
| Yes | 29, 31.9% |
| Tumor infiltrating lymphocytes | |
| No | 74, 81.3% |
| Yes | 17, 18.7% |
| Desmoplastic reaction | |
| Myxoid/immature | 28, 30.8% |
| Collagenous/mature | 32, 35.2% |
| Absent | 31, 34.1% |
| Lymphovascular invasion | 5, 5.5% |
| Perineural invasion | 2, 2.2% |
| Mitotic count/10HPF (mean, SD) | 28.2, 19.4 |
| Pathologic complete response | |
| No | 76, 83.5% |
| Yes | 15, 16.5% |
| CAP treatment response groups | |
| Group 0 | 15, 16.5% |
| Group 1 | 19, 20.9% |
| Group 2 | 28, 30.8% |
| Group 3 | 29, 31.9% |
| Percentage of residual tumor (mean, SD) | 38.3, 38.4 |
| ypT-stage | |
| T0 | 15, 16.5% |
| T1 | 5, 5.5% |
| T2 | 19, 20.9% |
| T3 | 44, 48.4% |
| T4 | 8, 8.8% |
| ypN-stage | |
| N0 | 64, 70.3% |
| N1 | 18, 19.8% |
| N2 | 9, 9.9% |
| ypTNM prognostic stage groups | |
| 0 | 14, 15.4% |
| I | 18, 19.8% |
| II | 30, 33.0% |
| III | 19, 20.9% |
| IV | 10, 11.0% |
| Mean follow-up, SD (years) | 4.1, 2.4 |
Abbreviations: T- Tumor; N- Node; M- Metastasis; SD - Standard Deviation; CEA - Carcinoembryonic Antigen; NAT - Neoadjuvant Treatment; Percentages may not add up to 100% due to rounding.
3.2. Tumor Characteristics
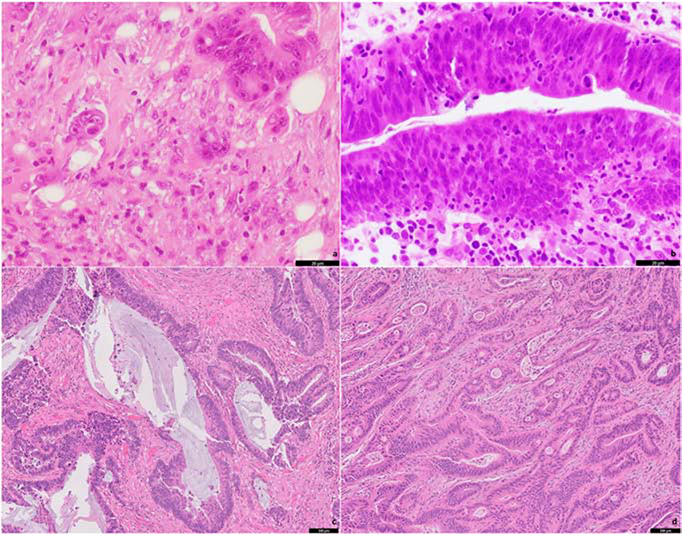
The majority of the RA were moderately-differentiated (76.9%), followed by well-, and poorly-differentiated, in 15.4% and 7.7% of the cases, respectively (Table-1). In 29.7% of the cases, a precursor lesion was not identified. The most common pre-neoplastic lesion identified was tubular adenoma (42.9%), followed by tubulovillous adenoma (20.9%) and villous adenoma (6.6%). TB was present in 29 cases (31.9%; Figure-1a), and TILs were present in 17 cases (18.7%; Figure-1b). LVI and PNI were identified in 5 (5.5%) and 2 (2.2%) of the cases, respectively. The mean number of mitoses in 10HPF was 28.2 ±19.4. Collagenous/mature desmoplastic stroma was present in 28 cases (30.8%; Figure-1c), myxoid/immature desmoplastic stroma in 32 cases (35.2%; Figure-1d), and in 31 cases (34.1%), prominent desmoplastic reaction was not identified.
Figure 1.
Separate cases of pre-treatment rectal adenocarcinoma biopsies showing intratumoral budding (a), tumor-infiltrating lymphocytes (b), collagenous stroma/mature fibrosis (c) and myxoid stroma/immature fibrosis (d).
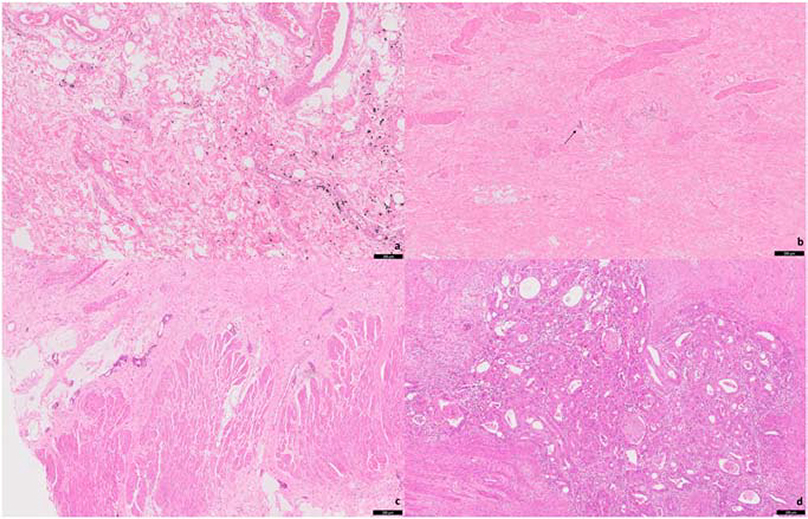
On review of the resection specimens, a pathologic complete response was achieved in 15 cases (16.5%; Figure-2a), and the mean percentage of residual tumor was 38.3 ±38.4% (Table-1). Nineteen cases (20.9%) were considered as pTR group 1 (Figure-2b), 28 cases (30.8%) group 2 (Figure-2c), and 29 cases (31.9%) group 3 (Figure-2d). The majority of the cases were post-treatment pathologic T-stage 3 (44 cases, 48.4%), ypN0 (64 cases, 70.3%), and post-treatment pathologic TNM prognostic stage group II (30 cases, 33.0%).
Figure 2.
Post-treatment rectal adenocarcinoma resections showing: complete pathologic response (a); a single group of small residual tumor (b, arrow) consistent with CAP treatment response group 1; evident tumor regression consistent with CAP treatment response group 2 (c); extensive residual tumor with no evident tumor regression consistent with CAP treatment response group 3 (d).
3.3. Correlation of Clinicopathologic Characteristics with CAP Treatment Response Groups
Patients in treatment response groups 0, 1 and 2 were associated with a longer time interval from the end of NAT to surgical resection (Table-2) than those with poor or no pathologic response (group 3, p = 0.022). Patients in treatment response groups 2 and 3 were associated with a higher pre-treatment clinical T-stage (p = 0.019) and pre-treatment clinical stage (p = 0.004) than those in treatment response groups 0 and 1. The presence of TILs in the pre-treatment biopsies was associated with a greater treatment response (p = 0.019). No other clinicopathologic variables were associated with treatment response (Table-2). There was no statistical significance difference between the treatment modalities and a complete response (p = 0.273).
Table-2.
Correlation of Clinicopathologic Features with CAP Pathologic Treatment Response Groups (N = 91)
| Group 0 (n = 15) | Group 1 (n = 19) | Group 2 (n = 28) | Group 3 (n = 29) | P | |
|---|---|---|---|---|---|
| Pre-treatment cT-stage | |||||
| T1 | 0 | 2, 10.5% | 0 | 0 | |
| T2 | 3, 20% | 5, 26.3% | 1, 3.6% | 2, 6.9% | 0.019 |
| T3 | 11, 73.3% | 8, 42.1% | 23, 82.1% | 25, 86.2% | |
| T4 | 1, 6.7% | 4, 21.1% | 4, 14.3% | 2, 6.9% | |
| Pre-treatment cN-stage | |||||
| N0 | 5, 33.3% | 6, 31.6% | 7, 25% | 16, 55.2% | |
| N1 | 9, 60% | 10, 52.6% | 15, 53.4% | 10, 34.5% | 0.295 |
| N2 | 1, 6.7% | 3, 15.8% | 6, 21.4% | 3, 10.3% | |
| Pre-treatment cTNM prognostic stage groups | |||||
| I | 1, 6.7% | 6, 31.6% | 0 | 2, 6.9% | |
| II | 5, 33.3% | 1, 5.3% | 8, 28.6% | 9, 31% | 0.004 |
| III | 9, 60% | 11, 57.9% | 18, 64.3% | 11, 37.9% | |
| IV | 0 | 1, 5.26% | 2, 7.1% | 7, 24.1% | |
| Pre-treatment CEA levels (mean, SD) | 8.4, 23.1 | 3.3, 3.6 | 6.3, 10.2 | 23.4, 55.5 | 0.120 |
| Time interval from end of NAT to resection, days (median, SD) | 88.5, 34.3 | 101.3, 86.9 | 123.6, 123.0 | 59.8, 68.5 | 0.022 |
| Histologic differentiation | |||||
| Well | 3, 20% | 4, 21.1% | 5, 17.9% | 2, 6.9% | |
| Moderate | 12, 80% | 14, 73.7% | 22, 78.6% | 22, 75.9% | 0.357 |
| Poor | 0 | 1, 5.3% | 1, 3.6% | 5, 17.2% | |
| Intratumoral budding | |||||
| No | 12, 80% | 12, 63.2% | 21, 75% | 17, 58.6% | 0.421 |
| Yes | 3, 20% | 7, 36.8% | 7, 25% | 12, 41.4% | |
| Tumor infiltrating lymphocytes | |||||
| No | 11, 73.3% | 12, 63.2% | 27, 96.4% | 24, 82.8% | 0.019 |
| Yes | 4, 26.7% | 7, 36.8% | 1, 3.6% | 5, 17.2% | |
| Desmoplastic reaction | |||||
| Myxoid/immature | 3, 20% | 5, 26.3% | 12, 42.9% | 12, 41.4% | 0.641 |
| Collagenous/mature | 6, 40% | 6, 31.6% | 9, 32.1% | 7, 24.1% | |
| Absent | 6, 40% | 8, 42.1% | 7, 25% | 10, 34.5% | |
| Lymphovascular invasion | |||||
| Yes | 0 | 0 | 4, 14.3% | 1, 3.5% | 0.172 |
| No | 15, 100% | 19, 100% | 24, 85.7% | 28, 96.5% | |
| Perineural invasion | |||||
| Yes | 0 | 1, 5.3% | 1, 3.6% | 0 | 0.667 |
| No | 15, 100% | 18, 94.7% | 27, 96.4% | 29, 100% | |
| Mitotic count/10HPF (mean, SD) | 32.3, 23.7 | 31.0, 17.4 | 24.1, 12.9 | 28.1, 23.3 | 0.566 |
Abbreviations: CEA - Carcinoembryonic Antigen; SD - Standard Deviation; NAT - Neoadjuvant Treatment. Percentages may not add up to 100% due to rounding.
3.4. Recurrence-Free Survival and Overall Survival
On Cox-univariate analysis there were no clinical or pathologic characteristics associated with RFS (Table-3). On Cox-multivariate analysis, pre-treatment TILs was significantly associated with RFS (p = 0.040). CAP treatment response groups was not associates with RFS on cox-multivariate analysis (p = 0.098). On Cox-univariate analysis, OS was associated with gender (p = 0.043), age (p = 0.023), pre-treatment CEA levels (p = 0.001), pre-treatment tumor differentiation (p = 0.033), percentage of residual tumor (p = 0.003), CAP treatment response groups (p = 0.029), and mitotic count in 10HPF (p = 0.052) (Table-4). On Cox-multivariate analysis, gender (p = 0.034), age (p = 0.002), mitotic count in 10HPF (p = 0.007), and CAP treatment response groups (p = 0.001) were associated with OS.
Table-3.
Cox Proportional Hazard Models of Recurrence-free Survival
| Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|
| P | HR (95% CI) | P | |
| Gender | 0.298 | ||
| Age | 0.601 | ||
| Pre-Tx CEA | 0.378 | ||
| Pre-Tx cT-stage | 0.474 | ||
| Pre-Tx cN-stage | 0.256 | ||
| Pre-Tx cTNM prognostic stage groups | 0.052 | ||
| Time from Tx to Sx | 0.372 | ||
| Intratumoral budding | 0.464 | ||
| Tumor differentiation | 0.065 | ||
| Tumor infiltrating lymphocytes | 0.159 | 3.085 (1.054 – 9.024) | 0.040 |
| Type of desmoplastic reaction | 0.422 | ||
| Pathologic complete response | 0.144 | ||
| Percentage of residual tumor | 0.054 | ||
| CAP treatment response groups | 0.197 | 0.098 | |
| 0 | 1 | ||
| 1 | 1.174 (0.195 – 7.082) | ||
| 2 | 4.219 (0.837 – 21.267) | ||
| 3 | 4.324 (0.911 – 20.517) | ||
| Mitotic count/10HPF | 0.553 | ||
| ypT-stage | 0.146 | ||
| ypN-stage | 0.243 | ||
| ypTNM prognostic stage groups | 0.240 | ||
Abbreviations: HR - Hazard ratio; CI - Confidence interval; CEA - Carcinoembryonic antigen; Tx - Treatment; Sx - Surgery; TB- Tumor budding; TILs - Tumor intraepithelial lymphocytes
Table-4.
Cox Proportional Hazard Models of Overall Survival
| Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|
| P | HR (95% CI) | P | |
| Gender | 0.043 | 0.034 | |
| Male | 1 | ||
| Female | 0.380 (0.155 – 0.929) | ||
| Age | 0.023 | 1.054 (1.019 – 1.091) | 0.002 |
| Pre-Tx CEA | 0.001 | ||
| Pre-Tx cT-stage | 0.975 | ||
| Pre-Tx cN-stage | 0.061 | ||
| Pre-Tx cTNM prognostic stage groups | 0.140 | ||
| Time from Tx to Sx | 0.774 | ||
| Intratumoral budding | 0.181 | ||
| Tumor differentiation | 0.033 | ||
| Tumor infiltrating lymphocytes | 0.868 | ||
| Type of desmoplastic reaction | 0.918 | ||
| Pathologic complete response | 0.095 | ||
| Percentage of residual tumor | 0.003 | ||
| CAP treatment response groups | 0.029 | 0.001 | |
| 0 | 1 | ||
| 1 | 3.806 (0.755 – 19.196) | ||
| 2 | 3.983 (1.027 – 15.446) | ||
| 3 | 14.604 (3.477 – 61.333) | ||
| Mitotic count/10HPF | 0.052 | 0.969 (0.948 – 0.991) | 0.007 |
| ypT-stage | 0.096 | ||
| ypN-stage | 0.609 | ||
| ypTNM prognostic stage groups | 0.245 | ||
Abbreviations: HR - Hazard ratio; CI - Confidence interval; CEA - Carcinoembryonic antigen; Tx - Treatment; Sx - Surgery
3.5. Correlation of Immunohistochemical Characteristics with Treatment Response
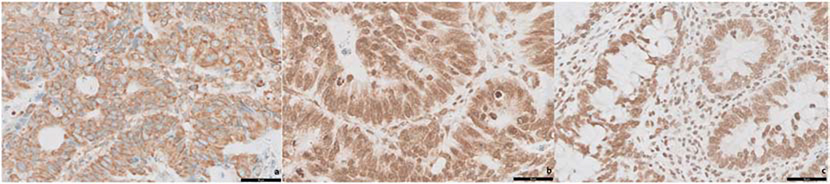
All tumors showed some degree of reactivity for CXCR4 in the pre-treatment biopsies. CXCR4 expression was divided into 3 groups: cytoplasmic (45 cases, 52.9%), cytoplasmic and nuclear (31 cases, 36.5%), and nuclear (9 cases, 10.6%) (Figure-3). There was no significant correlation between CXCR4 expression and pCR (p = 0.219), CAP treatment response group (p = 0.355), ypT-stage (p = 0.206), ypN-stage (p = 0.562), or ypTNM prognostic stage groups (p = 0.761) (Table-5). There was no significant association between CXCR4 expression and the percentage of residual tumor (p = 0.111).
Figure 3.
CXCR4 immunohistochemical reactivity in pre-treatment biopsies showing cytoplasmic (a), cytoplasmic and nuclear (b), and nuclear (c) reactivity.
Table-5.
Clinicopathologic features and CXCR4 Expression (N = 85)
| Cytoplasmic (n = 45) | Cytoplasmic and Nuclear (n = 31) | Nuclear (n = 9) | P | |
|---|---|---|---|---|
| Pathologic complete response | ||||
| No | 40, 88.9% | 23, 74.2% | 8, 88.9% | 0.220 |
| Yes | 5, 11.1% | 8, 25.8% | 1, 11.1% | |
| CAP treatment response groups | ||||
| Group 0 | 5, 11.1% | 7, 22.6% | 1, 11.1% | |
| Group 1 | 7, 15.6% | 8, 25.8% | 4, 44.4% | 0.355 |
| Group 2 | 16, 35.6% | 9, 29% | 2, 22.2% | |
| Group 3 | 17, 37.8% | 7, 22.6% | 2, 22.2% | |
| Percentage residual tumor (mean, SD) | 44.7, 37.6 | 30.0, 37.7 | 27.3%, 36.8 | 0.111 |
| ypT-stage | ||||
| T0 | 5, 11.1% | 7, 22.6% | 1, 11.1% | |
| T1 | 2, 4.4% | 2, 6.5% | 1, 11.1% | |
| T2 | 11, 24.4% | 6, 19.4% | 2, 22.2% | 0.206 |
| T3 | 19, 42.2% | 16, 51.6% | 5, 55.6% | |
| T4 | 8, 17.8% | 0 | 0 | |
| ypN-stage | ||||
| N0 | 29, 64.4% | 22, 70.9% | 8, 88.9% | |
| N1 | 11, 24.4% | 6, 19.4% | 0 | 0.562 |
| N2 | 5, 11.1% | 3, 9.7% | 1, 11.1% | |
| ypTNM prognostic stage groups | ||||
| 0 | 4, 8.9% | 7, 22.6% | 1, 11.1% | |
| I | 9, 20% | 6, 19.4% | 3, 33.3% | |
| II | 15, 33.3% | 9, 29% | 4, 44.4% | 0.761 |
| III | 11, 24.4% | 6, 19.4% | 1, 11.1% | |
| IV | 6, 13.3% | 3, 9.7% | 0 | |
Abbreviations: SD - standard deviation; yp – post-treated pathologic. Percentages may not add up to 100% due to rounding.
CXCR4 was further analyzed and divided in 2 groups: cytoplasmic (45 cases, 52.9%) and nuclear staining (40 cases, 47.1%). Nuclear CXCR4 expression was associated with a lower percentage of residual tumor compared to cytoplasmic expression (20.3 ±29.9% vs 34 ±36.3%, p = 0.036). Similarly, cases with cytoplasmic expression were associated with higher ypT-stage compared to nuclear expression (p = 0.039). No significant association was seen with pCR, CAP treatment response group, ypN-stage, and ypTNM prognostic stage groups.
4. DISCUSSION
In the treatment of rectal cancer with neoadjuvant therapy, pretreatment clinical T-stage and clinical TNM prognostic stage groups, therapy-to-surgery time interval, and TILs were associated with improved pathologic treatment response. TILs on pretreatment biopsy was also associated with a longer recurrence-free survival, and age, gender, treatment response, and mitotic rate were associated with improved overall-survival. CXCR4 nuclear expression was associated with a lower percentage of residual tumor.
In this study, we sought to evaluate the relationship between clinical and pathologic parameters and pathologic treatment response, recurrence-free survival, and overall survival. In our cohort, 16.5% of the cases achieved a pCR, which is similar to previously reported rates of 15 to 20%3,5,30–33. It’s well stablished that patients with pCR have significantly better 5-year RFS compare to those without a pCR32. Additionally, patients who achieve a cCR to NAT may be able to be treated without surgery7. While a cCR is determined by a combination of imaging and endoscopic evaluation, there are borderlines cases where it is difficult to differentiate treatment response from residual tumor on imaging. Perhaps in these borderline cases tumor characteristics associated with treatment response may guide clinicians.
A shorter time interval from end of NAT to surgical resection was significantly associated with a worse pTR (p = 0.022). The appropriate time interval between NAT and surgical resection has previously investigated. One of the first clinical trials to target this question was done by Francois Y. et al.34, where patients were divided into two groups based on the interval between the end of radiation therapy and resection (short: < 2 weeks, and long: 6 to 9 weeks). The long interval group had a significantly better pTR compared to the short interval group34. Another prospective study included 233 stage II and III rectal cancer patients and divided them into two groups: short (≤7 weeks) and long interval (>7 weeks). The long interval group was significantly associated with a better pTR and 3-year local recurrence rate35. It has been proposed that radiotherapy produces DNA damage with subsequent cellular death over subsequent weeks to months; therefore, a longer interval allows for continued therapy33,36.
Alternatively, some studies have shown that a longer neoadjuvant therapy-to-surgery interval is not always beneficial. Calvo FA et al. demonstrated that a surgical delay of ≥6 weeks was not associated with pCR or improved pTR37. Likewise, a multicenter clinical trial (GRECCAR-6) randomized patients into a short interval group (7 weeks) and long interval group (11 weeks) and found that the long interval group was associated with a higher morbidity and did not have a significant difference in achievement of pCR33. In our cohort, the mean time interval between NAT and resection in the pCR group was 12.6 weeks compared to 8.5 weeks in cases with poor or no response. Our findings suggest that a longer time interval is associated with a better pTR.
In our cohort, pre-treatment CEA level was not associated with pTR, however, a trend of a higher level of CEA was noted in the pTR group 3. In a recent study, a decreased CEA clearance pattern with NAT was associated with pTR and pCR in RA9. A similar study found no correlation between pre-treatment CEA and response; however, the post-treatment CEA was significantly lower in the pCR group10. A separate study with clinical stage II and III RA found that an elevated pre-treatment CEA level was associated with a worse pTR and OS13. Similar to these studies, in our cohort, a lower pre-treatment CEA was significantly associated with a better OS (p = 0.001). A limitation of our study is the unavailability of a post-treatment CEA level in all the cases.
Lower pre-treatment clinical T-stage and cTNM prognostic stage were associated with pCR and pTR. As expected, the majority of the clinical stage IV cases (70%) had a poor pTR (group 3) and none of them achieved a pCR. On the other hand, 77.8% of the cases with a pre-treatment clinical stage I were pTR groups 0 and 1. Similar results were noted in prior studies where cases with pCR were significantly associated with a lower pre-treatment clinical stage14,15. Patients with non-obstructive, well- or moderately-differentiated tumors and no clinically apparent nodal or distant metastatic disease have been reported to achieve the best pTR15.
TB has a well-established association with infiltrative tumor growth, perineural and lymphovascular invasion, lymph node metastasis, and advanced pathologic stage16–19. Recent studies have showed TB to be more prevalent in cecal adenocarcinomas, and associated with KRAS mutations and microsatellite stability18. TB has also been identified as an independent prognostic factor for lymph node involvement and as a poor prognostic factor38–44. One prior study evaluated TB in RA biopsies before NAT and found TB to be predictive of a poor pathologic response45. In our cohort, TB in the pre-treatment biopsies was not associated with pTR grade or pCR. Although not significant, the presence of TB was more predominant in cases with poor or no pTR (12 cases, 41.4%). TB is usually seen at the deep infiltrative edge of the tumor, and biopsy tissue from the luminal aspect is not an ideal site to assess for TB. Although we noted this finding as present or absent due to its prognostic associations, it might not reflect the TB status of the entire tumor and may explain our lack of association between TB and pTR.
TILs has been associated with a better RFS in stage II mismatch repair proficient (pMMR) colon cancer46 and with better RFS in node-negative colon cancer47. It is considered as an independent favorable prognostic marker in right colon and rectal cancers20. A decreased number of CD3+ or CD8+ TILs is associated with a worse RFS and OS, regardless of MMR status48. In the setting of NAT-RA one prior study evaluated the significance of CD3+ and CD8+ lymphocytes, defined as a percentage of tumor stromal positivity, in pre-treatment biopsy and the post-treatment resected specimens11. This study found a significantly higher lymphocyte density in the post-treatment tumors. In addition, a higher pre-treatment CD3+ and CD8+ was associated with better pTR, RFS, and OS11. In our study, TILs was significantly associated with a better pTR on univariate analysis and better RFS on multivariate analysis. A limitation in our study is the lack of MMR protein status for many of the included patients. Since our cohort included patients from 2008 to 2016, many of the patients from early on the study period did not have MMR testing performed, as it was not routinely performed on all colorectal tumors at that time. More studies with larger cohorts, the development of novel biomarkers, and advancement in imaging techniques are needed to better predict or detect a pCR, or near pCR.
In our study, we analyzed CXCR4 expression as a possible biomarker of prognosis in rectal cancer. A recent meta-analysis reported nuclear CXCR4 expression to correlate with a poor prognosis in older patients, advanced stage of disease, and poorly differentiated tumor grade21. CXCR4 expression is reported to be present in half of the CRCs in both a nuclear and/or cytoplasmic pattern, but only nuclear staining has been associated with worse survival29. A recent study analyzed CXCR4 and CXCL12 expression in metastatic rectal adenocarcinoma before and after local radiotherapy and systemic neoadjuvant treatment, and found no correlation between the expression of CXCR4 and CXCL12 and pTR23. Similarly, we found no association between CXCR4 expression and pCR, although there was a decrease in the percentage of residual tumor in cases with nuclear, or combined cytoplasmic and nuclear CXCR4 staining, compared to cases with only cytoplasmic staining (29.4% vs 44.7%; p = 0.036).
Pre-treatment assessment of tumor differentiation was significantly associated with OS. This is consistent with the established prognostic significance of tumor differentiation in CRC49. A lower percentage of residual tumor was also associated with better OS, which is concordant with previous investigations5. When controlling for multiple clinical and pathologic variables, CAP pTR group was associated with better OS, as was the mitotic count in 10HPF. A limitation of our study in terms of RFS and OS analysis is the small cohort of cases and a short follow-up period available for analysis.
In conclusion, pathologic factors such as TILs in combination with clinical staging can help predict response to neoadjuvant treatment and may also provide additional prognostic data for survival. These factors may help inform treatment decisions in patients with a borderline complete clinical response who wish to pursue non-operative management of their rectal cancer.
HIGHLIGHTS.
Lower pre-treatment cT-stage was associated with better treatment response
Lower AJCC cTNM stage groups was associated with better treatment response
Tumor-infiltrating lymphocytes was associated with better treatment response
CXCR4 nuclear expression was associated with a lower percentage of residual tumor
Acknowledgements
The authors thank members of the Anatomic and Molecular Pathology Laboratory at Washington University School of Medicine (Marina Platik) for immunohistochemistry staining.
Funding
This work was supported the Department of Pathology & Immunology, Washington University School of Medicine; Washington University School of Medicine Surgical Oncology Basic Science and Translational Research Training Program grant T32CA009621 from the National Cancer Institute (NCI) (WCC); and REDCap is supported by Clinical and Translational Science Award (CTSA) Grant [UL1 TR000448].
Footnotes
Disclosure
None of the authors have any relationships with, or financial interest in, any commercial companies pertaining to this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal Cancer Statistics, 2017. CA Cancer J Clin 2017;67(3):177–93. [DOI] [PubMed] [Google Scholar]
- 2.Gaertner WB, Kwaan MR, Madoff RD, et al. Rectal cancer: An evidence-based update for primary care providers. World J Gastroenterol 2015;21(25):7659–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nussbaum N, Altomare I. The Neoadjuvant Treatment of Rectal Cancer: A Review. Curr Oncol Rep 2015;17(11):1–9. [DOI] [PubMed] [Google Scholar]
- 4.Bedrosian I, Rodriguez-Bigas MA, Feig B, et al. Predicting the node-negative mesorectum after preoperative chemoradiation for locally advanced rectal carcinoma. J Gastrointest Surg 2004;8(1):56–63. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal A, Chang GJ, Hu CY, et al. Quantified Pathologic Response Assessed as Residual Tumor Burden is a Predictor of Recurrence-free Survival in Patients with Rectal Cancer who Undergo Resection after Neoadjuvant Chemoradiotherapy. Cancer 2013;119(24):4231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakuyama N, Kojima M, Kawano S, et al. Histological differences between preoperative chemoradiotherapy and chemotherapy for rectal cancer: A clinicopathological study. Pathol Int 2016;66(5):273–80. [DOI] [PubMed] [Google Scholar]
- 7.Smith JJ, Strombom P, Chow OS, et al. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncol 2019;e185896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith JJ, Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J Clin Oncol 2015;33(16):1797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu H, Huang J, Lan P, et al. CEA clearance pattern as a predictor of tumor response to neoadjuvant treatment in rectal cancer: a post-hoc analysis of FOWARC trial. BMC Cancer 2018;18:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleiman A, Al-Khamis A, Farsi A, et al. Normalization of CEA Levels Post-Neoadjuvant Therapy is a Strong Predictor of Pathologic Complete Response in Rectal Cancer. J Gastrointest Surg 2015;19(6):1106–12. [DOI] [PubMed] [Google Scholar]
- 11.Teng F, Mu D, Meng X, et al. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am J Cancer Res 2015;5(6):2064–74. [PMC free article] [PubMed] [Google Scholar]
- 12.Ueno H, Kanemitsu Y, Sekine S, et al. Desmoplastic Pattern at the Tumor Front Defines Poor-Prognosis Subtypes of Colorectal Cancer. Am J Surg Pathol 2017;41(11):1506–12. [DOI] [PubMed] [Google Scholar]
- 13.Probst CP, Becerra AZ, Aquina CT, et al. Watch and Wait?—Elevated Pretreatment CEA Is Associated with Decreased Pathological Complete Response in Rectal Cancer. J Gastrointest Surg 2016;20(1):43–52. [DOI] [PubMed] [Google Scholar]
- 14.Huh JW, Kim HR, Kim YJ. Clinical Prediction of Pathological Complete Response After Preoperative Chemoradiotherapy for Rectal Cancer. Dis Colon Rectum 2013;56(6):698–703. [DOI] [PubMed] [Google Scholar]
- 15.van der Sluis FJ, van Westreenen HL, van Etten B, van Leeuwen BL. Pretreatment identification of patients likely to have pathologic complete response after neoadjuvant chemoradiotherapy for rectal cancer. Int J Colorectal Dis 2018;33(2):149–57. [DOI] [PubMed] [Google Scholar]
- 16.Wang LM, Kevans D, Mulcahy H, et al. Tumor Budding is a Strong and Reproducible Prognostic Marker in T3N0 Colorectal Cancer. Am J Surg Pathol 2009;33(1):134–41. [DOI] [PubMed] [Google Scholar]
- 17.Lugli A, Kirsch R, Ajioka Y, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol 2017;30(9):1299–311. [DOI] [PubMed] [Google Scholar]
- 18.Graham RP, Vierkant RA, Tillmans LS, et al. Tumor Budding in Colorectal Carcinoma: Confirmation of Prognostic Significance and Histologic Cutoff in a Population-based Cohort. Am J Surg Pathol 2015;39(10):1340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan É, Khaw YL, Creavin B, et al. Tumor Budding and PDC Grade Are Stage Independent Predictors of Clinical Outcome in Mismatch Repair Deficient Colorectal Cancer. Am J Surg Pathol 2018;42(1):60–8. [DOI] [PubMed] [Google Scholar]
- 20.Berntsson J, Svensson MC, Leandersson K, et al. The clinical impact of tumour-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: A cohort study. Int J Cancer 2017;141(8):1654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv S, Yang Y, Kwon S, et al. The association of CXCR4 expression with prognosis and clinicopathological indicators in colorectal carcinoma patients: A meta-analysis. Histopathology 2014;64(5):701–12. [DOI] [PubMed] [Google Scholar]
- 22.Zhao H, Guo L, Zhao H, Zhao J, Weng H, Zhao B. CXCR4 over-expression and survival in cancer: A system review and meta-analysis. Oncotarget 2015;6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamas K, Domanska UM, van Dijk TH, et al. CXCR4 and CXCL12 Expression in Rectal Tumors of Stage IV Patients Before and After Local Radiotherapy and Systemic Neoadjuvant Treatment. Curr Pharm Des 2015;21(17):2276–83. [DOI] [PubMed] [Google Scholar]
- 24.Lippitz BE. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol 2013;14(6):218–28. [DOI] [PubMed] [Google Scholar]
- 25.González I, Goyal B, Xia MD, Pai RK, Ma C. DNA mismatch repair deficiency but not ARID1A loss is associated with prognosis in small intestinal adenocarcinoma. Hum Pathol 2019;85:18–26. [DOI] [PubMed] [Google Scholar]
- 26.Kakar S, Shi C; Berho EM, Driman DK,; Fitzgibbons P, Frankel WL, Hill KA, Jessup J, Krasinskas AMWMK. Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Colon and Rectum. Coll Am Pathol 2017;1–28. [Google Scholar]
- 27.American Joint Committee on Cancer Staging Manual. 8th Editio. Springer Nature; 2017. [Google Scholar]
- 28.Bai S, Wang D, Klein MJ, Siegal GP. Characterization of CXCR4 expression in chondrosarcoma of bone. Arch Pathol Lab Med 2011;135(6):753–8. [DOI] [PubMed] [Google Scholar]
- 29.Wang SC, Lin JK, Wang HS, Yang SH, Li AFY, Chang SC. Nuclear expression of CXCR4 is associated with advanced colorectal cancer. Int J Colorectal Dis 2010;25(10):1185–91. [DOI] [PubMed] [Google Scholar]
- 30.Kawai K, Ishihara S, Nozawa H, et al. Prediction of Pathological Complete Response Using Endoscopic Findings and Outcomes of Patients WhoUnderwent Watchfu Waiting After Chemoradiotherapy for Rectal Cancer. Dis Colon Rectum 2017;4(60):368–75. [DOI] [PubMed] [Google Scholar]
- 31.Shahab D, Gabriel E, Attwood K, et al. Adjuvant Chemotherapy Is Associated With Improved Overall Survival in Locally Advanced Rectal Cancer After Achievement of a Pathologic Complete Response to Chemoradiation. Clin Colorectal Cancer 2017;16(4):300–7. [DOI] [PubMed] [Google Scholar]
- 32.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer : a pooled analysis of individual patient data. Lancet Oncol 2010;11(9):835–44. [DOI] [PubMed] [Google Scholar]
- 33.Lefevre H, Mineur L, Kotti S, et al. Effect of Interval (7 or 11 weeks) Between Neoadjuvant Radiochemotherapy and Surgery on Complete Pathologic Response in Rectal Cancer : A Multicenter, Randomized, Controlled Trial (GRECCAR-6). J Clin Oncol 2016;34(31):3773–80. [DOI] [PubMed] [Google Scholar]
- 34.Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the Interval Between Preoperative Radiation Therpay and Surgery on Downstaging and on the Rate of Sphincter-Sparing Surgery for Rectal Cancer: The Lyon R90–01 Randomized Trial. J Clin Oncol 1999;17(8):2396–402. [DOI] [PubMed] [Google Scholar]
- 35.Zeng WG, Zhou ZX, Liang JW, et al. Impact of Interval Between Neoadjuvant Chemoradiotherapy and Surgery for Rectal Cancer on Surgical and Oncologic Outcome. J Surg Oncol 2014;110(4):463–7. [DOI] [PubMed] [Google Scholar]
- 36.Bujko K Timing of surgery following preoperative therapy in rectal cancer: there is no need for a prospective randomized trial. Dis Colon Rectum 2012;55(3):e31-author reply e31–2. [DOI] [PubMed] [Google Scholar]
- 37.Calvo FA, Morillo V, Santos M, et al. Interval between neoadjuvant treatment and definitive surgery in locally advanced rectal cancer: impact on response and oncologic outcomes. J Cancer Res Clin Oncol 2014;140(10):1651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kemi N, Eskuri M, Ikäläinen J, Karttunen TJ, Kauppila JH. Tumor Budding and Prognosis in Gastric Adenocarcinoma. Am J Surg Pathol 2018;00(00):1–6. [DOI] [PubMed] [Google Scholar]
- 39.O’Connor K, Li-Chang HH, Kalloger SE, et al. Tumor Budding Is an Independent Adverse Prognostic Factor in Pancreatic Ductal Adenocarcinoma. Am J Surg Pathol 2015;39(4):472–8. [DOI] [PubMed] [Google Scholar]
- 40.Lohneis P, Sinn M, Klein F, et al. Tumour buds determine prognosis in resected pancreatic ductal adenocarcinoma. Br J Cancer 2018;118(11):1485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohike N, Coban I, Kim GE, et al. Tumor Budding as a Strong Prognostic Indicator in Invasive Ampullary Adenocarcinomas. Am J Surg Pathol 2010;34(10):1417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landau MS, Hastings SM, Foxwell TJ, Luketich JD, Nason KS, Davison JM. Tumor budding is associated with an increased risk of lymph node metastasis and poor prognosis in superficial esophageal adenocarcinoma. Mod Pathol 2014;27(12):1578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boxberg M, Kuhn P, Reiser M, et al. Tumor Budding and Cell Nest Size are Highly Prognostic in Laryngeal and Hypopharyngeal Squamous Cell Carcinoma Further Evidence for a Unified Histopathologic Grading System for Squamous Cell Carcinomas of the Upper Aerodigestive Tract. Am J Surg Pathol 2018;00(00):1–11. [DOI] [PubMed] [Google Scholar]
- 44.Kadota K, Miyai Y, Katsuki N, et al. A Grading System Combining Tumor Budding and Nuclear Diameter Predicts Prognosis in Resected Lung Squamous Cell Carcinoma. Am J Surg Pathol 2017;41(6):750–60. [DOI] [PubMed] [Google Scholar]
- 45.Rogers AC, Gibbons D, Hanly AM, et al. Prognostic significance of tumor budding in rectal cancer biopsies before neoadjuvant therapy. Mod Pathol 2014;27(1):156–62. [DOI] [PubMed] [Google Scholar]
- 46.Turksma AW, Coupé VMH, Shamier MC, et al. Extent and Location of Tumor-Infiltrating Lymphocytes in Microsatellite-Stable Colon Cancer Predict Outcome to Adjuvant Active Specific Immunotherapy. Clin Cancer Res 2016;22(2):346–56. [DOI] [PubMed] [Google Scholar]
- 47.Markl B, Wieberneit J, Kretsinger H, et al. Number of Intratumoral T Lymphocytes Is Associated With Lymph Node Size, Lymph Node Harvest, and Outcome in Node-Negative Colon Cancer. Am J Clin Pathol 2016;145(6):826–36. [DOI] [PubMed] [Google Scholar]
- 48.Eriksen AC, Sørensen FB, Lindebjerg J, et al. The Prognostic Value of Tumor-Infiltrating lymphocytes in Stage II Colon Cancer. A Nationwide Population-Based Study. Transl Oncol 2018;11(4):979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hermanek P, Guggenmoos-Holzmann I, Gall FP. Prognostic factors in rectal carcinoma: a contribution to further development of tumor classification. Dis Colon Rectum 1989;32:593–9. [DOI] [PubMed] [Google Scholar]