Abstract
In Mauritania, obstetrical risk insurance (ORI) has been progressively implemented at the health district level since 2002 and was available in 25% of public healthcare facilities in 2015. The ORI scheme is based on pre-payment scheme principles and focuses on increasing the quality of and access to both maternal and perinatal healthcare. Compared with many community-based health insurance schemes, the ORI scheme is original because it is not based on risk pooling. For a pre-payment of 16–18 USD, women are covered during their pregnancy for antenatal care, skilled delivery, emergency obstetrical care [including caesarean section (C-section) and transfer] and a postnatal visit. The objective of this study is to evaluate the impact of ORI enrolment on maternal and child health services using data from the Multiple Indicator Cluster Survey (MICS) conducted in 2015. A total of 4172 women who delivered within the last 2 years before the interview were analysed. The effect of ORI enrolment on the outcomes was estimated using a propensity score matching estimation method. Fifty-eight per cent of the studied women were aware of ORI, and among these women, more than two-thirds were enrolled. ORI had a beneficial effect among the enrolled women by increasing the probability of having at least one prenatal visit by 13%, the probability of having four or more visits by 11% and the probability of giving birth at a healthcare facility by 15%. However, we found no effect on postnatal care (PNC), C-section rates or neonatal mortality. This study provides evidence that a voluntary pre-payment scheme focusing on pregnant women improves healthcare services utilization during pregnancy and delivery. However, no effect was found on PNC or neonatal mortality. Some efforts should be exerted to improve communication and accessibility to ORI.
Keywords: Maternal health, neonatal mortality, pre-payment scheme, universal health coverage, Mauritania, Sub-Saharan Africa
Key Messages
Enrolment in obstetrical risk insurance (ORI) increases the utilization of health services during pregnancy and delivery.
Women enrolled in ORI are more likely to give birth in a healthcare facility assisted by a midwife.
ORI has no impact on caesarean section rates, postnatal care or neonatal mortality.
Introduction
The total number of maternal deaths worldwide decreased by 29% from 390 155 in 1990 to 275 288 in 2013 (Kassebaum et al., 2016). Nevertheless, nearly 50% of these deaths occurred in Sub-Saharan Africa, and it has been estimated that only 10 countries achieved the Millennium Development Goal 5 by 2015 (Kassebaum et al., 2016). A key issue in reducing maternal mortality is increasing access to emergency obstetric and neonatal care (EmONC) available at healthcare centres (basic EmONC) and referral hospitals (comprehensive EmONC; Campbell et al., 2006). The problem is that access to these services is far from optimal in low-income and middle-income countries (LMICs) for many reasons. For example, many women delay their decision to seek care or forgo visiting a health facility due to fear of having to pay for excessive expenses (Borghi et al., 2003, 2006; Skordis-Worrall et al., 2011).
In September 2015, the Sustainable Development Goals were adopted by the United Nations, and a part of goal 3 includes achieving universal health coverage (UHC) by 2030 (United Nations Publications, 2016). The objective is for everybody to have access to health services without suffering financial issues because payment is required (World Health Organization, 2010; Sachs, 2012).
A common objective of different UHC programmes worldwide is to remove financial barriers, such as user fees, particularly for pregnant women and children who must have priority access to health services (Yates, 2009; Quick et al., 2014). Several financial reforms can be implemented and combined depending on the context to achieve UHC (Kutzin et al., 2016). Some countries in Sub-Saharan Africa have implemented user fee exemption policies for maternal healthcare services to improve access to emergency obstetric and neonatal care (EmONC; Kruk et al., 2008; Witter et al., 2010; Ridde et al., 2011; Bennis and De Brouwere, 2012; Ridde, 2015), whereas other countries have implemented targeted vouchers for delivery care (Kanya et al., 2014), national health insurance (Wang et al., 2014; Brugiavini and Pace, 2016) and community-based health insurance (CBHI; Smith and Sulzbach, 2008; Falisse et al., 2012; Odeyemi and Nixon, 2013; Alhassan et al., 2016; Ouattara and Ndiaye, 2017) or a performance-based financing scheme at the local and/or national level (Richard et al., 2010; Turcotte-Tremblay et al., 2016; Ridde et al., 2018b).
Among these mechanisms, pre-payment aims to increase healthcare utilization and provides financial risk protection (Smith and Sulzbach, 2008). Compared with CBHI, pre-payment schemes are not based on risk pooling and regular premium payments. Pre-payment schemes can focus on maternal healthcare and, theoretically, not only improve access to healthcare for pregnant women and reduce delays in seeking care by lowering direct payments but also improve women’s interactions with the formal healthcare system (Smith and Sulzbach, 2008). Pre-payment schemes for pregnant women are expected to have an effect on prenatal care, facility-based delivery (FBD), postnatal consultation and the appropriate management of obstetric complications, such as caesarean sections (C-sections; Smith and Sulzbach, 2008). In early 2000, Mauritania progressively implemented an original pre-payment scheme named obstetrical risk insurance (ORI) that specifically focuses on maternal healthcare services.
A previous study (Philibert et al., 2017) showed that, compared with districts without ORI, the availability of ORI at the district level significantly increased qualified antenatal care (ANC) and delivery rates in healthcare centres. However, due to the lack of available data, this study measured only the effect of the availability of ORI in districts and not the effect of enrolment. Therefore, in 2015, a new nationally representative survey collected information regarding ORI enrolment among women who gave birth over the last 2 years. This study adds a new element to the literature by measuring the impact of ORI enrolment (and not just its availability) on maternal and child health services using the new population health data available.
Materials and methods
Context
Mauritania
Mauritania is a West African country classed in 2015 as having low human development (ranked 157 of 186 countries; UNDP, 2016). In 2015, it was estimated that 810 women died during childbirth, and the maternal mortality rate (MMR) remains high in this country (602 per 100 000) compared with the MMR in Western Sub-Saharan Africa (542 per 100 000; WHO, 2015). Public healthcare facilities are divided into three levels. Health posts constitute the first level and provide primary healthcare, including antenatal visits, normal vaginal delivery and basic neonatal care. Health centres represent the intermediate level of care and provide the essential services cited above and some laboratory tests; however, few of these centres have a general surgical unit or radiology department. Moreover, health centres represent the highest level of care in most of the Moughatta (department; Dumont et al., 2017). Finally, regional or national hospitals represent the third level and constitute the reference level for emergency obstetric care that requires a higher technical platform, including transfusion and C-section. According to the most recent Multiple Indicator Cluster Survey (MICS) conducted in 2015, 85% of Mauritanian women had received at least one ANC visit during the last pregnancy and 69% gave birth in a health facility (ONS, 2017).
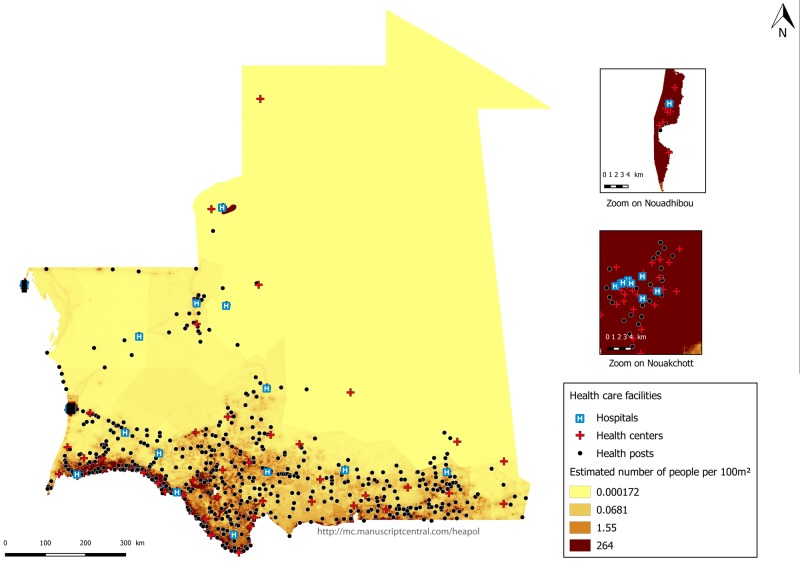
Figure 1 presents an estimation of the Mauritanian population and the locations of healthcare facilities in 2015. In terms of population size, Mauritania is low-density with an unequal distribution of the population throughout the territory. The most populated areas are in the capital Nouakchott, the coastal city of Nouadhibou and the southwest regions on the border with Senegal. The remainder of the country is rather arid and scarcely populated. Healthcare services are also unequally distributed across the country. Most healthcare facilities are located in the most densely populated areas. Therefore, access to care is still a vast problem for women living in area with the lowest-density of population.
Figure 1.
Estimation of the Mauritanian population density and locations of healthcare facilities in 2015. Source: Health map AECID (Spanish Agency for International Development Cooperation) consolidated 2015, OSM (OpenStreetMap).
Intervention
ORI in Mauritania has been widely described in previous studies (Renaudin et al., 2007; Dumont et al., 2017; Philibert et al., 2017).
ORI is offered to pregnant women upon their first contact with obstetric services at health facilities. The ORI enrolment cost is 6500 ouguiyas (∼18 USD according to the exchange rate in 2018) in Nouakchott and 5500 ouguiyas (∼16 USD) in other districts. The price is the same regardless of household income and can be paid in one or two instalments during pregnancy. ORI fees are lower than delivery costs at public maternity units. For example, a woman who receives four consultations during her pregnancy, a laboratory examination and an ultrasound and experiences an uncomplicated delivery would have to pay 9100 ouguiyas (∼26 USD) without the ORI scheme.). This cost would increase to 25 000 ouguiyas (∼71 USD) if the women had a C-section (Audibert et al., 2019).
Once the women are enrolled in ORI, they can benefit from an obstetric package that includes four antenatal visits; all prophylactic treatments during pregnancy; one blood test (haemoglobin level, blood group and rhesus); one urine test (proteinuria and glycosuria) at each antenatal visit; one ultrasound scan during the first trimester; treatment for any pathologies related to pregnancy and delivery; skilled delivery; treatment for any complications during pregnancy and delivery if needed, including C-section; ambulance transportation to a higher-level healthcare facility; hospital care if transferred; and one postnatal visit (Renaudin et al., 2007; Philibert et al., 2017).
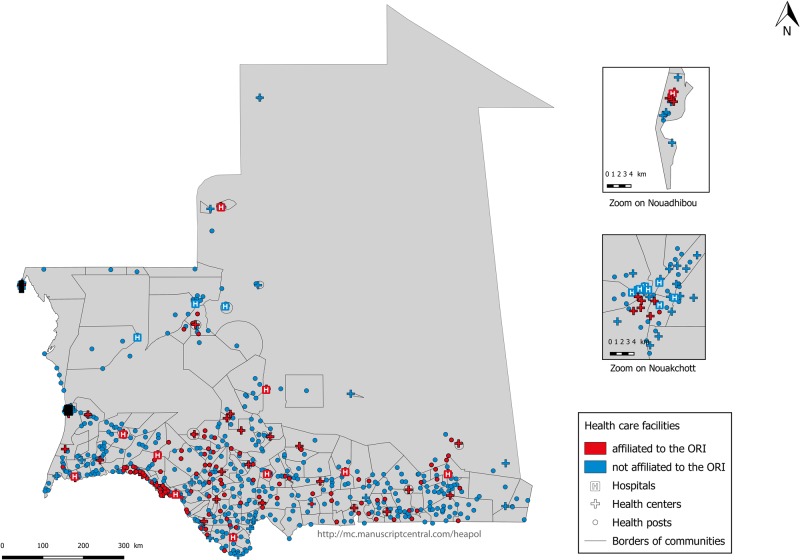
ORI was first implemented in November 2002 in Nouakchott and subsequently expanded to other districts outside of the capital. Quality was improved in the selected facilities by providing drugs and supplies, upgrading essential equipment and providing staff training at EmOC to ensure that these facilities can provide all the services included in the ORI package during pregnancy (Dumont et al., 2017). In 2015, ORI was available in 25% of public healthcare facilities [113/565 health posts, providing primary healthcare; 44/86 health centres, providing essential services and some laboratory tests; and 11/23 hospitals, constituting the reference level for emergency obstetric care; source: data from Health map AECID 2015 (Spanish Agency for International Development Cooperation)]. ORI implementation is unequally distributed across the country and located almost exclusively in the south of the country, in Nouakchott and the coastal city of Nouadhibou, where most of the population and health facilities are concentrated (Figure 2).
Figure 2.
Affiliation of public healthcare facilities with ORI in 2015. Source: Health map AECID (Spanish Agency for International Development Cooperation) consolidated 2015, OSM (OpenStreetMap).
Methods
Data
We used nationally representative household data from the final MICS carried out in 2015 by the National Office of Statistics (NOS) with the assistance of UNICEF for data collection and analysis (http://mics.unicef.org/surveys). MICSs are nationally representative with large sample sizes, and they have been conducted for more than 20 years in 116 developing countries (http://mics.unicef.org/surveys). These household surveys provide a wide range of information on the well-being of children and women, including child health, education, HIV prevalence and maternal health, and they are free and available on demand.
Women aged 15–49 years who were present in the surveyed households during the interviews and had delivered a live-born child in the 2 years before the interview were surveyed about their last pregnancy during these 2 years. Women who delivered a stillborn were not interviewed about their pregnancy and delivery. Data regarding household characteristics (demographic, socioeconomic and environmental conditions) and the final pregnancy, including information regarding the use of maternal health services, were extracted from relevant questionnaires. The socioeconomic status was evaluated using a principal components analysis as a relative wealth index based on the household conditions and assets (Sahn and Stifel, 2000; Rutstein and Johnson, 2004).
In addition to the usual information collected in the MICS (http://mics.unicef.org/tools? round=mics5), the interviewers asked the women the following questions: Have you heard about ORI during your previous pregnancy? If yes, were you enrolled in ORI? What is the reason for non-enrolment? If you did not enrol in ORI, do you think you would have attended at least one prenatal consultation? If you did not enrol in ORI, do you think you would still have a birth at a health facility? (ONS, 2017).
Outcomes
We studied the effects of ORI enrolment on maternal healthcare utilization and neonatal mortality. We considered one ANC visit and FBD to be the primary outcomes. ORI implementation should have a direct positive impact on women’s access to ANC and FBD. The secondary outcomes included the number of ANCs (at least four ANCs for those who reported an ANC visit), qualified ANC visits (antenatal visits performed by a doctor, a midwife or a nurse) and some exams performed during pregnancy (echography, blood sampling, urine sampling and blood pressure testing). Concerning delivery at healthcare facilities, we studied the level of care (regional hospital, district health centre and local health post), the qualification of the birth attendant and the mode of delivery (vaginal delivery or C-section). Finally, we studied postnatal care (PNC) before and after leaving the facility as well as early (<7 days) and late (<28 days) neonatal mortality. We used information regarding infant mortality (age at death in days, months or years) reported by the interviewed mothers. If a woman reported that her child died within the first 7 days of his/her life, we coded early neonatal mortality as 1; otherwise it was coded as 0. We implemented a similar approach for late neonatal mortality (<28 days). All measures were binary (coded as 0 or 1).
Exposure
The main independent variable of interest is ORI enrolment (Yes or No). Because the question in the MICS is only asked to women who have heard about ORI, we only have information for this subgroup of women.
Statistical analysis
We chose the propensity scoring matching (PSM) approach to estimate the effect of ORI enrolment on the selected outcomes (Gertler et al., 2016). We considered this approach as the best for our data because it is based on the observed sample.
We considered the following two groups of women: women who enrolled in ORI (Ti = 1, for woman i) and women who were not enrolled (Ti = 0) during their pregnancy within the previous 2 years. This approach consists of estimating the probability (a propensity score) of enrolling in ORI (T) conditional on observed characteristics (xi) as follows: P(xi) = Pr(T = 1|xi). Several assumptions are made when using PSM: positivity, consistency, exchangeability (i.e. no unmeasured confounding or selection bias); no measurement error; no interference; and correct specification of the model (Westreich and Cole, 2010). The precision of our estimates is also limited by the risk of unmeasured confounders such as the exact distance to a health facility. Despite this possible bias, the PSM method can improve covariate balance between treated and untreated subjects and is capable of reducing measurement errors (Austin, 2009; Elze et al., 2017).
Probit models were used to identify the determinants of the adhesion to ORI and calculate the propensity score. The variables to be included in the model used to calculate the propensity score must be related to the outcome variable or to the outcome variable and the exposure variable (Brookhart et al., 2006). We used a stepwise procedure to confirm that the variables chosen (determinants of enrolment in ORI) using the Heckman procedure are also linked to the outcomes being studied.
Then, the non-enrolled women were matched to the enrolled women based on this probability. Usually, PSM estimates the average treatment effect (ATE) on those treated, which is the effect of ORI enrolment among women who were enrolled. In this study, the intervention effect was estimated using the teffect command available in StataCorp L. (2013). This approach allows for an estimation of the ATE, which corresponds to the mean difference in outcomes across two groups, and the confidence interval is calculated using an estimate of the standard error (Abadie and Imbens, 2002, 2016; Abadie et al., 2004).
To match the enrolled and non-enrolled women, we used nearest-neighbour matching (Austin, 2011; Gertler et al., 2016). Each enrolled woman was matched to a comparison woman with the closest propensity score. The women did not have the same characteristics, although the distribution of the different determinants was broadly comparable between the two groups. We used a method based on matching with replacement as follows: a non-enrolled woman can be used several times to form a pair with an enrolled woman. We used complete cases for the matching and we took into account the structure of the questionnaire. For example, the examination during ANC is only available among women who have received at least one ANC.
Importantly, we accounted for the fact that the data are, by definition, truncated in our sample. Specifically, only women who have heard about ORI were asked about their enrolment. We used a maximum likelihood estimation based on Heckman’s (1977) method to account for selection bias. If the Mills ratio is significant in the model used to identify the determinants of the adherence to ORI (main equation), selection bias is confirmed.
In that situation, the model used to calculate the propensity score has to consider the determinants of hearing about ORI (selection equation) by introducing an inverse Mills ratio as a correction factor.
To test the matching quality, we calculated the pseudo-R2 by applying propensity score models to both matched and unmatched samples. The mean biases between the enrolled and non-enrolled women were compared with respect to the observed covariates included in the models. We verified that the mean absolute bias diminished after matching. We also calculated Rubin’s B, which is the absolute standardized difference of the means of the linear index of the propensity score between the enrolled and non-enrolled women, and Rubin’s R, which is the ratio of enrolled to non-enrolled women variances in the propensity score index. Rubin (2001) recommends that B should be < 25 and that R should be between 0.5 and 2 for the samples to be considered sufficiently balanced.
The analysis was conducted using Stata Statistical Software, release version 14.0 (Stata Corp. College Station, TX, USA).
Results
ORI coverage and background characteristics
Table 1 presents the characteristics of the women according to their knowledge and enrolment in ORI. We analysed 4172 women who delivered a live-born child within the 2 years before the interview. Among these women, 1766 women had not heard about ORI (42%) and 2401 women (58%) knew of its existence. Sixty-four per cent of the women who had heard about ORI were enrolled. Overall, 1528 women of the 4172 (37%) in our study were enrolled in the ORI scheme.
Table 1.
Socio-demographic characteristics of women who gave birth within the past 2 years before the interview according to their knowledge or enrolment in ORI
| All (n = 4172) | Knowledgeable about ORI (n = 4167) |
Enrolment among women who heard about ORI (n = 2400)c |
|||||
|---|---|---|---|---|---|---|---|
| No (n = 1766), %a | Yes (n = 2401), %a | P-valueb | No (n = 872), %a | Yes (n = 1528), %a | P-valueb | ||
| Age of women | |||||||
| Median [IQR] | 29 [24–34] | 29 [24–35] | 28[24–34] | 0.038 | 30[25–35] | 28 [23–34] | 0.001 |
| Women’s education | |||||||
| None | 27.3 | 28.4 | 26.5 | <0.001 | 25.6 | 27 | 0.018 |
| Coranic/Mahadra | 19.7 | 23.4 | 17.3 | 21.8 | 15.0 | ||
| Primary | 35.4 | 31.3 | 38.0 | 34.7 | 39.7 | ||
| Secondary and more | 17.6 | 16.8 | 18.1 | 17.9 | 18.2 | ||
| Education of the head of the household | |||||||
| None | 34.4 | 34.5 | 34.3 | 0.015 | 30.2 | 36.5 | 0.077 |
| Coranic/Mahadra | 32.0 | 35.3 | 29.9 | 32.7 | 28.4 | ||
| Primary | 18.6 | 16.8 | 19.8 | 19.4 | 20.0 | ||
| Secondary and more | 15.0 | 13.4 | 16.0 | 17.6 | 15.2 | ||
| Marital status | |||||||
| Currently married | 91.9 | 90.8 | 92.6 | 0.127 | 90.8 | 93.5 | 0.048 |
| Not married | 8.1 | 9.2 | 7.4 | 9.2 | 6.5 | ||
| Wealth quintiles of households | |||||||
| Q1 Poorest | 21.1 | 28.6 | 16.2 | <0.001 | 25.3 | 11.4 | <0.001 |
| Q2 Poorer | 21.0 | 22.7 | 19.8 | 21.4 | 19.0 | ||
| Q3 Middle | 19.9 | 16.6 | 21.9 | 16.7 | 24.7 | ||
| Q4 Richer | 20.1 | 15.3 | 23.2 | 17.7 | 26.2 | ||
| Q5 Richest | 18.0 | 16.8 | 18.8 | 18.9 | 18.7 | ||
| Zone of residence | |||||||
| Urban | 45.0 | 39.4 | 48.7 | 0.003 | 39.8 | 53.5 | <0.001 |
| Rural | 55.0 | 60.6 | 51.3 | 60.2 | 46.5 | ||
| Region | |||||||
| Nouakchott | 24.2 | 22.6 | 25.3 | <0.001 | 22.4 | 26.9 | <0.001 |
| Hodh charghy | 12.0 | 13.8 | 10.9 | 12.5 | 10.0 | ||
| Hodh Gharby | 9.9 | 14.6 | 6.8 | 9.5 | 5.3 | ||
| Assaba | 11.5 | 15.8 | 8.7 | 10.4 | 7.8 | ||
| Gorgol | 12.2 | 7.4 | 15.3 | 16.5 | 14.6 | ||
| Brakna | 10.3 | 4.4 | 14.0 | 7.6 | 17.5 | ||
| Trarza | 7.2 | 3.5 | 9.5 | 7.9 | 10.4 | ||
| Adrar | 0.5 | 1.0 | 0.1 | 0.3 | 0.0 | ||
| Dakhlet Nouadhibou | 3.3 | 2.9 | 3.5 | 1.3 | 4.7 | ||
| Tagant | 0.5 | 0.4 | 0.6 | 0.7 | 0.6 | ||
| Guidimaka | 7.9 | 12.6 | 4.8 | 10.2 | 2.0 | ||
| Tiris Zemmour | 0.5 | 0.9 | 0.2 | 0.4 | 0.0 | ||
| Inchiri | 0.1 | 0.2 | 0.1 | 0.1 | 0.0 | ||
| Parity | |||||||
| First child | 18.4 | 18.1 | 18.6 | 0.932 | 13.2 | 21.4 | 0.001 |
| Two or three | 32.6 | 32.4 | 32.6 | 32.3 | 32.8 | ||
| Four and more | 49.0 | 49.5 | 48.8 | 54.4 | 45.8 | ||
| Multiple pregnancy | |||||||
| Single | 98 | 97.7 | 98.2 | 0.323 | 98.2 | 98.3 | 0.885 |
| Multiple | 2.0 | 2.3 | 1.8 | 1.8 | 1.7 | ||
Percentages are adjusted based on the sampling weight, clustering and strata.
Chi-square test or quantile regression was used to compare the median age.
No information regarding the enrolment is available.
ORI, obstetric risk insurance.
The rates of women’s knowledge and enrolment differed widely according to the region of residence. The highest rates were observed in Nouakchott, which is the capital city. In some regions, such as Adrar, Tiris Zemmour and Inchiri, we found that almost no women knew about or were enrolled in ORI (see Supplementary Figure S1 for the location of the regions).
The level of education of the head of the household was positively related to the women’s knowledge about ORI but not for women’s enrolment. Non-married and high multiparous (four and more) women were less likely to be enrolled in ORI. Younger women with primary and secondary school education who were wealthier and urban were more aware of and more likely to be enrolled in ORI.
Selection bias
The Heckman model confirms selection bias by introducing the Mills ratio to the model to calculate the propensity score. The selection and principal equations are available in Supplementary Tables S1 and S2.
Propensity score estimation and matching quality
According to the probit model, the region, zone of residence (urban or rural), education level of the head of the household, wealth quintile of the household and total number of births are the main determinants of enrolment in ORI. These factors are used to calculate the propensity score.
Table 2 presents an evaluation of the propensity score models and matching quality for the main variables of interest in our study (at least one ANC and FBD).
Table 2.
Quality measurements of propensity score matching for primary outcomes
| Pseudo-R2 | P > Χ² | Mean bias | Rubin’s B | Rubin’s R | |
|---|---|---|---|---|---|
| At least one ANC | |||||
| Before matching | 0.134 | <0.001 | 13.342 | 90.497 | 0.744 |
| After matching | 0.003 | 1.000 | 2.176 | 13.567 | 0.947 |
| FBD | |||||
| Before matching | 0.134 | <0.001 | 13.366 | 90.505 | 0.745 |
| After matching | 0.006 | 0.997 | 2.854 | 17.727 | 0.832 |
ANC, antenatal care; FBD, facility-based delivery.
Supplementary Table S3 presents the median and maximum number of times women in the control group were used and the number of women excluded from the analysis, and Supplementary Table S4 presents the percentage of missing values and PSM performance for each variable of interest. We can observe that the pseudo-R2 is substantially reduced after matching. Thus, the distribution of these variables between the two subgroups (enrolled and non-enrolled) does not differ after matching. Furthermore, matching substantially reduced the mean biases in the observed covariates included in the models between the enrolled and non-enrolled women. The mean absolute bias was <5% in all models. As recommended, Rubin’s B is < 25 and Rubin’s R is between 0.5 and 2 for all variables (except for PNC after leaving the facility, with Rubin’s B equal to 25.608). Therefore, the samples can be considered sufficiently balanced.
Effect of ORI on maternal healthcare services utilization and neonatal mortality
Table 3 presents the differences in the proportion of all outcomes between the enrolled and non-enrolled women and the ATEs of ORI based on the matched samples.
Table 3.
ATE of ORI on maternal healthcare service utilization and neonatal mortality
| Proportions before matching |
ATE after matching |
||||
|---|---|---|---|---|---|
| Outcomes | Among non-enrolled women (%) | Among enrolled women (%) | ATE [95% CI]a | P-value | Number of pairs |
| ANC | |||||
| Total, n | 872 | 1528 | |||
| ANC1, n (%) | 729 (85.9) | 1507 (99.0) | +0.13 [0.10; 0.15] | <0.001 | 2361 |
| Total, nb | 841 | 1496 | |||
| ANC4, n (%) | 493 (62.4) | 1111 (76.7) | +0.11 [0.06; 0.16] | <0.001 | 2302 |
| Qualification of health professionals at ANCc | |||||
| Total, n | 728 | 1507 | |||
| Doctor, n (%) | 242 (33.5) | 230 (12.7) | −0.12 [−0.17; −0.07] | <0.001 | 2198 |
| Midwife, n (%) | 375 (52.0) | 1140 (78.0) | +0.12 [0.06; 0.17] | <0.001 | 2198 |
| Exam during ANCc | |||||
| Total, n | 728 | 1507 | |||
| Ultrasound, n (%) | 458 (62.3) | 1216 (81.1) | +0.09 [0.04; 0.14] | <0.001 | 2197 |
| Blood sample, n (%) | 494 (67.6) | 1302 (87.1) | +0.12 [0.07; 0.16] | <0.001 | 2197 |
| Urine sample | 564 (79.5) | 1366 (91.4) | +0.08 [0.03; 0.12] | <0.001 | 2198 |
| Blood pressure | 661 (89.9) | 1425 (94.5) | +0.04 [0.01; 0.08] | 0.009 | 2198 |
| FBD | |||||
| Total, n | 872 | 1528 | |||
| FBD, n (%) | 524 (60.8) | 1272 (84.3) | +0.15 [0.10; 0.19] | <0.001 | 2362 |
| Type of facility 3 | |||||
| Total, n | 501 | 1260 | |||
| Regional hospital | 353 (69.0) | 855 (65.7) | −0.02 [−0.08; 0.04] | 0.528 | 1733 |
| District health centre | 50 (11.3) | 167 (15.2) | +0.04 [0.00; 0.08] | 0.047 | 1733 |
| Local health post | 97 (19.4) | 238 (19.2) | −0.02 [−0.08; 0.04] | 0.514 | 1733 |
| Qualification of birth attendantd | |||||
| Total, n | 872 | 1528 | |||
| Skilled birth attendante | 551 (64.6) | 1260 (83.3) | +0.08 [0.04; 0.12] | <0.001 | 2362 |
| Doctor | 129 (14.4) | 176 (10.2) | −0.03 [−0.07; 0.01] | 0.180 | 2362 |
| Midwife | 370 (44.2) | 1006 (68.3) | +0.10 [0.06; 0.15] | <0.001 | 2362 |
| Mode of deliveryd | |||||
| Total, n | 524 | 1272 | |||
| C-section | 36 (8.4) | 71 (5.3) | −0.01 [−0.05; 0.03] | 0.563 | 1768 |
| Vaginal birth | 488 (91.6) | 1201 (94.7) | +0.01 [0.03; 0.05] | 0.563 | 1768 |
| PNCd | |||||
| Total, n | 524 | 1268 | |||
| Before leaving the facility | 430 (83.1) | 1094 (85.7) | 0.05 [−0.01; 0.10] | 0.105 | 1763 |
| After leaving the facility | 90 (16.4) | 264 (20.8) | 0.03 [−0.03; 0.09] | 0.353 | 1764 |
| Neonatal mortality | |||||
| Total, n | 872 | 1528 | |||
| Death <7 days, n (%) | 27 (3.6) | 37.2 (2.2) | −1% [−2%, 1%] | 0.510 | 2362 |
| Death <28 days, n (%) | 31 (4.0) | 41 (2.5) | −1% [−2%, 1%] | 0.514 | 2362 |
Risk differences between enrolled and non-enrolled women.
Among women whose exact ANC number is known.
Among women who had at least one ANC.
Among women who delivered at a facility.
Doctor, midwife or nurse.
Bold values indicate significant P-value.
ORI, obstetric risk insurance; ANC, antenatal care; FBD, facility-based delivery; PNC, postnatal care; ATE, average treatment effect.
Enrolment in ORI increases the probability of having at least one ANC by 13% (95% CI: 10–15%; P < 0.001) and the probability of having four or more ANCs by 11% (95% CI: 6–16%; P < 0.001). These consultations are 12% (95% CI: 6–17%; P < 0.001) more likely to be attended by a midwife and 12% (95% CI: −17 to −7%; P < 0.001) less likely to be attended by a doctor. Women with ORI benefit more often from a biological and ultrasound exam during their ANCs than women without ORI as follows: ultrasound (+9%; 95% CI: 4–14%; P < 0.001), blood test (+12%; 95% CI: 7–16%; P < 0.001), urine test (+8%; 95% CI: 3–12%; P < 0.001) and blood pressure check (+4%; 95% CI: 1–8%; P = 0.009).
Women who enrolled in ORI are 15% more likely (95% CI: 10–19%; P < 0.001) to give birth at a healthcare facility. Enrolment in ORI increases the likelihood of delivery at a health centre by 4% (95% CI: 0–8%; P = 0.047), delivery with qualified staff by 8% (95% CI: 4–12%; P < 0.001) and delivery with a midwife by 10% (95% CI: 6–15%; P < 0.001).
However, ORI enrolment was found to have no significant impact on the C-section rate or PNC.
Finally, concerning neonatal mortality, although the neonatal mortality rate was lower among the enrolled women (early and late neonatal mortality were 22/1000 and 25/1000 among the women who were enrolled, respectively, and 36/1000 and 40/1000 among those who were non- enrolled, respectively), no statistically significant effect of ORI enrolment was found.
Table 4 presents the reasons for non-enrolment among the 872 women who had heard about ORI but did not enrol. The main reasons were not listed during the interview (25%). Furthermore, 19% of these women said that they did not enrol in the ORI because the scheme is not available in their zone of residence. The remaining reasons included a lack of consideration of the importance of the ORI scheme (14%), poor information on the benefit package (12%), cost (12%) and distance (10%). About 4% of women indicated that the poor quality of the health services was a reason for non-adherence.
Table 4.
Reasons for non-enrolment in ORI among women who had heard about ORI (N = 872)
| Reasons | %a |
|---|---|
| Other (not in the list) | 24.5 |
| No ORI in the zone of residence | 19.1 |
| Do not consider ORI important | 14.2 |
| Poor information on the ORI | 12.2 |
| Costs are too high | 11.6 |
| Distance/transportation | 10.1 |
| Poor quality of health services | 4.3 |
| Do not know | 2.6 |
| Husband/family refused | 1.0 |
| No answer | 0.4 |
Percentages are adjusted based on the sampling weight, clustering and strata.
ORI, obstetric risk insurance.
Discussion
ORI benefits women who are enrolled by increasing their use of health services during pregnancy and childbirth without increasing hospital-based deliveries and C-section rates. These results are consistent with the primary objective of the ORI implementation in Mauritania, which is to increase access to maternal healthcare. However, we found no statistically significant effect of ORI on PNC or neonatal mortality.
A previous study that assesses the impact of ORI availability found that the scheme did not have an effect in general on the utilization of maternal health services except for delivery in local healthcare centres and qualified ANC (Philibert et al., 2017). Our results showed that availability of the ORI scheme is not sufficient and women have to enrol to improve the use of healthcare services during pregnancy and childbirth.
By increasing the number of prenatal visits, women significantly increased the use of a set of prenatal tests that are included in ORI (echography, blood and urine tests and blood pressure checks). A previous qualitative study showed that a main reason for women enrolling in the ORI is to benefit from a biological exam at a lower cost (Fauveau et al., 2018). Ultrasound was even qualified as a promotional tool for ORI enrolment as this examination is regularly and strongly demanded by pregnant women (Fauveau et al., 2018).
The effect of ORI enrolment on FBD was particularly apparent in terms of delivery at district health centres. This result confirms the findings of a previous study about this ORI (Philibert et al., 2017). Indeed, women who are enrolled in ORI at district health centres may prefer to deliver at the same centre to avoid additional payment if they should decide to deliver at a different healthcare facility, such as the referral hospital. This finding may also explain why only midwife-assisted deliveries have increased because midwives are first-line providers who attend births in these facilities. Moreover, the leading role of a health centre’s caregiver in decision-making may explain why the enrolled women are less likely to be referred to the hospital than non-enrolled women who preferentially go on their own to a hospital for delivery, taking into account the characteristics of the pregnancy or complications during the delivery. This finding may also explain why enrolled women are less likely to deliver by C-section than non-enrolled women (5.3% vs 8.4%; this difference is not statistically significant; P = 0.563). This unexpected effect on caesarean delivery is interesting and could potentially prevent an overuse of C-sections as is evident in other African countries (Kaboré et al., 2016). Moreover, women who enrolled in the ORI may not have the same potential risk of having a C-section. The fact that they have enrolled may also imply that they come earlier to the facility, thereby reducing the risk of a C-section.
We found that ORI enrolment has no impact on PNC. Thus, if women are attracted by ORI to perform ANC and delivery at a healthcare centre, once they leave the facility, they do not come back for postnatal visits any more often than those who are not enrolled. We show that only 16% of enrolled women and 21% of women who did not enrol return after leaving the facility for PNC. Interventions other than pre-payment schemes are needed to overcome the barriers to increasing PNC. These interventions may include improving the quality of postpartum care through strengthening postpartum services and care at facility and community levels (Duysburgh et al., 2015).
Although key intervention coverage rates, such as ANC attendance and skilled birth attendance, have been increasing among enrolled women, we found no statistically significant impact of ORI on neonatal mortality. This finding may be due to the fact that newborns with complications are born outside of healthcare facilities or the quality of care is not optimal. Another study on the fee subsidy policy in Burkina Faso also found no impact on neonatal mortality but increased FBD (Meda et al., 2018); thus, we suggest that a high quality of prepartum, intrapartum and postpartum care is essential for decreasing neonatal mortality, regardless of the availability of ORI. Although information regarding the quality of care is not available in our study, it is widely acknowledged that the quality of care provided to mothers and babies in most African countries falls short of current evidence-based practice (Kruk et al., 2018).
In our study, 37% of women who gave birth during the last 2 years were enrolled in ORI. A study using demographic data from 30 LMICs illustrated that more than one-third of interviewed people reported health insurance coverage in only four countries (Rwanda, Gabon, Ghana and Indonesia). However, these studies were interested in health insurance coverage and not pre-payment schemes. Furthermore, none of these schemes focused particularly on pregnancy and delivery care (Wang et al., 2014). We found no other similar schemes implemented in West Africa with which our results could be compared.
It is important to consider that 42% of the women in our study had never heard about ORI (>10 years after its implementation). We posit that if more women were aware of ORI, the enrolment rate could be greater, and the positive results found could be extended to more women. This hypothesis is consistent with a recent meta-analysis that suggested that knowledge and understanding of insurance and CBHI represent major enabling factors for enrolment (Dror et al., 2016).
Among the 21% of women who had heard of ORI but chose not to enrol, nearly 20% said it was because they believed that ORI was not available in their place of residence. The other reasons for non-adherence included a lack of understanding of ORI, poor information on the benefit package, excessively high costs and distance to the facility. These findings are consistent with the barriers to enrolment previously identified in the literature (Dror et al., 2016). Furthermore, a qualitative study has shown that health worker training on ORI is far from optimal when they are disseminating information about this scheme (Fauveau et al., 2018). We suggest extending ORI in Mauritania to improve the enrolment rate by increasing its availability and reducing the distances to health facilities by improving communication between facilities, improving transport systems or building new health facilities. As previously shown, improving communication regarding this scheme in both communities and health facilities where ORI is offered is highly important as increased information is a determinant of adhesion (Cofie et al., 2013; Ridde et al., 2018a).
This study has limitations. First, we studied only the impact of ORI among women who had heard about ORI because the question regarding their enrolment was asked only of this subgroup of women. To evaluate the impact of ORI, we should consider two comparable groups of enrolled and non-enrolled women without consideration of their individual characteristics. However, in the MICS carried out in Mauritania in 2015, the sample of enrolled women did not originate from random selection. To overcome this selection bias, we used matching based on the propensity score. The method we have chosen leads to the exclusion of some of the women from the matching sample (Supplementary Table S3). Indeed, the propensity scores of the women who were not enrolled were not close enough to those of the women who were enrolled. Therefore, they were not considered sufficiently comparable to be used as a control. These exclusions may impact the generalizability of the results.
In addition, the MICS only collects information regarding previous pregnancies within the previous 2 years for live births. We evaluated the impact of ORI on the neonatal mortality rate but not on the stillbirth rate, although these rates are high in Western Africa (Lawn et al., 2009).
Furthermore, the data resulting from these surveys are, by definition, based on the respondents’ statements. The quality of these data, therefore, depends primarily on the respondents’ knowledge of the health system as well as on their memory of what happened 2 years before the interview date—because women may have been being asked about a pregnancy that occurred 2 years ago (recall bias; Footman et al., 2015). Thus, the information concerning the person who performed the ANC, the exam received during these visits or the place of delivery may not have been exact. However, this recall bias likely did not differ between women who enrolled in the ORI and women who did not enrol and thus likely did not affect the estimation of the ORI effect.
Moreover, in this survey, only the type of place and not the exact place of birth is indicated. The institutions affiliated with the ORI scheme offered a standard set of services, although for those who were not affiliated with the ORI, the type of available care was not clear (e.g. whether all district health centres offer ultrasound was unknown).
Finally, we did not study who benefits from ORI and whether inequalities in healthcare utilization and neonatal mortality differ among women according to their enrolment. Further analyses are required to examine whether this pre-payment scheme is consistent with the principle of equity claimed by UHC.
Conclusions
This study provides evidence that a voluntary pre-payment scheme focusing on pregnant women improves healthcare service utilization during pregnancy and delivery without the overuse of these services, such as hospital-based delivery or C-section. However, an effect on PNC or neonatal mortality was not observed. We noted that only 42% of the women in our study had not heard about the ORI and suggest that the ORI and associated communication should be expanded to enrol more women and achieve a positive impact at the national level. However, a cost-effectiveness study of the ORI scheme is necessary before such an extension. The Mauritanian particularity is due to the wide area of the country and the low density of the population. It also seems important to consider access to healthcare facilities. Even if ORI were available throughout the country, not all women would be able to enrol.
Pre-payment schemes that focus on pregnant women could be considered a solution to achieving UHC and may allow all women to have access to maternal healthcare by removing financial barriers.
Supplementary data
Supplementary data are available at Health Policy and Planning online.
Supplementary Material
Acknowledgements
We thank the French Agency for Development (AFD) for funding the ORI scheme and the present evaluation. We especially thank Florent Bedecarrats for his important help and support throughout this study. We thank UNICEF for supporting the MICS in 2015 in Mauritania and the National Office of Statistics (NOS) for carrying out the survey and providing the data. We also thank the health workers involved in this study (midwives, nurses and doctors) for their input, as well as ORI partners in Mauritania, including the Ministry of Health and Regional Health Directorates, the Direction Régionale des Affaires Sociales (DRAS) and the Programme National de la Santé Reproductive (PNSR). This study was a part of the PhD work performed by Marion Ravit and was supported by the Université Pierre et Marie Curie (UPMC), France, and the Public Health Doctoral Network of the French School of Public Health (EHESP), France.
Conflict of interest statement. None declared.
Ethical approval. No ethical approval was required for this study.
References
- Abadie A, Drukker D, Herr JL. et al. 2004. Implementing matching estimators for average treatment effects in Stata. The Stata Journal: Promoting Communications on Statistics and Stata 4: 290–311. [Google Scholar]
- Abadie A, Imbens G.. 2002. Simple and Bias-Corrected Matching Estimators for Average Treatment Effects. No. t0283. Cambridge, MA: National Bureau of Economic Research. [Google Scholar]
- Abadie A, Imbens GW.. 2016. Matching on the estimated propensity score. Econometrica 84: 781–807. [Google Scholar]
- Alhassan RK, Nketiah-Amponsah E. et al. 2016. A review of the national health insurance scheme in Ghana: what are the sustainability threats and prospects? PLoS One 11: e0165151.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audibert M, Bonnet E, Dumont A, et al. 2019. Impacts du forfait obstétrical en Mauritanie sur l'offre, le recours et les inégalités d'accès aux soins - Synthèse du rapport final. Evaluation Ex-post (AFD) 79. [Google Scholar]
- Austin PC. 2009. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies McDonald KM (ed). Medical Decision Making 29: 661–77. [DOI] [PubMed] [Google Scholar]
- Austin PC. 2011. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharmaceutical Statistics 10: 150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennis I, De Brouwere V.. 2012. Fee exemption for caesarean section in Morocco. Archives of Public Health 70: 3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghi J, Ensor T, Neupane BD et al. 2006. Financial implications of skilled attendance at delivery in Nepal. Tropical Medicine and International Health 11: 228–37. [DOI] [PubMed] [Google Scholar]
- Borghi J, Hanson K, Acquah CA. et al. 2003. Costs of near-miss obstetric complications for women and their families in Benin and Ghana. Health Policy and Planning 18: 383–90. [DOI] [PubMed] [Google Scholar]
- Brookhart MA, Schneeweiss S, Rothman KJ et al. 2006. Variable selection for propensity score models. American Journal of Epidemiology 163: 1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugiavini A, Pace N.. 2016. Extending health insurance in Ghana: effects of the National Health Insurance Scheme on maternity care. Health Economics Review 6: 7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell OM, Graham WJ; Lancet Maternal Survival Series Steering Group. 2006. Strategies for reducing maternal mortality: getting on with what works. The Lancet 368: 1284–99. [DOI] [PubMed] [Google Scholar]
- Cofie P, De Allegri M, Kouyate B et al. 2013. Effects of information, education, and communication campaign on a community-based health insurance scheme in Burkina Faso. Global Health Action 6: 20791.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror DM, Hossain SS, Majumdar A et al. 2016. What factors affect voluntary uptake of community-based health insurance schemes in low- and middle-income countries? A systematic review and meta-analysis. PLoS One 11: e0160479.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont A, Philibert A, Ravit M, Dossa I, Bonnet E, Ridde V.. 2017. Impact du forfait obstétrical en Mauritanie: étude statistique à partir des données sociosanitaires de 2001 à 2011. Evaluation Ex-post (AFD) 66. [Google Scholar]
- Duysburgh E, Kerstens B, Kouanda S. et al. 2015. Opportunities to improve postpartum care for mothers and infants: design of context-specific packages of postpartum interventions in rural districts in four sub-Saharan African countries. BMC Pregnancy and Childbirth 15: 131.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elze MC, Gregson J, Baber U. et al. 2017. Comparison of propensity score methods and covariate adjustment. Journal of the American College of Cardiology 69: 345–57. [DOI] [PubMed] [Google Scholar]
- Falisse J, Meessen B, Ndayishimiye J et al. 2012. Community participation and voice mechanisms under performance‐based financing schemes in Burundi. Tropical Medicine & International Health 17: 674–82. [DOI] [PubMed] [Google Scholar]
- Fauveau V, Diarra A, Amar Z, Vinard P, Boillot F.. 2018. Evaluation du projet d'appui à l'extension de la forfait obstétrical en Mauritanie. Evaluation Ex-post (AFD) 74. [Google Scholar]
- Footman K, Benova L, Goodman C. et al. 2015. Using multi-country household surveys to understand who provides reproductive and maternal health services in low- and middle-income countries: a critical appraisal of the Demographic and Health Surveys. Tropical Medicine & International Health 20: 589–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler PJ, Martinez S, Premand P. et al. 2016. Impact Evaluation in Practice, Second Edition World Bank Publications: Washington, DC. United Nations Publications. 2016. Sustainable Development Goals Report 2016. UN: New York. [Google Scholar]
- Heckman JJ. 1977. Sample selection bias as a specification error (with an application to the estimation of labor supply functions). NBER Working Paper Series 172. [Google Scholar]
- Kaboré C, Ridde V, Kouanda S et al. 2016. Determinants of non-medically indicated cesarean deliveries in Burkina Faso. International Journal of Gynecology & Obstetrics 135: S58–63. [DOI] [PubMed] [Google Scholar]
- Kanya L, Obare F, Warren C et al. 2014. Safe motherhood voucher programme coverage of health facility deliveries among poor women in South-western Uganda. Health Policy and Planning 29: i4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassebaum NJ, Barber RM, Bhutta ZA. et al. 2016. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet 388: 1775–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk ME, Gage AD, Arsenault C. et al. 2018. High-quality health systems in the Sustainable Development Goals era: time for a revolution. The Lancet Global Health 6: e1196–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk ME, Mbaruku G, Rockers PC et al. 2008. User fee exemptions are not enough: out-of-pocket payments for ‘free’ delivery services in rural Tanzania. Tropical Medicine & International Health 13: 1442–51. [DOI] [PubMed] [Google Scholar]
- Kutzin J, Yip W, Cashin C.. 2016. Alternative financing strategies for universal health coverage In: World Scientific Handbook of Global Health Economics and Public Policy: Volume 1: The Economics of Health and Health Systems. Richard M Scheffler. World Scientific: UC Berkeley, 267–309. [Google Scholar]
- Lawn JE, Lee AC, Kinney M. et al. 2009. Two million intrapartum-related stillbirths and neonatal deaths: where, why, and what can be done? International Journal of Gynecology & Obstetrics 107: S5–19. [DOI] [PubMed] [Google Scholar]
- Meda BI, Dumont A, Kouanda S et al. 2018. Impact of fee subsidy policy on perinatal health in a low-resource setting: a quasi-experimental study. PLoS One 13: e0206978.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeyemi I, Nixon J.. 2013. Assessing equity in health care through the national health insurance schemes of Nigeria and Ghana: a review-based comparative analysis. International Journal for Equity in Health 12: 9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ONS. 2017. Enquête par Grappe à Indicateurs Multiples 2015 (MICS) --Mauritanie. Office National de la Statistique (ONS) -Mauritanie.
- Ouattara O, Ndiaye P.. 2017. Potentiel des mutuelles de santé la mise en œuvre de la Couverture Maladie Universelle au Mali et au Sénégal. Coordination MASMUT zone UEMOA.
- Philibert A, Ravit M, Ridde V. et al. 2017. Maternal and neonatal health impact of obstetrical risk insurance scheme in Mauritania: a quasi experimental before-and-after study. Health Policy and Planning 32: 405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick J, Jay J, Langer A.. 2014. Improving women’s health through universal health coverage. PLoS Medicine 11: e1001580.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudin P, Prual A, Vangeenderhuysen C et al. 2007. Ensuring financial access to emergency obstetric care: three years of experience with Obstetric Risk Insurance in Nouakchott, Mauritania. International Journal of Gynecology & Obstetrics 99: 183–90. [DOI] [PubMed] [Google Scholar]
- Richard F, Witter S, De Brouwere V.. 2010. Innovative approaches to reducing financial barriers to obstetric care in low-income countries. American Journal of Public Health 100: 1845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridde V. 2015. From institutionalization of user fees to their abolition in West Africa: a story of pilot projects and public policies. BMC Health Services Research 15: S6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridde V, Leppert G, Hien H, Robyn PJ, De Allegri M.. 2018a. Street-level workers’ inadequate knowledge and application of exemption policies in Burkina Faso jeopardize the achievement of universal health coverage: evidence from a cross-sectional survey. International Journal for Equity in Health 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridde V, Richard F, Bicaba A et al. 2011. The national subsidy for deliveries and emergency obstetric care in Burkina Faso. Health Policy and Planning 26: ii30–40. [DOI] [PubMed] [Google Scholar]
- Ridde V, Yaogo M, Zongo S et al. 2018b. Twelve months of implementation of health care performance‐based financing in Burkina Faso: a qualitative multiple case study. The International Journal of Health Planning and Management 33: e153–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB. 2001. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Services and Outcomes Research Methodology 2: 169–88. [Google Scholar]
- Rutstein SO, Johnson K.. 2004. The DHS wealth index. no. 6 DHS comparative reports6. [Google Scholar]
- Sachs JD. 2012. Achieving universal health coverage in low-income settings. The Lancet 380: 944–7. [DOI] [PubMed] [Google Scholar]
- Sahn DE, Stifel DC.. 2000. Poverty comparisons over time and across countries in Africa. World Development 28: 2123–55. [Google Scholar]
- Skordis-Worrall J, Pace N, Bapat U. et al. 2011. Maternal and neonatal health expenditure in Mumbai slums (India): a cross sectional study. BMC Public Health 11: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KV, Sulzbach S.. 2008. Community-based health insurance and access to maternal health services: evidence from three West African countries. Social Science & Medicine 66: 2460–73. [DOI] [PubMed] [Google Scholar]
- StataCorp L. 2013. STATA Treatment-Effects Reference Manual: Potential Outcomes/Counterfactual Outcomes Release 13. College Station, TX: A Stata Press Publication. [Google Scholar]
- Turcotte-Tremblay A-M, Spagnolo J, De Allegri M et al. 2016. Does performance-based financing increase value for money in low- and middle-income countries? A systematic review. Health Economics Review 6: 30.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDP (ed). 2016. Human Development for Everyone. New York: United Nations Development Programme. [Google Scholar]
- United Nations Publications. 2016. Sustainable Development Goals Report 2016. UN: New York. [Google Scholar]
- Wang W, Temsah G, Mallick L.. 2014. Health Insurance Coverage and Its Impact on Maternal Health Care Utilization in Low- and Middle-Income Countries. Rockville, MD: ICF International. [Google Scholar]
- Westreich D, Cole SR.. 2010. Invited commentary: positivity in practice. American Journal of Epidemiology 171: 674–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2015. Trends in maternal mortality: 1990-2015: estimates from WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division: executive summary. Geneva: World Health Organization. [Google Scholar]
- Witter S, Dieng T, Mbengue D et al. 2010. The national free delivery and caesarean policy in Senegal: evaluating process and outcomes. Health Policy and Planning 25: 384–92.. [DOI] [PubMed] [Google Scholar]
- World Health Organization (ed). 2010. Health Systems Financing: The Path to Universal Coverage. Geneva: WHO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates R. 2009. Universal health care and the removal of user fees. The Lancet 373: 2078–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.