Abstract
Animal models have been used to gain insight into the risk of noise-induced hearing loss (NIHL) and its potential prevention using investigational new drug agents. A number of compounds have yielded benefit in pre-clinical (animal) models. However, the acute traumatic injury models commonly used in pre-clinical testing are fundamentally different from the chronic and repeated exposures experienced by many human populations. Diverse populations that are potentially at risk and could be considered for enrollment in clinical studies include service members, workers exposed to occupational noise, musicians and other performing artists, and children and young adults exposed to non-occupational (including recreational) noise. Both animal models and clinical populations were discussed in this special issue, followed by discussion of individual variation in vulnerability to NIHL. In this final contribution, study design considerations for NIHL otoprotection in pre-clinical and clinical testing are integrated and broadly discussed with evidence-based guidance offered where possible, drawing on the contributions to this special issue as well as other existing literature. The overarching goals of this final paper are to (1) review and summarize key information across contributions and (2) synthesize information to facilitate successful translation of otoprotective drugs from animal models into human application.
I. INTRODUCTION
Noise-induced hearing loss (NIHL) is a major problem for active duty service members and veterans, as well as civilians. As per the preface to this special issue, the development of drugs that reduce or prevent NIHL is of significant interest, leading to the sponsorship of this special issue of the Journal of the Acoustical Society of America (JASA) by the Pharmaceutical Interventions for Hearing Loss (PIHL) group, which is housed under the umbrella of the U.S. Department of Defense (DoD) Hearing Center of Excellence (HCE) (see Le Prell et al., 2019). To facilitate insight into the drug development pathway, including aspects of the clinical test process, the series opened with detailed discussion of the drug development process by Cousins (2019). The rest of the articles included in this JASA special issue highlighted variation in the methodology used for pre-clinical (animal) testing of potential otoprotective drug agents, real-world noise and at-risk populations, and challenges associated with the translation from pre-clinical to clinical (human) test paradigms. Significant variability in pre-clinical test paradigms was revealed, and a lack of systematic data from many of the potentially at-risk populations was identified.
The overarching goal of this special issue was to provide a series of review papers addressing three specific themes. First, animal models used in noise injury research were reviewed, with particular emphasis on the species and noise injury models commonly used in otoprotection research. Second, real-world noise exposure and hearing loss observed in specific populations were reviewed in an effort to provide insight into the populations for whom pharmaceutical interventions might, or might not, be appropriate. Third, the factors that drive significant individual variability in humans were described; these factors decrease study power and confound study interpretations within human test paradigms.
With respect to pre-clinical study design, animal models routinely used to assess prevention of NIHL via investigational new drug agents most often use rodent models. Comprehensive discussion of rodent models was provided in papers discussing the mouse (Ohlemiller, 2019), rat (Escabi et al., 2019; Holt et al., 2019), chinchilla (Trevino et al., 2019; Radziwon et al., 2019), and guinea pig (Naert et al., 2019) as test species. A review of the use of non-human primates was also invited (Burton et al., 2019). Although non-human primates have not commonly been used in the assessment of otoprotective drug agents, they provide a useful model for the investigation of supra-threshold deficits. Moreover, the overall vulnerability of non-human primates to noise injury more closely parallels human vulnerability than rodent models; non-human primates and humans are both less vulnerable to NIHL than rodents. Additional papers within the first section of the special issue reviewed exposure paradigms commonly used in research laboratories, including exposures to impulse noise (Bielefeld et al., 2019), octave band noise (Gittleman et al., 2019), and blast exposure (Zhang, 2019).
Across the species-specific papers and noise-model papers, one of the major themes to emerge was significant reliance on models of acoustic trauma when initially assessing potential otoprotective agents in pre-clinical models. A second topic of discussion was the diverse measures used to assess noise injury in animal models. The two most common metrics are distortion product otoacoustic emissions (DPOAEs), which measure outer hair cell (OHC) function, and the auditory brainstem response (ABR), which is often used to measure the quietest tone levels that evoke a neural response. However, behavioral measures assessing both threshold and suprathreshold function have also been used in animal models.
In the second section of the special issue, the problems of real-world noise exposure and populations at risk for NIHL, who might be considered appropriate target populations for otoprotective therapies, were described. In an opening paper, Jokel et al. (2019) described the tremendous problem of noise exposure in the military. This was followed by the contribution from Hecht et al. (2019), which carefully discussed the challenges and ethics of investigating prevention of NIHL, including the prevention of temporary threshold shift (TTS). Noise exposure also commonly results in tinnitus, with or without NIHL, and thus the next paper in this series provided detailed discussion of the relationships between noise exposure and tinnitus in service members and veterans (Bramhall et al., 2019a). Relevant to both military and civilian populations is the issue of hazardous firearm noise exposure, which was discussed in two additional contributions presenting data on hazardous noise exposure and its mitigation at indoor (Murphy and Xiang, 2019) and outdoor (Wall et al., 2019) shooting ranges.
Firearm and other impulsive noise sources are well known to have the potential to result in acoustic trauma, but not all noise exposure is impulsive; the issue of non-impulse noise resulting in acoustic trauma was therefore discussed in the contribution by Berger and Dobie (2019). The remaining papers within section 2 largely discussed civilian populations exposed to repetitive noise. Workers exposed to occupational noise are a key at-risk population given their repetitive noise exposure; this population was discussed in detail by Themann and Masterson (2019). A second at-risk population of significant interest is music industry professionals repeatedly exposed to loud music; this at-risk population was discussed in detail by Wartinger et al. (2019). Finally, the issue of repetitive non-occupational noise exposure has emerged as a concern within the public health literature, and this was a topic of discussion as it impacts both adults (Neitzel and Fligor, 2019) and children (Roberts and Neitzel, 2019). Related discussions about the effects of non-occupational noise were provided in contributions from Feder et al. (2019) and Kamerer et al. (2019).
From the descriptions of populations that are potentially at risk for noise injury (section 2), it is readily apparent that the repetitive noise that causes slowly progressive changes in human hearing over time is systematically different from the acute traumatic noise exposure most often used in pre-clinical noise exposure models (section 1). Moving forwards, it is important to leverage the small number of chronic noise models described within the literature to more accurately mimic chronic, repeated human exposure during the development of new pre-clinical test paradigms. In parallel to increasing the real-world relevance of pre-clinical noise injury paradigms, investigators leading clinical trials will need to consider strategies for quantifying and recording information relevant to the variables that influence individual vulnerability, which were described in the third section of papers within this special issue.
It has long been known that some individuals develop more NIHL than others, despite common exposure histories. It is possible that some variation in hearing changes within workers in a given industry is related to specific shiftwork timing. Data from animal models have now firmly established that the time of day at which exposure occurs, relative to the circadian cycle, influences the degree of hearing loss observed (Fontana et al., 2019). Individual differences in vulnerability are likely also related to physical factors that differ from person to person, such as ear canal resonance (Grinn and Le Prell, 2019), sound-power transfer through the middle ear (Rosowski et al., 2019), and acoustic reflex strength (Deiters et al., 2019). Another non-modifiable factor that appears to influence vulnerability to NIHL (based on data from both animals and humans) is genetic variation (Clifford et al., 2019). Some risk factors are modifiable, however, such as hormone signaling (Shuster et al., 2019), inflammatory response (Frye et al., 2019), and nutrient intake (Spankovich and Le Prell, 2019). Additional data suggest that those workers that develop the most NIHL in early years continue to be more vulnerable to NIHL and they are thus also at increased risk for additional NIHL in later years (Cantley et al., 2019). There has been virtually no effort to account for any of these factors in clinical trials to date. Other factors that have not been commonly considered as part of the few clinical trials on NIHL prevention to date include extrinsic, environmental factors such as temperature, vibration, radiation, and exposure to chemicals and metals in the workplace, all of which have the potential to interact with noise and mediate hearing loss onset and progression. The remainder of this final discussion paper integrates and expands on the guidance offered across the contributions to this special issue with guidance organized according to study populations of potential interest.
II. NOISE-INDUCED HEARING LOSS (NIHL) IN THE MILITARY
NIHL is one of the most common injuries for Service members and one of the most common disabilities for veterans (Yankaskas, 2013; Gordon et al., 2017; Nelson et al., 2017; Swan et al., 2017); noise-induced tinnitus is also a major concern (Bramhall et al., 2019a). NIHL compromises the ability to detect and identify speech, particularly in noisy background conditions; thus, it can significantly impact operational readiness and fitness for duty (Tufts et al., 2009; Casto and Cho, 2012; Bevis et al., 2014; Semeraro et al., 2015; Sheffield et al., 2017). Because the U.S. Food and Drug Administration (FDA) has yet to approve any drugs for the purpose of preventing NIHL, or more broadly, acquired sensorineural hearing loss, the primary emphasis during hearing conservation efforts has been on hearing protection device (HPD) use (for additional discussion see Hecht et al., 2019; Jokel et al., 2019). The military hearing conservation program requires HPD use whenever noise exceeds 85 dBA (DoD Instruction 6055.12, 2010). Given the current regulatory language requiring HPD use, any drug developed for prevention of NIHL is likely to supplement, rather than replace, HPD use. Based on expectations that otoprotective drugs will supplement HPD use, it is worthwhile to understand the many ongoing efforts to improve functional performance while using HPDs.
A. Hearing protection devices
The important role of HPDs within hearing conservation programs in the military has driven systematic development of a series of tests assessing the impact of HPD use on sound detection, recognition/identification, localization, and communication (DRILCOM) (Lee and Casali, 2016, 2017, 2019). Electronic HPD products have significantly supplemented passive HPD products in recent years (Casali, 2010b,a); use of electronic HPD products may improve performance on some DRILCOM test elements (Robinson and Casali, 2003; Casali et al., 2009; Talcott et al., 2012; Clasing and Casali, 2014). Several long-term systematic efforts have sought to understand how passive HPD use (Lindeman, 1976; Chung and Gannon, 1979; Abel et al., 1980; Abel et al., 1982; Pekkarinen et al., 1990) and use of electronic HPD technology (Abel et al., 1991; Arlinger, 1992; Gower and Casali, 1994; Bockstael et al., 2011; Norin et al., 2011; Brown et al., 2015; Giguère et al., 2015; Hiselius et al., 2015; Giguère and Berger, 2016) impact speech understanding. Sound localization acuity during use of HPDs continues to be a topic of interest (Brungart et al., 2004; Brown et al., 2015; Joubaud et al., 2017), and the detection and identification of non-speech signals (Clasing and Casali, 2014) is of interest. Efforts are also ongoing to develop new education and outreach tools that support the correct and consistent use of HPDs by service members (Watts et al., 2018). As potential otoprotective agents begin to emerge, it will be important not only to assess threshold preservation, but also the preservation of sound detection, recognition/identification, localization, and communication ability; i.e., the suprathreshold functional assays contained within the DRILCOM test battery.
Although DoD instruction 6055.12 requires use of HPDs by service members exposed to hazardous sound levels (>85 dBA), command leadership may waive this requirement if mission success could be compromised (i.e., increased mortality, decreased lethality) due to factors such as the loss of situational awareness (DoD Instruction 6055.12, 2010). This has two critically important implications. First, continued advances are urgently needed in the design and adoption of electronic HPDs that preserve situational awareness and still protect hearing from dangerously high levels during military operations. Second, pharmaceutical agents that reduce or prevent cell death in the inner ear when HPDs are not worn, or do not provide adequate protection, are also urgently needed. With respect to dangerously high levels of impulse noise, which can cause immediate mechanical damage to the organ of Corti [see Bielefeld et al. (2019)], it will almost certainly be preferable to supplement HPDs with otoprotective agents, rather than replacing HPDs with otoprotective agents as unprotected exposure to military rifle discharge can cause immediate and permanent damage (Moon, 2007; Moon et al., 2011). Even with HPD use, rifles are a source of over-exposure for service members (Hecht et al., 2019; Jokel et al., 2019).
B. Firearm exposure
Rifles are typically fired in single or three-round bursts and sometimes in fully automatic mode. The combination of sound level suppressors with semi-automatic variants most often used by U.S. forces have been investigated to understand possible reductions in the hazards to shooters' hearing (Lobarinas et al., 2016; Meinke et al., 2017; Murphy et al., 2018). These reports supplement earlier literature more broadly discussing the hazards of noise exposure associated with various firearms (Kardous et al., 2003; Murphy and Tubbs, 2007; Meinke et al., 2013; Meinke et al., 2014; Lankford et al., 2016). In the current series of articles, noise abatement techniques that reduce exposure to hazardous firearm noise at both indoor (Murphy and Xiang, 2019) and outdoor (Wall et al., 2019) shooting ranges were reviewed and discussed. As well, the inclusion of the middle ear muscle contraction as an essential element within current and future damage risk criteria was examined in Deiters et al. (2019). The correspondence between animal models and human exposure is perhaps strongest for impulse noise research in animal models and human firearm users.
C. Animal models: Impulse noise
As reviewed by Bielefeld et al. (2019), there are a number of studies from animal models which support the potential for prevention of NIHL after impulse noise exposure using investigative drug agents (see also the comments from Zhang, 2019 regarding prevention of deficits secondary to blast exposure). The recent work by Chan et al. (2016) not only provides full noise-dose response curves for TTS and PTS with scaling factors provided for the transition from chinchilla to human, it also provides TTS recovery curves. After adjusting chinchilla shifts by a 28-dB scaling factor, there was a very good agreement between laboratory based chinchilla measurements and historic data from humans, with the chinchilla being more vulnerable than human (Chan et al., 2016). Data directly establishing differences in vulnerability across mammalian species are extremely limited. In their previous review and discussion of the limited literature, the NIOSH criteria document used a 20-dB adjustment to maximum unprotected peak exposure limits for impulse noise to account for differences between chinchillas and humans (NIOSH, 1998; see their discussion of ceiling limits for impulse noise, in their Sec. 3.2). While there are virtually no direct comparisons of vulnerability across species, as discussed by Gittleman et al. (2019), systematic review of the literature suggests the chinchilla is more vulnerable than both guinea pig and rat, with the rat being intermediate in vulnerability (more vulnerable than the guinea pig, less vulnerable than the chinchilla), at least for octave band noise exposures.
D. Clinical trials with firearm users: Outcomes and guidance
The very high sound exposure levels associated with firearm discharge have driven efforts to assess both the potential prevention of TTS observed to occur despite HPD use during short military training exercises (Le Prell et al., 2011; Lindblad et al., 2011), and the prevention of PTS associated with repeated exposure to firearm noise during multi-day firearm training experiences (Attias et al., 1994; Kopke et al., 2015; Campbell, 2016). Both the Le Prell et al. (2011) and Lindblad et al. (2011) studies were completed in partnership with the Swedish military during required firearm training exercises, with participants required to wear HPDs during training exercises whether or not they were enrolled in the clinical trials. There was little or no TTS in participants who took either placebo or active agents, precluding any significant insight into the potential for drug-based protection, in both of these investigations. Although neither clinical trial was successful in evaluating drug-mediated prevention of noise-induced injury, the lack of significant TTS in both study cohorts should be considered tremendously encouraging in that auditory injury was prevented by the correct and consistent use of HPDs during the firearm training exercises.
In contrast to the lack of TTS after short training exercises completed while using HPDs, permanent NIHL was observed in a subset of participants in the multi-day weapons training studies, despite the use of HPDs by participants (Attias et al., 1994; Kopke et al., 2015; Campbell, 2016). Three clinical trials assessing prevention of PTS associated with multi-day weapon training activities are summarized in Table I.
TABLE I.
Clinical trial design and outcomes in placebo cohorts in studies evaluating PTS prevention in service members.
| Attias et al. (1994) | Kopke et al. (2015) | Campbell (2016) | |
|---|---|---|---|
| Training description | 2 months military basic training; on average, each subject fired 420 shots from an M16 at a shooting range | 16 days of routine military noise during weapons training; every participant fired 325 M16 rounds during training; participants were also exposed to steady-state noise and simulated explosions | 2-weeks of Drill Sergeant Instructor training, including a minimum of 500 rounds of M16 weapons fire over an 11-day period |
| Sample size | N = 300 (Placebo, n = 150; Treated, n = 150) | N = 566 (Placebo, n = 289; Treated, n = 277) | N=318 (Placebo, n = 160; Treated, n = 158) |
| Investigational Treatment | 167 mg magnesium aspartate daily during 2 months of basic training | Three 900 mg dissolving effervescent tablets of N-acetylcysteine t.i.d., for a total daily dose of 2700 mg during first 13 days of weapons training; two 900 mg dissolving effervescent tablets of N-acetylcysteine b.i.d., for a total daily dose of 1800 mg during last 3 days of weapons training | Daily d-methionine during 3 days prior to exposure, 11 days of weapons training, and 4 days post-exposure for a total of 18 days per ClinicalTrials.gov record number NCT02903355; Daily dose not reported in Campbell (2016) or NCT02903355 |
| Primary STS Outcome | thresholds > 25 dB HL at one or more frequencies from 2 to 8 kHz | > 20 dB shift at any one test frequency, or >10 dB shift at any two consecutive test frequencies | > 20 dB shift at any one test frequency, or >10 dB shift at any two consecutive test frequencies |
| Rate of STS in left ear | Placebo: 21.5% | Placebo: 19.03% | Placebo: 8.33% |
| Treated: 11.2% | Treated: 21.30% | Treated: 6.78% | |
| P < 0.05 | P = 0.7816 | P = 0.4133 | |
| Rate of STS in right ear | Placebo: 28.5% | Placebo: 26.99% | Placebo: 9.02% |
| Treated: 11.2% | Treated: 20.94% | Treated: 7.69% | |
| P < 0.001 | P = 0.0562 | P = 0.4422 | |
| Rate of STS in either ear | Not reported | Placebo: 38.41% | Placebo: 15.38% |
| Treated: 36.82% | Treated: 13.91% | ||
| P = 0.3813 | P = 0.4439 | ||
| Rate of STS in both ears | Placebo: 11.5% | Placebo: 7.61% | Placebo: 2.31% |
| Treated: 1.2% | Treated: 5.42% | Treated: 0.88% | |
| P < 0.001 | P = 0.1877 | P = 0.3619 | |
| Rate of STS in trigger hand ear | Not reported | Placebo: 27.56% | Placebo: 8.27% |
| Treated: 21.56% | Treated: 6.84% | ||
| P = 0.0620 | P = 0.4276 | ||
| Rate of STS in non-trigger hand ear | Not reported | Placebo: 17.67% | Placebo: 9.09% |
| Treated: 21.56% | Treated: 7.63% | ||
| P = 0.8962 | P = 0.427 |
The first clinical trial shown in Table I, enrolling military recruits entering 2 months of basic training, was completed by Attias et al. (1994); here, the rate at which participants were observed to have thresholds greater than 25 dB hearing level (HL) at one or more frequencies from 2 to 8 kHz subsequent to basic training was calculated for each group. Because participants were required to have thresholds≤ 20 dB HL to enroll in the study, participants had ≥ 5 dB shift at one or more frequencies if they met the post-training criteria for hearing loss (thresholds >25 dB HL at one or more frequencies). Statistically significant decreases in the rate of right ear, left ear, and bilateral threshold elevations were reported for the treated condition.
The clinical trial by Kopke et al. (2015) enrolled U.S. Marine trainees at the U.S. Marine Corps Recruit Depot in San Diego CA; the clinical trial by Campbell (2016) enrolled Drill Sergeant instructor trainees at Fort Jackson. For both studies, the primary outcome was significant threshold shift (STS) defined as a threshold increase of 20 dB or greater at any test frequency, or an average increase of 10 dB or greater at any two consecutive test frequencies. Thus, the shift criteria was much larger than that used in the earlier investigation by Attias et al. (1994). This noise-induced STS criteria was selected to directly parallel ototoxic drug-induced change criteria, published by the American Speech-Language-Hearing Association (1994) and adopted by the American Academy of Audiology (2009). The groups were not statistically significantly different for the primary STS outcome measure in either study, although some secondary analyses assessing the rate at which smaller shifts or ear specific shifts were observed revealed statistically significant group differences (for complete reporting including additional analyses see Kopke et al., 2015; Campbell, 2016).
The clinical trial STS outcomes described above are very different from the gold standard metrics used in animal models, in which the absolute size of the threshold shift is compared across groups. Conclusions about drug efficacy in animal models are based on the observation of statistically significant decreases in mean PTS in experimentally treated animals relative to placebo controls (for review see Bielefeld et al., 2019). The success of any clinical trial is highly contingent on the use of primary outcome measures that are sensitive for detection and diagnosis of disease or injury in the control population. Given that large STS changes have occurred at relatively low rates in completed trials, it may be useful to compare average threshold shift for between group differences in future human clinical trials. Group comparisons for average threshold shift dependent variables are the typical convention that has been used in pre-clinical studies with animal models. If STS rates continue to be used as primary outcome measures, then the injury rates in control cohorts from the completed studies summarized in Table I should be carefully considered in combination with previous PIHL-sponsored guidance documents reviewing criteria for identification and monitoring of noise-induced TTS and PTS (Campbell et al., 2016) when selecting primary outcome measures for future clinical trials. Regardless of whether STS rate or average threshold shift is designated as the primary study outcome within the statistical analysis plan, it is possible to evaluate and report both STS rates and average threshold shift to more fully understand potential drug effects and guide future trial design (as in Kil et al., 2017).
E. Implications for clinical trials assessing populations exposed to impulse noise
It is possible that otoprotective agents that effectively reduce NIHL subsequent to impulse noise exposure in Service member populations will also be effective in other populations with firearm discharge exposure (i.e., police, sheriff, and other local law enforcement agencies requiring firearm training and qualification testing, or civilian populations exposed to firearm noise). Hearing loss is a common issue among recreational firearm users (Stewart et al., 2002), suggesting the potential that this population might be an appropriate target for interventions that supplement HPD use.
F. Standardization of pre-clinical impulse noise otoprotection paradigms
When populations with impulse noise exposure are the clinical target, pre-clinical testing of otoprotective agents using impulse noise based acoustic trauma paradigms are appropriate. The impulse noise used within laboratory settings should be modeled after firearm discharge, or other impulsive noise as appropriate based on the exposures to the population of interest. The review by Bielefeld et al. (2019) provides a comprehensive discussion of the characterization and use of impulse noise in laboratory studies including otoprotection assessments, and a number of other papers within this special issue comment at least briefly on the effects of impulse noise (Burton et al., 2019; Deiters et al., 2019; Escabi et al., 2019; Hecht et al., 2019; Holt et al., 2019; Jokel et al., 2019; Trevino et al., 2019; Naert et al., 2019; Ohlemiller, 2019; Radziwon et al., 2019). To illustrate the variability across impulse noise exposure models in otoprotection research paradigms, Table II provides a comprehensive list of impulse noise exposures used in otoprotection studies, with studies extracted from a systematic review by Hammill (2017). As observed in Table II, there is little consensus within either the pre-clinical or clinical research literature regarding standardized models for inducing acoustic trauma using impulse noise.
TABLE II.
Impulse noise exposure studies extracted from Hammill (2017), a systematic review of pharmaceutical interventions for noise-induced hearing loss investigations, excluding studies reporting blast or mixed exposures in the same recorded exposure. References are grouped by species to highlight study design variation not only within species but also across species.
| Reference | Species | Sample size (N) | Noise source | Sound level (NR = not recorded) | Duration (Time) | Duration (number of impulses) |
|---|---|---|---|---|---|---|
| Adelman et al. (2011) | Mouse | 57 | speaker system | 135 dB SPL peak | 1 min | 120 |
| simulated M16 rifle fire | 123 dB SPL peak | NR | NR | |||
| simulated M16 rifle fire in chamber | 155 dB SPL peak | NR | 10 | |||
| M16 fire - outdoor firing range | 155 dB SPL peak | NR | 10 | |||
| simulated M16 rifle fire | 155 dB SPL peak | NR | 700 | |||
| Duan et al. (2004) | Rat | 38 | speaker system | 160 dB SPL peak | NR | 50 |
| Hight et al. (2003) | Chinchilla | 60 | US Army M-16A1 rifle, 5.56 caliber round simulated fire | 145 dB SPL | NR | 100 |
| Harris et al. (2005) | Chinchilla | 48 | speaker system | 155 dB SPL | NR | 150 |
| Kopke et al. (2005) | Chinchilla | 18 | simulated M-16 rifle fire | 155 dB SPL peak | 2.5 min | 150 |
| Bielefeld et al. (2007) | Chinchilla | 46 | simulated gun fire (Speaker system) | 155 dB SPL | 75 s | 150 |
| speaker system | 123 dB SPL peak | 2 h | NR | |||
| Coleman et al. (2007) | Chinchilla | 48 | simulated M-16 rifle fire | 155 dB SPL peak | NR | 150 |
| Bielefeld et al. (2011) | Chinchilla | 17 (28 ears) | speaker system | 155 dB SPL peak | NR | 150 |
| Bielefeld (2013) | Chinchilla | 18 | simulated M-16 gunfire | 155 dB SPL peak | 0.1 s | 150 |
| Fetoni et al. (2014) | Chinchilla | 27 | speaker system | 155 dB SPL peak | 78.5 s | 150 |
| Haupt and Scheibe (2002) | Guinea pig | 55 | speaker system | 167 dB SPL peak | 38 min | 2280 |
| Scheibe et al. (2002) | Guinea pig | 104 | speaker system | 167 dB SPL peak | 38 min | 2280 |
| Attias et al. (2003) | Guinea pig | 25 | speaker system | 167 dB SPL peak | 1 min | 60 |
| Franzé et al. (2003) | Guinea pig | 72 | speaker system | 114 dB SPL | 2 h | NR |
| 5 h | NR | |||||
| Haupt et al. (2003) | Guinea pig | 26 | speaker system | 167 dB SPL peak | 4 min | 240 |
| Zhai et al. (2004) | Guinea pig | 20 | electronic-fire impulse generator (Shanghai Co.) | 172 dB SPL peak | NR | 100 |
| Sendowski et al. (2006) | Guinea pig | 32 | FAMAS F1 rifle gunshot | 170 dB SPL peak | NR | 3 |
| Heinrich et al. (2008) | Guinea pig | 54 | speaker system | 90 dB SPL | 1 h | |
| Abaamrane et al. (2009) | Guinea pig | 65 | blank FAMAS F1 rifle shot | 170 dB SPL peak | NR | 3 |
| Zhou et al. (2009) | Guinea pig | 55 | electric spark-gap impulse noise generator | 165 dB SPL peak | 2 s | NR |
| Abaamrane et al. (2011) | Guinea pig | 60 | FAMAS F1 rifle shots | 170 dB SPL peak | NR | 3 |
| Chi et al. (2011) | Guinea pig | 74 | an electric spark generator | 167 dB SPL peak | NR | 80 |
| Kansu et al. (2011) | Guinea pig | 21 | shooting range of the police department | 136 dB SPL peak | NR | 100 |
| Xiong et al. (2011b) | Guinea pig | 36 | 7.62 mm Chinese Army 81-1 type of assault rifle | 176 dB SPL | 1 s | 15 |
| Xiong et al. (2012b) | Guinea pig | 45 | 7.62 mm Chinese Army 81-1 type of assault rifle | 176 dB SPL | 1 s | 15 |
| Xiong et al. (2012a) | Guinea pig | 50 | 7.62 mm Chinese Army 81-1 type of assault rifle | 176 dB SPL | 1 s | 15 |
| Xiong et al. (2015) | Guinea pig | 30 | 7.62 mm Chinese Army 81-1 type of assault rifle | 176 dB SPL peak | NR | 15 |
| Müller et al. (2016, 2017) | Guinea pig | 225-270 | speaker system | 142 dB SPL | NR | 15 |
| 30 | ||||||
| 45 | ||||||
| 60 | ||||||
| 120 | ||||||
| Pilgramm and Schumann (1985) | Human | 122 | NR | NR | NR | NR |
| Markou et al. (2001) | Human | 72 | hand weapons; heavy weapons | NR | NR | NR |
| Markou et al. (2004) | Human | 108 | fire arm noise | NR | NR | NR |
| Suckfuell et al. (2007) | Human | 11 | firecrackers | NR | NR | NR |
| Psillas et al. (2008) | Human | 52 | G3 Rifle, 7.62 mm | NR | NR | NR |
| Le Prell et al. (2011) | Human | 31 | Ksp-58 automatic machine gun during military bunker training | 156 dB SPL peak | 1 min | 40 |
| Lindblad et al. (2011) | Human | 34 | Ksp-58 automatic machine gun during military bunker training, shot between two people | 165 dB SPL | NR | 40 |
| Xiong et al. (2011a) | Human | 75 | 7.62 mm Chinese Army 81-1 type assault rifle | NR | NR | NR |
| Zhou et al. (2013) | Human | 53 | fireworks | NA | NR | NR |
| Kopke et al. (2015) | Human | 634 | M-16 weapons fire | NR | 13 days | ∼325 rounds |
G. Non-impulsive exposures in the military
Other military populations should be considered as potential clinical trial populations, in addition to the soldiers enrolled in weapons training with small-caliber firearms as described above in Table I. Jokel et al. (2019) described the myriad of noise exposures that service members face during training and deployment; this discussion was significantly expanded by Hecht et al. (2019), who discuss enrollment of such noise-exposed individuals in clinical trials. When designing clinical trials with Service member participants, it is incumbent on the study team to understand the complete exposure of service members across duties and environments and potential difficulties achieving “noise-free” pre-testing windows to minimize the contributions of TTS to any measured deficits. Moreover, any potential operational impact must be carefully identified and mitigated for participation to be possible (for additional discussion see Hecht et al., 2019).
III. NOISE-INDUCED HEARING LOSS (NIHL) IN THE WORKPLACE
The World Health Organization (WHO) uses the pure-tone-average (PTA) threshold at the frequencies of 1, 2, 3, and 4 kHz (PTA1234), and specifies a criteria of PTA1234> 41 dB HL to define disabling hearing loss. Efforts to quantify the contributions of occupational noise exposure to disabling hearing loss suggest that worldwide, some 16% of disabling hearing may be attributable to occupational noise exposure (Nelson et al., 2005; see also the recent review by Graydon et al., 2019). The prevalence of disabling hearing loss due to occupational noise varies geographically, from about 7% to 21%, with developed countries such as the U.S. being at the lower end of this range (Nelson et al., 2005). A major limitation of this definition as that it is indicative of disability. It is now well known that the pure tone audiogram is not sensitive to inner hair cell loss (Lobarinas et al., 2013) or synaptic pathology (Kujawa and Liberman, 2009). Moreover, there are both persons with lower thresholds (less hearing loss) that nonetheless report significant hearing difficulty as well as persons with higher thresholds (more hearing loss) that deny having any hearing issues when questioned about hearing ability.
In this special issue, Themann and Masterson (2019) described tremendous differences in both exposure and hearing loss prevalence across civilian populations exposed to diverse occupational noise hazards, and they provided a comprehensive review of the critical public health problems associated with occupational noise exposure. This review builds on work that has specifically looked at the prevalence of NIHL in workers, which could perhaps be used to guide the selection of primary outcome measures in studies assessing NIHL prevention in workers exposed to occupational noise.
A. Pure tone average threshold greater than 25 dB HL at 1, 2, 3, and 4 kHz in either ear (PTA1234 ≥ 25 dB in either ear)
Masterson et al. (2015) analyzed the prevalence of hearing loss using the criteria of pure-tone average thresholds of 25 dB HL or greater at 1, 2, 3, and 4 kHz (PTA1234 ≥ 25 dB in either ear) by time period and industry sector. For the relatively recent period of 2006–2010, the hearing loss prevalence in many industry sectors was around 20% although for the Mining and Construction sectors, hearing loss prevalence was 25%. What is interesting is that the risk of incident hearing loss decreased by roughly 50% over that same period. Whereas prevalence data document the proportion of cases present at given time, incidence data provide insight into new cases; thus, the data from Masterson et al. (2015) document decreases in the rate at which new hearing loss cases are being detected.
From a clinical trial design perspective, it is critically important to know the expected prevalence of hearing loss using PTA1234 ≥ 25 dB in either ear is about 20%–25% depending on the industry of interest, and that the incidence of new hearing loss cases is decreasing, which will decrease study power for the detection of drug-mediated reductions in new hearing loss cases. In other words, if fewer workers are developing new hearing loss injuries, study sample sizes will need to be increased to provide adequate power for the detection of a potential drug-mediated decrease in new NIHL injuries.
Decreasing incidence of new NIHL cases raises significant questions about the utility of existing data sets, such as the noise-induced permanent threshold shift (NIPTS) data for 10th (least vulnerable), 50th (median), and 90th (most vulnerable) percentile populations at frequencies of 0.5, 1, 2, 3, 4, and 6 kHz which are printed in ISO-1999 (International Standard Organization, 2013). This standard includes tables summarizing the total hearing loss due to noise exposure after allowing for the effects of age, for workers exposed for 10, 20, 30, and 40 years to 8-h A-weighted sound exposure levels of 85 dBA (see their Table D.1), 90 dBA (see their Table D.2), 95 dBA (see their Table D.3), and 100 dBA (see their Table D.4).
Although these are among the most systematic data available, they do have some shortcomings. First and foremost, the recent report by Lempert (2019) notes that ISO 1999 does not very closely predict the patterns of hearing loss observed in either the Passchier-Vermeer (1968) or the Burns and Robinson (1970) reports. In addition, there is the issue that more recent generations appear to have better hearing than previous generations (Hoffman et al., 2017). Both generational differences (Hoffman et al., 2017) and decreased incidence of new NIHL injuries (Masterson et al., 2015) could explain at least in part why hearing loss in various worker populations assessed more recently has sometimes differed from that expected based on the ISO-1999 tables (Leensen et al., 2011; Leensen and Dreschler, 2015; Lie et al., 2016). Another factor influencing the value of the ISO-1999 tables for prediction of NIPTS was discussed by Dobie (2015b), who noted that the ISO 1999 tables were generated using data from studies conducted in the 1950s and 1960s, primarily enrolling American and European workers prior to the onset of governmental regulations limiting occupational noise exposure. Effects of ethnicity on vulnerability to NIPTS or age-related hearing loss (ARHL) would significantly confound predictive value as well, as these NIPTS data tables have had expected effects of aging subtracted out, in an effort to predict the effects of noise after accounting for aging. Indeed, significant ethnicity effects have been found within datasets related to ARHL, raising questions about the comparison of data from largely white populations to workers of other ethnicities (Deiters and Flamme, 2019; Flamme et al., 2019).
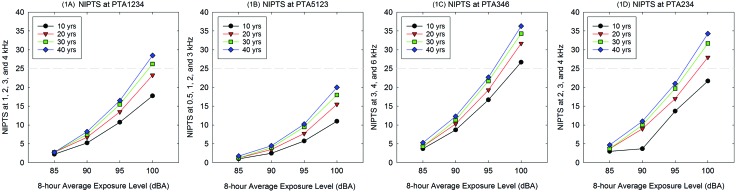
Although there are notable shortcomings, because the ISO-1999 data are the most systematic data available, the median NIPTS calculated using PTA1234 thresholds for workers within the ISO-1999 audiometric data are shown in Fig. 1(A) to illustrate median expected NIPTS for interested readers. As evident in Fig. 1(A), the median NIPTS will exceed 25 dB HL for PTA1234 only under the most extreme exposure conditions (30–40 years of exposure to 100 dBA noise levels). Although NIPTS ≥ 25 dB HL (i.e., dashed gray line) may not be a particularly sensitive clinical trial metric, NIPTS of 10 dB or greater is clearly expected for workers that are exposed to 95 dBA TWA or greater. The availability of populations with such exposures to participate in clinical trials should be extremely limited, however, as permissible exposure limits are set at 90 dBA TWA for most industries.
FIG. 1.
Noise-induced permanent threshold shift (NIPTS) is shown for pure-tone-average (PTA) thresholds at frequency combinations of 1, 2, 3, and 4 kHz (1 A), 0.5, 1, 3, and 3 kHz (1B), 3, 4, and 6 kHz (1 C), and 2, 3, and 4 kHz (1 D). All NIPTS PTA data are calculated using the single frequency shift data listed in ISO-1999 tables. Data are median NIPTS; those at the 10th percentile show smaller changes and those at the 90th percentile show larger changes.
B. Pure tone average threshold at 0.5, 1, 2, and 3 kHz (PTA5123) greater than 25 dB HL in either ear (PTA5123 ≥ 25 dB in either ear)
Other pure-tone threshold averages (PTAs) could also be designated for use as primary outcome measures. For example, the American Academy of Otolaryngology [AAO; now, the American Academy of Otolaryngology – Head and Neck Surgery (AAO-HNS)] advocates a formula for calculating hearing impairment based on PTA thresholds at the 0.5, 1, 2, and 3 kHz frequencies (PTA5123). The rationale for the selection of this PTA is the observation of correlations between speech-in-noise test outcomes and PTA5123 (Dobie, 2015a). The AAO-HNS specifies a low fence of 25 dB HL, and PTA thresholds above 25 dB HL accrue impairment at a rate of 1.5% impairment for each dB above 25 dB HL (American Academy of Otolaryngology Committee on Hearing and Equilibrium and American Council of Otolaryngology Commitee on the Medical Aspects of Noise, 1979). Based on this, it may be reasonable to propose using PTA5123 ≥ 25 dB HL in either ear as a primary outcome measure, however, PTA5123 will be even less likely to be affected by noise exposure than PTA 1234, given that noise predominantly affects the frequencies of 3, 4, and 6 kHz. Thus, the prevalence of hearing loss greater than 25 dB HL may be even less than the 20%–25% noted above for PTA1234 ≥ 25 dB HL.
The median NIPTS calculated using PTA5123 thresholds for workers within the ISO-1999 audiometric data tables are shown in Fig. 1(B); the median expected NIPTS for PTA5123 is less than that shown for PTA1234 [Fig. 1(A)]. As evident in Fig. 1(B), the median NIPTS does not exceed 25 dB HL for PTA5123 even under the most extreme exposure conditions (30–40 years of exposure to 100 dBA noise levels). Although NIPTS ≥ 25 dB HL (i.e., dashed gray line) would not be a particularly sensitive clinical trial metric, NIPTS of 10 dB is possible for workers that are exposed to 95 dBA TWA or greater. As previously noted, 95 dBA TWA exposures are not permitted under national occupational regulations.
C. Pure tone average threshold at 3, 4, and 6 kHz (PTA346)
Noise exposure predominantly affects the frequencies of 3, 4, and 6 kHz, typically but not always in a notched configuration with poorer hearing at 3, 4, and/or 6 kHz, relative to 0.5, 1, and 2 kHz, and 8 kHz (Niskar et al., 2001; Flamme et al., 2014). Consequently, both PTA5123 and PTA1234 will significantly underestimate the total impact of noise on hearing in workers exposed to occupational noise, relative to PTA346. The median NIPTS calculated using PTA346 thresholds for workers within the ISO-1999 audiometric data is shown in Fig. 1(C); median expected NIPTS for PTA346 is greater than that observed at PTA1234 [Fig. 1(A)] or PTA5123 [Fig. 1(B)]. As evident in Fig. 1(C), the median NIPTS exceeds 25 dB for PTA346 within the first ten years of exposure to 100 dBA TWA noise levels. Given that HPDs are mandatory at those sound levels, it is worth note that changes of 10 dB or greater are observed beginning at 90 dBA TWA exposure levels; 90 dBA TWA is the maximum exposure level permitted within the workplace without HPDs under OSHA regulations (OSHA, 1983). Under these regulations, HPDs must be provided beginning at 85 dBA TWA exposure, and HPDs must be worn by workers who have experienced an STS beginning at 85 dBA TWA. Workers who have not experienced an STS cannot exceed 90 dBA TWA in the workplace.
D. Change in pure tone average threshold at 2, 3, and 4 kHz > 10 dB (ΔPTA234 > 10 dB)
OSHA defines significant threshold shift (STS) using an average change at 2, 3, and 4 kHz of 10 dB or greater (ΔPTA234 > 10 dB). Figure 1(D) illustrates median NIPTS using PTA234 thresholds in workers with various exposure histories calculated from ISO-1999 median data. From these panels, it is clear that PTA234 will reveal greater NIPTS than PTA1234 [Fig. 1(A)] and PTA5123 [Fig. 1(B)], with slightly less sensitivity for change than PTA346 [Fig. 1(C)]. If selecting a criteria for change, for documenting differences in the prevalence of STS due to an experimental drug therapy, one could consider ΔPTA234 > 10 dB based on OSHA's definition of STS as an average change of 10 dB or greater. Comparing across the 10-year increments shown in Fig. 1(D), one can see that with exposures of 85 dBA, median NIPTS is 5 dB or less, even after 40 years, but with exposures of 100 dBA, the median NIPTS will increase from 20 dB (at 10 years of exposure) to 35 dB (at 40 years of exposure). With 90 dBA TWA exposure, the maximum permitted exposure, average changes of 10 dB at 2, 3, and 4 kHz emerge between 10 and 20 years of exposure. Understanding workplace sound levels and the use of HPDs in the workplace will be critical when trying to predict NIPTS in workers such that prevention of NIHL using pharmaceuticals can be considered as a study goal.
E. Threshold shift
As discussed above, animal models have routinely employed measurements of threshold shift, rather than comparing the prevalence of hearing loss meeting specific criteria. The average threshold shift at PTA5123, PTA1234, PTA346, or PTA234 could be evaluated in placebo and experimentally treated workers, instead of or in addition to assessing prevalence of a criterion threshold shift. The size of the group difference provides important additional information about the potential benefits of the drug agent.
F. Workplace threshold records
Longitudinal threshold data likely exist at many potential workplace study sites, allowing studies to be adequately powered based on review of historic data from untreated populations. Unfortunately, however, data regarding historic exposure levels, and changes in sound level as equipment is replaced, may not be readily available for every population. Moreover, even if detailed noise histories were maintained, historic data regarding correct and consistent wearing of HPDs is likely to be limited, as fit-testing of HPDs has not been broadly embraced across industry at this time. Fit-testing is a process through which individual user-achieved attenuation can be directly measured to determine the effectiveness of HPD use (Voix and Hager, 2009; Schulz, 2011; Murphy et al., 2016). Taken together, some caution in reliance on historic hearing loss prevalence data are required during the identification of possible clinical trial populations. An additional important caveat is that while historic data should be available and informative during development of the statistical analysis plan, investigators should work with their IRB to determine what permissions are necessary prior to systematic review of historic records. Historic threshold shift could be assessed as a metric for success of the hearing conservation program with few ethical concerns; however, reporting of this systematically analyzed data in grant applications or publications may not be possible in the absence of IRB approval.
G. Clinical trials in workers
Because NIHL in workers exposed to occupational noise typically accrues slowly over years, efforts to assess the potential drug-mediated prevention of hearing loss that occurs despite HPD use in the workplace have largely been based on prevention of TTS at the end of the work shift (Lin et al., 2010; Doosti et al., 2014). However, in these studies, temporary changes in hearing were on the order of 1–3 dB in both the participants who took placebo and those receiving active agents, and thus the protective benefits were very small, even when statistically significant, largely precluding any insight into the potential for drug-based protection against more significant acoustic exposure. For studies assessing prevention of PTS in workers, several possible outcome measures were noted above, with PTA346 assumed to be the most robust metric given the largest expected changes in hearing occur at these frequencies [see Fig. 1(C)].
A final criterion that could be considered is ASHA (American Speech-Language-Hearing Association) STS, which has already served as the primary outcome in two clinical trials enrolling service members as discussed above (Kopke et al., 2015; Campbell, 2016). Although this metric has been used previously, the development of STS meeting ASHA criteria (20 dB shift at one frequency or 10 dB shift at two adjacent frequencies) seems unlikely to be observed within many occupational cohorts. The NIPTS changes documented in ISO-1999 (International Standard Organization, 2013) generally do not meet the ASHA STS criteria as the changes in threshold at individual frequencies tend to be small even when time intervals are compared in 10-year increments. Although we did not plot single frequency data here, median NIPTS at 4 kHz with 85 dBA TWA exposure increases from 5 dB HL after 10 years of exposure to 7 dB after 40 years of exposure (International Standard Organization, 2013). Even for the most vulnerable 10% of the population, NIPTS at 4 kHz with 85 dBA TWA exposure increases from 7 dB HL after 10 years of exposure to 9 dB after 40 years of exposure. With 90 dBA TWA, the median NIPTS at 4 kHz increases from 11 dB HL after 10 years of exposure to 15 dB after 40 years of exposure (International Standard Organization, 2013), and for the most vulnerable 10% of the population, NIPTS at 4 kHz with 90 dBA TWA exposure increases from 15 dB HL after 10 years of exposure to 20 dB after 40 years of exposure. In other words, NIPTS reaching 20 dB at individual frequencies may be uncommon. This is not to say that total hearing loss occurring as consequence of both aging and noise will not reach 20 dB. If the effects of aging are considered, it should be noted that OSHA age correction tables point to expected ARHL increasing from 5 dB (20 year old male) to 33 dB (60 year old male) at the frequency of 4 kHz (see Appendix F in OSHA, 1983).
H. Threshold clinical trial metrics: Extended high frequency audiometry
In addition to threshold assessments within the conventional testing range of 250 Hz to 8 kHz, threshold measurements can be made at higher frequencies. Thus, another possible metric that could be considered is monitoring of threshold shift in the extended high frequency (EHF) range, which includes frequencies above 8 kHz. A variety of data show changes in EHF thresholds as a function of occupational noise exposure (Hallmo et al., 1995; Borchgrevink et al., 1996; Korres et al., 2008; Riga et al., 2010; Mehrparvar et al., 2014). The contribution by Kamerer et al. (2019) carefully discusses the importance of EHF testing and the interpretation of the observed relationships between EHF threshold sensitivity and the amplitude of sound evoked auditory potentials. The contribution by Wartinger et al. (2019) further provides discussion of changes in EHF hearing in musicians and other performing artists. Although the data is still emerging, a variety of data suggest potential deficits.
I. Non-threshold clinical trial metrics: Otoacoustic emissions
There has been significant discussion of the use of distortion product otoacoustic emissions (DPOAEs) as a clinical trial metric, as these reveal changes in the function of the outer hair cells (OHCs). The use of serial DPOAE tests to monitor OHC function during treatment with ototoxic medications is well established (Reavis et al., 2008; Dille et al., 2010; Reavis et al., 2011; McMillan et al., 2012) and serial DPOAE monitoring has been proposed for use both in hearing conservation programs (Konrad-Martin et al., 2012) and clinical trials (Konrad-Martin et al., 2016). Changes in DPOAE amplitude have been widely reported as a function of occupational noise (Seixas et al., 2004; Korres et al., 2009; Seixas et al., 2012; Boger et al., 2017). The contribution by Bramhall et al. (2019a) carefully discusses the importance of DPOAE testing and the interpretation of the observed relationships between DPOAE amplitude and the amplitude of sound evoked auditory potentials. A second type of otoacoustic emission worth mentioning here is the transient evoked otoacoustic emission (TEOAE) as the linear reflective component which dominates the TEOAE may be more sensitive to subtle cochlear pathology; data comparing the sensitivity of DPOAE and TEOAE metrics continue to emerge (Fraenkel et al., 2003; Sisto et al., 2007).
The recent guidance paper from the National Occupational Research Agenda (NORA) Hearing Loss Prevention Cross-Sector Council (2019) calls for research investigating the most sensitive and specific protocols for possible use in monitoring of DPOAE and EHF thresholds in workers exposed to occupational noise. In addition, the forthcoming chapter by Le Prell and Campbell (2019) discusses the potential use of both DPOAE and EHF metrics in clinical trials assessing potential otoprotective benefits of new drug agents in clinical trials.
J. An additional confounding factor: The impulsivity of noise (kurtosis)
In considering the factors that influence hearing loss in the workplace, one of the factors that should be considered is kurtosis, which is a measure of the impulsivity of noise, meaning the extent to which rapid temporal fluctuation in level is present. Exposure to impulsive noise has been demonstrated to result in greater amounts of hearing loss both in animals and in the human (Dunn et al., 1991; Zhao et al., 2010). While Earshen (1986) first proposed using the kurtosis metric to distinguish between noise exposures that contain various impulsive noise (impact and impulse), the most robust systematic research in this area was launched by Hamernik et al. (2003). Hamernik et al. (2003) completed a series of noise exposures in chinchillas where they varied the kurtosis of the exposure by adjusting the probability of an impulse occurring during a range of exposures, while keeping the total sound energy equivalent across exposures. These studies demonstrated that for a given noise energy, PTS increased with increasing kurtosis and then plateaued.
In addition to continuing accumulation of data from animal studies, this body of work has also been extended to human exposures. In a large employee population with evaluation of more than 1500 Chinese workers with a mixture of Gaussian and non-Gaussian (higher kurtosis) exposures, kurtosis has been carefully assayed for relationships with NIHL (Zhao et al., 2010; Davis et al., 2012; Xie et al., 2016). When the lifetime cumulative noise exposure is adjusted for kurtosis, estimated from full-shift recordings that are surrogates for the individual workers' exposures, the rate of hearing loss between the two exposure groups agrees well. That is, adjustment for kurtosis brings the disparate rates of hearing loss across individuals into agreement. Suter (2017) noted that some investigations recommended a 5 to 10 dB exposure penalty for more impulsive compared to continuous noise exposures. Adjustments based on kurtosis can increase the risk in a manner tailored to the noise exposure—greater kurtosis may require a larger adjustment (Lei et al., 1994).
If a clinical trial were to incorporate workers with different job titles and different patterns of exposure, it would be important to understand not only the average noise dose, which is based on time-weighted average exposure level, but also kurtosis adjusted exposure, so that workers could be stratified as a function of high kurtosis (higher risk) and low kurtosis (lower risk) exposure characteristics. In addition to considering kurtosis as a potential variable for stratification purposes, to assure equal numbers of experimentally treated participants within risk groups, one might also wish to stratify based on the amount of hearing loss already accrued. This is relevant not only to clinical trial design, but also to primary prevention efforts.
In this special issue, Cantley et al. (2019) suggest early identification and intervention is especially needed when annual testing indicates a rapid rate of hearing loss in the early part of a worker's career, as these same workers exhibit a more significant or substantial loss in later years. In other words, they report that workers with increased vulnerability in early work years appear to have increased vulnerability in later years as well. If workers with increased risk can be identified prior to the development of significant hearing loss, various interventions can be considered. As discussed in Le Prell and Spankovich (2013), primary intervention occurs before exposure to the hazard and can be either passive or active. An example of a passive protective intervention is engineering controls that attenuate sound levels; this is a passive protective strategy as no action is necessary on the employee's part. An example of an active prevention strategy is reliance on HPDs; consistent use of HPDs requires action by the employee. Interventions such as engineering controls and HPD use are labeled secondary interventions when they are implemented after hearing loss has already been observed, and the goal is prevention of additional hearing loss.
K. Consideration of HPD use as a confounding factor
The high rate of NIHL across workers has driven various efforts to reduce NIHL through engineering, administrative, and personal protection equipment use over many years (Kerr et al., 2017). In designing a clinical trial, the use of HPDs must therefore be considered as part of the study design and analysis, as noted above as part of the discussion of clinical trials enrolling service members. Here, it should be noted that the U.S. occupational regulations (OSHA, 1983) require HPD use when sound exposure exceeds the permissible exposure limit of 90 dBA TWA, or 85 dBA TWA for workers who have suffered a significant threshold shift (defined as an average shift of 10 dB or greater at the frequencies of 2, 3, and 4 kHz; i.e., ΔPTA234 ≥ 10 dB). Best practice guidance is more conservative, with NIOSH recommending HPD use when TWA exceeds 85 dBA (NIOSH, 1998). Most international regulations are more consistent with the more conservative guidance from NIOSH [for review see Suter (2007)], and there are many workplaces that have voluntarily adopted these more conservative exposure limits. Examples of employer-led efforts to reduce exposure are provided at the Safe-in-Sound Excellence in Hearing Loss Prevention website, where presentations made by the winners of this annual award program are housed. In designing a clinical trial, it is incumbent on the study team to understand the minimum standards for worker protection that must be complied with, as well as best practices for worker hearing loss prevention, so that risks and benefits can be accurately described during the IRB review process and fully disclosed to study participants.
For workers that wear HPDs, fit testing should be considered as part of the study design, to estimate the degree of protection achieved by study participants. Caution is warranted with respect to assumptions that more protection is always better than less protection. It is possible to provide “over-protection,” meaning that sound exposure is attenuated to the point that workers cannot readily detect signals that are important for their safety (Sayler et al., 2019). The impact of HPD use on listeners with existing hearing loss is similarly a significant concern, as these at-risk workers have reduced audibility and/or speech understanding even before accounting for the HPD attenuation and associated spectral distortion [see also Dolan and O'Loughlin (2005) and Themann and Masterson (2019)]. Accident and injury rates are elevated in workers with hearing loss (Woodcock and Pole, 2008; Cantley et al., 2015a; Cantley et al., 2015b; Palmer et al., 2015; Mick et al., 2018), including increases in the rate of injuries requiring hospitalization (Girard et al., 2015). Systematic review of the literature reveals that increased injury rates are associated with hearing loss across industries (Jadhav et al., 2015; Estill et al., 2017). Strategies for improving communication while wearing HPDs is of interest, and field studies assessing communication in noise are emerging for passive HPD products (Wagoner et al., 2007). Electronic HPDs are also of emerging interest for use by workers (Tufts et al., 2011).
L. Data from pre-clinical studies
For pre-clinical insights into the prevention of hearing loss secondary to workplace noise exposure, it is critical that animal models of NIHL employ repeated exposure paradigms. Given the major interest in prevention of hearing loss that occurs because of occupational noise exposure, it is surprising that there have been few, if any, efforts to identify prevention of hearing loss that slowly develops as a consequence of repeated noise exposure in pre-clinical (animal) models. The systematic review by Hammill (2017) identified a small number of studies with repeated exposures, none of which modeled daily exposure to occupational noise over the course of a working career. Moreover, none of the species-specific contributions within this special issue, which considered NIHL more broadly, identified any significant use of such paradigms within diverse animal species used to understand the effects of repetitive noise on the inner ear (Burton et al., 2019; Escabi et al., 2019; Holt et al., 2019; Trevino et al., 2019; Naert et al., 2019; Ohlemiller, 2019; Radziwon et al., 2019).
We used a series of targeted search terms and manually searched chapters in books within our personal libraries in order to identify a small number of studies employing repeated exposures, which we have listed in Table III. Table III identifies animal models that may be appropriate for the development of data addressing the potential for protection against NIHL that occurs as a consequence of workplace-like noise exposure. Demonstration of prevention of NIHL in animals that experience repeated daily noise exposure would be helpful in providing a rationale for the long-term investigation of potential benefits for human workers exposed to noise on a daily basis during the 5-day workweek. It will be particularly helpful if animal studies include interventions at both early times and later onset interventions, much like studies assessing prevention of ARHL have included both early intervention and later interventions, as some people will initiate therapy only after deficits have begun to emerge (see, for example, Heman-Ackah et al., 2010).
TABLE III.
Various search terms were used to identify studies employing repetitive noise exposures; the search criteria included multiple exposures per week, over periods lasting one week or longer. None of the identified studies included the assessment of an otoprotective agent for prevention of NIHL.
| Author | Species | Noise Type | Level (dB SPL) | Duration (h/day) | Frequency (days/wk) | Total duration of exposure cycle (weeks, months) |
|---|---|---|---|---|---|---|
| Lim et al. (1982) | Chinchilla | Impact | 125 dB peak SPL | 1 impulse every 2 s for 8 h/day | 5 | 1, 2, or 4 weeks |
| Erlandsson et al. (1987) | Guinea pig | Workshop exposure | 87–90 dBA | 8 h/day | 5 | 6 weeks |
| Davis et al. (1996) | Chinchilla | Continuous broadband noise | 115 dB peak SPL | 7.5 min | 7 | 26 days |
| Davis et al. (1996) | Chinchilla | 1 and 4 kHz narrowband impact | 115 dB peak SPL | 1/s for 6 h/day | 7 | 20 days |
| Carder and Miller (1972) | Chinchilla | 0.5 and 4 kHz octave band noise | 65 to 105 dB SPL | 24 h/day | 2, 7, 21 days | 3.5 to 7 weeks |
| Moody et al. (1978) | Macaca | 0.5, 2, 4, 8 kHz OBN | 117–120 SPL | 8 h/day | 5 | 20 days |
| 0.1-100 kHz BBN | ||||||
| Lonsbury-Martin et al. (1987) | Macaca Mulatta | 12 pure tones 0.354–16 kHz in 1/2 octave steps | 100 dB SPL | 3 mins/day | 5 | 6, 18 months |
| 75–85 dB background | ||||||
| Mills (1976) | Chinchilla | 4 kHz OBN | 80 dB SPL | 23.5 h/day | 7 | 90 days |
| Nielsen et al. (1986) | Monkey Saimiri Sciureus | 500, 750 Hz OBN | 89 dB SPL 90 dB SPL | 24 h/day | 7 | 60 days |
| 70 days | ||||||
| Harding and Bohne (2004) | Chinchilla | 4 kHz OBN | 47–108 dB SPL | 0.5 h to 24 days | 7 | 0.5 h to 36 days |
| Or | ||||||
| 500 Hz OBN | 65-128 dB SPL | 3.5 h to 24 days | 3.5 h to 432 days | |||
| Eldredge et al. (1973) | Chinchilla | 4 kHz OBN | 57,65,72,80 dB SPL | 24 | 7 | 24 |
Care to review the considerable literature on “toughening” or “conditioning” of the ear is warranted, as it is possible that repeated exposure to sound will alter vulnerability of the ear. In these classic and well established paradigms, repeated exposure to lower level noise has been shown to decrease vulnerability to a subsequent higher level sound exposure (Henselman et al., 1994; Canlon and Fransson, 1995; Canlon, 1997; Canlon and Fransson, 1998; Skellett et al., 1998; Canlon et al., 1999; Hamernik and Ahroon, 1999; Kujawa and Liberman, 1999; Peng et al., 2007).
M. Effects of occupational noise on synapses
The potential for pathology of the synapse; i.e., a loss of the synaptic connections between inner hair cells (IHCs) and the auditory nerve, as a consequence of occupational noise exposure has been discussed by Dobie and Humes (2017), with additional brief commentary by Murphy and Le Prell (2017). More recently, Le Prell (2019a) reviewed patterns of deficits in evoked potential amplitude and latency in combination with patterns of hearing loss in those exposed to occupational noise in an effort to gain insight into the potential for cochlear synaptopathy as a possible consequence of workplace noise injury. As discussed in that review, the presence of overt hearing loss confounds the interpretation of decreases in wave I amplitude. Nonetheless, the presence of wave I amplitude deficits at high stimulus levels, above the operating range for the cochlear amplifier, is consistent with a mixed pathology including both OHC and synapse loss as also discussed by Hickox et al. (2017). In this series of papers, this topic is discussed in detail by Themann and Masterson (2019), as well as by Bramhall et al. (2019a) and Kamerer et al. (2019). Bramhall et al. (2019a), for example, sought to identify an optimal stimulus paradigm that would stimulate the 3–6 kHz region of the human cochlea using sound levels high enough to drive the low-spontaneous rate fibers but not so high that the response is dominated by the altered response of the basilar membrane in the noise-damaged cochlea with concomitant OHC loss.
Careful investigation in workers with diverse work histories is needed to fully understand risk, and it is essential that work place noise monitoring records be accessed in order to identify exposure history as accurately as possible. In addition to workplace noise records, HPD use must be carefully surveyed, and fit testing data should be collected in an effort to estimate the attenuation currently achieved during HPD use by the participant. It is of course possible that HPD insertion varies from day to day, or even within a given day, and fit testing repeated across the duration of the study will provide the most accurate insight into achieved attenuation for the individual participants. For ethical reasons, we advise that workers be counseled and retrained on HPD use if fit testing reveals poor attenuation even though this counseling has the potential to decrease the incidence or progression of hearing loss across the course of a drug-intervention study. At a minimum, workers would need to be notified of changes in their hearing during annual testing, as this is a required element within 29 CFR 1910.95 (OSHA, 1983).
N. Acoustic trauma subsequent to non-impulsive noise
Much of the above text has focused on acoustic trauma after exposure to impulse noise, and progressive trauma that occurs after exposure to chronic (non-impulsive) noise. Acoustic trauma is specifically defined as an injury that occurs after a single acute exposure event and can be caused by an acute exposure to non-impulsive noise as well. The contribution by Berger and Dobie (2019) reviewed the literature on non-impulsive high level noise exposure resulting in acoustic trauma in humans to provide insight into other non-impulsive noise exposures that may be immediately hazardous to the ear. Berger and Dobie (2019) specifically update the early conference report by Ward (1991), with Berger and Dobie (2019) suggesting lower “safe” limits than previously suggested by Ward (1991) based on the identification of more recent case reports describing acoustic trauma in additional individuals. Particular attention should be paid to the comment by Berger and Dobie (2019) regarding the reliance of animal-based otoprotection research on acoustic trauma models. The vast majority of animal-based studies use a single high-level auditory exposure to induce PTS; i.e., acoustic trauma models. Animal studies that employ a single exposure to non-impulsive noise to induce acoustic trauma may be most directly applicable to the insults discussed by Berger and Dobie (2019). However, as discussed by Berger and Dobie (2019), participants with acute accidental exposure to traumatic sound may not be populations that can be readily recruited to participate in clinical trials as the exposures are typically unexpected. One of the examples identified in the review by Berger and Dobie (2019) was an accidental exposure to loud sound that occurred during workplace rupture of a steam line. As noted in their review, these populations were not likely to be useful as clinical trial populations given that by definition these were accidental exposures occurring only rarely.
IV. MUSIC-INDUCED HEARING LOSS (MIHL) IN PERFORMING ARTISTS
Exposure to loud music induces hearing loss (MIHL), and other related conditions such as tinnitus and hyperacusis, driving the use of broader terminology regarding music-induced hearing disorders (MIHD) by Wartinger et al. (2019). As reviewed by Wartinger et al. (2019), there is a wide range of values reported regarding hearing loss prevalence in musicians. As they note, one of the contributing factors is the pattern of exposure driven at least in part by genre. Classical musicians have been a population of significant interest, with many studies reporting evidence of MIHL but other studies reporting no increase in the prevalence of hearing loss in symphonic or orchestral musicians (Karlsson et al., 1983; Ostri et al., 1989; Royster et al., 1991; McBride et al., 1992; Obeling and Poulsen, 1999; Laitinen et al., 2003; Jansen et al., 2009; Pawlaczyk-Luszczynska et al., 2011; Schmidt et al., 2014).
Musicians who perform amplified music concerts are also a population of significant interest, again with differences in the prevalence of hearing loss reported across investigations (Lebo et al., 1967; Rintelmann and Borus, 1968; Reddell and Lebo, 1972; Axelsson and Lindgren, 1978; Axelsson et al., 1995; Santoni and Fiorini, 2010; Halevi-Katz et al., 2015). The specific instruments that are played by the performer also influence exposure, with brass (horn, trumpet, tuba) and percussion reported to carry the highest risk (Chesky and Henoch, 2000; Pawlaczyk-Luszczynska et al., 2011; Behar et al., 2018).
Although it is tempting to assume that repetitive exposure to music parallels repetitive exposure to occupational noise, there is some suggestion that unwanted noise and music performance may not have equivalent risk for the listener (or performer). As discussed by Wartinger et al. (2019) there are important differences not only in the acoustic signal (frequency, spectral pattern) but also the emotional response to these sounds. Whereas few industrial workers wish to experience high noise levels in the workplace, musicians create sound as a performing art, and performance attendees seek (and pay to experience) this sound creation. Differences in the acoustics and/or differences in the emotional response may explain previous findings in which TTS was smaller when music was used as an exposure stimulus, compared to exposure to noise, even though the total energy in the two exposures was equivalent (Lindgren and Axelsson, 1983; Strasser et al., 2003). There is also an interesting report in which the effects of the same noise exposure differed when the noise was delivered in the context of reward versus as a punishment, suggesting context of the sound influenced the physiological response to sound [Hörmann et al. (1970), as cited in Chasin (2010)]. Some caution is warranted with interpreting such results, however, given that randomly achieved distribution of factors that influence individual vulnerability could potentially result in erroneous conclusions regarding the seeming cause of a group difference. Individual variation in TTS after a four-hour exposure to music was described in detail by Le Prell et al. (2012), with no TTS in some participants and as much as 20 dB TTS in the most vulnerable participants. Finally, it must be acknowledged that there is some evidence that formal musical training may improve central auditory processing and therefore enhance ability to deal with mild peripheral deficits (White-Schwoch et al., 2013; Jantzen et al., 2014; Kraus et al., 2014; Strait et al., 2015; Tierney et al., 2015; Slater et al., 2017; Slater et al., 2018). What is less clear is whether this enhanced ability might also provide at least a partial explanation for increased awareness and distress related to of auditory dysfunction such as diplacusis or tinnitus. More research is warranted to fully understand risk relationships for music, hearing loss, and other MIHD.
To prevent MIHD, acoustic barriers are commonly recommended but may not be able to provide adequate protection (Wenmaekers et al., 2017); nonetheless, these remain an important component within hearing loss prevention programs (Ackermann et al., 2014; O'Brien et al., 2015). Earplug use similarly remains an important part of the hearing conservation program despite use that has been observed at low rates, with qualitative feedback suggesting musicians are concerned about compromised listening ability (for both their own and their colleagues playing), as well as changes in the timbre or dynamics of the music, and/or potential discomfort (Laitinen and Poulsen, 2008; Zander et al., 2008; Santoni and Fiorini, 2010; Huttunen et al., 2011; Ackermann et al., 2014; O'Brien et al., 2014; Bockstael et al., 2015; Beach and O'Brien, 2017). These protective devices are discussed in detail by Wartinger et al. (2019). In the context of a clinical trial, it would be helpful to monitor both individual exposure, which will be impacted by barrier devices, seating, size of the orchestra, etc., as well as HPD use, which will impact individual exposure.
Music students are a population in which there has been more recent interest (Barlow et al., 2016; Liberman et al., 2016; Skoe and Tufts, 2018). The discussion by Jin et al. (2013), who failed to detect evidence of hearing loss in University marching band members is timely in that they note the audiogram is relatively insensitive to the earliest effects of noise on the inner ear and thus a hearing conservation program is still recommended. Adopt-a-Band is an educational intervention program intended to support hearing loss prevention program through both increased knowledge and increased availability of HPDs (distributed to band members as part of the program). There are only a few systematic reports describing the effectiveness of this program and related variants (Auchter and Le Prell, 2014; Seever et al., 2018); more research is needed to document the effectiveness of this program. Another population of interest is that of sound technicians (El Dib et al., 2008). Hetu and Fortin (1995) provide a particularly interesting commentary, interviewing DJs and others about sound engineering procedures, and predicting TTS to be likely, with both the production team and those in the venue being deemed at risk [see also Mendes and Morata (2007)]. Other employees potentially at risk for sound overexposure include bartenders, waiters, cashiers, and security officers (Lee, 1999). Student workers at university entertainment venues have also been reported to be at significant risk (Sadhra et al., 2002).
For clinical trials enrolling musicians or music students as participants, the total accumulated dose during the clinical trial observation period and the potential use of HPDs will need to be carefully accounted for as part of the study design. When study participants choose to wear HPDs, the study team should consider incorporating fit testing into the study protocol, to obtain an estimate of the attenuation the participant achieved during the music event. Fit testing, which as noted above is a procedure for verification of the individual attenuation achieved by an HPD user, has been performed for custom molded high-fidelity (“musicians”) HPDs, with data suggesting only a small number of frequencies are necessary to validate the fidelity (“flatness”) of the HPD (Portnuff and Price, 2019).
From a clinical trial design perspective, musicians generally are not a population that is subject to significant regulatory oversight including mandatory HPD use and thus one can envision musicians being recruited for the assessment of investigational new drug agents for prevention of MIHD without as many confounds of variable HPD use by participants. However, we do not advocate any clinical trial design that precludes the use of HPDs; we do not yet have the tools to identify the most vulnerable subset of the population to know which participants may be at increased risk. Guidance for the care of musicians hearing health at this time includes recommendations for decreasing sound exposure level and duration, use of high fidelity HPDs, or perhaps use of in-ear monitors with careful control of in-ear levels [see Wartinger et al. (2019)]. Regardless of study participation, all musicians should receive consistent advice and guidance on safe listening behaviors that protect the health of the inner ear. Post-enrollment, careful collection of data about participant sound dose and HPD use (including effective attenuation) will be critical for accurate interpretation of both the effects of music on hearing in those receiving placebos and the drawing of correct conclusions regarding potential drug mediated protection. For those professional musicians that rely on-in-ear sound monitors for both acoustic awareness and isolation from some noise sources, documentation of in-ear sound levels should also be maintained as the levels set can vary significantly as a function of musician preference, training, and experience (Federman and Ricketts, 2008).
In the sections above, it was noted that HPDs are required for workers exposed to noise (OSHA, 1983) and for service members exposed to loud sound (DoD Instruction 6055.12, 2010), whereas musicians often are not subject to such requirements (although it is certainly audiological best practice to encourage safe performing behaviors). The contribution by Wartinger et al. (2019) therefore provides a careful discussion of the ethics of advocating a pharmaceutical intervention in lieu of an HPD. The importance of appropriately educating musicians on the degree and variability of protection is stressed, to decrease the risk of over-reliance on a pharmaceutical that may have variable results from individual to individual. Moreover, they caution that there is a real risk that those receiving treatment with a pharmaceutical may be complacent about the use of safe listening strategies, inadvertently increasing total exposure.
It should also of course be considered that medications can have unanticipated side effects, and a common side effect for many pharmaceuticals is tinnitus. Perhaps one of the best-known examples is that of aspirin, which is commonly used to induce tinnitus in animal models (Guitton et al., 2003). Because tinnitus can be career limiting for musicians (Schmidt et al., 2019), caution is warranted with safety and adverse side effects needing to be identified in Phase I safety studies even for common over the counter (OTC) agents, including those in the “generally regarded as safe” (GRAS) category. Increased incidence of transient tinnitus was observed after music player use in participants using a dietary supplement composed of beta-carotene, vitamins C and E, and magnesium (Le Prell et al., 2016). Even if tinnitus is temporary, side effects that include tinnitus would be a major concern for musician populations. Safety testing prior to use in populations that rely on the integrity of their hearing for their career is critical.
V. NIHL DUE TO NON-OCCUPATIONAL SOUND
A. Concert goers
The WHO recently suggested that some 1.1 × 109 young people world-wide may be at risk for NIHL based on both personal audio system (PAS) use and exposure to amplified music in bars, clubs, and concerts (World Health Organization, 2015). Attendee overexposure is a significant concern; Mercier and Hohmann (2002) report that 71% of young adult participants who had attended amplified music events (concerts, discotheques, raves) had tinnitus after attending such events. A number of investigations have provided documentation of TTS immediately after live music events (Kramer et al., 2006; Opperman et al., 2006; Derebery et al., 2012; Ramakers et al., 2016). Because TTS appears to be likely to recover within the first 24 h post exposure for many participants attending loud venues (see Grinn et al., 2017), any clinical trial data will necessarily need to emphasize threshold deficits at short post-exposure times. Interestingly, word-in-noise deficits may have a longer duration, lasting at least one day post exposure for some event attendees [i.e., those with higher noise doses, see Grinn et al. (2017)]. Surprisingly, however, other than threshold differences at the highest test frequencies within the EHF range, there has been little evidence of either overt hearing loss or supra-threshold functional deficits when those attending concerts frequently are compared to those participants only rarely attending such events (Grose et al., 2017). Indeed, the data from Grose et al. (2017) provide no evidence of permanent speech-in-noise deficits in frequent concert goers, despite the robust relationship between noise dose obtained at concerts (and other amplified music events) and temporary speech in noise deficits as measured by Grinn et al. (2017). It must of course be considered that use of more challenging speech in noise tests may be more sensitive for revealing subtle and previously undetected deficits that may emerge with frequent event attendance. The data from Liberman et al. (2016) for example reveal speech-in-noise deficits in music students, using a custom task in which words are presented at a relatively quiet level with reverberation, time compression, and background noise added, such that the target signal has a very poor signal to noise ratio.
For clinical trials enrolling young adults who visit loud musical venues as participants, the total accumulated dose during the event and the potential use of HPDs will need to be carefully accounted for as part of the study design. As noted above, when study participants choose to wear HPDs, the study team should consider incorporating fit testing into the study protocol, to obtain an estimate of the attenuation the participant may have achieved during the music event. Fit test data were recently collected for a variety of high-fidelity HPDs inserted in the ears of a sample of young adults (primarily female, and primarily students enrolled in a Doctor of Audiology training program) (Zaccardi et al., 2018; Zaccardi and Le Prell, 2019) following protocols similar to those developed by Portnuff and Price (2019). Those data revealed significant variation not only across HPDs, but also across individuals tested within a given high fidelity HPD condition. Historically, the use of HPDs by young adults attending loud music events has been low, with previous noise-induced hearing symptoms being one of the more common reasons for use according to the subset of participants reporting HPD use (Bogoch et al., 2005; Beach et al., 2011). HPD use increases when traditional foam HPDs are provided for free at loud events (Cha et al., 2015). The distribution of high-fidelity HPDs appears to be associated with even higher adoption rates, with high fidelity HPDs continuing to be used some 4 weeks after they were distributed to concert-goers (Beach et al., 2016). HPD use prevents TTS otherwise observed in concert-goers who chose not to wear HPDs (Opperman et al., 2006; Ramakers et al., 2016). From a hearing loss prevention point of view, it is promising that HPDs prevent TTS, but HPD use will add complexity to a clinical trial in that HPD use prevents (confounds) the primary outcome of interest.
From a clinical trial design perspective, recreational event attendees are not subject to regulatory mandates for HPD use, but, institutional review boards responsible for reviewing the risks to subjects might have some concerns about any clinical trial design that precludes the use of HPDs solely from the perspective that compensation to participants might bias participants not to use HPDs at loud events and an unknown subset of the population may be at increased risk given individual variation in vulnerability. Careful collection of data about participant sound dose and HPD use (including effective attenuation) will be critical for accurate interpretation of both the effects of music on hearing in those receiving placebos and the drawing of correct conclusions regarding potential protection if some concert goers choose to wear HPDs during some or all of the music events they attend. The Canadian Health Survey data presented by Feder et al. (2019) are perhaps particularly relevant here as they queried use of hearing protection as part of this national survey, with the highest use of HPDs reported during firearm use (44%) and the lowest use of HPDs reported during exposure to amplified music (2.5%) and sporting events (1.9%). If similarly low use was noted during a clinical trial, the impact of HPD use on the study outcomes would be relatively small. At a minimum, guidance on future HPD use should be provided as part of study exit interviews and/or final debriefing of the participants.
When the issue of sound exposure limits that would be protective for the entire population was discussed in detail for both adults (Neitzel and Fligor, 2019) and children (Roberts and Neitzel, 2019) exposed to recreational (leisure) noise, both papers arrived at a recommendation that exposures not exceed an 8-h average exposure of 80 dBA, which is equivalent to a 24-h exposure limit of 75 dBA. This limit would virtually eliminate the risk of recreational sound induced hearing loss, with adherence to an exposure limit of 70 dBA 24-h equivalent continuous level [LAEQ(24)] eliminating the risk of hearing loss even for the most vulnerable individuals. Additional discussion of the 70 dB LAEQ(24) recommended limit is available in the 1974 monograph from the U.S. Environmental Protection Agency (EPA) (Environmental Protection Agency, 1974). Anyone participating in a clinical trial involving concert attendance is likely to exceed these limits, although a single exposure to sound levels that exceed recommended limits is not necessarily hazardous. It is instead the repetition of sound exposure over multiple years that ultimately results in hearing loss for the majority of workers and others exposed to repeated loud sound events.
B. Pre-clinical (animal) data
Given significant interests in prevention of MIHD, including MIHL, it is surprising that there have not been more efforts to identify the effects of music on the inner ear in rodent models. Few efforts have been made to investigate the effects of music using rodent models. The small number of studies that we found in the literature, summarized in Table IV, were found to use either an acoustic trauma paradigm with a single exposure (Bohne et al., 1977; Okada et al., 1991) or two exposure series (Lamm et al., 2004) or a series of exposures over an extended time period to model repeated event attendance (Lipscomb, 1969a,b). We are not aware of any efforts to assess prevention of music-induced hearing loss in any of these animal models.
TABLE IV.
Paradigms in which rodents were exposed to music to induce acoustic trauma.
| Author | Species | Music Type | Level (dB SPL) | Duration (h) | Results |
|---|---|---|---|---|---|
| Lipscomb (1969a, 1969b) | Guinea pig | Rock ‘n roll | 122 dB peak (modeled after dance hall measurements) | Varied from 35 min to 227 min per session, for total of 88 h over two month period | IHC and OHC damage; loss of 20%–25% of cells in upper second and third cochlear turn |
| Lamm et al. (2004) | Guinea pig | Rock music | 106 dBA | Two sessions of 2.5 h | Significant TTS with partial recovery during first 24 h, resolving to stable PTS with no evident morphological trauma |
| Okada et al. (1991) | Guinea pig | Rock music | 110–130 dB SPL | 1–3 h | Dilated spiral vessels after 1–2 h; constricted spiral vessels after 3 h |
| Bohne et al. (1977) | Chinchilla | Rock music | Live Music at Discotheque, 107–117 dB SPL | 2.5 h | Small OHC losses in 3 of 4 ears; large losses in 1 of 4 ears. |
C. Personal audio system (PAS) use
A significant literature related to hearing loss in association with PAS use has been developed. Unfortunately, much of the data have been of generally low quality [for discussion see Sliwinska-Kowalska and Zaborowski (2017)], with one of the common issues being a failure to query other sources of exposure to loud sound, resulting in an allocation of any measured hearing loss to use of the PAS. Nonetheless, a subset of adolescents and young adults are clearly at risk for sound induced injury and hearing deficits because of such device use (Portnuff et al., 2011; Jiang et al., 2016; Portnuff, 2016; Park et al., 2017). These findings were replicated in the report by Feder et al. (2019), who found that 46.7% of the Canadian survey respondents reported PAS use, and of those, 41.8% reported loud PAS use. The data from by Feder et al. (2019) are compelling in that they surveyed a large variety of non-occupational activities ultimately finding that 28.5% of the total respondents could be at risk for NIHL if their current recreational exposure patterns continue over time. Several clinical trials have assessed prevention of TTS that occurs subsequent to controlled music exposure delivered using a PAS, with mixed results across agents and investigations (Le Prell et al., 2016; Kil et al., 2017). In both of these investigations, the sound levels were calibrated such that the PAS delivered approximately 100 dBA sound levels when measured in an acoustic coupler.
D. Cochlear synaptopathy, non-occupational exposure, and aging
The issue of cochlear synaptopathy, as discussed above and across papers in this special issue, is of significant recent interest. Although studies that assess those with firearm exposure (Bramhall et al., 2017) and significant lifetime noise exposure (Valderrama et al., 2018) suggest the possibility of noise induced cochlear synaptopathy, other studies assessing the relationships between lifetime noise exposure data and auditory evoked potentials (Prendergast et al., 2017a; Prendergast et al., 2017b) have not detected these relationships. A series of studies assessing relationships between recreational noise exposure (estimated based on exposures in the past year) and auditory evoked potentials have not revealed significant relationships (Fulbright et al., 2017; Grinn et al., 2017; Spankovich et al., 2017). Several detailed reviews of this literature are available (Barbee et al., 2018; Bramhall et al., 2019b; Le Prell, 2019a), with all three reviews concluding that differences in the patterns of exposure may drive differences in findings across investigations.
In this special issue, Kamerer et al. (2019) carefully built on previous findings by including both normal hearing individuals and individuals with some hearing loss, including individuals with significant lifetime noise exposure as measured using the LENS-Q survey. As in the recent work by Johannesen et al. (2019), Kamerer et al. (2019) reports that age was associated with ABR wave I amplitude, but noise exposure was not. Based on EHF hearing loss, they conclude that OHC loss in basal regions of the cochlea may be driving the decreased ABR wave I amplitudes observed as a function of aging and note that diagnosing cochlear synaptopathy in patients with hearing loss may prove difficult if OHC loss induces wave I changes mimicking those expected to occur with synaptic loss. While cochlear synaptopathy remains a challenging clinical target, it is certain to remain of interest for potential drug interventions as metrics for diagnosis of this pathology improve. Because cochlear synaptopathy cannot be directly measured in living patients or participants, we encourage language specific to the measurable study outcomes; i.e., prevention of speech in noise deficits is a more precise label than prevention of cochlear synaptopathy, as prevention of the pathology can only be inferred in human participants whereas functional protection can be measured and confirmed in additional investigations.
VI. FACTORS INFLUENCING INDIVIDUAL VULNERABILITY
Studies in animals have the significant advantage of using animals with less genetic diversity, controlled housing, controlled sound exposure, controlled diet, controlled health history, etc. Animals tend to be the same age (at least within any given study), and may even all be drawn from a pool of same sex animals (commonly male although sometimes female), further reducing possible variation in head size and body weight. Successful demonstration of otoprotective benefit in these highly controlled samples has driven significant interest in translation of therapeutics from animal models to human clinical trials (Le et al., 2017; Wang and Puel, 2018; Kujawa and Liberman, 2019; Le Prell, 2019b) as well as interest in therapeutic benefits following different types of noise injury (Wada et al., 2017). However, as studies move from animal models to human testing, sources of variability that can confound interpretations of drug effects significantly increase. Indeed, it has long been known that some individuals are more vulnerable than others, meaning, they develop more NIHL despite common exposure histories.
Some of these individual differences may be related to peripheral factors, such as the external and middle ear (Grinn and Le Prell, 2019; Rosowski et al., 2019). Significant differences exist across species with respect to ear canal gain as a function of frequency with the chinchilla having more gain than the human, and the guinea pig having more gain than the chinchilla [for review and illustration, see Saunders and Tilney (1982)]. Tympanic membrane velocity curves also differ, suggesting that the total amount of energy reaching the ear will be species-dependent (Saunders and Tilney, 1982). These differences may at least in part explain differences in vulnerability such as those documented by Decory et al. (1992) who showed that with equivalent exposures, chinchillas were somewhat more vulnerable than guinea pigs, and both rodent species were significantly more vulnerable than cats, with species differences explained at least in part by differences in the transmission of energy to the middle ear. These factors also matter in humans, with different gain measured in different ear canals (Grinn, 2019; Grinn and Le Prell, 2019) and different power transfer across the middle ear as can occur for example in the presence of conductive hearing loss (Rosowski et al., 2019).
The strength of the acoustic reflex may also mediate individual vulnerability, with those with a stronger muscle reflex presumably having less overall exposure due to the stiffening of the middle ear conductive system. Flamme et al. (2017) and McGregor et al. (2018) found that middle ear muscle contractions (MEMC) were not pervasive (i.e., observed in less than 95% of the population at a 95% confidence level). Deiters et al. (2019) found detection rates in 190 normal hearing subjects with clinically present MEMC were between 20% and 80% depending upon the stimulus. White noise was most likely to elicit a reflexive response, and the noise from a recorded discharge from a 0.22 caliber rifle, which has a broad frequency spectrum, was substantially less likely to elicit an MEMC.
Other contributing factors that were discussed in detail within the special issue included individual genetic variation (Clifford et al., 2019), hormone signaling (Shuster et al., 2019), inflammatory response (Frye et al., 2019), nutritional status (Spankovich and Le Prell, 2019), and the time of day at which the exposure occurs (Fontana et al., 2019). Another factor that may influence the effects of music on hearing is the stress response, based on relationships between self-reported hearing disorders and stress (Hasson et al., 2009). Efforts to incorporate some of these factors into human clinical trials are emerging. For example, efforts to look at human genes that influence NIHL and responsiveness to treatment were included within a recent drug intervention study (Lin et al., 2009; Lin et al., 2010). As we continue to identify factors that importantly mediate NIHL we will hopefully be increasingly able to identify those most at risk for noise injury, and we will also be able to better control confounds that introduce variability into the outcomes of clinical trials evaluating drugs that have the potential to mediate NIHL. At this time, we cannot readily quantify the effects of many of these factors in animals yet alone in humans, but the accumulating body of evidence clearly shows that many factors each have small effects on vulnerability of the inner ear to noise injury.
VII. SUMMARY
The articles in this special issue of the Journal of the Acoustical Society of America highlight challenges related to pre-clinical testing of potential otoprotective drug agents, and challenges in their translation to human clinical trials, which may have contributed to some of the mixed results across the small number of completed clinical studies to date. Otoprotective drugs will ultimately be developed for and targeted to specific populations and markets, and the current series of articles has furthered the understanding of who the at-risk populations are such that clinical trial paradigms can be improved. The current series includes detailed discussion of pre-clinical paradigms as well, highlighting variation across animal models and noise injury paradigms. The current series of articles was specifically organized so as to describe the most common animal models used when a drug is initially developed for potential application, and contrast these animal models with the real-world noise hazards and patterns of injury observed in humans, which poorly match most of the animal models used to date. The comments throughout this final manuscript have tried to marry information across animal and human contributions to provoke discussion and, we hope, better fidelity of the animal paradigms used to model human risk for noise injury. The editors and authors of these articles are grateful to the DoD HCE not only for sponsoring the PIHL committee but also for supporting this broad partnership between the DoD and the VA (Veterans Affairs) as well as academic and industry partners. Improved clinical and translational study designs, specifically intended to further the development of otoprotective drug agents that will decrease the prevalence, impact, and cost of NIHL not only for the DoD and the VA, but also for industry and the general public, continue to be urgently needed.
ACKNOWLEDGEMENTS
C.G.L. is currently supported by USAMRAA Grant Nos. W81XWH-19-C-0054, JPC-8/SRMRP W81XWH1820014, NIH-NIDCD 1R01DC014088, 3M Inc., and the Emilie and Phil Schepps Professorship in Hearing Science. C.G.L. has previously received contract funding and/or clinical trial material from industry partners including Sound Pharmaceuticals, Inc., Edison Pharmaceuticals, Inc., and Hearing Health Science, Inc. The authors gratefully acknowledge administrative support provided by J. R. Stefanson (HCE), and the editorial support of Liz Bury, Kelly Quigley, and James Lynch (Acoustical Society of America). The authors thank Chris Spankovich for helpful comments and suggestions on an earlier version of this manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the U.S. Department of Defense, Centers for Disease Control and Prevention, or the National Institute for Occupational Safety and Health.
References
- 1. Abaamrane, L. , Raffin, F. , Gal, M. , Avan, P. , and Sendowski, I. (2009). “ Long-term administration of magnesium after acoustic trauma caused by gunshot noise in guinea pigs,” Hear. Res. 247, 137–145. 10.1016/j.heares.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 2. Abaamrane, L. , Raffin, F. , Schmerber, S. , and Sendowski, I. (2011). “ Intracochlear perfusion of leupeptin and z-VAD-FMK: Influence of antiapoptotic agents on gunshot-induced hearing loss,” Eur. Arch. Otorhinolaryngol. 268, 987–993. 10.1007/s00405-011-1487-0 [DOI] [PubMed] [Google Scholar]
- 3. Abel, S. M. , Alberti, P. W. , Haythornthwaite, C. , and Riko, K. (1982). “ Speech intelligibility in noise: Effects of fluency and hearing protector type,” J. Acoust. Soc. Am. 71, 708–715. 10.1121/1.387547 [DOI] [PubMed] [Google Scholar]
- 4. Abel, S. M. , Alberti, P. W. , and Riko, K. (1980). “ Speech intelligibility in noise with ear protectors,” J. Otolaryngol. 9, 256–265. [PubMed] [Google Scholar]
- 5. Abel, S. M. , Krever, E. M. , Giguere, C. , and Alberti, P. W. (1991). “ Signal detection and speech perception with level-dependent hearing protectors,” J. Otolaryngol. 20, 46–53. [PubMed] [Google Scholar]
- 6. Ackermann, B. J. , Kenny, D. T. , O'Brien, I. , and Driscoll, T. R. (2014). “ Sound Practice—Improving occupational health and safety for professional orchestral musicians in Australia,” Front. Psychol. 5, 973. 10.3389/fpsyg.2014.00973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adelman, C. , Weinberger, J. M. , Kriksunov, L. , and Sohmer, H. (2011). “ Effects of furosemide on the hearing loss induced by impulse noise,” J. Occ. Med. Toxicol. (Lond.) 6, 14. 10.1186/1745-6673-6-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Academy of Audiology (2009). “ Position statement and clinical practice guidelines: Ototoxicity monitoring,” http://audiology-web.s3.amazonaws.com/migrated/OtoMonGuidelines.pdf_539974c40999c1.58842217.pdf (Last viewed July 5, 2016).
- 9.American Academy of Otolaryngology Committee on Hearing and Equilibrium, and American Council of Otolaryngology Commitee on the Medical Aspects of Noise (1979). “ Guide for the evaluation of hearing handicap,” JAMA 241, 2055–2059. 10.1001/jama.1979.03290450053025430800 [DOI] [Google Scholar]
- 10.American Speech-Language-Hearing Association (1994). “ Guidelines for the audiologic management of individuals receiving cochleotoxic drug therapy,” ASHA 36(Suppl. 12), 11–19. [Google Scholar]
- 11. Arlinger, S. (1992). “ Speech recognition in noise when wearing amplitude-sensitive ear-muffs,” Scand. Audiol. 21, 123–126. 10.3109/01050399209045992 [DOI] [PubMed] [Google Scholar]
- 12. Attias, J. , Bresloff, I. , Haupt, H. , Scheibe, F. , and Ising, H. (2003). “ Preventing noise induced otoacoustic emission loss by increasing magnesium (Mg2+) intake in guinea-pigs,” J. Basic Clin. Physiol. Pharmacol. 14, 119–136. 10.1515/JBCPP.2003.14.2.119 [DOI] [PubMed] [Google Scholar]
- 13. Attias, J. , Weisz, G. , Almog, S. , Shahar, A. , Wiener, M. , Joachims, Z. , Netzer, A. , Ising, H. , Rebentisch, E. , and Guenther, T. (1994). “ Oral magnesium intake reduces permanent hearing loss induced by noise exposure,” Am. J. Otolaryngol. 15, 26–32. 10.1016/0196-0709(94)90036-1 [DOI] [PubMed] [Google Scholar]
- 14. Auchter, M. , and Le Prell, C. G. (2014). “ Hearing loss prevention education using adopt-a-band: Changes in self-reported earplug use in two high school marching bands,” Am. J. Audiol. 23, 211–226. 10.1044/2014_AJA-14-0001 [DOI] [PubMed] [Google Scholar]
- 15. Axelsson, A. , Eliasson, A. , and Israelsson, B. (1995). “ Hearing in pop/rock musicians: A follow-up study,” Ear Hear. 16, 245–253. 10.1097/00003446-199506000-00001 [DOI] [PubMed] [Google Scholar]
- 16. Axelsson, A. , and Lindgren, F. (1978). “ Hearing in pop musicians,” Acta Otolaryngol. (Stockh). 85, 225–231. 10.3109/00016487809121444 [DOI] [PubMed] [Google Scholar]
- 17. Barbee, C. M. , James, J. A. , Park, J. H. , Smith, E. M. , Johnson, C. E. , Clifton, S. , and Danhauer, J. L. (2018). “ Effectiveness of auditory measures for detecting hidden hearing loss and/or cochlear synaptopathy: A systematic review,” Semin. Hearing 39, 172–209. 10.1055/s-0038-1641743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barlow, N. , Purdy, S. C. , Sharma, M. , Giles, E. , and Narne, V. (2016). “ The effect of short-term auditory training on speech in noise perception and cortical auditory evoked potentials in adults with cochlear implants,” Semin. Hearing 37, 84–98. 10.1055/s-0035-1570335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beach, E. F. , Nielsen, L. , and Gilliver, M. (2016). “ Providing earplugs to young adults at risk encourages protective behaviour in music venues,” Glob. Health Promot. 23, 45–56. 10.1177/1757975914558887 [DOI] [PubMed] [Google Scholar]
- 20. Beach, E. F. , and O'Brien, I. (2017). “ In their own words: Interviews with musicians reveal the advantages and disadvantages of wearing earplugs,” Med. Probl. Perform. Ar. 32, 101–110. 10.21091/mppa.2017.2017 [DOI] [PubMed] [Google Scholar]
- 21. Beach, E. F. , Williams, W. , and Gilliver, M. (2011). “ A qualitative study of earplug use as a health behavior: The role of noise injury symptoms, self-efficacy and an affinity for music,” J. Hlth. Psychol. 17, 237–246. 10.1177/1359105311412839 [DOI] [PubMed] [Google Scholar]
- 22. Behar, A. , Chasin, M. , Mosher, S. , Abdoli-Eramaki, M. , and Russo, F. A. (2018). “ Noise exposure and hearing loss in classical orchestra musicians: A five-year follow-up,” Noise Health 20, 42–46. 10.4103/nah.NAH_39_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berger, E. H. , and Dobie, R. A. (2019). “ Acoustic trauma from continuous noise: Minimum exposures, issues in clinical trial design, and comments on MRI exposures,” J. Acoust. Soc. Am. 146, 3873–3878. 10.1121/1.5132712 [DOI] [PubMed] [Google Scholar]
- 24. Bevis, Z. L. , Semeraro, H. D. , van Besouw, R. M. , Rowan, D. , Lineton, B. , and Allsopp, A. J. (2014). “ Fit for the frontline? A focus group exploration of auditory tasks carried out by infantry and combat support personnel,” Noise Health 16, 127–135. 10.4103/1463-1741.132101 [DOI] [PubMed] [Google Scholar]
- 25. Bielefeld, E. C. (2013). “ Reduction in impulse noise-induced permanent threshold shift with intracochlear application of an NADPH oxidase inhibitor,” J. Am. Acad. Audiol. 24, 461–473. 10.3766/jaaa.24.6.3 [DOI] [PubMed] [Google Scholar]
- 26. Bielefeld, E. C. , Hangauer, D. , and Henderson, D. (2011). “ Protection from impulse noise-induced hearing loss with novel Src-protein tyrosine kinase inhibitors,” Neurosci. Res. 71, 348–354. 10.1016/j.neures.2011.07.1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bielefeld, E. C. , Harrison, R. T. , and DeBacker, J. R. (2019). “ Pharmaceutical otoprotection strategies to prevent impulse noise-induced hearing loss,” J. Acoust. Soc. Am. 146, 3790–3799. 10.1121/1.5132285 [DOI] [PubMed] [Google Scholar]
- 28. Bielefeld, E. C. , Kopke, R. D. , Jackson, R. L. , Coleman, J. K. , Liu, J. , and Henderson, D. (2007). “ Noise protection with N-acetyl-l-cysteine (NAC) using a variety of noise exposures, NAC doses, and routes of administration,” Acta Otolaryngol. (Stockh). 127, 914–919. 10.1080/00016480601110188 [DOI] [PubMed] [Google Scholar]
- 29. Bockstael, A. , De Coensel, B. , Botteldooren, D. , D'Haenens, W. , Keppler, H. , Maes, L. , Philips, B. , Swinnen, F. , and Bart, V. (2011). “ Speech recognition in noise with active and passive hearing protectors: A comparative study,” J. Acoust. Soc. Am. 129, 3702–3715. 10.1121/1.3575599 [DOI] [PubMed] [Google Scholar]
- 30. Bockstael, A. , Keppler, H. , and Botteldooren, D. (2015). “ Musician earplugs: Appreciation and protection,” Noise Health 17, 198–208. 10.4103/1463-1741.160688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boger, M. E. , Sampaio, A. L. L. , and Oliveira, C. (2017). “ Analysis of hearing and tinnitus in workers exposed to occupational noise,” Int. Tinnitus J. 20, 88–92. 10.5935/0946-5448.20160017 [DOI] [PubMed] [Google Scholar]
- 32. Bogoch, I. I. , House, R. A. , and Kudla, I. (2005). “ Perceptions about hearing protection and noise-induced hearing loss of attendees of rock concerts,” Can. J. Public Health. 96, 69–72. 10.1007/BF03404022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bohne, B. A. , Ward, P. H. , and Fernandez, C. (1977). “ Rock music and inner ear damage,” Am. Fam. Physician 15, 117–118. [PubMed] [Google Scholar]
- 34. Borchgrevink, H. M. , Hallmo, P. , and Mair, I. W. S. (1996). “ Extended high freqeuncy hearing loss form noise exposure,” in Scientific Basis of Noise-Induced Hearing Loss, edited by Axelsson A., Borchgrevink H. M., Hamernik R. P., Hellstrom P. A., Henderson D., and Salvi R. J. ( Thieme, New York: ), pp. 299–312. [Google Scholar]
- 35. Bramhall, N. F. , Beach, E. F. , Epp, B. , Le Prell, C. G. , Lopez-Poveda, E. A. , Plack, C. J. , Schaette, R. , Verhulst, S. , and Canlon, B. (2019b). “ The search for noise-induced cochlear synaptopathy in humans: Mission impossible?,” Hear. Res. 377, 88–103. 10.1016/j.heares.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 36. Bramhall, N. F. , Konrad-Martin, D. , McMillan, G. P. , and Griest, S. E. (2017). “ Auditory brainstem response altered in humans with noise exposure despite normal outer hair cell function,” Ear Hear. 38, e1–e12. 10.1097/AUD.0000000000000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bramhall, N. F. , McMillan, G. P. , Gallun, F. J. , and Konrad-Martin, D. (2019a). “ Auditory brainstem response demonstrates that reduced peripheral auditory input is associated with self-report of tinnitus,” J. Acoust. Soc. Am. 146, 3849–3862. 10.1121/1.5132708 [DOI] [PubMed] [Google Scholar]
- 38. Brown, A. D. , Beemer, B. T. , Greene, N. T. , Argo, T. t. , Meegan, G. D. , and Tollin, D. J. (2015). “ Effects of active and passive hearing protection devices on sound source localization, speech recognition, and tone detection,” PLoS One 10, e0136568. 10.1371/journal.pone.0136568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brungart, D. S. , Kordik, A. J. , and Simpson, B. D. (2004). “ The effects of single and double hearing protection on the localization and segregation of spatially-separated speech signals,” J. Acoust. Soc. Am. 116, 1897–1900. 10.1121/1.1786812 [DOI] [PubMed] [Google Scholar]
- 40. Burns, W. , and Robinson, D. W. (1970). Hearing and Noise in Industry ( Her Majesty's Stationery Office, London: ). [Google Scholar]
- 41. Burton, J. A. , Valero, M. D. , Hackett, T. A. , and Ramachandran, R. (2019). “ The use of nonhuman primates in studies of noise injury and treatment,” J. Acoust. Soc. Am. 146, 3770–3789. 10.1121/1.5132709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Campbell, K. C. M. (2016). “ Phase 2 clinical trials: d-methionine to reduce noise-induced hearing loss. Final report for award W81XWH-11-C-0033, 2016,” http://www.dtic.mil/dtic/tr/fulltext/u2/1028742.pdf (Last viewed February 28, 2019).
- 43. Campbell, K. C. M. , Hammill, T. , Hoffer, M. , Kil, J. , and Le Prell, C. G. (2016). “ Guidelines for auditory threshold measurement for significant threshold shift (STS),” Otol. Neurotol. 37, e263–e270. 10.1097/MAO.0000000000001135 [DOI] [PubMed] [Google Scholar]
- 44. Canlon, B. (1997). “ Protection against noise trauma by sound conditioning,” Ear. Nose. Throat J. 76, 248–250. 10.1177/014556139707600413 [DOI] [PubMed] [Google Scholar]
- 45. Canlon, B. , and Fransson, A. (1995). “ Morphological and functional preservation of the outer hair cells from noise trauma by sound conditioning,” Hear. Res. 84, 112–124. 10.1016/0378-5955(95)00020-5 [DOI] [PubMed] [Google Scholar]
- 46. Canlon, B. , and Fransson, A. (1998). “ Reducing noise damage by using a mid-frequency sound conditioning stimulus,” Neuroreport 9, 269–274. 10.1097/00001756-199801260-00017 [DOI] [PubMed] [Google Scholar]
- 47. Canlon, B. , Ryan, A. F. , and Boettcher, F. A. (1999). “ On the factors required for obtaining protection against noise trauma by prior acoustic experience,” Hear. Res. 127, 158–161. 10.1016/S0378-5955(98)00170-1 [DOI] [PubMed] [Google Scholar]
- 48. Cantley, L. F. , Galusha, D. , Cullen, M. R. , Dixon-Ernst, C. , Rabinowitz, P. M. , and Neitzel, R. L. (2015a). “ Association between ambient noise exposure, hearing acuity, and risk of acute occupational injury,” Scand. J. Work. Environ. Health 41, 75–83. 10.5271/sjweh.3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cantley, L. F. , Galusha, D. , Cullen, M. R. , Dixon-Ernst, C. , Tessier-Sherman, B. , Slade, M. D. , Rabinowitz, P. M. , and Neitzel, R. L. (2015b). “ Does tinnitus, hearing asymmetry, or hearing loss predispose to occupational injury risk?,” Int. J. Audiol. 54, S30–S36. 10.3109/14992027.2014.981305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cantley, L. F. , Slade, M. D. , and Galusha, D. (2019). “ Early hearing slope as a predictor of subsequent hearing trajectory in a noise-exposed occupational cohort,” J. Acoust. Soc. Am. 146, 4044–4050. 10.1121/1.5132542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carder, H. M. , and Miller, J. D. (1972). “ Temporary threshold shifts from prolonged exposure to noise,” J. Speech Hear. Res. 15, 603–623. 10.1044/jshr.1503.603 [DOI] [PubMed] [Google Scholar]
- 52. Casali, J. G. (2010a). “ Passive augmentations in hearing protection technology circa 2010 including flat-attenuation, passive level-dependent, passive wave resonance, passive adjustable attenuation, and adjustable-fit devices: Review of design, testing, and research,” Int. J. Acoust. Vib. 15, 187–195. [Google Scholar]
- 53. Casali, J. G. (2010b). “ Powered electronic augmentations in hearing protection technology circa 2010 including active noise reduction, electronically-modulated sound transmission, and tactical communications devices: Review of design, testing, and research,” Int. J. Acoust. Vib. 15, 168–186. [Google Scholar]
- 54. Casali, J. G. , Ahroon, W. A. , and Lancaster, J. A. (2009). “ A field investigation of hearing protection and hearing enhancement in one device: For soldiers whose ears and lives depend upon it,” Noise Health 11, 69–90. 10.4103/1463-1741.48564 [DOI] [PubMed] [Google Scholar]
- 55. Casto, K. L. , and Cho, T. H. (2012). “ In-flight speech intelligibility evaluation of a service member with sensorineural hearing loss: Case report,” Mil. Med. 177, 1114–1116. 10.7205/MILMED-D-12-00184 [DOI] [PubMed] [Google Scholar]
- 56. Cha, J. , Smukler, S. R. , Chung, Y. , House, R. , and Bogoch II (2015). “ Increase in use of protective earplugs by Rock and Roll concert attendees when provided for free at concert venues,” Int. J. Audiol. 54, 984–986. 10.3109/14992027.2015.1080863 [DOI] [PubMed] [Google Scholar]
- 57. Chan, P. , Ho, K. , and Ryan, A. F. (2016). “ Impulse noise injury model,” Mil. Med. 181, 59–69. 10.7205/MILMED-D-15-00139 [DOI] [PubMed] [Google Scholar]
- 58. Chasin, M. (2010). Hear the Music: Hearing Loss Prevention for Musicians ( Musicians' Clinics of Canada; ). [Google Scholar]
- 59. Chesky, C. , and Henoch, M. A. (2000). “ Instrument-specific reports of hearing loss: Differences between classical and nonclassical musicians,” Med. Probl. Perform. Ar. 15, 35. [Google Scholar]
- 60. Chi, F. L. , Yang, M. Q. , Zhou, Y. D. , and Wang, B. (2011). “ Therapeutic efficacy of topical application of dexamethasone to the round window niche after acoustic trauma caused by intensive impulse noise in guinea pigs,” J. Laryngol. Otol. 125, 673–685. 10.1017/S0022215111000028 [DOI] [PubMed] [Google Scholar]
- 61. Chung, D. Y. , and Gannon, R. P. (1979). “ The effect of ear protectors on word discrimination in subject with normal hearing and subjects with noise-induced hearing loss,” J. Am. Aud. Soc. 5, 11–16. [PubMed] [Google Scholar]
- 62. Clasing, J. E. , and Casali, J. G. (2014). “ Warfighter auditory situation awareness: Effects of augmented hearing protection/enhancement devices and TCAPS for military ground combat applications,” Int. J. Audiol. 53, S43–S52. 10.3109/14992027.2013.860489 [DOI] [PubMed] [Google Scholar]
- 63. Clifford, R. E. , Hertzano, R. , and Ohlemiller, K. K. (2019). “ Untangling the genomics of noise-induced hearing loss and tinnitus: Contributions of mus musculus and homo sapiens,” J. Acoust. Soc. Am. 146, 4007–4019. 10.1121/1.5132552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Coleman, J. K. , Littlesunday, C. , Jackson, R. , and Meyer, T. (2007). “ AM-111 protects against permanent hearing loss from impulse noise trauma,” Hear. Res. 226, 70–78. 10.1016/j.heares.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 65. Cousins, R. P. (2019). “ Medicines discovery for auditory disorders: Challenges for industry,” J. Acoust. Soc. Am. 146, 3652–3667. 10.1121/1.5132706 [DOI] [PubMed] [Google Scholar]
- 66. Davis, R. I. , Hamernik, R. P. , Ahroon, W. A. , and Underwood, K. A. (1996). “ Threshold shift dynamics following interrupted impact or continuous noise exposure: A review,” in Scientific Basis of Noise-Induced Hearing Loss, edited by Axelsson A., Borchgrevink H. M., Hamernik R. P., Hellstrom P. A., Henderson D., and Salvi R. J. ( Thieme, New York: ), pp. 134–158. [Google Scholar]
- 67. Davis, R. I. , Qiu, W. , Heyer, N. J. , Zhao, Y. , Qiuling Yang, M. S. , Li, N. , Tao, L. , Zhu, L. , Zeng, L. , and Yao, D. (2012). “ The use of the kurtosis metric in the evaluation of occupational hearing loss in workers in China: Implications for hearing risk assessment,” Noise Health 14, 330–342. 10.4103/1463-1741.104903 [DOI] [PubMed] [Google Scholar]
- 68. Decory, L. , Dancer, A. L. , and Aran, J. M. (1992). “ Species differences and mechanisms of damage,” in Noise-Induced Hearing Loss, edited by Dancer A. L., Henderson D., Salvi R., and Hamernik R. P. ( Mosby Year Book, St. Louis: ), pp. 229–248. [Google Scholar]
- 69. Deiters, K. K. , and Flamme, G. A. (2019). “SASRAC Technical Report #1904_4 Update of NIOSH Age Adjustment Tables,” SASRAC, Loveland, OH. [Google Scholar]
- 70. Deiters, K. K. , Flamme, G. A. , Tasko, S. M. , Murphy, W. J. , Greene, N. T. , Jones, H. G. , and Ahroon, W. A. (2019). “ Generalizability of clinically-measured acoustic reflexes to brief sounds,” J. Acoust. Soc. Am. 146, 3993–4006. 10.1121/1.5132705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Derebery, M. J. , Vermiglio, A. , Berliner, K. I. , Potthoff, M. , and Holguin, K. (2012). “ Facing the music: Pre- and postconcert assessment of hearing in teenagers,” Otol. Neurotol. 33, 1136–1141. 10.1097/MAO.0b013e31825f2328 [DOI] [PubMed] [Google Scholar]
- 72. Dille, M. F. , McMillan, G. P. , Reavis, K. M. , Jacobs, P. , Fausti, S. A. , and Konrad-Martin, D. (2010). “ Ototoxicity risk assessment combining distortion product otoacoustic emissions with a cisplatin dose model,” J. Acoust. Soc. Am. 128, 1163–1174. 10.1121/1.3473693 [DOI] [PubMed] [Google Scholar]
- 73. Dobie, R. A. (2015a). “ Impairment, handicap, and disability,” in Medical-Legal Evaluation of Hearing Loss, 3rd ed., edited by Dobie R. A. ( Plural Publishing, San Diego: ), pp. 83–139. [Google Scholar]
- 74. Dobie, R. A. (2015b). “ Is this STS work-related? ISO 1999 predictions as an adjunct to clinical judgment,” Am. J. Ind. Med. 58, 1311–1318. 10.1002/ajim.22534 [DOI] [PubMed] [Google Scholar]
- 75. Dobie, R. A. , and Humes, L. E. (2017). “ Commentary on the regulatory implications of noise-induced cochlear neuropathy,” Int. J. Audiol. 56, 74–78. 10.1080/14992027.2016.1255359 [DOI] [PubMed] [Google Scholar]
- 76.DoD Instruction 6055.12 (2010). “ Department of Defense Instruction NUMBER 6055.12, Hearing Conservation Program (HCP),” December 3, 2010.
- 77. Dolan, T. G. , and O'Loughlin, D. (2005). “ Amplified earmuffs: Impact on speech intelligibility in industrial noise for listeners with hearing loss,” Am. J. Audiol. 14, 80–85. 10.1044/1059-0889(2005/007) [DOI] [PubMed] [Google Scholar]
- 78. Doosti, A. , Lotfi, Y. , Moossavi, A. , Bakhshi, E. , Talasaz, A. H. , and Hoorzad, A. (2014). “ Comparison of the effects of N-acetyl-cysteine and ginseng in prevention of noise induced hearing loss in male textile workers,” Noise Health 16, 223–227. 10.4103/1463-1741.137057 [DOI] [PubMed] [Google Scholar]
- 79. Duan, M. , Qiu, J. , Laurell, G. , Olofsson, A. , Counter, S. A. , and Borg, E. (2004). “ Dose and time-dependent protection of the antioxidant N-L-acetylcysteine against impulse noise trauma,” Hear. Res. 192, 1–9. 10.1016/j.heares.2004.02.005 [DOI] [PubMed] [Google Scholar]
- 80. Dunn, D. E. , Davis, R. R. , Merry, C. J. , and Franks, J. R. (1991). “ Hearing loss in the chinchilla from impact and continuous noise exposure,” J. Acoust. Soc. Am. 90, 1979–1985. 10.1121/1.401677 [DOI] [PubMed] [Google Scholar]
- 81. Earshen, J. J. (1986). “ Sound measurement: Instrumentation and devices,” in The Noise and Hearing Conservation Manual, edited by Berger E. H., Ward W. D., Morrill J. C., and Royster L. H. ( American Industrial Hygiene Association, Akron: ), pp. 41–100. [Google Scholar]
- 82. El Dib, R. P. , Silva, E. M. , Morais, J. F. , and Trevisani, V. F. (2008). “ Prevalence of high frequency hearing loss consistent with noise exposure among people working with sound systems and general population in Brazil: A cross-sectional study,” BMC Public Health 8, 151. 10.1186/1471-2458-8-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Eldredge, D. H. , Mills, J. H. , and Bohne, B. A. (1973). “ Anatomical, behavioral, and electrophysiological observations on chinchillas after long exposures to noise,” Adv. Otorhinolaryngol. 20, 64–81. 10.1159/000393089 [DOI] [PubMed] [Google Scholar]
- 84.Environmental Protection Agency (1974). “ Information on levels of environmental noise requisite to protect public health and welfare with an adequate margin of safety,” EPA Report 550/9-74-004.
- 85. Erlandsson, B. , Hakanson, H. , Ivarsson, A. , Nilsson, P. , and Wersall, J. (1987). “ Hair cell damage in the inner ear of the guinea pig due to noise in a workshop,” Acta Otolaryngol. (Stockh). 103, 204–211. 10.3109/00016488709107274 [DOI] [PubMed] [Google Scholar]
- 86. Escabi, C. D. , Frye, M. , and Lobarinas, E. (2019). “ The rat animal model for noise-induced hearing loss,” J. Acoust. Soc. Am. 146, 3692–3709. 10.1121/1.5132553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Estill, C. F. , Rice, C. H. , Morata, T. , and Bhattacharya, A. (2017). “ Noise and neurotoxic chemical exposure relationship to workplace traumatic injuries: A review,” J. Saf. Res. 60, 35–42. 10.1016/j.jsr.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Feder, K. P. , Marro, L. , McNamee, J. , Keith, S. E. , and Michaud, D. (2019). “ Prevalence of loud leisure noise activities among a representative sample of Canadians aged 6 to 79,” J. Acoust. Soc. Am. 146, 3934–3946. 10.1121/1.5132949 [DOI] [PubMed] [Google Scholar]
- 89. Federman, J. , and Ricketts, T. (2008). “ Preferred and minimum acceptable listening levels for musicians while using floor and in-ear monitors,” J. Speech. Lang. Hear. Res. 51, 147–159. 10.1044/1092-4388(2008/011) [DOI] [PubMed] [Google Scholar]
- 90. Fetoni, A. R. , Bielefeld, E. C. , Paludetti, G. , Nicotera, T. , and Henderson, D. (2014). “ A putative role of p53 pathway against impulse noise induced damage as demonstrated by protection with pifithrin-alpha and a Src inhibitor,” Neurosci. Res. 81-82, 30–37. 10.1016/j.neures.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 91. Flamme, G. A. , Deiters, K. K. , Tasko, S. M. , and Ahroon, W. A. (2017). “ Acoustic reflexes are common but not pervasive: Evidence from the National Health and Nutrition Examination Survey, 1999-2012,” Int. J. Audiol. 56, 52–62. 10.1080/14992027.2016.1257164 [DOI] [PubMed] [Google Scholar]
- 92. Flamme, G. A. , Goldfarb, D. , Zeig-Owens, R. , Hall, C. , Vaeth, B. , Schwartz, T. , Yip, J. , Vossbrinck, M. , Stein, C. , Friedman, L. , Cone, J. , and Prezant, D. (2019). “ Hearing loss among World Trade Center firefighters and emergency medical service workers,” J. Occup. Environ. Med. (in press). [DOI] [PubMed] [Google Scholar]
- 93. Flamme, G. A. , Stephenson, M. R. , Deiters, K. K. , Hessenauer, A. , VanGessel, D. K. , Geda, K. , Wyllys, K. , and McGregor, K. D. (2014). “ Short-term variability of pure-tone thresholds obtained with TDH-39P earphones,” Int. J. Audiol. 53, S5–S15. 10.3109/14992027.2013.857435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fontana, J. M. , Tserga, E. , Sarlus, H. , Canlon, B. , and Cederroth, C. (2019). “ Impact of noise exposure on the circadian clock in the auditory system,” J. Acoust. Soc. Am. 146, 3960–3966. 10.1121/1.5132290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fraenkel, R. , Freeman, S. , and Sohmer, H. (2003). “ Use of ABR threshold and OAEs in detection of noise induced hearing loss,” J. Basic Clin. Physiol. Pharmacol. 14, 95–118. 10.1515/jbcpp.2003.14.2.95 [DOI] [PubMed] [Google Scholar]
- 96. Franzé, A. , Sequino, L. , Saulino, C. , Attanasio, G. , and Marciano, E. (2003). “ Effect over time of allopurinol on noise-induced hearing loss in guinea pigs,” Int. J. Audiol. 42, 227–234. 10.3109/14992020309101318 [DOI] [PubMed] [Google Scholar]
- 97. Frye, M. D. , Ryan, A. F. , and Kurabi, A. (2019). “ Inflammation associated with noise-induced hearing loss,” J. Acoust. Soc. Am. 146, 4020–4032. 10.1121/1.5132545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fulbright, A. N. C. , Le Prell, C. G. , Griffiths, S. K. , and Lobarinas, E. (2017). “ Effects of recreational noise on threshold and supra-threshold measures of auditory function,” Semin. Hearing 38, 298–318. 10.1055/s-0037-1606325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Giguère, C. , and Berger, E. H. (2016). “ Speech recognition in noise under hearing protection: A computational study of the combined effects of hearing loss and hearing protector attenuation,” Int. J. Audiol. 55, S30–S40. 10.3109/14992027.2015.1129460 [DOI] [PubMed] [Google Scholar]
- 100. Giguère, C. , Laroche, C. , and Vaillancourt, V. (2015). “ The interaction of hearing loss and level-dependent hearing protection on speech recognition in noise,” Int. J. Audiol. 54, S9–S18. 10.3109/14992027.2014.973540 [DOI] [PubMed] [Google Scholar]
- 101. Girard, S. A. , Leroux, T. , Courteau, M. , Picard, M. , Turcotte, F. , and Richer, O. (2015). “ Occupational noise exposure and noise-induced hearing loss are associated with work-related injuries leading to admission to hospital,” Inj. Prev. 21, e88–e92. 10.1136/injuryprev-2013-040828 [DOI] [PubMed] [Google Scholar]
- 102. Gittleman, S. , Le Prell, C. G. , and Hammill, T. (2019). “ Octave band noise exposure: Laboratory models and otoprotection efforts,” J. Acoust. Soc. Am. 146, 3800–3810. 10.1121/1.5133393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gordon, J. S. , Griest, S. E. , Thielman, E. J. , Carlson, K. F. , Helt, W. J. , Lewis, M. S. , Blankenship, C. , Austin, D. F. , Theodoroff, S. M. , and Henry, J. A. (2017). “ Audiologic characteristics in a sample of recently-separated military veterans: The noise outcomes in servicemembers epidemiology study (NOISE study),” Hear. Res. 349, 21–30. 10.1016/j.heares.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 104. Gower, D. W., Jr. , and Casali, J. G. (1994). “ Speech intelligibility and protective effectiveness of selected active noise reduction and conventional communications headsets,” Hum. Factors 36, 350–367. 10.1177/001872089403600214 [DOI] [PubMed] [Google Scholar]
- 105. Graydon, K. , Waterworth, C. , Miller, H. , and Gunasekera, H. (2019). “ Global burden of hearing impairment and ear disease,” J. Laryngol. Otol. 133, 18–25. 10.1017/S0022215118001275 [DOI] [PubMed] [Google Scholar]
- 106. Grinn, S. K. (2019). “ Modeling individual noise-induced hearing loss risk with proxy metrics of external ear amplification [Dissertation],” in School of Behavioral and Brain Sciences ( University of Texas at Dallas, Dallas: ). [Google Scholar]
- 107. Grinn, S. K. , and Le Prell, C. G. (2019). “ Noise-dose estimated with and without pre-cochlear amplification,” J. Acoust. Soc. Am. 146, 3967–3977. 10.1121/1.5132546 [DOI] [PubMed] [Google Scholar]
- 108. Grinn, S. K. , Wiseman, K. B. , Baker, J. A. , and Le Prell, C. G. (2017). “ Hidden Hearing Loss? No effect of common recreational noise exposure on cochlear nerve response amplitude in humans,” Front. Neurosci. 11, 465. 10.3389/fnins.2017.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Grose, J. H. , Buss, E. , and Hall, J. W., 3rd (2017). “ Loud music exposure and cochlear synaptopathy in young adults: Isolated auditory brainstem response effects but no perceptual consequences,” Trends Hear. 21, 2331216517737417. 10.1177/2331216517737417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Guitton, M. J. , Caston, J. , Ruel, J. , Johnson, R. M. , Pujol, R. , and Puel, J. L. (2003). “ Salicylate induces tinnitus through activation of cochlear NMDA receptors,” J. Neurosci. 23, 3944–3952. 10.1523/JNEUROSCI.23-09-03944.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Halevi-Katz, D. N. , Yaakobi, E. , and Putter-Katz, H. (2015). “ Exposure to music and noise-induced hearing loss (NIHL) among professional pop/rock/jazz musicians,” Noise Health 17, 158–164. 10.4103/1463-1741.155848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hallmo, P. , Borchgrevink, H. M. , and Mair, I. W. (1995). “ Extended high-frequency thresholds in noise-induced hearing loss,” Scand. Audiol. 24, 47–52. 10.3109/01050399509042209 [DOI] [PubMed] [Google Scholar]
- 113. Hamernik, R. P. , and Ahroon, W. A. (1999). “ Sound-induced priming of the chinchilla auditory system,” Hear. Res. 137, 127–136. 10.1016/S0378-5955(99)00131-8 [DOI] [PubMed] [Google Scholar]
- 114. Hamernik, R. P. , Qiu, W. , and Davis, B. (2003). “ The effects of the amplitude distribution of equal energy exposures on noise-induced hearing loss: The kurtosis metric,” J. Acoust. Soc. Am. 114, 386–395. 10.1121/1.1582446 [DOI] [PubMed] [Google Scholar]
- 115. Hammill, T. L. (2017). “ An evidence-base and implementation framework for promoting best practices in pharmaceutical interventions for hearing loss (PIHL) [Dissertation],” The University of Texas at Austin, Austin, TX. [Google Scholar]
- 116. Harding, G. W. , and Bohne, B. A. (2004). “ Noise-induced hair-cell loss and total exposure energy: Analysis of a large data set,” J. Acoust. Soc. Am. 115, 2207–2220. 10.1121/1.1689961 [DOI] [PubMed] [Google Scholar]
- 117. Harris, K. C. , Hu, B. , Hangauer, D. , and Henderson, D. (2005). “ Prevention of noise-induced hearing loss with Src-PTK inhibitors,” Hear. Res. 208, 14–25. 10.1016/j.heares.2005.04.009 [DOI] [PubMed] [Google Scholar]
- 118. Hasson, D. , Theorell, T. , Liljeholm-Johansson, Y. , and Canlon, B. (2009). “ Psychosocial and physiological correlates of self-reported hearing problems in male and female musicians in symphony orchestras,” Int. J. Psychophysiol. 74, 93–100. 10.1016/j.ijpsycho.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 119. Haupt, H. , and Scheibe, F. (2002). “ Preventive magnesium supplement protects the inner ear against noise-induced impairment of blood flow and oxygenation in the guinea pig,” Magnes. Res. 15, 17–25. [PubMed] [Google Scholar]
- 120. Haupt, H. , Scheibe, F. , and Mazurek, B. (2003). “ Therapeutic efficacy of magnesium in acoustic trauma in the guinea pig,” ORL. J. Otorhinolaryngol. Relat. Spec. 65, 134–139. 10.1159/000072250 [DOI] [PubMed] [Google Scholar]
- 121. Hecht, Q. A. , Hammill, T. L. , Calamia, P. T. , Smalt, C. J. , and Brungart, D. S. (2019). “ Characterization of acute hearing changes in United States military populations,” J. Acoust. Soc. Am. 146, 3839–3848. 10.1121/1.5132710 [DOI] [PubMed] [Google Scholar]
- 122. Heinrich, U. R. , Fischer, I. , Brieger, J. , Rumelin, A. , Schmidtmann, I. , Li, H. , Mann, W. J. , and Helling, K. (2008). “ Ascorbic acid reduces noise-induced nitric oxide production in the guinea pig ear,” Laryngoscope 118, 837–842. 10.1097/MLG.0b013e31816381ae [DOI] [PubMed] [Google Scholar]
- 123. Heman-Ackah, S. E. , Juhn, S. K. , Huang, T. C. , and Wiedmann, T. S. (2010). “ A combination antioxidant therapy prevents age-related hearing loss in C57BL/6 mice,” Otolaryngol. Head Neck Surg. 143, 429–434. 10.1016/j.otohns.2010.04.266 [DOI] [PubMed] [Google Scholar]
- 124. Henselman, L. W. , Henderson, D. , Subramaniam, M. , and Sallustio, V. (1994). “ The effect of ‘conditioning’ exposures on hearing loss from impulse noise,” Hear. Res. 78, 1–10. 10.1016/0378-5955(94)90038-8 [DOI] [PubMed] [Google Scholar]
- 125. Hetu, R. , and Fortin, M. (1995). “ Potential risk of hearing damage associated with exposure to highly amplified music,” J. Am. Acad. Audiol. 6, 378–386. [PubMed] [Google Scholar]
- 126. Hickox, A. E. , Larsen, E. , Heinz, M. G. , Shinobu, L. , and Whitton, J. P. (2017). “ Translational issues in cochlear synaptopathy,” Hear. Res. 349, 164–171. 10.1016/j.heares.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hight, N. G. , McFadden, S. L. , Henderson, D. , Burkard, R. F. , and Nicotera, T. (2003). “ Noise-induced hearing loss in chinchillas pre-treated with glutathione monoethylester and R-PIA,” Hear. Res. 179, 21–32. 10.1016/S0378-5955(03)00067-4 [DOI] [PubMed] [Google Scholar]
- 128. Hiselius, P. , Edvall, N. , and Reimers, D. (2015). “ To measure the impact of hearing protectors on the perception of speech in noise,” Int. J. Audiol 54, S3–S8. 10.3109/14992027.2014.973539 [DOI] [PubMed] [Google Scholar]
- 129. Hoffman, H. J. , Dobie, R. A. , Losonczy, K. G. , Themann, C. L. , and Flamme, G. A. (2017). “ Declining prevalence of hearing loss in US adults aged 20 to 69 years,” JAMA Otolaryngol. Head Neck Surg. 143, 274–285. 10.1001/jamaoto.2016.3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Holt, A. G. , Kallakuri, S. , Braun, R. , and Altschuler, R. A. (2019). “ The rat as a model for studying noise injury and otoprotection,” J. Acoust. Soc. Am. 146, 3681–3691. 10.1121/1.5131344 [DOI] [PubMed] [Google Scholar]
- 131. Hörmann, H. , Mainka, G. , and Gummlich, H. (1970). “ Psychological and physiological reactions to noise of different subjective valence (TTS and EMG),” Psychol. Forsch. 33, 289–309. 10.1007/BF00424556 [DOI] [PubMed] [Google Scholar]
- 132. Huttunen, K. H. , Sivonen, V. P. , and Poykko, V. T. (2011). “ Symphony orchestra musicians' use of hearing protection and attenuation of custom-made hearing protectors as measured with two different real-ear attenuation at threshold methods,” Noise Health 13, 176–188. 10.4103/1463-1741.77210 [DOI] [PubMed] [Google Scholar]
- 133.International Standard Organization (2013). “Acoustics: Estimation of noise-induced hearing loss (ISO-1999),” International Standard Organization, Geneva, Switzerland. [Google Scholar]
- 134. Jadhav, R. , Achutan, C. , Haynatzki, G. , Rajaram, S. , and Rautiainen, R. (2015). “ Risk factors for agricultural injury: A systematic review and meta-analysis,” J. Agromed. 20, 434–449. 10.1080/1059924X.2015.1075450 [DOI] [PubMed] [Google Scholar]
- 135. Jansen, E. J. , Helleman, H. W. , Dreschler, W. A. , and de Laat, J. A. (2009). “ Noise induced hearing loss and other hearing complaints among musicians of symphony orchestras,” Int. Arch. Occup. Environ. Health 82, 153–164. 10.1007/s00420-008-0317-1 [DOI] [PubMed] [Google Scholar]
- 136. Jantzen, M. G. , Howe, B. M. , and Jantzen, K. J. (2014). “ Neurophysiological evidence that musical training influences the recruitment of right hemispheric homologues for speech perception,” Front. Psychol. 5, 171. 10.3389/fpsyg.2014.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Jiang, W. , Zhao, F. , Guderley, N. , and Manchaiah, V. (2016). “ Daily music exposure dose and hearing problems using personal listening devices in adolescents and young adults: A systematic review,” Int. J. Audiol. 55, 197–205. 10.3109/14992027.2015.1122237 [DOI] [PubMed] [Google Scholar]
- 138. Jin, S. H. , Nelson, P. B. , Schlauch, R. , and Carney, E. (2013). “ Hearing conservation program for marching band members: A risk for noise-induced hearing loss?,” Am. J. Audiol. 22, 26–39. 10.1044/1059-0889(2012/11-0030) [DOI] [PubMed] [Google Scholar]
- 139. Johannesen, P. T. , Buzo, B. C. , and Lopez-Poveda, E. A. (2019). “ Evidence for age-related cochlear synaptopathy in humans unconnected to speech-in-noise intelligibility deficits,” Hear. Res. 374, 35–48. 10.1016/j.heares.2019.01.017 [DOI] [PubMed] [Google Scholar]
- 140. Jokel, C. , Yankaskas, K. , and Robinette, M. B. (2019). “ Noise of military weapons, ground vehicles, planes and ships,” J. Acoust. Soc. Am. 146, 3832–3838. 10.1121/1.5134069 [DOI] [PubMed] [Google Scholar]
- 141. Joubaud, T. , Zimpfer, V. , Garcia, A. , and Langrenne, C. (2017). “ Sound localization models as evaluation tools for tactical communication and protective systems,” J. Acoust. Soc. Am. 141, 2637. 10.1121/1.4979693 [DOI] [PubMed] [Google Scholar]
- 142. Kamerer, A. M. , Kopun, J. G. , Fultz, S. E. , Allen, C. , Neely, S. T. , and Rasetshwane, D. M. (2019). “ Examining physiological and perceptual consequences of noise exposure,” J. Acoust. Soc. Am. 146, 3947–3959. 10.1121/1.5132291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kansu, L. , Ozkarakas, H. , Efendi, H. , and Okar, I. (2011). “ Protective effects of pentoxifylline and nimodipine on acoustic trauma in Guinea pig cochlea,” Otol. Neurotol. 32, 919–925. 10.1097/MAO.0b013e3182267e06 [DOI] [PubMed] [Google Scholar]
- 144. Kardous, C. A. , Willson, R. D. , Hayden, C. S. , Szlapa, P. , Murphy, W. J. , and Reeves, E. R. (2003). “ Noise exposure assessment and abatement strategies at an indoor firing range,” Appl. Occup. Environ. Hyg. 18, 629–636. 10.1080/10473220301409 [DOI] [PubMed] [Google Scholar]
- 145. Karlsson, K. , Lundquist, P. G. , and Olaussen, T. (1983). “ The hearing of symphony orchestra musicians,” Scand. Audiol. 12, 257–264. 10.3109/14992028309044429 [DOI] [PubMed] [Google Scholar]
- 146. Kerr, M. J. , Neitzel, R. L. , Hong, O. , and Sataloff, R. T. (2017). “ Historical review of efforts to reduce noise-induced hearing loss in the United States,” Am. J. Ind. Med. 60, 569–577. 10.1002/ajim.22627 [DOI] [PubMed] [Google Scholar]
- 147. Kil, J. , Lobarinas, E. , Spankovich, C. , Griffiths, S. , Antonelli, P. J. , Lynch, E. D. , and Le Prell, C. G. (2017). “ Safety and efficacy of Ebselen for the prevention of noise-induced hearing loss: A randomized double blind placebo-controlled phase 2 clinical trial,” The Lancet 390, 969–979. 10.1016/S0140-6736(17)31791-9 [DOI] [PubMed] [Google Scholar]
- 148. Konrad-Martin, D. , Poling, G. L. , Dreisbach, L. E. , Reavis, K. M. , McMillan, G. P. , Lapsley Miller, J. A. , and Marshall, L. (2016). “ Serial monitoring of otoacoustic emissions in clinical trials,” Otol. Neurotol. 37, e286–e294. 10.1097/MAO.0000000000001134 [DOI] [PubMed] [Google Scholar]
- 149. Konrad-Martin, D. , Reavis, K. , McMillan, G. , and Dille, M. (2012). “ Multivariate DPOAE metrics for identifying changes in hearing: Perspectives from ototoxicity monitoring,” Int. J. Audiol. 1, S51–S62. 10.3109/14992027.2011.635713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kopke, R. , Bielefeld, E. , Liu, J. , Zheng, J. , Jackson, R. , Henderson, D. , and Coleman, J. K. (2005). “ Prevention of impulse noise-induced hearing loss with antioxidants,” Acta Otolaryngol. (Stockh). 125, 235–243. 10.1080/00016480410023038 [DOI] [PubMed] [Google Scholar]
- 151. Kopke, R. , Slade, M. D. , Jackson, R. , Hammill, T. , Fausti, S. , Lonsbury-Martin, B. , Sanderson, A. , Dreisbach, L. , Rabinowitz, P. , Torre, P., 3rd , and Balough, B. (2015). “ Efficacy and safety of N-acetylcysteine in prevention of noise induced hearing loss: A randomized clinical trial,” Hear. Res. 323, 40–50. 10.1016/j.heares.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 152. Korres, G. S. , Balatsouras, D. G. , Tzagaroulakis, A. , Kandiloros, D. , and Ferekidis, E. (2008). “ Extended high-frequency audiometry in subjects exposed to occupational noise,” B-ENT 4, 147–155. [PubMed] [Google Scholar]
- 153. Korres, G. S. , Balatsouras, D. G. , Tzagaroulakis, A. , Kandiloros, D. , Ferekidou, E. , and Korres, S. (2009). “ Distortion product otoacoustic emissions in an industrial setting,” Noise Health 11, 103–110. 10.4103/1463-1741.50695 [DOI] [PubMed] [Google Scholar]
- 154. Kramer, S. , Dreisbach, L. , Lockwood, J. , Baldwin, K. , Kopke, R. D. , Scranton, S. , and O'Leary, M. (2006). “ Efficacy of the antioxidant N-acetylcysteine (NAC) in protecting ears exposed to loud music,” J. Am. Acad. Audiol. 17, 265–278. 10.3766/jaaa.17.4.5 [DOI] [PubMed] [Google Scholar]
- 155. Kraus, N. , Slater, J. , Thompson, E. C. , Hornickel, J. , Strait, D. L. , Nicol, T. , and White-Schwoch, T. (2014). “ Music enrichment programs improve the neural encoding of speech in at-risk children,” J. Neurosci. 34, 11913–11918. 10.1523/JNEUROSCI.1881-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kujawa, S. G. , and Liberman, M. C. (1999). “ Long-term sound conditioning enhances cochlear sensitivity,” J. Neurophysiol. 82, 863–873. 10.1152/jn.1999.82.2.863 [DOI] [PubMed] [Google Scholar]
- 157. Kujawa, S. G. , and Liberman, M. C. (2009). “ Adding insult to injury: Cochlear nerve degeneration after” temporary “noise-induced hearing loss,” J. Neurosci. 29, 14077–14085. 10.1523/JNEUROSCI.2845-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kujawa, S. G. , and Liberman, M. C. (2019). “ Translating animal models to human therapeutics in noise-induced and age-related hearing loss,” Hear. Res. 377, 44–52. 10.1016/j.heares.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Laitinen, H. , and Poulsen, T. (2008). “ Questionnaire investigation of musicians' use of hearing protectors, self reported hearing disorders, and their experience of their working environment,” Int. J. Audiol. 47, 160–168. 10.1080/14992020801886770 [DOI] [PubMed] [Google Scholar]
- 160. Laitinen, H. M. , Toppila, E. M. , Olkinuora, P. S. , and Kuisma, K. (2003). “ Sound exposure among the Finnish National Opera personnel,” Appl. Occup. Environ. Hyg. 18, 177–182. 10.1080/10473220301356 [DOI] [PubMed] [Google Scholar]
- 161. Lamm, K. , Michaelis, C. , Deingruber, K. , Scheler, R. , Steinhoff, H. J. , Grober, I. , Huth, M. , Kutscher, C. , and Arnold, W. (2004). “ Inner ear damage due to leisure and broadband noise. An experimental study on initial and permanent functional and morphological damage,” Hno 52, 301–310. 10.1007/s00106-003-1042-4 [DOI] [PubMed] [Google Scholar]
- 162. Lankford, J. E. , Meinke, D. K. , Flamme, G. A. , Finan, D. S. , Stewart, M. , Tasko, S. , and Murphy, W. J. (2016). “ Auditory risk of air rifles,” Int. J. Audiol. 55, S51–S58. 10.3109/14992027.2015.1131851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Le, T. N. , Straatman, L. V. , Lea, J. , and Westerberg, B. (2017). “ Current insights in noise-induced hearing loss: A literature review of the underlying mechanism, pathophysiology, asymmetry, and management options,” J. Otolaryngol. Head Neck Surg. 46, 41. 10.1186/s40463-017-0219-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Lebo, C. P. , Oliphant, K. S. , and Garrett, J. (1967). “ Acoustic trauma from rock- and-roll music,” Calif. Med. 107, 378–380. [PMC free article] [PubMed] [Google Scholar]
- 165. Lee, K. , and Casali, J. G. (2016). “ Effects of low speed wind on the recognition/identification and pass-through communication tasks of auditory situation awareness afforded by military hearing protection/enhancement devices and tactical communication and protective systems,” Int. J. Audiol. 55, S21–S29. 10.3109/14992027.2015.1129073 [DOI] [PubMed] [Google Scholar]
- 166. Lee, K. , and Casali, J. G. (2017). “ Development of an auditory situation awareness test battery for advanced hearing protectors and TCAPS: Detection subtest of DRILCOM (detection-recognition/identification-localization-communication),” Int. J. Audiol. 56, 22–33. 10.1080/14992027.2016.1256505 [DOI] [PubMed] [Google Scholar]
- 167. Lee, K. , and Casali, J. G. (2019). “ Learning to localize a broadband tonal complex signal with advanced hearing protectors and TCAPS: The effectiveness of training on open-ear vs. device-occluded performance,” Int. J. Audiol. 58, S65–S73. 10.1080/14992027.2018.1532118 [DOI] [PubMed] [Google Scholar]
- 168. Lee, L. T. (1999). “ A study of the noise hazard to employees in local discotheques,” Singapore Med. J. 40, 571–574. [PubMed] [Google Scholar]
- 169. Leensen, M. C. , and Dreschler, W. A. (2015). “ Longitudinal changes in hearing threshold levels of noise-exposed construction workers,” Int. Arch. Occup. Environ. Health 88, 45–60. 10.1007/s00420-014-0932-y [DOI] [PubMed] [Google Scholar]
- 170. Leensen, M. C. , van Duivenbooden, J. C. , and Dreschler, W. A. (2011). “ A retrospective analysis of noise-induced hearing loss in the Dutch construction industry,” Int. Arch. Occup. Environ. Health 84, 577–590. 10.1007/s00420-010-0606-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Lei, S. F. , Ahroon, W. A. , and Hamernik, R. P. (1994). “ The application of frequency and time domain kurtosis to the assessment of hazardous noise exposures,” J. Acoust. Soc. Am. 96, 1435–1444. 10.1121/1.410287 [DOI] [PubMed] [Google Scholar]
- 172. Lempert, B. (2019). “ ISO estimates of noise-induced hearing impairment,” J. Acoust. Soc. Am. 145, 3640. 10.1121/1.5111862 [DOI] [PubMed] [Google Scholar]
- 173. Le Prell, C. G. (2019a). “ Effects of noise exposure on auditory brainstem response and speech-in-noise tasks: A review of the literature,” Int. J. Audiol. 58, S3–S32. 10.1080/14992027.2018.1534010 [DOI] [PubMed] [Google Scholar]
- 174. Le Prell, C. G. (2019b). “ Otoprotectants: From research to clinical application,” Semin. Hearing 40, 162–176. 10.1055/s-0039-1684045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Le Prell, C. G. , and Campbell, K. C. M. (2019). “ Clinical test paradigms and problems: Human otoprotection studies,” in Advances in Audiology, Speech Pathology, and Hearing Science, Volume 2: Otoprotection, Regeneration and Telemedicine, edited by Hatzopoulos S., Ciorba A., and Krumm M. ( Apple Academic, Pal Bay, FL: ), pp. 233–269. [Google Scholar]
- 176. Le Prell, C. G. , Dell, S. , Hensley, B. N. , Hall, J. W. I. , Campbell, K. C. M. , Antonelli, P. A. , Green, G. E. , Miller, J. M. , and Guire, K. (2012). “ Digital music exposure reliably induces temporary threshold shift (TTS) in normal hearing human subjects,” Ear Hear. 33, e44–e58. 10.1097/AUD.0b013e31825f9d89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Le Prell, C. G. , Fulbright, A. , Spankovich, C. , Griffiths, S. , Lobarinas, E. , Campbell, K. C. M. , Antonelli, P. J. , Green, G. E. , Guire, K. , and Miller, J. M. (2016). “ Dietary supplement comprised of β-carotene, vitamin C, vitamin E, and magnesium: Failure to prevent music-induced temporary threshold shift,” Audiol. Neurootol. EXTRA 6, 20–39. 10.1159/000446600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Le Prell, C. G. , Hamill, T. L. , and Murphy, W. J. (2019). “ Noise-induced hearing loss: Translating risk from animal models to real-world environments,” J. Acoust. Soc. Am. 146, 3646–3651. 10.1121/1.5133385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Le Prell, C. G. , Johnson, A.-C. , Lindblad, A.-C. , Skjönsberg, A. , Ulfendahl, M. , Guire, K. , Green, G. E. , Campbell, K. C. M. , and Miller, J. M. (2011). “ Increased vitamin plasma levels in Swedish military personnel treated with nutrients prior to automatic weapon training,” Noise Health 13, 432–443. 10.4103/1463-1741.90317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Le Prell, C. G. , and Spankovich, C. (2013). “ Noise-induced hearing loss: Detection, prevention and management,” in Otology and Neurotology, edited by Kirtane M. V., de Souza C. E., Sanna M., and Devaiah A. K. ( Thieme, Stuttgart, Germany: ), pp. 268–284. [Google Scholar]
- 181. Liberman, M. C. , Epstein, M. J. , Cleveland, S. S. , Wang, H. , and Maison, S. F. (2016). “ Toward a differential diagnosis of hidden hearing loss in humans,” PLoS One 11, e0162726. 10.1371/journal.pone.0162726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Lie, A. , Skogstad, M. , Johnsen, T. S. , Engdahl, B. , and Tambs, K. (2016). “ Noise-induced hearing loss in a longitudinal study of Norwegian railway workers,” BMJ Open 6, e011923. 10.1136/bmjopen-2016-011923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Lim, D. J. , Dunn, D. E. , Ferraro, J. A. , and Lempert, B. L. (1982). “ Anatomical changes found in the cochleas of animals exposed to typical industrial noise,” in New Perspectives on Noise-Induced Hearing Loss, edited by Hamernik R. P., Henderson D., and Salvi R. ( Raven Press, New York: ), pp. 23–48. [Google Scholar]
- 184. Lin, C. Y. , Wu, J. L. , Shih, T. S. , Tsai, P. J. , Sun, Y. M. , and Guo, Y. L. (2009). “ Glutathione S-transferase M1, T1, and P1 polymorphisms as susceptibility factors for noise-induced temporary threshold shift,” Hear. Res. 257, 8–15. 10.1016/j.heares.2009.07.008 [DOI] [PubMed] [Google Scholar]
- 185. Lin, C. Y. , Wu, J. L. , Shih, T. S. , Tsai, P. J. , Sun, Y. M. , Ma, M. C. , and Guo, Y. L. (2010). “ N-Acetyl-cysteine against noise-induced temporary threshold shift in male workers,” Hear. Res. 269, 42–47. 10.1016/j.heares.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 186. Lindblad, A. C. , Rosenhall, U. , Olofsson, A. , and Hagerman, B. (2011). “ The efficacy of N-acetylcysteine to protect the human cochlea from subclinical hearing loss caused by impulse noise: A controlled trial,” Noise Health 13, 392–401. 10.4103/1463-1741.90293 [DOI] [PubMed] [Google Scholar]
- 187. Lindeman, H. E. (1976). “ Speech intelligibility and the use of hearing protectors,” Audiology 15, 348–356. 10.3109/00206097609071794 [DOI] [PubMed] [Google Scholar]
- 188. Lindgren, F. , and Axelsson, A. (1983). “ Temporary threshold shift after exposure to noise and music of equal energy,” Ear Hear. 4, 197–201. 10.1097/00003446-198307000-00004 [DOI] [PubMed] [Google Scholar]
- 189. Lipscomb, D. M. (1969a). “ Ear damage from exposure to rock and roll music,” Arch. Otolaryngol. 90, 545–555. 10.1001/archotol.1969.00770030547003 [DOI] [PubMed] [Google Scholar]
- 190. Lipscomb, D. M. (1969b). “ High intensity sounds in the recreational environment. Hazard to young ears,” Clin. Pediatr. (Phila). 8, 63–68. 10.1177/000992286900800205 [DOI] [PubMed] [Google Scholar]
- 191. Lobarinas, E. , Salvi, R. , and Ding, D. (2013). “ Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas,” Hear. Res. 302, 113–120. 10.1016/j.heares.2013.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Lobarinas, E. , Scott, R. , Spankovich, C. , and Le Prell, C. G. (2016). “ Differential effects of suppressors on hazardous sound pressure levels generated by AR-15 rifles: Considerations for recreational shooters, law enforcement, and the military,” Int. J. Audiol. 55, S59–S71. 10.3109/14992027.2015.1122241 [DOI] [PubMed] [Google Scholar]
- 193. Lonsbury-Martin, B. L. , Martin, G. K. , and Bohne, B. A. (1987). “ Repeated TTS exposures in monkeys: Alterations in hearing, cochlear structure, and single-unit thresholds,” J. Acoust. Soc. Am. 81, 1507–1518. 10.1121/1.394503 [DOI] [PubMed] [Google Scholar]
- 194. Markou, K. , Lalaki, P. , Barbetakis, N. , Tsalighopoulos, M. G. , and Daniilidis, I. (2001). “ The efficacy of medication on tinnitus due to acute acoustic trauma,” Scand. Audiol. Suppl. 2001, 180–184. 10.1080/010503901300007461 [DOI] [PubMed] [Google Scholar]
- 195. Markou, K. , Nikolaou, A. , Petridis, D. G. , Vlachtsis, K. C. , Kouloulas, A. , and Daniilidis, I. C. (2004). “ Evaluation of various therapeutic schemes in the treatment of tinnitus due to acute acoustic trauma,” Kulak Burun Boğaz Ihtisas Dergisi (Turk. J. Ear Nose Throat) 12, 107–114, available at https://www.ncbi.nlm.nih.gov/pubmed/16020985. [PubMed] [Google Scholar]
- 196. Masterson, E. A. , Deddens, J. A. , Themann, C. L. , Bertke, S. , and Calvert, G. M. (2015). “ Trends in worker hearing loss by industry sector, 1981–2010,” Am. J. Ind. Med. 58, 392–401. 10.1002/ajim.22429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. McBride, D. , Gill, F. , Proops, D. , Harrington, M. , Gardiner, K. , and Attwell, C. (1992). “ Noise and the classical musician,” BMJ 305, 1561–1563. 10.1136/bmj.305.6868.1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. McGregor, K. D. , Flamme, G. A. , Tasko, S. M. , Deiters, K. K. , Ahroon, W. A. , Themann, C. L. , and Murphy, W. J. (2018). “ Acoustic reflexes are common but not pervasive: Evidence using a diagnostic middle ear analyser,” Int. J. Audiol. 57, S42–S50. 10.1080/14992027.2017.1416189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. McMillan, G. P. , Konrad-Martin, D. , and Dille, M. F. (2012). “ Accuracy of distortion-product otoacoustic emissions-based ototoxicity monitoring using various primary frequency step-sizes,” Int. J. Audiol. 51, 689–696. 10.3109/14992027.2012.688143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Mehrparvar, A. H. , Mirmohammadi, S. J. , Davari, M. H. , Mostaghaci, M. , Mollasadeghi, A. , Bahaloo, M. , and Hashemi, S. H. (2014). “ Conventional audiometry, extended high-frequency audiometry, and DPOAE for early diagnosis of NIHL,” Iran. Red Crescent Med. J. 16, e9628. 10.5812/ircmj.9628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Meinke, D. K. , Finan, D. S. , Flamme, G. A. , Murphy, W. J. , Stewart, M. , Lankford, J. E. , and Tasko, S. (2017). “ Prevention of noise-induced hearing loss from recreational firearms,” Semin. Hearing 38, 267–281. 10.1055/s-0037-1606323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Meinke, D. K. , Finan, D. S. , Soendergaard, J. , Flamme, G. A. , Murphy, W. J. , Lankford, J. E. , and Stewart, M. (2013). “ Impulse noise generated by starter pistols,” Int. J. Audiol. 52, S9–S19. 10.3109/14992027.2012.745650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Meinke, D. K. , Murphy, W. J. , Finan, D. S. , Lankford, J. E. , Flamme, G. A. , Stewart, M. , Soendergaard, J. , and Jerome, T. W. (2014). “ Auditory risk estimates for youth target shooting,” Int. J. Audiol. 53, S16–S25. 10.3109/14992027.2013.865845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Mendes, M. H. , and Morata, T. C. (2007). “ Occupational exposure to music: A review,” Rev. Soc. Bras. Fonoaudiol. 12, 63–69. 10.1590/S1516-80342007000100012 [DOI] [Google Scholar]
- 205. Mercier, V. , and Hohmann, B. W. (2002). “ Is electronically amplified music too loud? What do young people think?,” Noise Health 4, 47–55. [PubMed] [Google Scholar]
- 206. Mick, P. , Foley, D. , Lin, F. , and Pichora-Fuller, M. K. (2018). “ Hearing difficulty is associated with injuries requiring medical care,” Ear Hear. 39, 631–644. 10.1097/AUD.0000000000000535 [DOI] [PubMed] [Google Scholar]
- 207. Mills, J. H. (1976). “ Threshold shifts produced by a 90-day exposure to noise,” in Effects of Noise on Hearing, edited by Henderson D., Hamernik R. P., Dosanjh D. S., and Mills J. H. ( Raven Press, New York: ), pp. 265–275. [Google Scholar]
- 208. Moody, D. B. , Stebbins, W. C. , Hawkins, J. E., Jr. , and Johnsson, L. G. (1978). “ Hearing loss and cochlear pathology in the monkey (Macaca) following exposure to high levels of noise,” Arch. Otorhinolaryngol. 220, 47–72. 10.1007/BF00456301 [DOI] [PubMed] [Google Scholar]
- 209. Moon, I. S. (2007). “ Noise-induced hearing loss caused by gunshot in South Korean military service,” Mil. Med. 172, 421–425. 10.7205/MILMED.172.4.421 [DOI] [PubMed] [Google Scholar]
- 210. Moon, I. S. , Park, S. Y. , Park, H. J. , Yang, H. S. , Hong, S. J. , and Lee, W. S. (2011). “ Clinical characteristics of acoustic trauma caused by gunshot noise in mass rifle drills without ear protection,” J. Occup. Env. Hyg. 8, 618–623. 10.1080/15459624.2011.609013 [DOI] [PubMed] [Google Scholar]
- 211. Müller, M. , Tisch, M. , Maier, H. , and Lowenheim, H. (2016). “ Reduction of permanent hearing loss by local glucocorticoid application: Guinea pigs with acute acoustic trauma,” HNO 64, 831–840. 10.1007/s00106-016-0256-1 [DOI] [PubMed] [Google Scholar]
- 212. Müller, M. , Tisch, M. , Maier, H. , and Lowenheim, H. (2017). “ Reduction of permanent hearing loss by local glucocorticoid application: Guinea pigs with acute acoustic trauma,” HNO 65, 59–67. 10.1007/s00106-016-0266-z [DOI] [PubMed] [Google Scholar]
- 213. Murphy, W. J. , Flamme, G. A. , Campbell, A. R. , Zechmann, E. L. , Tasko, S. M. , Lankford, J. E. , Meinke, D. K. , Finan, D. S. , and Stewart, M. (2018). “ The reduction of gunshot noise and auditory risk through the use of firearm suppressors and low-velocity ammunition,” Int. J. Audiol. 57, S28–S41. 10.1080/14992027.2017.1407459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Murphy, W. J. , and Le Prell, C. G. (2017). “ Making sound waves: Selected papers from the 2016 annual conference of the National Hearing Conservation Association,” Int. J. Audiol. 56, 1–3. 10.1080/14992027.2016.1271145 [DOI] [PubMed] [Google Scholar]
- 215. Murphy, W. J. , Themann, C. L. , and Murata, T. K. (2016). “ Hearing protector fit testing with off-shore oil-rig inspectors in Louisiana and Texas,” Int. J. Audiol. 55, 688–698. 10.1080/14992027.2016.1204470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Murphy, W. J. , and Tubbs, R. L. (2007). “ Assessment of noise exposure for indoor and outdoor firing ranges,” J. Occup. Environ. Hyg. 4, 688–697. 10.1080/15459620701537390 [DOI] [PubMed] [Google Scholar]
- 217. Murphy, W. J. , and Xiang, N. (2019). “ Room acoustic modeling and auralization at an indoor firing range,” J. Acoust. Soc. Am. 146, 3868–3872. 10.1121/1.5132286 [DOI] [PubMed] [Google Scholar]
- 218. Naert, G. , Pasdelou, M.-P. , and Le Prell, C. G. (2019). “ Use of the Guinea pig in studies on the development and prevention of acquired sensorineural hearing loss, with an emphasis on noise,” J. Acoust. Soc. Am. 146, 3743–3769. 10.1121/1.5132711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.National Occupational Research Agenda (NORA) Hearing Loss Prevention Cross-Sector Council (2019). “ National occupational research agenda for hearing loss prevention,” available at https://www.cdc.gov/nora/councils/hlp/pdfs/National_Occupational_Research_Agenda_for_HLP_July_2019-2508.pdf (Last viewed 9/17/2019).
- 220. Neitzel, R. L. , and Fligor, B. J. (2019). “ Risk of noise-induced hearing loss due to recreational sound: Review and recommendations,” J. Acoust. Soc. Am. 146, 3911–3921. 10.1121/1.5132287 [DOI] [PubMed] [Google Scholar]
- 221. Nelson, D. I. , Nelson, R. Y. , Concha-Barrientos, M. , and Fingerhut, M. (2005). “ The global burden of occupational noise-induced hearing loss,” Am. J. Ind. Med. 48, 446–458. 10.1002/ajim.20223 [DOI] [PubMed] [Google Scholar]
- 222. Nelson, J. T. , Swan, A. A. , Swiger, B. , Packer, M. , and Pugh, M. J. (2017). “ Hearing testing in the U.S. Department of Defense: Potential impact on Veterans Affairs hearing loss disability awards,” Hear. Res. 349, 13–20. 10.1016/j.heares.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 223. Nielsen, D. W. , Bauman, M. J. , and Brandt, D. K. (1986). “ Changes in auditory threshold during and after long duration noise exposure: Species differences,” in Basic and Applied Aspects of Noise-Induced Hearing Loss, edited by Salvi R. J., Henderson D., Hamernik R. P., and Colletti V. ( Plenum Press, New York: ), pp. 281–293. [Google Scholar]
- 224.NIOSH (1998). Criteria for a Recommended Standard, Occupational Noise Exposure, DHHS (NIOSH) Publication No. 98-126 ( DHHS/CDC/NIOSH, Cincinnati, OH: ). [Google Scholar]
- 225. Niskar, A. S. , Kieszak, S. M. , Holmes, A. E. , Esteban, E. , Rubin, C. , and Brody, D. J. (2001). “ Estimated prevalence of noise-induced hearing threshold shifts among children 6 to 19 years of age: The Third National Health and Nutrition Examination Survey, 1988-1994, United States,” Pediatrics 108, 40–43. 10.1542/peds.108.1.40 [DOI] [PubMed] [Google Scholar]
- 226. Norin, J. A. , Emanuel, D. C. , and Letowski, T. R. (2011). “ Speech intelligibility and passive, level-dependent earplugs,” Ear Hear. 32, 642–649. 10.1097/AUD.0b013e31821478c8 [DOI] [PubMed] [Google Scholar]
- 227. O'Brien, I. , Driscoll, T. , and Ackermann, B. (2015). “ Description and evaluation of a hearing conservation program in use in a professional symphony orchestra,” Ann. Occup. Hyg. 59, 265–276. 10.1093/annhyg/meu092 [DOI] [PubMed] [Google Scholar]
- 228. O'Brien, I. , Driscoll, T. , Williams, W. , and Ackermann, B. (2014). “ A clinical trial of active hearing protection for orchestral musicians,” J. Occup. Env. Hyg. 11, 450–459. 10.1080/15459624.2013.875187 [DOI] [PubMed] [Google Scholar]
- 229. Obeling, L. , and Poulsen, T. (1999). “ Hearing ability in Danish symphony orchestra musicians,” Noise Health 1, 43–49. [PubMed] [Google Scholar]
- 230. Ohlemiller, K. K. (2019). “ Mouse methods and models for studies in hearing,” J. Acoust. Soc. Am. 146, 3668–3680. 10.1121/1.5132550 [DOI] [PubMed] [Google Scholar]
- 231. Okada, H. , Yamane, H. , and Nakai, Y. (1991). “ Morphological changes of the spiral vessel after rock music exposure,” Acta Otolaryngol. Suppl. (Stockh). 486, 61–65. 10.3109/00016489109134983 [DOI] [PubMed] [Google Scholar]
- 232. Opperman, D. A. , Reifman, W. , Schlauch, R. , and Levine, S. (2006). “ Incidence of spontaneous hearing threshold shifts during modern concert performances,” Otolaryngol. Head Neck Surg. 134, 667–673. 10.1016/j.otohns.2005.11.039 [DOI] [PubMed] [Google Scholar]
- 233.OSHA (1983). 29 CFR 1910.95, Occupational Noise Exposure; Hearing Conservation Amendment; Final Rule, effective 8 March 1983.
- 234. Ostri, B. , Eller, N. , Dahlin, E. , and Skylv, G. (1989). “ Hearing impairment in orchestral musicians,” Scand. Audiol. 18, 243–249. 10.3109/01050398909042202 [DOI] [PubMed] [Google Scholar]
- 235. Palmer, K. T. , D'Angelo, S. , Harris, E. C. , Linaker, C. , and Coggon, D. (2015). “ Sensory impairments, problems of balance and accidental injury at work: A case-control study,” Occup. Environ. Med. 72, 195–199. 10.1136/oemed-2014-102422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Park, Y. , Guercio, D. , Ledon, V. , and Le Prell, C. G. (2017). “ Variation in music player listening level as a function of campus location,” J. Am. Acad. Audiol. 28, 295–313. 10.3766/jaaa.16011 [DOI] [PubMed] [Google Scholar]
- 237. Passchier-Vermeer, W. (1968). “Hearing loss due to exposure to steadystate broadband noise,” Report No. 35 and Supplement to Report No. 35 ( Institute for Public Health Engineering, The Netherlands: ). [Google Scholar]
- 238. Pawlaczyk-Luszczynska, M. , Dudarewicz, A. , Zamojska, M. , and Sliwinska-Kowalska, M. (2011). “ Evaluation of sound exposure and risk of hearing impairment in orchestral musicians,” Int. J. Occup. Saf. Ergonomics 17, 255–269. 10.1080/10803548.2011.11076892 [DOI] [PubMed] [Google Scholar]
- 239. Pekkarinen, E. , Viljanen, V. , Salmivalli, A. , and Suonpaa, J. (1990). “ Speech recognition in a noisy and reverberant environment with and without earmuffs,” Audiology 29, 286–293. 10.3109/00206099009072859 [DOI] [PubMed] [Google Scholar]
- 240. Peng, J. H. , Tao, Z. Z. , and Huang, Z. W. (2007). “ Long-term sound conditioning increases distortion product otoacoustic emission amplitudes and decreases olivocochlear efferent reflex strength,” Neuroreport 18, 1167–1170. 10.1097/WNR.0b013e32820049a8 [DOI] [PubMed] [Google Scholar]
- 241. Pilgramm, M. , and Schumann, K. (1985). “ Hyperbaric oxygen therapy for acute acoustic trauma,” Arch. Otorhinolaryngol. 241, 247–257. 10.1007/BF00453696 [DOI] [PubMed] [Google Scholar]
- 242. Portnuff, C. D. (2016). “ Reducing the risk of music-induced hearing loss from overuse of portable listening devices: Understanding the problems and establishing strategies for improving awareness in adolescents,” Adolesc. Health Med. Ther. 7, 27–35. 10.2147/AHMT.S74103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Portnuff, C. D. , Fligor, B. J. , and Arehart, K. H. (2011). “ Teenage use of portable listening devices: A hazard to hearing?,” J. Am. Acad. Audiol. 22, 663–677. 10.3766/jaaa.22.10.5 [DOI] [PubMed] [Google Scholar]
- 244. Portnuff, C. D. F. , and Price, D. (2019). “ Validation of clinical techniques for verification of uniform attenuation earplugs,” Int. J. Audiol. 58, S33–S39. 10.1080/14992027.2018.1532119 [DOI] [PubMed] [Google Scholar]
- 245. Prendergast, G. , Guest, H. , Munro, K. , Hall, D. , Kluk, K. , Léger, A. , Hall, D. A. , Heinz, M. G. , and Plack, C. J. (2017a). “ Effects of noise exposure on young adults with normal audiograms I: Electrophysiology,” Hear. Res. 344, 68–81. 10.1016/j.heares.2016.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Prendergast, G. , Millman, R. E. , Guest, H. , Munro, K. J. , Kluk, K. , Dewey, R. S. , Hall, D. A. , Heinz, M. G. , and Plack, C. J. (2017b). “ Effects of noise exposure on young adults with normal audiograms II: Behavioral measures,” Hear. Res. 356, 74–86. 10.1016/j.heares.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Psillas, G. , Pavlidis, P. , Karvelis, I. , Kekes, G. , Vital, V. , and Constantinidis, J. (2008). “ Potential efficacy of early treatment of acute acoustic trauma with steroids and piracetam after gunshot noise,” Eur. Arch. Otorhinolaryngol. 265, 1465–1469. 10.1007/s00405-008-0689-6 [DOI] [PubMed] [Google Scholar]
- 248. Radziwon, K. , Sheppard, A. , and Salvi, R. J. (2019). “ Psychophysical changes in temporal processing in chinchillas with noise-induced hearing loss,” J. Acoust. Soc. Am. 146, 3733–3742. 10.1121/1.5132292 [DOI] [PubMed] [Google Scholar]
- 249. Ramakers, G. G. , Kraaijenga, V. J. , Cattani, G. , van Zanten, G. A. , and Grolman, W. (2016). “ Effectiveness of earplugs in preventing recreational noise-induced hearing loss: A randomized clinical trial,” JAMA Otolaryngol. Head Neck Surg. 142, 551–558. 10.1001/jamaoto.2016.0225 [DOI] [PubMed] [Google Scholar]
- 250. Reavis, K. M. , McMillan, G. , Austin, D. , Gallun, F. , Fausti, S. A. , Gordon, J. S. , Helt, W. J. , and Konrad-Martin, D. (2011). “ Distortion-product otoacoustic emission test performance for ototoxicity monitoring,” Ear Hear. 32, 61–74. 10.1097/AUD.0b013e3181e8b6a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Reavis, K. M. , Phillips, D. S. , Fausti, S. A. , Gordon, J. S. , Helt, W. J. , Wilmington, D. , Bratt, G. W. , and Konrad-Martin, D. (2008). “ Factors affecting sensitivity of distortion-product otoacoustic emissions to ototoxic hearing loss,” Ear Hear. 29, 875–893. 10.1097/AUD.0b013e318181ad99 [DOI] [PubMed] [Google Scholar]
- 252. Reddell, R. C. , and Lebo, C. P. (1972). “ Ototraumatic effects of hard rock music,” Calif. Med. 116, 1–4. [PMC free article] [PubMed] [Google Scholar]
- 253. Riga, M. , Korres, G. , Balatsouras, D. , and Korres, S. (2010). “ Screening protocols for the prevention of occupational noise-induced hearing loss: The role of conventional and extended high frequency audiometry may vary according to the years of employment,” Med. Sci. Monit. 16, CR352–CR356. 10.12659/MSM.880932 [DOI] [PubMed] [Google Scholar]
- 254. Rintelmann, W. F. , and Borus, J. F. (1968). “ Noise-induced hearing loss and rock and roll music,” Arch. Otolaryngol. 88, 377–385. 10.1001/archotol.1968.00770010379010 [DOI] [PubMed] [Google Scholar]
- 255. Roberts, B. , and Neitzel, R. L. (2019). “ Noise exposure limit for children in recreational settings: Review of available evidence,” J. Acoust. Soc. Am. 146, 3922–3933. 10.1121/1.5132540 [DOI] [PubMed] [Google Scholar]
- 256. Robinson, G. S. , and Casali, J. G. (2003). “ Speech communications and signal detection in noise,” in The Noise Manual, 5th ed., edited by Berger E. H., Royster L. H., Royster J. D., Driscoll D. P., and Layne M. ( American Industrial Hygiene Association, Fairfax: ), pp. 567–600. [Google Scholar]
- 257. Rosowski, J. J. , Remenschneider, A. K. , and Cheng, J. T. (2019). “ Limitations of present models of blast-induced sound power conduction through the external and middle ear,” J. Acoust. Soc. Am. 146, 3978–3992. 10.1121/1.5132288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Royster, J. D. , Royster, L. H. , and Killion, M. C. (1991). “ Sound exposures and hearing thresholds of symphony orchestra musicians,” J. Acoust. Soc. Am. 89, 2793–2803. 10.1121/1.400719 [DOI] [PubMed] [Google Scholar]
- 259. Sadhra, S. , Jackson, C. A. , Ryder, T. , and Brown, M. J. (2002). “ Noise exposure and hearing loss among student employees working in university entertainment venues,” Ann. Occup. Hyg. 46, 455–463. [PubMed] [Google Scholar]
- 260. Santoni, C. B. , and Fiorini, A. C. (2010). “ Pop-rock musicians: Assessment of their satisfaction provided by hearing protectors,” Braz. J. Otorhinolaryngol. 76, 454–461. 10.1590/S1808-86942010000400009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Saunders, J. C. , and Tilney, L. G. (1982). “ Species differences in susceptability to noise exposure,” in New perspectives on Noise-Induced Hearing Loss, edited by Hamernik R. P., Henderson D., and Salvi R. ( Raven Press, New York: ), pp. 229–248. [Google Scholar]
- 262. Sayler, S. K. , Rabinowitz, P. M. , Galusha, D. , Sun, K. , and Neitzel, R. L. (2019). “ Hearing Protector Attenuation and Noise Exposure Among Metal Manufacturing Workers,” Ear Hear. 40, 680–689. 10.1097/AUD.0000000000000650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Scheibe, F. , Haupt, H. , Ising, H. , and Cherny, L. (2002). “ Therapeutic effect of parenteral magnesium on noise-induced hearing loss in the guinea pig,” Magnes. Res. 15, 27–36. [PubMed] [Google Scholar]
- 264. Schmidt, J. H. , Paarup, H. M. , and Baelum, J. (2019). “ Tinnitus severity is related to the sound exposure of symphony orchestra musicians independently of hearing impairment,” Ear Hear. 40, 88–97. 10.1097/AUD.0000000000000594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Schmidt, J. H. , Pedersen, E. R. , Paarup, H. M. , Christensen-Dalsgaard, J. , Andersen, T. , Poulsen, T. , and Baelum, J. (2014). “ Hearing loss in relation to sound exposure of professional symphony orchestra musicians,” Ear Hear. 35, 448–460. 10.1097/AUD.0000000000000029 [DOI] [PubMed] [Google Scholar]
- 266. Schulz, T. Y. (2011). “ Individual fit-testing of earplugs: A review of uses,” Noise Health 13, 152–162. 10.4103/1463-1741.77216 [DOI] [PubMed] [Google Scholar]
- 267. Seever, K. L. , Johnson, C. E. , Baldwin, J. , Danhauer, J. L. , Wolfe, B. , and Jeannont, S. (2018). “ Effects of including information about hidden hearing loss in an Adopt-A-Band Program on college band members' attitudes toward healthy hearing behaviors,” Semin. Hearing 39, 210–220. 10.1055/s-0038-1641744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Seixas, N. S. , Kujawa, S. G. , Norton, S. , Sheppard, L. , Neitzel, R. , and Slee, A. (2004). “ Predictors of hearing threshold levels and distortion product otoacoustic emissions among noise exposed young adults,” Occup. Environ. Med. 61, 899–907. 10.1136/oem.2003.009209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Seixas, N. S. , Neitzel, R. , Stover, B. , Sheppard, L. , Feeney, P. , Mills, D. , and Kujawa, S. (2012). “ 10-Year prospective study of noise exposure and hearing damage among construction workers,” Occup. Environ. Med. 69, 643–650. 10.1136/oemed-2011-100578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Semeraro, H. D. , Bevis, Z. L. , Rowan, D. , van Besouw, R. M. , and Allsopp, A. J. (2015). “ Fit for the frontline? identification of mission-critical auditory tasks (MCATs) carried out by infantry and combat-support personnel,” Noise Health 17, 98–107. 10.4103/1463-1741.153401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Sendowski, I. , Abaamrane, L. , Raffin, F. , Cros, A. , and Clarencon, D. (2006). “ Therapeutic efficacy of intra-cochlear administration of methylprednisolone after acoustic trauma caused by gunshot noise in guinea pigs,” Hear. Res. 221, 119–127. 10.1016/j.heares.2006.08.010 [DOI] [PubMed] [Google Scholar]
- 272. Sheffield, B. , Brungart, D. , Tufts, J. , and Ness, J. (2017). “ The effects of elevated hearing thresholds on performance in a paintball simulation of individual dismounted combat,” Int. J. Audiol. 56, 34–40. 10.1080/14992027.2016.1255360 [DOI] [PubMed] [Google Scholar]
- 273. Shuster, B. Z. , Depireux, D. A. , Mong, J. A. , and Hertzano, R. (2019). “ Sex differences in hearing: Probing the role of estrogen signaling,” J. Acoust. Soc. Am. 145, 3656–3663. 10.1121/1.5111870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Sisto, R. , Chelotti, S. , Moriconi, L. , Pellegrini, S. , Citroni, A. , Monechi, V. , Gaeta, R. , Pinto, I. , Stacchini, N. , and Moleti, A. (2007). “ Otoacoustic emission sensitivity to low levels of noise-induced hearing loss,” J. Acoust. Soc. Am. 122, 387–401. 10.1121/1.2737668 [DOI] [PubMed] [Google Scholar]
- 275. Skellett, R. A. , Cullen, J. K., Jr. , Fallon, M. , and Bobbin, R. P. (1998). “ Conditioning the auditory system with continuous vs. interrupted noise of equal acoustic energy: Is either exposure more protective?,” Hear. Res. 116, 21–32. 10.1016/S0378-5955(97)00199-8 [DOI] [PubMed] [Google Scholar]
- 276. Skoe, E. , and Tufts, J. (2018). “ Evidence of noise-induced subclinical hearing loss using auditory brainstem responses and objective measures of noise exposure in humans,” Hear. Res. 361, 80–91. 10.1016/j.heares.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 277. Slater, J. , Ashley, R. , Tierney, A. , and Kraus, N. (2018). “ Got rhythm? Better inhibitory control is linked with more consistent drumming and enhanced neural tracking of the musical beat in adult percussionists and nonpercussionists,” J. Cogn. Neurosci. 30, 14–24. 10.1162/jocn_a_01189 [DOI] [PubMed] [Google Scholar]
- 278. Slater, J. , Azem, A. , Nicol, T. , Swedenborg, B. , and Kraus, N. (2017). “ Variations on the theme of musical expertise: Cognitive and sensory processing in percussionists, vocalists and non-musicians,” Eur. J. Neurosci. 45, 952–963. 10.1111/ejn.13535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Sliwinska-Kowalska, M. , and Zaborowski, K. (2017). “ WHO environmental noise guidelines for the European region: A systematic review on environmental noise and permanent hearing loss and tinnitus,” Int. J. Environ. Res. Public Health 14, E1139. 10.3390/ijerph14101139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Spankovich, C. , and Le Prell, C. G. (2019). “ The role of diet in vulnerability to noise-induced cochlear injury and hearing loss,” J. Acoust. Soc. Am. 146, 4033–4043. 10.1121/1.5132707 [DOI] [PubMed] [Google Scholar]
- 281. Spankovich, C. , Le Prell, C. G. , Hood, L. J. , and Lobarinas, E. (2017). “ Noise history and auditory function in young adults with and without Type-1 diabetes,” Ear Hear. 38, 724–735. 10.1097/AUD.0000000000000457 [DOI] [PubMed] [Google Scholar]
- 282. Stewart, M. , Pankiw, R. , Lehman, M. E. , and Simpson, T. H. (2002). “ Hearing loss and hearing handicap in users of recreational firearms,” J. Am. Acad. Audiol. 13, 160–168. [PubMed] [Google Scholar]
- 283. Strait, D. L. , Slater, J. , O'Connell, S. , and Kraus, N. (2015). “ Music training relates to the development of neural mechanisms of selective auditory attention,” Developmental cognitive neuroscience 12, 94–104. 10.1016/j.dcn.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Strasser, H. , Irle, H. , and Legler, R. (2003). “ Temporary hearing threshold shifts and restitution after energy-equivalent exposures to industrial noise and classical music,” Noise Health 5, 75–84. [PubMed] [Google Scholar]
- 285. Suckfuell, M. , Canis, M. , Strieth, S. , Scherer, H. , and Haisch, A. (2007). “ Intratympanic treatment of acute acoustic trauma with a cell-permeable JNK ligand: A prospective randomized phase I/II study,” Acta Otolaryngol. 127, 938–942. 10.1080/00016480601110212 [DOI] [PubMed] [Google Scholar]
- 286. Suter, A. H. (2007). “ Development of standards and regulations for occupational noise,” in Handbook of Noise and Vibration Control, edited by Crocker M. ( John Wiley and Sons, Inc, Hoboken: ), pp. 377–382. [Google Scholar]
- 287. Suter, A. H. (2017). “ Occupational hearing loss from non-Gaussian noise,” Semin. Hear. 38, 225–262. 10.1055/s-0037-1603726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Swan, A. A. , Nelson, J. T. , Swiger, B. , Jaramillo, C. A. , Eapen, B. C. , Packer, M. , and Pugh, M. J. (2017). “ Prevalence of hearing loss and tinnitus in Iraq and Afghanistan veterans: A chronic effects of neurotrauma consortium study,” Hear. Res. 349, 4–12. 10.1016/j.heares.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 289. Talcott, K. A. , Casali, J. G. , Keady, J. P. , and Killion, M. C. (2012). “ Azimuthal auditory localization of gunshots in a realistic field environment: Effects of open-ear versus hearing protection-enhancement devices (HPEDs), military vehicle noise, and hearing impairment,” Int. J. Audiol. 51, S20–S30. 10.3109/14992027.2011.631591 [DOI] [PubMed] [Google Scholar]
- 290. Themann, C. L. , and Masterson, E. A. (2019). “ Review: Occupational noise exposure and hearing loss,” J. Acoust. Soc. Am. 146, 3879–3905. 10.1121/1.5134465 [DOI] [PubMed] [Google Scholar]
- 291. Tierney, A. T. , Krizman, J. , and Kraus, N. (2015). “ Music training alters the course of adolescent auditory development,” Proc. Natl. Acad. Sci. U.S.A. 112, 10062–10067. 10.1073/pnas.1505114112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Trevino, M. , Lobarinas, E. , Maulden, A. C. , and Heinz, M. G. (2019). “ The chinchilla animal model for hearing science and noise-induced hearing loss,” J. Acoust. Soc. Am. 146, 3710–3732. 10.1121/1.5132950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Tufts, J. B. , Hamilton, M. A. , Ucci, A. J. , and Rubas, J. (2011). “ Evaluation by industrial workers of passive and level-dependent hearing protection devices,” Noise Health 13, 26–36. 10.4103/1463-1741.73998 [DOI] [PubMed] [Google Scholar]
- 294. Tufts, J. B. , Vasil, K. A. , and Briggs, S. (2009). “ Auditory fitness for duty: A review,” J. Am. Acad. Audiol. 20, 539–557. 10.3766/jaaa.20.9.3 [DOI] [PubMed] [Google Scholar]
- 295. Valderrama, J. T. , Beach, E. F. , Yeend, I. , Sharma, M. , Van Dun, B. , and Dillon, H. (2018). “ Effects of lifetime noise exposure on the middle-age human auditory brainstem response, tinnitus and speech-in-noise intelligibility,” Hear. Res. 365, 36–48. 10.1016/j.heares.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 296. Voix, J. , and Hager, L. D. (2009). “ Individual fit testing of hearing protection devices,” Int. J. Occup. Saf. Ergonomics 15, 211–219. 10.1080/10803548.2009.11076802 [DOI] [PubMed] [Google Scholar]
- 297. Wada, T. , Sano, H. , Nishio, S. Y. , Kitoh, R. , Ikezono, T. , Iwasaki, S. , Kaga, K. , Matsubara, A. , Matsunaga, T. , Murata, T. , Naito, Y. , Suzuki, M. , Takahashi, H. , Tono, T. , Yamashita, H. , Hara, A. , and Usami, S. I. (2017). “ Differences between acoustic trauma and other types of acute noise-induced hearing loss in terms of treatment and hearing prognosis,” Acta Otolaryngol. (Stockh). 137, S48–S52. 10.1080/00016489.2017.1297899 [DOI] [PubMed] [Google Scholar]
- 298. Wagoner, L. , McGlothlin, J. , Chung, K. , Strickland, E. , Zimmerman, N. , and Carlson, G. (2007). “ Evaluation of noise attenuation and verbal communication capabilities using three ear insert hearing protection systems among airport maintenance personnel,” J. Occup. Environ. Hyg. 4, 114–122. 10.1080/15459620601126377 [DOI] [PubMed] [Google Scholar]
- 299. Wall, A. T. , Wagner, C. M. , Rasband, R. D. , Gee, K. L. , and Murphy, W. J. (2019). “ Cumulative noise exposure model for outdoor shooting ranges,” J. Acoust. Soc. Am. 146, 3863–3867. 10.1121/1.5132289 [DOI] [PubMed] [Google Scholar]
- 300. Wang, J. , and Puel, J. L. (2018). “ Toward cochlear therapies,” Physiol. Rev. 98, 2477–2522. 10.1152/physrev.00053.2017 [DOI] [PubMed] [Google Scholar]
- 301. Ward, W. D. (1991). “ Hearing loss from noise and music,” presented at Audio Engineering Society, New York: (Oct. 4–8, 1991). [Google Scholar]
- 302. Wartinger, F. , Malyuk, H. , and Portnuff, C. D. (2019). “ Human exposures and their associated hearing loss profiles: Music industry professionals,” J. Acoust. Soc. Am. 146, 3906–3910. 10.1121/1.5132541 [DOI] [PubMed] [Google Scholar]
- 303. Watts, K. L. , Welles, R. , and Zurek, P. (2018). “ Development of the warfighter's hearing health instructional (WHHIP) primer app,” Mil. Med. 183, 231–236. 10.1093/milmed/usx177 [DOI] [PubMed] [Google Scholar]
- 304. Wenmaekers, R. , Nicolai, B. , Hornikx, M. , and Kohlrausch, A. (2017). “ Why orchestral musicians are bound to wear earplugs: About the ineffectiveness of physical measures to reduce sound exposure,” J. Acoust. Soc. Am. 142, 3154. 10.1121/1.5012689 [DOI] [PubMed] [Google Scholar]
- 305. White-Schwoch, T. , Woodruff Carr, K. , Anderson, S. , Strait, D. L. , and Kraus, N. (2013). “ Older adults benefit from music training early in life: Biological evidence for long-term training-driven plasticity,” J. Neurosci. 33, 17667–17674. 10.1523/JNEUROSCI.2560-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Woodcock, K. , and Pole, J. D. (2008). “ Educational attainment, labour force status and injury: A comparison of Canadians with and without deafness and hearing loss,” Int. J. Rehabil. Res. 31, 297–304. 10.1097/MRR.0b013e3282fb7d4d [DOI] [PubMed] [Google Scholar]
- 307.World Health Organization (2015). “ Hearing loss due to recreational exposure to loud sounds: A review,” http://apps.who.int/iris/bitstream/10665/154589/1/9789241508513_eng.pdf (Last viewed 9/17/2019).
- 308. Xie, H. W. , Qiu, W. , Heyer, N. J. , Zhang, M. B. , Zhang, P. , Zhao, Y. M. , and Hamernik, R. P. (2016). “ The use of the kurtosis-adjusted cumulative noise exposure metric in evaluating the hearing loss risk for complex noise,” Ear Hear. 37, 312–323. 10.1097/AUD.0000000000000251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Xiong, M. , He, Q. , Lai, H. , Huang, W. , Wang, L. , Yang, C. , and Wang, J. (2011a). “ Radix astragali injection enhances recovery from acute acoustic trauma,” Acta Otolaryngol. (Stockh). 131, 1069–1073. 10.3109/00016489.2011.591823 [DOI] [PubMed] [Google Scholar]
- 310. Xiong, M. , He, Q. , Lai, H. , and Wang, J. (2012a). “ Astragaloside IV inhibits apoptotic cell death in the guinea pig cochlea exposed to impulse noise,” Acta Otolaryngol. (Stockh). 132, 467–474. 10.3109/00016489.2011.643457 [DOI] [PubMed] [Google Scholar]
- 311. Xiong, M. , Lai, H. , He, Q. , and Wang, J. (2011b). “ Astragaloside IV attenuates impulse noise-induced trauma in guinea pig,” Acta Otolaryngol. (Stockh). 131, 809–816. 10.3109/00016489.2011.568524 [DOI] [PubMed] [Google Scholar]
- 312. Xiong, M. , Lai, H. , Yang, C. , Huang, W. , Wang, J. , Fu, X. , and He, Q. (2012b). “ Comparison of the protective effects of radix astragali, alpha-lipoic acid, and vitamin E on acute acoustic trauma,” Ear. Nose. Throat J. 5, 25–31. 10.4137/CMENT.S10711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Xiong, M. , Zhu, Y. , Lai, H. , Fu, X. , Deng, W. , Yang, C. , He, Q. , and Zheng, G. (2015). “ Radix astragali inhibits the down-regulation of connexin 26 in the stria vascularis of the guinea pig cochlea after acoustic trauma,” Eur. Arch. Otorhinolaryngol. 272, 2153–2160. 10.1007/s00405-014-3093-4 [DOI] [PubMed] [Google Scholar]
- 314. Yankaskas, K. (2013). “ Prelude: Noise-induced tinnitus and hearing loss in the military,” Hear. Res. 295, 3–8. 10.1016/j.heares.2012.04.016 [DOI] [PubMed] [Google Scholar]
- 315. Zaccardi, T. , and Le Prell, C. G. (2019). “ Sound level attenuation using high fidelity hearing protection devices: Human ears,” NHCA Spectrum 36, 42. [Google Scholar]
- 316. Zaccardi, T. , Palmer, K. , Grinn, S. K. , and Le Prell, C. G. (2018). “ Attenuation using high fidelity hearing protection devices,” NHCA Spectrum 35, 40. [Google Scholar]
- 317. Zander, M. F. , Spahn, C. , and Richter, B. (2008). “ Employment and acceptance of hearing protectors in classical symphony and opera orchestras,” Noise Health 10, 14–26. 10.4103/1463-1741.39004 [DOI] [PubMed] [Google Scholar]
- 318. Zhai, S. Q. , Wang, D. J. , Wang, J. L. , Han, D. Y. , and Yang, W. Y. (2004). “ Basic fibroblast growth factor protects auditory neurons and hair cells from glutamate neurotoxicity and noise exposure,” Acta Otolaryngol 124, 124–129. 10.1080/00016480310015939 [DOI] [PubMed] [Google Scholar]
- 319. Zhang, J. (2019). “ Blast-induced tinnitus: Animal models,” J. Acoust. Soc. Am. 146, 3811–3831. 10.1121/1.5132551 [DOI] [PubMed] [Google Scholar]
- 320. Zhao, Y. M. , Qiu, W. , Zeng, L. , Chen, S. S. , Cheng, X. R. , Davis, R. I. , and Hamernik, R. P. (2010). “ Application of the kurtosis statistic to the evaluation of the risk of hearing loss in workers exposed to high-level complex noise,” Ear Hear. 31, 527–532. 10.1097/AUD.0b013e3181d94e68 [DOI] [PubMed] [Google Scholar]
- 321. Zhou, Y. , Zheng, G. , Zheng, H. , Zhou, R. , Zhu, X. , and Zhang, Q. (2013). “ Primary observation of early transtympanic steroid injection in patients with delayed treatment of noise-induced hearing loss,” Audiol. Neurootol. 18, 89–94. 10.1159/000345208 [DOI] [PubMed] [Google Scholar]
- 322. Zhou, Y. , Zheng, H. , Shen, X. , Zhang, Q. , and Yang, M. (2009). “ Intratympanic administration of methylprednisolone reduces impact of experimental intensive impulse noise trauma on hearing,” Acta Otolaryngol. (Stockh). 129, 602–607. 10.1080/00016480802342424 [DOI] [PubMed] [Google Scholar]