Abstract
With advances in the understanding of mechanisms of noise injury, the past 30 years have brought numerous efforts to identify drugs that prevent noise-induced hearing loss (NIHL). The diverse protocols used across investigations have made comparisons across drugs difficult. A systematic review of the literature by Hammill [(2017). Doctoral thesis, The University of Texas at Austin] identified original reports of chemical interventions to prevent or treat hearing loss caused by noise exposure. An initial search returned 3492 articles. After excluding duplicate articles and articles that did not meet the systematic review inclusion criteria, a total of 213 studies published between 1977 and 2016 remained. Reference information, noise exposure parameters, species, sex, method of NIHL assessment, and pharmaceutical intervention details for these 213 studies were entered into a database. Frequency-specific threshold shifts in control animals (i.e., in the absence of pharmaceutical intervention) are reported here. Specific patterns of hearing loss as a function of species and noise exposure parameters are provided to facilitate the selection of appropriate pre-clinical models. The emphasis of this report is octave band noise exposure, as this is one of the most common exposure protocols across pharmacological otoprotection studies.
I. INTRODUCTION
The effects of noise on the inner ear are a topic of long-standing interest, with some of the earliest descriptions of human noise-induced hearing loss (NIHL) occurring in blacksmiths and boilermakers, and some of the earliest work in animal models emerging in the early 1900s (for review, see Hawkins and Schacht, 2005). Early efforts to explore noise-induced “temporary deafness” in humans revealed the greatest vulnerability at 4 kHz, larger changes in hearing with higher level and longer duration exposures, slower recovery after larger hearing changes, and significant individual differences in noise-induced changes in hearing (Davis et al., 1950). These early studies included exposures to 0.5, 1, 2, and 4 kHz tones and band spectrum noise at levels of 110, 120, and 130 dB for periods of 1–64 min, and temporary changes in hearing were 60 dB or greater in the most vulnerable individuals when tested in some exposure conditions (Davis et al., 1950).
Temporary changes in hearing, now termed temporary threshold shifts (TTS), recover subsequent to the noise exposure; lasting changes that do not recover within a period of several weeks to one month are termed permanent threshold shifts (PTS; for review, see Ryan et al., 2016). Although NIHL is generally considered preventable (i.e., with the implementation of engineering controls, administrative controls, or the use of hearing protection devices), NIHL is still reported in many populations, including, for example, service members (Hecht and Hammill, 2019; Jokel et al., 2019), firearm users (Wall et al., 2019), individuals exposed to occupational noise (Themann and Masterson, 2019), professional musicians (Wartinger et al., 2019), individuals exposed to recreational sound (Neitzel and Fligor, 2019), and children exposed to loud sound (Roberts and Neitzel, 2019).
The large number of affected individuals, the costs of compensation and rehabilitation, and adverse effects on quality of life have driven multiple efforts to identify mechanisms of injury underlying NIHL. In addition to mechanical trauma (for examples, see Henderson and Hamernik, 1986; Wang et al., 2002), there has been significant effort to identify the potential contributions of metabolic stress, activation of JNK pathways, activation of TNF-α, and calcium-induced excitotoxicity (for reviews and discussion, see Le Prell et al., 2007b; Abi-Hachem et al., 2010; Poirrier et al., 2010; Le Prell and Bao, 2012). Furthermore, recent efforts have targeted the prevention of inflammation as a potential therapeutic for prevention of NIHL (see Frye et al., 2019). Improved understanding of the multiple mechanisms underlying noise injury has driven widespread research efforts seeking to identify agents that prevent noise injury and resulting NIHL (for reviews and discussion, see Abi-Hachem et al., 2010; Poirrier et al., 2010; Le Prell and Bao, 2012). Positive results were obtained in several early clinical investigations (for review, see Le Prell and Lobarinas, 2015), and results from several other clinical trials have become available in recent years (Kopke et al., 2015; Le Prell et al., 2016; Kil et al., 2017). One barrier to the development of otoprotective agents is the difficulty of benchmarking the efficacy of novel agents relative to other agents based on the diversity of pre-clinical research models and clinical trial paradigms (for discussion, see Lynch et al., 2016).
The recent systematic review of otoprotection research methodologies by Hammill (2017) documents the diversity of pre-clinical and clinical research models with respect to species, noise exposure paradigm, method of dosing, and agent of interest. A subset of the paradigms used in otoprotection research use impulsive noise to induce trauma (Bielefeld et al., 2019), based on the importance of this clinical issue and its relevance to military populations. However, by far, the most common paradigm is the use of octave band noise to induce NIHL. In the chinchilla, which has an audiogram similar to that of humans (see Trevino et al., 2019; Radziwon et al., 2019), octave band noise exposures commonly contain energy from approximately 2 to 6 kHz. The guinea pig has a slightly higher frequency audiogram, and thus octave band noise exposures used for this species commonly contain energy from approximately 4 to 8 kHz. The rat (Escabi et al., 2019; Holt et al., 2019) and the mouse (Ohlemiller, 2019) have better hearing at higher frequencies than humans, chinchillas, and guinea pigs, and thus octave band noise exposures for these species commonly contain energy from approximately 8 to 16 kHz. Data drawn specifically from previous otoprotection research designs are presented here, with data from control animals extracted and used to illustrate similarities and differences in NIHL subsequent to the diverse octave band noise exposures commonly used in rodents.
II. METHODS
A. Systematic review strategy
The development of the study database using a systematic review strategy is described in detail by Hammill (2017). In brief, the systematic review protocol was developed and registered with PROSPERO (registration number CRD42015027009, 2015). Original reports of chemical interventions to prevent or treat hearing loss or peripheral tinnitus caused by noise or blast exposure in any setting were included; pre-clinical animal investigations and human controlled trials were included. A comprehensive literature search strategy was used; there were no date limitations, but the inclusion criteria required studies be published in the English language or as English translations. Studies that described hearing regeneration, rehabilitation with hearing aid devices, or acupuncture interventions were excluded. Additionally, studies focused on drug-induced hearing loss (DIHL), Meniere's disease, congenital deafness, sudden sensorineural hearing loss (SSNHL), age-related hearing loss (ARHL), or other diseases of the ear (i.e., otitis media, otosclerosis, etc.) were excluded. Conference proceedings, editorials, non-original research (i.e., reviews or duplicative publications of the same study), and retrospective or case studies were also excluded.
The automated search employed the University of Texas Health Science Center San Antonio (UTHSCSA), University of Texas at Austin (UT), and the U.S. Air Force 59th Medical Wing, Wilford Hall Ambulatory Surgical Center (WHASC) library databases, and their inherent database search engines. A Boolean/phrase search mode with no limiting/exclusion terms was used in the database search. Databases were searched for the period January 1950–January 12, 2017.1 The search did not include “grey” nor more robustly international, non-English literature.
In addition to the automated search, a personal collection of reports written by or for the Department of Defense (DoD), amassed over ten years through the Pharmaceutical Interventions for Hearing Loss (PIHL) Group of the Department of Defense Hearing Center of Excellence (DoD HCE), was identified. All article bibliographies were searched for additional studies worthy of inclusion. Hand-searched, bibliography, and search update garnered articles were all added to the same database for final article count and PRISMA flow chart development (San Francisco).
As described by Hammill (2017), this project employed a single-reviewer coding strategy for all studies. Data were entered directly into a Microsoft (MS) Access database (Redmond, VA) created for the study. Data captured included eight categories of information, with a ninth category available in the codebook for future research efforts (quality) as detailed in Table I.
TABLE I.
Correlation assessment matrix of variables. D, descriptive statistics; C, correlation statistics possible; N/A, non-applicable.
| Citation (C) | Study design (SA or SC) | Exposure (E) | Drug/biologic (D) | Measures (M) | Intervention arm (I) | Outcome (O) | Analytics (A) | |
|---|---|---|---|---|---|---|---|---|
| Citation (C) | D | C | C | C | C | C | C | C |
| Study design (SA or SC) | D | C | C | C | C | C | C | |
| Exposure (NE or OE) | D | C | C | N/A | C | C | ||
| Drug/biologic (D) | D | C | N/A | C | C | |||
| Measures (M) | D | N/A | C | C | ||||
| Intervention arm (I) | D | C | C | |||||
| Outcome (O) | D | C | ||||||
| Analytics (A) | D |
Because of the high level of variability in study arm designs, the coder identified each exposure type (E) employed and measure (M) utilized, and then matched those up per intervention arm (I) with the specific drug administration protocol (D) used in that arm. This allowed analysis of the various combinations of these three variable categories (E, M, and D) created across studies. Reporting quality was noted among primary variables (i.e., when elements were not reported, “NR” was captured for quantitative assessment), but also subjectively assessed for general trends. All coded data, collected in MS Access, were exported into separate MS Excel® (2013) tabs and compared for compliance to the study aims and codebook instructions when finalizing (i.e., correcting typos and syntax) the closed data set. All final coded data were transferred into SAsoftware (version 9.4; Cary, NC) database for additional analysis.
The database was sorted by type of noise exposure (broadband, octave band noise, impulse, pure tone, other) and species (guinea pig, chinchilla, rat, mouse, human). All articles meeting the inclusion criteria of octave band noise exposure and rodent model were accessed through the University of Texas at Dallas electronic journal subscription or inter-library loan service. Threshold shift at all reported times and frequencies was entered into a spreadsheet; furthermore, the strategy for quantifying variance [standard deviation (SD), standard error of the mean (SEM)] and the sample size were recorded. A small number of studies reported pre-exposure thresholds and post-exposure thresholds; for those studies, threshold shift was calculated as the difference in mean thresholds at pre- and post-noise test times, but variance was not extracted. Additional exclusionary criteria included reporting of post-noise thresholds in the absence of pre-noise thresholds, and lack of auditory brainstem response (ABR) threshold data reporting. Studies in the rat often included distortion product otoacoustic emission (DPOAE) measurements in lieu of ABR measurements. To extract data, graphs were printed and data points estimated using linear interpolation of the plots. Study-specific data are plotted as extracted, with articles referenced using the article identifications (IDs) established in the original database. Where averages for a noise exposure are reported across studies, a weighted average was calculated by weighting each study mean and variance by the total number of animals within the original study group.
III. RESULTS
A. Guinea pig
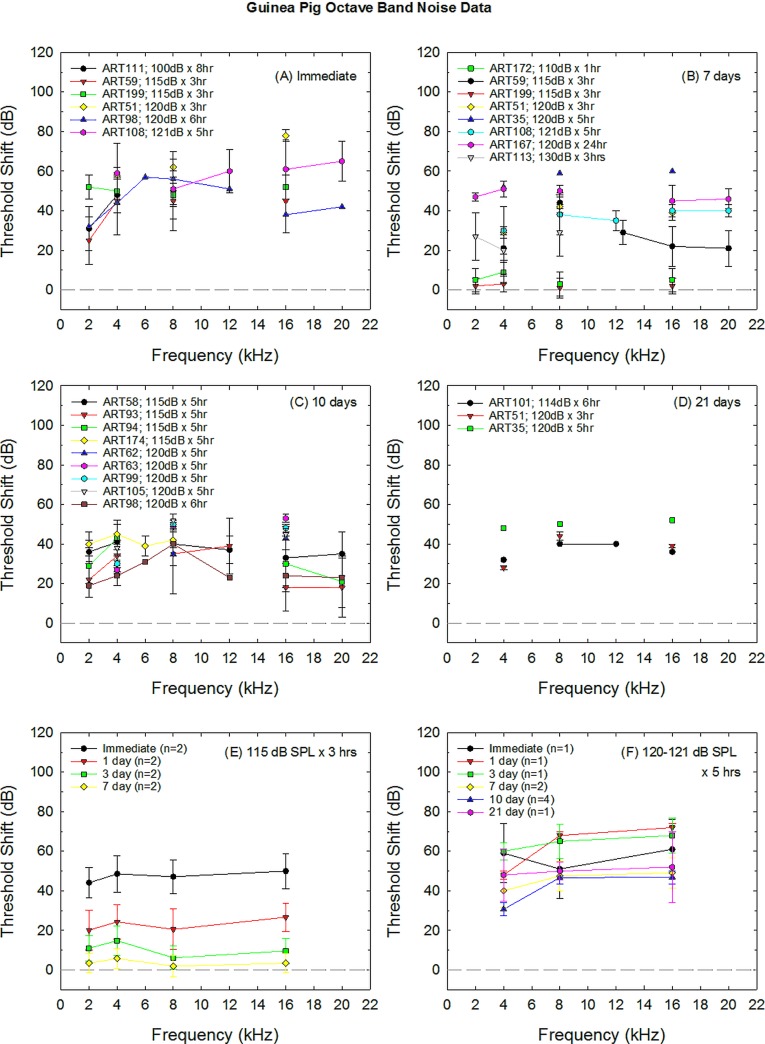
Threshold shift data collected from control animals were extracted from 22 of the otoprotection studies using guinea pigs as subjects (Table II). Data from several studies included in the original database (Hammill, 2017) were excluded from the analysis shown in Fig. 1 as ABR threshold shift was not available in all reports (Pirvola et al., 2000; Fakhry et al., 2007; Pourbakht, 2011, 2013; Wen et al., 2017). The effects of increasing the sound exposure level and/or exposure duration are shown at several common post-noise test times in Figs. 1(A) (immediate), 1(B) (7 days), 1(C) (10 days), and 1(D) (21 days). In general, increasing either sound exposure level or duration results in a larger threshold shift. Interestingly, PTS measured at day 21 is generally equivalent for exposures of 114 dB sound pressure level (SPL) × 6 h (McFadden et al., 2005) and 120 dB SPL × 3 h (Inaoka et al., 2009), with increasing hearing loss on day 21 when the exposure increases to 120 dB SPL × 5 h [Hori et al., 2013; Fig. 1(D)]. In general, studies with less traumatic noise exposure emphasize immediate, 1-day, and 3-day post-noise test times, with 7-day test times used to document recovery of TTS [for example, see Fig. 1(E)], whereas studies with more traumatic exposures routinely include 10 days post-noise test times, and sometimes 14 or 21 days post-noise test times. There appears to be relatively little additional recovery from days 7 to 21 after 120–121 dB SPL × 5 h exposures [see Fig. 1(F)].
TABLE II.
Otoprotection paradigms in which guinea pigs were exposed to octave band noise. NR, not reported.
| Article ID (from Hammill, 2017) | Reference | Sample size | Level (dB SPL) | Duration (hr:min) | Strain | Age | Weight range (grams) | Notes |
|---|---|---|---|---|---|---|---|---|
| 143 | Arpornchayanon et al. (2013) | 6 | 106 | 00:30 | Hartley albino | NR | 250 | SEM |
| 172 | Chen et al. (2003) | 8 | 110 | 01:00 | Pigmented | NR | 300–400 | SD |
| 101 | McFadden et al. (2005) | 8 | 114 | 06:00 | Outbred Dunkin Hartley albino | 2 weeks | 205–269 | Shift calculated as difference between pre- and post-noise thresholds |
| 59 | Yamasoba et al. (2005) | 5 | 115 | 03:00 | Albino | NR | 250–350 | SD 14 controls; 5 with ABR data |
| 199 | Lin et al. (2011) | 12 | 115 | 03:00 | Hartley | NR | 250–300 | SD |
| 174 | Diao et al. (2007) | 20 | 115 | 05:00 | Long–Evans pigmented | 4 weeks | 300–350 | SD |
| 58 | Yamasoba et al. (1999) | 6 | 115 | 05:00 | Pigmented | NR | 250–350 | SD |
| 94 | Ohinata et al. (2003) | 16 | 115 | 05:00 | Pigmented | NR | 250–300 | SD |
| 209 | Takeda et al. (2016) | Not specified | 116 | 02:00 | Hartley | 4 weeks | NR | SD/SEM not specified |
| 98 | Mohammadkhani et al. (2013) | 10 | 120 | 06:00 | Albino | 6 weeks | 280–300 | SD/SEM not provided |
| 35 | Hori et al. (2013) | 5 | 120 | 05:00 | Hartley | NR | 350–400 | SD |
| 51 | Inaoka et al. (2009) | 6 | 120 | 03:00 | Hartley | 4 weeks | 300–350 | SD/SEM not specified |
| 62 | Yamashita et al. (2008) | 7 | 120 | 05:00 | Hartley | NR | 250–300 | SD |
| 108 | Kurioka et al. (2014b) | 6 | 121 | 05:00 | Hartley | NR | 300–350 | SEM |
| 87 | Pourbakht and Yamasoba (2003) | 6 | 125 | 05:00 | Pigmented | NR | 250–300 | SD |
| 113 | Hirose et al. (2016) | 4 | 130 | 03:00 | Hartley | NR | 350–400 | SD/SEM not specified |
| 111 | Hou et al. (2003) | 8 | 100 | 08:00 | Pigmented | NR | 250–300 | SD |
| 93 | Ohinata et al. (2000) | 5 | 115 | 05:00 | Pigmented | NR | 250–300 | SD |
| 63 | Yamashita et al. (2005) | 6 | 120 | 05:00 | Pigmented | NR | 250–300 | SEM |
| 105 | Le Prell et al. (2007a) | 9 | 120 | 05:00 | Pigmented | NR | 250–300 | SEM |
| 99 | Minami et al. (2007) | 6 | 120 | 05:00 | Pigmented | 2–4 weeks | 200–400 | SD |
| 167 | Takemura et al. (2004) | 5 | 120 | 24:00 | Hartley | 5–8 weeks | 300–500 | SEM |
FIG. 1.
Noise-induced threshold shift in guinea pigs has been induced by a variety of different noise exposures. Deficits measured in various otoprotection studies using various exposure paradigms are shown at different post-noise durations, including immediate (A), 7 days (B), 10 days (C), and 21 days (D) post-noise durations. ART# refers to the article identification (IDs) provided in Table II. In (E), temporary NIHL is averaged across two studies using 115 dB SPL × 3 h exposures (Yamasoba et al., 2005; Lin et al., 2011). In F, permanent NIHL is averaged across studies using 120–121 dB SPL × 5 h exposures. Sample sizes shown in each legend entry are the number of studies contributing data to the weighted average. Where n = 1, only one study included data at that exposure × time combination; where n is greater than one, the weighted averages were calculated using sample sizes within studies to weight datasets. Deficits shown in (E) were temporary; deficits shown in (F) were permanent and showed little recovery from 7 to 21 days.
B. Chinchilla
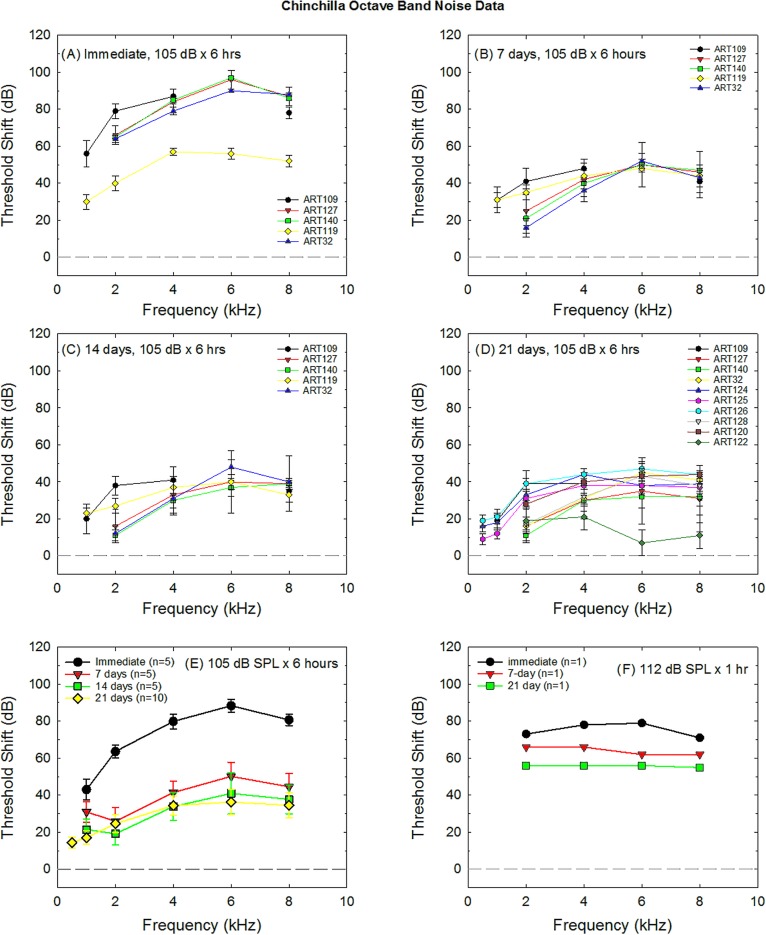
Threshold shift data collected from control animals were extracted from 18 of the otoprotection studies using chinchillas as subjects (Table III). Data from several studies included in the original database (Hammill, 2017) were excluded as ABR threshold shift was not available (Hu et al., 1997; Wang et al., 1999). The majority of otoprotection investigations in chinchillas have used a 105 dB SPL × 6 h exposure. Both the compound threshold shift measured immediately post-exposure [Fig. 2(A)] and the PTS measured at 21 days post-noise [Fig. 2(D)] have been variable in the control animals used across investigations. Figure 2(E) illustrates weighted threshold shift averages across studies. There appears to be relatively little additional recovery from days 14 to 21 after 106 dB SPL × 6 h exposures [see Fig. 2(E)]. A small number of studies have shown PTS generally equivalent to that induced by the 105 dB SPL × 6 h exposure when using octave band noise at 105 dB SPL × 4 h (Hight et al., 2003), 106 dB SPL × 4 h (Harris et al., 2005), or 107 dB SPL × 2 h (Bielefeld et al., 2011). The larger PTS, induced using a 1 h exposure to 112 dB SPL noise (Bielefeld et al., 2011), is illustrated in Fig. 2(F). Prevention of TTS in the chinchilla was not evaluated within any of the studies identified as part of the systematic review by Hammill (2017) and is not illustrated in Fig. 2.
TABLE III.
Otoprotection paradigms in which chinchillas were exposed to octave band noise.
| Article ID (from Hammill, 2017) | Reference | Sample size | Level (dB SPL) | Duration (hr:min) | Strain | Age | Weight range (grams) | Notes |
|---|---|---|---|---|---|---|---|---|
| 124 | Choi et al. (2011) | 6 | 105 | 06:00 | Laniger | 3–5 years | 500–850 | SD/SEM not specified |
| 125 | Choi et al. (2008) | 12 | 105 | 06:00 | Laniger | 3–5 years | 500–850 | SEM |
| 126 | Choi et al. (2014) | 12 | 105 | 06:00 | Laniger | 3–5 years | 500–850 | SEM |
| 128 | Coleman et al. (2007) | 10 | 105 | 06:00 | Laniger | NR | NR | SEM |
| 131 | Du et al. (2011) | 12 | 105 | 06:00 | Laniger | 3–5 years | 500–850 | SEM |
| 109 | Kopke et al. (2000) | 5 | 105 | 06:00 | Laniger | NR | NR | SEM |
| 122 | Campbell et al. (2007) | 10 | 105 | 06:00 | Laniger | NR | NR | SD/SEM not specified |
| 127 | Clifford et al. (2011) | 105 ± 0.5 | 06:00 | Laniger | NR | NR | SD; 26 chinchillas total, group sizes not reported | |
| 140 | Kopke et al. (2002) | 6 | 105 ± 0.5 | 06:00 | Laniger | Adult | NR | SEM |
| 119 | Bielefeld et al. (2005) | 5 | 100 | 06:00 | NR | Adult | 400–700 | SEM |
| 120 | Bielefeld et al. (2007) | 6 | 105 | 06:00 | NR | Adult | 400–700 | SEM |
| 130 | Du et al. (2012) | 6 | 105 | 06:00 | NR | NR | NR | SEM |
| 136 | Hight et al. (2003) | 10 | 105 | 04:00 | NR | Adult | NR | SD |
| 32 | Coleman et al. (2010) | 6 | 105 | 06:00 | NR | Adult | NR | SEM |
| 135 | Harris et al. (2005) | 8 | 106 | 04:00 | NR | Adult | 400–600 | SD |
| 177 | Bielefeld (2013) | 10 | 106 | 06:00 | NR | Adult | 400–700 | SD/SEM not specified |
| 121 | Bielefeld et al. (2011) | 6 | 107 | 02:00 | NR | Adult | 400–700 | SD/SEM not specified |
| 121 | Bielefeld et al. (2011) | 6 | 112 | 01:00 | NR | Adult | 400–700 | SD/SEM not specified |
FIG. 2.
Noise-induced threshold shift in chinchillas is commonly induced by 105 dB SPL × 6 h exposure. Deficits measured in various studies using this exposure paradigm are shown at different post-noise durations, including immediate (A), 7 days (B), 14 days (C), and 21 days (D) post-noise durations. ART# refers to the article IDs provided in Table III. Deficits shown in (D) at 21 days post-noise are assumed to be permanent. In (E), NIHL is averaged across studies. Sample sizes shown in each legend entry are the number of studies contributing data to the weighted average. (F) illustrates NIHL subsequent to a shorter but higher level exposure (112 dB SPL × 1 h; Bielefeld et al., 2011).
C. Mouse
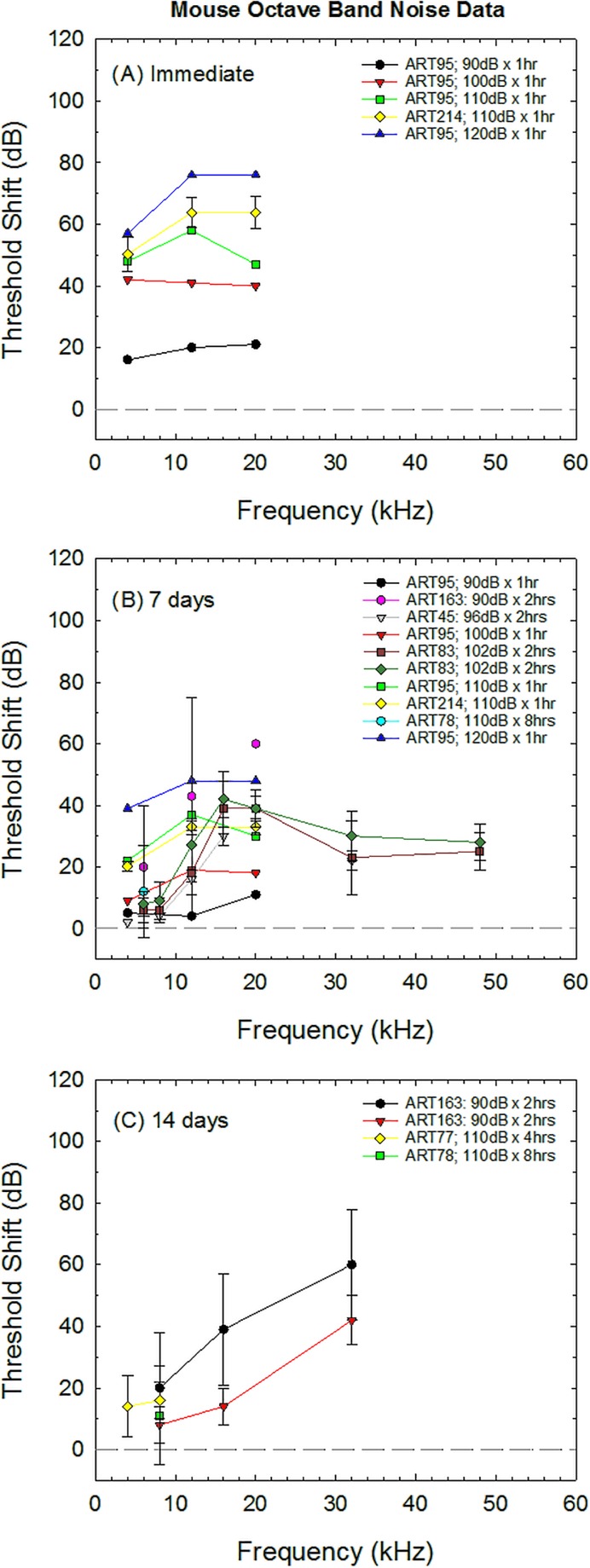
Threshold shift data collected from control animals were extracted from 14 of the otoprotection studies using mice as subjects (Table IV). Data from several studies included in the original database (Hammill, 2017) were excluded because average shift was the only reported value (Qu et al., 2015), post-exposure thresholds were provided without pre-exposure thresholds and thus shift could not be calculated (Horie et al., 2010), or control animal data were not included (Brown et al., 2014). The most common strain used in otoprotection studies was the C57/BL6J (Samson et al., 2008; Peppi et al., 2011; Rewerska et al., 2013; Brown et al., 2014; Honkura et al., 2016), although the CBA/CaJ (Le Prell et al., 2011; Peppi et al., 2011), and Std-ddy (Nagashima et al., 2010; Yamaguchi et al., 2014) have also been used in otoprotection research with octave band noise models. There are significant differences in both auditory threshold sensitivity (Zheng et al., 1999) and vulnerability to NIHL across different strains of mice (Myint et al., 2016), so variation between studies may be greater across studies using mice (Fig. 3) compared to those using guinea pigs (Fig. 1) and chinchillas (Fig. 2). Because there was little overlap in the exposure parameters used in each investigation, there is little opportunity to systematically probe these factors in this review. The most important outcomes shown in Fig. 3 are the increase in hearing loss as noise exposure levels increase [Figs. 3(A) and 3(B)] and the termination of study follow-up at earlier time points [final post-noise test measures collected 7–14 days post-noise; Figs. 3(B) and 3(C)] relative to guinea pigs and chinchillas (final post-noise test measures typically collected 14–21 days post-noise). None of the studies identified in the systematic review by Hammill (2017) included data collection in mice beyond 14 days after exposure to octave band noise. Interestingly, although the mouse is a high frequency hearing animal and the greatest noise injuries appear to be located at the highest test frequencies [see Fig. 3(C)], many of the studies assessing protection of the mouse cochlea did not collect threshold shift measurements above 20 kHz.
TABLE IV.
Otoprotection paradigms in which mice were exposed to octave band noise.
| Article ID (from Hammill, 2017) | Reference | Sample size | Level (dB SPL) | Duration (hr:min) | Strain | Age | Weight range (grams) | Notes |
|---|---|---|---|---|---|---|---|---|
| 83 | Peppi et al. (2011) | 4–7 | 100 | 02:00 | C57/BL/6J (B6) | 6 weeks | 18–25 | SD/SEM not specified |
| 163 | Brown et al. (2014) | Not reported | 90 | 02:00 | C57BL/6 | 8–10 weeks | NR | SD |
| 78 | Rewerska et al. (2013) | 80 | 110 | 08:00 | C57BL/6 | 6 weeks | NR | SD |
| 77 | Samson et al. (2008) | Not reported | 110 | 04:00 | C57BL/6 | 12 weeks | NR | SEM |
| 83 | Peppi et al. (2011) | Not reported | 102 | 02:00 | CBA/CaJ (CB) | 10–12 weeks | NR | SEM |
| 38 | Le Prell et al. (2011) | 16 | 113–116 | 02:00 | CBA/J | 5–6 weeks | 25–35 | SEM |
| 45 | Honkura et al. (2016) | 7–8 | 96 | 02:00 | Nrf2 knockout (Nrf2-/-) (C57BL/6) | 6–7 weeks | 17–20 | SEM |
| 95 | Nagashima et al. (2010) | 4 | 90 | 01:00 | Std-ddY | adult | 26–28 | SD/SEM not specified |
| 95 | Nagashima et al. (2010) | 4 | 100 | 01:00 | Std-ddY | adult | 26–28 | SD/SEM not specified |
| 95 | Nagashima et al. (2010) | 4 | 110 | 01:00 | Std-ddY | adult | 26–28 | SD/SEM not specified |
| 214 | Yamaguchi et al. (2014) | Not reported | 110 | 01:00 | Std-ddY | adult | 26–28 | SEM |
| 95 | Nagashima et al. (2010) | 4 | 120 | 01:00 | Std-ddY | adult | 26–28 | SD/SEM not specified |
| 45 | Honkura et al. (2016) | 7–8 | 96 | 02:00 | Wild | 6–7 weeks | 17–20 | SEM |
| 163 | Brown et al. (2014) | Not reported | 90 | 02:00 | Wild type | 8–10 weeks | NR | SD |
FIG. 3.
Noise-induced threshold shift in mouse induced by various exposure conditions. Deficits measured in various studies are shown at different post-noise durations including immediate (A), 7 days (B), and 14 days (C) post-noise durations. ART# refers to the article IDs provided in Table IV. Deficits shown in (C) at 14 days post-noise were likely permanent based on the lack of recovery beyond day 14 shown in chinchilla in Fig. 2.
D. Rat
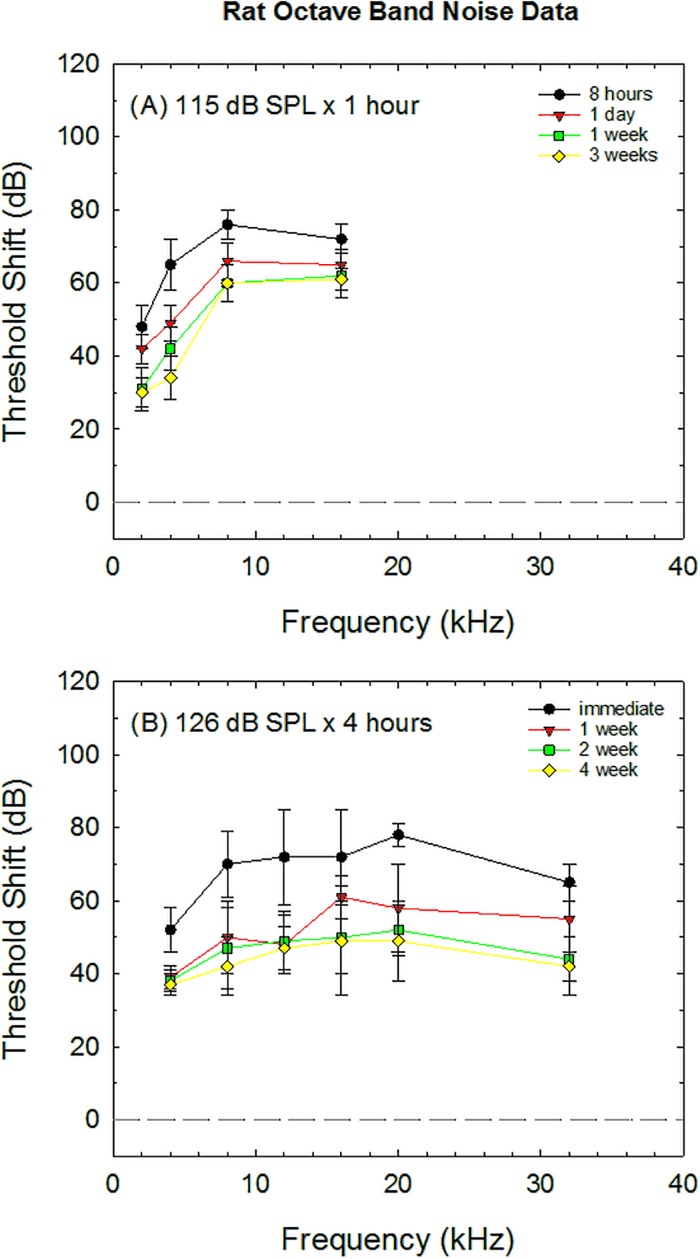
Threshold shift data collected from control animals were extracted from five otoprotection studies using rats as subjects (Table V). Data from several studies included in the original database (Hammill, 2017) were excluded as ABR threshold shift was not available (Rao and Fechter, 2000; Lorito et al., 2006; Pouyatos et al., 2007; Guthrie et al., 2011; Loukzadeh et al., 2015). Indeed, DPOAE measurements have often been used in place of ABR threshold measurements in studies using the rat. For the studies in which ABR threshold shift was assessed, the post-noise test times were highly variable, including 1 week (Lorito et al., 2008); 3, 6, and 9 weeks (Kil et al., 2007); 8 h, 1 day, 1 week, and 3 weeks (Lu et al., 2014); and immediately, 1, 2, and 4 weeks (Kurioka et al., 2014a). Figure 4 illustrates the results from two studies that included multiple frequencies at multiple test times. Both studies showed little additional recovery beyond the 1–2-week test times. Although it seems anomalous that the longer, higher level exposure [126 dB SPL × 4 h, Fig. 4(B)] resulted in less PTS than the shorter, lower level exposure [115 dB SPL × 1 h, Fig. 4(A)], strain differences (Sprague-Dawley and Long–Evans, respectively) and age differences (5 weeks and 10–11 weeks, respectively) make it difficult to interpret differences in the effects of noise across these two studies. Compared to the guinea pig and chinchilla, the rat model is less well developed for studies assessing prevention of NIHL induced by octave band noise.
TABLE V.
Otoprotection paradigms in which rats were exposed to octave band noise.
| Article ID (from Hammill, 2017) | Reference | Sample size | Level (dB SPL) | Duration (hr:min) | Strain | Age | Weight range (grams) | Notes |
|---|---|---|---|---|---|---|---|---|
| 138 | Kil et al. (2007) | 4 | 113 | 04:00 | F-344 | 6 weeks; 10–12 weeks | NR | SEM |
| 148 | Lu et al. (2014) | 18 | 115 | 01:00 | Long–Evans pigmented | 10–11 weeks | 310–340 | SEM |
| 203 | Lorito et al. (2008) | 4 | 105 | 04:00 | Sprague Dawley albino | NR | 190–210 | SD |
| 107 | Kurioka et al. (2014a) | 4 | 126 | 05:00 | Sprague-Dawley | 5 weeks | 150–200 | SEM |
| 48 | Ogurlu et al. (2017) | Not provided | 120 | 04:00 | Spraque Dawley albino | Adult | 250–350 | Not specified |
FIG. 4.
Noise-induced threshold shift in rat induced by two different exposure conditions, as reported in Lu et al. (2014) (A) and Kurioka et al. (2014a) (B). Studies using the rat model are listed in Table V.
IV. DISCUSSION
PTS in control animals used in the most common guinea pig otoprotection model (120 dB SPL × 5 h) results in about 50 dB PTS at the most affected frequencies (8–16 kHz; see Fig. 1). PTS in control animals used in the most common chinchilla otoprotection model (105 dB SPL × 6 h) results in about 40 dB PTS at the most affected frequencies (6–8 kHz; see Fig. 2). There is not a single most common exposure paradigm in the mouse (see Fig. 3). Across noise exposure models, exposures range from little or no threshold shift (90 dB SPL × 1 h) to as much as 50–60 dB threshold shift (90 dB SPL × 2 h; 120 dB SPL × 1 h) at the 1-week test time, beyond which there is not likely to be significant additional recovery. Data collected from two strains of mice 14 days after exposure to 90 dB SPL octave band noise revealed 40–60 dB PTS with the greatest shifts at and above 30 kHz. It was surprising that only a small number of studies using mice as a model included frequencies of 30 kHz or above. Data from the rat model were the most limited, with only two studies reporting thresholds at multiple frequencies across time. Although relatively lower frequencies were less affected, PTS ranged from 40 to 60 dB across a wide range of frequencies, a finding that is consistent with data from other species reviewed here. Similar patterns of results are well established within the primary literature, outside of otoprotection research (Wang et al., 2002).
Although the emphasis of this review was PTS induced by octave band noise, review of Tables II and III reveal other differences across studies using different species. Specifically, chinchillas tend to be older (3–5 years, or “adult”) at study onset, whereas guinea pigs tend to be younger, based on weights that are under 500 g. There is not a consistent reporting convention for age, although weights are consistently reported across species.
Differences in vulnerability are well known and illustrated here. It clearly took more noise to induce larger PTS changes in the guinea pig (120 dB SPL × 5 h) than in the chinchilla (105 dB SPL × 6 h). A single study using 112 dB SPL × 1 h in the chinchilla documented PTS of 70–80 dB. Hearing loss in the mouse tended to be on the order of 20–40 dB for most noise exposures, but exposures of 120 dB × 1 h did produce 30–40 dB PTS. Hearing loss in the two rat studies identified here ranged from 40 to 60 dB PTS, on par with the guinea pig and chinchilla, and was induced by noise exposures including 115 dB SPL × 1 h, an exposure that is slightly higher than the exposures resulting in 70–80 dB PTS in the chinchilla, and 126 dB SPL × 4 h, which is generally similar to the guinea pig exposure of 120 dB SPL × 5 h. Taken together, the data suggest it takes more noise to induce hearing loss in a guinea pig than a rat, with the most vulnerable animal model being the chinchilla. Data from the mouse were variable enough that it is difficult to rank them relative to guinea pig, rat, and chinchilla. Which is the best model for human hearing loss is a key question. The answer to that question may be driven by metabolism of drug agents of interest, the degree of hearing change in a clinical population, and species-specific vulnerability.
Stebbins et al. (1982) identified major challenges in the understanding of NIHL, including the use of diverse species across studies, diverse protocols for threshold measurement, diverse noise exposures (many of which do not necessarily model human exposures), and overall lack of consideration of supra-threshold measures of sensitivity. Although there has since been a wealth of research into the effects of noise on the inner ear, there is still little consensus on what noise models should be used during pre-clinical assessment of potential otoprotective agents. Currently, there is tremendous variation not only in the specific agents of interest and which species they are evaluated in, but also how drugs are delivered (orally, by injection, or by transtympanic delivery), when drug dosing is initiated relative to the onset of noise, and how long dosing continues after noise exposure (Le Prell and Bao, 2012; Le Prell and Miller, 2016). The systematic review by Hammill (2017) provides detailed descriptions and descriptive statistics on these issues. Here, we have leveraged that comprehensive database to assess the effects of octave band noise, the most common noise model, on hearing thresholds in the most commonly used rodent species (guinea pig, chinchilla, rat, mouse). To the extent that investigators can select common species and noise models, comparisons across studies will be greatly facilitated. When other species must be selected, selection of models that yield a common degree of trauma will be helpful in facilitating comparisons across agents. Both TTS and PTS models are urgently needed to facilitate the identification and perhaps even a relative ranking of promising agents. Given the state of the science today, it is difficult if not impossible to draw conclusions regarding the relative promise of diverse pharmaceutical agents proposed for clinical testing based on pre-clinical research. Although this review did not compare efficacy of agents as a function of the noise model in which they are assessed, it is reasonable to speculate that the noise model may influence the relative benefits of the otoprotective agent. As the exposure level and duration increase, mechanical damage to the hair cells is increasingly likely. However, the majority of drugs of interest for prevention of NIHL act on metabolic and other biochemical events. Otoprotective agents that target biochemical pathways are not likely to prevent acute mechanical injury, including, for example, disruption of the reticular lamina. Thus, to compare relative efficacy of different drugs for otoprotective benefit, it is critical that noise models be consistent across investigations.
ACKNOWLEDGMENTS
Support for this review was provided by the Emilie and Phil Schepps Professorship in Hearing Science. C.L. is currently supported by USAMRAA Nos. W81XWH-19-C-0054, JPC-8/SRMRP W81XWH1820014, NIH-NIDCD 1R01DC014088, 3M Inc., and the Emilie and Phil Schepps Professorship in Hearing Science. C.L. has previously received contract funding and/or clinical trial material from industry partners including Sound Pharmaceuticals, Inc., Edison Pharmaceuticals, Inc., and Hearing Health Science, Inc.
Footnotes
Databases searched include PubMed (National Center for Biotechnology Information, U.S. National Library of Medicine, Bethesda), CINAHLVR Plus with Full Text (Cumulative Index of Nursing and Allied Health Literature, EBSCO Information Services HQ, Ipswich, MA), PsycINFO (American Psychological Association, Washington, DC), EBSCO Military & Government Collection database (EBSCO Information Services HQ, Ipswich, MA), Agricola (United States Department of Agriculture, National Agricultural Library), eBook Collection (EBSCOhost; EBSCO Information Services HQ, Ipswich, MA), Cochrane Library (John Wiley & Sons, Inc., Hoboken, NJ), and ClinicalTrials.gov (U.S. National Library of Medicine, Bethesda, MD).
References
- 1. Abi-Hachem, R. N. , Zine, A. , and Van De Water, T. R. (2010). “ The injured cochlea as a target for inflammatory processes, initiation of cell death pathways and application of related otoprotectives strategies,” Recent Pat. CNS Drug Discov. 5, 147–163. 10.2174/157488910791213121 [DOI] [PubMed] [Google Scholar]
- 2. Arpornchayanon, W. , Canis, M. , Ihler, F. , Settevendemie, C. , and Strieth, S. (2013). “ TNF-α inhibition using etanercept prevents noise-induced hearing loss by improvement of cochlear blood flow in vivo,” Int. J. Audiol. 52, 545–552. 10.3109/14992027.2013.790564 [DOI] [PubMed] [Google Scholar]
- 3. Bielefeld, E. C. (2013). “ Reduction in impulse noise-induced permanent threshold shift with intracochlear application of an NADPH oxidase inhibitor,” J. Am. Acad. Audiol. 24, 461–473. 10.3766/jaaa.24.6.3 [DOI] [PubMed] [Google Scholar]
- 4. Bielefeld, E. C. , Harrison, R. T. , and DeBacker J. R. (2019). “ Pharmaceutical otoprotection strategies to prevent impulse noise-induced hearing loss,” J. Acoust. Soc. Am. 146, 3790–3799. 10.1121/1.5132285 [DOI] [PubMed] [Google Scholar]
- 5. Bielefeld, E. C. , Hynes, S. , Pryznosch, D. , Liu, J. , Coleman, J. K. , and Henderson, D. (2005). “ A comparison of the protective effects of systemic administration of a pro-glutathione drug and a Src-PTK inhibitor against noise-induced hearing loss,” Noise Health 7, 24–30. 10.4103/1463-1741.31875 [DOI] [PubMed] [Google Scholar]
- 6. Bielefeld, E. C. , Kopke, R. D. , Jackson, R. L. , Coleman, J. K. , Liu, J. , and Henderson, D. (2007). “ Noise protection with N-acetyl-l-cysteine (NAC) using a variety of noise exposures, NAC doses, and routes of administration,” Acta Otolaryngol. 127, 914–919. 10.1080/00016480601110188 [DOI] [PubMed] [Google Scholar]
- 7. Bielefeld, E. C. , Wantuck, R. , and Henderson, D. (2011). “ Postexposure treatment with a Src-PTK inhibitor in combination with N-l-acetyl cysteine to reduce noise-induced hearing loss,” Noise Health 13, 292–298. 10.4103/1463-1741.82962 [DOI] [PubMed] [Google Scholar]
- 8. Brown, K. D. , Maqsood, S. , Huang, J. Y. , Pan, Y. , Harkcom, W. , Li, W. , Sauve, A. , Verdin, E. , and Jaffrey, S. R. (2014). “ Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss,” Cell Metab. 20, 1059–1068. 10.1016/j.cmet.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campbell, K. C. , Meech, R. P. , Klemens, J. J. , Gerberi, M. T. , Dyrstad, S. S. , Larsen, D. L. , Mitchell, D. L. , El-Azizi, M. , Verhulst, S. J. , and Hughes, L. F. (2007). “ Prevention of noise- and drug-induced hearing loss with D-methionine,” Hear. Res. 226, 92–103. 10.1016/j.heares.2006.11.012 [DOI] [PubMed] [Google Scholar]
- 10. Chen, Z. , Ulfendahl, M. , Ruan, R. , Tan, L. , and Duan, M. (2003). “ Acute treatment of noise trauma with local caroverine application in the guinea pig,” Acta Otolaryngol. 123, 905–909. 10.1080/00016480310000638 [DOI] [PubMed] [Google Scholar]
- 11. Choi, C. H. , Chen, K. , Du, X. , Floyd, R. A. , and Kopke, R. D. (2011). “ Effects of delayed and extended antioxidant treatment on acute acoustic trauma,” Free Radic. Res. 45, 1162–1172. 10.3109/10715762.2011.605360 [DOI] [PubMed] [Google Scholar]
- 12. Choi, C. H. , Chen, K. , Vasquez-Weldon, A. , Jackson, R. L. , Floyd, R. A. , and Kopke, R. D. (2008). “ Effectiveness of 4-hydroxy phenyl N-tert-butylnitrone (4-OHPBN) alone and in combination with other antioxidant drugs in the treatment of acute acoustic trauma in chinchilla,” Free Radic. Biol. Med. 44, 1772–1784. 10.1016/j.freeradbiomed.2008.02.005 [DOI] [PubMed] [Google Scholar]
- 13. Choi, C. H. , Du, X. , Floyd, R. A. , and Kopke, R. D. (2014). “ Therapeutic effects of orally administrated antioxidant drugs on acute noise-induced hearing loss,” Free Radic. Res. 48, 264–272. 10.3109/10715762.2013.861599 [DOI] [PubMed] [Google Scholar]
- 14. Clifford, R. E. , Coleman, J. K. , Balough, B. J. , Liu, J. , Kopke, R. D. , and Jackson, R. L. (2011). “ Low-dose D-methionine and N-acetyl-L-cysteine for protection from permanent noise-induced hearing loss in chinchillas,” Otolaryngol. Head Neck Surg. 145, 999–1006. 10.1177/0194599811414496 [DOI] [PubMed] [Google Scholar]
- 15. Coleman, J. , Huang, X. , Liu, J. , Kopke, R. , and Jackson, R. (2010). “ Dosing study on the effectiveness of salicylate/N-acetylcysteine for prevention of noise-induced hearing loss,” Noise Health 12, 159–165. 10.4103/1463-1741.64972 [DOI] [PubMed] [Google Scholar]
- 16. Coleman, J. K. , Kopke, R. D. , Liu, J. , Ge, X. , Harper, E. A. , Jones, G. E. , Cater, T. L. , and Jackson, R. L. (2007). “ Pharmacological rescue of noise induced hearing loss using N-acetylcysteine and acetyl-L-carnitine,” Hear. Res. 226, 104–113. 10.1016/j.heares.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 17. Davis, H. , Morgan, C. T. , Hawkins, J. E., Jr. , Galambos, R. , and Smith, F. W. (1950). “ Temporary deafness following exposure to loud tones and noise,” Acta Otolaryngol. Suppl. (Stockh). 88, 1–57. [PubMed] [Google Scholar]
- 18. Diao, M. , Gao, W. , and Sun, J. (2007). “ Nitric oxide synthase inhibitor reduces noise-induced cochlear damage in guinea pigs,” Acta Otolaryngol. 127, 1162–1167. 10.1080/00016480701242436 [DOI] [PubMed] [Google Scholar]
- 19. Du, X. , Chen, K. , Choi, C. H. , Li, W. , Cheng, W. , Stewart, C. , Hu, N. , Floyd, R. A. , and Kopke, R. D. (2012). “ Selective degeneration of synapses in the dorsal cochlear nucleus of chinchilla following acoustic trauma and effects of antioxidant treatment,” Hear. Res. 283, 1–13. 10.1016/j.heares.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 20. Du, X. , Choi, C. H. , Chen, K. , Cheng, W. , Floyd, R. A. , and Kopke, R. D. (2011). “ Reduced formation of oxidative stress biomarkers and migration of mononuclear phagocytes in the cochleae of chinchilla after antioxidant treatment in acute acoustic trauma,” Int. J. Otolaryngol. 2011, 612690. 10.1155/2011/612690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Escabi, C. D. , Frye, M. , and Lobarinas, E. (2019). “ The rat animal model for noise-induced hearing loss,” J. Acoust. Soc. Am. 146, 3692–3709. 10.1121/1.5132553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fakhry, N. , Rostain, J. C. , and Cazals, Y. (2007). “ Hyperbaric oxygenation with corticoid in experimental acoustic trauma,” Hear. Res. 230, 88–92. 10.1016/j.heares.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 23. Frye, M. D. , Ryan A. F., and Kurabi, A. (2019). “ Inflammation associated with noise-induced hearing loss,” J. Acoust. Soc. Am. 146, 4020–4032. 10.1121/1.5132545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guthrie, O. W. , Gearhart, C. A. , Fulton, S. , and Fechter, L. D. (2011). “ Carboxy alkyl esters of Uncaria tomentosa augment recovery of sensorineural functions following noise injury,” Brain Res. 1407, 97–106. 10.1016/j.brainres.2011.06.044 [DOI] [PubMed] [Google Scholar]
- 25. Hammill, T. L. (2017). “ An evidence-base and implementation framework for promoting best practices in pharmaceutical interventions for hearing loss (PIHL) research,” Doctoral thesis, The University of Texas at Austin. [Google Scholar]
- 26. Harris, K. C. , Hu, B. , Hangauer, D. , and Henderson, D. (2005). “ Prevention of noise-induced hearing loss with Src-PTK inhibitors,” Hear. Res. 208, 14–25. 10.1016/j.heares.2005.04.009 [DOI] [PubMed] [Google Scholar]
- 27. Hawkins, J. E. , and Schacht, J. (2005). “ Sketches of otohistory. Part 10: Noise-induced hearing loss,” Audiol. Neurootol. 10, 305–309. 10.1159/000087347 [DOI] [PubMed] [Google Scholar]
- 28. Hecht, Q. A. , Hammill, T. L. , Calamia, P. T. , Smalt, C. J. , and Brungart, D. S. (2019). “ Characterization of acute hearing changes in United States military populations,” J. Acoust. Soc. Am. 146, 3839–3848. 10.1121/1.5132710 [DOI] [PubMed] [Google Scholar]
- 29. Henderson, D. , and Hamernik, R. P. (1986). “ Impulse noise: Critical review,” J. Acoust. Soc. Am. 80, 569–584. 10.1121/1.394052 [DOI] [PubMed] [Google Scholar]
- 30. Hight, N. G. , McFadden, S. L. , Henderson, D. , Burkard, R. F. , and Nicotera, T. (2003). “ Noise-induced hearing loss in chinchillas pre-treated with glutathione monoethylester and R-PIA,” Hear. Res. 179, 21–32. 10.1016/S0378-5955(03)00067-4 [DOI] [PubMed] [Google Scholar]
- 31. Hirose, Y. , Sugahara, K. , Kanagawa, E. , Takemoto, Y. , Hashimoto, M. , and Yamashita, H. (2016). “ Quercetin protects against hair cell loss in the zebrafish lateral line and guinea pig cochlea,” Hear. Res. 342, 80–85. 10.1016/j.heares.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 32. Holt, A. G. , Kallakuri, S. , Braun, R. , and Altschuler, R. A. (2019). “ The rat as a model for studying noise injury and otoprotection,” J. Acoust. Soc. Am. 146, 3681–3691. 10.1121/1.5131344 [DOI] [PubMed] [Google Scholar]
- 33. Honkura, Y. , Matsuo, H. , Murakami, S. , Sakiyama, M. , Mizutari, K. , Shiotani, A. , Yamamoto, M. , Morita, I. , Shinomiya, N. , Kawase, T. , Katori, Y. , and Motohashi, H. (2016). “ NRF2 is a key target for prevention of noise-induced hearing loss by reducing oxidative damage of cochlea,” Sci. Rep. 6, 19329. 10.1038/srep19329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hori, R. , Nakagawa, T. , Yamamoto, N. , Hamaguchi, K. , and Ito, J. (2013). “ Prostaglandin E receptor subtype EP4 agonist serves better to protect cochlea than prostaglandin E1,” Auris. Nasus. Larynx 40, 539–542. 10.1016/j.anl.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 35. Horie, R. T. , Sakamoto, T. , Nakagawa, T. , Ishihara, T. , Higaki, M. , and Ito, J. (2010). “ Stealth-nanoparticle strategy for enhancing the efficacy of steroids in mice with noise-induced hearing loss,” Nanomedicine (London, England) 5, 1331–1340. 10.2217/nnm.10.88 [DOI] [PubMed] [Google Scholar]
- 36. Hou, F. , Wang, S. , Zhai, S. , Hu, Y. , Yang, W. , and He, L. (2003). “ Effects of alpha-tocopherol on noise-induced hearing loss in guinea pigs,” Hear. Res. 179, 1–8. 10.1016/S0378-5955(03)00065-0 [DOI] [PubMed] [Google Scholar]
- 37. Hu, B. H. , Zheng, X. Y. , McFadden, S. L. , Kopke, R. D. , and Henderson, D. (1997). “ R-phenylisopropyladenosine attenuates noise-induced hearing loss in the chinchilla,” Hear. Res. 113, 198–206. 10.1016/S0378-5955(97)00143-3 [DOI] [PubMed] [Google Scholar]
- 38. Inaoka, T. , Nakagawa, T. , Kikkawa, Y. S. , Tabata, Y. , Ono, K. , Yoshida, M. , Tsubouchi, H. , Ido, A. , and Ito, J. (2009). “ Local application of hepatocyte growth factor using gelatin hydrogels attenuates noise-induced hearing loss in guinea pigs,” Acta Otolaryngol. 129, 453–457. 10.1080/00016480902725197 [DOI] [PubMed] [Google Scholar]
- 39. Jokel, C. , Yankaskas, K. , and Robinette, M. B. (2019). “ Noise of military weapons, ground vehicles, planes and ships,” J. Acoust. Soc. Am. 146, 3832–3838. 10.1121/1.5134069 [DOI] [PubMed] [Google Scholar]
- 40. Kil, J. , Lobarinas, E. , Spankovich, C. , Griffiths, S. , Antonelli, P. J. , Lynch, E. D. , and Le Prell, C. G. (2017). “ Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: A randomized double blind placebo-controlled phase 2 clinical trial,” The Lancet 390, 969–979. 10.1016/S0140-6736(17)31791-9 [DOI] [PubMed] [Google Scholar]
- 41. Kil, J. , Pierce, C. , Tran, H. , Gu, R. , and Lynch, E. D. (2007). “ Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase,” Hear. Res. 226, 44–51. 10.1016/j.heares.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 42. Kopke, R. , Slade, M. D. , Jackson, R. , Hammill, T. , Fausti, S. , Lonsbury-Martin, B. , Sanderson, A. , Dreisbach, L. , Rabinowitz, P. , Torre, P. 3rd , and Balough, B. (2015). “ Efficacy and safety of N-acetylcysteine in prevention of noise induced hearing loss: A randomized clinical trial,” Hear. Res. 323, 40–50. 10.1016/j.heares.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 43. Kopke, R. D. , Coleman, J. K. , Liu, J. , Campbell, K. C. , and Riffenburgh, R. H. (2002). “ Candidate's thesis: Enhancing intrinsic cochlear stress defenses to reduce noise-induced hearing loss,” Laryngoscope 112, 1515–1532. 10.1097/00005537-200209000-00001 [DOI] [PubMed] [Google Scholar]
- 44. Kopke, R. D. , Weisskopf, P. A. , Boone, J. L. , Jackson, R. L. , Wester, D. C. , Hoffer, M. E. , Lambert, D. C. , Charon, C. C. , Ding, D. L. , and McBride, D. (2000). “ Reduction of noise-induced hearing loss using L-NAC and salicylate in the chinchilla,” Hear. Res. 149, 138–146. 10.1016/S0378-5955(00)00176-3 [DOI] [PubMed] [Google Scholar]
- 45. Kurioka, T. , Matsunobu, T. , Niwa, K. , Tamura, A. , Satoh, Y. , and Shiotani, A. (2014a). “ Activated protein C rescues the cochlea from noise-induced hearing loss,” Brain Res. 1583, 201–210. 10.1016/j.brainres.2014.07.052 [DOI] [PubMed] [Google Scholar]
- 46. Kurioka, T. , Matsunobu, T. , Satoh, Y. , Niwa, K. , and Shiotani, A. (2014b). “ Inhaled hydrogen gas therapy for prevention of noise-induced hearing loss through reducing reactive oxygen species,” Neurosci. Res. 89, 69–74. 10.1016/j.neures.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 47. Le Prell, C. G. , and Bao, J. (2012). “ Prevention of noise-induced hearing loss: Potential therapeutic agents,” in Noise-Induced Hearing Loss: Scientific Advances, Springer Handbook of Auditory Research, edited by Le Prell C. G., Henderson D., Fay R. R., and Popper A. N. ( Springer Science and Business Media, LLC, New York: ), pp. 285–338. [Google Scholar]
- 48. Le Prell, C. G. , Fulbright, A. , Spankovich, C. , Griffiths, S. , Lobarinas, E. , Campbell, K. C. M. , Antonelli, P. J. , Green, G. E. , Guire, K. , and Miller, J. M. (2016). “ Dietary supplement comprised of β-carotene, vitamin C, vitamin E, and magnesium: Failure to prevent music-induced temporary threshold shift,” Audiol. Neurotol. Extra 6, 20–39. 10.1159/000446600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Le Prell, C. G. , Gagnon, P. M. , Bennett, D. C. , and Ohlemiller, K. K. (2011). “ Nutrient-enhanced diet reduces noise-induced damage to the inner ear and hearing loss,” Transl. Res. 158, 38–53. 10.1016/j.trsl.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Le Prell, C. G. , Hughes, L. F. , and Miller, J. M. (2007a). “ Free radical scavengers vitamins A, C, and E plus magnesium reduce noise trauma,” Free Radic. Biol. Med. 42, 1454–1463. 10.1016/j.freeradbiomed.2007.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Le Prell, C. G. , and Lobarinas, E. (2015). “ Strategies for assessing antioxidant efficacy in clinical trials,” in Oxidative Stress in Applied Basic Research and Clinical Practice: Free Radicals in ENT Pathology, edited by Miller J. M., Le Prell C. G., and Rybak L. P. ( Humana, New York: ), pp. 163–192. [Google Scholar]
- 52. Le Prell, C. G. , and Miller, J. M. (2016). “ The role of oxidative stress in hearing loss,” in Oxidative Stress and Antioxidant Protection: The Science of Free Radical Biology and Disease, edited by Armstrong D. and Stratton R. D. ( Wiley, NJ: ), pp. 115–131. [Google Scholar]
- 53. Le Prell, C. G. , Yamashita, D. , Minami, S. B. , Yamasoba, T. , and Miller, J. M. (2007b). “ Mechanisms of noise-induced hearing loss indicate multiple methods of prevention,” Hear. Res. 226, 22–43. 10.1016/j.heares.2006.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lin, Y. , Kashio, A. , Sakamoto, T. , Suzukawa, K. , Kakigi, A. , and Yamasoba, T. (2011). “ Hydrogen in drinking water attenuates noise-induced hearing loss in guinea pigs,” Neurosci. Lett. 487, 12–16. 10.1016/j.neulet.2010.09.064 [DOI] [PubMed] [Google Scholar]
- 55. Lorito, G. , Giordano, P. , Petruccelli, J. , Martini, A. , and Hatzopoulos, S. (2008). “ Different strategies in treating noiseinduced hearing loss with N-acetylcysteine,” Med. Sci. Monit. 14, Br159–164. [PubMed] [Google Scholar]
- 56. Lorito, G. , Giordano, P. , Prosser, S. , Martini, A. , and Hatzopoulos, S. (2006). “ Noise-induced hearing loss: A study on the pharmacological protection in the Sprague Dawley rat with N-acetyl-cysteine,” Acta Otorhinolaryngol. Ital. 26, 133–139. [PMC free article] [PubMed] [Google Scholar]
- 57. Loukzadeh, Z. , Hakimi, A. , Esmailidehaj, M. , and Mehrparvar, A. H. (2015). “ Effect of ascorbic acid on noise induced hearing loss in rats,” Iran. J. Otorhinolaryngol. 27, 267–272. [PMC free article] [PubMed] [Google Scholar]
- 58. Lu, J. , Li, W. , Du, X. , Ewert, D. L. , West, M. B. , Stewart, C. , Floyd, R. A. , and Kopke, R. D. (2014). “ Antioxidants reduce cellular and functional changes induced by intense noise in the inner ear and cochlear nucleus,” J. Assoc. Res. Otolaryngol. 15, 353–372. 10.1007/s10162-014-0441-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lynch, E. D. , Kil, J. , and Le Prell, C. G. (2016). “ Human clinical studies in noise-induced hearing loss,” in Translational Research in Audiology and the Hearing Sciences, Springer Handbook of Auditory Research, edited by Le Prell C. G., Lobarinas E., Fay R. R., and Popper A. N. ( Springer, New York: ), pp. 105–139. [Google Scholar]
- 60. McFadden, S. L. , Woo, J. M. , Michalak, N. , and Ding, D. (2005). “ Dietary vitamin C supplementation reduces noise-induced hearing loss in guinea pigs,” Hear. Res. 202, 200–208. 10.1016/j.heares.2004.10.011 [DOI] [PubMed] [Google Scholar]
- 61. Minami, S. B. , Yamashita, D. , Ogawa, K. , Schacht, J. , and Miller, J. M. (2007). “ Creatine and tempol attenuate noise-induced hearing loss,” Brain Res. 1148, 83–89. 10.1016/j.brainres.2007.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mohammadkhani, G. , Pourbakht, A. , Khanavi, M. , and Faghihzadeh, S. (2013). “ Protective effect of silymarin on noise-induced hearing loss in guinea pigs,” Iran. Red Crescent Med. J. 15, e8890. 10.5812/ircmj.8890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Myint, A. , White, C. H. , Ohmen, J. D. , Li, X. , Wang, J. , Lavinsky, J. , Salehi, P. , Crow, A. L. , Ohyama, T. , and Friedman, R. A. (2016). “ Large-scale phenotyping of noise-induced hearing loss in 100 strains of mice,” Hear. Res. 332, 113–120. 10.1016/j.heares.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nagashima, R. , Yamaguchi, T. , Tanaka, H. , and Ogita, K. (2010). “ Mechanism underlying the protective effect of tempol and Nomega-nitro-L-arginine methyl ester on acoustic injury: Possible involvement of c-Jun N-terminal kinase pathway and connexin26 in the cochlear spiral ligament,” J. Pharmacol. Sci. 114, 50–62. 10.1254/jphs.10113FP [DOI] [PubMed] [Google Scholar]
- 65. Neitzel, R. L. , and Fligor, B. J. (2019). “ Risk of noise-induced hearing loss due to recreational sound: Review and recommendations,” J. Acoust. Soc. Am. 146, 3911–3921. 10.1121/1.5132287 [DOI] [PubMed] [Google Scholar]
- 66. Ogurlu, M. , Celebi Erdivanli, O. , Tumkaya, L. , Ozgur, A. , Ozergin Coskun, Z. , Terzi, S. , Demirci, M. , and Dursun, E. (2017). “ The therapeutic effect of thymoquinone on acoustic trauma-induced hearing loss in rats,” Eur. Arch. Otorhinolaryngol. 274, 743–749. 10.1007/s00405-016-4319-4 [DOI] [PubMed] [Google Scholar]
- 67. Ohinata, Y. , Miller, J. M. , and Schacht, J. (2003). “ Protection from noise-induced lipid peroxidation and hair cell loss in the cochlea,” Brain Res. 966, 265–273. 10.1016/S0006-8993(02)04205-1 [DOI] [PubMed] [Google Scholar]
- 68. Ohinata, Y. , Yamasoba, T. , Schacht, J. , and Miller, J. M. (2000). “ Glutathione limits noise-induced hearing loss,” Hear. Res. 146, 28–34. 10.1016/S0378-5955(00)00096-4 [DOI] [PubMed] [Google Scholar]
- 69. Ohlemiller, K. K. (2019). “ Mouse methods and models for studies in hearing,” J. Acoust. Soc. Am. 146, 3668–3680. 10.1121/1.5132550 [DOI] [PubMed] [Google Scholar]
- 70. Peppi, M. , Kujawa, S. G. , and Sewell, W. F. (2011). “ A corticosteroid-responsive transcription factor, promyelocytic leukemia zinc finger protein, mediates protection of the cochlea from acoustic trauma,” J. Neurosci. 31, 735–741. 10.1523/JNEUROSCI.3955-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pirvola, U. , Xing-Qun, L. , Virkkala, J. , Saarma, M. , Murakata, C. , Camoratto, A. M. , Walton, K. M. , and Ylikoski, J. (2000). “ Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibitor of c-Jun N-terminal kinase activation,” J. Neurosci. 20, 43–50. 10.1523/JNEUROSCI.20-01-00043.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Poirrier, A. L. , Pincemail, J. , Van Den Ackerveken, P. , Lefebvre, P. P. , and Malgrange, B. (2010). “ Oxidative stress in the cochlea: An update,” Curr. Med. Chem. 17, 3591–3604. 10.2174/092986710792927895 [DOI] [PubMed] [Google Scholar]
- 73. Pourbakht, A. (2011). “ Effect of N-acetylcysteine in protecting from simultaneous noise and carbon monoxide induced hair cell loss,” Audiology 20, 107–107. [Google Scholar]
- 74. Pourbakht, A. (2013). “ The effect of celecoxib, a cyclooxygenase-2 inhibitor on noise- induced hearing loss,” Iran. J. Basic Med. Sci. 16, 726–730. [PMC free article] [PubMed] [Google Scholar]
- 75. Pourbakht, A. , and Yamasoba, T. (2003). “ Ebselen attenuates cochlear damage caused by acoustic trauma,” Hear. Res. 181, 100–108. 10.1016/S0378-5955(03)00178-3 [DOI] [PubMed] [Google Scholar]
- 76. Pouyatos, B. , Gearhart, C. , Nelson-Miller, A. , Fulton, S. , and Fechter, L. (2007). “ Oxidative stress pathways in the potentiation of noise-induced hearing loss by acrylonitrile,” Hear. Res. 224, 61–74. 10.1016/j.heares.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 77. Qu, J. , Liao, Y. H. , Kou, Z. Z. , Wei, Y. Y. , Huang, J. , Chen, J. , Yanagawa, Y. , Wu, S. X. , Shi, M. , and Li, Y. Q. (2015). “ Puerarin alleviates noise-induced hearing loss via affecting PKCγ and GABAB receptor expression,” J. Neurol. Sci. 349, 110–116. 10.1016/j.jns.2014.12.038 [DOI] [PubMed] [Google Scholar]
- 78. Radziwon, K. , Sheppard, A. , and Salvi, R. J. (2019). “ Psychophysical changes in temporal processing in chinchillas with noise-induced hearing loss,” J. Acoust. Soc. Am. 146, 3733–3742. 10.1121/1.5132292 [DOI] [PubMed] [Google Scholar]
- 79. Rao, D. , and Fechter, L. D. (2000). “ Protective effects of phenyl-N-tert-butylnitrone on the potentiation of noise-induced hearing loss by carbon monoxide,” Toxicol. Appl. Pharmacol. 167, 125–131. 10.1006/taap.2000.8995 [DOI] [PubMed] [Google Scholar]
- 80. Rewerska, A. , Pawelczyk, M. , Rajkowska, E. , Politanski, P. , and Sliwinska-Kowalska, M. (2013). “ Evaluating D-methionine dose to attenuate oxidative stress-mediated hearing loss following overexposure to noise,” Eur. Arch. Otorhinolaryngol. 270, 1513–1520. 10.1007/s00405-012-2265-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Roberts, B. , and Neitzel, R. L. (2019). “ Noise exposure limit for children in recreational settings: Review of available evidence,” J. Acoust. Soc. Am. 146, 3922–3933. 10.1121/1.5132540 [DOI] [PubMed] [Google Scholar]
- 82. Ryan, A. F. , Kujawa, S. G. , Hammill, T. , Le Prell, C. , and Kil, J. (2016). “ Temporary and permanent noise-induced threshold shifts: A review of basic and clinical observations,” Otol. Neurotol. 37, e271–e275. 10.1097/MAO.0000000000001071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Samson, J. , Wiktorek-Smagur, A. , Politanski, P. , Rajkowska, E. , Pawlaczyk-Luszczynska, M. , Dudarewicz, A. , Sha, S. H. , Schacht, J. , and Sliwinska-Kowalska, M. (2008). “ Noise-induced time-dependent changes in oxidative stress in the mouse cochlea and attenuation by D-methionine,” Neuroscience 152, 146–150. 10.1016/j.neuroscience.2007.11.015 [DOI] [PubMed] [Google Scholar]
- 84. Stebbins, W. C. , Moody, D. B. , and Serafin, J. V. (1982). “ Some principal issues in the analysis of noise effects on hearing in experimental animals,” Am. J. Otolaryngol. 3, 295–304. 10.1016/S0196-0709(82)80069-0 [DOI] [PubMed] [Google Scholar]
- 85. Takeda, H. , Kurioka, T. , Kaitsuka, T. , Tomizawa, K. , Matsunobu, T. , Hakim, F. , Mizutari, K. , Miwa, T. , Yamada, T. , Ise, M. , Shiotani, A. , Yumoto, E. , and Minoda, R. (2016). “ Protein transduction therapy into cochleae via the round window niche in guinea pigs,” Mol. Ther. Methods Clin. Dev. 3, 16055. 10.1038/mtm.2016.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Takemura, K. , Komeda, M. , Yagi, M. , Himeno, C. , Izumikawa, M. , Doi, T. , Kuriyama, H. , Miller, J. M. , and Yamashita, T. (2004). “ Direct inner ear infusion of dexamethasone attenuates noise-induced trauma in guinea pig,” Hear. Res. 196, 58–68. 10.1016/j.heares.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 87. Themann, C. L. , and Masterson, E. A. (2019). “ Review: Occupational noise exposure and hearing loss,” J. Acoust. Soc. Am. 146, 3879–3905. 10.1121/1.5134465 [DOI] [PubMed] [Google Scholar]
- 88. Trevino, M. , Lobarinas, E. , Maulden, A. C. , and Heinz, M. G. (2019). “ The chinchilla animal model for hearing science and noise-induced hearing loss,” J. Acoust. Soc. Am. 146, 3710–3732. 10.1121/1.5132950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wall, A. T. , Wagner, C. M. , Rasband, R. D. , Gee, K. L. , and Murphy, W. J. (2019). “ Cumulative noise exposure model for outdoor shooting ranges,” J. Acoust. Soc. Am. 146, 3863–3867. 10.1121/1.5132289 [DOI] [PubMed] [Google Scholar]
- 90. Wang, J. , Ding, D. , Shulman, A. , Stracher, A. , and Salvi, R. J. (1999). “ Leupeptin protects sensory hair cells from acoustic trauma,” Neuroreport 10, 811–816. 10.1097/00001756-199903170-00027 [DOI] [PubMed] [Google Scholar]
- 91. Wang, Y. , Hirose, K. , and Liberman, M. C. (2002). “ Dynamics of noise-induced cellular injury and repair in the mouse cochlea,” J. Assoc. Res. Otolaryngol. 3, 248–268. 10.1007/s101620020028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wartinger, F. , Malyuk, H. , and Portnuff, C. D. (2019). “ Human exposures and their associated hearing loss profiles: Music industry professionals,” J. Acoust. Soc. Am. 146, 3906–3910. 10.1121/1.5132541 [DOI] [PubMed] [Google Scholar]
- 93. Wen, J. , Duan, N. , Wang, Q. , Jing, G. X. , and Xiao, Y. (2017). “ Protective effect of propofol on noise-induced hearing loss,” Brain Res. 1657, 95–100. 10.1016/j.brainres.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 94. Yamaguchi, T. , Nagashima, R. , Yoneyama, M. , Shiba, T. , and Ogita, K. (2014). “ Disruption of ion-trafficking system in the cochlear spiral ligament prior to permanent hearing loss induced by exposure to intense noise: Possible involvement of 4-hydroxy-2-nonenal as a mediator of oxidative stress,” PloS One 9, e102133. 10.1371/journal.pone.0102133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yamashita, D. , Jiang, H. Y. , Le Prell, C. G. , Schacht, J. , and Miller, J. M. (2005). “ Post-exposure treatment attenuates noise-induced hearing loss,” Neuroscience 134, 633–642. 10.1016/j.neuroscience.2005.04.015 [DOI] [PubMed] [Google Scholar]
- 96. Yamashita, D. , Shiotani, A. , Kanzaki, S. , Nakagawa, M. , and Ogawa, K. (2008). “ Neuroprotective effects of T-817MA against noise-induced hearing loss,” Neurosci. Res. 61, 38–42. 10.1016/j.neures.2008.01.009 [DOI] [PubMed] [Google Scholar]
- 97. Yamasoba, T. , Pourbakht, A. , Sakamoto, T. , and Suzuki, M. (2005). “ Ebselen prevents noise-induced excitotoxicity and temporary threshold shift,” Neurosci. Lett. 380, 234–238. 10.1016/j.neulet.2005.01.047 [DOI] [PubMed] [Google Scholar]
- 98. Yamasoba, T. , Schacht, J. , Shoji, F. , and Miller, J. M. (1999). “ Attenuation of cochlear damage from noise trauma by an iron chelator, a free radical scavenger and glial cell line-derived neurotrophic factor in vivo,” Brain Res. 815, 317–325. 10.1016/S0006-8993(98)01100-7 [DOI] [PubMed] [Google Scholar]
- 99. Zheng, Q. Y. , Johnson, K. R. , and Erway, L. C. (1999). “ Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses,” Hear. Res. 130, 94–107. 10.1016/S0378-5955(99)00003-9 [DOI] [PMC free article] [PubMed] [Google Scholar]